-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Transformative Innovations in Reproductive, Maternal, Newborn, and Child Health over the Next 20 Years
As part of the "Grand Convergence:
Aligning Technologies and Realities in Global Health" Collection, Cyril Engmann and colleagues discuss promising innovations that have the potential to move the RMNCH agenda forward.
Published in the journal: Transformative Innovations in Reproductive, Maternal, Newborn, and Child Health over the Next 20 Years. PLoS Med 13(3): e32767. doi:10.1371/journal.pmed.1001969
Category: Collection Review
doi: https://doi.org/10.1371/journal.pmed.1001969Summary
As part of the "Grand Convergence:
Aligning Technologies and Realities in Global Health" Collection, Cyril Engmann and colleagues discuss promising innovations that have the potential to move the RMNCH agenda forward.Summary Points
Accelerating progress in reproductive, maternal, newborn, and child health (RMNCH) over the past 30 years has resulted in significant decreases in mortality, as well as shifts in causes of death. For example, deaths from diarrhea among children under age 5 have significantly declined. This increased survival means an increasing fraction of under-5 deaths occur in the first 4 weeks of life, the neonatal period.
Transformative changes, including advances such as the development of immunizations, wide uptake of contraception, and the availability of medications such as oxytocin, have contributed to an improved morbidity and mortality curve. Such advances are set against a broader backdrop of increasing national wealth, stronger health systems, aligned political agendas, and advocacy systems.
Global mechanisms and strategies such as the Global Strategy for Women’s, Children’s, and Adolescents’ Health, Global Alliance for the Vaccine Initiative (GAVI), the United Nations Commission on Life-Saving Commodities for Women and Children, Family Planning 2020, and the Every Newborn Action Plan, among others, are serving to drive the global agenda forward, although stubborn gaps remain.
In this paper, we discuss promising innovations that in our opinion have significant promise in moving the RMNCH agenda forward. While some of these are technologies, others are efforts aimed at improving commodities, increasing demand for services, and promoting equity in access.
Introduction
Reproductive, maternal, newborn, and child health (RMNCH) was a pivotal focus of the Millennium Development Goals (MDGs). On the cusp of the Sustainable Development Goals’ (SDGs’) era to guide progress for the next 20 years, RMNCH continues to be central to the SDG targets that have been set. Building on the Lancet “Commission on Investing in Health” publication, we reflect on major levers that have resulted in increased RMNCH survival over the past 25–30 years [1] and examine promising and important innovations in RMNCH that have transformative potential for the survival and well-being of mothers and children worldwide.
RMNCH Yesterday and Today
The epidemiology of reproductive, maternal, newborn, and child mortality has changed significantly over the past 25 years [2,3]. In high-income settings, maternal mortality has more than halved, while the decline has been less in low - and middle-income countries. Especially in sub-Saharan Africa, maternal mortality rates plateaued and even increased during the HIV-AIDS epidemic [4,5]. In child health, global under-5 deaths nearly halved from 12.2 million in 1990 to 6.3 million in 2013 [3]. Closer inspection suggests that nine countries (India, China, Pakistan, Bangladesh, Indonesia, Afghanistan, Brazil, Nigeria, and Ethiopia) were responsible for two-thirds of these declines. While overall global trends show a steady decline, the causes and distribution of deaths have changed significantly over time [6]. Diarrhea and pneumonia, once leading causes of under-5 mortality, continue to decrease at remarkable rates in certain settings, and neonatal mortality now accounts for more than 44% of all under-5 deaths [7]. Among neonatal deaths, prematurity is the most common cause of mortality [8].
The landscape of global RMNCH today is very different from what it was 30 years ago. Thirty years ago, the MDGs were not articulated, and neither the Global Fund nor the Global Alliance for Vaccine and Immunization (GAVI) existed. “Mega-billanthropy,” through vehicles such as the Giving Pledge, in which billionaires commit to giving away half of their wealth during their lifetime, had yet to be conceptualized. Official development assistance (ODA) for health stood at US$6.7 billion in 1990, compared to US$28.4 billion in 2011 [9]. Within maternal, newborn, and child health (MNCH) alone, the total volume of worldwide ODA more than doubled between 2003 and 2010, rising from US$2.6 billion in 2003 to US$6.5 billion in 2010 [10].
Country-level improvements have also been significant over the past 30 years. Many countries have undergone remarkable economic improvements, which in many cases led to more robust health systems. Leaders of African Union countries signed the Abuja Declaration in 2001, pledging to spend 15% of their annual budgets on health by 2015. While only a minority of African countries appear to have realized that pledge as of 2015, there are powerful examples of success: Rwanda spends nearly 22% of its national budget on health [11]. In India, net ODA received as a percentage of gross national income was 0.1% in 2013, compared to 2.1% in 1964, a 20-fold difference [12]. In March 2015, the British Parliament became only the sixth of the 29 wealthy nations who are members of the Development Assistance Committee (DAC) to enact the promise made in 1970 to spend 0.7% of its gross national products on international aid, and the United Arab Emirates now gives 1.25% of its gross national product, a 4-fold increase since the previous year [13]. The United States contribution to ODA is now estimated at US$31 billion, a significant increase over previous years, although short of the UN target of 0.7% (Figs 1 and 2).
Fig. 1. Development Assistance Committee (DAC) members' official development assistance (ODA) in 2013. 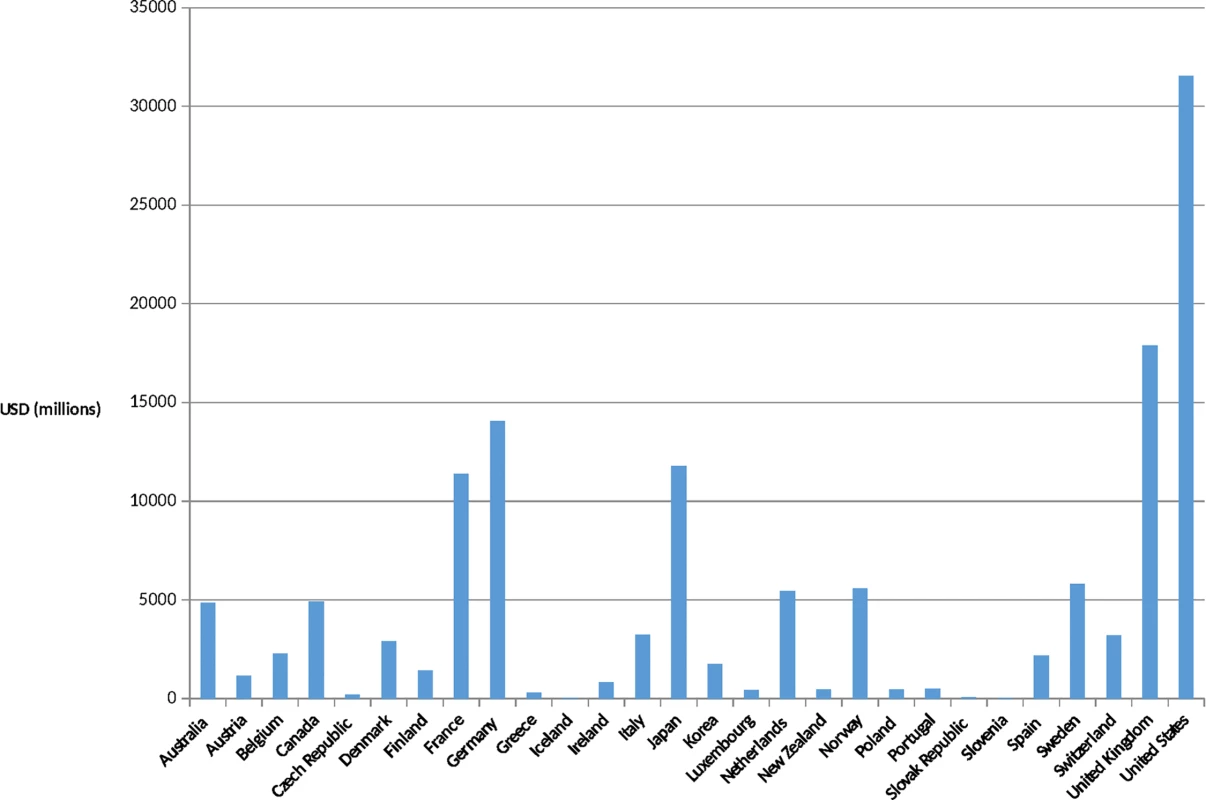
Source: OECD. Development Co-operation Report 2014: Mobilising Resources for Sustainable Development. Paris: OECD Publishing; 2014. Available at http://observ-ocd.org/sites/observ-ocd.org/files/publicacion/docs/informe_coop.desen_._2014_ocde.pdf. Accessed January 14, 2016. Fig. 2. ODA as a percentage of gross national income (GNI) in (2013). 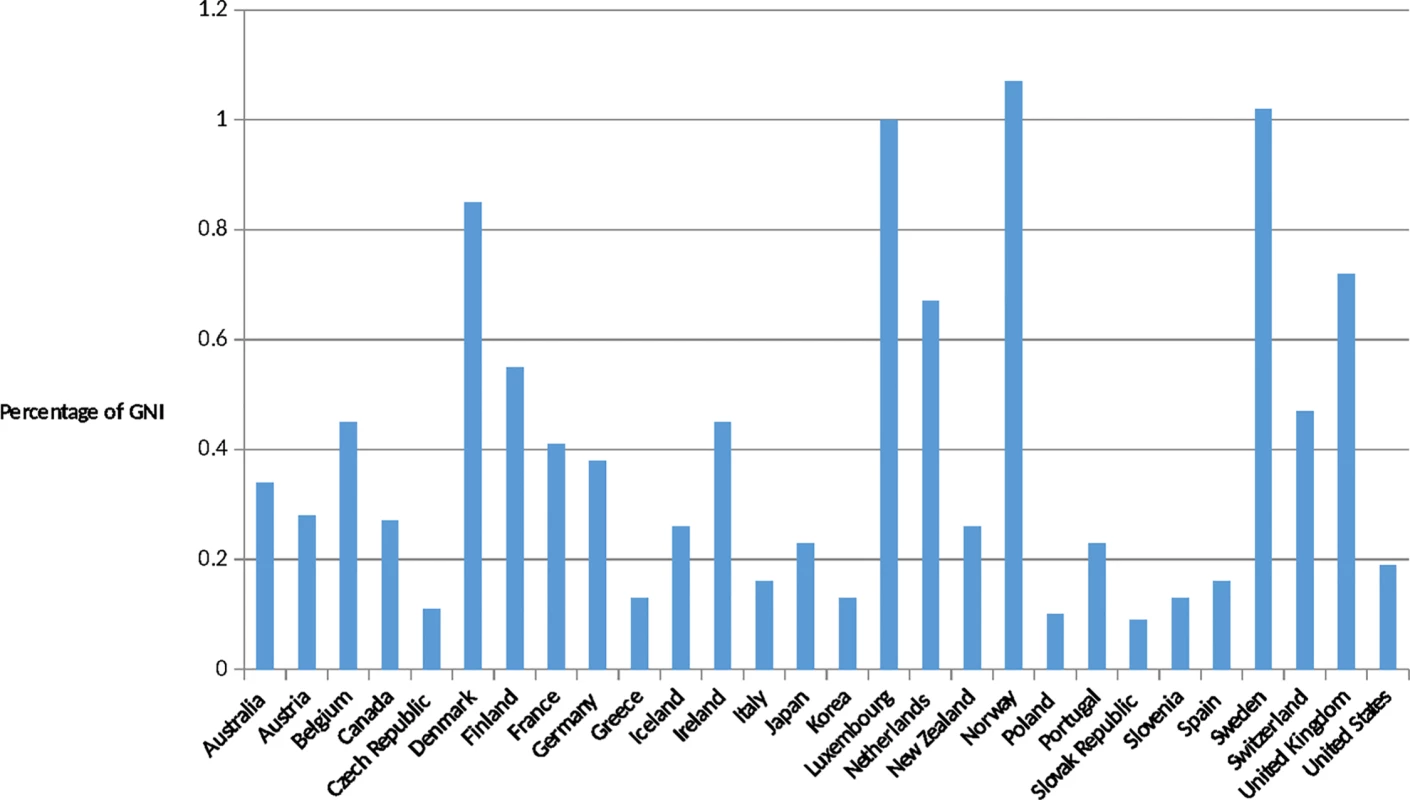
Source: OECD. Development Co-operation Report 2014: Mobilising Resources for Sustainable Development. Paris: OECD Publishing; 2014. Available at http://observ-ocd.org/sites/observ-ocd.org/files/publicacion/docs/informe_coop.desen_._2014_ocde.pdf. Accessed January 14, 2016. However, many successes in RMNCH cannot be attributed to condition-specific interventions alone, i.e., interventions solely and specifically directed at one disease or condition. Among many other factors, there have been accompanying improvements in country revenues, health financing, education and food security systems, and alignment of global and national political and advocacy purposes, as well as an absence of war/strife, that together have successfully accelerated progress or “bent the curve” in reducing reproductive maternal, newborn, and child mortality. Some examples are instructive to consider, particularly because until their development, a huge toll in mortality and morbidity was exacted.
Increased access to and uptake of contraception and family planning services and prevention of unsafe abortion practices
Major reductions in maternal and newborn morbidity and mortality have been noted with uptake of family planning services and increased birth spacing. Estimations are that fulfilling the unmet need for modern family planning methods will avert 70,000 maternal deaths (18,000 attributable to unsafe abortion and 53,000 from complications of pregnancy and childbirth) and 500,000 newborn deaths every year [14]. In addition, improvements in training, care provision, and methods have reduced mortality due to unsafe abortion from 69,000 in 1990 to 47,000 in 2008 [15]. This trend is likely to continue, given the increasingly wide availability of drugs to induce a pharmacologic abortion, diminished need for surgical interventions post delivery and post abortion, and the increasing thrust of the global community to make family planning options available to millions of women worldwide.
Increasing immunization coverage
The introduction of immunizations over the past century has been one of the biggest successes of public health and a major lever in reducing under-5 mortality. Vaccination with diphtheria-tetanus-pertussis (DPT), polio, measles, and Bacillus Calmette–Guérin (BCG) currently saves an estimated 3 million lives each year [16,17], smallpox has been eradicated, and the world stands on the brink of eradicating polio. Certain galvanizing programs such as the expanded program on immunizations (EPI) and organizations such as GAVI and the Global Fund have further spurred on these wins [18]. The success of maternal tetanus administration in reducing the incidence of neonatal tetanus (and more recent data on the benefits of maternal immunization with influenza vaccine on newborn mortality and morbidity) has initiated a promising line of inquiry into broader applications of maternal immunization [19].
Improvements in obstetric care and improved understanding and management of intrapartum birth asphyxia among newborns
Over the past 40 years, ultrasound and doppler use have revolutionized obstetric practice and produced a clearer understanding of the pathophysiology of intrapartum birth asphyxia. An increasing number of standardized neonatal resuscitation protocols assist in promptly identifying and addressing the nonbreathing baby [20]. In many countries, these protocols have been applied by skilled birth attendants (and in some cases by nonskilled birth attendants), whose presence early in the process can dramatically improve neonatal outcomes [21].
Though we have highlighted these interventions for their health impact in RMNCH, many of these interventions gained traction against a backdrop of significant social and economic changes, such as increasing labor force participation of women and the need for birth spacing and limiting.
Looking Forward
While these interventions have changed the RMNCH landscape over the past 25 years, what might be “game-changing innovations” over the next 25 years to not only increase survival but to also improve healthy development, well-being, and thriving, the combination described programmatically by some as “thrival”? We posit innovations within the following categories: technology, commodities, demand-side barriers to care seeking and care provision, supply-side issues (especially for marginalized populations), financing, and monitoring and evaluation to ensure accountability and real-time feedback for quality assurance and continuous quality improvement. We recognize that the success of the technological and nontechnological interventions discussed in the ensuing sections is and will often be dependent on nonbiomedical factors. For the purposes of this paper and its scope, we will restrict the discussion to a biomedical focus.
Technological
By 2035, the technological advances in health care provision are likely to be myriad. Within RMNCH, a few key innovations already in the development pipeline today could dramatically increase survival and well-being. In low-income countries, two conditions that affect both the mother and her baby during pregnancy and result in maternal and perinatal mortality and morbidity are infections and preeclampsia. In many of these settings, antenatal screening and treatment for these infections and preeclampsia are often unavailable or late, inconsistently or incorrectly administered, and poorly resourced.
Innovations in diagnostics might include simplified, rapid, multiplexed point-of-care tests [22], especially those that definitively identify and differentiate bacterial and viral sepsis [23–25], or routine use of biomarkers in low-resource settings to identify which women might experience premature labor [26] or predict who might suffer from preeclampsia/eclampsia [27,28]. Similarly, screening tests such as genome sequencing [29] may allow for more rapid and accurate identification of high-risk disease conditions [30–32]. In settings where there are significant physical barriers to accessing continuing care, telemonitoring may provide a potential solution. Telemonitoring is already being explored in high-income countries [33], and biometrics may be used to monitor ongoing care for sick infants and mothers. Tools designed to facilitate remote screening and monitoring will include noninvasive devices [34] for detection of conditions such as hypoxia in children [35] or hypertension in pregnant women [36]. If such tools can be designed to be both affordable and easily administered by minimally trained health workers, they may prove pivotal in encouraging prompt care seeking and thus reduce the burden of preventable or easily treatable illnesses.
Other technological innovations with the potential to have a major impact on mortality and morbidity relate to the use of safe blood products [37–39]. The supply of safe blood products is often a rate-limiting step to survival in many low - and middle-income countries, and supply often falls far short of demand. Work is currently underway on synthetic blood substitutes [40,41] that could be stored at room temperature for extended periods and may be universally compatible with all blood types [42,43]. Such products could thus be safely administered in emergency situations and in communities where supply, screening, typing, and storage of blood are a challenge.
Commodities and Supply Chain
Innovative technologies to improve the quality, longevity, and usability of pharmaceuticals and the vaccine cold chain will likely play a major role in improving survival.
For example, the World Health Organization recommends oxytocin as the drug of choice for addressing postpartum hemorrhage, the leading cause of maternal death in low - and middle-income countries [44,45]. However, given the heat-labile nature of oxytocin, there are ongoing concerns about the stability and quality of oxytocin in tropical or subtropical climates in countries, where there may be challenges to keeping oxytocin in the cold chain consistently during transport and storage. Oxytocin is an injectable drug, and in-country policies restricting administering injections to certain categories of providers serve as an additional barrier to its use. There is work underway to produce heat-stable formulations of oxytocin, as well as research exploring alternative delivery mechanisms for oxytocin, such as the sublingual and inhaled route. This circumvents the current need where only providers trained in injection techniques can administer this. In addition, to help providers assess the potency of oxytocin at the point of use, the UN Commission on Life-Saving Commodities (UNCoLSC) is supporting efforts to pilot the inclusion of time-temperature indicators (TTIs) on vials of oxytocin [46]. The TTIs, similar to vaccine vial monitors, change color under cumulative exposure to heat and light, indicating potential issues with potency, as well as providing cues for which vials of oxytocin should be utilized first [47].
Another drug, magnesium sulphate (MgSO4) has been identified by the World Health Organization as the most effective, low-cost anticonvulsant for the treatment of severe preeclampsia or eclampsia [48], one of the major causes of maternal morbidity and mortality globally, yet this intervention remains widely underused [49,50]. In part, intravenous or intramuscular administration and a complex series of calculations and dosing have been key barriers to widespread use. To address this, PATH has identified a range of promising technology solutions that are currently being tested, including the use of a dilution bottle, dosing and dilution mobile app use, simplified regimens including ready-to-use MgSO4 packs separately containing 20% and 50% drug concentrations, and a reusable, electricity-free, low-cost infusion delivery system. Additionally, one new drug delivery platform, via rectally administered gel, is being explored and trialed [51,52].
Addressing Demand-Side Barriers to Care Seeking and Care Provision
Demand-side barriers refer to those barriers that affect women and constrain their ability to seek care. These include, among others, poverty, poor health status, illiteracy, language, customs, lack of information regarding the availability of health services and providers, and limited control over household resources [53]. There are many innovations attempting to address demand-side issues, including the use of community health workers, community-based volunteers, peer support groups, and even electronic health (e-health: the use of information technology and communications within the health system, including laptops, netbooks, personal digital assistants [PDAs], mobile cell phones, or patient monitors), and mobile health (m-health: used mainly to describe how a healthl professional is supported by a mobile device such as a phone or PDA, which provides treatment and public health information—e.g., using short message service [SMS] or wireless technology) technologies [54,55]. These latter two are likely to play a prominent role in the post-2015 RMNCH agenda. According to Agarwal and Labrique, “MHealth strategies may have the potential to improve neonatal (and maternal) survival by catalyzing and improving the delivery of interventions of known efficacy, improving access to information and modifying demand for quality services, and enabling the provision of targeted care, where and when these benefits are needed the most” [56]. For example, Rwanda was able to demonstrate a 27% increase in facility deliveries after the introduction of SMS text messages to community health workers in the case of emergencies [57]. In Kenya, the use of mobile phones to monitor and document birth weight within 7 days of delivery significantly increased timely infant weight monitoring [58]. M-health strategies also include such things as smart phone-based treatment algorithms for remote health workers [59], cell phone-based chronic disease management [60], and the use of health management information systems (HMIS) to provide feedback for quality improvement. The potential for m-health strategies to change the face of MNCH is enormous; however, few m-health initiatives have moved beyond the pilot stage. Challenges include an insufficient evidence base for scale-up, issues of compatibility with existing systems, absence of standards for context-specific adoption, and a lack of ownership [61]. Similarly, Piette et al. conclude that “preliminary evidence shows that e-health systems can have a beneficial impact on the process of clinical care in low - and middle-income countries. However, more studies, particularly to examine the key information needs of health-care workers as well as the effects of e-health services on patient outcomes, are required in resource-poor settings” [62].
Improving Supply-Side Issues, Especially for Marginalized Populations
Supply-side issues in RMNCH typically refer to such things as the availability of trained, culturally sensitive providers in well-supplied facilities that are physically accessible to women seeking care. Poor quality provision, maltreatment, inadequate referral systems for emergency obstetric care, lack of transportation, and the disconnect between communities and facilities often serve as barriers to care seeking [53,63]. One aspect of supply-side innovation involves bringing care to women where they are, rather than expecting women to obtain all of their care in a facility. While this is challenging and rather inefficient for complex issues requiring specialized care, it is an important area of exploration for some of the less complex issues in MNCH.
Sri Lanka more than halved its maternal and neonatal mortality by ensuring access to midwives, particularly for the rural communities [64]. Ghana developed “CHPS compounds” (community-based health planning and services programs) where an auxiliary nurse trained in basic delivery care is available in a community-based compound. Results suggest that such a model has increased the percent of women obtaining skilled delivery [65]. Many other countries have introduced similar user-centric measures, such as Ethiopia with the Health Development Army [66] and India with accredited social health activists [67].
One supply-side issue that is essential for addressing future global health needs is identifying mechanisms that simplify the process of administering medications to patients in need. The recent publication of effective simplified antibiotic regimens for infants aged 2 months and less with presumed sepsis and pneumonia [68] is stimulating enquiry into alternative dosing, route, and duration that antibiotics can be given in the treatment of presumed newborn sepsis. Such changes may allow for outpatient oral administration—or single intramuscular injections—of medications that could once only be administered via continuous intravenous (IV) administration, for example.
Financing
Innovations in the financing of health care currently occur at several altitudes. Macrolevel innovations include such things as the Vaccine Independence Initiative (VII), which was launched in 1991 to assist low - and middle-income countries by decoupling procurement of vaccines from their payment. The VII effectively allows countries to use UNICEF as a purchasing agent, creating higher volume orders and minimizing the impact of individual nations’ poor credit [69]. The Global Fund’s Debt2Health initiative allows nations to swap existing debts for grants and convert loans to grants when certain performance targets are met [70]. While initially used mostly in HIV, tuberculosis, and malaria programs, such a financing mechanism has enormous potential for addressing deficits in MNCH as governments seek debt forgiveness.
Perhaps the most commonly discussed “innovation” in financing is the need for universal health care (UHC), primarily to reduce or eliminate user fees for pregnant and postpartum mothers. UHC is increasingly being regarded as an overarching goal for health in the post-2015 development agenda [71], yet the challenges in defining, funding, and implementing UHC require creative solutions that may vary by country. For example, the BRICS nations of Brazil, Russia, India, China, and South Africa have all signed on to the idea of UHC, but implementation has proven extremely variable and country specific [72]. Nonetheless, in countries where UHC or other programs have reduced or eliminated user fees, service utilization has increased significantly, although not always in a “pro-poor” manner and not always in a way that has translated to measurable improved outcomes among mothers and their babies [73–75].
Smaller-scale innovations in financing have included examples such as vouchers for pregnant women to visit health facilities, conditional cash transfers for mothers who engage in target behaviors [76], and even performance-based incentives for traditional birth attendants who bring their clients to facilities for antenatal, delivery, and postnatal care [77]. All of these efforts have demonstrated improvements in antenatal care attendance, facility delivery, vaccinations, and, in some cases, incidence of low birth weight [76]. One issue that such schemes typically lack is a plan for sustainability. Who will pay for the vouchers when the bilateral donor shifts priorities or when ODA falters? Thus, an important innovation for the next 20 years will be to develop models for sustaining the financial incentives that appear to be effective in getting women to seek reproductive health care.
One interesting financial innovation is the Global Financing Facility (GFF), a new mechanism with a goal to be the principal financing mechanism to accelerate and end preventable MNCH mortality by 2030. Although still in its formative phase, preliminary themes for its utilization include financing that is (1) scaled up, with 3–5 year investments supported by broader and longer term investment strategies; (2) smart, leveraging value for money; (3) sustainable, with a focus on helping countries seek innovative ways of mobilizing resources; and (4) accountable, such that every pregnancy, every birth, and every death is registered and counted. The GFF is a partnership formed initially by the governments of Norway, Canada, and the US and housed within the World Bank [12].
Better Monitoring and Measurement, Linked to Accountability and Real-Time Feedback for Quality Assurance
Measurement is critical to the success and failure of any RMNCH endeavor. Without it, we are unable to assess the impact of programmatic efforts, care provision, or interventions, nor can we be assured that we are working toward desired targets. While there have been improvements over the past decade in global data collection and availability, there remains a paucity of robust MNCH data [78,79]. For example, it is estimated that as of 2007, only 30% of the global population lived in countries with complete vital registration systems [80]. By 2012, 57 million children (or 40% of all births) remained nonregistered by their first birthday [81], and another 15% of births occurred in countries with no vital registration data at all. Virtually all data being examined to inform national - and global-level policy change are retrospective, and virtually all are being examined 3–7 years after collection. Thus, the global community is effectively driving while looking through the rear-view mirror, rather than having real-time feedback and accurate projections to ensure more effective and efficient forward motion. Testing of real-time data collection with immediate feedback is underway, and modalities to optimally synthesize, analyze, and test these modalities to strengthen system-level, real-time data utilization are critical to progress in global health.
Challenges to Maximum Uptake and Effectiveness of Innovations
There are many challenges to maximum uptake and effectiveness of the RMNCH innovations described here. A one-size-fits-all approach to introduction and scale-up is unlikely to be successful, given the heterogeneous nature of the innovations themselves. However, innovations will only reach their potential if they take a deliberate user-centric focus that includes an understanding of care-seeking behaviors and social and cultural preferences of women and communities, the skill, capabilities, and resources available to health care providers, and the current national and local policies regarding medical procedures that can be conducted by these providers [63,82–85]. A second challenge relates to how innovations are integrated with existing programs and within the continuum of care and beyond. Integrating approaches across the lifespan, across platforms for intervention (e.g., reinforcing the same messages at schools, churches, community centers, and health centers), and across the community-to-facility continuum will be critical for eventual success. A final challenge relates to the environment in which innovations are introduced. Only interventions which occur in an enabling environment and address the current social, medical, and logistic context can be effectively implemented and successfully scaled up for maximum public health impact [86–88].
Many of the challenges delineated above are magnified during humanitarian situations. The Ebola outbreak strained already fragile health care systems, further complicating access and delivery of routine services [89]. Even formerly robust health care systems are strained by conflict, with reversal of earlier successes; for example, the polio outbreak in Syria in 2013 triggered an outbreak response in a region that had been free of the disease for 15 years [90]. Yet, it is in precisely such environments that innovations are most crucially needed and where, counter to the challenges described above, they may be most readily adopted. Innovations which reduce complexity, streamline service delivery, minimize human resource workload, and offset infrastructural demands [91] can fundamentally alter the impact of humanitarian situations on mothers and their children.
Conclusion
Over the next 20 years, additional emerging areas in which these transformative innovations will likely have a deep impact will include completing the unfinished MDG agenda [92], tackling stillbirth, adolescent health and preconception care, mental health, ensuring exclusive breast milk feeding, promoting integrated early childhood development practices from birth, and focusing on care involving populations such as the urban poor and displaced peoples in emergency settings.
Innovations matter. Innovations have powerful potential to result in transformative change when technologies are coupled with system-level, condition-sensitive enabling environments that support advocacy and political will, sufficient investment, a large and well-trained workforce, opportunity for real-time feedback, and quality improvement.
Given the trajectory and lessons learned from the MDGs, there is reason for cautious optimism for the future of RMNCH.
Zdroje
1. Verguet S, Norheim O, Olson Z, Yamey G, Jamison D. Annual rates of decline in child, maternal, HIV, and tuberculosis mortality across 109 countries of low and middle income from 1990 to 2013: an assessment of the feasibility of post-2015 goals. Lancet Glob Health. 2014;2(12): e698–e709. doi: 10.1016/S2214-109X(14)70316-X 25433625
2. Kassebaum N, Bertozzi-Villa A, Coggeshall M, Shackelford K, Steiner C, Heuton K, et al. Global, Regional, and National Levels and Causes of Maternal Mortality During 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9947): 980–1004.
3. Wang H, Liddell C, Coates M, Mooney M, Levitz C, Schumacher A et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9947): 957–979.
4. Lozano R, Wang H, Foreman K, Rajaratnam J, Naghavi M, Marcus J et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. The Lancet. 2011;378(9797): 1139–1165.
5. Viner R, Coffey C, Mathers C, Bloem P, Costello A, Santelli J et al. 50-year mortality trends in children and young people: a study of 50 low-income, middle-income, and high-income countries. The Lancet. 2011;377(9772): 1139–1165.
6. Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385(9963): 117–71.
7. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn J et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. The Lancet. 2015;385(9966): 430–440.
8. Lawn J, Blencowe H, Oza S, You D, Lee A, Waiswa P et al. Every Newborn: progress, priorities, and potential beyond survival. The Lancet. 2014;384(9938): 189–205.
9. Compareyourcountry.org. Official Development Assistance 2014 (by OECD) [Internet]. 2015 [cited 22 December 2015]. http://www.compareyourcountry.org/oda?cr=20001&cr1=oecd&lg=en&page=1
10. Hsu J, Pitt C, Greco G, Berman P, Mills A. Countdown to 2015: changes in official development assistance to maternal, newborn, and child health in 2009–10, and assessment of progress since 2003. The Lancet. 2012;380(9848): 1157–1168.
11. WHO Global Health Expenditure Database. Rwanda 2013 [Internet]. 2015 [cited 16 November 2015]. http://apps.who.int/nha/database/Country_Profile/Index/en
12. World Bank | World Development Indicators. Data [Internet]. 2015 [cited 16 November 2015]. http://data.worldbank.org/data-catalog/world-development-indicators
13. OECD. Development Co-operation Report 2014: Mobilising Resources for Sustainable Development. Paris: OECD Publishing; 2014.
14. Singh S, Darroch J, Ashford L. Adding It Up: The Costs and Benefits of Investing in Sexual and Reproductive Health 2014. New York: Guttmacher Institute; 2014.
15. Ahman E, Shah I. New estimates and trends regarding unsafe abortion mortality. Int J Gynaecol Obstet. 2011;115(2): 121–126. doi: 10.1016/j.ijgo.2011.05.027 21885049
16. Nara P, Nara D, Chaudhuri R, Lin G, Tobin G. Perspectives on advancing preventative medicine through vaccinology at the comparative veterinary, human and conservation medicine interface: Not missing the opportunities. Vaccine. 2008;26(49): 6200–6211. doi: 10.1016/j.vaccine.2008.07.094 18708109
17. Pilishvili T, Lexau C, Farley M, Hadler J, Harrison L, Bennett N et al. Sustained Reductions in Invasive Pneumococcal Disease in the Era of Conjugate Vaccine. J Infect Dis. 2010;201(1): 32–41. doi: 10.1086/648593 19947881
18. Bustreo F, Okwo-Bele J, Kamara L. World Health Organization perspectives on the contribution of the Global Alliance for Vaccines and Immunization on reducing child mortality. Arch Dis Child. 2015;100(Suppl 1): S34–S37. doi: 10.1136/archdischild-2013-305693 25613965
19. Omer S, Goodman D, Steinhoff M, Rochat R, Klugman K, Stoll B et al. Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study. PLoS Med. 2011;8(5): e1000441. doi: 10.1371/journal.pmed.1000441 21655318
20. Carlo W, Goudar S, Jehan I, Chomba E, Tshefu A, Garces A et al. Newborn-Care Training and Perinatal Mortality in Developing Countries. N Engl J Med. 2010;362(7): 614–623. doi: 10.1056/NEJMsa0806033 20164485
21. Goudar S, Somannavar M, Clark R, Lockyer J, Revankar A, Fidler H et al. Stillbirth and Newborn Mortality in India After Helping Babies Breathe Training. Pediatrics. 2013;131(2): e344–e352. doi: 10.1542/peds.2012-2112 23339215
22. Pai NP, Dhurat R, Potter M, Behlim T, Landry G, Vadnais C, et al. Will a quadruple multiplexed point-of-care screening strategy for HIV-related co-infections be feasible and impact detection of new co-infections in at-risk populations? Results from cross-sectional studies. BMJ Open. 2014;4(12): e005040. doi: 10.1136/bmjopen-2014-005040 25510882
23. Lucignano B, Ranno S, Liesenfeld O, Pizzorno B, Putignani L, Bernaschi P et al. Multiplex PCR Allows Rapid and Accurate Diagnosis of Bloodstream Infections in Newborns and Children with Suspected Sepsis. J Clin Microbiol. 2011;49(6): 2252–2258. doi: 10.1128/JCM.02460-10 21471340
24. Zumla A, Al-Tawfiq J, Enne V, Kidd M, Drosten C, Breuer J et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—needs, advances, and future prospects. Lancet Infect Dis. 2014;14(11): 1123–1135. doi: 10.1016/S1473-3099(14)70827-8 25189349
25. Rasanen J, Quinn M, Laurie A, Bean E, Roberts C, Nagalla S et al. Maternal serum glycosylated fibronectin as a point-of-care biomarker for assessment of preeclampsia. Am J Obstet Gynecol. 2015;212(1): 82.e1–82.e9.
26. Chan R. Biochemical Markers of Spontaneous Preterm Birth in Asymptomatic Women. BioMed Res Int. 2014;2014 : 1–8.
27. Polsani S, Phipps E, Jim B. Emerging New Biomarkers of Preeclampsia. Adv Chronic Kidney Dis. 2013;20(3): 271–279. doi: 10.1053/j.ackd.2013.01.001 23928393
28. Acestor N, Goett J, Lee A, Herrick T, Engelbrecht S, Harner-Jay C et al. Towards biomarker-based tests that can facilitate decisions about prevention and management of preeclampsia in low-resource settings. Clin Chem Lab Med. 2015.
29. Ma Y, Vilanova D, Atalar K, Delfour O, Edgeworth J, Ostermann M et al. Genome-Wide Sequencing of Cellular microRNAs Identifies a Combinatorial Expression Signature Diagnostic of Sepsis. PLoS ONE. 2013;8(10): e75918. doi: 10.1371/journal.pone.0075918 24146790
30. Berg J, Khoury M, Evans J. Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time. Genet Med. 2011;13(6): 499–504. doi: 10.1097/GIM.0b013e318220aaba 21558861
31. Bhattacharjee A, Sokolsky T, Wyman S, Reese M, Puffenberger E, Strauss K et al. Development of DNA Confirmatory and High-Risk Diagnostic Testing for Newborns Using Targeted Next-Generation DNA Sequencing. Genet Med. 2014;17(5): 337–347. doi: 10.1038/gim.2014.117 25255367
32. Wlodarska M, Johnston J, Gardy J, Tang P. A Microbiological Revolution Meets an Ancient Disease: Improving the Management of Tuberculosis with Genomics. Clin Microbiol Rev. 2015;28(2): 523–539. doi: 10.1128/CMR.00124-14 25810419
33. Piumelli R, Nassi N, Liccioli G, Ernst C, Donzelli G. Telemonitoring for infants at risk of apnoea, bradycardia and hypoxaemia: transmission of data improves the family compliance during home monitoring. J Telemed Telecare. 2012;18(6): 344–347. doi: 10.1258/jtt.2012.120405 22933479
34. Liu H, Wang Y, Wang L. A review of non-contact, low-cost physiological information measurement based on photoplethysmographic imaging. Conf Proc IEEE Eng Med Biol Soc. 2012;2012 : 2088–91. doi: 10.1109/EMBC.2012.6346371 23366332
35. Petersen CL, Chen TP, Ansermino JM, Dumont GA. Design and evaluation of a low-cost smartphone pulse oximeter. Sensors (Basel). 2013;13(12): 16882–93.
36. Abo-Zahhad M, Ahmed SM, Elnahas O. A wireless emergency telemedicine system for patients monitoring and diagnosis. Int J Telemedicine Appl. 2014;2014 : 1–11.
37. Kim HW, Greenburg AG: Toward 21st century blood component replacement therapeutics: artificial oxygen carriers, platelet substitutes, recombinant clotting factors, and others. Artif Cells Blood Subsit Immobil Biotechnol. 2006; 34(6): 537–550.
38. Grethlein S, Rajan A. Blood Substitutes [Internet]. Emedicine.medscape.com. 2015 [cited 3 February 2015]. http://emedicine.medscape.com/article/207801-overview#showall
39. PATH. Blood Substitutes: Technology Opportunities Assessment [Internet]. Seattle: PATH; 2012 p. 1–5. http://sites.path.org/mnhtech/files/2013/07/Blood-Substitutes_FINAL_17June2013.pdf
40. Jahr JS, Akha AS, Holtby RJ. Crosslinked, polymerized, and PEG-conjugated hemoglobin-based oxygen carriers: clinical safety and efficacy of recent and current products. Curr Drug Discov Technol. 2012;9(3): 158–165. 21745179
41. Varnado CL, Mollan TL, Birukou I, Smith BJ, Henderson DP, Olson JS. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013;18(17):2314–2328. doi: 10.1089/ars.2012.4917 23025383
42. HaemO2.com. HaemO2—Creating a safe blood substitute for the 21st century [Internet]. 2015 [cited 22 March 2015]. http://www.haemo2.com/.
43. Reeder BJ, Grey M, Silaghi-Dumitrescu RL, Svistunenko DA, Bulow L, Cooper CE, et al. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem. 2008;283(45): 30780–30787. doi: 10.1074/jbc.M804709200 18728007
44. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A, Daniels J et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6): e323–e333. doi: 10.1016/S2214-109X(14)70227-X 25103301
45. World Health Organization (WHO). WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage [Internet]. Geneva: World Health Organization (WHO); 2012. http://apps.who.int/iris/bitstream/10665/75411/1/9789241548502_eng.pdf
46. PATH. Technical Reference Team Recommendation 10: Product Innovation [Internet]. Seattle: PATH; 2012. http://www.path.org/publications/files/APP_un_comm_rec10.pdf
47. Hodgins S. Oxytocin: taking the heat. Glob Health Sci Pract. 2014;2(3):259–260. doi: 10.9745/GHSP-D-14-00102 25276584
48. Duley L, Gülmezoglu A, Henderson-Smart D, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010.
49. Lumbiganon P, Metin Gulmezoglu A, Piaggio G, Langer A, Grimshaw J. Magnesium sulfate is not used for pre-eclampsia and eclampsia in Mexico and Thailand as much as it should be. Bull World Health Organ. 2007;85(10): 763–767. 18038057
50. Bigdeli M, Zafar S, Assad H, Ghaffar A. Health System Barriers to Access and Use of Magnesium Sulfate for Women with Severe Pre-Eclampsia and Eclampsia in Pakistan: Evidence for Policy and Practice. PLoS ONE. 2013;8(3): e59158. doi: 10.1371/journal.pone.0059158 23555626
51. RMNCH Strategy & Coordination team. UN Commission on Life Saving Commodities: 2014 Progress Report [Internet]. independent Expert Review Group (iERG), World Health Organization; 2015. http://www.who.int/woman_child_accountability/ierg/reports/UNCOLSC_submission_iERG_2015.pdf
52. PATH. Saving Women's Lives by Simplifying the Treatment for Preeclampsia/Eclampsia with Rectally Delivered Magnesium Sulfate [Internet]. Saving Lives at Birth: A Grand Challenge for Development. 2013 [cited 7 December 2015]. https://savinglivesatbirth.net/summaries/267
53. Ensor T, Cooper S. Overcoming barriers to health service access: influencing the demand side. Health Policy Plan. 2004;19(2): 69–79. 14982885
54. Tamrat T, Kachnowski S. Special Delivery: An Analysis of mHealth in Maternal and Newborn Health Programs and Their Outcomes Around the World. Matern Child Health J. 2011;16(5):1092–1101.
55. Kallander K, Tibenderana JK, Akpogheneta OJ, Strachan DL, Hill Z, ten Asbroek AH, et al. Mobile health (mHealth) approaches and lessons for increased performance and retention of community health workers in low - and middle-income countries: a review. J Med Internet Res. 2013;15(1): e17. doi: 10.2196/jmir.2130 23353680
56. Agarwal S, Labrique A. Newborn health on the line: the potential mHealth applications. JAMA. 2014;312(3): 229–230. doi: 10.1001/jama.2014.6371 24953141
57. Ngabo F, Nguimfack J, Nwaigwe F, Mugeni C, Muhoza D, Wilson DR, et al. Designing and Implementing an Innovative SMS-based alert system (RapidSMS-MCH) to monitor pregnancy and reduce maternal and child deaths in Rwanda. Pan Afr Med J. 2012;13 : 31. 23330022
58. Gisore P, Shipala E, Otieno K, Rono B, Marete I, Tenge C, et al. Community based weighing of newborns and use of mobile phones by village elders in rural settings in Kenya: a decentralised approach to health care provision. BMC Pregnancy Childbirth. 2012;12 : 15. doi: 10.1186/1471-2393-12-15 22429731
59. McNabb M, Chukwu E, Ojo O, Shekhar N, Gill CJ, Salami H, et al. Assessment of the quality of antenatal care services provided by health workers using a mobile phone decision support application in northern Nigeria: a pre/post-intervention study. PLoS ONE. 2015;10(5): e0123940. doi: 10.1371/journal.pone.0123940 25942018
60. Piette JD, Marinec N, Gallegos-Cabriales EC, Gutierrez-Valverde JM, Rodriguez-Saldana J, Mendoz-Alevares M, et al. Spanish-speaking patients' engagement in interactive voice response (IVR) support calls for chronic disease self-management: data from three countries. J Telemed Telecare. 2013;19(2): 89–94. doi: 10.1177/1357633X13476234 23532005
61. Tomlinson M, Rotheram-Borus M, Swartz L, Tsai A. Scaling Up mHealth: Where Is the Evidence?. PLoS Med. 2013;10(2): e1001382. doi: 10.1371/journal.pmed.1001382 23424286
62. Piette J, Lun K, Moura L, Fraser H, Mechael P, Powell J et al. Impacts of e-health on the outcomes of care in low - and middle-income countries: where do we go from here?. Bull World Health Organ. 2012;90(5): 365–372. doi: 10.2471/BLT.11.099069 22589570
63. Moyer CA, Adongo PB, Aborigo RA, Hodgson A, Engmann CM, Devries R. "It's up to the woman's people": how social factors influence facility-based delivery in Rural Northern Ghana. Matern Child Health J. 2014;18(1): 109–119. doi: 10.1007/s10995-013-1240-y 23423857
64. Pathmanathan I, Liljestrand J, Martins J, Rajapaksa L, Lissne C, de Silva A. Investing effectively in maternal health in Malaysia and Sri Lanka. Washington (DC): The World Bank. 2003.
65. Sakeah E, Doctor HV, McCloskey L, Bernstein J, Yeboah-Antwi K, Mills S. Using the community-based health planning and services program to promote skilled delivery in rural Ghana: socio-demographic factors that influence women utilization of skilled attendants at birth in northern Ghana. BMC Public Health. 2014;14 : 344. doi: 10.1186/1471-2458-14-344 24721385
66. Ramundo K. The Female "Army" Leading Ethiopia's Health Revolution. Frontlines, USAID [Internet]. 2012 [cited 22 December 2015];(May/June 2012): 28–31. https://www.usaid.gov/sites/default/files/frontlines/FL_MAYJUN12.pdf
67. Saprii L, Richards E, Kokho P, Theobald S. Community health workers in rural India: analysing the opportunities and challenges Accredited Social Health Activists (ASHAs) face in realising their multiple roles. Human Resources for Health. 2015;13(1).
68. Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. The Lancet. 2015;385(9979): 1767–1776.
69. Furtwangler T. Innovative health sector financing: the Vaccine Independence Initiative [Internet]. PATH blog. 2015 [cited 22 December 2015]. http://blog.path.org/2015/01/innovative-health-sector-financing/
70. Fryatt R, Mills A, Nordstrom A. Financing of health systems to achieve the health Millennium Development Goals in low-income countries. The Lancet. 2010;375(9712): 419–426.
71. Vega J. Universal health coverage: the post-2015 development agenda. The Lancet. 2013;381(9862): 179–180.
72. Marten R, McIntyre D, Travassos C, Shishkin S, Longde W, Reddy S, et al. An assessment of progress towards universal health coverage in Brazil, Russia, India, China, and South Africa (BRICS). The Lancet. 2014;384(9960): 2164–2171.
73. McKinnon B, Harper S, Kaufman JS, Bergevin Y. Removing user fees for facility-based delivery services: a difference-in-differences evaluation from ten sub-Saharan African countries. Health Policy Plan. 2015;30(4): 432–441. doi: 10.1093/heapol/czu027 24816570
74. Amoako Johnson F, Frempong-Ainguah F, Padmadas S. Two decades of maternity care fee exemption policies in Ghana: have they benefited the poor?. Health Policy Plan. 2015;31(1):46–55. doi: 10.1093/heapol/czv017 25862731
75. Dzakpasu S, Powell-Jackson T, Campbell OM. Impact of user fees on maternal health service utilization and related health outcomes: a systematic review. Health Policy Plan. 2014;29(2): 137–150. doi: 10.1093/heapol/czs142 23372035
76. Glassman A, Duran D, Fleisher L, Singer D, Sturke R, Angeles G, et al. Impact of conditional cash transfers on maternal and newborn health. J Health Popul Nutr. 2013;31(4 Suppl 2): 48–66. 24992803
77. Oyebola BC, Muhammad F, Otunomeruke A, Galadima A. Effect of performance-based incentives for traditional birth attendants on access to maternal and newborn health-care facilities in Gombe State, Nigeria: a pilot study. The Lancet. 2014;384: S10.
78. Setel PW, Macfarlane SB, Szreter S, Mikkelsen L, Jha P, Stout S, et al. A scandal of invisibility: making everyone count by counting everyone. The Lancet. 2007;370(9598): 1569–1577.
79. Engmann C, Garces A, Jehan I, Ditekemena J, Phiri M, Thorsten V, et al. Birth attendants as perinatal verbal autopsy respondents in low - and middle-income countries: a viable alternative?. Bull World Health Organ. 2012;90(3): 200–208. doi: 10.2471/BLT.11.092452 22461715
80. Mahapatra P, Shibuya K, Lopez AD, Coullare F, Notzon FC, Rao C, et al. Civil registration systems and vital statistics: successes and missed opportunities. The Lancet. 2007;370(9599):1653–1663.
81. UNICEF, UNICEF. Every Child’s Birth Right: Inequities and trends in birth registration. New York: UNICEF. 2013.
82. Aborigo RA, Moyer CA, Gupta M, Adongo PB, Williams J, Hodgson A, Allote P, Engmann CM. Obstetric danger signs and factors affecting health seeking behaviour among the Kassena-Nankani of northern Ghana: a qualitative study: original research article. Afr J Reprod Health. 2014;18(3): 78–86. 25438512
83. Hill E, Hess R, Aborigo R, Adongo P, Hodgson A, Engmann C, et al. "I don't know anything about their culture": the disconnect between allopathic and traditional maternity care providers in rural northern Ghana. Afr J Reprod Health. 2014;18(2): 36–45. 25022140
84. Engmann C. Improving neonatal mortality in sub-Saharan Africa: any cause for optimism?. J Perinatol. 2011;31(12):745–748. doi: 10.1038/jp.2011.53 22124515
85. Owais A, Sultana S, Stein AD, Bashir NH, Awaldad R, Zaidi AK. Why do families of sick newborns accept hospital care? A community-based cohort study in Karachi, Pakistan. J Perinatol. 2011;31(9): 586–592. doi: 10.1038/jp.2010.191 21273989
86. Mangham LJ, Hanson K. Scaling up in international health: what are the key issues?. Health Policy Plan. 2010;25(2): 85–96. doi: 10.1093/heapol/czp066 20071454
87. Engmann C, Garces A, Jehan I, Ditekemena J, Phiri M, Mazariegos M, et al. Causes of community stillbirths and early neonatal deaths in low-income countries using verbal autopsy: an International, Multicenter Study. J Perinatol. 2012;32(8): 585–592. doi: 10.1038/jp.2011.154 22076413
88. Engmann C, Wall S, Darmstadt G, Valsangkar B, Claeson M. Consensus on kangaroo mother care acceleration. The Lancet. 382(9907):e26–e27.
89. Hayden EC. Maternal health: Ebola's lasting legacy. Nature. 2015;519(7541): 24–26. doi: 10.1038/519024a 25739614
90. World Health Organization (WHO). Strategic plan for polio outbreak response in the Middle East November, 2013. The Syrian Arab Republic, Iraq, Jordan, Lebanon, Turkey, West Bank and Gaza Strip. [Internet]. Geneva: World Health Organization (WHO); 2013. http://polioeradication.org/Portals/0/Document/InfectedCountries/MiddleEast/ME_StrategicPlan.pdf
91. Emerging economies drive frugal innovation. Bull World Health Organ. 2013;91(1): 6–7. doi: 10.2471/BLT.13.020113 23397344
92. Requejo J, Bhutta Z. The post-2015 agenda: staying the course in maternal and child survival. Arch Dis Child. 2015;100(Suppl 1): S76–S81. doi: 10.1136/archdischild-2013-305737 25613979
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Trans-Pacific Partnership Provisions in Intellectual Property, Transparency, and Investment Chapters Threaten Access to Medicines in the US and Elsewhere
- An Uninformative Truth: The Logic of Amarin’s Off-Label Promotion
- Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study
- Pragmatic Trials for Noncommunicable Diseases: Relieving Constraints
- Antibiotic Resistance in India: Drivers and Opportunities for Action
- Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis
- Routine Pediatric Enterovirus 71 Vaccination in China: a Cost-Effectiveness Analysis
- Increased Duration of Paid Maternity Leave Lowers Infant Mortality in Low- and Middle-Income Countries: A Quasi-Experimental Study
- Planned Repeat Cesarean Section at Term and Adverse Childhood Health Outcomes: A Record-Linkage Study
- Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea
- A Novel Brief Therapy for Patients Who Attempt Suicide: A 24-months Follow-Up Randomized Controlled Study of the Attempted Suicide Short Intervention Program (ASSIP)
- Comparison of the Schwartz and CKD-EPI Equations for Estimating Glomerular Filtration Rate in Children, Adolescents, and Adults: A Retrospective Cross-Sectional Study
- Cardiovascular and Renal Outcomes of Renin–Angiotensin System Blockade in Adult Patients with Diabetes Mellitus: A Systematic Review with Network Meta-Analyses
- Length of Stay After Childbirth in 92 Countries and Associated Factors in 30 Low- and Middle-Income Countries: Compilation of Reported Data and a Cross-sectional Analysis from Nationally Representative Surveys
- Transformative Innovations in Reproductive, Maternal, Newborn, and Child Health over the Next 20 Years
- Compassionate and Proactive Interventions by Health Workers in the United Kingdom: A Better Approach to Prevent and Respond to Female Genital Mutilation?
- Translational Research for Tuberculosis Elimination: Priorities, Challenges, and Actions
- The Community As the Patient in Malaria-Endemic Areas: Preempting Drug Resistance with Multiple First-Line Therapies
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea
- Comparison of the Schwartz and CKD-EPI Equations for Estimating Glomerular Filtration Rate in Children, Adolescents, and Adults: A Retrospective Cross-Sectional Study
- Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study
- A Novel Brief Therapy for Patients Who Attempt Suicide: A 24-months Follow-Up Randomized Controlled Study of the Attempted Suicide Short Intervention Program (ASSIP)
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



