-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
Background:
Human immunodeficiency virus (HIV) infection is the strongest risk factor for developing tuberculosis and has fuelled its resurgence, especially in sub-Saharan Africa. In 2010, there were an estimated 1.1 million incident cases of tuberculosis among the 34 million people living with HIV worldwide. Antiretroviral therapy has substantial potential to prevent HIV-associated tuberculosis. We conducted a systematic review of studies that analysed the impact of antiretroviral therapy on the incidence of tuberculosis in adults with HIV infection.Methods and Findings:
PubMed, Embase, African Index Medicus, LILACS, and clinical trial registries were systematically searched. Randomised controlled trials, prospective cohort studies, and retrospective cohort studies were included if they compared tuberculosis incidence by antiretroviral therapy status in HIV-infected adults for a median of over 6 mo in developing countries. For the meta-analyses there were four categories based on CD4 counts at antiretroviral therapy initiation: (1) less than 200 cells/µl, (2) 200 to 350 cells/µl, (3) greater than 350 cells/µl, and (4) any CD4 count.
: Eleven studies met the inclusion criteria. Antiretroviral therapy is strongly associated with a reduction in the incidence of tuberculosis in all baseline CD4 count categories: (1) less than 200 cells/µl (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.07 to 0.36), (2) 200 to 350 cells/µl (HR 0.34, 95% CI 0.19 to 0.60), (3) greater than 350 cells/µl (HR 0.43, 95% CI 0.30 to 0.63), and (4) any CD4 count (HR 0.35, 95% CI 0.28 to 0.44). There was no evidence of hazard ratio modification with respect to baseline CD4 count category (p = 0.20).Conclusions:
Antiretroviral therapy is strongly associated with a reduction in the incidence of tuberculosis across all CD4 count strata. Earlier initiation of antiretroviral therapy may be a key component of global and national strategies to control the HIV-associated tuberculosis syndemic.Review Registration:
International Prospective Register of Systematic Reviews CRD42011001209
Please see later in the article for the Editors' Summary.
Published in the journal: Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis. PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001270
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001270Summary
Background:
Human immunodeficiency virus (HIV) infection is the strongest risk factor for developing tuberculosis and has fuelled its resurgence, especially in sub-Saharan Africa. In 2010, there were an estimated 1.1 million incident cases of tuberculosis among the 34 million people living with HIV worldwide. Antiretroviral therapy has substantial potential to prevent HIV-associated tuberculosis. We conducted a systematic review of studies that analysed the impact of antiretroviral therapy on the incidence of tuberculosis in adults with HIV infection.Methods and Findings:
PubMed, Embase, African Index Medicus, LILACS, and clinical trial registries were systematically searched. Randomised controlled trials, prospective cohort studies, and retrospective cohort studies were included if they compared tuberculosis incidence by antiretroviral therapy status in HIV-infected adults for a median of over 6 mo in developing countries. For the meta-analyses there were four categories based on CD4 counts at antiretroviral therapy initiation: (1) less than 200 cells/µl, (2) 200 to 350 cells/µl, (3) greater than 350 cells/µl, and (4) any CD4 count.
: Eleven studies met the inclusion criteria. Antiretroviral therapy is strongly associated with a reduction in the incidence of tuberculosis in all baseline CD4 count categories: (1) less than 200 cells/µl (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.07 to 0.36), (2) 200 to 350 cells/µl (HR 0.34, 95% CI 0.19 to 0.60), (3) greater than 350 cells/µl (HR 0.43, 95% CI 0.30 to 0.63), and (4) any CD4 count (HR 0.35, 95% CI 0.28 to 0.44). There was no evidence of hazard ratio modification with respect to baseline CD4 count category (p = 0.20).Conclusions:
Antiretroviral therapy is strongly associated with a reduction in the incidence of tuberculosis across all CD4 count strata. Earlier initiation of antiretroviral therapy may be a key component of global and national strategies to control the HIV-associated tuberculosis syndemic.Review Registration:
International Prospective Register of Systematic Reviews CRD42011001209
Please see later in the article for the Editors' Summary.Introduction
Tuberculosis and human immunodeficiency virus (HIV) are major threats to global public health. HIV infection is the strongest risk factor for tuberculosis and has fuelled its resurgence [1]. In 2010 there were an estimated 1.1 million incident cases of tuberculosis among the 34 million people living with HIV worldwide; 900,000 of these cases were among the 22.9 million Africans living with HIV [2],[3]. The 350,000 deaths among incident HIV-positive tuberculosis cases comprised 19% of all HIV-related deaths [2] and 24% of all tuberculosis deaths globally [3].
As part of the Millennium Development Goals, all 192 United Nations member states agreed to halt and decrease the annual mortality, incidence, and prevalence of tuberculosis and to increase the proportion of tuberculosis cases detected and cured under the DOTS strategy by 2015 [4]. The World Health Organization (WHO) and the Stop TB Partnership have endorsed the Millennium Development Goal targets and also aim to reduce the global annual incidence of active tuberculosis to less than one case per million population by 2050 [5]. While latest estimates indicate that the world is on track to achieve the Millennium Development Goal targets [3], achieving elimination will require a shift in strategy [6]–[8].
The DOTS strategy was largely developed in the pre-HIV era, and its implementation between 1995 and 2010 helped successfully treat 46 million people with tuberculosis and save 6.8 million lives [3]. While the DOTS strategy is essential for people with and without HIV, it is unlikely to reduce the incidence and prevalence of tuberculosis in countries where HIV is highly prevalent [9]. Given the importance of HIV as a driver of the tuberculosis epidemic in many regions, especially in Africa, where approximately 40% of incident tuberculosis cases in 2010 were associated with HIV [3], WHO recommends a range of collaborative activities through which HIV and tuberculosis programmes can address HIV-associated tuberculosis [10]. These include the Three I's for HIV/TB: intensified tuberculosis case-finding [11], isoniazid preventive therapy [11], and tuberculosis infection control [12]. Unfortunately only 178,144 people, a small fraction of the millions eligible, received isoniazid preventive therapy in 2010 [3]. The barriers contributing to this low coverage of isoniazid preventive therapy are complex and underscore the need for complementary interventions to prevent tuberculosis in adults with HIV [1],[11],[13].
In 2009, WHO recommended antiretroviral therapy for all adults with CD4 counts less than 350 cells/µl and for all tuberculosis patients irrespective of CD4 count [14]. In more recent years, accumulating evidence has pointed towards the potential of antiretroviral therapy scale-up to further contribute to control of the HIV-associated tuberculosis syndemic [15],[16],[17]. However, the evidence regarding antiretroviral therapy's preventive impact on tuberculosis has not undergone formal systematic review or synthesis. The objective of this study was to systematically review the effect of antiretroviral therapy on incident tuberculosis in developing countries across a range of CD4 cell count strata.
Methods
Conduct of Systematic Review
This systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) statement (Text S1) [18]. The investigators wrote a protocol and registered it with the International Prospective Register of Systematic Reviews (identification number: CRD42011001209) in March 2011 [19]. PubMed and Embase were systematically searched without language, publication, or date restrictions in August 2011, while African Index Medicus and LILACS (Latin American and Caribbean Health Science Literature Database) were systematically searched without language, publication, or date restrictions in February 2012.
Search Strategy, Selection Criteria, and Data Extraction
The search strategies (Table S1) were designed with a librarian to identify studies reporting on the effect of antiretroviral therapy in preventing HIV-associated tuberculosis. Per recommendations from the PRISMA Group, eligibility criteria were based on key study characteristics: population, intervention, comparator, outcome, design, and length of follow-up [18]. Specifically, studies were included when (1) the study population was composed of adults (≥13 y) with HIV; (2) the intervention was antiretroviral therapy (defined as three or more antiretroviral drugs used in combination); (3) the comparator was no antiretroviral drugs; (4) the outcome was an incident case of tuberculosis; (5) the study design was a randomised trial, prospective cohort study, or retrospective cohort study; and (6) participants were followed for more than 6 mo (since viral suppression, immune recovery, and associated tuberculosis risk reduction is a time-dependent process [20]–[24] and tuberculosis rates during early antiretroviral therapy depend highly upon the intensity of screening for prevalent tuberculosis prior to antiretroviral therapy initiation [25]). The WHO International Clinical Trials Registry Platform, the Cochrane Central Register of Controlled Trials, the International Standard Randomised Controlled Trial Number Register, and ClinicalTrials.gov were searched for future and ongoing studies using the terms “antiretroviral” and “tuberculosis”. Experts in the field were also contacted to identify unpublished research or ongoing studies.
Tuberculosis transmission is complex and is influenced by biological, social, and economic factors [26]. Data from 134 countries indicate that development, measured by the Human Development Index, correlates with national tuberculosis incidence [27]. The Human Development Index is a composite national score of health (life expectancy at birth), education (expected years of schooling), and living standards (per capita gross national income) [28]. The Human Development Index categorises developed countries as those scoring in the top quartile and developing countries as those scoring below the top quartile [28]. Since developed countries collectively contributed less than 0.5% of all HIV-positive tuberculosis cases globally (Table S2), the scope of this systematic review was limited to developing countries to maximise the generalisability of the meta-analyses to countries facing the highest burden of HIV-associated tuberculosis.
Two of the investigators, A. B. S. and D. S., independently screened abstracts of all retrieved articles from PubMed and Embase and then matched the full texts of all articles selected during screening against the inclusion criteria. A. B. S. and R. M. G. conducted this same process for the African Index Medicus and LILACS databases. Disagreements on which articles met the inclusion criteria were resolved by discussion. Articles meeting inclusion criteria were included in the review (Figure 1). A. B. S. and J. d. A. completed the data extraction using a standardised spreadsheet that collected information on the first author, year of publication, methods and design, study population, intervention and control, duration of follow-up, inclusion and exclusion criteria, outcomes, and losses to follow-up.
Fig. 1. Flow of information through different phases of the review. 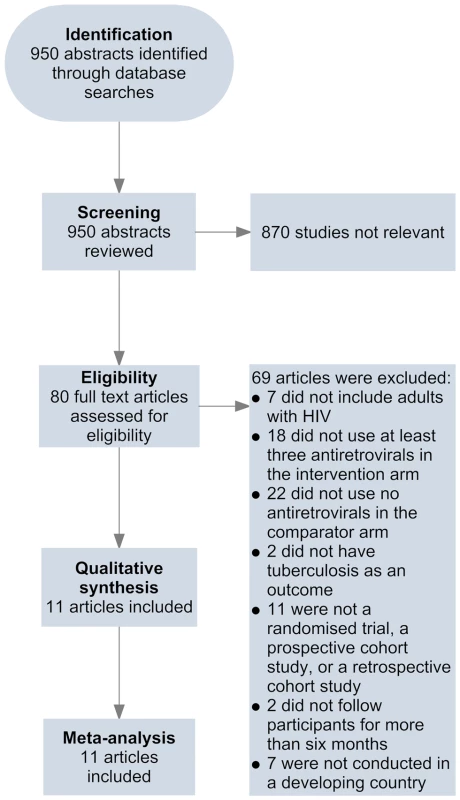
Quality Assessment
Per recommendations from the Cochrane Collaboration [29], the Newcastle-Ottawa quality assessment scale was used to assess bias in studies included in this review [30]. This scale rates studies on three sources of bias based on eight criteria. Each criterion is worth one point except confounding, which is worth two points. Selection bias was assessed using four criteria: (1) representativeness of the cohort on antiretroviral therapy to the average adult on antiretroviral therapy in the community from which study participants were drawn, (2) representativeness of the cohort off antiretroviral therapy to the cohort on antiretroviral therapy, (3) ascertainment of antiretroviral therapy use, and (4) demonstration that prevalent tuberculosis was not present at the start of follow-up. To judge whether appropriate methods were used to address confounding, adjustment for baseline CD4 count was used for studies not reporting analyses in CD4 strata. Since a low body mass index is a key risk factor for developing tuberculosis in adults, irrespective of HIV status [8],[21],[31]–[34], adjustment for body mass index was used to judge whether appropriate methods were used to address confounding for analyses within CD4 strata. Measurement bias was assessed with three criteria: (1) microbiological (i.e., culture or acid-fast bacilli smear) confirmation of tuberculosis cases, (2) adequate follow-up to detect antiretroviral therapy's long-term preventive effect on tuberculosis (i.e., median follow-up of at least 1 y [20]–[24]), and (3) ≤30% of participants lost to follow-up during the study. Based on these criteria, studies were scored out of 100%. For this systematic review, studies scoring ≥67% were arbitrarily considered high methodological quality, those scoring 34%–66% were arbitrarily considered moderate methodological quality, and those ≤33% were arbitrarily considered low methodological quality.
Per recommendations from the Cochrane Collaboration [29], the Collaboration's Risk of Bias tool was used to assess bias in randomised trials meeting eligibility criteria. This tool rates studies on four sources of bias based on six criteria: (1) adequate sequence generation to gauge selection bias; (2) allocation concealment to gauge selection bias; (3) blinding of participants, personnel, and outcome assessors to gauge performance and detection bias; (4) incomplete outcome data to gauge attrition bias; (5) selective reporting to gauge reporting bias; and (6) a criterion for other forms of bias. Based on these criteria, trials were scored out of 100%.
Statistical Analyses
Past WHO guidelines have used a CD4 threshold of 200 cells/µl [35] and 350 cells/µl [14] for initiation of antiretroviral therapy in asymptomatic adults. Given that there is considerable heterogeneity among different populations regarding CD4 counts directly after seroconversion and the subsequent rate of CD4 decline, the need for multiple strata above 350 cells/µl is population-specific [36]–[38]. Therefore, four categories based on CD4 at antiretroviral therapy initiation were used for the analytical component of this review: less than 200 cells/µl, 200 to 350 cells/µl, greater than 350 cells/µl, and any CD4 count. A funnel plot with the effect measures on the x-axis and standard error of the log for the effect measures on the y-axis was created to assess publication bias, and the Egger and Begg tests were used to test the funnel plot's symmetry. Since studies were similar enough to combine, meta-analyses were performed and statistical heterogeneity was assessed. Effect measures were entered as the natural log of the effect measure, and standard error as the natural log of (95% upper limit÷95% lower limit)÷3.92 [39]. Fixed-effects models assume that the magnitude and direction of an intervention's effect is identical across studies and that observed differences among study results are due solely to chance [29]. Random-effects models assume that the magnitude and direction of an intervention's effect is not identical across studies but follows a distribution [29]. Since it is possible that the magnitude and direction of antiretroviral therapy's preventive impact on tuberculosis could differ for reasons other than chance, random-effects models were used for all meta-analyses. χ and τ statistics require the number of events in each study arm to assess heterogeneity in the magnitude of effect across studies. Since these data were not available for all studies meeting inclusion criteria, I2 statistics were used to measure heterogeneity [40]. I2 values near 25% indicate low heterogeneity, values near 50% indicate moderate heterogeneity, and those above 75% indicate high heterogeneity [41]. The χ2 test, against the null hypothesis that there is no difference in the hazard ratio (HR) with respect to baseline CD4 count category, was used to test for hazard ratio modification. STATA version 10.0 was used for all analyses.
Results
Search Results
Eleven studies met the inclusion criteria for this systematic review (Tables 1 and 2) [42]–[52]. Four of these studies were from sub-Saharan Africa [42],[44],[46],[49], four were from South America [45],[47],[48],[50], one was from the Caribbean [51], one was from Asia [52], and one was from a combination of regions in sub-Saharan Africa, South America, and Asia [43]. Two studies reported effect estimates for baseline CD4 counts less than 200 cells/µl [42],[47], four studies reported effect estimates for baseline CD4 counts from 200 to 350 cells/µl [42],[45],[47],[51], and three studies reported effect estimates for baseline CD4 counts greater than 350 cells/µl [42],[43],[45] (Figure 2). One ongoing randomised study was identified in the Cochrane Central Register of Controlled Trials [53], three additional ongoing trials were identified in ClinicalTrials.gov [54]–[56], while no additional studies were found in the International Standard Randomised Controlled Trial Number Register or the WHO International Clinical Trials Registry Platform. Results on antiretroviral therapy's preventive impact on tuberculosis are not yet available from the ongoing trials [53]–[56].
Fig. 2. Antiretroviral therapy use and hazard of tuberculosis by baseline CD4 count. 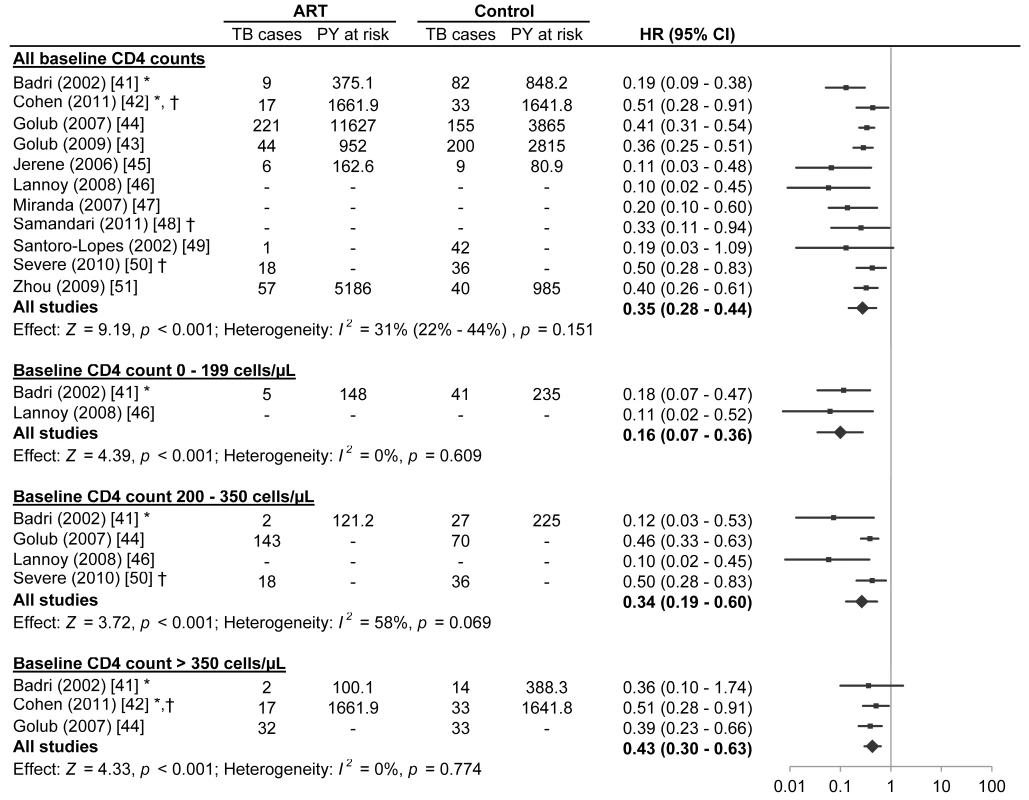
The centres of the squares represent study estimates, the centres of the quadrilaterals represent summary estimates, and the horizontal lines represent 95% confidence intervals. PY, person-years; –, data not reported; *, study effect measure is an incidence rate ratio; †, data are from a randomised controlled trial. Tab. 1. Characteristics of participants in studies meeting inclusion criteria. 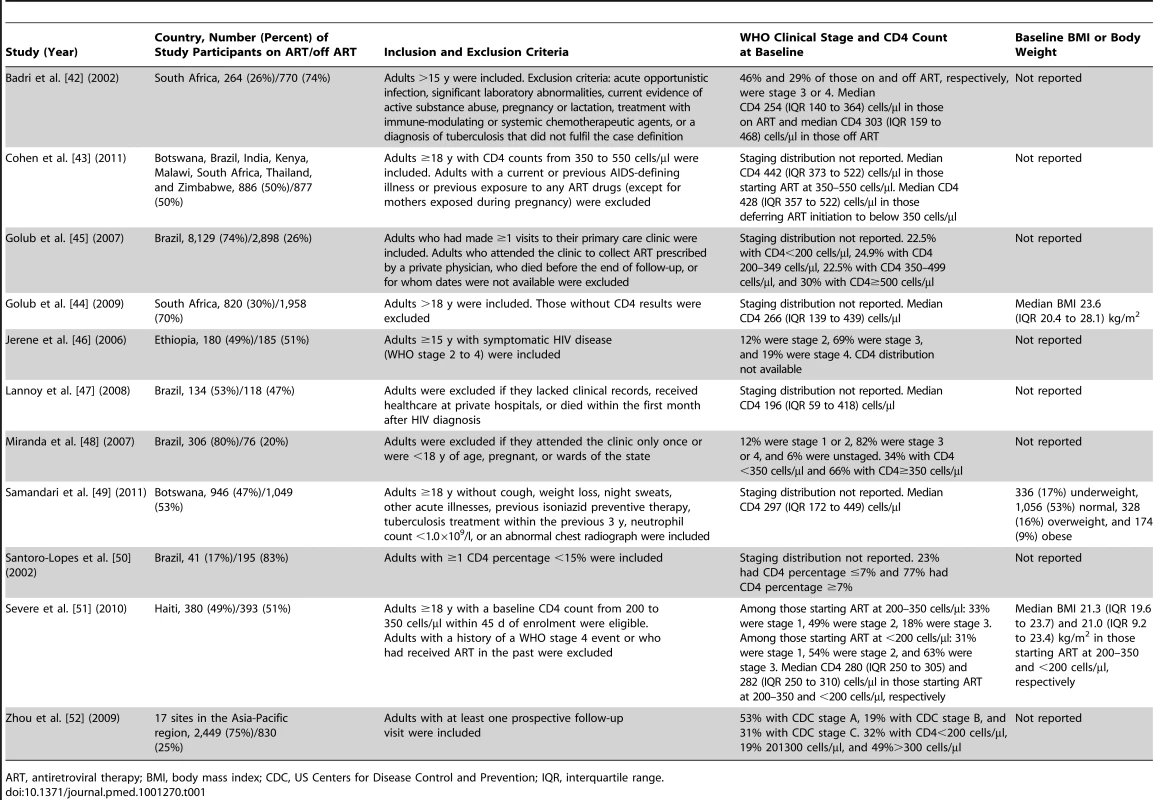
ART, antiretroviral therapy; BMI, body mass index; CDC, US Centers for Disease Control and Prevention; IQR, interquartile range. Tab. 2. Methods of studies meeting inclusion criteria. 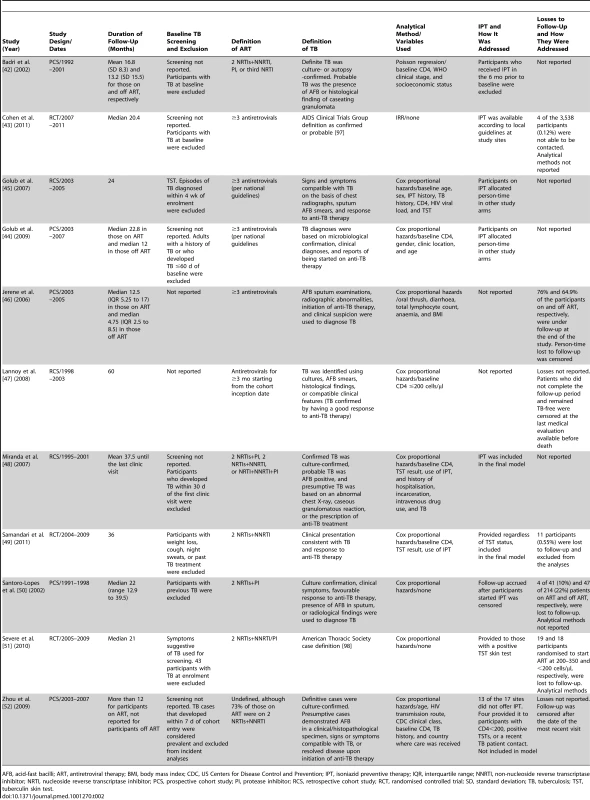
AFB, acid-fast bacilli; ART, antiretroviral therapy; BMI, body mass index; CDC, US Centers for Disease Control and Prevention; IPT, isoniazid preventive therapy; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PCS, prospective cohort study; PI, protease inhibitor; RCS, retrospective cohort study; RCT, randomised controlled trial; SD, standard deviation; TB, tuberculosis; TST, tuberculin skin test. Quality Assessment
The assessment of bias indicated that four studies were of high methodological quality [43],[48],[49],[51], five studies were of moderate methodological quality [42],[44],[45],[47],[50], and two studies were of low methodological quality [46],[52] (Table 3). There appeared to be limited bias in the three randomised controlled trials identified [43],[49],[51] (Table 4).
Tab. 3. Newcastle-Ottawa quality assessment scale for studies meeting inclusion criteria. 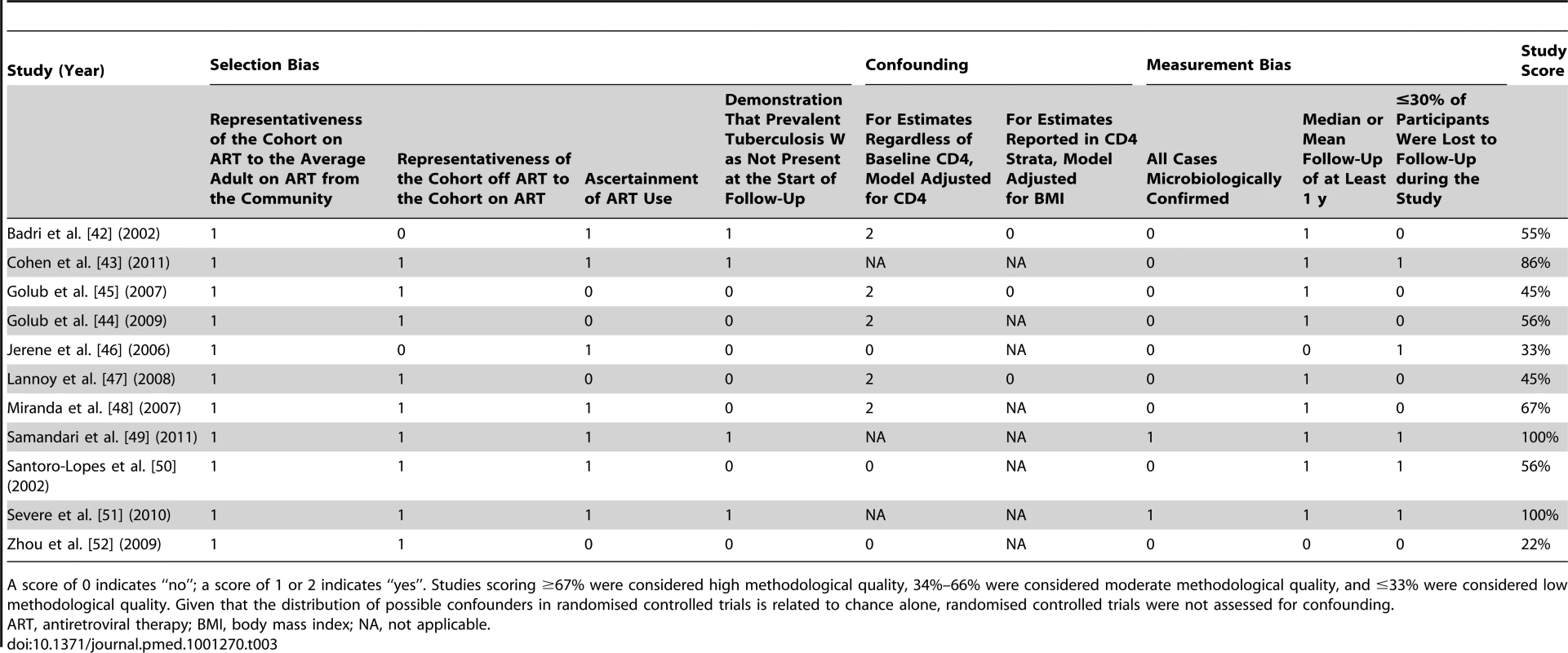
A score of 0 indicates “no”; a score of 1 or 2 indicates “yes”. Studies scoring ≥67% were considered high methodological quality, 34%–66% were considered moderate methodological quality, and ≤33% were considered low methodological quality. Given that the distribution of possible confounders in randomised controlled trials is related to chance alone, randomised controlled trials were not assessed for confounding. Tab. 4. Bias assessment for randomised controlled trials meeting inclusion criteria. 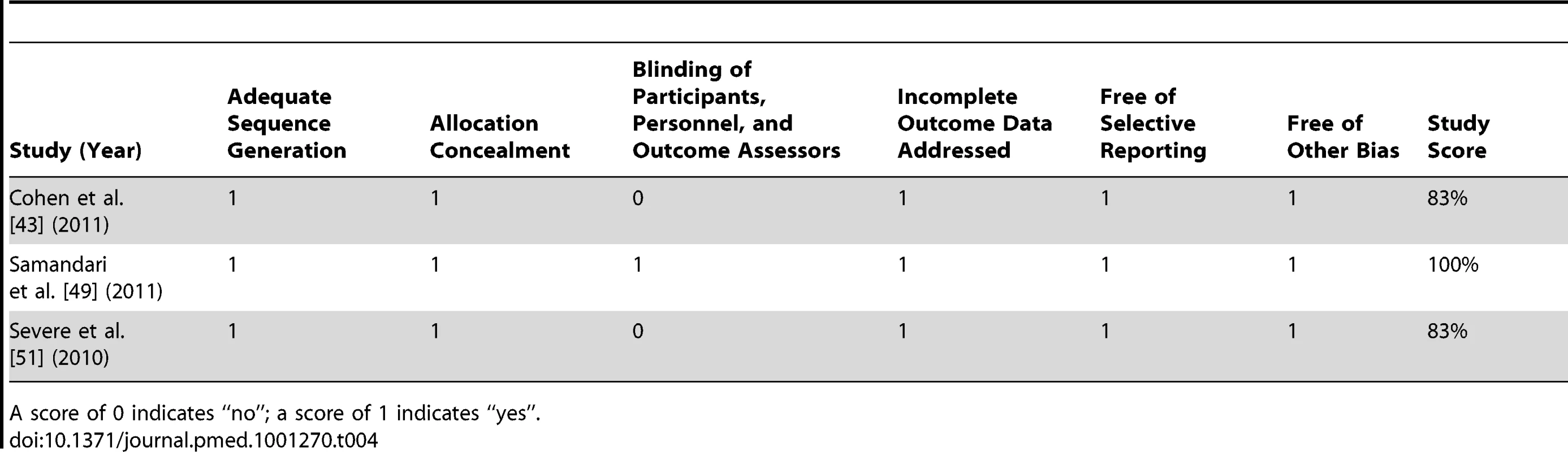
A score of 0 indicates “no”; a score of 1 indicates “yes”. Meta-Analyses
A meta-analysis of all eleven studies meeting inclusion criteria found that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence across all baseline CD4 counts (HR 0.35, 95% confidence interval [CI] 0.28 to 0.44; p-value for effect <0.001; p-value for heterogeneity = 0.151). Inspection of the funnel plot (Figure S1) suggested possible publication bias (Begg test p = 0.12; Egger test p = 0.02).
Two studies reported on participants with baseline CD4 counts less than 200 cells/µl. Badri et al. [42] (adjusted incidence rate ratio [IRR] 0.18, 95% CI 0.07 to 0.47) and Lannoy et al. [47] (IRR 0.11, 95% CI 0.02 to 0.52; Text S2) reported that antiretroviral therapy was associated with a reduction in tuberculosis incidence. A meta-analysis of these two studies found that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence in adults with baseline CD4 counts less than 200 cells/µl (HR 0.16, 95% CI 0.07 to 0.36; p-value for effect <0.001; p-value for heterogeneity = 0.609).
Four studies reported on participants with baseline CD4 counts from 200 to 350 cells/µl. Badri et al. [42] (adjusted IRR 0.12, 95% CI 0.03 to 0.53), Lannoy et al. [47] (adjusted HR 0.10, 95% CI 0.02 to 0.45), Golub et al. [45] (adjusted HR 0.46, 95% CI 0.33 to 0.63), and Severe et al. [51] (HR 0.50, 95% CI 0.29 to 0.83) reported that antiretroviral therapy was associated with a reduction in tuberculosis incidence. The meta-analysis of these four studies found that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence in adults with baseline CD4 counts from 200 to 350 cells/µl (HR 0.34, 95% CI 0.19 to 0.60; p-value for effect <0.001; p-value for heterogeneity = 0.069).
Three studies reported on participants with baseline CD4 counts above 350 cells/µl. Cohen et al. [43] (IRR 0.51, 95% CI 0.28 to 0.91; Text S2), Badri et al. [42] (adjusted IRR 0.36, 95% CI 0.10 to 1.74), and Golub et al. [45] (adjusted HR 0.39, 95% CI 0.23 to 0.66) reported that antiretroviral therapy was associated with a reduction in tuberculosis incidence, although Badri's estimate lacked statistical significance. The meta-analysis of these three studies indicated that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence in adults with CD4 counts above 350 cells/µl (HR 0.43, 95% CI 0.30 to 0.63; p-value for effect <0.001; p-value for heterogeneity = 0.774).
Visual inspection of the hazard ratios and confidence intervals for the three CD4 categories suggested a possible gradient in antiretroviral therapy's effect in relation to baseline CD4 count (Figure 3); however, there was no evidence of hazard ratio modification with respect to baseline CD4 count category using the χ2 test (p = 0.20).
Fig. 3. Antiretroviral therapy use and pooled hazard ratios of tuberculosis by baseline CD4 count. 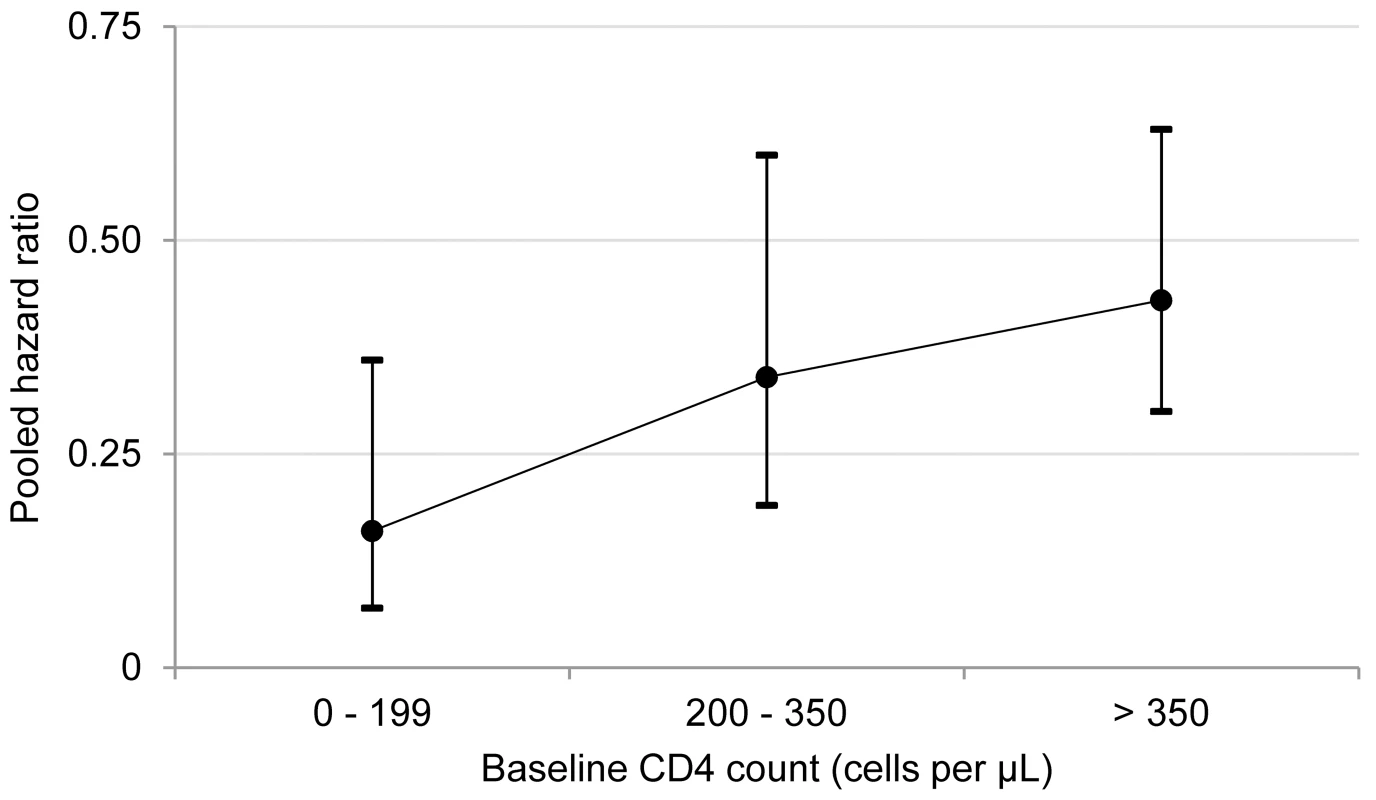
The circles represent pooled estimates, and the vertical lines represent 95% confidence intervals. The p-value for hazard ratio modification by baseline CD4 count category is 0.20. I2 values for the 0–200, 201–350, and greater than 350 cells/µl categories are 0%, 58%, and 0%, respectively. Discussion
This systematic review indicates that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence in adults with CD4 counts (1) less than 200 cells/µl (HR 0.16, 95% CI 0.07 to 0.36), (2) from 200 to 350 cells/µl (HR 0.34, 95% CI 0.19 to 0.60), (3) greater than 350 cells/µl (HR 0.43, 95% CI 0.30 to 0.63), and (4) at any level (HR 0.35, 95% CI 0.28 to 0.45). This study was a rigorous systematic literature review that focused exclusively on studies from developing countries and included very recent studies that provided data on adults with high baseline CD4 cell counts. These factors enabled what is, to our knowledge, the first ever estimate of antiretroviral therapy impact stratified by baseline CD4 category. The finding that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence across all CD4 counts is consistent with an earlier meta-analysis that included studies from developed and developing countries [13]. That meta-analysis, the meta-analyses reported here, and a previous comparative analysis of data from developed and developing countries [57] support the conclusion that tuberculosis risk reduction is similar regardless of country.
Nine of the 11 studies meeting the inclusion criteria were of moderate or high methodological quality (Tables 3 and 4). However, there were some methodological limitations that need to be considered when evaluating the strong association between antiretroviral therapy and the reduction of tuberculosis incidence. Since diagnostic capabilities differed by country and study site, some studies did not microbiologically confirm tuberculosis cases, which could induce measurement bias. Moreover, one of the studies found that earlier antiretroviral therapy was associated with a decrease only in extrapulmonary tuberculosis, while the others did not make a distinction between pulmonary and extrapulmonary disease [43]. Stratifying by disease site in future studies may be useful in explaining the contribution of antiretroviral therapy in preventing different types of tuberculosis. Since tuberculosis incidence rates during early antiretroviral therapy depend highly upon the efficiency of tuberculosis screening prior to antiretroviral therapy initiation [25], prevalent cases of tuberculosis are often unmasked soon after antiretroviral therapy initiation [20],[58]. Despite efforts to screen for prevalent tuberculosis at study baseline, it is difficult for investigators to establish definitively whether tuberculosis cases that develop soon after antiretroviral therapy initiation are truly incident cases. This uncertainly could introduce measurement bias into studies with a short period of follow-up. Furthermore, the complexity and expense of conducting randomised controlled trials means that most of our data were derived from observational studies. Although our analyses included data from randomised controlled trials, the potential for unmeasured confounding in prospective and retrospective cohort studies makes attempts to reliably establish causal effect more difficult. For example, in some studies there is potential for unmeasured confounding due to isoniazid preventive therapy (Table 2). Nonetheless, our finding that there is no hazard ratio modification with respect to baseline CD4 count is consistent with the randomised controlled trials, in which the reduction in tuberculosis incidence when initiating antiretroviral therapy at 200 to 350 cells/µl (HR 0.50, 95% CI 0.28 to 0.83) [51] was nearly identical to the reduction in tuberculosis incidence when initiating antiretroviral therapy above 350 cells/µl (IRR 0.51, 95% CI 0.28 to 0.91) [43]. These randomised controlled trial stratum estimates were also very similar to the 63% and 57% reductions obtained in the meta-analyses for the categories 200–350 cells/µl and greater than 350 cells/µl, respectively.
The meta-analyses may have limitations in the statistical methodology used. Since laboratory capabilities differed by country and study site, some of the studies did not adjust for baseline CD4 count, body mass index, smoking, and/or diabetes, which could confound results. Both incidence rate ratios and hazard ratios calculate events over person-time at risk; however, they rely on different methodologies depending on the nature of the data that are collected [59]. Given similarities in study methods (Table 2), the meta-analyses in this systematic review combined hazard ratios and incidence rate ratios from randomised controlled trials and cohort studies. A meta-regression of all studies included in the meta-analysis for all CD4 counts found that the type of effect measure (i.e., hazard ratio or incidence rate ratio) did not explain the heterogeneity in the magnitude of effect (p = 0.80). Although the χ2 test suggested no hazard ratio modification, inclusion of more strata and additional study estimates could improve this assessment. Since some studies contributed tuberculosis cases to CD4-stratum estimates and to estimates across all CD4 counts, the data used for the meta-analyses are not independent. Although there was mixed evidence of publication bias in this systematic review, the power to detect publication bias increases as the number of studies included in meta-analyses increases, and additional studies could strengthen the assessment of publication bias for antiretroviral therapy's preventive impact on tuberculosis [60]. While heterogeneity for the meta-analysis including all CD4 counts was calculated using I2 statistics and a 95% confidence interval, calculating I2 95% confidence intervals for CD4 categories was not possible because of the limited number of studies within CD4 strata. Although the meta-analyses included antiretroviral therapy status and baseline CD4 count, other analyses exploring community tuberculosis incidence, community tuberculosis prevalence, participant history of tuberculosis, CD4 cell count recovery, and viral suppression might have provided additional insight into antiretroviral therapy's preventive impact on tuberculosis if these variables had been collected systematically in all studies. Finally, the validity of meta-analyses is subject to proper analyses by investigators in included studies. Two of the studies' 95% confidence intervals [46],[48] have asymmetry on the logarithmic scale. These two studies were included in the meta-analysis for all CD4 counts. In order to determine whether these studies introduced bias into our results, we ran a sensitivity analysis without them and found the results to be nearly identical (HR 0.35, 95% CI 0.28 to 0.44, with all studies, versus HR 0.38, 95% CI 0.31 to 0.46, without [46] and [48]).
While there are many potential benefits to providing earlier antiretroviral therapy, one risk of providing antiretroviral therapy to people with CD4 counts above 350 cells/µl is that it may compromise high adherence rates and potentially lead to widespread antiretroviral resistance. While this is plausible, a randomised trial has shown that adherence counselling facilitated greater than 95% adherence to antiretroviral therapy in 79% of participants initiating antiretroviral therapy above 350 cells/µl and 74% of participants initiating antiretroviral therapy below 350 cells/µl [43]. Additionally, observational data indicate that the risk of drug resistance is higher among people who started antiretroviral therapy below 350 cells/µl relative to those who started antiretroviral therapy above 350 cells/µl [61]. There is also concern that the risk of life-threatening antiretroviral therapy toxicity could be higher among people with CD4 counts above 350 cells/µl; however, a randomised trial indicates that the risk of life-threatening adverse events is similar in those initiating antiretroviral therapy above 350 cells/µl and those initiating antiretroviral therapy below 350 cells/µl (14% of participants in each study arm experienced such an event, p = 0.64) [43]. Results from surveillance and future trials [54]–[56] are awaited to confirm or refute these adherence and toxicity findings. Meanwhile, it is important to continue to scale up antiretroviral therapy to achieve universal access goals while also carefully conducting national surveillance of antiretroviral toxicity [62] and antiretroviral resistance [63].
While our analyses clearly show that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence in adults with HIV, its role in long-term tuberculosis elimination is more complex [6]–[8],[15]. Antiretroviral therapy's effect on the population incidence of tuberculosis depends on HIV prevalence and the extent to which antiretroviral therapy (1) reduces HIV transmission, (2) increases patient life expectancy, (3) reduces the annual risk of tuberculosis, and (4) reduces subsequent tuberculosis transmission. Dynamic models have suggested that antiretroviral therapy reduces new HIV infections and that increasing antiretroviral therapy coverage in people living with HIV will lower the population tuberculosis incidence [15]. Indeed, programmatic data thus far indicate that antiretroviral therapy scale-up is associated with reductions in tuberculosis incidence of 33% and 24% in high-burden Malawian and South African communities [64],[65]. Earlier antiretroviral therapy initiation could lead to a more substantial reduction in population tuberculosis incidence [15]. Expansion of antiretroviral therapy may also reduce HIV incidence at the city [66],[67], district [68],[69], and national levels [70],[71], while decreasing tuberculosis mortality [72]–[74] and HIV-related mortality [75]–[78].
Operationally, antiretroviral therapy's impact on tuberculosis control depends on (1) changes that facilitate access to HIV testing and linkage to care earlier in the course of HIV infection, (2) when national guidelines and programme implementation allow people to initiate antiretroviral therapy, (3) sustaining high adherence to antiretroviral therapy, and (4) improving long-term retention rates [16],[17],[79]. WHO recommends provider-initiated HIV testing and counselling in all health facilities in generalised (i.e., antenatal HIV prevalence ≥1%) epidemics [80]. Unfortunately, Demographic and Health Surveys indicate that only approximately 11% of people aged 15–49 y in generalised epidemics reported receiving an HIV test in the previous year [81], and that many people with HIV enrol onto antiretroviral therapy many years after HIV seroconversion, after the development of tuberculosis and other life-threatening illnesses, and after transmitting HIV to others [82]. A cluster-randomised trial recently found that community-based HIV testing detects approximately four times as many people with HIV as health-facility-based testing alone [83], and a 1-wk community-based multi-disease campaign recently tested 47,311 Kenyans (87% of the target sexually active population 15–49 y of age) and found that HIV-positive participants tested positive earlier in the course of their HIV infection (median 541 cells/µl in the campaign, [84]) than patients identified via health-facility-based approaches [82]. In order to harness the lifespan, HIV transmission, and tuberculosis prevention benefits of antiretroviral therapy, HIV programmes in countries with a high HIV prevalence need to expand HIV testing coverage and could consider offering community-based HIV testing, with linkage to antiretroviral therapy for those eligible, regularly to the general public [85].
WHO's Policy on HIV/TB Collaborative Activities currently recommends the Three I's for HIV/TB: intensified tuberculosis case-finding [11], isoniazid preventive therapy [11], and infection control [12] to prevent tuberculosis in people with HIV. WHO infection control guidelines recommend administrative, managerial, engineering, and personal respiratory methods to avoid nosocomial tuberculosis transmission, such as logistical changes to avoid patient congestion, and early identification and diagnosis of tuberculosis patients in healthcare facilities, congregate settings, and households [12]. Intensified tuberculosis case-finding involves screening people with HIV for current cough, night sweats, fever, and weight loss at every clinical encounter [11]. Those without any of these symptoms have a very low probability of having tuberculosis (98% negative predictive value in settings with a tuberculosis prevalence of 5% [86]) and should be initiated on isoniazid preventive therapy [11].
Isoniazid stops Mycobacterium tuberculosis replication during latent infection and reduces tuberculosis incidence by 33% [87]. WHO has recommended isoniazid preventive therapy for prevention of tuberculosis in adults with HIV since 1993 [11],[88],[89]; however, only a small fraction of the millions eligible received isoniazid preventive therapy in 2010 [3]. Antiretroviral therapy causes viral suppression and immune recovery, which reduces tuberculosis incidence by 65% across all CD4 counts. Initiating antiretroviral therapy as early as possible strengthens the WHO Three I's for HIV/TB strategy by building upon antiretroviral therapy's synergy with isoniazid preventive therapy. Indeed, observational studies from South Africa [44],[90], Brazil [45], and 16 other countries [91] indicate that combined isoniazid preventive therapy and antiretroviral therapy was superior to antiretroviral therapy or isoniazid preventive therapy alone in reducing tuberculosis incidence among adults with HIV. This finding was recently confirmed through a cluster-randomised trial in Brazil, where isoniazid preventive therapy reduced tuberculosis incidence among Brazilians who remained in care and received antiretroviral therapy [92]. These data suggest that antiretroviral therapy and isoniazid preventive therapy work by complementary mechanisms and that simultaneous use substantially decreases tuberculosis incidence in adults with HIV. Results from other ongoing trials assessing the synergy between antiretroviral therapy and isoniazid preventive therapy are eagerly awaited [54],[56], and ecological, operational, and clinical research on the impact of scaling up antiretroviral therapy and the Three I's for HIV/TB on community and/or national tuberculosis incidence rates is needed [93].
In conclusion, antiretroviral therapy is a potentially safe, well-tolerated, and HIV-transmission-interrupting intervention [43],[94] necessary to increase life expectancy in people with HIV [75]–[78]. There has been considerable debate on the optimal timing to start antiretroviral therapy in asymptomatic adults with HIV. Published results from ongoing randomised trials are expected in 2016 and are eagerly awaited [54],[55]. This review found that antiretroviral therapy is strongly associated with a reduction in tuberculosis incidence in adults with HIV across all CD4 cell counts. Our key finding that antiretroviral therapy has a significant impact on preventing tuberculosis in adults with CD4 counts above 350 cells/µl is consistent with studies from developed countries [95],[96] and will need to be considered by healthcare providers, researchers, policymakers, and people living with HIV when weighing the benefits and risks of initiating antiretroviral therapy above 350 cells/µl.
Supporting Information
Zdroje
1. HarriesAD, ZachariahR, CorbettEL, LawnSD, Santos-FilhoET, et al. (2010) The HIV-associated tuberculosis epidemic—when will we act? Lancet 375 : 1906–1919.
2. Joint United Nations Programme on HIV/AIDS (2011) World AIDS Day report 2011. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf. Accessed 23 November 2011.
3. World Health Organization (2011) Global tuberculosis control 2011. Available: http://www.who.int/entity/tb/publications/global_report/2011/gtbr11_full.pdf. Accessed 14 October 2011.
4. The United Nations (2011) The Millenium Development Goals report. Available: http://mdgs.un.org/unsd/mdg/Resources/Static/Products/Progress2011/11-31339%20(E)%20MDG%20Report%202011_Book%20LR.pdf. Accessed 18 July 2011.
5. Stop TB Partnership (2010) The global plan to stop TB: 2011–2015. Available: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf. Accessed 7 February 2011.
6. BorgdorffMW, FloydK, BroekmansJF (2002) Interventions to reduce tuberculosis mortality and transmission in low - and middle-income countries. Bull World Health Organ 80 : 217–227.
7. Abu-RaddadLJ, SabatelliL, AchterbergJT, SugimotoJD, LonginiIMJr, et al. (2009) Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A 106 : 13980–13985.
8. LonnrothK, CastroKG, ChakayaJM, ChauhanLS, FloydK, et al. (2010) Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet 375 : 1814–1829.
9. De CockKM, ChaissonRE (1999) Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis 3 : 457–465.
10. World Health Organization (2012) WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Available: http://whqlibdoc.who.int/publications/2012/9789241503006_eng.pdf. Accessed 12 March 2012.
11. World Health Organization (2011) Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Available: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. Accessed 11 April 2011.
12. World Health Organization (2009) WHO policy on TB infection control in health-care facilities, congregate settings, and households. Available: http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf. Accessed 10 September 2010.
13. LawnSD, WoodR, De CockKM, KranzerK, LewisJJ, et al. (2010) Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 10 : 489–498.
14. World Health Organization (2010) Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Available: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed 11 April 2011.
15. WilliamsBG, GranichR, De CockKM, GlaziouP, SharmaA, et al. (2010) Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci U S A 107 : 19485–19489.
16. LawnSD, HarriesAD, WilliamsBG, ChaissonRE, LosinaE, et al. (2011) Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis 15 : 571–581.
17. World Health Organization (2012 June) Programmatic update: antiretroviral therapy as prevention (TasP) of HIV and TB. Available: http://whqlibdoc.who.int/hq/2012/WHO_HIV_2012.12_eng.pdf. Accessed 25 June 2012.
18. LiberatiA, AltmanDG, TetzlaffJ, MulrowC, GotzschePC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6: e1000100 doi:10.1371/journal.pmed.1000100.
19. Suthar AB (2011) Antiretroviral therapy for prevention of HIV-associated tuberculosis: a systematic review and meta-analysis. PROSPERO. Available: http://www.crd.york.ac.uk/PROSPEROFILES/1209_PROTOCOL.pdf. Accessed 22 June 2012.
20. LawnSD, MyerL, EdwardsD, BekkerLG, WoodR (2009) Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 23 : 1717–1725.
21. Van RieA, WestreichD, SanneI (2011) Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr 56 : 349–355.
22. GirardiE, SabinCA, d'Arminio MonforteA, HoggB, PhillipsAN, et al. (2005) Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 41 : 1772–1782.
23. SterlingTR, LauB, ZhangJ, FreemanA, BoschRJ, et al. (2011) Risk factors for tuberculosis after highly active antiretroviral therapy initiation in the United States and Canada: implications for tuberculosis screening. J Infect Dis 204 : 893–901.
24. DembeleM, SaleriN, CarvalhoAC, SaouadogoT, HienAD, et al. (2010) Incidence of tuberculosis after HAART initiation in a cohort of HIV-positive patients in Burkina Faso. Int J Tuberc Lung Dis 14 : 318–323.
25. LawnSD, KranzerK, EdwardsDJ, McNallyM, BekkerLG, et al. (2010) Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS 24 : 1323–1328.
26. DyeC, WilliamsBG (2010) The population dynamics and control of tuberculosis. Science 328 : 856–861.
27. DyeC, LonnrothK, JaramilloE, WilliamsBG, RaviglioneM (2009) Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ 87 : 683–691.
28. The United Nations Development Programme (2011) Human development report. sustainability and equity: a better future for all. Available: http://hdr.undp.org/en/media/HDR_2011_EN_Complete.pdf. Accessed 14 March 2012.
29. The Cochrane Collaboration (2011) Cochrane handbook for systematic reviews of interventions. Available: http://www.cochrane-handbook.org/. Accessed 19 April 2011.
30. Wells G, Shea B, O'Connell D, Peterson J, Welch V, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 23 February 2011.
31. HanrahanCF, GolubJE, MohapiL, TshabanguN, ModisenyaneT, et al. (2010) Body mass index and risk of tuberculosis and death. AIDS 24 : 1501–1508.
32. SeylerC, ToureS, MessouE, BonardD, GabillardD, et al. (2005) Risk factors for active tuberculosis after antiretroviral treatment initiation in Abidjan. Am J Respir Crit Care Med 172 : 123–127.
33. NakanjakoD, Mayanja-KizzaH, OumaJ, WanyenzeR, MwesigireD, et al. (2010) Tuberculosis and human immunodeficiency virus co-infections and their predictors at a hospital-based HIV/AIDS clinic in Uganda. Int J Tuberc Lung Dis 14 : 1621–1628.
34. LonnrothK, WilliamsBG, CegielskiP, DyeC (2010) A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 39 : 149–155.
35. GilksCF, CrowleyS, EkpiniR, GoveS, PerriensJ, et al. (2006) The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 368 : 505–510.
36. WilliamsBG, KorenrompEL, GouwsE, SchmidGP, AuvertB, et al. (2006) HIV infection, antiretroviral therapy, and CD4+ cell count distributions in African populations. J Infect Dis 194 : 1450–1458.
37. KorenrompEL, WilliamsBG, SchmidGP, DyeC (2009) Clinical prognostic value of RNA viral load and CD4 cell counts during untreated HIV-1 infection—a quantitative review. PLoS ONE 4: e5950 doi:10.1371/journal.pone.0005950.
38. WandelS, EggerM, RangsinR, NelsonKE, CostelloC, et al. (2008) Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect 84 (Suppl 1) i31–i36.
39. Cooper H, Hedges L (1994) The handbook of research synthesis. New York: Russell Sage Foundation.
40. HigginsJP, ThompsonSG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21 : 1539–1558.
41. HigginsJP, ThompsonSG, DeeksJJ, AltmanDG (2003) Measuring inconsistency in meta-analyses. BMJ 327 : 557–560.
42. BadriM, WilsonD, WoodR (2002) Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 359 : 2059–2064.
43. CohenMS, ChenYQ, McCauleyM, GambleT, HosseinipourMC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 : 493–505.
44. GolubJE, PronykP, MohapiL, ThsabanguN, MoshabelaM, et al. (2009) Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS 23 : 631–636.
45. GolubJE, SaraceniV, CavalcanteSC, PachecoAG, MoultonLH, et al. (2007) The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 21 : 1441–1448.
46. JereneD, NaessA, LindtjornB (2006) Antiretroviral therapy at a district hospital in Ethiopia prevents death and tuberculosis in a cohort of HIV patients. AIDS Res Ther 3 : 10.
47. LannoyLH, Cortez-EscalanteJJ, Evangelista MdoS, RomeroGA (2008) Tuberculosis incidence and risk factors among patients living with HIV/AIDS in public health service institutions in Brasilia, Federal District. Rev Soc Bras Med Trop 41 : 549–555.
48. MirandaA, MorganM, JamalL, LasersonK, BarreiraD, et al. (2007) Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS ONE 2: e826 doi:10.1371/journal.pone.0000826.
49. SamandariT, AgizewTB, NyirendaS, TedlaZ, SibandaT, et al. (2011) 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet 377 : 1588–1598.
50. Santoro-LopesG, de PinhoAM, HarrisonLH, SchechterM (2002) Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis 34 : 543–546.
51. SevereP, JusteMA, AmbroiseA, EliacinL, MarchandC, et al. (2010) Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med 363 : 257–265.
52. ZhouJ, ElliottJ, LiPC, LimPL, KiertiburanakulS, et al. (2009) Risk and prognostic significance of tuberculosis in patients from The TREAT Asia HIV Observational Database. BMC Infect Dis 9 : 46.
53. MoultonLH, GolubJE, DurovniB, CavalcanteSC, PachecoAG, et al. (2007) Statistical design of THRio: a phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials 4 : 190–199.
54. French National Agency for Research on AIDS and Viral Hepatitis (2007) Early antiretroviral treatment and/or early isoniazid prophylaxis against tuberculosis in HIV-infected adults (ANRS 12136 TEMPRANO). Available: http://clinicaltrials.gov/ct2/show/NCT00495651. Accessed 5 August 2011.
55. University of Minnesota Clinical and Translational Science Institute (2009) Strategic timing of antiretroviral treatment (START). Available: http://clinicaltrials.gov/ct2/show/NCT00867048. Accessed 5 August 2011.
56. University of Cape Town (2007) Isoniazid plus (highly active antiretroviral therapy) HAART to prevent tuberculosis (TB) in HIV-infected persons (HAART-IPT). Available: http://clinicaltrials.gov/ct2/show/NCT00463086. Accessed 5 August 2011.
57. BrinkhofMW, EggerM, BoulleA, MayM, HosseinipourM, et al. (2007) Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis 45 : 1518–1521.
58. LawnSD, WilkinsonRJ, LipmanMC, WoodR (2008) Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med 177 : 680–685.
59. Rothman K, GreenlandS, LashT (2008) Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins.
60. SterneJA, GavaghanD, EggerM (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53 : 1119–1129.
61. UyJ, ArmonC, BuchaczK, WoodK, BrooksJT (2009) Initiation of HAART at higher CD4 cell counts is associated with a lower frequency of antiretroviral drug resistance mutations at virologic failure. J Acquir Immune Defic Syndr 51 : 450–453.
62. World Health Organization (2009) A practical handbook on the pharmacovigilance of antiretroviral medicines. Available: http://www.who.int/hiv/topics/pharmacovigilance/arv_pharmacovigilance_handbook.pdf. Accessed 8 November 2011.
63. JordanMR, BennettDE, WainbergMA, HavlirD, HammerS, et al. (2012) Update on World Health Organization HIV drug resistance prevention and assessment strategy: 2004–2011. Clin Infect Dis 54 (Suppl 4) S245–S249.
64. ZachariahR, BemelmansM, AkessonA, GomaniP, PhiriK, et al. (2011) Reduced tuberculosis case notification associated with scaling up antiretroviral treatment in rural Malawi. Int J Tuberc Lung Dis 15 : 933–937.
65. MiddelkoopK, BekkerLG, MyerL, JohnsonLF, KloosM, et al. (2011) Antiretroviral therapy and TB notification rates in a high HIV prevalence South African community. J Acquir Immune Defic Syndr 56 : 263–269.
66. DasM, ChuPL, SantosGM, ScheerS, VittinghoffE, et al. (2010) Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE 5: e11068 doi:10.1371/journal.pone.0011068.
67. CowanSA, GerstoftJ, HaffJ, Hartvig ChristiansenA, NielsenJ, et al. (2012) Stable incidence of HIV diagnoses among Danish MSM despite increased engagement in unsafe sex. J Acquir Immune Defic Syndr. E-pub ahead of print doi:10.1097/QAI.0b013e31825af890.
68. MontanerJS, LimaVD, BarriosR, YipB, WoodE, et al. (2010) Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 376 : 532–539.
69. Tanser F, Bärnighausen T, Grapsa E, Newell M-L (2012) Effect of ART coverage on rate of new HIV infections in a hyper-endemic, rural population: South Africa [abstract 136LB]. 19th Conference on Retroviruses and Opportunistic Infections; 5–8 March 2012; Seattle, Washington, United States.
70. Egger M (2011) Community viral load and newly reported HIV infections in Switzerland [IAS satellite session 17 July 2011]. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 July 2011; Rome, Italy.
71. GranichRM, GilksCF, DyeC, De CockKM, WilliamsBG (2009) Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373 : 48–57.
72. Abdool KarimSS, NaidooK, GroblerA, PadayatchiN, BaxterC, et al. (2010) Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 362 : 697–706.
73. BlancFX, SokT, LaureillardD, BorandL, RekacewiczC, et al. (2011) Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 365 : 1471–1481.
74. HavlirDV, KendallMA, IveP, KumwendaJ, SwindellsS, et al. (2011) Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 365 : 1482–1491.
75. KitahataMM, GangeSJ, AbrahamAG, MerrimanB, SaagMS, et al. (2009) Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 360 : 1815–1826.
76. SterneJA, MayM, CostagliolaD, de WolfF, PhillipsAN, et al. (2009) Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 373 : 1352–1363.
77. The HIV-CAUSAL Collaboration (2011) When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries. Ann Intern Med 154 : 509–515.
78. The CASCADE Collaboration (2011) Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med 171 : 1560–1569.
79. BärnighausenT, TanserF, DabisF, NewellML (2012) Interventions to improve the performance of HIV health systems for treatment-as-prevention in sub-Saharan Africa: the experimental evidence. Curr Opin HIV AIDS 7 : 140–150.
80. World Health Organization (2007) Guidance on provider-initiated HIV testing and counselling in health facilities. Available: http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf. Accessed 7 February 2011.
81. World Health Organization (2010) Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Available: http://whqlibdoc.who.int/publications/2010/9789241500395_eng.pdf. Accessed 11 April 2011.
82. Mugglin C, Althoff K, Wools-Kaloustian K, Sterne J, Nash D, et al. (2012) Immunodeficiency at the start of ART: global view [abstract 100]. 19th Conference on Retroviruses and Opportunistic Infections; 5–8 March 2012; Seattle, Washington, United States.
83. SweatM, MorinS, CelentanoD, MulawaM, SinghB, et al. (2011) Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis 11 : 525–532.
84. LugadaE, MillarD, HaskewJ, GrabowskyM, GargN, et al. (2010) Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PLoS ONE 5: e12435 doi:10.1371/journal.pone.0012435.
85. SutharAB, KlinkenbergE, RamsayA, GargN, BennettR, et al. (2012) Community-based multi-disease prevention campaigns for controlling HIV-associated tuberculosis. Int J Tuberc Lung Dis 16 : 430–436.
86. GetahunH, KittikraisakW, HeiligCM, CorbettEL, AylesH, et al. (2011) Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med 8: e1000391 doi:10.1371/journal.pmed.1000391.
87. AkoloC, AdetifaI, ShepperdS, VolminkJ (2010) Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010: CD000171.
88. Tuberculosis preventive therapy in HIV-infected individuals: a joint statement of the WHO Tuberculosis Programme and the Global Programme on AIDS, and the International Union Against Tuberculosis and Lung Disease (IUATLD). Wkly Epidemiol Rec 68 : 361–364.
89. World Health Organization, Joint United Nations Programme on HIV/AIDS (1998) Policy statement on preventive therapy against tuberculosis. Available: http://whqlibdoc.who.int/hq/1998/WHO_TB_98.255.pdf. Accessed 30 June 2010.
90. CharalambousS, GrantAD, InnesC, HoffmannCJ, DowdeswellR, et al. (2010) Association of isoniazid preventive therapy with lower early mortality in individuals on antiretroviral therapy in a workplace programme. AIDS 24 (Suppl 5) S5–S13.
91. FennerL, ForsterM, BoulleA, PhiriS, BraitsteinP, et al. (2011) Tuberculosis in HIV programmes in lower-income countries: practices and risk factors. Int J Tuberc Lung Dis 15 : 620–627.
92. Durovni B, Saraceni V, Pacheco A, Cavalcante S, Cohn S, et al. (2011) Impact of tuberculosis (TB) screening and isoniazid preventive therapy (IPT) on incidence of TB and death in the TB/HIV in Rio de Janeiro (THRio) study [abstract WELBB02]. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 17–20 July 2011; Rome, Italy.
93. GranichR, GuptaS, SutharAB, SmythC, HoosD, et al. (2011) Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res 9 : 446–469.
94. AnglemyerA, RutherfordGW, EggerM, SiegfriedN (2011) Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev 2011: CD009153.
95. PodlekarevaD, MocroftA, DragstedUB, LedergerberB, BeniowskiM, et al. (2006) Factors associated with the development of opportunistic infections in HIV-1-infected adults with high CD4+ cell counts: a EuroSIDA study. J Infect Dis 194 : 633–641.
96. The HIV-CAUSAL Collaboration (2012) Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin Infect Dis 54 : 1364–1372.
97. AIDS Clinical Trials Group (2007) Appendix 60: diagnoses appendix. Available: http://www.hptn.org/web%20documents/HPTN052/Appendix60V1.123Feb2007.pdf. Accessed 23 August 2011.
98. American Thoracic Society (1990) Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis 142 : 725–735.
Štítky
Interné lekárstvo
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2012 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



