-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Sharing Individual Participant Data (IPD) within the Context of the Trial Reporting System (TRS)
Deborah Zarin and Tony Tse of ClinicalTrials.Gov consider how sharing individual participant data can and cannot help improve the reporting of clinical trials.
Published in the journal: Sharing Individual Participant Data (IPD) within the Context of the Trial Reporting System (TRS). PLoS Med 13(1): e32767. doi:10.1371/journal.pmed.1001946
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.1001946Summary
Deborah Zarin and Tony Tse of ClinicalTrials.Gov consider how sharing individual participant data can and cannot help improve the reporting of clinical trials.
Summary Points
The role of individual participant data (IPD) sharing can best be understood as part of an overall three-level trial reporting system (TRS) framework.
Different “types” of IPD, which reflect varying degrees of information granularity, have different potential benefits and harms.
Study 329 of Paxil (paroxetine) in children with depression is used as a case study to highlight the potential value of different components of the TRS.
The Institute of Medicine (IOM) [1], journal editors [2,3], and many others [4–6] have called for more widespread, third-party access to the individual participant data (IPD) and associated documentation from clinical trials (i.e., “IPD sharing”). Advocates assert that access to trial IPD will help to address well-established flaws in the current system of communicating trial results, including nonpublication, selective reporting, and lack of reproducibility [7]. Additional proposed benefits include the ability to reanalyze study data (e.g., validation and/or correction of previously published findings [8]) and to combine data from multiple studies (e.g., IPD-level meta-analyses [9]). Others note the burdens and costs associated with preparing IPD and associated documentation for sharing, the need to ensure participant privacy, and the risk of invalid analyses [10].
We do not attempt to replicate the more comprehensive analysis of IPD sharing that was conducted by the recent IOM panel [1]. However, we believe that it would be helpful at this pivotal time to consider the implications of IPD sharing within the context of the “trial reporting system” (TRS), which encompasses existing efforts to enhance access to information about trials and their findings and to improve the transparency of the clinical research enterprise (CRE) [11]. In this essay, we attempt to add precision to the ongoing discussion by examining the range of information granularity associated with different types of IPD. We then consider IPD sharing within a three-level TRS framework and illustrate the roles of these levels with a case study.
What Is the Nature of IPD?
As attention shifts to IPD sharing, it is instructive to consider the mechanism by which initial “raw” data collected from each trial participant are analyzed, transformed, and aggregated into the summary data reported in the results sections of journal articles, conference abstracts, press releases, and package inserts and as entries in results databases (Fig 1).
Fig. 1. Schematic depicting information granularity for different types of data [<em class="ref">12</em>]. ![Schematic depicting information granularity for different types of data [<em class="ref">12</em>].](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/4918afc41222533a165cba0c47316c26.png)
Each arrow in Fig 1 indicates a transformation of trial data. While some transformations are based on procedures prespecified in study documents (e.g., detailed criteria or algorithms in the protocol or statistical analysis plan), others likely rely on ad hoc expert judgments. For example, analyzing IPD collected for the primary outcome measure of “change in tumor size from baseline at 3 months” might involve the following decisions:
choosing a specific imaging approach (e.g., fluorodeoxyglucose (FDG)-positron emission tomography (PET) using a specific device);
determining a particular method for transforming 2 - or 3-D images into tumor size measurements (e.g., Digital Imaging and Communications in Medicine [DICOM] standard using autocontouring to calculate the volume for the region of interest);
applying these methods to measure tumor size for each individual at baseline and at 3 months; and
calculating and recording the changes in size per participant.
Additional decisions must be made by the researchers about the handling of missing data, unreadable images, and other data deficiencies; determining the analysis population (e.g., all who started the study [including those who discontinued] or only those who received the full course of treatment); and aggregating the IPD for purposes of reporting and analysis (e.g., mean change in size versus proportion with a change over a certain size). The most granular data (far left in Fig 1) would provide insight into these decisions and allow independent researchers to examine the implications of alternative analytic decisions. On the other hand, the least granular IPD (far right) would obscure some of these decisions and would not allow for testing the impact of different analytic methods.
Most discussions of IPD sharing policies sidestep the issue of matching IPD types with anticipated benefits and burdens. For example, third-party researchers interested in independently recoding the IPD would need access to uncoded data (i.e., data types to the left of “Coded” on the x-axis in Fig 1). In contrast, users who intend to replicate and confirm the reproducibility of aggregate data published in a journal article may only require access to the analyzable IPD (i.e., final type of IPD before undergoing transformation into aggregated data in Fig 1). While not an insurmountable barrier for IPD sharing policies, we believe that consideration of various data types and their uses is a timely issue for discussion within the research community, including questions such as the following:
What standard terminology or classification should be used to describe the different data types?
Which types of IPD should be made available systematically?
When more than one type is available for sharing, how should they be uniquely identified and tracked (e.g., cited) within the research community?
Where Does IPD Fit in the TRS?
The TRS framework encompasses key existing and proposed efforts and is designed to increase trial transparency systematically. Fig 2 depicts the TRS as a pyramid with prospective registration at its base, summary or aggregate trial results reporting in the middle, and the sharing of trial IPD and relevant documents at its apex.
Fig. 2. Schematic depicting the functions of the three key components of the TRS. 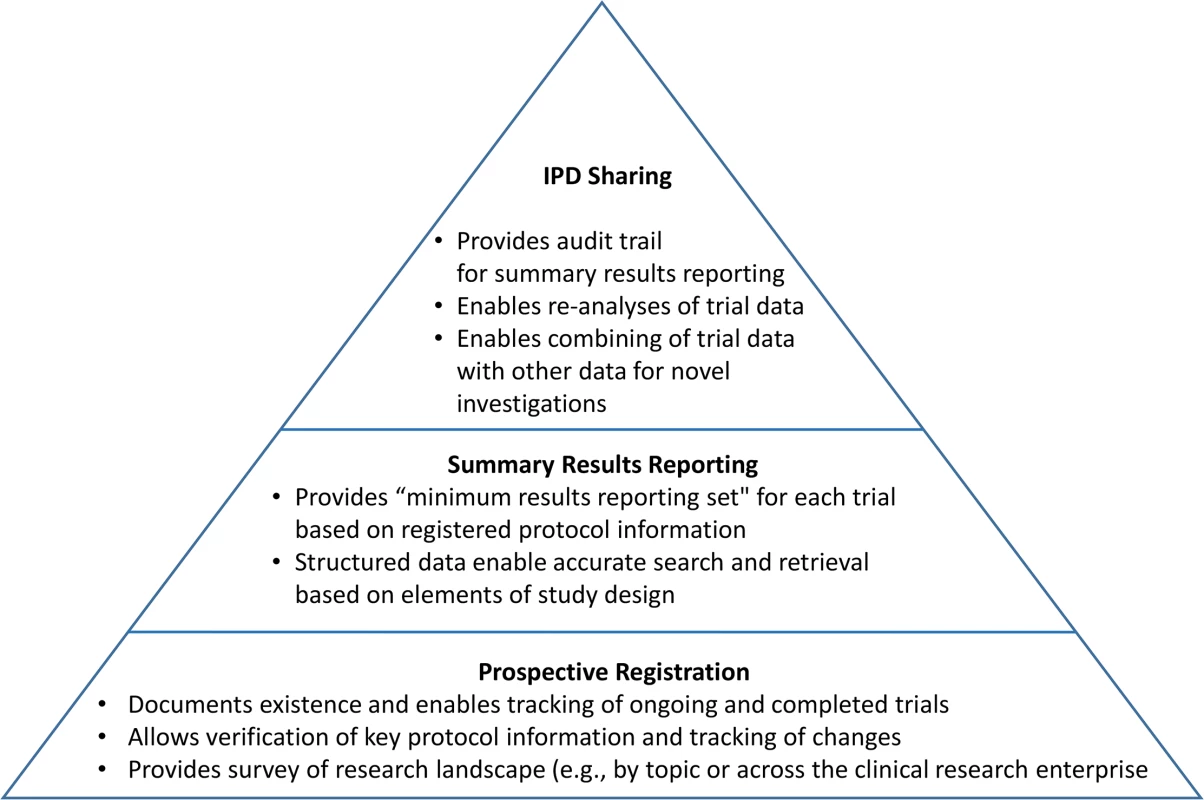
At its base, prospective registration provides a public listing of all ongoing and completed trials, along with key protocol and administrative details to allow people to identify the full set of trials conducted within a research area (e.g., antidepressant trials in children). Trial registration, if done and used appropriately, also allows for the assessment of fidelity to key protocol details, such as definition of the prespecified primary outcome measure [13]. Summary results reporting in trial registries, currently implemented at ClinicalTrials.gov and the European Union Clinical Trials Registry [14], is the next level of the TRS. Results databases—designed to ensure that aggregate trial results are reported systematically in a timely, structured, and complete manner based in part on expert trial-reporting guidelines such as the Consolidated Standards of Reporting Trials (CONSORT) statement [15] and its extensions—call attention to unacknowledged deviations from the registered protocol details [13]. Current policies are generally intended to address these two foundational levels of the TRS.
Registration information and summary results displayed as a single trial record provide the minimal, essential information needed to understand a trial and its findings. Each record also uses a format that is highly structured and searchable by a range of criteria. Ideally, users could easily retrieve information about all completed or ongoing trials for a particular clinical or policy question (e.g., to identify a need for additional research or conduct a systematic review), avoiding the biases imposed by incomplete and selective publication. Trial registration and results records are also linked, via unique registry identifiers, to relevant peer-reviewed journal publications [16]. As the use of unique registry identifiers expands (e.g., systematic reviews and press releases), an extensive network of automated, explicit linkages can provide an even more useful way to identify publicly available information about a trial from the trial record itself (Fig 3).
Fig. 3. Schematic depicting <a href="http://ClinicalTrials.gov">ClinicalTrials.gov</a> as an “information scaffold” using the record unique identifier (NCT number) to link to various online resources. 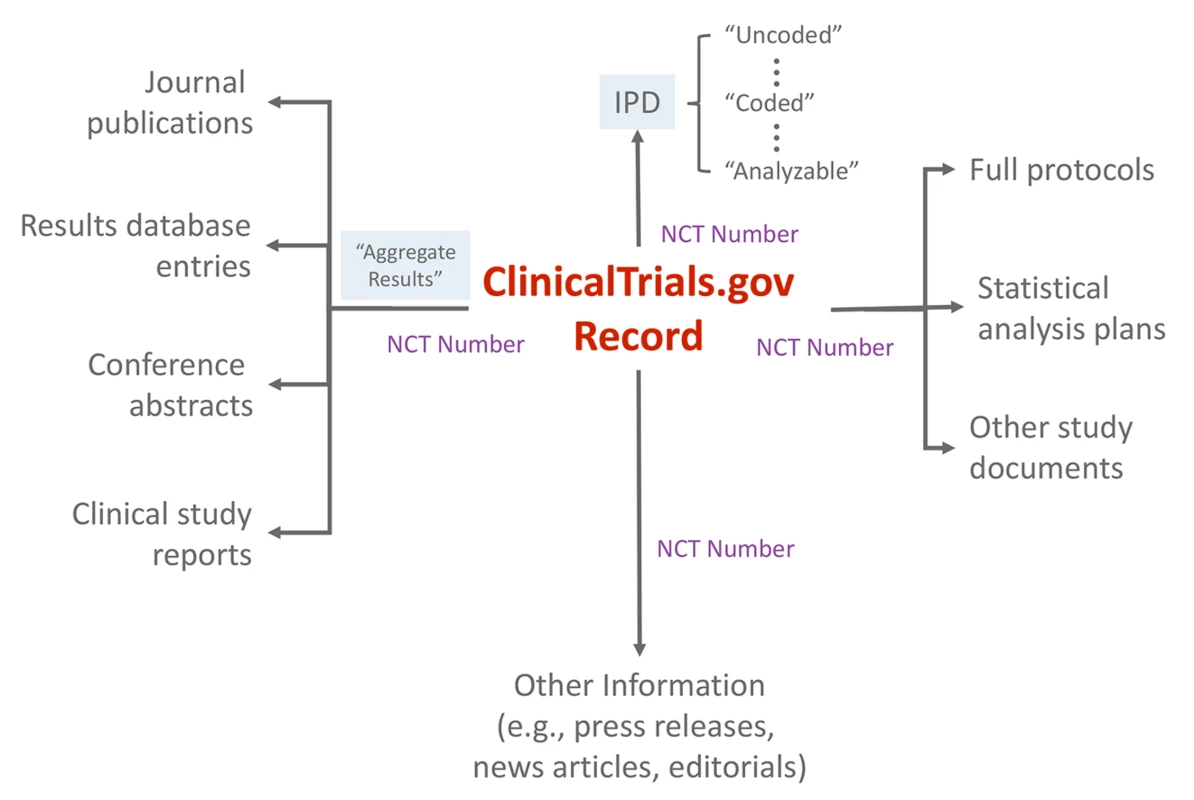
IPD and related documents reside at the apex of this pyramid because they are most useful within the context of the two lower levels, which serve as the foundation. Without careful use of trial registries and summary results databases, access to IPD might simply recreate or amplify existing reporting biases [17]. For example, analysis of trial IPD cannot mitigate biases that stem from selective release of data from only one trial among a “family” of trials for the studied population, intervention, and condition (e.g., a likely result of proposals to require the release of IPD only upon journal publication).
How Would the Three Key Components of TRS Work Together?
Case Study: Recent Reanalysis of Study 329
Study 329, sponsored by SmithKline Beecham (now GlaxoSmithKline [GSK]), was one of several studies conducted to examine the use of Paxil (paroxetine) in children with depression and the first with results to be published. The original publication of Study 329 in 2001 implied that the study results showed the safety and efficacy of Paxil in children [18]. In 2004, the New York State attorney general filed a consumer fraud lawsuit against GSK, alleging that the suppression and misreporting of trial data created the false impression that Paxil was safe and effective in depressed children [19].
A newly published reanalysis, part of the Restoring Invisible and Abandoned Trials (RIAT) initiative [20], was based on access to original case report forms (CRFs) for 34% of the 275 participants [21]. These highly granular IPD datasets enabled the researchers to recategorize certain adverse events that they determined had been miscategorized originally (e.g., “mood lability” rather than the more serious “suicidality”). The reanalysis concluded that Study 329 did not show either efficacy or safety.
How Would the Problems of Study 329 Be Addressed by the Current TRS?
It would be an oversimplification to conclude that this reanalysis demonstrates the need to make IPD for all trials available. A more nuanced look at the specific problems is useful. Many of the concerns about Study 329 and the other Paxil studies might have been addressed if current policies regarding registration and results reporting had been in existence (Table 1, [22–24]). The key issue that specifically required access to IPD was the detection of miscategorization of some adverse events in the original report.
Tab. 1. Key issues with trials of antidepressant use in children for depression and the role of the TRS. 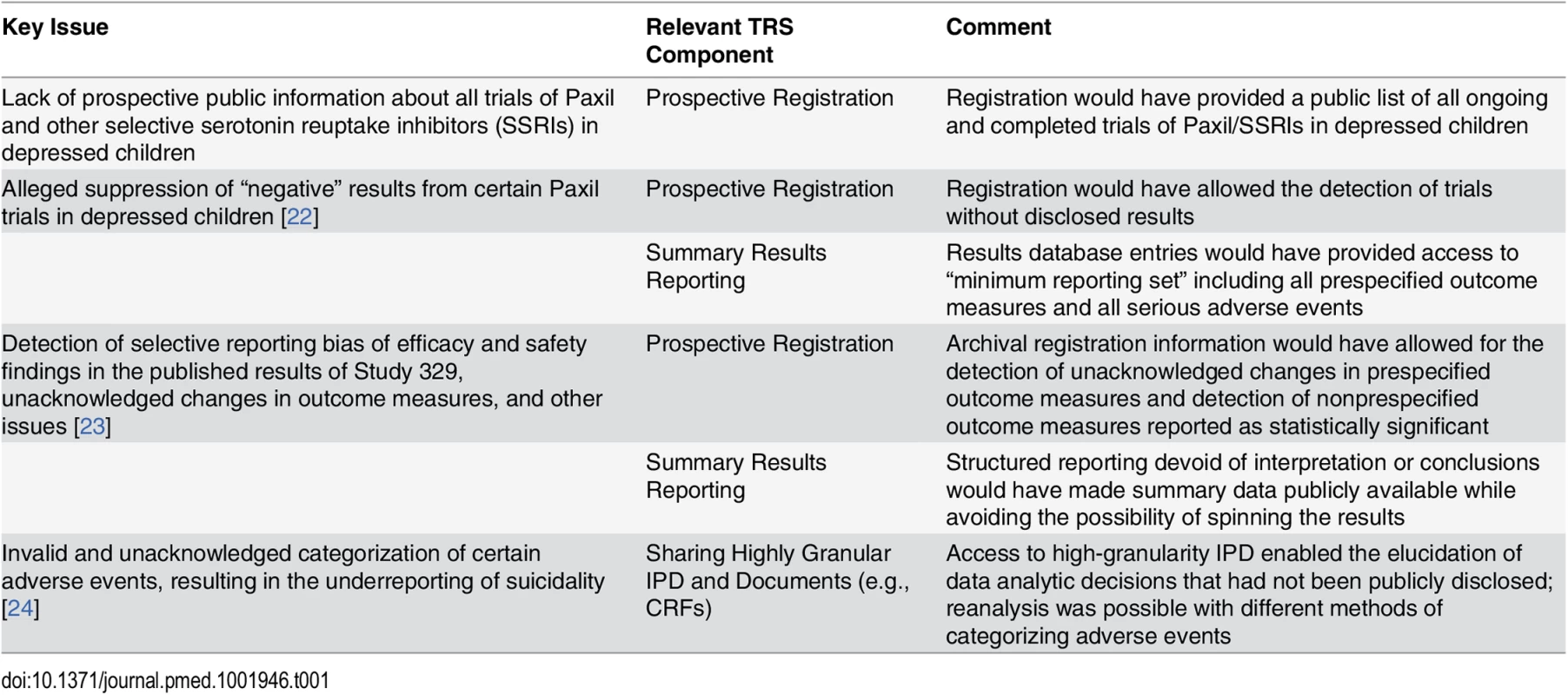
It is important to note that this illuminating reanalysis required access to the highly detailed IPD available in the original CRFs, represented by the far-left side of the x-axis in Fig 1. However, recent high-profile proposals for the sharing of IPD might not have added any clarity in the case of the Paxil studies in children beyond what could have been achieved with the optimal use of a registry and results database (i.e., two foundational levels of the pyramid in Fig 2). The reason is that journal publication serves as the “trigger” for IPD release in many of these proposals [1]), which could not possibly mitigate biases resulting from selective publication in the first place (i.e., IPD from unpublished trials would be exempt from sharing requirements). In addition, such proposed IPD policies call for the release of only the “coded” or “analyzable” dataset, which would not have allowed for the detection of miscategorization or the recategorization of the adverse events. Finally, such proposals would only require the sharing of a subset of IPD and documents for those aggregate data reported in the publication and not the full dataset, precluding secondary analyses intended to go beyond validation and reproducibility of the original publication.
Conclusion
The evolving TRS can be thought of as a pyramid, with each successive layer being dependent on the layer(s) below it. We should not allow the prospects for providing access to IPD and relevant documents to divert attention from the continuing need to ensure complete, accurate, and timely trial registration and summary results reporting—as well as attentive and consistent use of these tools by key stakeholders. In addition, IPD sharing policies and systems must consider the different benefits and burdens that would be expected from third-party access to data types of varying levels of granularity.
Zdroje
1. Institute of Medicine (2015) Sharing clinical trial data: maximizing benefits, minimizing risks. Washington DC: The National Academies Press.
2. Drazen JM (2015) Sharing individual patient data from clinical trials. N Engl J Med 372 : 201–202. doi: 10.1056/NEJMp1415160 25587944
3. Data Availability Policy. PLoS Med; 2016 [cited 2016 Jan 6]. http://journals.plos.org/plosmedicine/s/data-availability#loc-acceptable-data-sharing-methods.
4. Eichler HG, Abadie E, Breckenridge A, Leufkens H, Rasi G (2012) Open clinical trial data for all? A view from regulators. PLoS Med 9: e1001202. doi: 10.1371/journal.pmed.1001202 22505851
5. Krumholz HM, Peterson ED (2014) Open access to clinical trials data. JAMA 312 : 1002–1003. doi: 10.1001/jama.2014.9647 25203080
6. Mello MM, Francer JK, Wilenzick M, Teden P, Bierer BE, et al. (2013) Preparing for responsible sharing of clinical trial data. N Engl J Med 369 : 1651–1658. doi: 10.1056/NEJMhle1309073 24144394
7. Song F, Parekh S, Hooper L, Loke YK, Ryder J, et al. (2010) Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess 14: iii, ix-xi, 1–193. doi: 10.3310/hta14080 20181324
8. Ebrahim S, Sohani ZN, Montoya L, Agarwal A, Thorlund K, et al. (2014) Reanalyses of randomized clinical trial data. JAMA 312 : 1024–1032. doi: 10.1001/jama.2014.9646 25203082
9. Tierney JF, Vale C, Riley R, Smith CT, Stewart L, et al. (2015) Individual Participant Data (IPD) Meta-analyses of Randomised Controlled Trials: Guidance on Their Use. PLoS Med 12: e1001855. doi: 10.1371/journal.pmed.1001855 26196287
10. Tudur Smith C, Dwan K, Altman DG, Clarke M, Riley R, et al. (2014) Sharing individual participant data from clinical trials: an opinion survey regarding the establishment of a central repository. PLoS ONE 9: e97886. doi: 10.1371/journal.pone.0097886 24874700
11. Zarin DA, Tse T (2008) Medicine. Moving toward transparency of clinical trials. Science 319 : 1340–1342. doi: 10.1126/science.1153632 18323436
12. Zarin DA (2012) Presentation at IOM Workshop on Sharing Clinical Research Data. Washington DC.
13. Tse T, Zarin DA, Williams RJ, Ide NC (2012) The role and importance of clinical trial registries and results databases. In: Gallin JI, Ognibene FP, editors. Principles and Practice of Clinical Research 3rd ed Amsterdam: Academic Press. pp. 171–181.
14. EU Clinical Trials Register. European Medicines Agency. [cited 2016 Jan 6] https://www.clinicaltrialsregister.eu/ctr-search/search.
15. Schulz KF, Altman DG, Moher D, Group C (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 7: e1000251. doi: 10.1371/journal.pmed.1000251 20352064
16. Clinical Trial Registry Numbers in MEDLINE®/PubMed® Records. U.S. National Library of Medicine. [cited 2016 Jan 6] https://www.nlm.nih.gov/bsd/policy/clin_trials.html.
17. Zarin DA (2013) Participant-level data and the new frontier in trial transparency. N Engl J Med 369 : 468–469. doi: 10.1056/NEJMe1307268 23902488
18. Keller MB, Ryan ND, Strober M, Klein RG, Kutcher SP, et al. (2001) Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 40 : 762–772. 11437014
19. The People of the State of New York v. GlaxoSmithKline, Complaint, filed 2 June 2004. [cited 2016 Jan 6] http://news.findlaw.com/wsj/docs/glaxo/nyagglaxo60204cmp.pdf.
20. Doshi P, Dickersin K, Healy D, Vedula SS, Jefferson T (2013) Restoring invisible and abandoned trials: a call for people to publish the findings. BMJ 346: f2865. doi: 10.1136/bmj.f2865 23766480
21. Le Noury J, Nardo JM, Healy D, Jureidini J, Raven M, et al. (2015) Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence. BMJ 351: h4320. doi: 10.1136/bmj.h4320 26376805
22. GSK Assurance of Discontinuance, 2004. [cited 2016 Jan 6] http://www.ag.ny.gov/sites/default/files/press-releases/archived/aug26a_04_attach2.pdf.
23. Jureidini JN, McHenry LB, Mansfield PR (2008) Clinical trials and drug promotion: Selective reporting of study 329. International Journal of Risk & Safety in Medicine 20 : 76–81.
24. Leslie LK, Newman TB, Chesney PJ, Perrin JM (2005) The Food and Drug Administration's deliberations on antidepressant use in pediatric patients. Pediatrics 116 : 195–204. 15995053
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Sharing Clinical Trial Data: A Proposal from the International Committee of Medical Journal Editors
- Pharmaceutical Industry Off-label Promotion and Self-regulation: A Document Analysis of Off-label Promotion Rulings by the United Kingdom Prescription Medicines Code of Practice Authority 2003–2012
- Can Data Sharing Become the Path of Least Resistance?
- Between Openness and Privacy in Genomics
- Developing Global Norms for Sharing Data and Results during Public Health Emergencies
- Advancing Medical Professionalism in US Military Detainee Treatment
- Resuscitating the Dying Autopsy
- Data Sharing as Part of the Normal Scientific Process: A View from the Pharmaceutical Industry
- Evidence for Community Transmission of Community-Associated but Not Health-Care-Associated Methicillin-Resistant Strains Linked to Social and Material Deprivation: Spatial Analysis of Cross-sectional Data
- Intimate Partner Violence and Depression Symptom Severity among South African Women during Pregnancy and Postpartum: Population-Based Prospective Cohort Study
- Intramuscular Artesunate for Severe Malaria in African Children: A Multicenter Randomized Controlled Trial
- Strategies to Prevent Cholera Introduction during International Personnel Deployments: A Computational Modeling Analysis Based on the 2010 Haiti Outbreak
- Preventing Weight Gain in Women in Rural Communities: A Cluster Randomised Controlled Trial
- Cotrimoxazole Prophylaxis Discontinuation among Antiretroviral-Treated HIV-1-Infected Adults in Kenya: A Randomized Non-inferiority Trial
- Sharing Individual Participant Data (IPD) within the Context of the Trial Reporting System (TRS)
- “Asymptomatic” Malaria: A Chronic and Debilitating Infection That Should Be Treated
- “Real-Time” Monitoring of Under-Five Mortality: A Vision Tempered by Reality
- “Real-Time” Monitoring of Under-Five Mortality: Lessons for Strengthened Vital Statistics Systems
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Cotrimoxazole Prophylaxis Discontinuation among Antiretroviral-Treated HIV-1-Infected Adults in Kenya: A Randomized Non-inferiority Trial
- Sharing Individual Participant Data (IPD) within the Context of the Trial Reporting System (TRS)
- Pharmaceutical Industry Off-label Promotion and Self-regulation: A Document Analysis of Off-label Promotion Rulings by the United Kingdom Prescription Medicines Code of Practice Authority 2003–2012
- Between Openness and Privacy in Genomics
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



