-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Effect of Water, Sanitation, and Hygiene on the Prevention of Trachoma: A Systematic Review and Meta-Analysis
Background:
Trachoma is the world's leading cause of infectious blindness. The World Health Organization (WHO) has endorsed the SAFE strategy in order to eliminate blindness due to trachoma by 2020 through “surgery,” “antibiotics,” “facial cleanliness,” and “environmental improvement.” While the S and A components have been widely implemented, evidence and specific targets are lacking for the F and E components, of which water, sanitation, and hygiene (WASH) are critical elements. Data on the impact of WASH on trachoma are needed to support policy and program recommendations. Our objective was to systematically review the literature and conduct meta-analyses where possible to report the effects of WASH conditions on trachoma and identify research gaps.Methods and Findings:
We systematically searched PubMed, Embase, ISI Web of Knowledge, MedCarib, Lilacs, REPIDISCA, DESASTRES, and African Index Medicus databases through October 27, 2013 with no restrictions on language or year of publication. Studies were eligible for inclusion if they reported a measure of the effect of WASH on trachoma, either active disease indicated by observed signs of trachomatous inflammation or Chlamydia trachomatis infection diagnosed using PCR. We identified 86 studies that reported a measure of the effect of WASH on trachoma. To evaluate study quality, we developed a set of criteria derived from the GRADE methodology. Publication bias was assessed using funnel plots. If three or more studies reported measures of effect for a comparable WASH exposure and trachoma outcome, we conducted a random-effects meta-analysis. We conducted 15 meta-analyses for specific exposure-outcome pairs. Access to sanitation was associated with lower trachoma as measured by the presence of trachomatous inflammation-follicular or trachomatous inflammation-intense (TF/TI) (odds ratio [OR] 0.85, 95% CI 0.75–0.95) and C. trachomatis infection (OR 0.67, 95% CI 0.55–0.78). Having a clean face was significantly associated with reduced odds of TF/TI (OR 0.42, 95% CI 0.32–0.52), as were facial cleanliness indicators lack of ocular discharge (OR 0.42, 95% CI 0.23–0.61) and lack of nasal discharge (OR 0.62, 95% CI 0.52–0.72). Facial cleanliness indicators were also associated with reduced odds of C. trachomatis infection: lack of ocular discharge (OR 0.40, 95% CI 0.31–0.49) and lack of nasal discharge (OR 0.56, 95% CI 0.37–0.76). Other hygiene factors found to be significantly associated with reduced TF/TI included face washing at least once daily (OR 0.76, 95% CI 0.57–0.96), face washing at least twice daily (OR 0.85, 95% CI 0.80–0.90), soap use (OR 0.76, 95% CI 0.59–0.93), towel use (OR 0.65, 95% CI 0.53–0.78), and daily bathing practices (OR 0.76, 95% CI 0.53–0.99). Living within 1 km of a water source was not found to be significantly associated with TF/TI or C. trachomatis infection, and the use of sanitation facilities was not found to be significantly associated with TF/TI.Conclusions:
We found strong evidence to support F and E components of the SAFE strategy. Though limitations included moderate to high heterogenity, low study quality, and the lack of standard definitions, these findings support the importance of WASH in trachoma elimination strategies and the need for the development of standardized approaches to measuring WASH in trachoma control programs.
Please see later in the article for the Editors' Summary
Published in the journal: Effect of Water, Sanitation, and Hygiene on the Prevention of Trachoma: A Systematic Review and Meta-Analysis. PLoS Med 11(2): e32767. doi:10.1371/journal.pmed.1001605
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001605Summary
Background:
Trachoma is the world's leading cause of infectious blindness. The World Health Organization (WHO) has endorsed the SAFE strategy in order to eliminate blindness due to trachoma by 2020 through “surgery,” “antibiotics,” “facial cleanliness,” and “environmental improvement.” While the S and A components have been widely implemented, evidence and specific targets are lacking for the F and E components, of which water, sanitation, and hygiene (WASH) are critical elements. Data on the impact of WASH on trachoma are needed to support policy and program recommendations. Our objective was to systematically review the literature and conduct meta-analyses where possible to report the effects of WASH conditions on trachoma and identify research gaps.Methods and Findings:
We systematically searched PubMed, Embase, ISI Web of Knowledge, MedCarib, Lilacs, REPIDISCA, DESASTRES, and African Index Medicus databases through October 27, 2013 with no restrictions on language or year of publication. Studies were eligible for inclusion if they reported a measure of the effect of WASH on trachoma, either active disease indicated by observed signs of trachomatous inflammation or Chlamydia trachomatis infection diagnosed using PCR. We identified 86 studies that reported a measure of the effect of WASH on trachoma. To evaluate study quality, we developed a set of criteria derived from the GRADE methodology. Publication bias was assessed using funnel plots. If three or more studies reported measures of effect for a comparable WASH exposure and trachoma outcome, we conducted a random-effects meta-analysis. We conducted 15 meta-analyses for specific exposure-outcome pairs. Access to sanitation was associated with lower trachoma as measured by the presence of trachomatous inflammation-follicular or trachomatous inflammation-intense (TF/TI) (odds ratio [OR] 0.85, 95% CI 0.75–0.95) and C. trachomatis infection (OR 0.67, 95% CI 0.55–0.78). Having a clean face was significantly associated with reduced odds of TF/TI (OR 0.42, 95% CI 0.32–0.52), as were facial cleanliness indicators lack of ocular discharge (OR 0.42, 95% CI 0.23–0.61) and lack of nasal discharge (OR 0.62, 95% CI 0.52–0.72). Facial cleanliness indicators were also associated with reduced odds of C. trachomatis infection: lack of ocular discharge (OR 0.40, 95% CI 0.31–0.49) and lack of nasal discharge (OR 0.56, 95% CI 0.37–0.76). Other hygiene factors found to be significantly associated with reduced TF/TI included face washing at least once daily (OR 0.76, 95% CI 0.57–0.96), face washing at least twice daily (OR 0.85, 95% CI 0.80–0.90), soap use (OR 0.76, 95% CI 0.59–0.93), towel use (OR 0.65, 95% CI 0.53–0.78), and daily bathing practices (OR 0.76, 95% CI 0.53–0.99). Living within 1 km of a water source was not found to be significantly associated with TF/TI or C. trachomatis infection, and the use of sanitation facilities was not found to be significantly associated with TF/TI.Conclusions:
We found strong evidence to support F and E components of the SAFE strategy. Though limitations included moderate to high heterogenity, low study quality, and the lack of standard definitions, these findings support the importance of WASH in trachoma elimination strategies and the need for the development of standardized approaches to measuring WASH in trachoma control programs.
Please see later in the article for the Editors' SummaryIntroduction
Trachoma is the world's leading cause of infectious blindness, responsible for visual impairment of an estimated 2.2 million people, of whom 1.2 million are irreversibly blind [1],[2]. Repeated infection with the bacteria C. trachomatis results in scarring of the conjunctiva of the upper eyelid that inverts the eyelids (entropion) leading to eyelashes touching the cornea and conjunctiva (trichiasis). Abrasions and secondary infections of the cornea then lead, without preventive surgery, to irreversible blindness [3],[4]. Although great progress has been made, the disease remains endemic in 53 countries, primarily in sub-Saharan Africa, the Middle East, and Asia [2]. Approximately 334,000 disability-adjusted life years are currently lost due to trachoma infection [5]. An estimated 229 million people live in endemic areas, including 176 million in Africa, though 80% of the global burden is now limited to 14 countries [6].
Intensive effort since the founding of the Global Alliance for Elimination of Blinding Trachoma by 2020 (GET 2020) has led to considerable reduction in the global burden of trachoma from 84 million cases of active trachoma in 2003 to 21.4 million in 2012 [7]–[10]. Success of trachoma control has been in part due to the World Health Organization (WHO)-endorsed SAFE strategy: a simple, low-cost “surgery” for patients with advanced stages of the disease, treatment with the “antibiotics” azithromycin or tetracycline eye ointment, promotion of “facial” cleanliness, and “environmental” improvement, which encompasses promotion of sanitation construction and increased water access [9].
The World Health Assembly (WHA) in resolution 51.11 has called for the elimination of blinding trachoma by 2020 [8],[9]. Donation of Zithromax (azithromycin) by Pfizer Inc. has enabled scale-up of efforts to reduce disease burden in relation to these targets. Annual treatments have increased from 1 million doses in 1998 to 47.8 million doses in 2012 [2] and more than 30 countries have articulated national trachoma strategies and undertaken trachoma elimination programs [11]. However, these efforts alone will not lead to sustainable elimination of blinding trachoma, and it is widely recognized that scale-up of the full SAFE strategy is needed to reach 2020 targets [12]. In particular, improvements in environmental conditions, most notably hygiene and sanitation, are needed for sustained reductions in disease burden, and no single tool can be recommended for the F and E components of SAFE [13],[14]. As pointed out by the editors of the Lancet in 2012 in response to the launch of Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases and reiterated by key stakeholders in the trachoma control community [15], the inclusion of water, sanitation, and hygiene as a strategic goal within the global strategy did not include specific goals or targets [16].
There is a need for increased intersectoral collaboration between neglected tropical disease (NTD) and water, sanitation, and hygiene (WASH) sectors, as well as for increased financial resources for WASH interventions in the context of trachoma control [17]. However, there is little evidence to support specific WASH interventions within the SAFE framework. While there have been successes in integrating WASH into trachoma control [15], a need remains for empirical guidance on the impact of the F and E components and for guidance on how to monitor progress. Recent Cochrane reviews on the environmental improvement (sanitation) component [18] and on face washing [19] revealed few rigorous randomized trials. The most recent comprehensive reviews of the impact of sanitation access and face washing on trachoma were conducted in 2000 [13],[14]. We found no previous reviews of all facets of WASH on trachoma.
We conducted a systematic review and meta-analysis to quantify the relationship between WASH exposures and C. trachomatis infection and active disease. Our purpose was to assess and present the available evidence in order to inform policy for effective and cost-effective integration of WASH for trachoma control, as well as to inform WASH monitoring indicators for trachoma control programs.
Methods
Search Strategy
We performed a systematic review and meta-analysis of the literature to address the effects of WASH exposures on infection and clinical signs of trachoma. We systematically searched PubMed, Embase, ISI Web of Knowledge, MedCarib, Lilacs, REPIDISCA, DESASTRES, and African Index Medicus databases with no restrictions on language or year of publication. Our search was performed through October 27, 2013 with no restriction of start date. We employed a broad set of search terms, pairing the term [trachom*] with the following WASH-related keywords: [clean* fac*], [environment*], [excre*], [face washing], [facewashing], [faec*], [fec*], [hand washing], [handwashing], [hygiene], [latrine*], [sanitation], [toilet*], [towel*], [wash cloth*], [waste], and [water]. In addition, we examined seven previous reviews pertaining to trachoma and some aspect of WASH [3],[14],[18]–[22], and hand searched the bibliographies of all relevant publications. Any additional articles found to be pertinent during this process were included.
Studies were included in the systematic review only if they measured WASH exposure, trachoma infection, and attempted to quantify the association between a WASH exposure, condition, or risk factor on trachoma. All study types were eligible if they met these inclusion criteria, and we employed no additional exclusion criteria in our approach. If an article was considered relevant, but data were not available in the format needed for our meta-analysis, the corresponding authors were contacted by e-mail and asked to supply the relevant data. We conducted meta-analyses for specific exposure-outcome relationships based on available data. Meta-analyses were conducted in adherence to the PRISMA statement (Text S1) and the MOOSE guidelines for reporting meta-analyses of observational studies [23]. Our complete protocol is available in Text S2.
Selection Criteria and Data Extraction
Articles were selected for inclusion using a two-step review process. First, the titles and abstracts of all identified studies were examined, and studies that failed to meet the inclusion criteria after this step were excluded. Second, two reviewers (MES and CM) independently examined the full text of potentially relevant articles using a standard protocol developed by MES and MCF. In the event of disagreement regarding the eligibility of a study during this phase, the opinion of a third reviewer (MCF) was sought, and the parameters of the study's inclusion were discussed until consensus was reached.
Once a set of eligible studies was agreed upon, relevant data were extracted from each study by MES using a standard protocol. To ensure extraction reliability, CM also extracted data from a subset of 10% of identified studies, and no discrepancies were found. Data extracted included a brief description of the study (e.g., study design, setting, year, and sample size), details of the study population, and measures of trachoma. Measures of trachoma included C. trachomatis infection assessed by laboratory analysis of ocular swabs, most commonly using PCR or ELISA techniques, and clinical signs of active trachoma. Clinical signs of trachoma were diagnosed by trained observers using torchlights and 2.5× magnifying loupes [24]. In this approach, eyes are graded according to the WHO simplified grading system and assigned one or more of the following grades: trachomatous inflammation-follicular (TF), trachomatous inflammation-intense (TI), trachomatous scarring (TS), trachomatous trichiasis (TT), and corneal opacity (CO) [25]. Detailed descriptions of all WASH-related conditions, risk factors, or interventions were assessed and coded on the basis of the descriptions in Table 1. Primary WASH components were defined on the basis of the data available from the review.
Tab. 1. Summary of literature density results for overall systematic review. 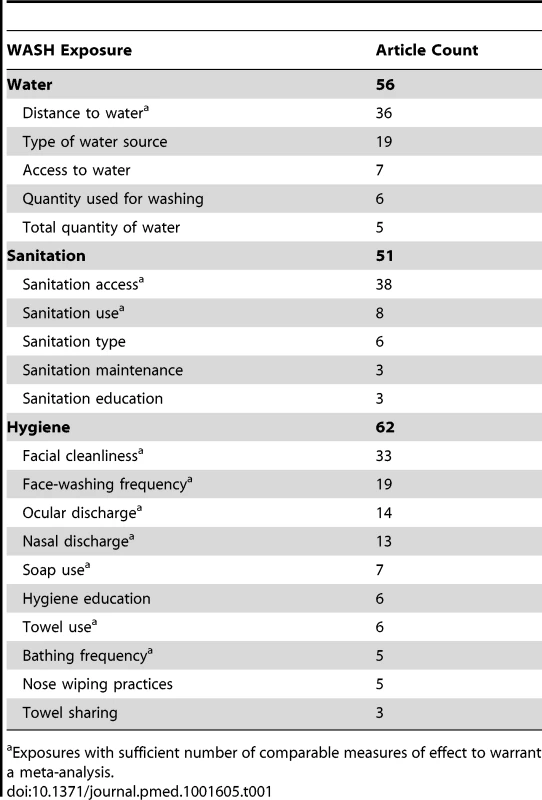
Exposures with sufficient number of comparable measures of effect to warrant a meta-analysis. Quality Issues
In order to determine the quality of identified studies, we developed a set of criteria derived from the GRADE methodology [26]. Our criteria took into account diagnostic features, assessment of WASH-related risk factors, study design, and overall strengths and limitations of the studies. Studies could obtain an overall score ranging between −1 and +6 points for each meta-analysis. Studies that employed a rigorous diagnostic approach (e.g., PCR or other laboratory technique used to assess infection status) received +1 point, while studies that relied only upon clinical diagnosis of trachoma through eye examinations were given 0 points. A study was given +1 point if WASH conditions were investigated directly by the research team (e.g., directly observed the presence of sanitation facility or distance to water source). However, no point was assigned if this was assessed using only a questionnaire. Studies with quasi-experimental or experimental designs were awarded +1 point, while observational studies were awarded 0 points. Studies that purposively calculated sample size to address trachoma as a key outcome of WASH characteristics were awarded +1 point. Studies that attempted to control for confounding factors were assigned an additional +1 point. A list of the confounders each study attempted to control for is available in Text S3. Other strengths and limitations of each study were assessed, and the study was assigned +1 point for additional strengths, and −1 points for additional limitations. MES performed the quality assessment independently and documented the results in separate tables. All relevant studies were included in the review regardless of their overall quality rating. Quality ratings did not affect the meta-analysis or subsequent summary of effect measures, but help to demonstrate the overall quality of individual studies and to identify research gaps. The worksheet used in grading studies for each meta-analysis is available in Text S4.
Meta-Analysis
We conducted meta-analyses for all WASH-related exposures for which three or more studies reported comparable odds ratios (ORs) for the same WASH–trachoma association (i.e., ORs describing the effect of similarly defined exposures on the same measure of trachoma). All ORs used in meta-analyses reflected the results of cross-sectional risk factor analyses. WASH conditions included sanitation access, sanitation use, distance to water of less than 1 km, clean face, lack of ocular discharge, lack of nasal discharge, washing face at least once daily, washing face at least twice daily, bathing at least once daily, towel use, and soap use. Data were stratified by clinically relevant trachoma measures: C. trachomatis infection and the most commonly reported measure of active trachoma, trachomatous inflammation-follicular and/or trachomatous inflammation-intense (TF/TI). Reported ORs served as effect measures. Where a study reported more than one OR for the same WASH exposure (i.e., one for the entire population and one for a sub-population), we chose for inclusion the OR most similar to others in the meta-analysis. When both unadjusted and adjusted ORs were reported, adjusted ORs were included in meta-analysis [27], and adjusted ORs are indicated with asterisks within the forest plots. When ORs were not reported, they were calculated from 2×2 contingency tables; ORs that were calculated using data provided by contacted authors are indicated as such by a footnote in the forest plot. Findings of those studies identified in the systematic review, but not included in meta-analyses were summarized and examined for patterns within and between WASH subcomponent measures, as recommended by the Cochrane Collaboration [27].
We used Microsoft Excel (Microsoft Corporation) to conduct meta-analyses and to develop forest plots [28]. Funnel plots were utilized to investigate the existence of publication bias [29]. Heterogeneity between studies was determined using Higgins' I2 and Cochran's Q-tests [27]. When heterogeneity was moderate to high (I2>50%), sub-group analyses were performed to identify potential sources of heterogeneity. Random effects models were used throughout to enhance generalizability of results [30], and pooled ORs for the effect of the selected WASH conditions on trachoma were employed [27]. Associations between WASH conditions and trachoma were reoriented so that all ORs reflect the relationship between improved WASH conditions on trachoma (e.g., all facial cleanliness ORs were converted to reflect the effect of having a clean face, rather than dirty face, on odds of trachoma).
Results
Characteristics of Identified Studies
Our initial search yielded 5,425 publications (Figure 1). Ninety-nine publications were deemed relevant after review of titles and, when available, abstracts. These articles were fully screened by MES and CM. Following this screening, 86 articles were determined to meet systematic review inclusion criteria (Tables 2–8). We conducted a total of 15 meta-analyses. A summary of the calculated pooled ORs and 95% CIs is provided in Figure 2 and Table 9. Forty-six studies were included in at least one meta-analysis, and 33 appeared in two or more meta-analyses.
Fig. 1. Flow chart of publications identified and excluded for this review. 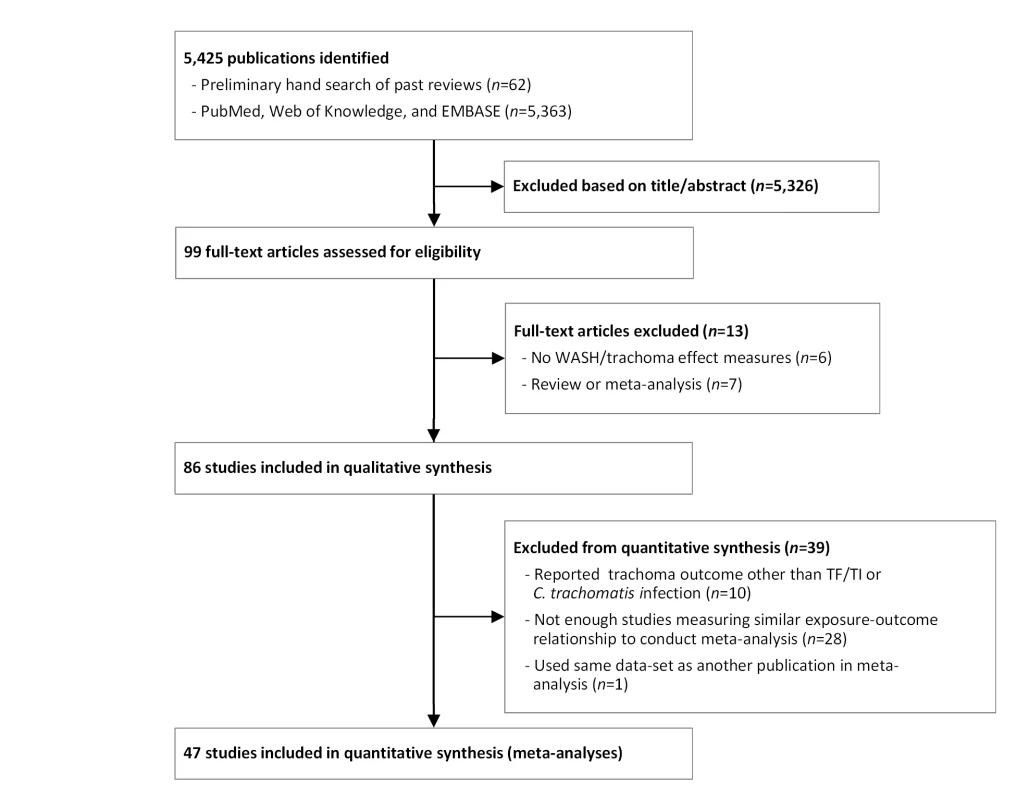
Fig. 2. Summary of meta-analyses examining association of WASH exposures with active trachoma (TF/TI) and C. trachomatis infection. 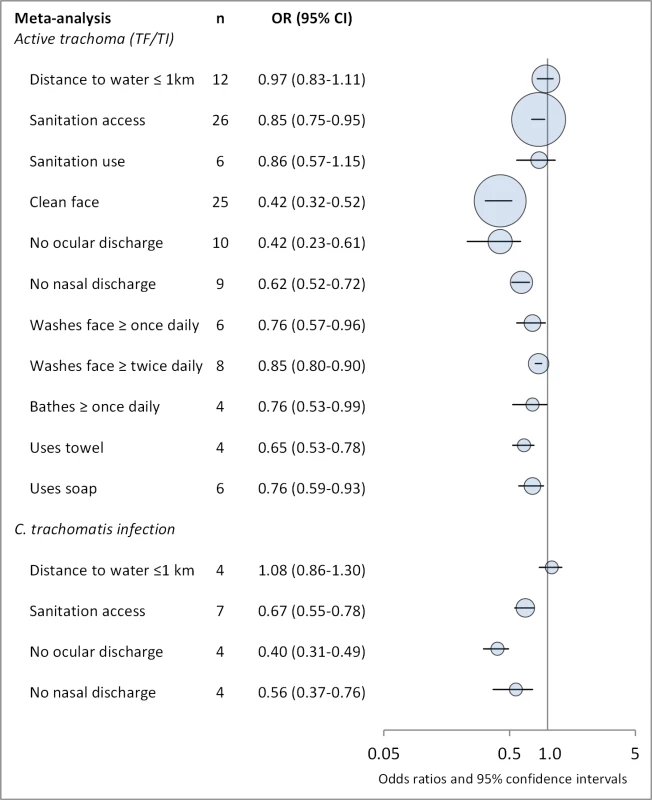
Circles indicate ORs, while the size of circles represents the number of studies included in the meta-analysis (n). Horizontal lines represent 95% CIs. Tab. 2. Summary of publications reporting only on water-related risk factors. 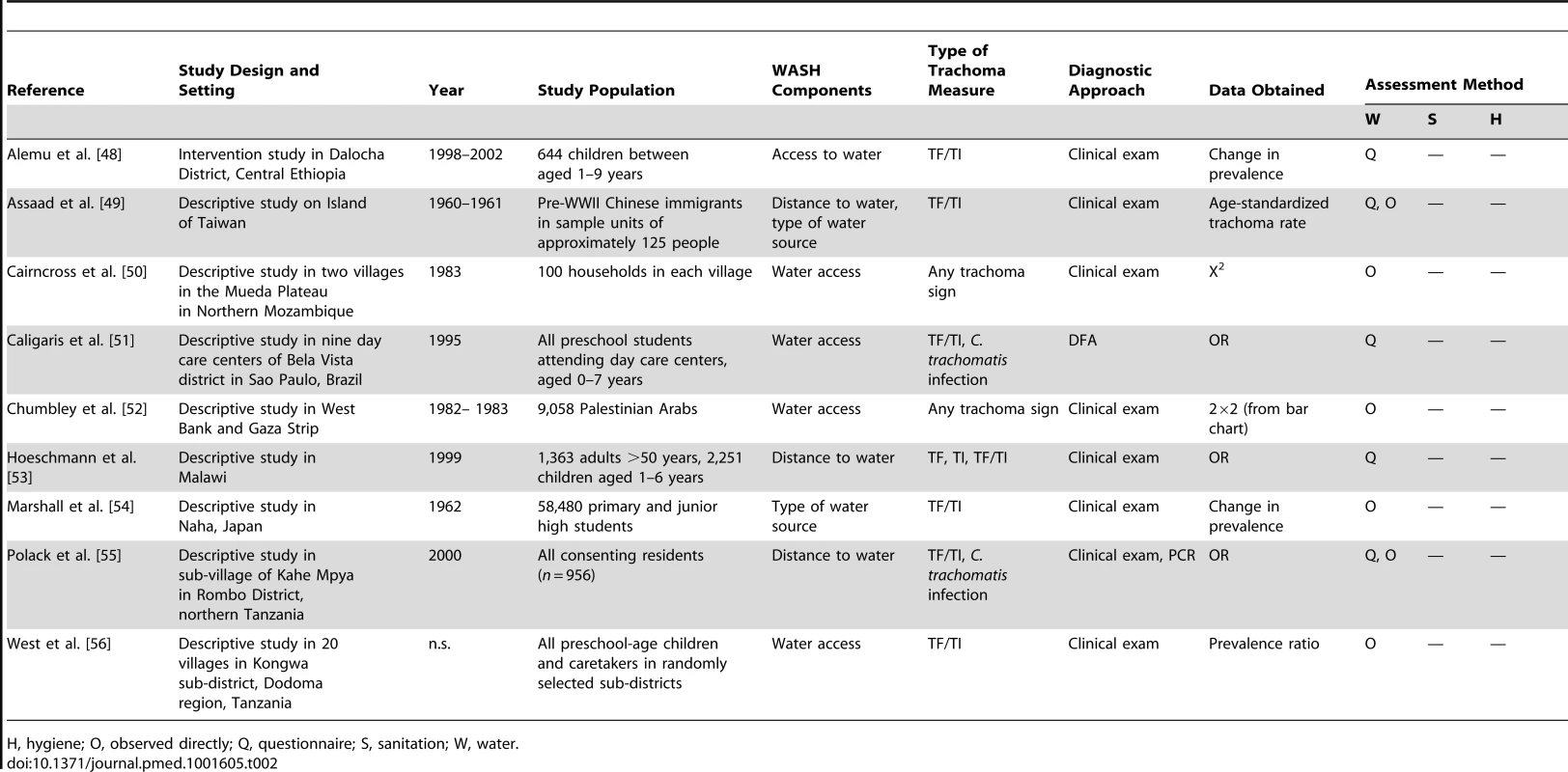
H, hygiene; O, observed directly; Q, questionnaire; S, sanitation; W, water. Tab. 3. Summary of publications reporting only on sanitation-related risk factors. 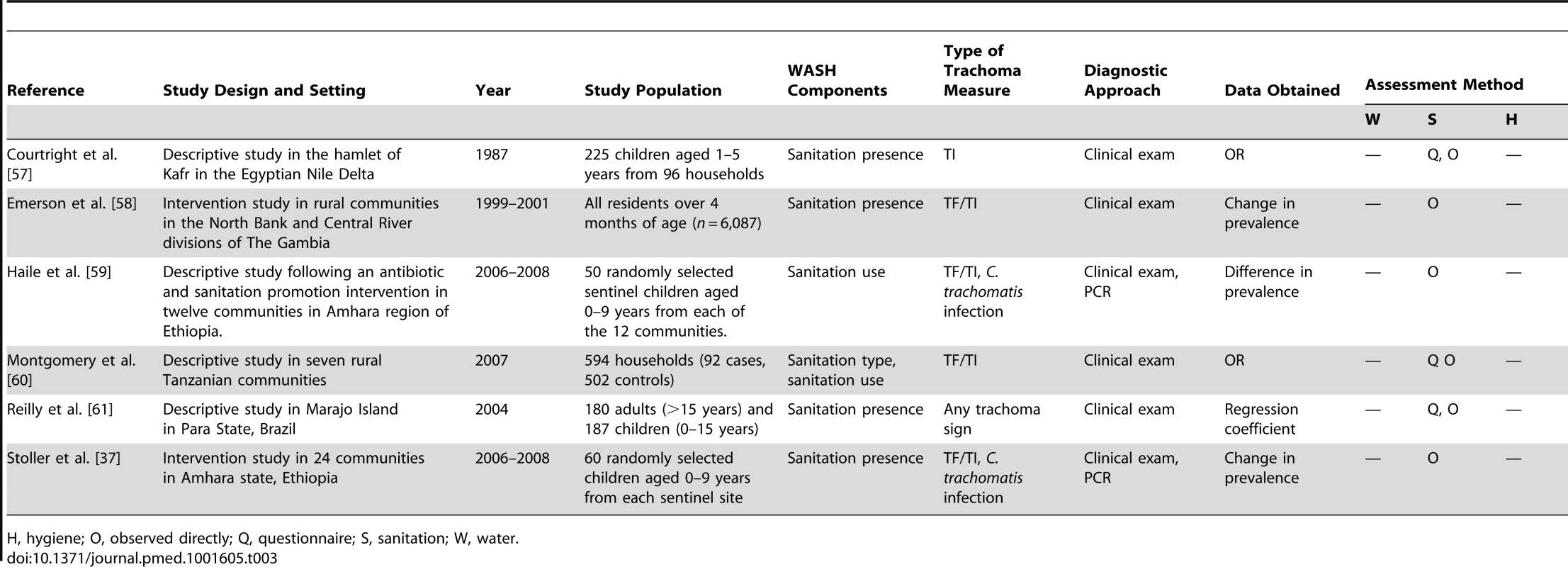
H, hygiene; O, observed directly; Q, questionnaire; S, sanitation; W, water. Tab. 4. Summary of publications reporting only on hygiene-related risk factors. 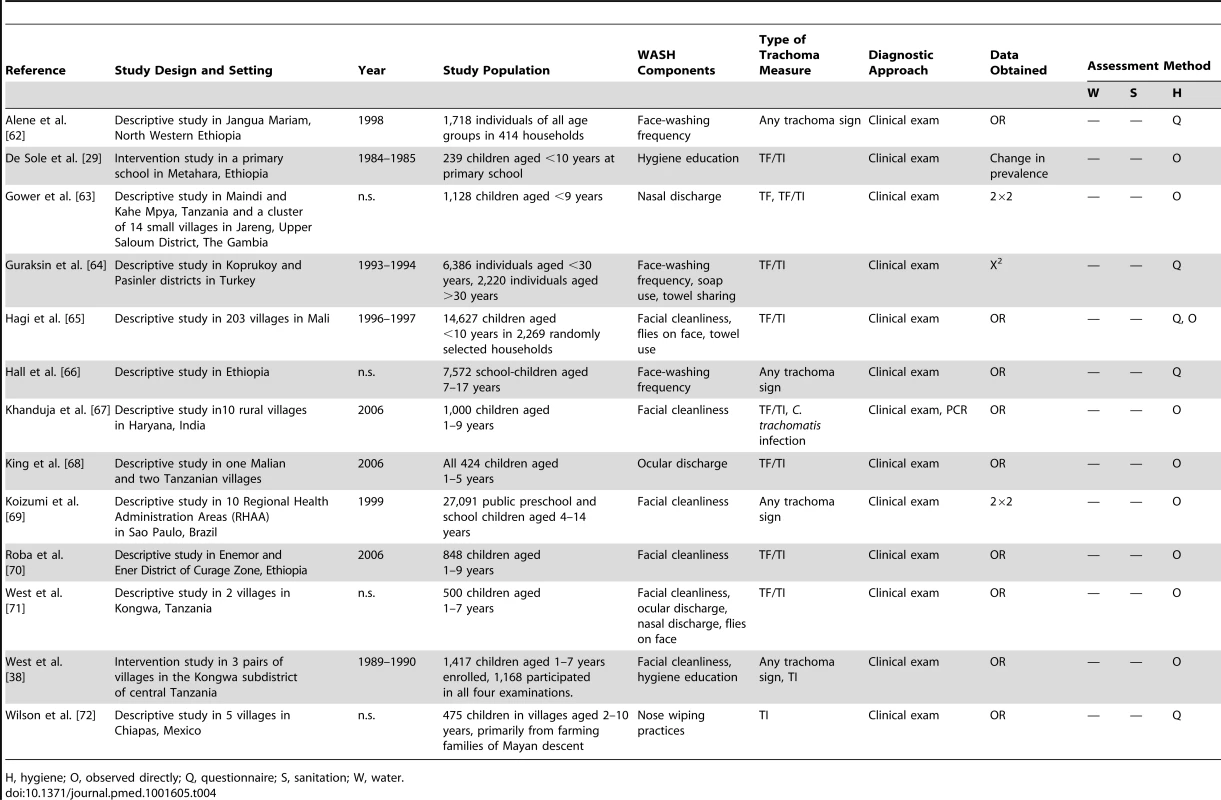
H, hygiene; O, observed directly; Q, questionnaire; S, sanitation; W, water. Tab. 5. Summary of publications reporting on water- and sanitation-related risk factors. 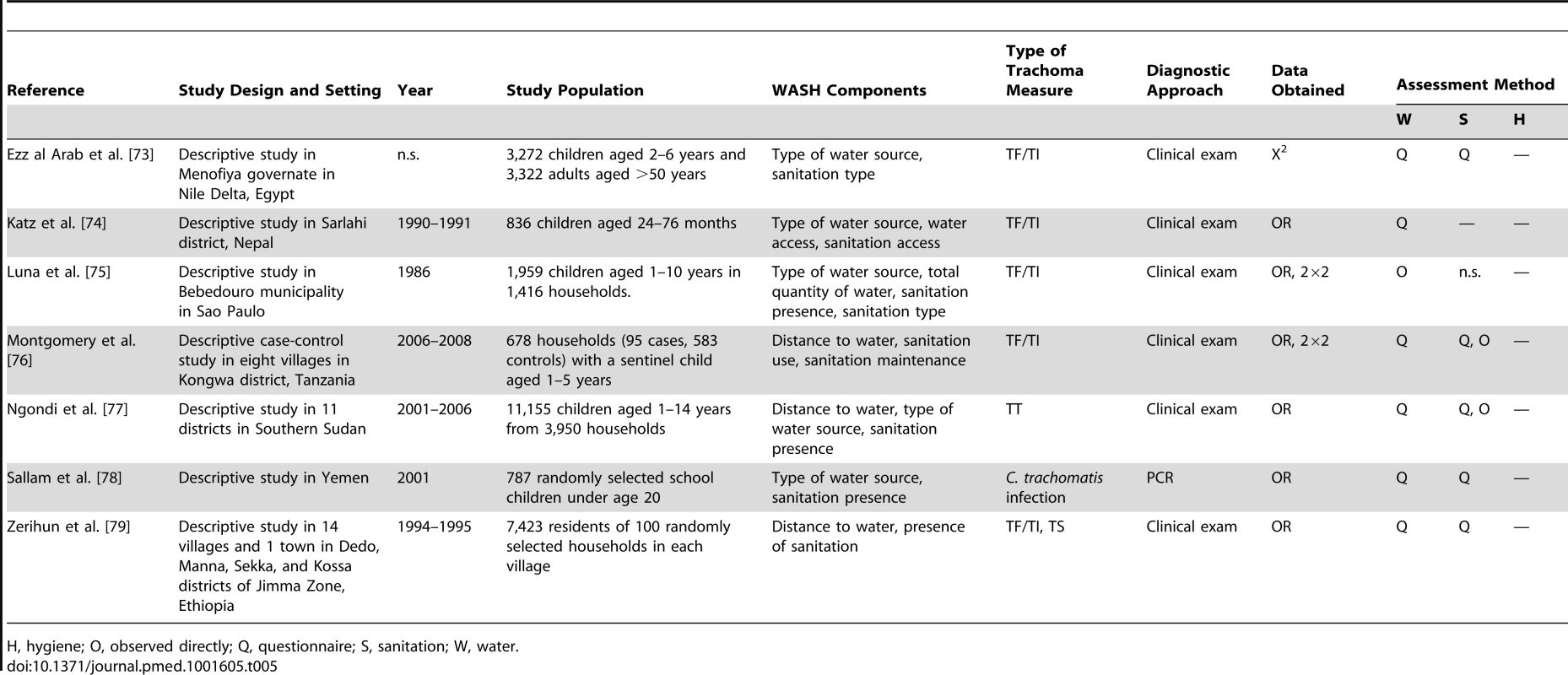
H, hygiene; O, observed directly; Q, questionnaire; S, sanitation; W, water. Tab. 6. Summary of publications reporting on water- and hygiene-related risk factors. 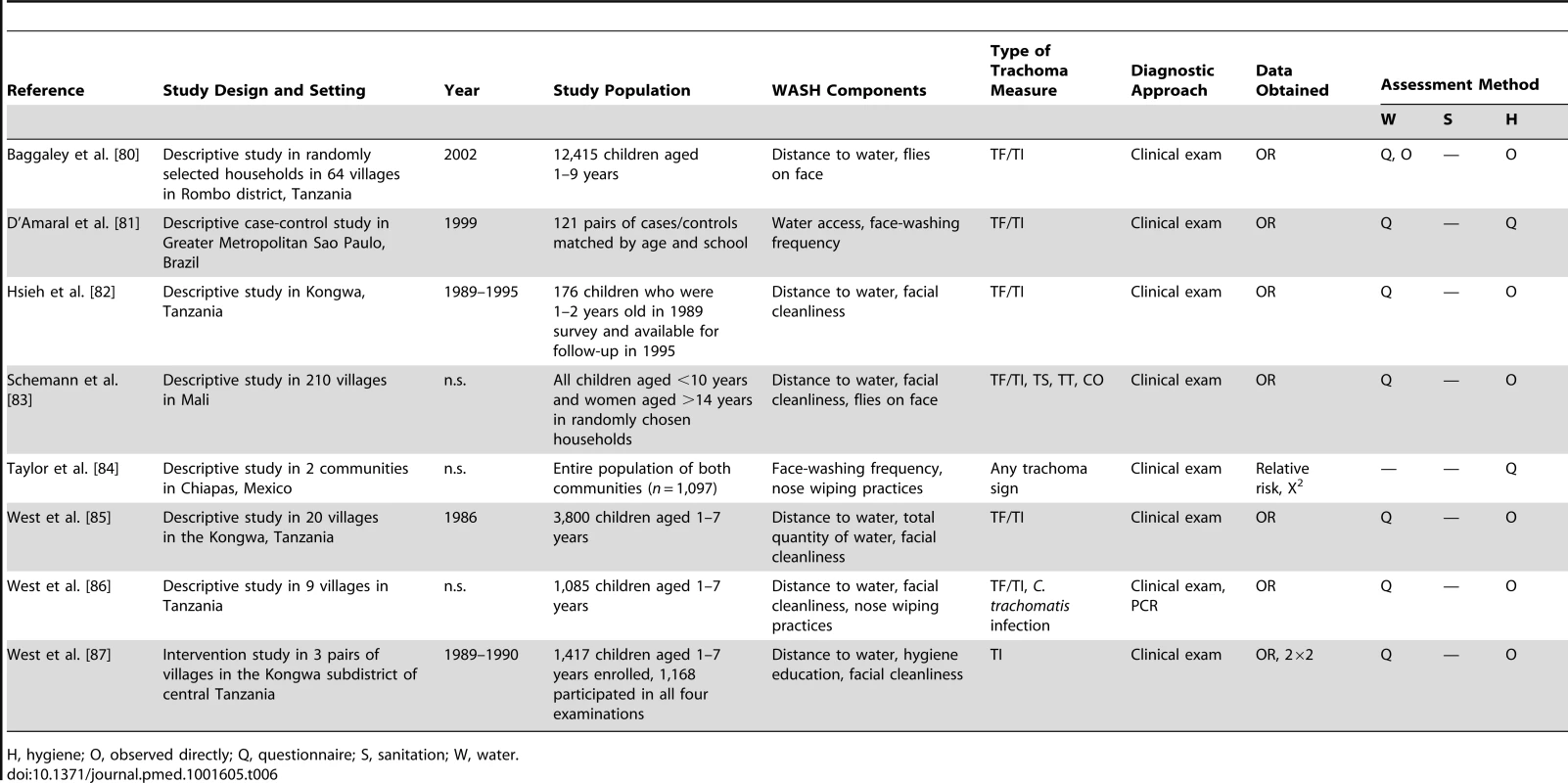
H, hygiene; O, observed directly; Q, questionnaire; S, sanitation; W, water. Tab. 7. Summary of publications reporting only on sanitation- and hygiene-related risk factors. 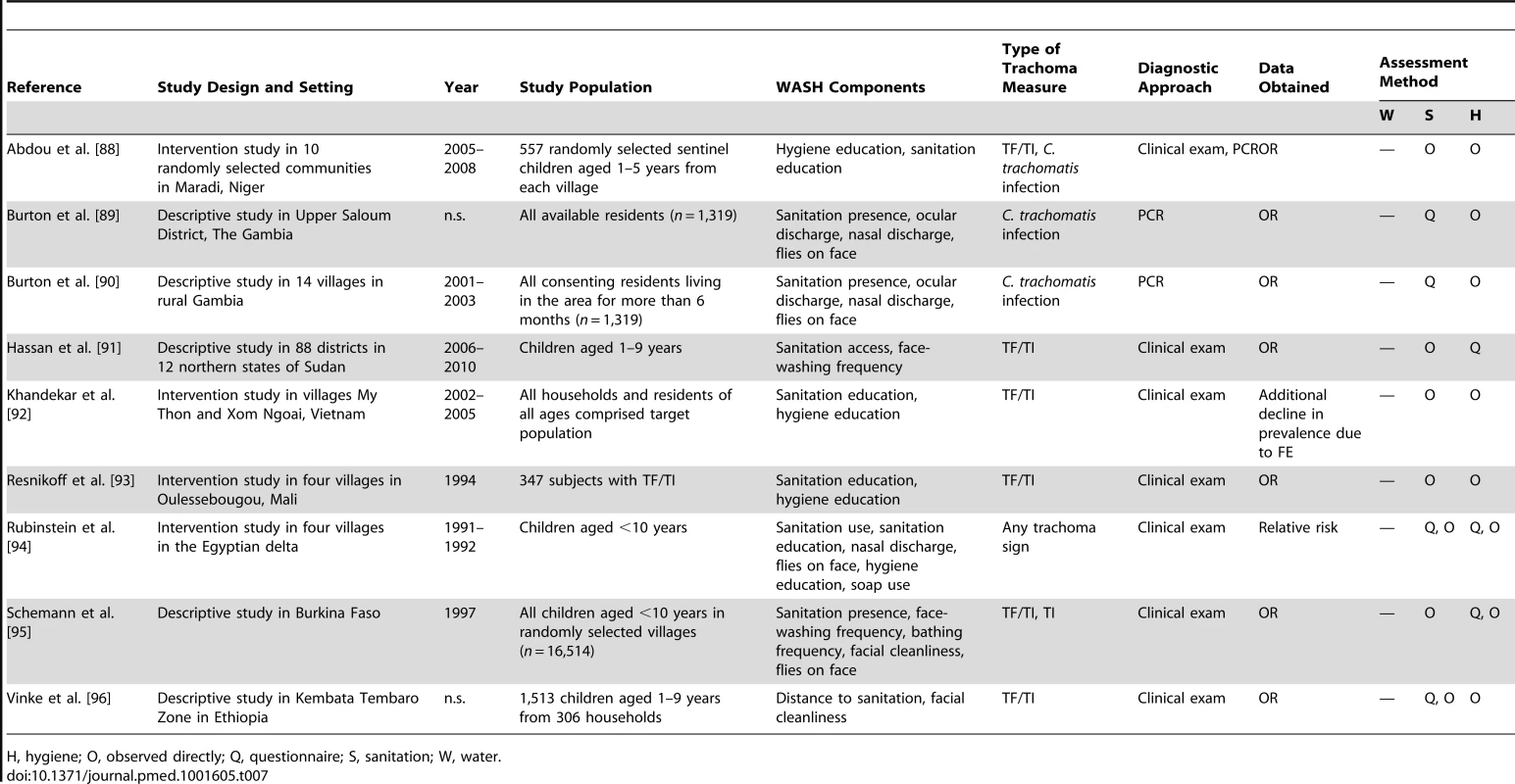
H, hygiene; O, observed directly; Q, questionnaire; S, sanitation; W, water. Tab. 8. Summary of publications reporting on water-, sanitation-, and hygiene-related risk factors. 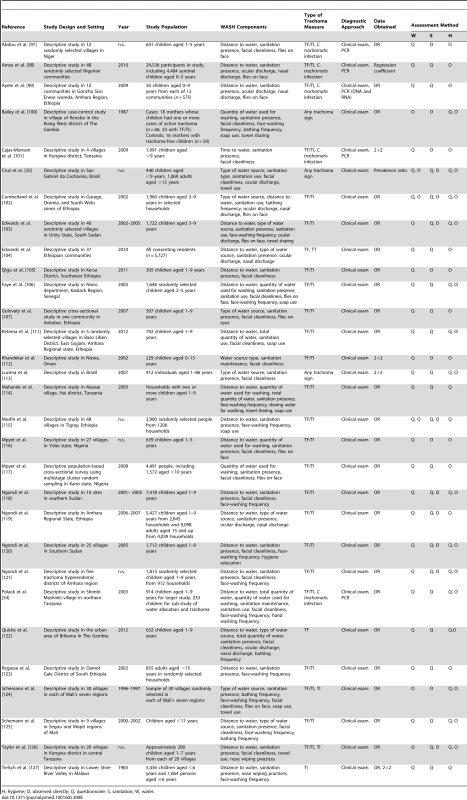
H, hygiene; O, observed directly; Q, questionnaire; S, sanitation; W, water. Tab. 9. Summary of meta-analyses examining association of WASH exposures with active trachoma (TF/TI) and <i>C. trachomatis</i> infection. 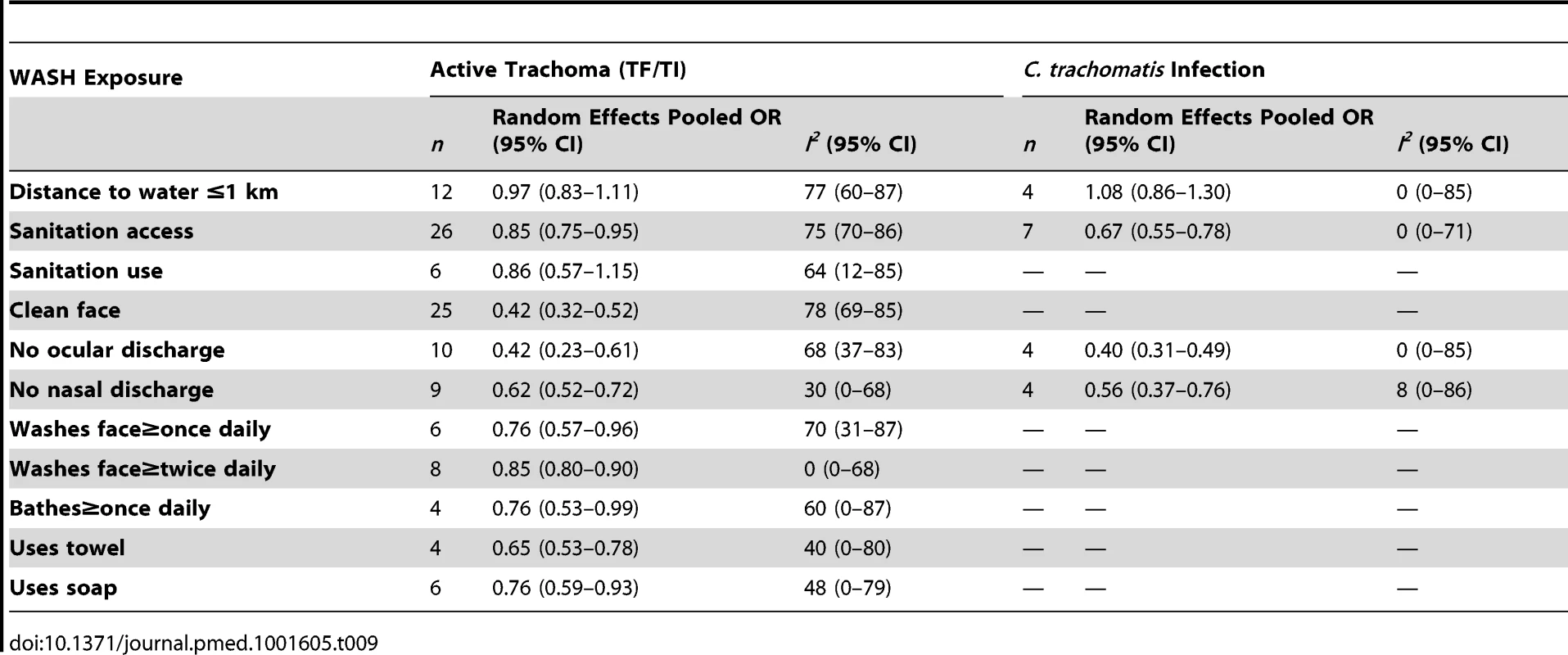
Seventy-six of the included publications were descriptive cross-sectional surveys, assessing at least one WASH-related condition or risk factor for C. trachomatis infection or clinical disease; ten publications involved some WASH-related intervention. Of the 453 effect measures from the included studies, 127 were reported as adjusted ORs, 269 were reported as unadjusted ORs, 20 were unadjusted ORs calculated from raw data using 2×2 tables, and 37 were reported as some other measure of effect (age-standardized rates, risk ratios, regression coefficients, etc.). Tansy Edwards, Joanne Katz, Jeremy Keenan, Caleb Mpyet, and Candace Vinke sent raw data in the form of 2×2 tables in response to a data request. Eighty percent of the studies were conducted in Africa, 10% were conducted in Asia, and 10% were conducted in Latin America. In general, the quality was low for studies included in the meta-analyses (see forest plots). These quality scores were due in part to the observational nature of nearly all studies, as well as the fact that WASH was the primary exposure of interest in only 32 of the studies.
Definitions of WASH Characteristics
“Sanitation access” was generally assessed through interviews or questionnaires given to household head or child caretaker, sometimes verified through direct observation of the presence of a sanitation facility, which typically meant presence of a household-level, onsite, toilet or latrine facility. Access does not necessarily take into account use of the facility, which was generally assessed independently using questionnaires given to the head-of-household or a child's caregiver and, in some cases, verified through observation of indirect sanitation usage indicators (e.g., the presence of a beaten path to the door of the sanitation facility or the presence of feces). “Sanitation type” mainly referred to shared status or the location of a toilet or latrine (within house versus within compound). Each study assessing “facility maintenance” defined a different method of assessing and grading maintenance. All “sanitation education” was part of larger programs that also included a hygiene education component.
The time and distance increments used in examining the effect of “distance to water” varied from study to study, although the baseline for comparison was most commonly <30 min, or within 1 km to source. Because households often estimate distance to source in terms of travel time, WASH household surveys commonly equate a 30 min round trip from household to source (15 min each way) to a distance of 1 km (average walking speed of 4 km/hr) [31],[32]. As such, we standardized distance-to-source and time-to-source metrics where possible, according to this commonly used conversion, in order to include related ORs in a single meta-analysis. Similarly, there were no common volume increments used when evaluating the effect of “quantity of water used for washing” or “total quantity of water” collected by a household. The definition of “quantity of water used for washing” also varied by study (e.g., overall volume of water used for washing for the entire household versus volume used for individual children). “Water source type” most commonly examined the relative effects of community-specific water sources (e.g., tap versus draw-well versus hand-pump versus “other”, or indoor plumbing versus outdoor plumbing, comparing different types of wells, etc.) or compared “safe” and “unsafe” water sources as defined in each study. “Water access” as an exposure was poorly defined. In general, studies measured the presence of some specific water source within a village or the reported year-round availability of water.
The definition of “facial cleanliness” varied slightly from study to study, but generally was defined as the lack of ocular discharge, nasal discharge, and/or flies on face at the time of clinical examination. “Ocular discharge” included any discharge in or surrounding the eye, including “sleep” in eyes, and “nasal discharge” included any discharge present from the nose. “Face washing frequency” and “bathing frequency” were assessed using questionnaires and were most commonly reported as at least once daily (versus not daily) and at least twice daily (versus only daily or not daily) for face-washing frequency, and at least once daily (versus not daily) for bathing frequency. “Soap use” and “towel use” were also assessed using questionnaires and were reported as either use or no use. “Towel sharing” was defined as self-reported towel sharing or the availability of less than one towel per person. “Nose wiping practices” were also self-reported and compared children who wipe or blow nose using clothing or handkerchief and those who used neither. “Hygiene education” included both cross-sectional assessments of exposure or uptake of hygiene education and interventions involving hygiene education and promotion, including combined programs that also included sanitation education.
Literature Density
Studies often examined more than one WASH exposure or condition and sometimes reported multiple effect measures (e.g., incremental measures of distance or face-washing frequency). Table 1 provides the total number of articles reporting on each WASH exposure, and Figure 3 shows the distribution of articles reporting on various combinations of WASH components. Effect measures, the number of parameters reported in the literature to compare a WASH exposure and trachoma outcome, are reported throughout the manuscript.
Fig. 3. Publications reporting on the association between trachoma and water, sanitation, and hygiene exposures. 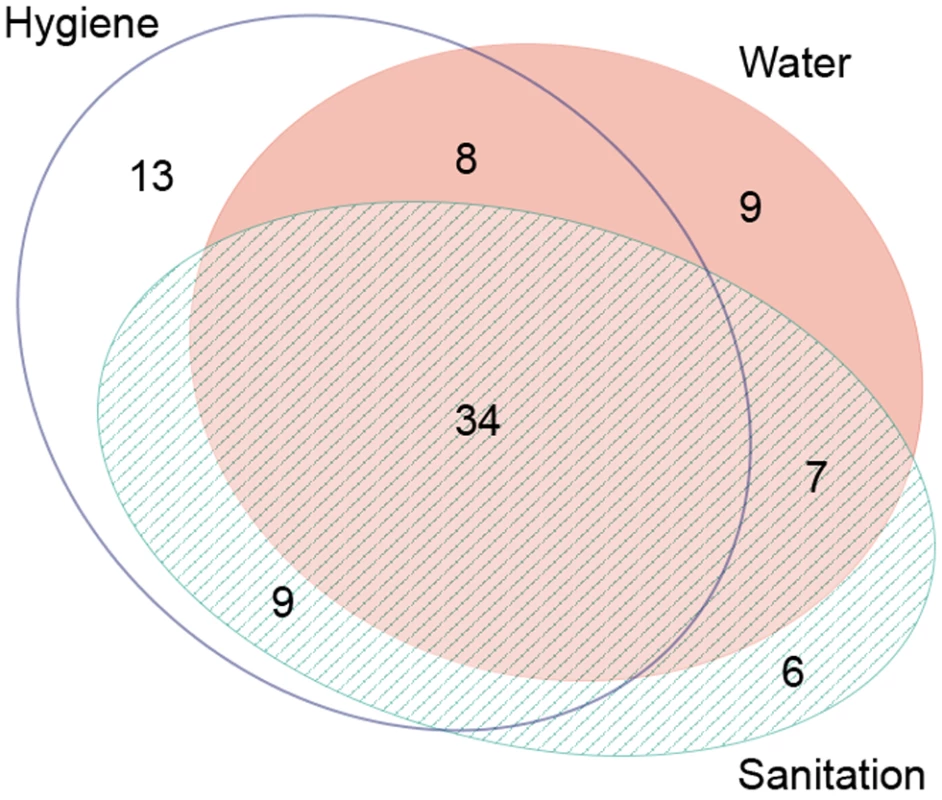
Created using eulerAPE software. Water-Related Exposures or Risk Factors
Fifty-eight of the studies identified in our systematic review reported at least one measure of effect for a water-related exposure or risk factor on trachoma, with a total 151 reported effect measures overall. Two intervention studies that improved access to water by installing water sources were identified; the remaining studies were observational. Study participants were chosen at random, either at individual or at household level in 69% of the relevant studies. In 14 studies, all individuals of a particular community, village, or special population group were enrolled, whereas no selection criteria for study participation were specified in four studies.
Figures 4 and 5 illustrate the association between distance to water and TF/TI and C. trachomatis infection, respectively. Distance to water was reported in 38 publications, with a total reported 86 measures of effect. We included articles reporting on <1 km to water (or <30 min to collect water) in our meta-analyses [33]. We found no significant association between <1 km to water and TF/TI (OR 0.97, 95% CI 0.83–1.11) nor <1 km to water and C. trachomatis infection (OR 1.08, 95% CI 0.86–1.30).
Fig. 4. Meta-analysis examining the association of distance to water (≤1 km) with TF/TI. 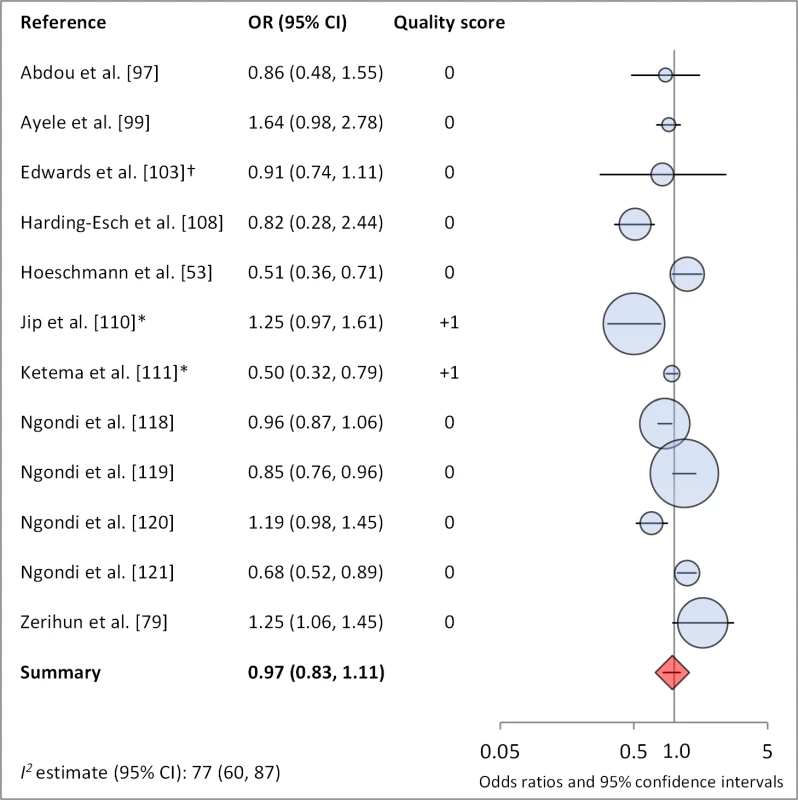
Circles indicate ORs, while the size of circles represents the sample size. Horizontal lines represent 95% confidence intervals. The diamond and corresponding line represent the random effects pooled OR and 95% confidence interval. *OR was adjusted for possible confounders. †OR was calculated using data sent from author. Fig. 5. Meta-analysis examining the association of distance to water (≤1 km) with C. trachomatis infection. 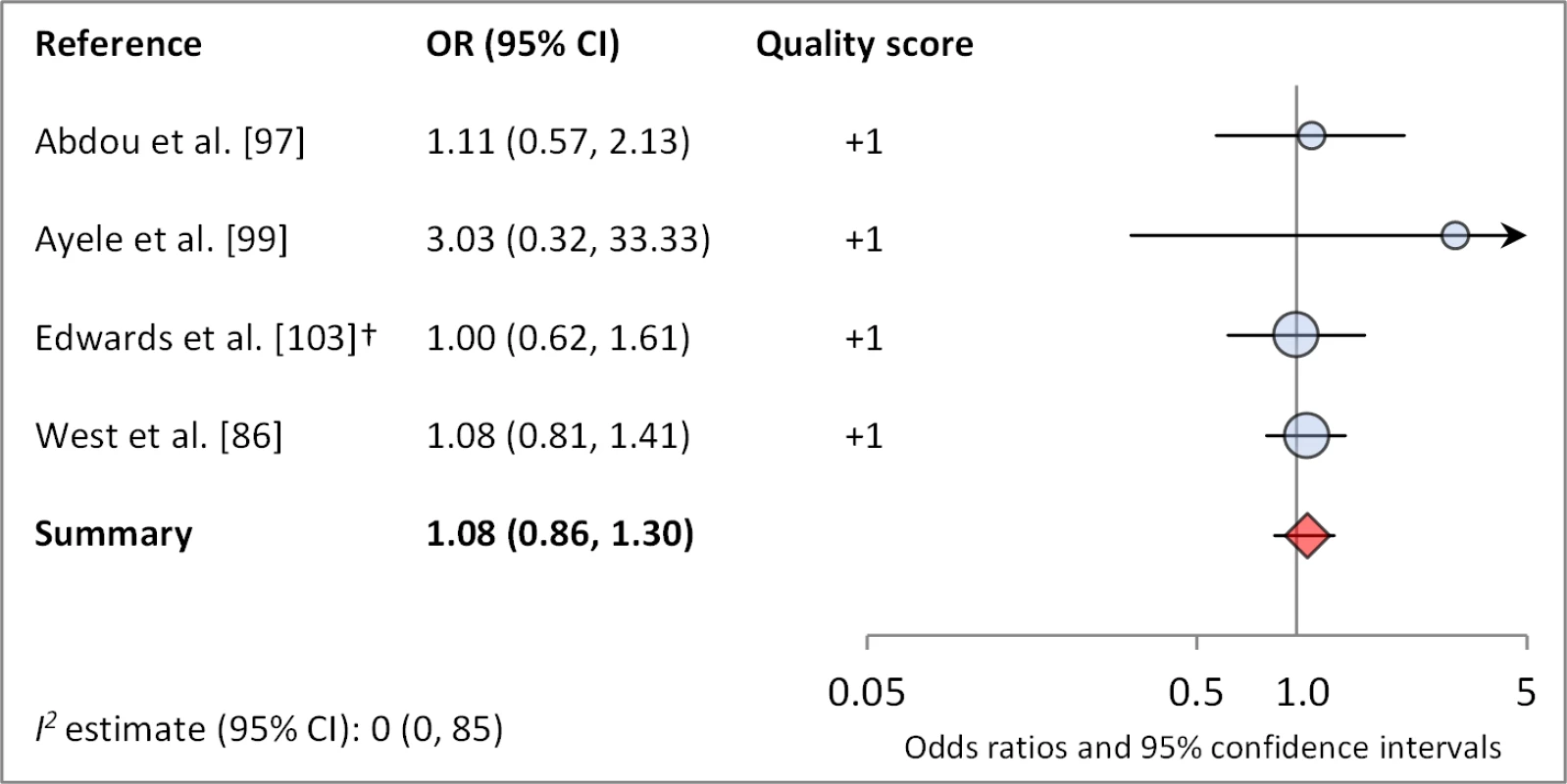
†OR was calculated using data sent from author. Meta-analyses were not performed for other water-related conditions or risk factors owing to the lack of sufficient number of studies with comparable ORs and the variation in how exposures were measured and defined across studies (e.g., the type of water source reported in a study varied depending on context, and there were no common increments in measuring quantity of water collected or used for washing). The components for which meta-analyses were not conducted included: type of water source (20 articles, 37 effect measures), access to water (seven articles, seven effect measures), total quantity of water used for washing (six articles, 16 effect measures), and total quantity of water used daily within a household (six articles, nine effect measures). Despite the high number of water source-related studies identified, no meta-analyses were conducted because water source types tended to be poorly defined, and individual studies generally examined the relative effects of site-specific water sources, making comparison of across studies difficult. No clear significant effect of water source type was found across the articles, but, in general, those that showed a significant association between water source and trachoma found that “safer” water sources (those defined as “safe or protected” or piped water versus various types of wells versus an unprotected source) were protective against trachoma. “Access to water” was defined as either the presence of an improved water source or self-reported access to water year-round versus sporadically; most effect measures assessing access to water were not statistically significant (four effect measures), but those did find a significant effect suggest improved access is protective (three effect measures). Additionally, neither intervention that included the component improving water access led to a significant reduction in trachoma. Seven reported ORs suggest that using more water for washing is associated with significantly lower odds of trachoma, but the remaining nine reported ORs did not show a statistically significant relationship. Four of six studies reported a significant association between increased total quantity of water and lowered odds of trachoma. One study determined that, regardless of total water collection, using a higher proportion of the total household water for hygiene is protective [34].
Sanitation-Related Exposures or Risk Factors
Fifty-six of the studies identified in our systematic review reported at least one measure of effect for a sanitation-related risk factor on trachoma, with a total of 96 reported effect measures overall. Four of these studies involved interventions to improve sanitation or sanitation/hygiene promotion; the remaining studies were observational. Study participants were chosen at random, either at individual or at household level in 68% of relevant studies. In 17 studies, all individuals of a particular community, village, or special population group were enrolled, whereas no selection criteria for study participation were specified in one study.
Figures 6 and 7 illustrate the association between sanitation and trachoma. We found that access to sanitation was associated with lower trachoma as measured by presence of TF/TI (OR 0.85, 95% CI 0.75–0.95) and C. trachomatis infection (OR 0.67, 95% CI 0.55–0.78). TF/TI was not significantly lower for those that reported higher levels of sanitation use (OR 0.86, CI 95% 0.57–1.15) (Figure 8).
Fig. 6. Meta-analysis examining the association of sanitation access with TF/TI. 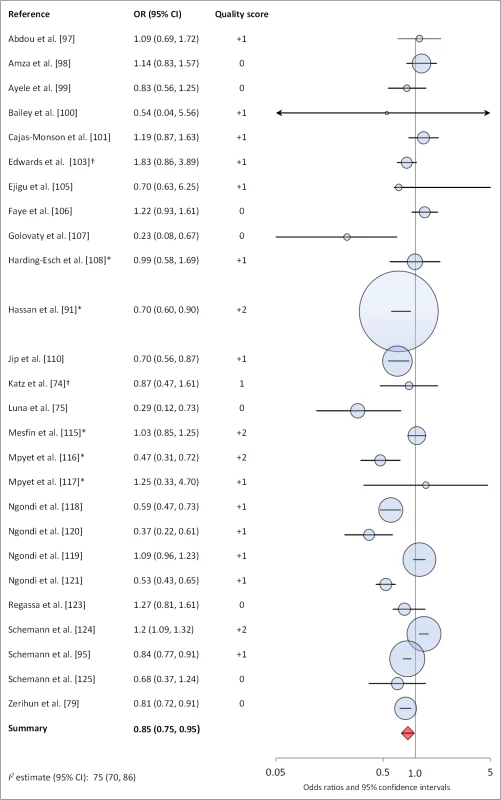
*OR was adjusted for possible confounders. †OR was calculated using data sent from author. Fig. 7. Meta-analysis examining the association of sanitation access with C. trachomatis infection. 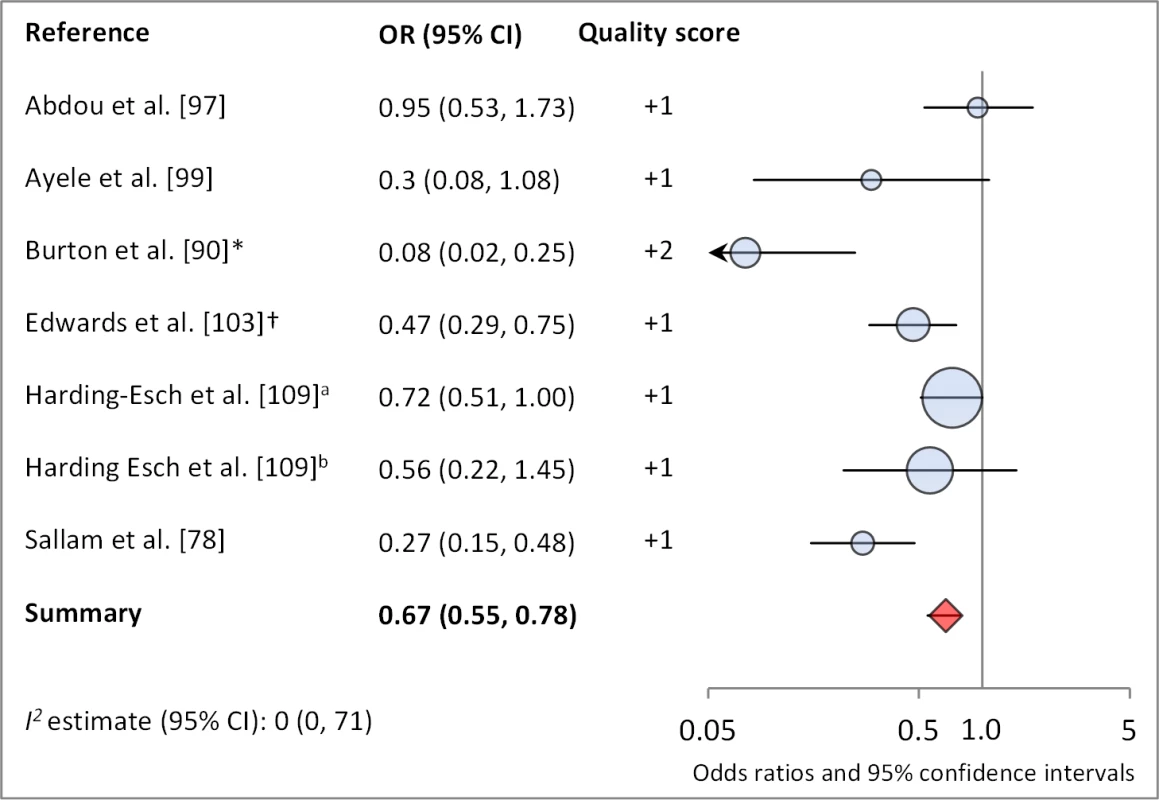
Results reported separately for aThe Gambia population, bTanzania population. *OR was adjusted for possible confounders. †OR was calculated using data sent from author. Fig. 8. Meta-analysis examining the association of sanitation use with TF/TI. 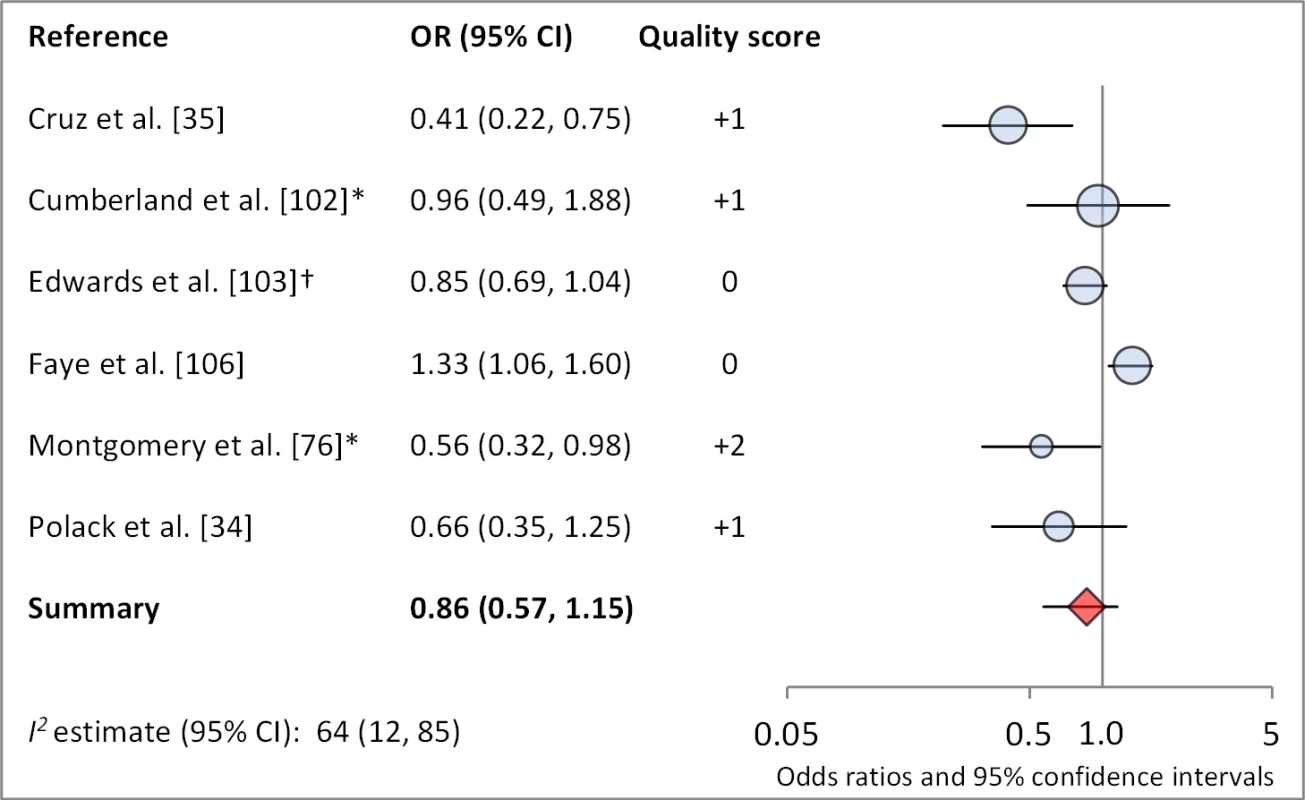
*OR was adjusted for possible confounders. †OR was calculated using data sent from author. Meta-analyses were not performed for other sanitation-related conditions or risk factors owing to a lack of sufficient number of studies with comparable ORs and the variation in how exposures were measured and defined across studies (e.g., there was no standard definition of either “facility maintenance” or “sanitation type”). These exposures included sanitation type (six articles, 11 effect measures), sanitation education (three articles, six effect measures), and facility maintenance (three articles, five effect measures). One study found the presence of a sanitation facility within the house to be significantly protective against TF/TI compared to facilities located outside of the house [35], and another found that a facility with no privacy may be associated with increased odds of TF [36]. The remaining four studies found no association between type of sanitation and trachoma. Three studies examined the effect of sanitation education or promotion on trachoma, but these analyses were part of larger programs also addressing hygiene and face washing, thus sanitation education could not be isolated. Two of three studies showed FE combined education intervention villages had significantly lower trachoma at follow-up. One study examined the effect of sanitation promotion without hygiene education but found no statistically significant effect [37].
Hygiene-Related Exposures or Risk Factors
Sixty-four studies identified in our systematic review reported some association between a hygiene-related exposure and trachoma, with a total of 213 reported measures of effect. Six studies assessed the effect of a hygiene education intervention on trachoma; all other studies were observational. Study participants were chosen at random, either at individual or at household level in 61% of relevant studies. In 23 studies, all individuals of a particular community, village, or special population group were enrolled, whereas no selection criteria for study participation were specified in two studies.
Facial cleanliness status was reported in 35 publications, with a total reported 49 measures of effect. We found that having a clean face was protective against TF/TI (OR 0.42, 95% CI 0.32–0.52) (Figure 9). No ocular discharge at the time of examination was associated with reduced odds of both TF/TI (OR 0.42, 95% CI 0.23–0.61) (Figure 10) and C. trachomatis infection (OR 0.40, 95% CI 0.31–0.49) (Figure 11). We also found significant associations between lack of nasal discharge and reduced odds of both TF/TI (OR 0.62, 95% CI 0.52–0.72) (Figure 12) and C. trachomatis infection (OR 0.56, 95% CI 0.37–0.76) (Figure 13). The relationship between TF/TI and face washing at least once a day (OR 0.76, 95% CI 0.57–0.96) (Figure 14) was stronger than the relationship between TF/TI and washing at least twice a day (OR 0.85, 95% CI 0.80–0.90) (Figure 15), and soap use was significantly associated with reduced TF/TI (OR 0.76, 95% CI 0.59–0.93) (Figure 16). Few studies reported measures of effect between TF/TI and bathing at least once a day (OR 0.76, 95% CI 0.53–0.99) (Figure 17) or towel use (OR 0.65, 95% CI 0.53–0.78) (Figure 18).
Fig. 9. Meta-analysis examining the association of clean face with TF/TI. 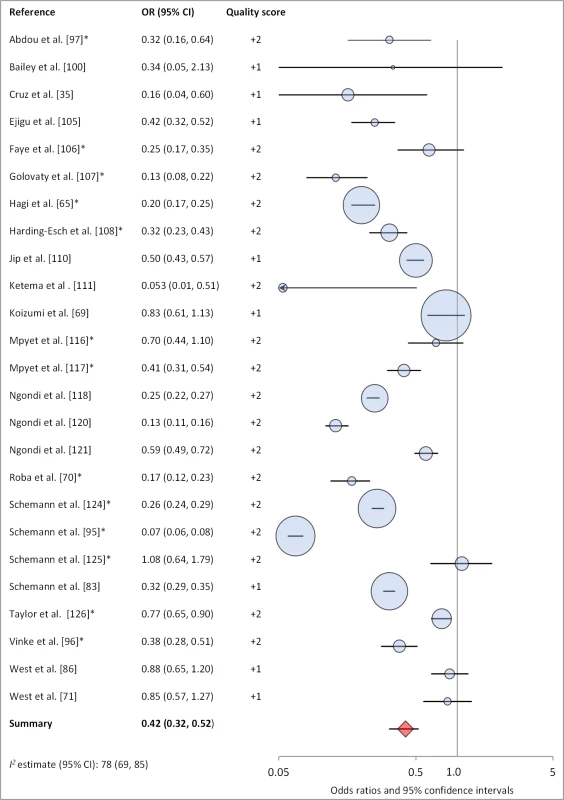
*OR was adjusted for possible confounders. Fig. 10. Meta-analysis examining the association of no ocular discharge with TF/TI. 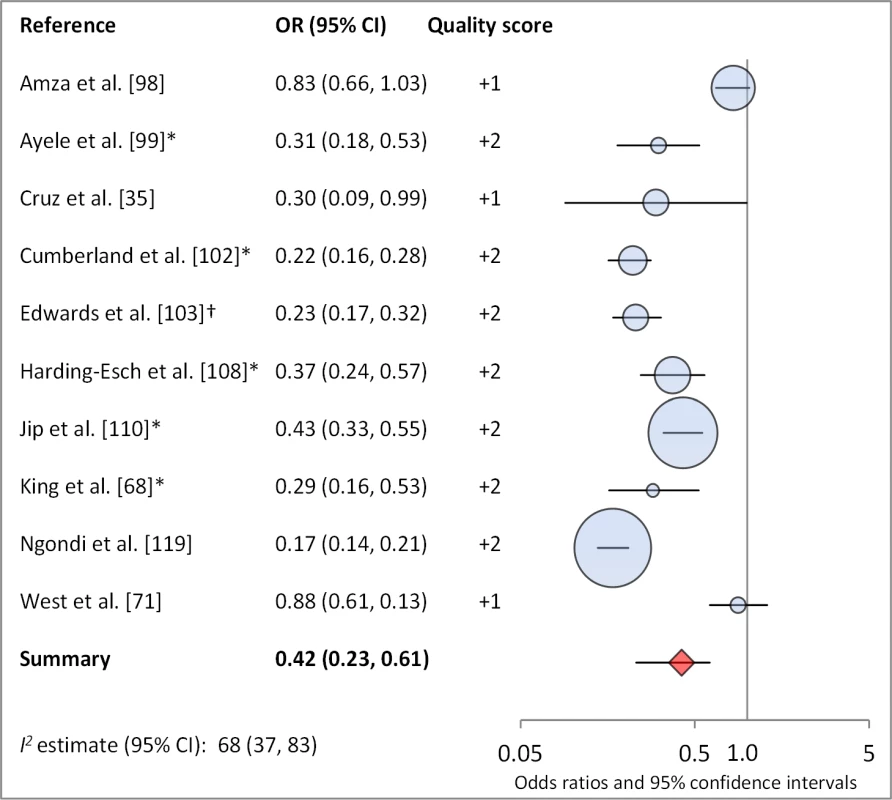
*OR was adjusted for possible confounders. †OR was calculated using data sent from author. Fig. 11. Meta-analysis examining the association of no ocular discharge with C. trachomatis infection. 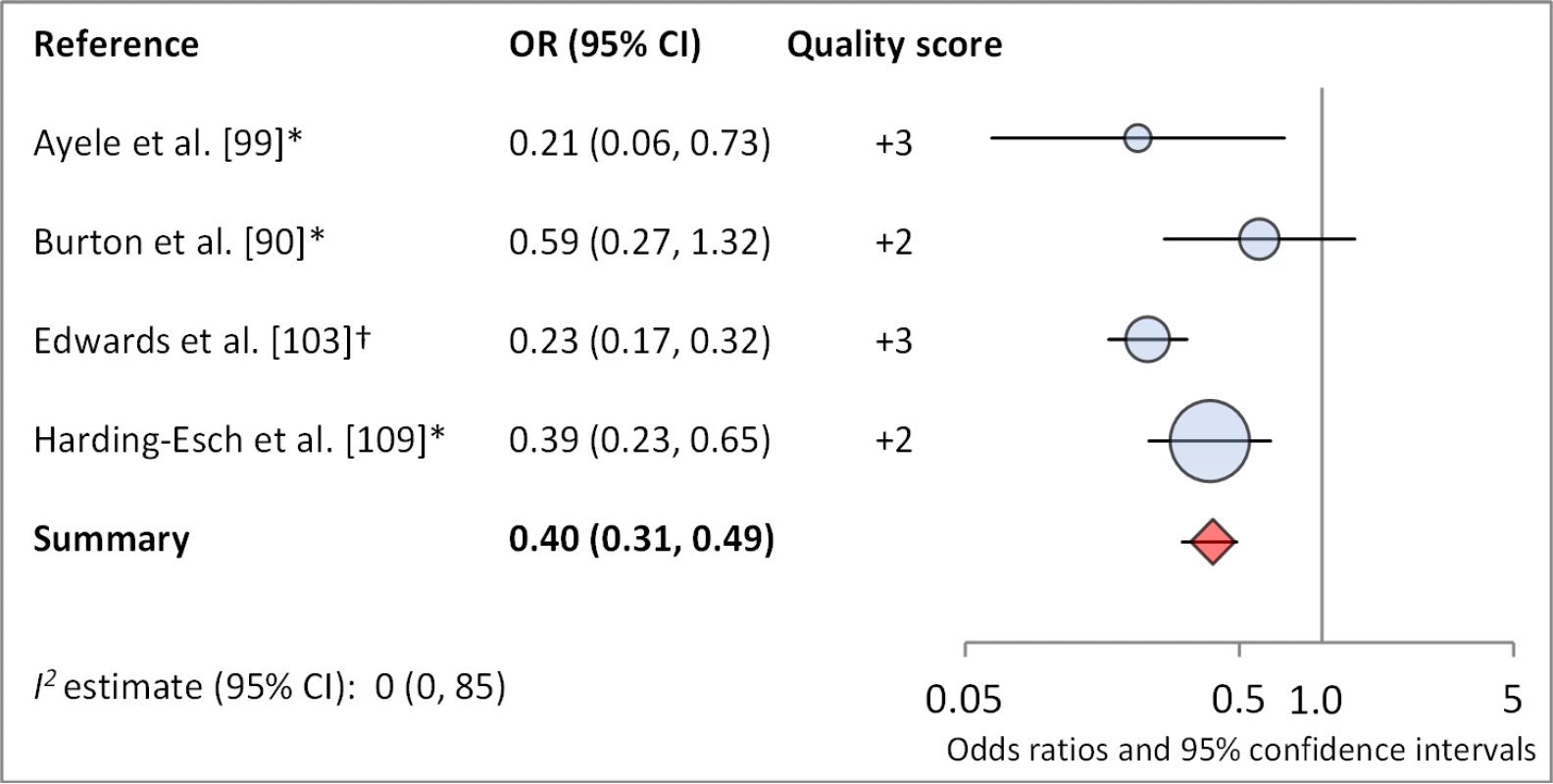
*OR was adjusted for possible confounders. †OR was calculated using data sent from author. Fig. 12. Meta-analysis examining the association of no nasal discharge with TF/TI. 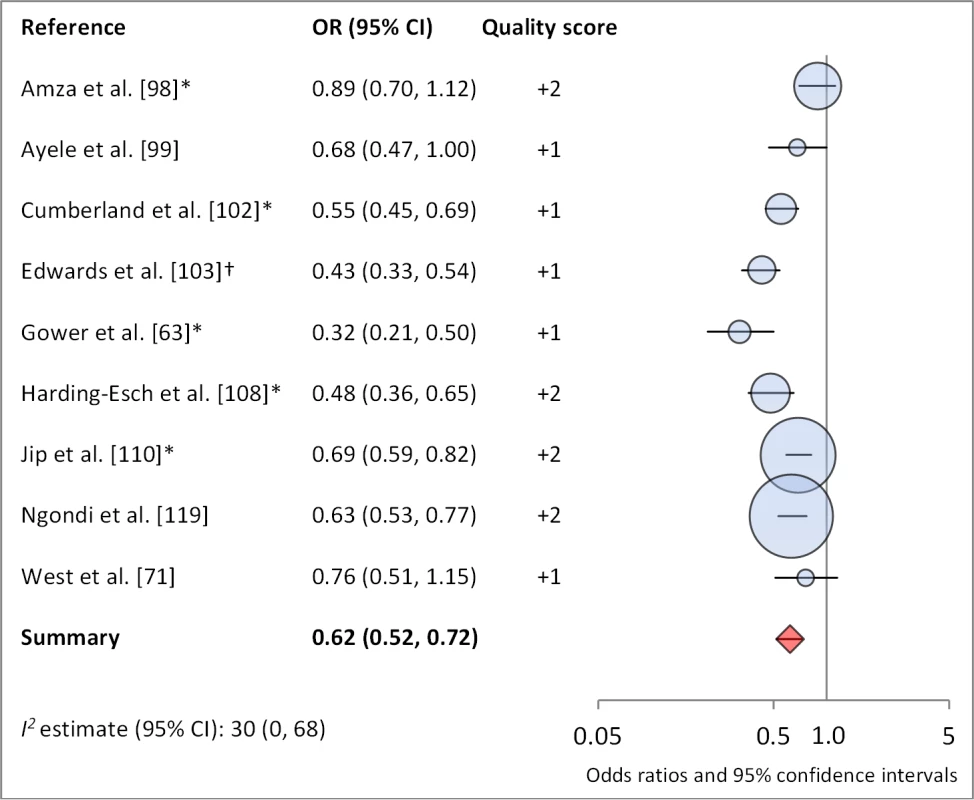
*OR was adjusted for possible confounders. †OR was calculated using data sent from author. Fig. 13. Meta-analysis examining the association of no nasal discharge with C. trachomatis infection. 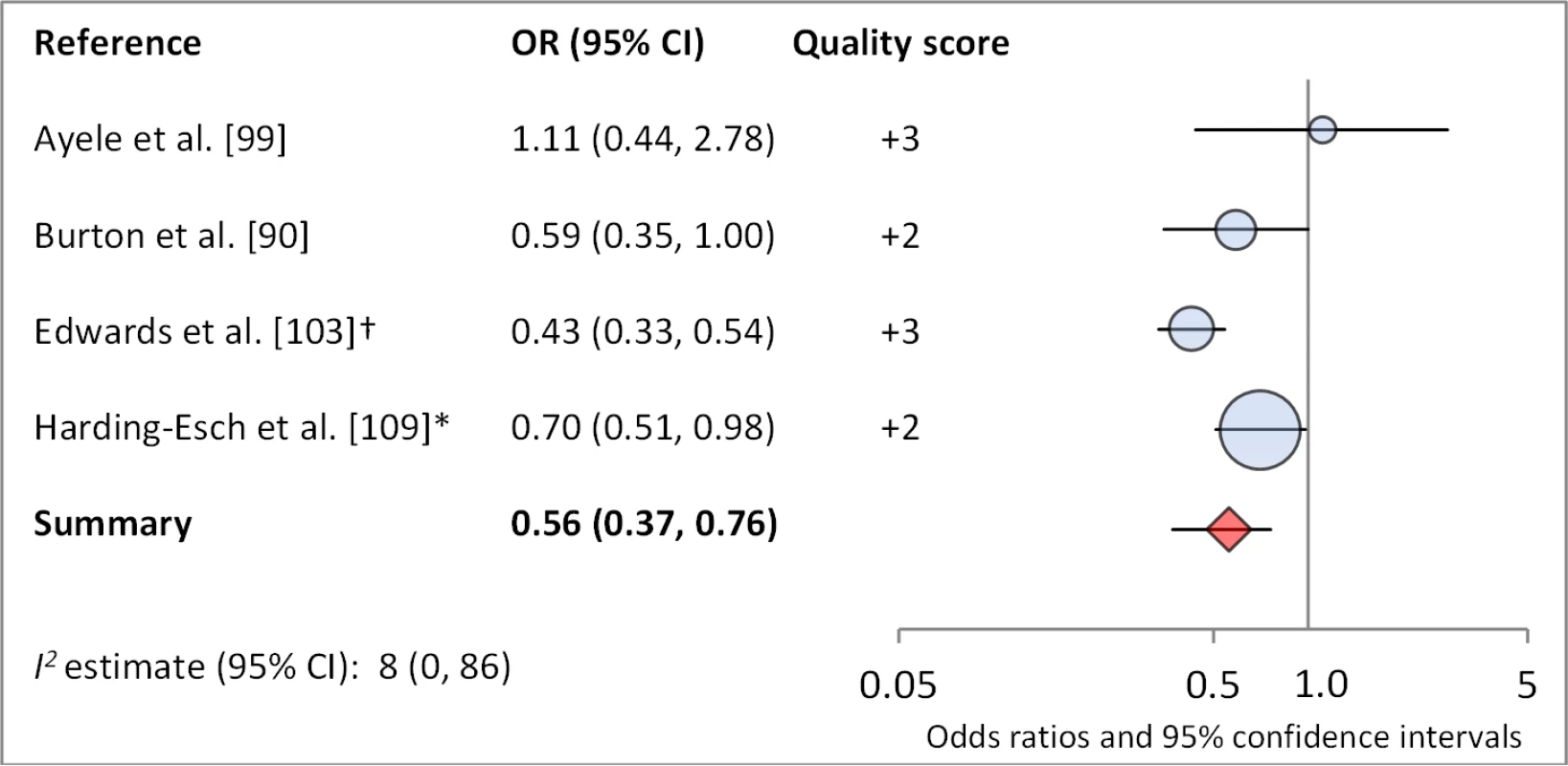
*OR was adjusted for possible confounders. †OR was calculated using data sent from author. Fig. 14. Meta-analysis examining the association of washing face ≥1 time per day (versus <1 time per day) with TF/TI. 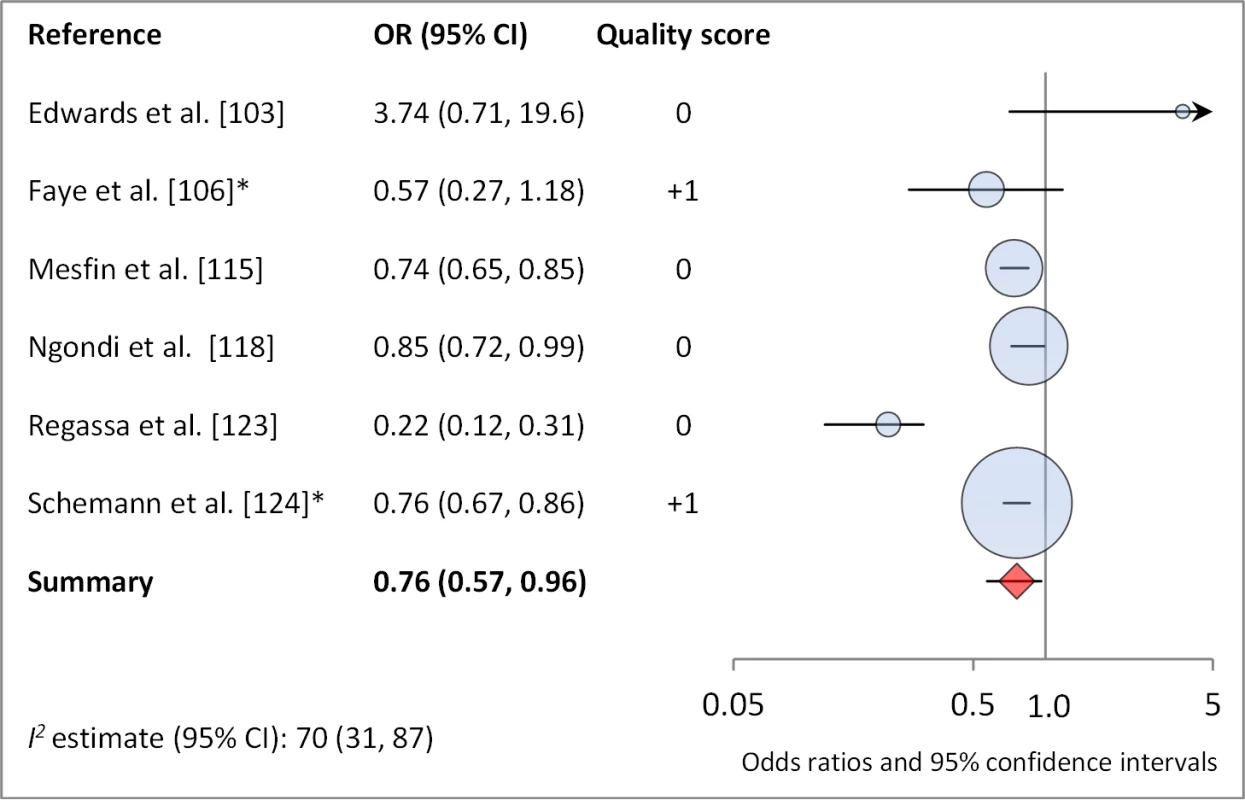
*OR was adjusted for possible confounders. Fig. 15. Meta-analysis examining the association of washing face ≥2 times per day (versus <2× per day) with TF/TI. 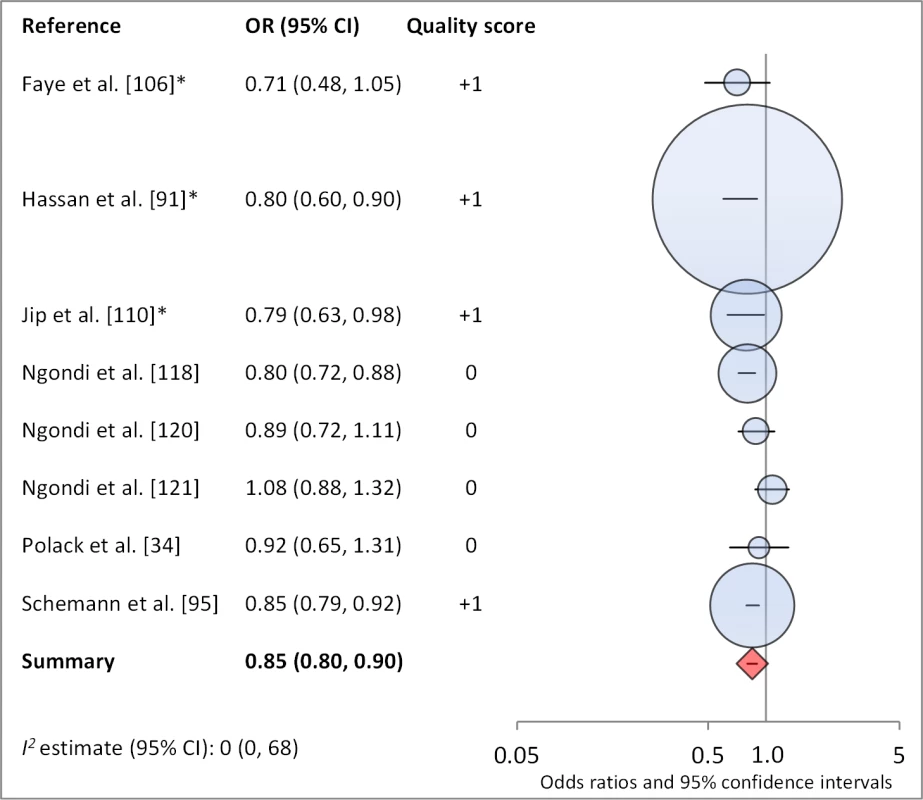
*OR was adjusted for possible confounders. Fig. 16. Meta-analysis examining the association of towel use with TF/TI. 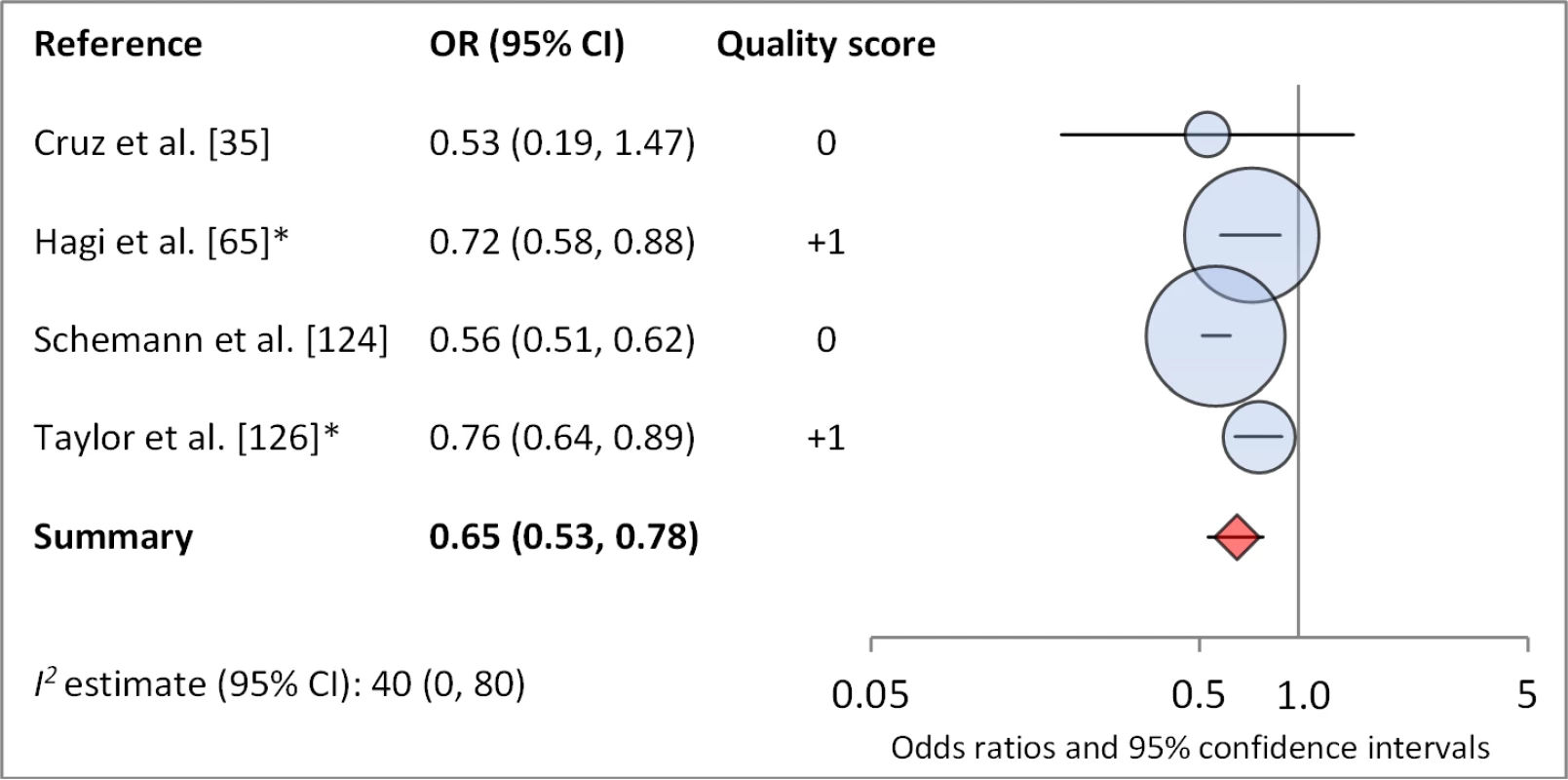
*OR was adjusted for possible confounders. Fig. 17. Meta-analysis examining the association of soap use with TF/TI. 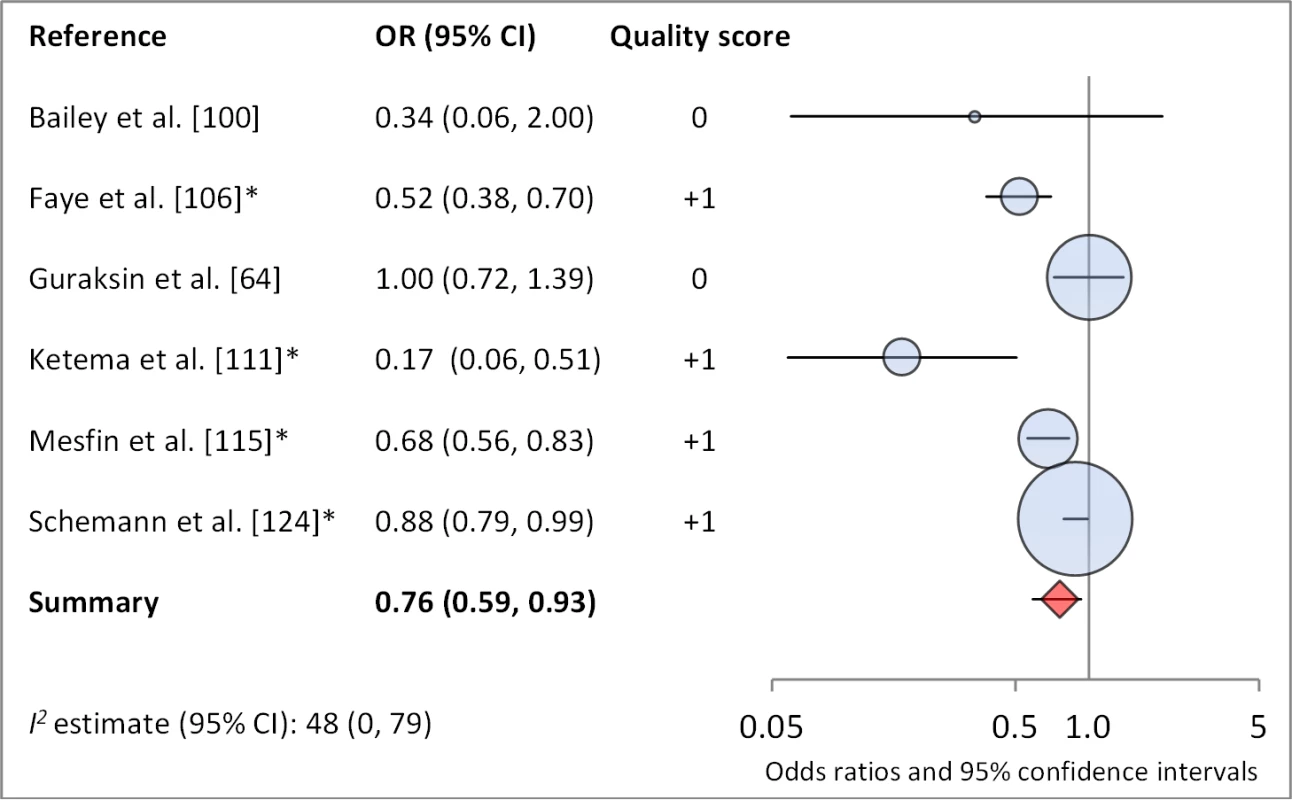
*OR was adjusted for possible confounders. Fig. 18. Meta-analysis examining the association of bathing at least once daily with TF/TI. 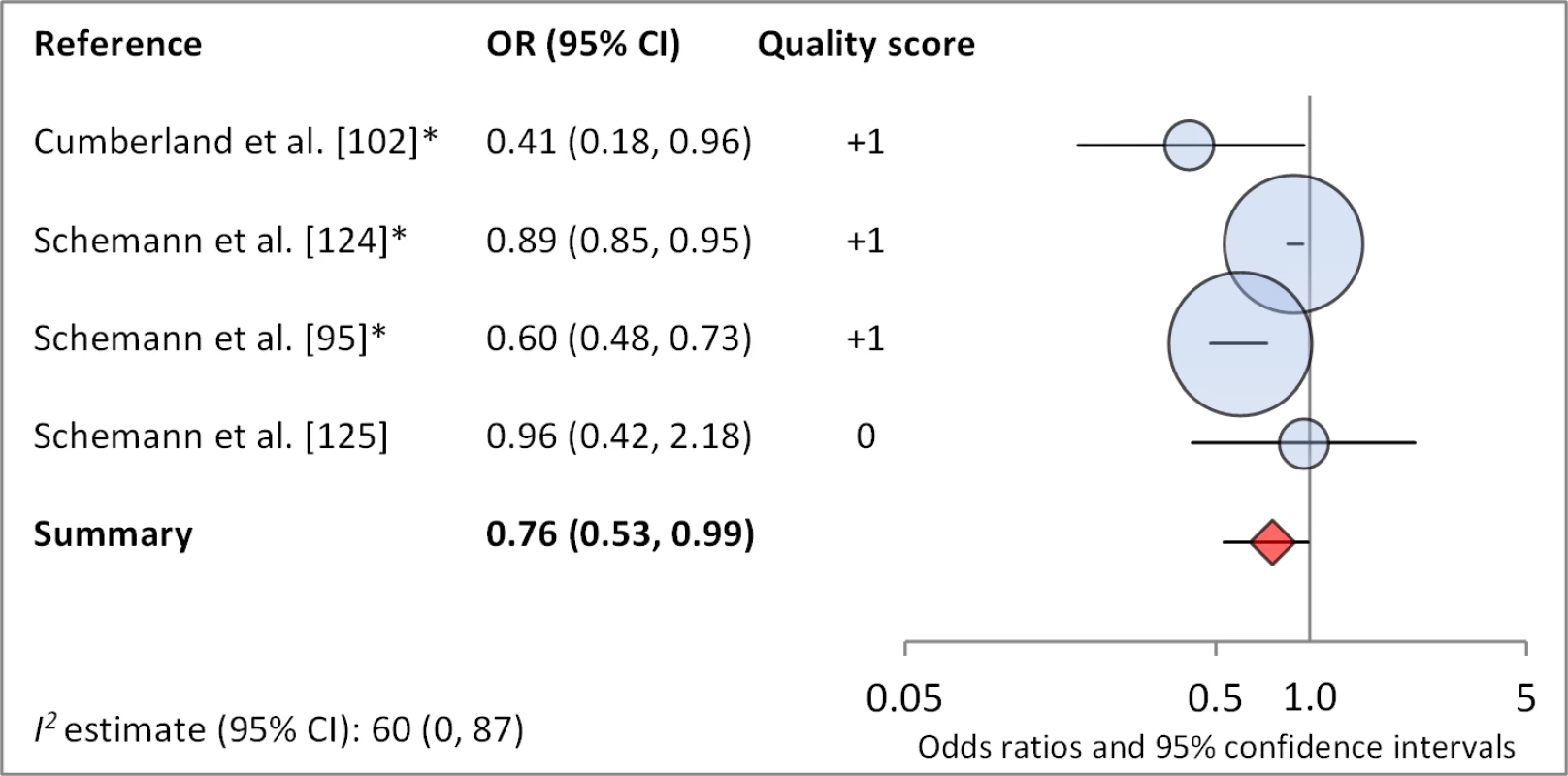
*OR was adjusted for possible confounders. Meta-analyses were not performed for other hygiene-related conditions or risk factors due to a lack of sufficient number of studies with comparable ORs, often resulting from variation in how exposures were measured and defined across studies (e.g., the nose-wiping practices of interest varied from study to study, hygiene education interventions may or may not have included a sanitation component). These exposures included towel sharing (three articles, four measures of effect), hygiene education (six articles and 12 measures of effect), and nose-wiping practices (five articles, seven measures of effect). Towel sharing among family members was not found to be significantly associated with trachoma in any study. One of two interventions focused solely on face-washing education reported a significant reduction in severe trachoma (TI) compared to control villages [38]. Two of the three interventions that combined hygiene and sanitation education components showed a significant reduction in TF/TI in villages receiving education. All five studies addressing nose-wiping practices reported significant effects: three studies showed that using clothing to blow nose is a significant risk factor for some form of clinical trachoma; the remaining two studies showed significant associations between handkerchief use and lower odds of TF/TI, TI, and C. trachomatis infection.
Discussion
Our analysis revealed evidence of an association between improved WASH conditions and exposures and reduced trachoma in 11 of the 15 meta-analyses conducted with the available literature. Pooled estimates of effect for the relationship between a WASH exposure and clinical trachoma (TF/TI) ranged from strong effects associated with a clean face (OR 0.42) to little evidence of an association for distance to water source <1 km (OR 0.97). Three of the four meta-analyses assessing trachoma through PCR found associations between WASH conditions and lower infection with C. trachomatis. Access to sanitation was strongly associated with lower levels of trachoma, both TF/TI and C. trachomatis. The relative strength of the association between sanitation access and C. trachomatis infection compared to TF/TI may be due in part to the persistence of clinical signs of trachoma for some time after infection is eliminated [39]. Only seven studies reported estimates of the relationship between sanitation use and TF/TI, and while the meta-analysis suggested an association that was not statistically significant.
We found the strongest evidence of the association between hygiene factors and trachoma. Our analyses suggest that the presence of a clean face, the lack of ocular and nasal discharge, increased frequency of face washing, towel use, the use of soap, and daily bathing were all associated with lower odds of trachoma. The relationship between a clean face and reduced odds of trachoma was one of the strongest associations. The shared use of towels, which has long been thought to be a key risk factor in the transmission of trachoma [40]–[42], was not found to be significantly associated with increased risk of trachoma in any of the identified studies, though too few studies were available to conduct a meta-analysis. Our meta-analysis suggest that facial cleanliness and the absence of either ocular or nasal discharge are highly associated with decreased odds of infection with C. trachomatis. However, there is a potentially tautological effect, as trachoma causes inflammation of the eyes, which results in lacrimation and ocular discharge that contains contagion [43]. It stands to reason that removing ocular discharge removes contagion, but ocular discharge itself may also be a symptom of trachoma, and so ocular discharge and active trachoma are both risk factors and effects. However, considering the strong association between facial cleanliness and nasal discharge and trachoma infection, our results suggest that health behaviors that result in clean faces may reduce the prevalence of trachoma. Our meta-analysis examining daily face-washing practices suggests that washing once a day may decrease odds of TF/TI, although washing more than once a day does not appear to result in an additional decrease. The use of soap was associated with lower levels of trachoma, which is particularly relevant as current strategies for trachoma control advocate for face washing with water, but do not consistently emphasize the use of soap [41].
Our analysis did not reveal any evidence of an assocation between the distance to drinking water source and C. trachomatis infection or TF/TI. The lack of an observed effect may be due in part to the 1 km cutpoint used in most studies, which may not be a meaningful standard with respect to trachoma control. A more proximate drinking water source may serve as an indicator for increased quantity of water [32], but is not a guarantee of improved water quality [44]. Given the strong association found between hygiene and trachoma, a lower cutoff value for distance to water source, which ensures more assuredly adequate water quantity for household uses, may be preferable. We found a number of studies that reported an association between improved water quantity and reduced odds of trachoma, but there was an insufficient number of articles to conduct a meta-analysis. Data on water access were limited by lack of standardization of definitions and metrics and the resulting low comparability across studies.
Our review identified relationships between WASH conditions and trachoma that require additional research. While we found several studies assessing the association of household sanitation access and facial cleanliness on signs of trachoma, there are comparatively fewer exploring the relationship between water use and trachoma, as well as other characteristics of sanitation, such as maintenance of sanitation facilities and use of facilities shared by more than one household. As a result, the association of these variables with trachoma and the potential role of these variables in trachoma control remains unknown.
Strengths and Limitations
We searched three widely used databases—EMBASE, Web of Science, and PubMed—as well as MedCarib, Lilacs, REPIDISCA, DESASTRES, and African Index Medicus using a pre-specified, systematic search protocol. In addition, we hand-searched the bibliographies of available reviews. We adhered to the MOOSE guidelines for reporting meta-analysis of observational studies. The lack of standard definitions and methods of measuring WASH components in the available studies made meta-analysis for certain exposures infeasible. In addition, variations in the definitions and methods could have introduced error in our meta-analyses. Study design and setting varied, and heterogeneity was moderate to high in some meta-analyses, particularly those examining the relationship between WASH and TF/TI (refer to I2 values and 95% confidence intervals in Table 9). Although infection with C. trachomatis is a more objective measure of trachoma and is likely associated with more temporal access to WASH, it was the outcome of interest in only four of our 15 meta-analyses, as most studies reported only on clinically evident signs of TF/TI, which could be the result of past infection and more related to cumulative exposure to risk factors. Overall, funnel plots did not appear to show high publication bias (Figure S1). However, it is clear from our review that the majority of studies that report on trachoma prevalence do not report on associations with WASH. Many studies only reported the statistically significant ORs from multivariable analysis. In these cases, ORs available from the univariable were used in meta-analyses; however, as these univariable ORs are not adjusted for possible confounders, they may not be as reliable as ORs from multivariable analyses. It is difficult to draw solid conclusions for those WASH exposures for which meta-analyses were not possible due to scarcity of literature (e.g., facility maintenance and quality, water source type), and we could be missing important insights into the relationship between trachoma and other aspects of WASH. Finally, there is a lack of independence between our meta-analyses estimates, since many studies report multiple measures of effect that contribute to several analyses.
All studies included in meta-analyses had relatively low quality scores. These low-quality grades were due in part to the fact that only observational studies were included in the meta-analyses, and all ORs reflected cross-sectional data. WASH exposures were often assessed as secondary risk factors of interest (e.g., sanitation access could be included in a model focused on assessing an antibiotic intervention), meaning that there is a potential for publication bias if non-significant associations were not included in final published manuscripts.
We found many different measures of WASH exposures and many of these parameters were poorly described in the methods. This lack of specificity and standardization of measures limits the harmonization of findings into a meta-analysis; this creates challenges to amassing a consistent and useable body of evidence, and effectively demonstrating impact across the WASH and disease control sectors. In addition, we only reported measures of TF/TI together. Since TI has been dropped from some surveys in recent years, our measures of effect may represent small differences in the definition of the outcome.
Policy Implications
While there has been considerable progress in global access to improved drinking water supply, specifically in East and South Asia, there is evidence that these gains have systematically ignored the poorest and most marginalized populations [45],[46], which are precisely the most vulnerable to trachoma. In addition, the world is far from achieving the Millennium Development Goal target of reducing by half the global population without access to sanitation. In sub-Saharan Africa, which has the world's highest burden of trachoma, fewer than 50% of households have access to an improved sanitation facility, and more than 25% of households practice open defecation [47]. If elimination of trachoma is to be achieved, additional resources must be targeted towards sustainable access to WASH in trachoma endemic areas. Recent discourse between the neglected tropical disease (NTD) control programs and those in the WASH sector may lead to more tangible efforts at collaboration, coordination, and cooperation between the sectors [17], which are essential to achieving articulated WASH strategies, and targeted WASH implementation for trachoma reduction [12].
Face washing has long been an elemental component of the SAFE strategy. Among NGOs not specifically targeting trachoma control, however, improving WASH access is typically promoted to reduce diarrheal diseases, and as such few promote face washing alongside handwashing with soap. Our data support the importance, found by others, of daily face washing to reduce trachoma. Increasing attention to and investment in improved hygiene practices that include daily face washing will support the achievement of trachoma control and elimination targets.
Access to household sanitary facility is an important factor in trachoma, and continued use of program indicators that record progress toward improving household access to sanitation is recommended. Though sanitation use, in addition to access, is theoretically important to control trachoma, our review found no significant association between measures of sanitation use and trachoma. This may suggest that current measures of sanitation use, which vary between observation of sanitation characteristics and direct household surveys related to defecation practices, are not sufficiently accurate or consistent on the whole. Additionally, despite a scarcity of literature examining the role of sanitation facility quality or maintenance, interventions to improve the usability and acceptability of existing sanitation facilities may be effective. Further research is needed to understand whether sanitation access at a household level, or whether reaching high sanitation coverage within the community as a whole is more important to achieving trachoma reduction. None of these studies focused on schools, revealing a gap in the literature of the importance of school-based WASH promotion.
Conclusions
Our review finds that the F and E components of the SAFE strategy—specifically face washing, facial cleanliness, and sanitation access—but also general hygiene behaviors for trachoma control are well supported by evidence. Facial cleanliness and environmental improvement are, in general, highly associated with trachoma infection and disease. Lack of evidence of the association between water access and trachoma may be due to the low number of studies available or a parameterization of water access (<1 km distance to source) in a majority of studies that does not best capture the impact of increased access to water for hygiene and cleanliness. These data support the need for a more harmonized approach to monitoring WASH exposures as they relate to trachoma, better monitoring and reporting of these associations, and increased attention to and funds for the use of WASH to meet trachoma elimination targets as part of the full SAFE strategy. Further studies should employ WASH definitions compatible with those used in the WASH sector, such as the Joint Monitoring Program definitions for improved and unimproved water sources and infrastructure [47]. There is, furthermore, a clear need for more robust evidence of WASH impact on trachoma that may be achieved by including baseline and follow-up measurements of trachoma in impact evaluations of WASH programs and activities.
Supporting Information
Zdroje
1. PascoliniD, MariottiSP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96 : 614–618.
2. WHO (2013) Weekly Epidemiological Record. 24 : 241–256.
3. HuVH, Harding-EschEM, BurtonMJ, BaileyRL, KadimpeulJ, et al. (2010) Epidemiology and control of trachoma: systematic review. Trop Med Int Health 15 : 673–691.
4. BurtonMJ, MabeyDC (2009) The global burden of trachoma: a review. PLoS Negl Trop Dis 3: e460.
5. MurrayCJ, VosT, LozanoR, NaghaviM, FlaxmanAD, et al. (2013) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 : 2197–2223.
6. International Coalition for Trachoma Control (2011) 2020 INSight: the end in sight. Atlanta. 16 p.
7. MariottiSP, PascoliniD, Rose-NussbaumerJ (2009) Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol 93 : 563–568.
8. WHO (2003) Report of the second scientific meeting on trachoma. Geneva: Alliance for the Global Elimination of Blinding Trachoma by 2020.
9. WHO (1998) World Health Assembly Resolution WHA 51.11. Geneva: World Health Organization.
10. WHO (2012) Weekly Epidemiological Record. 17 : 161–168.
11. International Coalition for Trachoma Control (2011) 2020 INSight: the end in sight. Atlanta.
12. LavettDK, LansinghVC, CarterMJ, EckertKA, SilvaJC (2013) Will the SAFE strategy be sufficient to eliminate trachoma by 2020? Puzzlements and possible solutions. Sci World J 2013
13. PrüssA, MariottiSP (2000) Preventing trachoma through environmental sanitation: a review of the evidence base. Bull World Health Organ 78 : 267–273.
14. EmersonPM, CairncrossS, BaileyRL, MabeyD (2000) Review of the evidence base for the ‘F’and ‘E’components of the SAFE strategy for trachoma control. Trop Med Int Health 5 : 515–527.
15. EmersonP, KollmannM, MacArthurC, BushS, HaddadD (2012) SAFE strategy for blinding trachoma addresses sanitation, the other half of MDG7. Lancet 380 : 27–28.
16. Anonymous (2012) Progress in sanitation needed for neglected tropical diseases. Lancet 379 : 978.
17. FreemanMC, OgdenS, JacobsonJ, AbbottD, AddissDG, et al. (2013) Integration of water, sanitation, and hygiene for the prevention and control of neglected tropical diseases: a rationale for inter-sectoral collaboration. PLoS Negl Trop Dis 7: e2439.
18. RabiuM, AlhassanMB, EjereHO, EvansJR (2012) Environmental sanitary interventions for preventing active trachoma. Cochrane Database Syst Rev 2: CD004003.
19. EjereHO, AlhassanMB, RabiuM (2012) Face washing promotion for preventing active trachoma. Cochrane Database Syst Rev 4: CD003659.
20. EsreySA, PotashJB, RobertsL, ShiffC (1991) Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ 69 : 609–621.
21. KuperH, SolomonAW, BuchanJ, ZondervanM, FosterA, et al. (2003) A critical review of the SAFE strategy for the prevention of blinding trachoma. Lancet Infect Dis 3 : 372–381.
22. PrussA, MariottiSP (2000) Preventing trachoma through environmental sanitation: a review of the evidence base. Bull World Health Organ 78 : 258–266.
23. StroupDF, BerlinJA, MortonSC, OlkinI, WilliamsonGD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283 : 2008–2012.
24. ThyleforsB, DawsonCR, JonesBR, WestS, TaylorHR (1987) A simple system for the assessment of trachoma and its complications. Bull World Health Organ 65 : 477.
25. ThyleforsB, DawsonCR, JonesBR, WestSK, TaylorHR (1987) A simple system for the assessment of trachoma and its complications. Bull World Health Organ 65 : 477–483.
26. AtkinsD, BestD, BrissPA, EcclesM, Falck-YtterY, et al. (2004) Grading quality of evidence and strength of recommendations. BMJ 328 : 1490.
27. Higgins JP, Green S, Collaboration C (2008) Cochrane handbook for systematic reviews of interventions: Wiley Online Library.
28. NeyeloffJL, FuchsSC, MoreiraLB (2012) Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes 5 : 52.
29. De SoleG, MartelE (1988) Test of the prevention of blindness health education programme for Ethiopian primary schools. Int Ophthalmol 11 : 255–259.
30. HedgesLV, VeveaJL (1998) Fixed - and random-effects models in meta-analysis. Psychol Methods 3 : 486–504.
31. White G, Bradley D, White A (1972) Drawers of water: domestic water use in East Africa. Chicago: The University of Chicago Press.
32. Cairncross S, Feachem R (1993) Environmental health engineering in the tropics: an introductory text. Chichester: John Wiley & Sons Ltd.
33. UNICEF, WHO (2011) Drinking water equity, safety and sustainability: thematic report on drinking water 2011. New York: WHO/UNICEF Joint Monitoring Program for Water Supply and Sanitation.
34. PolackS, KuperH, SolomonAW, MassaePA, AbueloC, et al. (2006) The relationship between prevalence of active trachoma, water availability and its use in a Tanzanian village. Trans R Soc Trop Med Hyg 100 : 1075–1083.
35. CruzAA, MedinaNH, IbrahimMM, SouzaRM, GomesUA, et al. (2008) Prevalence of trachoma in a population of the upper Rio Negro basin and risk factors for active disease. Ophthalmic Epidemiol 15 : 272–278.
36. KaluaK, ChirwaT, KalilaniL, AbbenyiS, MukakaM, et al. (2010) Prevalence and risk factors for trachoma in central and southern Malawi. PLoS One 5: e9067.
37. StollerNE, GebreT, AyeleB, ZerihunM, AssefaY, et al. (2011) Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial. Int Health 3 : 75–84.
38. WestS, MunozB, LynchM, KayongoyaA, ChilangwaZ, et al. (1995) Impact of face-washing on trachoma in Kongwa, Tanzania. Lancet 345 : 155–158.
39. KeenanJD, LakewT, AlemayehuW, MeleseM, HouseJI, et al. (2011) Slow resolution of clinically active trachoma following successful mass antibiotic treatments. Arch Ophthalmol 129 : 512–513.
40. Boldt J (1904) Trachoma. Harvard University: Hodder and Stoughton.
41. Solomon AW (2006) Trachoma control: a guide for programme managers: World Health Organization, London School of Hygiene and Tropical Medicine, International Trachoma Initiative.
42. Taylor HR (2008) Trachoma: a blinding scourge from the Bronze age to the twenty-first century. Australia: Center for Eye Research.
43. BurtonMJ (2007) Trachoma: an overview. Br Med Bull 84 : 99–116.
44. WrightJ, GundryS, ConroyR (2004) Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health 9 : 106–117.
45. UN (2012) The Millenium Development Goals Report 2012. New York: United Nations.
46. WHO (2012) UN-water global analysis and assessment of sanitation and drinking-water (GLAAS) 2012 report: the challenge of extending and sustaining services. Geneva: World Health Organization.
47. WHO/UNICEF (2013) Progress on sanitation and drinking-water: 2013 update. New York: WHO/UNICEF Joint Monitoring Program for Water Supply and Sanitation.
48. AlemuY, BejigaA (2004) The impact of water supply on trachoma prevalence. Ethiop Med J 42 : 179–184.
49. AssaadFA, Maxwell-LyonsF, SundaresanT (1969) Use of local variations in trachoma endemicity in depicting interplay between socio-economic conditions and disease. Bull World Health Organ 41 : 181–194.
50. CairncrossS, CliffJL (1987) Water use and health in Mueda, Mozambique. Trans R Soc Trop Med Hyg 81 : 51–54.
51. CaligarisLSA, MorimotoWTM, MedinaNH, WaldmanEA (2006) Trachoma prevalence and risk factors among preschool children in a central area of the city of Sao Paulo, Brazil. Ophthalmic Epidemiol 13 : 365–370.
52. ChumbleyLC, ThomsonIM (1988) Epidemiology of trachoma in the West Bank and Gaza Strip. Eye (Lond) 2 (Pt 5) 463–470.
53. HoechsmannA, MetcalfeN, KanjalotiS, GodiaH, MtamboO, et al. (2001) Reduction of trachoma in the absence of antibiotic treatment: evidence from a population-based survey in Malawi. Ophthalmic Epidemiol 8 : 145–153.
54. MarshallCL (1968) The relationship between trachoma and piped water in a developing area. Arch Environ Health 17 : 215–220.
55. PolackSR, SolomonAW, AlexanderND, MassaePA, SafariS, et al. (2005) The household distribution of trachoma in a Tanzanian village: an application of GIS to the study of trachoma. Trans R Soc Trop Med Hyg 99 : 218–225.
56. WestSK, MunozB, TurnerVM, MmbagaBB, TaylorHR (1991) The epidemiology of trachoma in central Tanzania. Int J Epidemiol 20 : 1088–1092.
57. CourtrightP, SheppardJ, LaneS, SadekA, SchachterJ, et al. (1991) Latrine ownership as a protective factor in inflammatory trachoma in Egypt. Br J Ophthalmol 75 : 322–325.
58. EmersonPM, LindsaySW, AlexanderN, BahM, DibbaSM, et al. (2004) Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet 363 : 1093–1098.
59. HaileM, TadesseZ, GebreselassieS, AyeleB, GebreT, et al. (2013) The association between latrine use and trachoma: a secondary cohort analysis from a randomized clinical trial. Am J Trop Med Hyg 89 : 717–720.
60. MontgomeryMA, DesaiMM, ElimelechM (2010) Comparing the effectiveness of shared versus private latrines in preventing trachoma in rural Tanzania. Am J Trop Med Hyg 82 : 693–695.
61. ReillyLA, FavachoJ, GarcezLM, CourtenayO (2007) Preliminary evidence that synanthropic flies contribute to the transmission of trachoma - causing Chlamydia trachomatis in Latin America. Cad Saude Publica 23 : 1682–1688.
62. AleneGD, AbebeS (2000) Prevalence of risk factors for trachoma in a rural locality of north-western Ethiopia. East Afr Med J 77 : 308–312.
63. GowerEW, SolomonAW, BurtonMJ, AguirreA, MunozB, et al. (2006) Chlamydial positivity of nasal discharge at baseline is associated with ocular chlamydial positivity 2 months following azithromycin treatment. Invest Ophthalmol Vis Sci 47 : 4767–4771.
64. GuraksinA, GulluluG (1997) Prevalence of trachoma in eastern Turkey. Int J Epidemiol 26 : 436–442.
65. HagiM, SchemannJF, MaunyF, MomoG, SackoD, et al. (2010) Active trachoma among children in Mali: Clustering and environmental risk factors. PLoS Negl Trop Dis 4: e583.
66. HallA, KassaT, DemissieT, DegefieT, LeeS (2008) National survey of the health and nutrition of schoolchildren in Ethiopia. Trop Med Int Health 13 : 1518–1526.
67. KhandujaS, JhanjiV, SharmaN, VashistP, MurthyGVS, et al. (2009) Rapid assessment of trachoma among children living in rural northern India. Ophthalmic Epidemiol 16 : 206–211.
68. KingJD, NgondiJ, KastenJ, DialloMO, ZhuH, et al. (2011) Randomised trial of face-washing to develop a standard definition of a clean face for monitoring trachoma control programmes. Trans R Soc Trop Med Hyg 105 : 7–16.
69. KoizumiIK, MedinaNH, D'AmaralRKK, MorimotoWTM, CaligarisLSA, et al. (2005) Prevalence of trachoma in preschool and schoolchildren in the city of Sao Paulo. Revista de Saude Publica 39 : 937–942.
70. RobaAA, PatelD, ZondervanM (2012) Risk of trachoma in a SAFE intervention area. Int Ophthalmol 1–7.
71. WestSK, CongdonN, KatalaS, MeleL (1991) Facial cleanliness and risk of trachoma in families. Arch Ophthalmol 109 : 855–857.
72. WilsonM, Keyvan-LarijaniE, Millan-VelascoF, TielschJM, TaylorHR (1987) The epidemiology of trachoma in Chiapas (Mexico). Revue international du trachome et de pathologie oculaire tropicale et subtropicale et de sante publique : organe de la Ligue contre le trachome avec la collaboration de l'International Organization against Trachoma ...
159–174.
73. Ezz al ArabG, TawfikN, El GendyR, AnwarW, CourtrightP (2001) The burden of trachoma in the rural Nile Delta of Egypt: a survey of Menofiya governorate. Br J Ophthalmol 85 : 1406–1410.
74. KatzJ, WestKPJr, KhatrySK, LeClerqSC, PradhanEK, et al. (1996) Prevalence and risk factors for trachoma in Sarlahi district, Nepal. Br J Ophthalmol 80 : 1037–1041.
75. LunaEJ, MedinaNH, OliveiraMB, de BarrosOM, VranjacA, et al. (1992) Epidemiology of trachoma in Bebedouro State of Sao Paulo, Brazil: prevalence and risk factors. Int J Epidemiol 21 : 169–177.
76. MontgomeryMA, DesaiMM, ElimelechM (2010) Assessment of latrine use and quality and association with risk of trachoma in rural Tanzania. Trans R Soc Trop Med Hyg 104 : 283–289.
77. NgondiJ, ReacherMH, MatthewsFE, BrayneC, GatpanG, et al. (2009) Risk factors for trachomatous trichiasis in children: cross-sectional household surveys in Southern Sudan. Trans R Soc Trop Med Hyg 103 : 305–314.
78. SallamTA, Raja'aYA, Al-ZubieryTK, Al-ShaibaniKS, Al-ShamiriAA, et al. (2003) Chlamydia trachomatis infections among Yemeni school pupils in relation to environmental conditions. Saudi Med J 24 : 84–87.
79. ZerihunN (1997) Trachoma in Jimma zone, south western Ethiopia. Trop Med Int Health 2 : 1115–1121.
80. BaggaleyRF, SolomonAW, KuperH, PolackS, MassaePA, et al. (2006) Distance to water source and altitude in relation to active trachoma in Rombo district, Tanzania. Trop Med Int Health 11 : 220–227.
81. D'AmaralRK, CardosoMR, MedinaNH, CunhaIC, WaldmanEA (2005) [Factors associated with trachoma in a low-endemic area in southeast Brazil]. Cad Saude Publica 21 : 1701–1708.
82. HsiehYH, BoboLD, QuinnTC, WestSK (2000) Risk factors for trachoma: 6-year follow-up of children aged 1 and 2 years. Am J Epidemiol 152 : 204–211.
83. SchemannJF, LafflyD, SackoD, ZephakG, MalvyD (2007) Trichiasis and geoclimatic factors in Mali. Trans R Soc Trop Med Hyg 101 : 996–1003.
84. TaylorHR, VelascoFM, SommerA (1985) The ecology of trachoma: an epidemiological study in southern Mexico. Bull World Health Organ 63 : 559–567.
85. WestS, LynchM, TurnerV, MunozB, RapozaP, et al. (1989) Water availability and trachoma. Bull World Health Organ 67 : 71–75.
86. WestSK, RapozaP, MunozB, KatalaS, TaylorHR (1991) Epidemiology of ocular chlamydial infection in a trachoma-hyperendemic area. J Infect Dis 163 : 752–756.
87. WestSK, MunozB, LynchM, KayongoyaA, MmbagaBB, et al. (1996) Risk factors for constant, severe trachoma among preschool children in Kongwa, Tanzania. Am J Epidemiol 143 : 73–78.
88. AbdouA, MunozBE, NassirouB, KadriB, MoussaF, et al. (2010) How much is not enough? A community randomized trial of a Water and Health Education programme for Trachoma and Ocular C. trachomatis infection in Niger. Trop Med Int Health 15 : 98–104.
89. BurtonMJ, HollandMJ, FaalN, AryeeEAN, AlexanderNDE, et al. (2003) Which members of a community need antibiotics to control trachoma? Conjunctival Chlamydia trachomatis infection load in Gambian villages. Invest Ophthalmol Vis Sci 44 : 4215–4222.
90. BurtonMJ, HollandMJ, MakaloP, AryeeEA, AlexanderND, et al. (2005) Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet 365 : 1321–1328.
91. HassanA, NgondiJM, KingJD, ElshafieBE, GinaidGA, et al. (2011) The prevalence of blinding trachoma in northern states of Sudan. PLoS Negl Trop Dis 5.
92. KhandekarR, TonTK, Do ThiP (2006) Impact of face washing and environmental improvement on reduction of active trachoma in Vietnam-a public health intervention study. Ophthalmic Epidemiol 13 : 43–52.
93. ResnikoffS, PeyramaureF, BagayogoCO, HuguetP (1995) Health education and antibiotic therapy in trachoma control. Revue international du trachome et de pathologie oculaire tropicale et subtropicale et de sante publique : organe de la Ligue contre le trachome avec la collaboration de l'International Organization against Trachoma ... 72 : 89–110, 89-98, 101-110.
94. RubinsteinRA (2006) Controlling blinding trachoma in the Egyptian Delta: integrating clinical, epidemiological, and anthropological understandings. Anthropology & Medicine 13 : 99–118.
95. SchemannJF, GuinotC, IlboudoL, MomoG, KoB, et al. (2003) Trachoma, flies and environmental factors in Burkina Faso. Trans R Soc Trop Med Hyg 97 : 63–68.
96. VinkeC, LonerganS (2011) Social and environmental risk factors for trachoma: a mixed methods approach in the Kembata Zone of southern Ethiopia. Can J Dev Stud 32 : 254–268.
97. AbdouA, NassirouB, KadriB, MoussaF, MunozBE, et al. (2007) Prevalence and risk factors for trachoma and ocular Chlamydia trachomatis infection in Niger. Br J Ophthalmol 91 : 13–17.
98. AmzaA, KadriB, NassirouB, StollerNE, YuSN, et al. (2012) Community risk factors for ocular Chlamydia infection in Niger: pre-treatment results from a cluster-randomized trachoma trial. PLoS Negl Trop Dis 6: e1586.
99. AyeleB, GebreT, MoncadaJ, HouseJI, StollerNE, et al. (2011) Risk factors for ocular chlamydia after three mass azithromycin distributions. PLoS Negl Trop Dis 5: e1441.
100. BaileyR, DownesB, DownesR, MabeyD (1991) Trachoma and water use; a case control study in a Gambian village. Trans R Soc Trop Med Hyg 85 : 824–828.
101. Cajas-MonsonLC, MkochaH, MunozB, QuinnTC, GaydosCA, et al. (2011) Risk factors for ocular infection with Chlamydia trachomatis in children 6 months following mass treatment in Tanzania. PLoS Negl Trop Dis 5: e978.
102. CumberlandP, HailuG, ToddJ (2005) Active trachoma in children aged three to nine years in rural communities in Ethiopia: prevalence, indicators and risk factors. Trans R Soc Trop Med Hyg 99 : 120–127.
103. EdwardsT, Harding-EschEM, HailuG, AndreasonA, MabeyDC, et al. (2008) Risk factors for active trachoma and Chlamydia trachomatis infection in rural Ethiopia after mass treatment with azithromycin. Trop Med Int Health 13 : 556–565.
104. EdwardsT, SmithJ, SturrockHJ, KurLW, SabasioA, et al. (2012) Prevalence of trachoma in unity state, South Sudan: results from a large-scale population-based survey and potential implications for further surveys. PLoS Negl Trop Dis 6: e1585.
105. EjiguM, KariukiMM, IlakoDR, GelawY (2013) Rapid trachoma assessment in kersa district, southwest ethiopia. Ethiop J Health Sci 23 : 1–9.
106. FayeM, KuperH, DineenB, BaileyR (2006) Rapid assessment for prioritisation of trachoma control at community level in one district of the Kaolack Region, Senegal. Trans R Soc Trop Med Hyg 100 : 149–157.
107. GolovatyI, JonesL, GelayeB, TilahunM, BeleteH, et al. (2009) Access to water source, latrine facilities and other risk factors of active trachoma in Ankober, Ethiopia. PLoS One 4: e6702.
108. Harding-EschEM, EdwardsT, SillahA, Sarr-SissohoI, AryeeEA, et al. (2008) Risk factors for active trachoma in The Gambia. Trans R Soc Trop Med Hyg 102 : 1255–1262.
109. Harding-EschEM, EdwardsT, MkochaH, MunozB, HollandMJ, et al. (2010) Trachoma prevalence and associated risk factors in the gambia and Tanzania: baseline results of a cluster randomised controlled trial. PLoS Negl Trop Dis 4: e861.
110. JipNF, KingJD, DialloMO, MiriES, HamzaAT, et al. (2008) Blinding trachoma in katsina state, Nigeria: population-based prevalence survey in ten local government areas. Ophthalmic Epidemiol 15 : 294–302.
111. KetemaK, TirunehM, WoldeyohannesD, MuluyeD (2012) Active trachoma and associated risk factors among children in Baso Liben District of East Gojjam, Ethiopia. BMC Public Health 12 : 1105.
112. KhandekarR, MabryR, Al HadramiK, SarvananN (2005) Active trachoma, face washing (F) and environmental improvement (E) in a high-risk population in Oman. East Mediterr Health J 11 : 402–409.
113. LucenaAD, CruzAAVE, AkaishiP (2010) Epidemiology of trachoma in the village of Araripe plateau - Ceara State. Arq Bras Oftalmol 73 : 271–275.
114. MahandeMJ, KwekaEJ (2011) Association between water related factors and active trachoma in Hai district, northern Tanzania. Trop Med Int Health 16 : 218.
115. MesfinMM, de la CameraJ, TarekeIG, AmanualG, ArayaT, et al. (2006) A community-based trachoma survey: prevalence and risk factors in the Tigray region of northern Ethiopia. Ophthalmic Epidemiol 13 : 173–181.
116. MpyetC, GoyolM, OgoshiC (2010) Personal and environmental risk factors for active trachoma in children in Yobe state, north-eastern Nigeria. Trop Med Int Health 15 : 168–172.
117. MpyetC, LassBD, YahayaHB, SolomonAW (2012) Prevalence of and risk factors for trachoma in kano state, Nigeria. PLoS One 7: e40421.
118. NgondiJ, MatthewsF, ReacherM, OnsarigoA, MatendeI, et al. (2007) Prevalence of risk factors and severity of active trachoma in southern Sudan: an ordinal analysis. Am J Trop Med Hyg 77 : 126–132.
119. NgondiJ, GebreT, ShargieEB, GravesPM, EjigsemahuY, et al. (2008) Risk factors for active trachoma in children and trichiasis in adults: a household survey in Amhara Regional State, Ethiopia. Trans R Soc Trop Med Hyg 102 : 432–438.
120. NgondiJ, MatthewsF, ReacherM, BabaS, BrayneC, et al. (2008) Associations between active trachoma and community intervention with Antibiotics, Facial cleanliness, and Environmental improvement (A,F,E). PLoS Negl Trop Dis 2: e229.
121. NgondiJ, GebreT, ShargieEB, AdamuL, TeferiT, et al. (2010) Estimation of effects of community intervention with antibiotics, facial cleanliness, and environmental improvement (A,F,E) in five districts of Ethiopia hyperendemic for trachoma. Br J Ophthalmol 94 : 278–281.
122. QuickeE, SillahA, Harding-EschEM, LastA, JoofH, et al. (2013) Follicular trachoma and trichiasis prevalence in an urban community in The Gambia, West Africa: is there a need to include urban areas in national trachoma surveillance? Trop Med Int Health In press.
123. RegassaK, TeshomeT (2004) Trachoma among adults in Damot Gale District, South Ethiopia. Ophthalmic Epidemiol 11 : 9–16.
124. SchemannJF, SackoD, MalvyD, MomoG, TraoreL, et al. (2002) Risk factors for trachoma in Mali. Int J Epidemiol 31 : 194–201.
125. SchemannJF, GuinotC, TraoreL, ZefackG, DembeleM, et al. (2007) Longitudinal evaluation of three azithromycin distribution strategies for treatment of trachoma in a sub-Saharan African country, Mali. Acta Trop 101 : 40–53.
126. TaylorHR, WestSK, MmbagaBB, KatalaSJ, TurnerV, et al. (1989) Hygiene factors and increased risk of trachoma in central Tanzania. Arch Ophthalmol 107 : 1821–1825.
127. TielschJM, WestKPJr, KatzJ, Keyvan-LarijaniE, TizazuT, et al. (1988) The epidemiology of trachoma in southern Malawi. Am J Trop Med Hyg 38 : 393–399.
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2014 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Data Access for the Open Access Literature: PLOS's Data Policy
- Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap
- Effect of Water, Sanitation, and Hygiene on the Prevention of Trachoma: A Systematic Review and Meta-Analysis
- Biomarker Profiling by Nuclear Magnetic Resonance Spectroscopy for the Prediction of All-Cause Mortality: An Observational Study of 17,345 Persons
- Physicians as Fundraisers: Medical Philanthropy and the Doctor-Patient Relationship
- Molecular Diagnostics for Gonorrhoea: Implications for Antimicrobial Resistance and the Threat of Untreatable Gonorrhoea
- Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis
- Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study
- Quantitative Measurement of Melanoma Spread in Sentinel Lymph Nodes and Survival
- Effect of an Educational Toolkit on Quality of Care: A Pragmatic Cluster Randomized Trial
- Patterns of Obesity Development before the Diagnosis of Type 2 Diabetes: The Whitehall II Cohort Study
- Long-Term Survival and Dialysis Dependency Following Acute Kidney Injury in Intensive Care: Extended Follow-up of a Randomized Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study
- Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap
- Quantitative Measurement of Melanoma Spread in Sentinel Lymph Nodes and Survival
- Physicians as Fundraisers: Medical Philanthropy and the Doctor-Patient Relationship
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



