-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
Sarah Wild and colleagues investigate whether a monitoring system that shares results with a patient's physician or nurse can help reduce HbA1c measurements for patients with type 2 diabetes.
Published in the journal: Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial. PLoS Med 13(7): e32767. doi:10.1371/journal.pmed.1002098
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002098Summary
Sarah Wild and colleagues investigate whether a monitoring system that shares results with a patient's physician or nurse can help reduce HbA1c measurements for patients with type 2 diabetes.
Introduction
The International Diabetes Federation has estimated that the number of people with diagnosed diabetes in the world in 2013 was 206 million [1]. Annual health care costs of a patient with type 2 diabetes are approximately two to three times higher than the costs of a person of similar age and sex without diabetes, and approximately 80% of the cost of diabetes to health services in developed countries is spent on complications. Control of blood glucose and blood pressure and management of dyslipidemia reduces complications and mortality among people with diabetes [2,3]. The increasing prevalence of diabetes and other chronic conditions means that traditional models of management in family practice are under extreme pressure and there is considerable interest in developing effective approaches to self-management across the world.
The traditional clinician-led model with regular face-to-face consultations for managing diabetes and hypertension is costly in terms of health care professionals’ time, rarely supports self-management by patients, and is often not very effective, partly because therapeutic inertia may result in reluctance to change treatments [4]. Systematic reviews indicate that engaging patients in self-monitoring and management can improve clinical outcomes in some chronic disorders (for example, asthma), but the evidence that self-monitoring of blood glucose is beneficial in people with type 2 diabetes is less clear [5,6]. This may be due to therapeutic inertia, poor adherence to both lifestyle advice and prescribed medication among people with diabetes [7,8], and the fact that patients become anxious when faced with self-monitored evidence of poor control when feedback from clinicians is infrequent [9].
The aim of the Telescot Diabetes Trial was to investigate the effect of supervised, self-monitoring of glycemic control, blood pressure, and weight with telemetric transmission of measurements (hereafter described as supported telemonitoring) among people with poorly controlled diabetes compared with a control group receiving usual care. The trial was performed within a program of similar trials in patients with chronic conditions described further at www.telescot.org and included a previous hypertension trial [10].
Methods
Study Design
We performed a randomized, parallel, investigator-blind, controlled trial in family practices in four regions of the United Kingdom (UK). The South-East Scotland Research Ethics Committee (reference number: 10/S1102160) approved the study, which was overseen by an Independent Trial Steering Committee. All participants provided written informed consent. The protocol provides details of the design and methods and is available as S1 Text and in the published version [11].
Participants
We recruited family practices caring for socially diverse populations from Borders, Glasgow, and Lothian in Scotland and Kent in England through primary care research networks. Participating practices searched their registers of people with type 2 diabetes (assisted as necessary by researchers from Primary Care Research Networks) to identify and send an information sheet and letter inviting potential participants to respond to the research team if they were interested in participating in the trial.
Volunteers attended a screening visit at which the intervention and trial was explained, written informed consent was obtained, a blood sample for HbA1c and lipid measurement was collected and daytime ambulatory blood pressure monitoring (ABPM) was initiated. Inclusion criteria were the following: diagnosis of type 2 diabetes managed in family practice, age over 17 years, availability of a mobile telephone signal at home, and poor glycemic control, defined as HbA1c >58 mmol/mol. The original protocol included an inclusion criterion of poorly controlled blood pressure (based on average daytime self-monitored blood pressure ≥130 mmHg), but this criterion was removed and the protocol [11] amended in the first month of recruitment because the majority of potential participants had well-controlled blood pressure. After 15 months of recruitment the initial upper age limit was removed and we clarified that exclusion on the basis of surgery within the last 3 mo implied major surgery and that treated atrial fibrillation was not an exclusion criterion. The protocol changes mean that trial participants are more representative of the population with type 2 diabetes with poor glycemic control than if the inclusion requirements for poor blood pressure control and maximum age had remained.
Exclusion criteria were blood pressure >210/135 mmHg, hypertension or renal disease managed in secondary care, treatment for a cardiac event or other life-threatening illness within the previous 6 mo, major surgery within the last 3 mo, atrial fibrillation unless successfully treated or cardioverted, inability to use self-monitoring equipment, and pregnancy.
Randomization and Masking
People meeting the eligibility criteria were randomized at a second visit using an allocated treatment code generated by a computer from a minimization procedure based on age (<70 or ≥70 years of age), sex, geographic area, prescription of two or more diabetes treatments, prescription of three or more anti-hypertensive medications, and whether they had previously monitored their blood sugars, as well as a 1 : 1 ratio for intervention to control group. Minimization was used to ensure that important baseline variables were balanced between intervention and control groups. To ensure unpredictability of the minimization procedure, there was a 1 in 10 chance that the determined treatment allocation was reversed; the corresponding random numbers list was stored securely at the Edinburgh Clinical Trials Unit and concealed from participants and research nurses. Random allocations were obtained by research nurses who enrolled participants through a secure web-based system prepared and maintained by the Edinburgh Clinical Trials Unit.
It was not possible to blind participants, clinicians, or research nurses to allocation group for all participants. In Lothian and Borders a different research nurse collected data at baseline and follow-up, although this was not possible for participants in Kent and Glasgow. The primary and principal secondary outcomes were based on objective measurements derived from collection of blood samples, ABPM, and weight measurement by research nurses such that lack of blinding is unlikely to introduce measurement bias. In addition, participants were asked not to reveal allocation until after final data collection was complete.
Procedures
Research nurses undertook baseline measurements prior to randomization in all participants. Baseline measurements included HbA1c, the average daytime blood pressure (BP) from ABPM based on a minimum of 12 measurements recorded every 20 min between 7 a.m. and 11 p.m., average of second and third measurements of office BP measured by research nurses, smoking history, height and weight, exhaled carbon monoxide, questionnaire data on anxiety/depression (Hospital Anxiety and Depression Scale) [12], quality of life (EQ-5D) [13], self-efficacy [14], medication adherence [15], physical activity [16], knowledge of managing diabetes (based on responses from the first 14 items of the diabetes knowledge test) [17], and ethnic group based on categories included in the 2011 UK Census.
A full description of the intervention is provided in S3 Text. In brief, participants in the intervention group were given instructions for use of blood pressure, blood glucose, and weight monitors, that used Bluetooth technology to transmit readings via a supplied modem to a remote secure server by research nurses. The participant and their family practice professionals were able to access password-protected records on the server. Participants were asked to measure one fasting and one nonfasting blood glucose at least twice weekly and measure BP and weight at least weekly (with increased testing as recommended by the clinician for people treated with insulin). Participants were given advice on lifestyle modification, on lag time for effects of lifestyle and medication change on glucose and blood pressure, and when and how to contact their family practice team via research nurses. Primary care nurses were asked to check participants’ results weekly and to organize treatment changes based on national guidelines for diabetes and hypertension management (see protocol and S4 Text) [11]. The intervention lasted 9 mo, when patients were asked to attend for follow-up. The comparison group received usual care. Usual diabetes care in family practice is financially incentivized in the UK with targets set on a sliding scale of rewards for glycemic and blood pressure control (see [18] for more detail). Well-controlled patients are reviewed at least once a year, but more frequent reviews are performed for people who have poor glycemic or blood pressure control.
Outcomes
The follow-up appointment included similar measurements and questionnaires to those performed at baseline. The primary outcome was adjusted mean difference in HbA1c, and secondary outcomes were adjusted mean differences in daytime ambulatory systolic and diastolic blood pressures and weight between treatment groups (see statistical analysis for further details of adjustments). HbA1c (that reflects blood glucose control over the previous 2–3 mo) recorded within 6 wk either side of the 9 mo follow-up point were used in order to maximize completeness of follow-up data. Standard blood pressure measures, serum total cholesterol, HDL cholesterol, urinary sodium/creatinine ratio, renal function (eGFR), United Kingdom Prospective Diabetes Study (UKPDS) risk score, anxiety and depression, quality of life, self-efficacy, self-reported physical activity, self-reported exercise tolerance, self-reported alcohol intake and diabetes knowledge, number of attendances at accident and emergency, at out-of-hours care, with practice nurse, with general practitioner (GP), hospital admissions, and number of telephone/email contacts with practice nurses and GPs were also compared between groups as pre-specified additional outcome measures. Use of health care resources (number and duration of hospital admissions, practice and out-of-hours consultations, routine reviews for BP or diabetes, prescriptions for antihypertensive and diabetes drugs) and adverse events were extracted from participants’ electronic family practice records by research nurses.
Statistical Analysis
We used distribution of HbA1c and BP from the Lothian population-based diabetes register for 2008 (mean [SD] HbA1c of 68 [15] mmol/mol and mean [SD] systolic blood pressure of 149 [13] mmHg) when planning the trial (see protocol for further details). Using this information, we estimated that a randomized controlled trial with 125 people completing each arm with no change in values in the control group at the end of the trial would have 80% power at 5% significance to detect a 6 mmol/mol fall in HbA1c and a 5 mmHg fall in systolic BP in the intervention group. We aimed to recruit a total of 320 trial participants to allow for 20% attrition.
Data were analyzed on an intention-to-treat basis using a complete case analysis assuming missing outcome data were missing completely at random. Difference in mean HbA1c between intervention and control groups, the primary outcome, was estimated using linear regression with a 95% confidence interval for the difference, after adjusting for baseline HbA1c and with the addition of minimization strata (for which adjustment was accidentally omitted from the protocol) using analysis of covariance. Clustering by practice was investigated for the primary outcome by calculating the intra-cluster correlation coefficient. A priori planned sensitivity analyses involved (i) exclusion of participants with values of the primary outcome variable more than four standard deviations from the mean and (ii) a multiple imputation analysis using the same variables as in the main analysis (including the outcome variable) with the addition of weight, baseline ABPM systolic blood pressure, baseline ABPM diastolic blood pressure, baseline HDL cholesterol, and baseline total cholesterol, based on 30 imputation datasets, and combining results using Rubin’s rules [19]. Similar linear regression methods were used for systolic and diastolic BPs and weight and the other continuous secondary outcomes listed in the protocol. Negative binomial regression was used for outcomes measured as counts, adjusting for baseline and minimization strata, where possible. Logistic regression was used for binary outcomes, adjusting for the minimization strata. Subgroup analyses and tests for interactions were performed as described in the protocol and S5 Text [11]. Analyses were performed using version 9.3 of the SAS system for Windows (SAS Institute Inc., Cary, NC, United States). There was no data monitoring committee. The trial had the registration number ISRCTN71674628 in Current Controlled Trials.
Results
Forty-four practices were invited to participate, of which 42 recruited participants (with a mean of 8 participants per practice, min 1, max 46). The intra-cluster correlation coefficient was calculated to be below zero (-0.0012) and so there was no evidence of clustering by practice. We invited 2,680 potential participants, 500 were assessed for eligibility, and 321 were randomized, 160 to the intervention and 161 to the control arms, respectively (see Fig 1: CONSORT flow chart). Just over half of the participants were recruited from Lothian and Borders. The first participant was randomized on June 6, 2011, and the last was randomized on July 19, 2013, with the last follow-up visit on May 21, 2014. Data for the primary outcome were available for 146 people (91%) in the intervention group and 139 people (86%) in the control group.
Fig. 1. CONSORT flow chart for the Telescot diabetes trial. 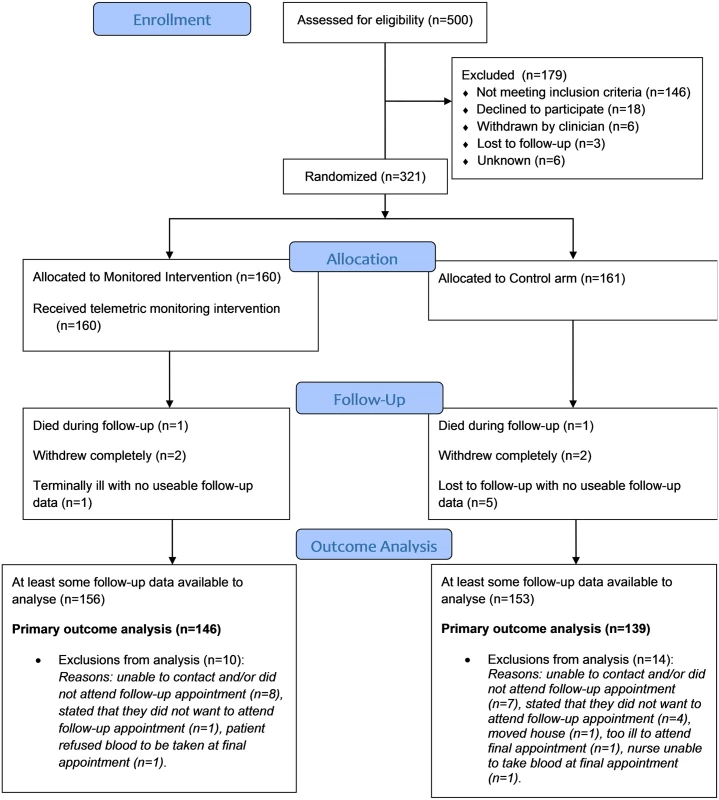
The two groups had similar distributions of relevant characteristics at baseline as shown in Tables 1 and 2 (summarizing distribution of minimization criteria for all randomized participants and participants contributing to the primary outcome analyses respectively) and Tables 3 and 4 (summarizing clinical characteristics for all randomized participants and participants contributing to the primary outcome analyses respectively). The mean (SD) age of participants was 61.0 (9.8) years with a range of 33.7 to 86.3 years; two-thirds were men and mean (SD) duration of diabetes was 7.4 (5.8) years. Twenty-five percent of participants in the intervention group submitted blood glucose readings as requested (one fasting and one nonfasting blood glucose at least twice weekly) with 21% of participants submitting less than a quarter of requested measurements.
Tab. 1. Distribution of minimization criteria in all trial participants by trial arm (supported telemonitoring compared to usual care). 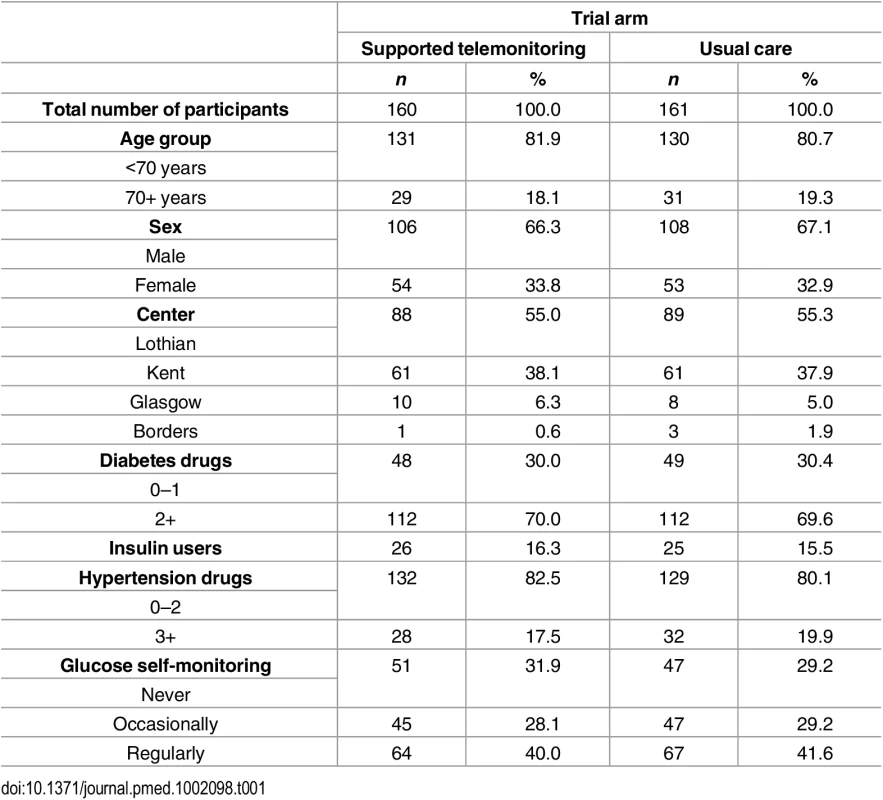
Tab. 2. Distribution of minimization criteria in trial participants included in the primary endpoint analysis by trial arm (supported telemonitoring compared to usual care). 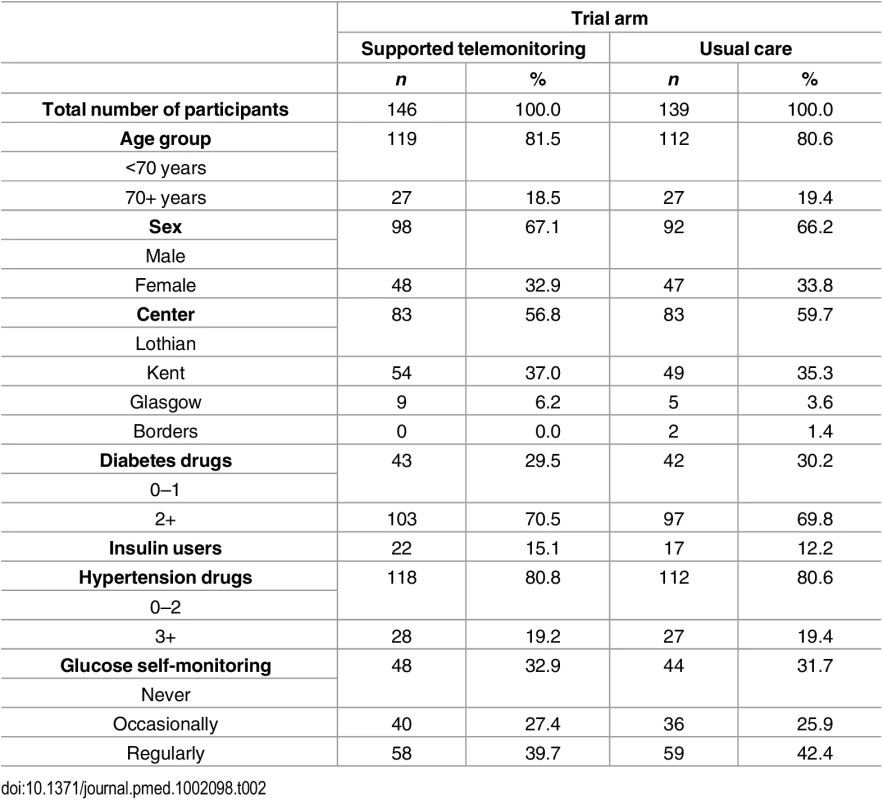
Tab. 3. Characteristics of all trial participants at baseline by trial arm (supported telemonitoring compared to usual care). 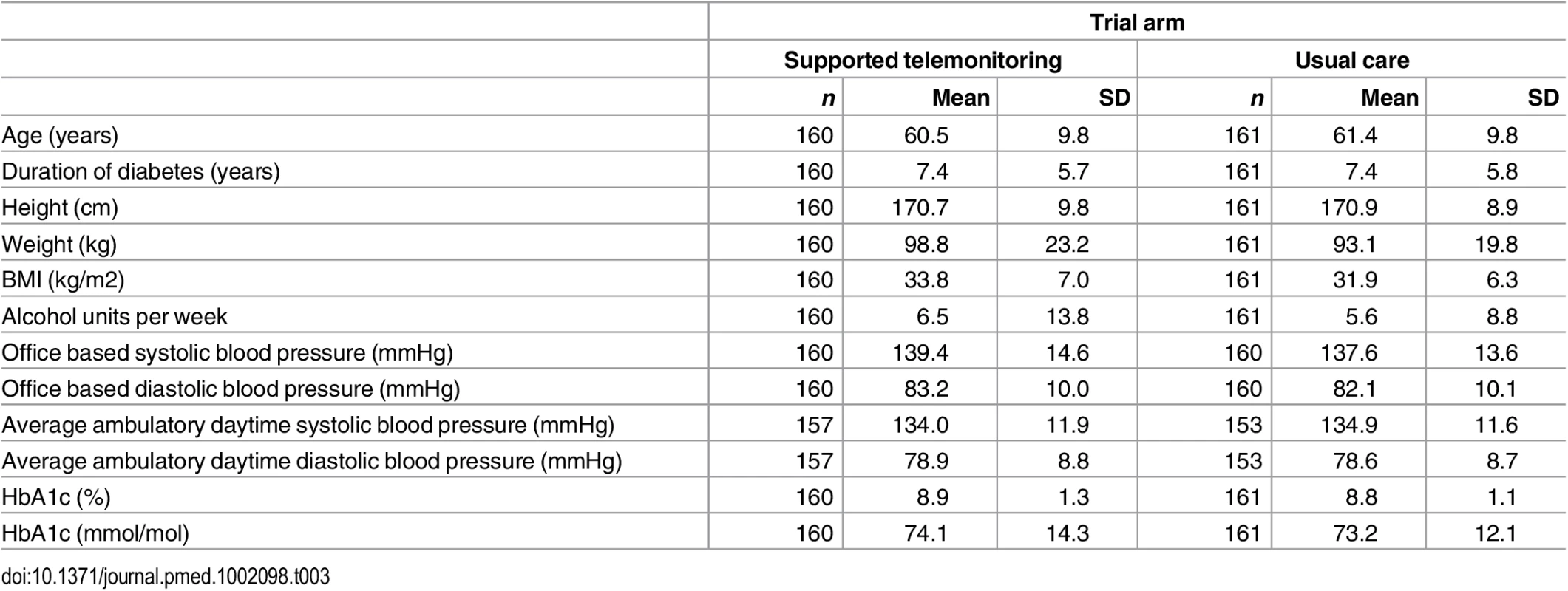
Tab. 4. Characteristics of trial participants included in the primary endpoint analysis at baseline by trial arm (supported telemonitoring compared to usual care). 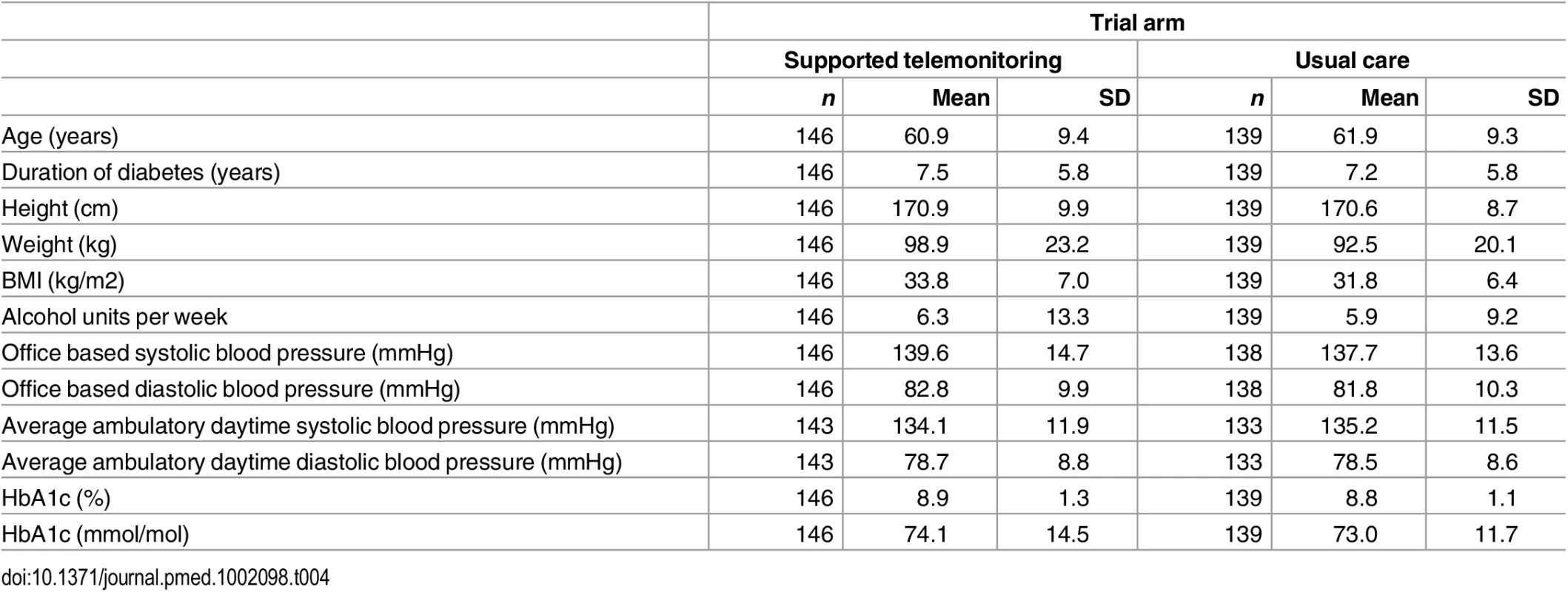
Primary and secondary outcomes are described in detail in Table 5. The absolute mean difference in HbA1c between groups was 5.60 mmol/mol; (95% CI 2.38 to 8.81 mmol/mol; p = 0.0007) lower in the monitored compared with the control arm. The effect size was similar after removing outliers more than four standard deviations away from the mean in the primary outcome variable in a sensitivity analysis: 5.32 mmol/mol 95% CI 2.12 to 8.52 mmol/mol, p = 0.0012. After performing multiple imputation, the difference was estimated to be 4.75 95% CI 1.55 to 7.95 mmol/mol, p = 0.0036.
Tab. 5. Baseline and follow-up values for primary and secondary outcomes in the trial by trial arm. 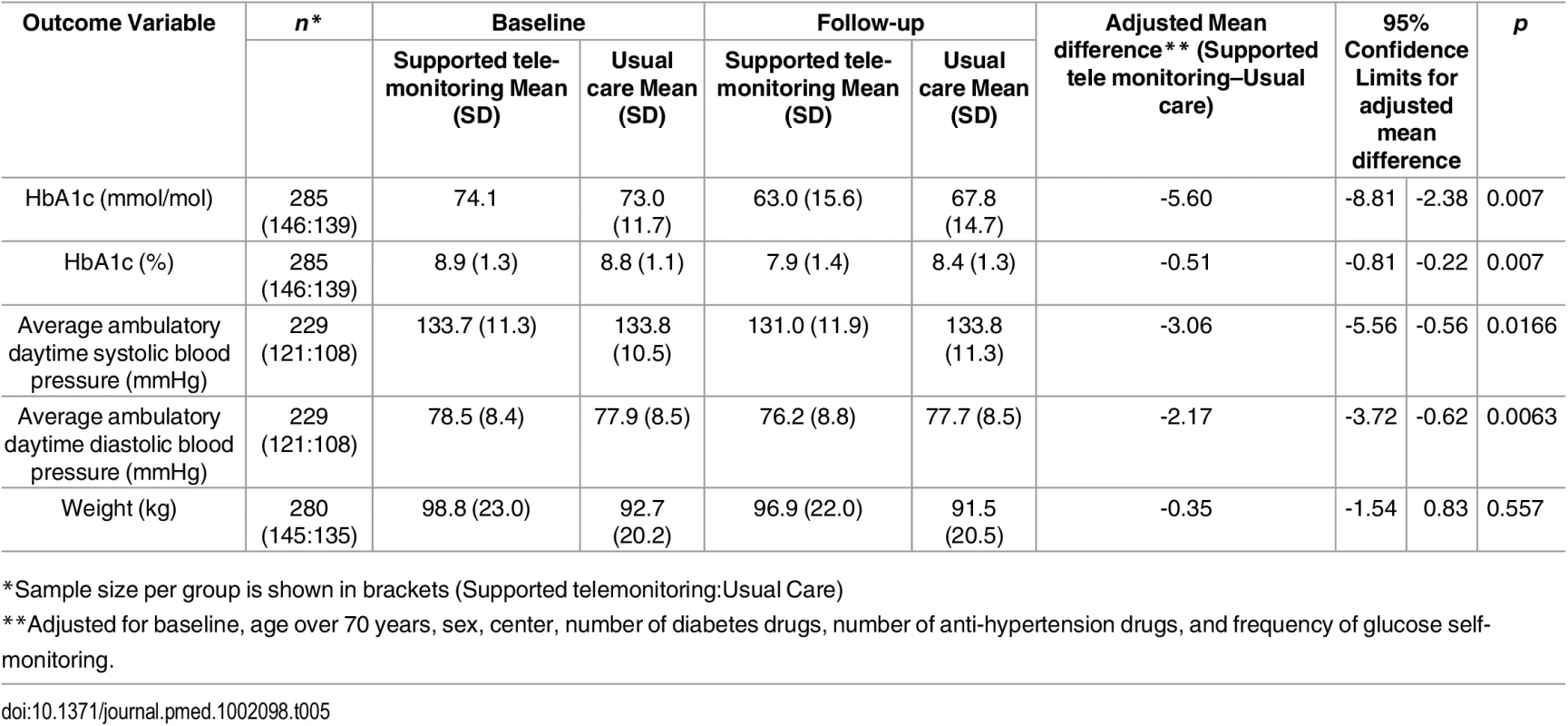
*Sample size per group is shown in brackets (Supported telemonitoring:Usual Care) Ambulatory BP data were available at follow-up for 229 people, and BP was lower in the intervention than control group: systolic BP by 3.06 mmHg; 95% CI 0.56 to 5.56 mm Hg; p = 0.017 and diastolic BP by 2.17 mmHg; 95% CI 0.62 to 3.72 mmHg; p = 0.006. Weight at follow-up was not significantly different between trial arms (0.35 kg lower for the 145 people in the intervention compared to the 135 people in control group for whom data were available; 95% CI -0.83 to 1.54 kg, p = 0.557).
Additional outcome measures are reported in S1 and S2 Tables. Prescribing patterns for diabetes treatments or anti-hypertensive drugs were similar between intervention and control groups as demonstrated in Tables 6 and 7. Insulin treatment was started during follow-up for eight people in the intervention group and six people in the control group. In addition, post-hoc linear regression models adjusted for baseline defined daily doses (DDDs) [20] and minimization variables did not identify statistically significant differences in mean DDD at follow-up of either diabetes treatment (0.003 [95% CI -0.21 to 0.21]) or anti-hypertensive treatment (0.18 [95% CI -0.04 to 0.39]) between groups. In particular, there was no evidence that the intervention increased anxiety levels. The only statistically significant difference between randomization groups in use of health services was a greater number of telephone calls between practice nurses and patients in the intervention compared with control group (rate ratio 7.50 [95% CI 4.45 to 12.65, p < 0.0001]) (S3 Table). The intervention cost significantly more than usual care (mean difference per patient £286.00 [95% CI £154.27 to £409.62]) due to telemonitoring service costs and additional nurse phone consultations. Health economic modelling of the cost-effectiveness of the intervention is in progress and will be reported separately. There were few adverse events that could be attributed to diabetes or BP control and these were equally distributed between the intervention and control groups (S4 Table provides further detail).
Tab. 6. Distribution of differences in numbers of diabetes treatments prescribed between baseline and follow-up in Telescot diabetes trial participants by trial arm and initiation of insulin during follow-up. 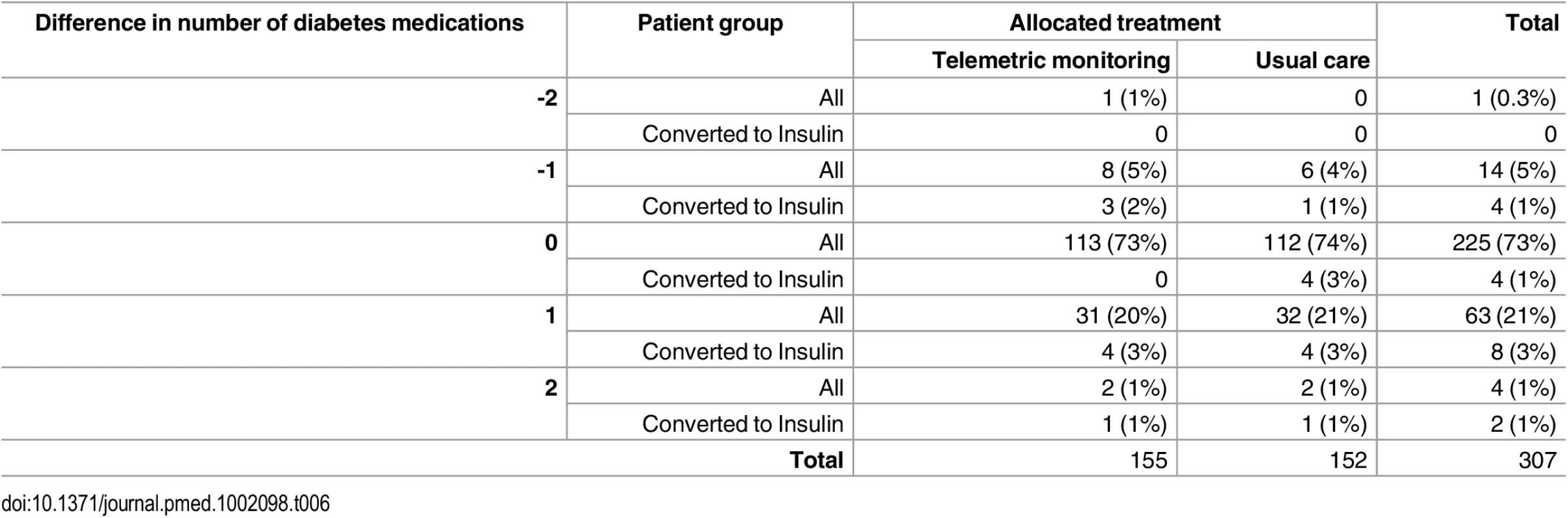
Tab. 7. Distribution of differences in numbers of anti-hypertensive medications prescribed between baseline and follow-up in Telescot diabetes trial participants by trial arm and initiation of insulin during follow-up. 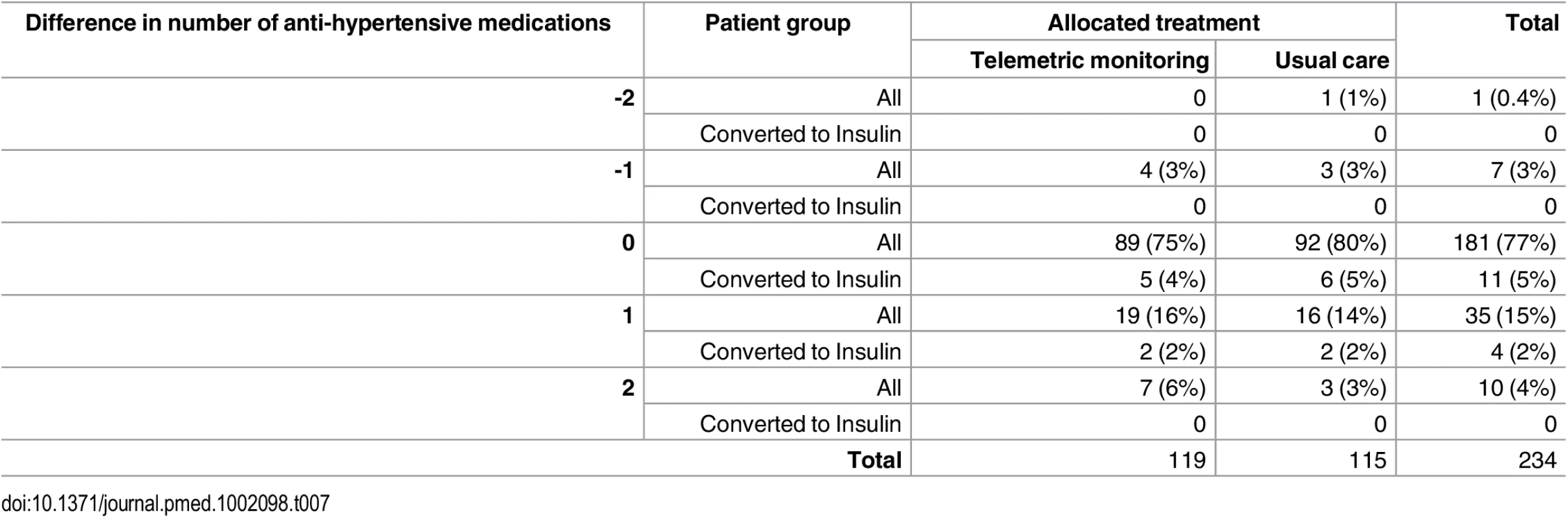
There were no statistically significant interactions with group allocation for any of the pre-specified sub-groups of age, sex, socioeconomic status, baseline HbA1c, monitored systolic blood pressure, or body mass index (see S5 Text and S5–S10 Tables).
Discussion
In this multicenter pragmatic trial based in UK family practice we observed clinically and statistically significantly greater improvements in glycemic control among people with poor glycemic control of type 2 diabetes who were offered supported telemonitoring over 9 mo than among the comparison group offered usual care. There were also significant reductions in blood pressure. The key feature of our trial is robust evidence suggesting that blood glucose monitoring with relatively little additional support from health professionals can be of value in terms of improving glycemic control in people who have previously had poor glycemic control. This finding contrasts with that of a systematic review published previously that suggested that unsupported self-monitoring of blood glucose does not have clinically significant beneficial effects [21].
The effect size (5·5 mmol/mol) we observed in our trial is the same as that used to establish efficacy of new drugs by the National Institute for Health and Care Excellence (NICE) algorithms and guidelines [22]. Similar improvements in systolic and diastolic BP to those observed in this trial of 3.3 and 1.4 mmHg were reported in the Heart Outcome Prevention Evaluation (HOPE) study and were associated with a 22% reduction in relative risk of cardiovascular mortality, myocardial infarction, or stroke [23]. In contrast to trials of strict glycemic control, mean weight did not increase in the intervention group. The lack of change in weight may reflect dietary changes or may be related to shorter follow-up in this trial compared to trials of intensive glycemic control [24]. In contrast to many other non-pharmacological interventions, this was achieved with a relatively small increase in researcher or health professional workload.
The strengths of this trial include recruitment from family practice of people with type 2 diabetes who have poor glycemic control despite the incentives offered to family practitioners for controlling diabetes and who constitute a more representative group than people participating in trials at specialist centers. In addition, diabetes management continued to take place in normal family practice rather than in a research setting. Objective measures were used for primary and principal secondary outcomes of glycemic control (HbA1c), BP (ABPM) and weight. There was limited loss to follow-up, and the sensitivity analyses and tests for interaction suggest that the reported findings are robust. However, the nature of trials is that although the majority of family practices who were invited agreed to participate, only 12% of people identified as potentially eligible from this challenging group of patients were randomized in this trial. It is not possible to determine what proportion of the general population of people with type 2 diabetes would wish to use telemonitoring if it were provided as a service and whether they would all be able to achieve similar results. Further work is required to determine what barriers there may be to implement telehealth in this group at scale, given the large numbers of people with poorly controlled type 2 diabetes (almost 100,000 people, or 2% of the population in Scotland alone). Additionally it is important to identify whether a shorter intervention may be equally clinically effective (and potentially more cost-effective than a longer intervention) or whether ongoing monitoring is required to maintain the effect.
The results of previous similar studies are conflicting, which may reflect the quality of the research, variation in settings between research clinics and normal clinical practice and the diverse nature of complex interventions. In a systematic review and meta-analysis of data from 5,069 patients participating in 26 studies of home telehealth for diabetes, the weighted mean difference in HbA1c between home telemonitoring and comparison groups was -0.21% (95% CI -0.35 to -0.08) in the available 21 studies, which were noted to be of variable methodological quality [25]. A meta-analysis of data from 2,552 participants in six clinical trials of self-monitoring of blood glucose among people with non-insulin treated type 2 diabetes reported a mean reduction in HbA1c of 2.7 (95% CI 1.6 to 3.9) mmol/mol at 6 mo in the intervention compared with control group, which was consistent across all strata and was described as a statistically significant, but not clinically meaningful result [21]. The results of the type 2 diabetes component of the Whole Systems Demonstrator cluster randomized controlled trial with 12 mo follow-up were similar in that HbA1c was lower by 0.21% or 2.3 mmol/mol (95% CI, 0.04% to 0.38, p = 0.013) in the intervention [26]. However a meta-analysis of 13 randomized controlled trials of telemedicine among 4,207 people with diabetes reported a statistically significant and clinically relevant absolute decline in HbA1c level in the intervention compared to control groups similar to our finding (mean difference -4.8 mmol/mol and 95% CI 6.7 to -2.8 mmol/mol; p < 0.001), but with evidence of heterogeneity between trials [27].
The International Diabetes Federation has identified seven essential elements of self-monitoring of blood glucose (SMBG): patient education, provider education, structured SMBG profile, SMBG goals, feedback, data used to modify treatment, and interactive communication or shared decision making [28]. Our intervention included all of these elements and, as for all complex interventions, it is not clear which component had the most effect and what effect telemonitoring had relative to more frequent contacts (e.g., office visits and telephone calls) or better tailored advice. A recent systematic review found that none of 15 studies of telemonitoring interventions in type 2 diabetes, all of which were performed outside the UK, included all of these elements, but those that included five or more achieved significant improvements in HbA1c [29].
A further meta-analysis of 23 trials with 7,037 participants and high levels of heterogeneity reported that home BP telemonitoring improved office systolic blood pressure by 4.71 (95% CI 3.24 to 6.18) and diastolic blood pressure by 2.45 (1.57 to 3.33) mmHg [30]. One of the meta-analyses mentioned above also summarized the effect of telemedicine on systolic BP from eight trials performed among people with diabetes, reporting no significant effect (-1.6 mmHg; 95% CI -7.2 to 4.1 mmHg; p = 0.585) [27]. It is important to note that these trials were performed in people with poorly controlled blood pressure, whereas our trial participants on average had reasonable blood pressure control at baseline and that we used the gold standard of ambulatory blood pressure monitoring as the outcome.
We were surprised that prescribing patterns did not differ markedly between the two arms of trial participants as our, and other, blood pressure telemonitoring studies reported increased anti-hypertensive prescribing in the intervention arm [10,30]. Improved glycemic control is frequently associated with increased weight, possibly due to failure to lose calories through glycosuria [24], but we observed no difference in weight change by trial arm. These findings suggest that the effect may have been mediated by improved self-management, resulting in changes in diet and lifestyle or increased adherence to drug treatment, rather than intensification of treatment. This explanation is supported by the findings of the nested qualitative study performed within the trial, in which participants reported that both the information they received from self-monitoring and knowledge that their glycemic control was being observed encouraged them to make lifestyle changes [31]. Unpublished data from the qualitative research suggest that reasons for not submitting glucose measurements included technical problems from the start and forgetting to take readings, particularly when away from home. In contrast, the responses to relevant questionnaires did not provide any evidence of improved concordance with lifestyle recommendations or treatment, but it is possible that the questionnaires may not be sensitive to such changes, as has been suggested by other studies of the performance of some of the questionnaires [32,33].
In summary, telemonitoring and supported self-management of blood glucose can result in clinically meaningful improvements in blood glucose among people with poorly controlled type 2 diabetes managed in routine family practice while requiring relatively small increases in clinician workload. There are also additional benefits for control of blood pressure in this population. Further research is required to determine if these changes are sustained over time and can be achieved if the intervention is used more widely or when targeted at specific patient groups.
Supporting Information
Zdroje
1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49. doi: 10.1016/j.diabres.2013.11.002 24630390
2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470 18784090
3. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–15. doi: 10.1001/jama.2014.18574 25668264
4. Simon D. Therapeutic inertia in type 2 diabetes: insights from the PANORAMA study in France. Diabetes Metab. 2012;38 Suppl 3:S47–S52. doi: 10.1016/S1262-3636(12)71534-3 22541602
5. Gibson PG, Coughlan J, Wilson AJ, Abramson M, Bauman A, Hensley MJ, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2000(2):CD001117. 10796600
6. Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. 17591623
7. Egede LE. Lifestyle modification to improve blood pressure control in individuals with diabetes: is physician advice effective? Diabetes Care. 2003;26(3):602–7. 12610008
8. Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19(4):279–84. 11942998
9. Peel E, Parry O, Douglas M, Lawton J. Blood glucose self-monitoring in non-insulin-treated type 2 diabetes: a qualitative study of patients' perspectives. Br J Gen Pract. 2004;54(500):183–8. 15006123
10. McKinstry B, Hanley J, Wild S, Pagliari C, Paterson M, Lewis S, et al. Telemonitoring based service redesign for the management of uncontrolled hypertension: multicentre randomised controlled trial. BMJ. 2013;346:f3030. doi: 10.1136/bmj.f3030 23709583
11. Wild S, Hanley J, Lewis S, McKnight J, McCloughan L, Padfield P, et al. The impact of supported telemetric monitoring in people with type 2 diabetes: study protocol for a randomised controlled trial. Trials. 2013;14(1):198.
12. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. 6880820
13. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective: Evidence from the EuroQol BIO MED Research Programme: Kluwer Academic Publishers; 2003 2003.
14. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–62. 11769298
15. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. 3945130
16. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. 12900694
17. Fitzgerald JT, Funnell MM, Hess GE, Barr PA, Anderson RM, Hiss RG, et al. The reliability and validity of a brief diabetes knowledge test. Diabetes Care. 1998;21(5):706–10. 9589228
18. Do Health. The NHS Outcomes Framework 2015/6. 2015 2015. Report No.
19. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 2004 2004.
20. Methodology WCCfDS. ATC/DDD Index 2016. http://www.whocc.no/atc_ddd_index/.
21. Farmer AJ, Perera R, Ward A, Heneghan C, Oke J, Barnett AH, et al. Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ. 2012;344:e486. doi: 10.1136/bmj.e486 22371867
22. Excellence NIfHaC. NICE guideline CG87 Type 2 diabetes: newer agents. London: 2009 2009. Report No.
23. Sleight P, Yusuf S, Pogue J, Tsuyuki R, Diaz R, Probstfield J. Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet. 2001;358(9299):2130–1. 11784631
24. Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187–92. doi: 10.2337/dc08-9026 19092168
25. Polisena J, Tran K, Cimon K, Hutton B, McGill S, Palmer K. Home telehealth for diabetes management: a systematic review and meta-analysis. Diabetes Obes Metab. 2009;11(10):913–30. doi: 10.1111/j.1463-1326.2009.01057.x 19531058
26. Steventon A, Bardsley M, Doll H, Tuckey E, Newman SP. Effect of telehealth on glycaemic control: analysis of patients with type 2 diabetes in the Whole Systems Demonstrator cluster randomised trial. BMC Health Serv Res. 2014;14 : 334. doi: 10.1186/1472-6963-14-334 25100190
27. Marcolino MS, Maia JX, Alkmim MB, Boersma E, Ribeiro AL. Telemedicine application in the care of diabetes patients: systematic review and meta-analysis. PLoS ONE. 2013;8(11):e79246. doi: 10.1371/journal.pone.0079246 24250826
28. International Diabetes Federation. Self-monitoring of blood glucose in non-insulin treated type 2 diabetes. Brussels: 2009
29. Greenwood DA, Young HM, Quinn CC. Telehealth Remote Monitoring Systematic Review: Structured Self-monitoring of Blood Glucose and Impact on A1C. J Diabetes Sci Technol. 2014;8(2):378–89. 24876591
30. Omboni S, Gazzola T, Carabelli G, Parati G. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013;31(3):455–67. doi: 10.1097/HJH.0b013e32835ca8dd 23299557
31. Hanley J, Fairbrother P, McCloughan L, Pagliari C, Paterson M, Pinnock H, et al. Qualitative study of telemonitoring of blood glucose and blood pressure in type 2 diabetes. BMJ Open. 2015;5(12):e008896. doi: 10.1136/bmjopen-2015-008896 26700275
32. Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8 : 115. doi: 10.1186/1479-5868-8-115 22018588
33. Lee WY, Ahn J, Kim JH, Hong YP, Hong SK, Kim YT, et al. Reliability and validity of a self-reported measure of medication adherence in patients with type 2 diabetes mellitus in Korea. J Int Med Res. 2013;41(4):1098–110. doi: 10.1177/0300060513484433 23860015
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- The Clinical and Public Health Challenges of Diabetes Prevention: A Search for Sustainable Solutions
- Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
- Diabetes: A Cinderella Subject We Can’t Afford to Ignore
- Prescribing Exercise and Lifestyle Training for High Risk Women in Pregnancy and Early Post-partum—Is It Worth It?
- Population Approaches to Prevention of Type 2 Diabetes
- First-Year Evaluation of Mexico’s Tax on Nonessential Energy-Dense Foods: An Observational Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
- Leveraging Genetics to Advance Type 2 Diabetes Prevention
- Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
- Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
- Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
- Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients
- Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
- Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women
- Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study
- Engagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Cycling and Diabetes Prevention: Practice-Based Evidence for Public Health Action
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



