-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Salivary Gland NK Cells Are Phenotypically and Functionally Unique
Natural killer (NK) cells and CD8+ T cells play vital roles in containing and eliminating systemic cytomegalovirus (CMV). However, CMV has a tropism for the salivary gland acinar epithelial cells and persists in this organ for several weeks after primary infection. Here we characterize a distinct NK cell population that resides in the salivary gland, uncommon to any described to date, expressing both mature and immature NK cell markers. Using RORγt reporter mice and nude mice, we also show that the salivary gland NK cells are not lymphoid tissue inducer NK-like cells and are not thymic derived. During the course of murine cytomegalovirus (MCMV) infection, we found that salivary gland NK cells detect the infection and acquire activation markers, but have limited capacity to produce IFN-γ and degranulate. Salivary gland NK cell effector functions are not regulated by iNKT or Treg cells, which are mostly absent in the salivary gland. Additionally, we demonstrate that peripheral NK cells are not recruited to this organ even after the systemic infection has been controlled. Altogether, these results indicate that viral persistence and latency in the salivary glands may be due in part to the presence of unfit NK cells and the lack of recruitment of peripheral NK cells.
Published in the journal: Salivary Gland NK Cells Are Phenotypically and Functionally Unique. PLoS Pathog 7(1): e32767. doi:10.1371/journal.ppat.1001254
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001254Summary
Natural killer (NK) cells and CD8+ T cells play vital roles in containing and eliminating systemic cytomegalovirus (CMV). However, CMV has a tropism for the salivary gland acinar epithelial cells and persists in this organ for several weeks after primary infection. Here we characterize a distinct NK cell population that resides in the salivary gland, uncommon to any described to date, expressing both mature and immature NK cell markers. Using RORγt reporter mice and nude mice, we also show that the salivary gland NK cells are not lymphoid tissue inducer NK-like cells and are not thymic derived. During the course of murine cytomegalovirus (MCMV) infection, we found that salivary gland NK cells detect the infection and acquire activation markers, but have limited capacity to produce IFN-γ and degranulate. Salivary gland NK cell effector functions are not regulated by iNKT or Treg cells, which are mostly absent in the salivary gland. Additionally, we demonstrate that peripheral NK cells are not recruited to this organ even after the systemic infection has been controlled. Altogether, these results indicate that viral persistence and latency in the salivary glands may be due in part to the presence of unfit NK cells and the lack of recruitment of peripheral NK cells.
Introduction
Human herpesvirus 5, also known as human cytomegalovirus (HCMV), is a prototypical β-herpesvirus. HCMV infection is widespread with 50–95% of the adult population being seropositive. CMVs are opportunistic pathogens that promote their survival by exploiting a defective immune response. Primary HCMV infection is usually asymptomatic and controlled by the immune system, but is never completely cleared from the host and can cause recurrent infections especially at times of immunosuppression [1]. Consequently, CMV is a serious medical concern for organ transplant recipients, immunocompromised individuals and neonates, where CMV disease ranges from mild to fatal. In fact, CMV is one of the most serious viral complications for solid organ transplantation, is frequently associated with loss of sight in HIV patients, and is a major cause of congenital infection resulting in microcephaly, deafness, blindness and mental retardation [2].
The CMV family members have strict species specificity, precluding the use of an HCMV animal model. As a result, murine cytomegalovirus (MCMV) has been advantageous for advancing CMV immune research. Both HCMV and MCMV have 240kb double stranded DNA encoding more than 200 ORFs and show similarity in disease progression, as well as dissemination, persistence in salivary glands, and reactivation following immunosuppression [3]. Much is known about the numerous defensive strategies human and mouse CMV employ to evade immune detection [4], however, we have little understanding of how viral propensity for the epithelial cells of the salivary gland contributes to viral persistence. MCMV is cleared efficiently by cytotoxic lymphocytes in all organs of the infected host, except the submandibular gland (SMG) where it persists for several weeks to months depending on host, route of entry, and dose, eventually becoming latent for the life of the host [5], [6]. MCMV primarily replicates in the acinar glandular epithelial cells of the SMG where viral particles are first detectable around day 5 post-infection (p.i.). The SMG is considered a privileged site where dissemination of the virus to other tissues as well as transmission to naïve individuals is possible. The SMG provides a peculiar barrier where mucosal tissues are typically poised to respond in a Th2 fashion and stimulate the production of IgA, while denying access to commensal microbes and other environmental pathogens. This proclivity may contribute to the ability of CMV to take advantage of compartmental distinctions and evade the immune system in the SMG.
Natural killer (NK) cells are a widely distributed heterogenous population capable of responding to numerous pathogens and providing tumor surveillance. NK cells are thought to develop from CLPs in the bone marrow, although individual tissues contain phenotypically and functionally distinct subsets [7]. At present, it is not known whether these differences stem from developmental regulation or homing and retention signals unique to the tissue environment. However, the investigation of NK cells in mucosal tissue has gained attention recently as the frequency of NK cells compared to other lymphocytes is highest in non-lymphoid tissue. NK cell deficiency in both mice and humans results in severe viral infections. NK cells are critical to the early containment of CMV and their loss results in uncontrolled infection in both mice and humans [8], [9], [10]. Here, we investigate the phenotype of NK cells in the naïve SMG and their response to MCMV infection. The NK cell response to MCMV has been well characterized in the liver and spleen, however their contribution in the SMG has not been examined. Using the resistant C57BL/6 mouse strain, we found the resident SMG NK cell population has a unique phenotype. Importantly, during infection, the SMG NK cells acquire activation markers, yet have limited effector functions in vivo and ex vivo.
Results
Phenotype of resident SMG NK cells
NK cells are critical for the early containment of CMV, and a population of NK cells reportedly resides in the SMG of mice and rats [11], [12]. Despite the presence of a significant NK cell population in MCMV resistant C57BL/6 mice (Figure 1A), high viral titers persist in the salivary gland for several weeks post-infection [5], [6]. We first examined the phenotype of naïve resident SMG NK cells. NK1.1+CD3− cells compose a larger percentage of lymphocytes in the SMG vs. the liver, spleen or blood. Depending on the method of preparation, the NK cell population ranged from ∼9%–30% (Figure 1A and data not shown). These numbers are consistent with other reports of mouse and rat NK cells being more prevalent in non-lymphoid tissues, including the naïve SMG as well as during diseases like Sjogren syndrome (SS) [12], [13], [14]. Enzymatic treatment of the SMG is necessary in order to obtain adequate numbers of lymphocytes for analysis. However, we report that both Ly49H and CD27 are sensitive to both collagenase and liberase treatment (not shown). In order to account for any other changes in cell-surface markers, we compared enzymatic treatment to no treatment and found that out of all the other receptors analyzed there were no significant alterations. A modified protocol was performed which eliminated the loss of cell surface markers (see material and methods).
Fig. 1. Phenotype of NK1.1+CD3− cells in the SMG. 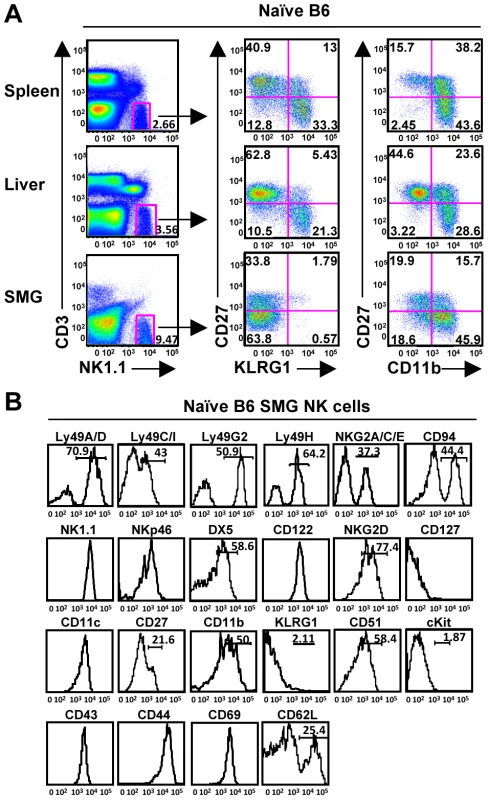
(A) Naïve NK cells represent a significant population of lymphocytes within the SMG and display reduced levels of KLRG1 and CD27 compared to the spleen and liver. (B) Naïve NK1.1+CD3− cells from the SMG of B6 mice were examined for other NK cell markers. Expression of CD27 and CD11b subdivides mouse splenic NK cells into 4 subsets from the least mature to the most mature: CD11blowCD27low, CD11blowCD27high, CD11bhighCD27high, and CD11bhighCD27low [15], [16]. KLRG1 is expressed on the most mature NK cells and is upregulated on the majority of NK cells responding to MCMV infection in the liver, spleen and blood [17], [18]. Here, we show the naïve SMG NK cells are lacking KLRG1 expression and have a significantly reduced expression of CD27, yet express mature NK cell markers CD11b, DX5, Ly49s and CD43 (Figure 1B). Furthermore, the SMG NK cells express CD51 but not CD117 (c-kit), receptors typically found on immature NK cells or NK cell precursors [18], [19]. The mean fluorescence intensity for all of these makers is provided in supplementary data (Figure S1).
The NKp46 receptor is expressed on NK cells commencing at an early stage of differentiation and has been used as a NK cell marker for mice and humans [20], [21], [22]. The SMG NK1.1+CD3− cells express NKp46, although at slightly lower intensity than NK cells of the spleen or liver (Figure 1B and data not shown). Taken together, these data demonstrate that in naïve mice, SMG NK cells have a unique phenotype uncommon to any described to date.
SMG NKp46+CD3− cells are present in Rag−/− mice, are not thymic derived and do not express RORγt
Salivary gland NK cells are highly positive for CD69, an early activation receptor. Since subsets of NKT cells [11] constitutively express CD69 and other rare T cell subsets exhibit NK cell-like features, including expression of NKp46 [23], we wanted to verify that the NK1.1+CD3−NKp46int population was not a novel population of NK-like T cells. Using Rag−/− mice, deficient of both T and B cells, we found an identical population of NK1.1+CD3−NKp46int cells, excluding the possibility that this cell population is derived from the T cell lineage (Figure 2A). Similarly, the Rag−/− NK cells are predominantly absent for the KLRG1 marker, express high levels of CD69 and consist of a distribution of Ly49H+ and Ly49H− subsets (Figure 2A).
Fig. 2. SMG NK cells are present in Rag−/− mice and are not thymic or LTi derived. 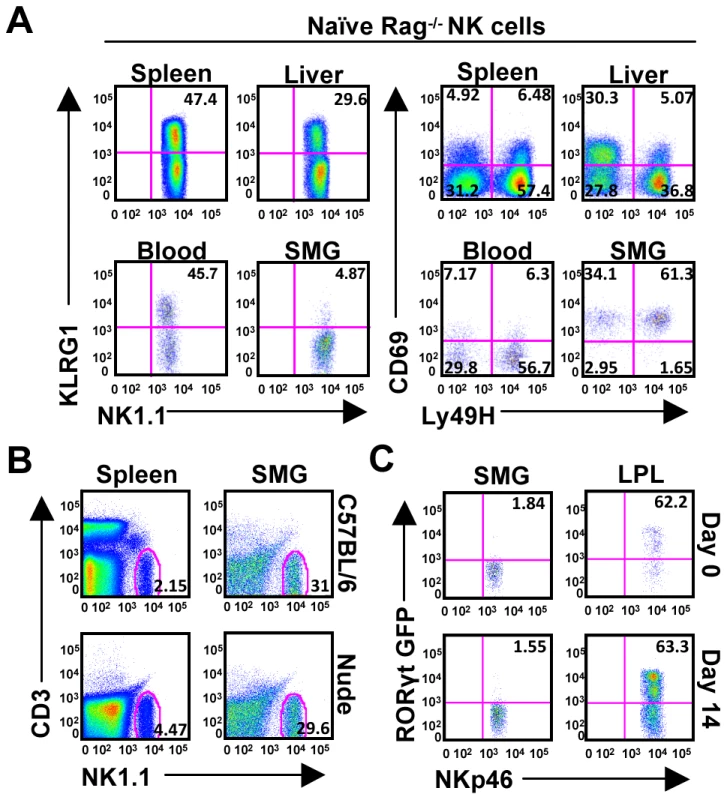
(A) Organs from naïve Rag−/− mice were examined to confirm the presence of NK cells in the SMG vs. spleen, liver, and blood. NK cells from the SMG were comparably lacking cell surface KLRG1, but expressed increased levels of CD69. (B) Organs from naïve nude mice were examined to confirm the presence of NK cells in the SMG and spleen. (C) NKp46+CD3− cells from the SMG and gut lamina propria of naïve or D14 MCMV infected RORcγt+/GFP reporter mice were examined for GFP expression. Despite the proximity to the thymus, the SMG NK cell phenotype is quite different from the recently reported thymic-derived NK cell subset [24]. In contrast to thymic-derived NK cells, SMG NK cells express Ly49 receptors A/D, C/I, G2 and are mostly CD11b positive (Figure 1B). Most importantly, the SMG NK cells lack CD127 (Figure 1B) and are present in nude mice (Figure 2B) ruling out a thymic contribution for SMG NK cell development.
Recently, a mucosal population of NK-like cells has been described [25], [26], [27], [28], [29]. These cells produce IL-22 and their development requires the retinoic acid receptor-related orphan receptor gamma t (RORγt) transcription factor. In order to determine whether the SMG contains these NK-like cells we used RORcγt+/GFP reporter mice. Although we did find NKp46+/RORγt+ cells in the gut lamina propria as previously reported, we did not detect a significant population of these cells in the SMG (Figure 2C). Altogether, these results demonstrate that the SMG NK cells are not thymic derived and do not depend on RORγt for their development.
SMG NK cells express activation markers during MCMV infection
We next investigated possible changes in the SMG NK cell population during MCMV infection that could contribute to viral persistence. Mice were infected with MCMV for 7, 14 or 21 days and analyzed for expression of KLRG1 as an indicator of activation. KLRG1 expression appears on the SMG NK cells prior to D7 MCMV and by D14 is expressed at levels comparable to that of liver NK cells, a>10 fold increase (Figure 3A). Notably, Rag−/− mice show a similar expression at D14 in the SMG, indicating that this phenotype occurs independently of the adaptive immune cells (Figure S2).
Fig. 3. SMG NK cells become activated and induce the expression of KLRG1 during MCMV infection. 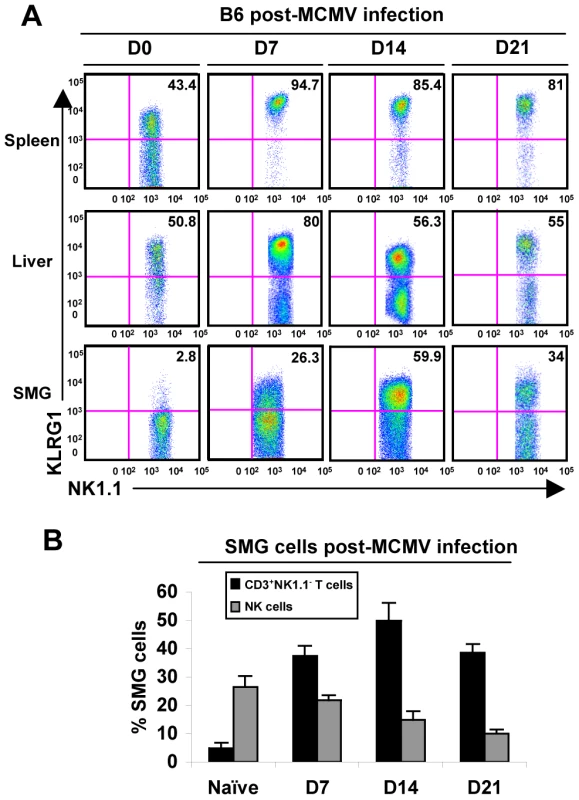
(A) B6 mice were infected with 5×104 pfu/mouse MCMV and sacrificed on D0, D7, 14 or 21 p.i. NK1.1+CD3− cells of the SMG were compared to spleen and liver for expression of KLRG1. (B) The graph represents the percentage of B6 NK cells and CD8+ T cells in the SMG during MCMV infection at D0, 7, 14 and 21. 3–5 experiments of 2–3 mice per group is shown. Additionally, CD69 expression intensifies on the NK cell population during infection (not shown), further indicating that the SMG NK cells are capable of recognizing MCMV infection. Therefore, SMG NK cells acquire activation markers during MCMV infection with a delayed kinetic compared to splenic or liver NK cells. Notably, NKp46+RORγt+ cells were not detected in the SMG during viral infection (Figure 2C).
NK cells are not recruited to the SMG during the acute stage of MCMV
During the course of infection the frequency of NK cells in the SMG decreases while the T cell population increases dramatically (Figure 3B). It is known that T cells infiltrate the salivary gland during MCMV ([11] and Figure S3A), but it has not been shown whether NK cells are actively recruited. NK cells were purified from CD45.2+ mice (>99%) and injected into B6 congenic recipients. To investigate whether infection alters NK cell migration, NK cells were adoptively transferred into naïve recipient mice (Figure S3B) or infected recipient mice either at the time of MCMV infection or 10 days post-infection. This time point was chosen because visceral viral replication drops below the level of detectability by D10, while MCMV replication is peaking in the SMG and typically reaches titers that are several times higher than in other organs [11]. At D7 post-MCMV infection with concurrent NK cell transfer, donor NK cells were visible in spleen, liver and blood, showing an increased percentage of infiltration in all three organs in comparison to NK cell transfer in uninfected hosts (Figure 4). At D7 post-transfer with prior MCMV infection, the NK cell percentage was further increased in spleen, liver and blood. However, under neither condition were significant numbers of transferred NK cells detectable in the SMG indicating that the resident SMG NK cells expand in situ but do not repopulate via a blood pathway. Therefore, it seems likely that the systemic NK cells do not receive appropriate chemotactic signals necessary to migrate to and reside in the SMG during MCMV infection, contributing to insufficient viral control in this organ.
Fig. 4. NK cells from the periphery are not recruited to the SMG during MCMV infection. 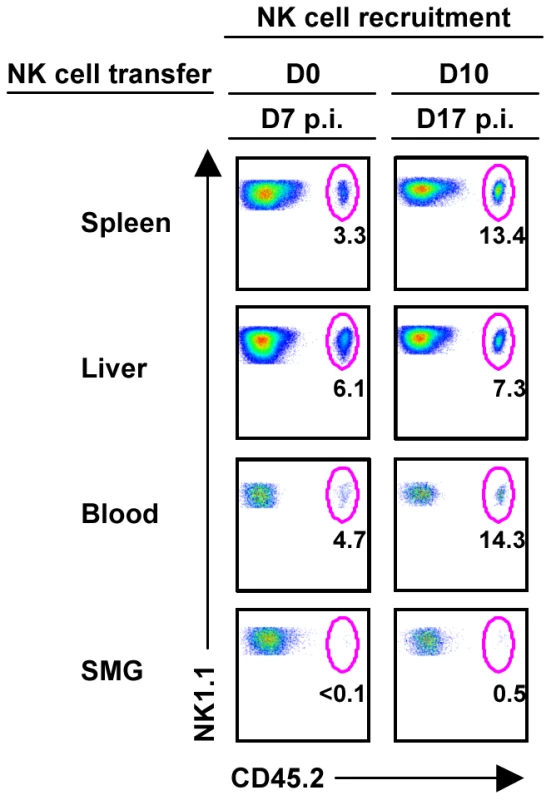
B6 splenic lymphocytes were depleted of CD19+ and CD5+ cells to enrich for NK cells. Remaining cells were stained with non-reducing amounts of NK1.1 and CD3 and sorted using a FACSAria. NK1.1+CD3− cells were of > 98% purity before i.v. injection into congenic B6 mice at 2×106 cells/mouse. Recipient congenic B6 mice were either infected with 5×104 pfu/mouse MCMV two hours or ten days prior to adoptive transfer. On D7 post-NK cell transfer, mice were analyzed for CD45.2+ NK cell trafficking. The figures represent 1 of 2 separate experiments with 2 mice per group. SMG NK cells respond weakly to MCMV in vivo
In order to study the function of SMG NK cells in vivo, C57BL/6 mice were infected with MCMV and NK cell IFN-γ measured at various times post-infection. The production of NK cell IFN-γ reaches its maximum in the spleen at D2 post-infection. During this time the percentage of splenic IFN-γ+ NK cells is around 40% ([17], [30] and Figure 5). In contrast, less than 4% of the SMG NK cells produce IFN-γ at this time point (Figure 5). At D6, D7, D9, D10, D12 and D14 post-infection, when MCMV replication in the SMG is active, no significant NK cell IFN-γ was detected in any organs (Figure 5 and Figure S4). Therefore during MCMV infection, SMG NK cells acquire activation markers such as KLRG1 with a delayed kinetic but the IFN-γ response is modest in this organ.
Fig. 5. SMG NK cells are hyporesponsive in vivo during MCMV infection. 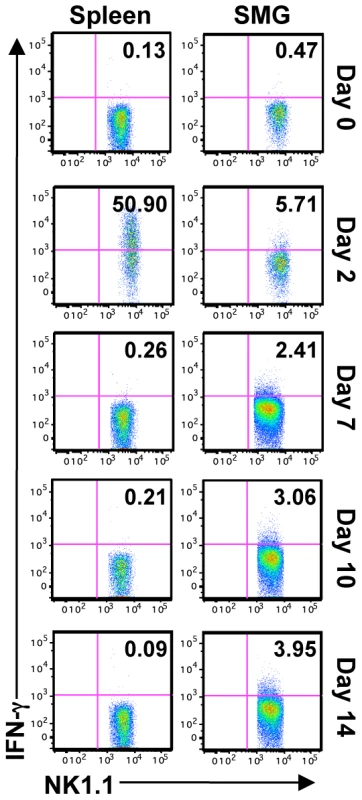
B6 mice were infected with 5×104 pfu/mouse MCMV and sacrificed on D2, 7, 10 or 14 p.i. IFN-γ production from NK1.1+CD3− cells was determined by intracellular staining. Representative of 5 separate experiments with 2–4 mice pooled per group. SMG NK cells are hyporesponsive ex vivo
Naïve NK cells do not acquire optimal effector functions unless they are primed with TLR ligands such as poly(I∶C) [31]. To measure and compare effector functions from splenic and SMG primed NK cells, B6 mice were primed in vivo for 24 hours with poly(I∶C). Lymphocytes from pooled salivary glands or spleens were then stimulated with anti-Ly49H, anti-NKG2D or IL-12/IL-18 for 6 hours. We found that SMG NK cells are significantly impaired in their effector functions. Poly(I∶C) primed SMG NK cells produce significantly less IFN-γ than splenic NK cells in all the conditions tested (Figure 6A and Figure S5B). In addition, their capacity to degranulate, as measured by lysosomal-associated membrane protein 1 (LAMP1), or CD107α expression, is also significantly decreased (Figure 6B and Figure S5D).
Fig. 6. Poly(I∶C) primed SMG NK cells have impaired IFN-γ and degranulation compared with splenic NK cells. 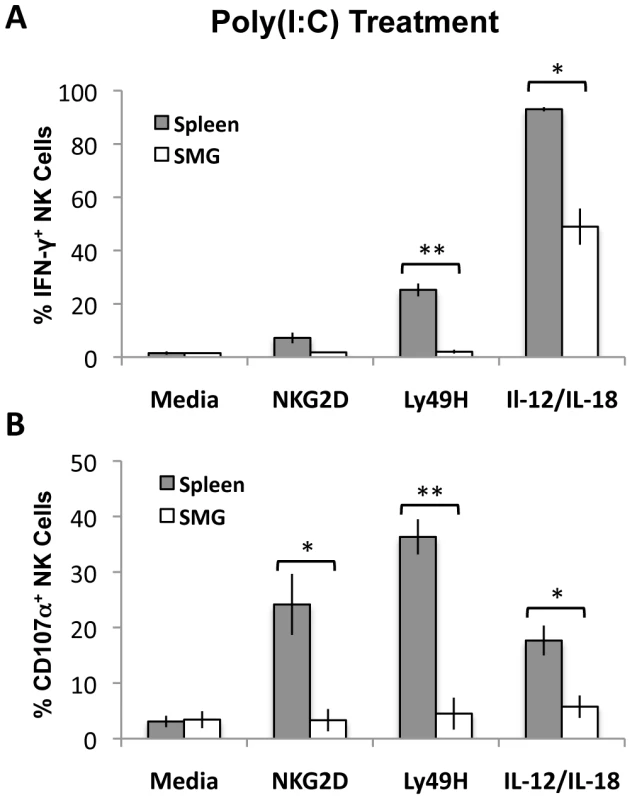
Lymphoid cells from the SMG and spleens were isolated from 24 hour Poly(I∶C) treated mice. Both organs were processed and incubated for 6 hours with no treatment, crosslinked with anti-NKG2D or anti-Ly49H, or stimulated with IL-12/IL-18. Cells were treated with Monensin and stained for IFN-γ (A) and CD107α (B) and analyzed by flow cytometry. The bar graph shows a compilation of 3 experiments with 9 mice pooled for each experiment. *p = 0.004−0.003 **p = <0.003. SMG NK cell functions are impaired at the peak of MCMV replication
MCMV replication in mice is not synchronized and peaks at D2 in the spleen and D10 in the SMG. To circumvent this issue, we measured and compared NK cell effector functions in response to MCMV at the peak of replication in the respective tissues. Lymphocytes from pooled salivary glands (D10 post-infection) and spleens (D2 post-infection) were then stimulated with anti-Ly49H, anti-NKG2D or IL-12/IL-18 for 6 hours. We found that SMG NK cell production of IFN-γ is significantly decreased (Figure 7A and Figure S5A) and they have an impaired capacity to degranulate at the peak of replication (Figure 7B and Figure S5C). Altogether these data suggest that SMG NK cells are hyporesponsive upon either cytokine stimulation or activating receptor crosslinking.
Fig. 7. MCMV activated SMG NK cells have impaired IFN-γ and degranulation compared with splenic NK cells. 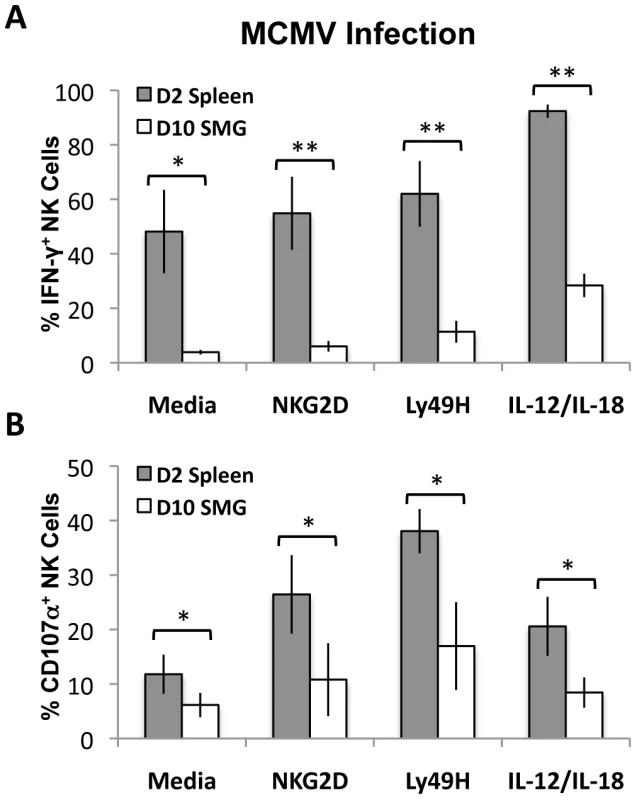
Lymphoid cells from the SMG and spleens were isolated from D10 and D2 MCMV infected mice, respectively. Both organs were processed and incubated for 6 hours as described in Figure 6. Cells were treated with Monensin and stained for IFN-γ (A) and CD107α (B) and analyzed by flow cytometry. The bar graph shows a compilation of 4 experiments with 3 mice pooled for each experiment. *p = 0.03−0.003 **p = <0.003. T-regs and iNKT cells are absent from naïve and MCMV infected SMG
We speculated that an increase in Tregs might regulate the NK effector functions, influencing their unique phenotype. CD4+CD25+Foxp3+ Tregs are involved with maintaining immune homeostasis, self-tolerance and limiting tissue damage. However, using mice expressing a GFP-Foxp3 fusion-protein reporter [32], we did not detect a naïve population of resident Tregs nor an infiltration of GFP+ T cells post-MCMV infection (Figure 8A). Additionally, the naïve B6 SMG contains a population of less than 0.2% that are positive for TCRβ/CD3, NK1.1 and CD1d tetramer, indicating that iNKT cells are not a significant population in this organ (Figure 8B and data not shown).
Fig. 8. T-regs and iNKT cells are absent from naïve and MCMV infected SMG. 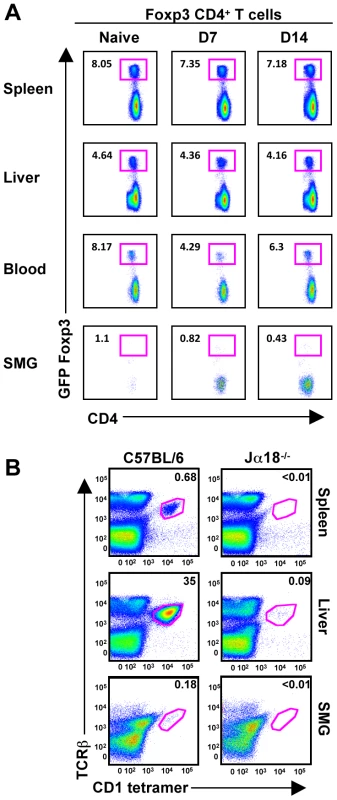
(A) Mice expressing a GFP-Foxp3 fusion-protein reporter were infected with MCMV for the time points shown and analyzed for accumulation of Tregs in the spleen, liver, blood and SMG as shown by CD4+GFP+ T cells. Representative of 3 separate experiments with 3–4 mice per group. (B) Splenic, hepatic and SMG leukocytes were isolated from naïve B6 and Jα18 deficient mice. The iNKT cell compartment was analyzed by staining with TCR-β and α-GalCer-loaded CD1d tetramer. Representative of 3 separate experiments with 2–3 mice per group. Discussion
Development of the salivary glands requires a process called branching morphogenesis to create the compact encapsulated glands with draining ducts. Branching morphogenesis is a complex developmental pathway involving cell-cell and cell-matrix interactions, cellular migration and timely proliferation as well as the appropriate response to growth factors and environmental changes. This process forms the salivary glands, lungs, kidneys and mammary glands, all places involved with potential dissemination and horizontal infection of numerous pathogens including CMV. The immune cells that organize and develop in these organs have been mostly overlooked. A need to investigate immune system recognition and responses to pathogens at barrier locations such as epithelial and mucosal tissue is becoming increasingly important with regard to understanding viral dissemination and transmission.
Significant questions involving CMV disease remain as to whether persistent viral replication in the SMG is a contributing factor to the development of latency. The existence of resident NK cells and the ability to recruit T cells to the SMG are somehow insufficient to control and eliminate virus in comparison to other organs of the host. This discrepancy implicates the SMG microenvironment and its homeostatic condition as contributing factors to viral evasion. To investigate possible reasons for viral evasion in the SMG, we examined the phenotype of the resident lymphoid cells.
We first identified a phenotypically distinct population of NK1.1+CD3−KLRG1−CD69+ cells that appears to deviate from known developmental stages. Newly described subsets of NK cells have been reported for the thymus, lymph node, lung, gut, skin, uterus, and pancreas [24], [25], [26], [27], [28], [29], [33], [34], [35]. NK cell precursors migrate to distal locations where local cytokines and receptor interactions are likely to elicit specific environmental differentiation (for review see [36], [37]). For instance, SMG expresses low amounts of MHC class I ([4]), which is required for normal NK cell maturation. Therefore, NK cell development is likely to be influenced by the unique SMG mucosal microenvironment. This is supported by a recent report from Caligiuri and colleagues who identified a novel hematopoietic precursor that predominates in the human lymph node and differentiates into CD56bright NK cells [33].
We also found that SMG NK cells respond weakly to MCMV in vivo. Several mechanisms, not mutually exclusive, could explain this phenotype. First, low MHC class I could potentially lead to the development of hyporesponsive NK cells in the SMG, due to inefficient “licensing” or “disarming” [38], [39]. Second, inhibitory receptors could play a role in raising the threshold of activation. For instance, the KLRG1 ligands, E - and N-cadherin, are expressed in SMG [40], [41] and could potentially inhibit KLRG1+ NK cells during infection. Third, we noticed that SMG NK1.1+CD3− cells express NKp46 at a lower intensity than splenic NK cells. NKp46 is a member of the Ig superfamily whose engagement initiates the activation pathway [22]. In human, a correlation between surface density of NKp46 and natural cytotoxicity has been shown [42]. In corroboration of these findings, we observed that NKp46low NK cells of the SMG also have diminished cytolytic potential. Notably, SMG NK cell effector functions are also impaired after poly(I∶C) treatment suggesting their hyporesponsive status is independent of the infection. Importantly, although NK cell depletion prior to infection results in significant higher viral titer in the salivary glands [43], it has been shown that NK cell depletion at days 6 to 9 post infection has no effect on SMG MCMV titers [44] further reinforcing the findings described here.
The inability of the influxing CD8+ T cells and resident NK cells to provide efficient viral elimination in the SMG is puzzling. We found that Treg and iNKT cells are unlikely to regulate NK cell functions as they are nearly absent from the SMG in both naïve and infected animals. Humphreys et al. found CD4+ T cells expressing IL-10 only localized to the SMG during MCMV infection [45]. This discovery causes speculation that the function of this population may be involved in limiting tissue injury [45]. Interestingly, although we never detected IL-10 in the serum of MCMV infected wild-type animals at any time point tested [46] we found CD3−NK1.1+GFP+ cells in both spleens and SMG of infected IL-10 reporter animals (Figure S6). Given the low NK cell IFN-γ production observed in SMG, it is tempting to speculate that the net outcome of the response might be in favor of the immunosuppressive function of IL-10 in this organ as suggested by others [47], [48]. IL-10−/− mice show reduced serum viral titers, but greater pathology along with increased CD4+ T cell IFN-γ production and increased susceptibility to MCMV infection [49]. It is well known that the early inflammatory milieu, IL-12 and/or type I IFN, dictates the rate at which CD8+ T cells acquire memory characteristics [50], [51] and conditions NK cell proliferation and effector functions [52]. It is therefore conceivable that during MCMV infection, IL-10 not only limits Ag specific contraction resulting in increased numbers of memory CD8+ T cells in the SMG but also controls NK cell responses. Therefore, while MCMV utilizes an IL-10 dependent mechanism to persist in salivary gland, it could also potentially favor the development of memory CD8+ T cells and perhaps memory NK cells [53] preventing reactivation of the virus while limiting tissue injury. In support of this, MCMV is capable of replicating in the SMG without causing tissue damage [54], further indicating that decreased NK cell cytotoxicity could ultimately benefit the host.
Materials and Methods
Mice
C57BL/6, C57B6.SJL (Taconic Laboratory Animals and Services, Germantown, NY) and B6.Cg-Foxp3tm2Tch/J, B6.129S7-Rag1Tm1Mom/J, B6.129P2(Cg)-Rorctm2Litt/J, B6.129S6-Il10tm1Flv/J (Jackson Laboratory, Bar Harbor, ME) were purchased for these studies. C57BL/6NTac-Foxn1<nu>N9 (nude mice) were purchased from Taconic. B6.Ja18−/− mice (kindly provided by Dr. M. Taniguchi, Riken Research Center for Allergy and Immunology, Yokohoma, Japan) were bred, crossed to B6 (>12 generations). All mice were maintained in pathogen-free breeding facilities at Brown University (Providence, RI). All mice were between 6 and 10 wks of age. Experiments were conducted in accordance with institutional guidelines for animal care.
Infection protocols
Stocks of Smith strain MCMV salivary gland extracts or clone RVG-102 (a gift of Dr. Hamilton, Duke University) recombinant for GFP under the promoter of the immediate early gene-1 (ie-1) were prepared as previously described [17]. Infections were initiated on day 0 with 5×104 plaque-forming units (PFU) of MCMV delivered i.p. For Rag−/− mice 2.5×104 PFU of MCMV was used.
Adoptive transfer
Splenic lymphocytes from CD45.2+ B6 mice were isolated and depleted of CD5 and CD19 positive cells following AutoMACS protocol. The remaining cells were stained for CD3 and NK1.1. NK cells were sorted using a FACSAria to >98% purity. Approximately 2×106 NK cells were injected i.v. into CD45.1+ SJL mice that had been infected with MCMV 10 days prior to transfer, 2 hours prior to transfer or left uninfected. CD8+ T cells were positively selected and injected i.v. into CD45.1+ SJL mice that were infected with MCMV 3 hours after transfer or left uninfected. Mice were sacrificed 7 days post-transfer and analyzed for NK or CD8+ T cell trafficking.
NK cell effector function assay
96-well tissue culture plates were coated with anti-NKG2D or anti-Ly49H [5µg/mL]. Lymphocytes were isolated from spleen and SMG and pooled respectively. Cells were plated at 2×106 cells/well and incubated with CD107α or isotype, in RPMI with Monensin (BD Biosciences) for 6 hours. As a control, cells were treated with IL-12/IL-18 [10ng/mL]. Cells were then harvested and analyzed for expression of CD107α, TCR-β or CD3, NK1.1 and intracellular IFN-γ by flow cytometry.
Isolation of lymphocytes
To obtain splenic lymphocytes, spleens were minced, passed through nylon mesh (Tetko, Kansas City, MO), washed once in 1% PBS-serum and cell suspensions were layered on lympholyte-M (Cedarlane Laboratories Ltd., Canada). Hepatic lymphocytes were prepared by mincing and passage through a 70 mm nylon cell strainer (Falcon, Franklin Lakes, NJ). After washing 3 times in 1% PBS-serum, cell suspensions were layered on a two-step discontinuous Percoll gradient (Pharmacia Fine Chemicals, Piscataway, NJ). Salivary gland lymphocytes were prepared as described [55] with some modifications. Briefly, SMGs were removed of all lymph nodes and connective tissue, followed by mincing. Single cell dissociation was performed using one incubation with digestion medium (RPMI 1640 containing 1mg/ml of collagenase IV (Sigma), 5mM CaCl2 50µg of DNase I (Sigma) and 8% FBS) with continuous shaking at room temperature. The digestion mixture was pipetted vigorously to dissociate remaining cells. Supernatant was collected, passed through nylon mesh and the lymphocytes purified by layering on a lympholyte-M gradient. Alternatively, salivary glands were prepared, minced and incubated with liberase for 20 min. at room temperature, pipetted vigorously, washed in PBS and cell suspensions were layered on lympholyte-M. To determine possible cleavage of molecular markers by enzymatic digestion, salivary glands were also processed without digestion agents, by pressing through a 70 mm nylon cell strainer, pooled and layered on a lympholyte-M gradient. Splenocytes, hepatic lymphocytes and salivary gland lymphocytes were collected after centrifugation for 30 min at 900× g. Blood was collected by cardiac puncture and mixed with heparin sulfate. Red blood cells were lysed through incubation with NH4Cl for 10 min. on ice.
Antibodies and reagents
Ly49H-APC, KLRG1-APC, CD3-PerCP-Cy7, CD3-pacific blue, CD3-APC, CD4-PE, CD8-PerCP, CD8-pacific blue, CD11b-PE, CD11b-efluor450, CD11c-APC, CD27-FITC, CD27-PE, CD43-PE, CD44-FITC, CDC45.2-FITC, CD51-PE, CD62L-APC, CD62L-PE-Cy7, CD69-PE, CD94-FITC, NKG2A/C/E-PE, CD122-PE, CD127-APC, NK1.1-APC, NK1.1-PerCP-Cy5.5, NKp46-PE, DX5-PE, CD107α-FITC, IFN-γ-PE and Ly49A/D-FITC were purchased from eBioscience (San Diego, CA). CD1d Tetramer-PE was obtained from the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility at Emory University (Atlanta, GA). NKp46-Alexa Fluor 647 was a generous gift from Dr. Vivier (Centre d'Immunologie Marseille-Luminy). Ly49H-FITC was a generous gift from Wayne Yokayama (Washington University School of Medicine, St Louis, MO) The above mentioned antibodies were used for FACS analysis in this study.
Flow cytometric analysis
Cells were suspended in buffer comprised of PBS containing 1% FCS. Cells were first incubated with 2.4G2 mAb and stained with mAbs specific for cell surface markers for 30 min at 4°C. For intracellular staining, cells were fixed with cytofix/cytoperm and then stained for 30 min in perm/wash buffer for 30 min. Events were collected on a FACSAria, and the data was analyzed using FlowJo (Tree Star Inc.).
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals as defined by the National Institutes of Health (PHS Assurance #A3284-01). Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Brown University. All animals were housed in a centralized and AAALAC-accredited research animal facility that is fully staffed with trained husbandry, technical, and veterinary personnel.
Supporting Information
Zdroje
1. ZanghelliniF
BoppanaSB
EmeryVC
GriffithsPD
PassRF
1999 Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis 180 702 707
2. BrittWJ
AlfordCA
1996 Cytomegalovirus.
FieldsBN
FieldsDN
KnipeDM
HolleyPM
Fields Virology, 3rd ed Philadelphia Lippincott-Raven Publishers 2493 2523
3. JordanMC
ShanleyJD
StevensJG
1977 Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J Gen Virol 37 419 423
4. HengelH
BruneW
KoszinowskiUH
1998 Immune evasion by cytomegalovirus–survival strategies of a highly adapted opportunist. Trends Microbiol 6 190 197
5. HensonD
StranoAJ
1972 Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am J Pathol 68 183 202
6. JonjicS
MutterW
WeilandF
ReddehaseMJ
KoszinowskiUH
1989 Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med 169 1199 1212
7. Di SantoJP
2008 Natural killer cells: diversity in search of a niche. Nat Immunol 9 473 475
8. BironCA
ByronKS
SullivanJL
1989 Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 320 1731 1735
9. LohJ
ChuDT
O'GuinAK
YokoyamaWM
VirginHWt
2005 Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 79 661 667
10. WelshRM
BrubakerJO
Vargas-CortesM
O'DonnellCL
1991 Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med 173 1053 1063
11. CavanaughVJ
DengY
BirkenbachMP
SlaterJS
CampbellAE
2003 Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J Virol 77 1703 1717
12. O'SullivanNL
SkanderaCA
MontgomeryPC
2001 Lymphocyte lineages at mucosal effector sites: rat salivary glands. J Immunol 166 5522 5529
13. MontgomeryPC
SkanderaCA
O'SullivanNL
1996 Phenotypic profiles of lymphocyte populations isolated from rat major salivary glands. Oral Microbiol Immunol 11 248 253
14. NaritaJ
KawamuraT
MiyajiC
WatanabeH
HondaS
1999 Abundance of NKT cells in the salivary glands but absence thereof in the liver and thymus of aly/aly mice with Sjogren syndrome. Cell Immunol 192 149 158
15. HayakawaY
SmythMJ
2006 CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 176 1517 1524
16. ChiossoneL
ChaixJ
FuseriN
RothC
VivierE
2009 Maturation of mouse NK cells is a 4-stage developmental program. Blood 113 5488 5496
17. RobbinsSH
NguyenKB
TakahashiN
MikayamaT
BironCA
2002 Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol 168 2585 2589
18. RobbinsSH
TessmerMS
MikayamaT
BrossayL
2004 Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J Immunol 173 259 266
19. KimS
IizukaK
KangHS
DokunA
FrenchAR
2002 In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 3 523 528
20. SivoriS
VitaleM
MorelliL
SanseverinoL
AugugliaroR
1997 p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med 186 1129 1136
21. BiassoniR
PessinoA
BottinoC
PendeD
MorettaL
1999 The murine homologue of the human NKp46, a triggering receptor involved in the induction of natural cytotoxicity. Eur J Immunol 29 1014 1020
22. WalzerT
BleryM
ChaixJ
FuseriN
ChassonL
2007 Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A 104 3384 3389
23. StewartCA
WalzerT
RobbinsSH
MalissenB
VivierE
2007 Germ-line and rearranged Tcrd transcription distinguish bona fide NK cells and NK-like gammadelta T cells. Eur J Immunol 37 1442 1452
24. VosshenrichCA
Garcia-OjedaME
Samson-VillegerSI
PasqualettoV
EnaultL
2006 A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol 7 1217 1224
25. Satoh-TakayamaN
VosshenrichCA
Lesjean-PottierS
SawaS
LochnerM
2008 Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29 958 970
26. LuciC
ReyndersA
IvanovII
CognetC
ChicheL
2009 Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 10 75 82
27. SanosSL
BuiVL
MorthaA
OberleK
HenersC
2009 RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol 10 83 91
28. CupedoT
CrellinNK
PapazianN
RomboutsEJ
WeijerK
2009 Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol 10 66 74
29. CellaM
FuchsA
VermiW
FacchettiF
OteroK
2009 A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457 722 725
30. KrugA
FrenchAR
BarchetW
FischerJA
DzionekA
2004 TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21 107 119
31. LucasM
SchachterleW
OberleK
AicheleP
DiefenbachA
2007 Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26 503 517
32. FontenotJD
RasmussenJP
WilliamsLM
DooleyJL
FarrAG
2005 Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22 329 341
33. FreudAG
BecknellB
RoychowdhuryS
MaoHC
FerketichAK
2005 A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22 295 304
34. YadiH
BurkeS
MadejaZ
HembergerM
MoffettA
2008 Unique receptor repertoire in mouse uterine NK cells. J Immunol 181 6140 6147
35. BraunerH
ElemansM
LemosS
BrobergerC
HolmbergD
2010 Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J Immunol 184 2272 2280
36. ColonnaM
2009 Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 31 15 23
37. VivierE
SpitsH
CupedoT
2009 Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol 9 229 234
38. KimS
Poursine-LaurentJ
TruscottSM
LybargerL
SongYJ
2005 Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436 709 713
39. FernandezNC
TreinerE
VanceRE
JamiesonAM
LemieuxS
2005 A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105 4416 4423
40. DavisMA
ReynoldsAB
2006 Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell 10 21 31
41. MenkoAS
ZhangL
SchianoF
KreidbergJA
KukuruzinskaMA
2002 Regulation of cadherin junctions during mouse submandibular gland development. Dev Dyn 224 321 333
42. SimonaS
DanielaP
CristinaB
EmanuelaM
AnnaP
1999 NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol 29 1656 1666
43. PolicB
HengelH
KrmpoticA
TrgovcichJ
PavicI
1998 Hierarchical and Redundant Lymphocyte Subset Control Precludes Cytomegalovirus Replication during Latent Infection. J Exp Med 188 1047 1054
44. BukowskiJF
WodaBA
WelshRM
1984 Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol 52 119 128
45. HumphreysIR
de TrezC
KinkadeA
BenedictCA
CroftM
2007 Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med 204 1217 1225
46. WesleyJD
TessmerMS
ChaukosD
BrossayL
2008 NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog 4 e1000106
47. Perona-WrightG
MohrsK
SzabaFM
KummerLW
MadanR
2009 Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe 6 503 512
48. VivierE
UgoliniS
2009 Regulatory natural killer cells: new players in the IL-10 anti-inflammatory response. Cell Host Microbe 6 493 495
49. OakleyOR
GarvyBA
HumphreysS
QureshiMH
PomeroyC
2008 Increased weight loss with reduced viral replication in interleukin-10 knock-out mice infected with murine cytomegalovirus. Clin Exp Immunol 151 155 164
50. JoshiNS
CuiW
ChandeleA
LeeHK
UrsoDR
2007 Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27 281 295
51. HartyJT
BadovinacVP
2008 Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol 8 107 119
52. VivierE
TomaselloE
BaratinM
WalzerT
UgoliniS
2008 Functions of natural killer cells. Nat Immunol 9 503 510
53. SunJC
BeilkeJN
LanierLL
2009 Adaptive immune features of natural killer cells. Nature 457 557 561
54. LussierG
BerthiaumeL
PaymentP
1974 Electron microscopy of murine cytomegalovirus: development of the virus in vivo and in vitro. Arch Gesamte Virusforsch 46 269 280
55. MegaJ
McGheeJR
KiyonoH
1992 Cytokine - and Ig-producing T cells in mucosal effector tissues: analysis of IL-5 - and IFN-gamma-producing T cells, T cell receptor expression, and IgA plasma cells from mouse salivary gland-associated tissues. J Immunol 148 2030 2039
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Salivary Gland NK Cells Are Phenotypically and Functionally Unique
- Genetic Epidemiology of Tuberculosis Susceptibility: Impact of Study Design
- Early Target Cells of Measles Virus after Aerosol Infection of Non-Human Primates
- Multiple Plant Surface Signals are Sensed by Different Mechanisms in the Rice Blast Fungus for Appressorium Formation
- Biofilm Development on by Is Facilitated by Quorum Sensing-Dependent Repression of Type III Secretion
- Distinct Patterns of IFITM-Mediated Restriction of Filoviruses, SARS Coronavirus, and Influenza A Virus
- Molecular Basis of Increased Serum Resistance among Pulmonary Isolates of Non-typeable
- A Helminth Immunomodulator Exploits Host Signaling Events to Regulate Cytokine Production in Macrophages
- HCMV Spread and Cell Tropism are Determined by Distinct Virus Populations
- Characteristics of the Earliest Cross-Neutralizing Antibody Response to HIV-1
- Induction of a Peptide with Activity against a Broad Spectrum of Pathogens in the Salivary Gland, following Infection with Dengue Virus
- Structural Basis for the Recognition of Cellular mRNA Export Factor REF by Herpes Viral Proteins HSV-1 ICP27 and HVS ORF57
- Identification and Characterization of the Host Protein DNAJC14 as a Broadly Active Flavivirus Replication Modulator
- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of
- Selective C-Rel Activation via Malt1 Controls Anti-Fungal T-17 Immunity by Dectin-1 and Dectin-2
- Imaging Single Retrovirus Entry through Alternative Receptor Isoforms and Intermediates of Virus-Endosome Fusion
- Aerosols Transmit Prions to Immunocompetent and Immunodeficient Mice
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



