-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Selective C-Rel Activation via Malt1 Controls Anti-Fungal T-17 Immunity by Dectin-1 and Dectin-2
C-type lectins dectin-1 and dectin-2 on dendritic cells elicit protective immunity against fungal infections through induction of TH1 and TH-17 cellular responses. Fungal recognition by dectin-1 on human dendritic cells engages the CARD9-Bcl10-Malt1 module to activate NF-κB. Here we demonstrate that Malt1 recruitment is pivotal to TH-17 immunity by selective activation of NF-κB subunit c-Rel, which induces expression of TH-17-polarizing cytokines IL-1β and IL-23p19. Malt1 inhibition abrogates c-Rel activation and TH-17 immunity to Candida species. We found that Malt1-mediated activation of c-Rel is similarly essential to induction of TH-17-polarizing cytokines by dectin-2. Whereas dectin-1 activates all NF-κB subunits, dectin-2 selectively activates c-Rel, signifying a specialized TH-17-enhancing function for dectin-2 in anti-fungal immunity by human dendritic cells. Thus, dectin-1 and dectin-2 control adaptive TH-17 immunity to fungi via Malt1-dependent activation of c-Rel.
Published in the journal: Selective C-Rel Activation via Malt1 Controls Anti-Fungal T-17 Immunity by Dectin-1 and Dectin-2. PLoS Pathog 7(1): e32767. doi:10.1371/journal.ppat.1001259
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001259Summary
C-type lectins dectin-1 and dectin-2 on dendritic cells elicit protective immunity against fungal infections through induction of TH1 and TH-17 cellular responses. Fungal recognition by dectin-1 on human dendritic cells engages the CARD9-Bcl10-Malt1 module to activate NF-κB. Here we demonstrate that Malt1 recruitment is pivotal to TH-17 immunity by selective activation of NF-κB subunit c-Rel, which induces expression of TH-17-polarizing cytokines IL-1β and IL-23p19. Malt1 inhibition abrogates c-Rel activation and TH-17 immunity to Candida species. We found that Malt1-mediated activation of c-Rel is similarly essential to induction of TH-17-polarizing cytokines by dectin-2. Whereas dectin-1 activates all NF-κB subunits, dectin-2 selectively activates c-Rel, signifying a specialized TH-17-enhancing function for dectin-2 in anti-fungal immunity by human dendritic cells. Thus, dectin-1 and dectin-2 control adaptive TH-17 immunity to fungi via Malt1-dependent activation of c-Rel.
Introduction
Fungal infections are a major health threat and incidence of both superficial and invasive infections by Candida species are growing throughout the world due to increasing numbers of at-risk immunocompromised patients, such as transplant recipients and those infected with HIV-1/AIDS, as well as the emergence of strains that are resistant to antimycotic drugs [1]. Anti-fungal adaptive immunity requires both T helper cell type 1 (TH1) and TH-17 immune responses; IL-17 secreted by TH-17 cells mobilizes neutrophils required for anti-fungal responses [2], [3], whereas TH1-produced IFNγ optimally activates neutrophils and subsequent phagocytosis of fungi [4]. Dendritic cells (DCs) are crucial for the induction of T helper cell differentiation [5], [6]. Although the requirements for TH-17 differentiation by human DCs are not completely clear, it is evident that secretion of IL-23, IL-1β and IL-6 are important for TH-17 development [7], [8], whereas IL-12p70 skews T helper cell differentiation towards TH1 responses [9]. Little is known about the molecular mechanisms that underlie the induction of the TH-17-promoting cytokines by DCs after fungal infections.
Pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and C-type lectins, sense pathogens through conserved pattern-associated molecular patterns (PAMPs), which induce signaling pathways to regulate gene transcription. C-type lectins are important in fungal recognition by DCs and in induction of anti-fungal TH1 and TH-17 immune responses [5], [10]. The cell-wall of many fungi, including Candida species (spp), consists of carbohydrate structures such as chitin, mannan and β-glucan that are recognized by C-type lectins like dectin-1, dectin-2, DC-SIGN and mannose receptor [5], [11], [12]. Triggering of β-glucan receptor dectin-1 by C. albicans induces both TH1 and TH-17 immune responses by DCs through Syk-dependent NF-κB activation [10], [13], [14]. Syk induces the assembly of a scaffold consisting of the caspase recruitment domain 9 (CARD9) protein, B cell lymphoma 10 (Bcl10) and mucosa-associated lymphoid-tissue lymphoma-translocation gene 1 (Malt1) [13], [15]. This CARD9-Bcl10-Malt1 scaffold couples dectin-1 in human to the canonical NF-κB pathway by activating NF-κB subunit p65 and c-Rel [10], [13], whereas dectin-1 triggering also leads to activation of the non-canonical NF-κB RelB pathway [10]. The balance between p65 and RelB activity is controlled by a distinct Raf-1-dependent pathway that thereby dictates expression of IL-12p70, IL-1β and IL-23 [10]. It is unclear how the CARD9-Bcl10-Malt1 complex is involved in the activation of the different NF-κB subunits and how this affects TH-17 differentiation. Although dectin-1-deficient people are more susceptible to mucocutaneous fungal infection, CARD9 deficiency in human causes a more pronounced phenotype with chronic mucoctaneous as well as invasive fungal infections [16], [17]. These studies suggest that dectin-1 is not the only receptor that couples CARD9-Bcl10-Malt1 to the defense against fungi. Indeed, dectin-2 interacts with C. albicans through mannan structures present on both yeast and hyphal forms [18], [19] and a recent study shows that dectin-2 is involved in the induction of TH-17 responses to C. albicans in mice [19], [20]. Dectin-2 indirectly activates Syk through association with the FcRγ chain [12] which results in CARD9-dependent expression of IL-2, IL-10 and TNF [20]. Thus, both dectin-1 and dectin-2 are involved in TH-17 development through Syk and CARD9 but the underlying mechanisms and involvement of Bcl10 and Malt1 remain unclear. It is also unclear whether dectin-1 and dectin-2 are required for a general anti-fungal response to all Candida species.
Here we demonstrate that dectin-1 and dectin-2 convergently contribute to anti-fungal TH-17 immunity by inducing IL-1β and IL-23 production. Both dectin-1 and dectin-2 triggering leads to Malt1 activation, which specifically activates NF-κB subunit c-Rel that is pivotal to the transcriptional activation of the Il1b and Il23p19 genes. Syk-dependent recruitment of CARD9 and Bcl10 upon dectin-1 triggering is crucial for activation of all NF-κB subunits, while recruitment of Malt1 and activation of its paracaspase activity is distinctively required for c-Rel but not p65 or RelB activation. In contrast to dectin-1, dectin-2 triggering activates only c-Rel, which is also dependent on Malt1 signaling, signifying a specialized function for dectin-2 in TH-17 immunity. Simultaneous triggering of dectin-1 and dectin-2 by pathogenic fungi promotes the expression of IL-1β and IL-23 to boost TH-17-mediated cellular responses, whereas Malt1 inhibition after Candida infection markedly reduces TH-17 polarization. Thus, Malt1 activation links dectin-1 and dectin-2 to the c-Rel-dependent expression of IL-1β and IL-23 and directs adaptive anti-fungal immunity.
Results
Dectin-1 signaling via Malt1 affects IL-1β, IL-23p19, IL-6 and IL-12p35 expression
The recruitment of the CARD9-Bcl10-Malt1 complex by Syk links dectin-1 on DCs to NF-κB activation, thereby controlling anti-fungal TH-17 immunity [10], [13]–[15]. In mice, the pivotal role for Syk and CARD9 in dectin-1 signaling has been established using knock-out models [13], [14], while, in contrast, little is known about their role in regulating human adaptive immunity. Here we investigated the role of the CARD9-Bcl10-Malt1 module in relaying signals from dectin-1 in human primary DCs to induce cytokine responses. We used the β-glucan curdlan, which is a specific ligand for dectin-1 and induces Syk activation in both mice and human [10], [14]. We silenced Syk, CARD9, Bcl10 and Malt1 by RNA interference (Figure S1) and analyzed expression of cytokines involved in TH1 and TH-17 polarization. Expression of IL-1β, IL-23p19, IL-6, IL-12p35 and IL-12p40 mRNA was completely abrogated by Syk, CARD9 as well as Bcl10 silencing (Figure 1A and 1B). Notably, Malt1 silencing had distinct effects on the different cytokines; IL-1β and IL-23p19 mRNA expression was strongly decreased, whereas IL-6 and IL-12p35 mRNA was enhanced and IL-12p40 mRNA expression was unaffected by Malt1 silencing (Figure 1B). TLR4-dependent cytokine expression was unaffected by silencing of either Syk, CARD9, Bcl10 or Malt1 (Figure S2). These data show that Malt1 has a very distinctive function by inducing the TH-17-polarizing cytokines IL-23 and IL-1β, whereas both CARD9 and Bcl10 are more generally required for all dectin-1-induced cytokine responses.
Fig. 1. Dectin-1-induced cytokine expression requires Syk, CARD9 and Bcl10, whereas Malt1-mediated signaling enhances IL-1β and IL-23p19, but decreases IL-6 and IL-12p35 expression. 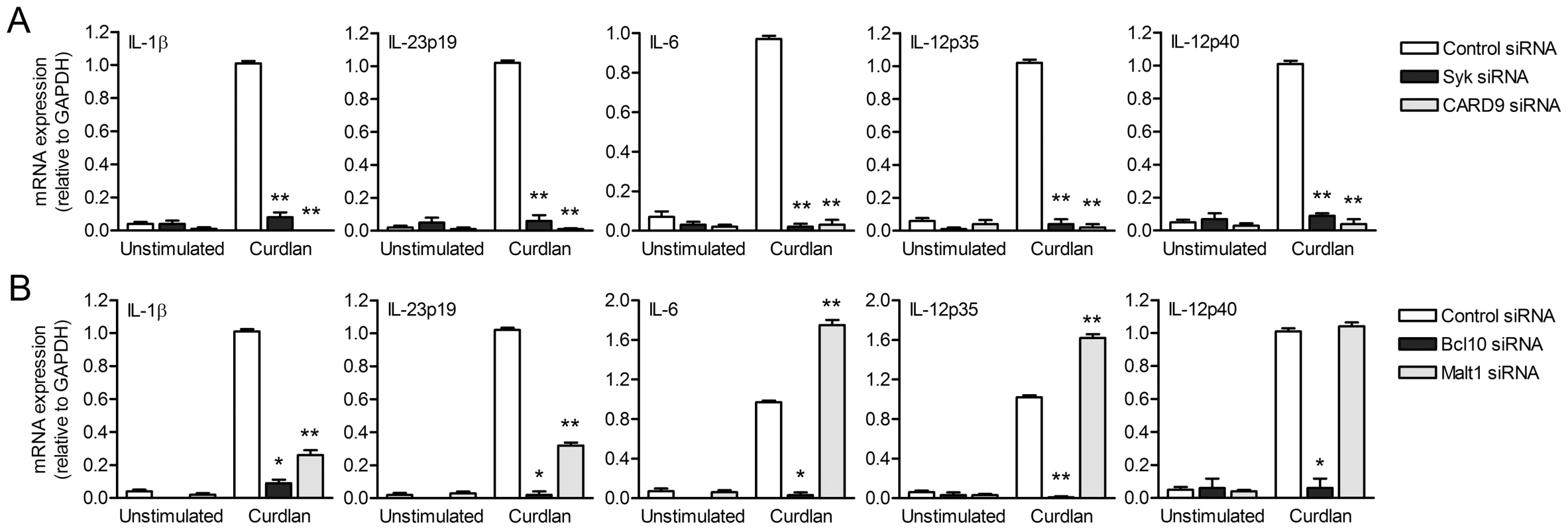
(A and B) Quantitative real-time PCR for indicated mRNAs in curdlan-stimulated DCs after Syk, CARD9 (A), Bcl10 and Malt1 (B) silencing by RNA interference (siRNA). Expression is normalized to GAPDH and set at 1 in curdlan-stimulated cells. Data are mean ± s.d. of four independent experiments, *p<0.05 and **p<0.01 (Student's t-test). Malt1 controls c-Rel activation by dectin-1
The distinct functions of CARD9, Bcl10 and Malt1 in cytokine induction after dectin-1 triggering led us to investigate their functions in the activation of NF-κB. Dectin-1 triggering activates all NF-κB subunits in a Syk-dependent manner, which is crucial to dectin-1-induced cytokine responses [10]. We first determined nuclear translocation and subsequent DNA binding of the different subunits after dectin-1 triggering. NF-κB dimers are normally retained inactive in the cytoplasm and translocate into the nucleus upon activation [21]. In control-silenced DCs, dectin-1 triggering by curdlan resulted in activation of p65, c-Rel, p52 and RelB, while p50 DNA binding could already be detected in unstimulated cells (Figure 2A). Silencing of either CARD9 or Bcl10 in DCs completely impaired activation of p65, c-Rel, RelB and p52 after curdlan stimulation (Figure 2A). Strikingly, Malt1 silencing specifically abrogated c-Rel activation (Figure 2A), whereas nuclear translocation of the other subunits was unaffected (Figure 2A). Immunofluorescence stainings showed that Malt1 silencing interfered with the nuclear translocation of c-Rel but neither with p65 nor RelB (Figure 2B). These data strongly suggest that Malt1 is required for selective activation of c-Rel-containing NF-κB dimers, whereas recruitment of CARD9 and Bcl10 are an absolute requirement for activation of all NF-κB subunits.
Fig. 2. Malt1 signaling by dectin-1 is specifically required for c-Rel-dependent cytokine expression. 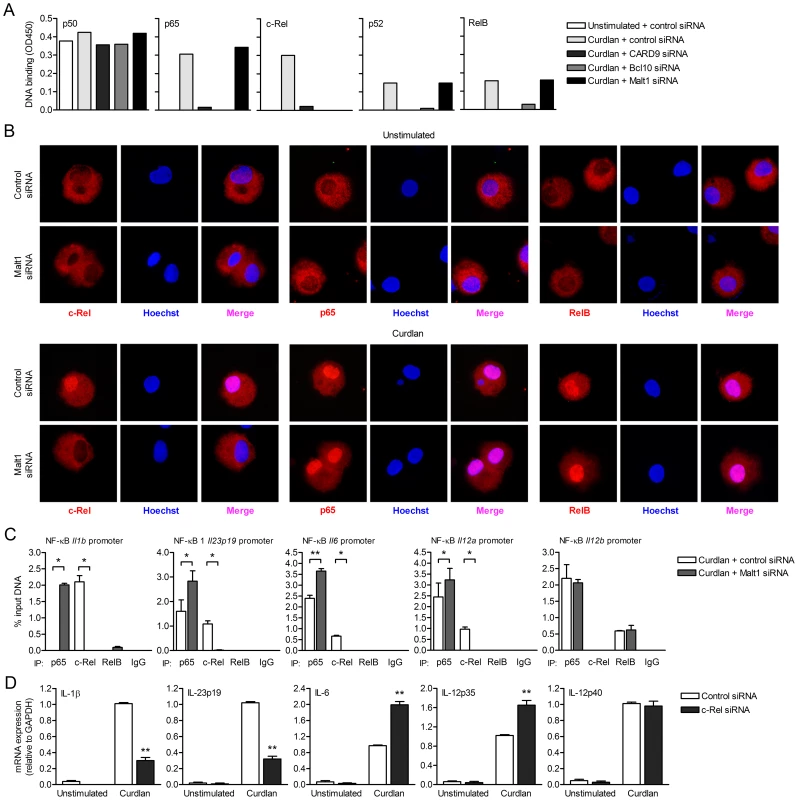
(A) DNA binding of NF-κB subunits in nuclear extracts of curdlan-stimulated DCs after Malt1 silencing by RNA interference (siRNA). Graphs are representative of three independent experiments. (B) Translocation of c-Rel, p65 or RelB (red) into the nucleus (Hoechst staining, blue; colocalization (Merge, pink)) in curdlan-stimulated DCs after Malt1 silencing. Stainings are representative of two independent experiments. (C) ChIP assays were performed to determine binding of p65, c-Rel and RelB to NF-κB binding motifs of the Il1b, Il23p19, Il12a, Il12b and Il6 promoters. Protein-DNA complexes were immunoprecipitated from sheared chromatin isolated from para-formaldehyde-fixed curdlan-stimulated DCs after Malt1 silencing by RNA interference (siRNA). Immunoprecipitation with mouse IgG served as a negative control. Quantitative real-time PCR reactions for indicated regions were performed. Levels are normalized with respect to the ‘input DNA’ sample, which had not undergone immunoprecipitation; results are expressed as the % input DNA. Data are mean ± s.d. of two independent experiments, *p<0.05 and **p<0.01 (Student's t-test). (D) Quantitative real-time PCR for indicated mRNAs in curdlan-stimulated DCs after c-Rel silencing by RNA interference (siRNA). Expression is normalized to GAPDH and set at 1 in curdlan-stimulated cells. Data are mean ± s.d. of four independent experiments, **p<0.01 (Student's t-test). c-Rel activation by Malt1 induces TH-17-polarizing cytokines IL-1β and Il-23
We next used chromatin immunoprecipitation (ChIP) assays to investigate the effect of Malt1-induced c-Rel activation on the DNA binding of the NF-κB subunits to different cytokine promoters. Our data show that the NF-κB site of the Il1b promoter was solely occupied by c-Rel, while both c-Rel and p65 were bound to the Il23, Il6 and Il12a promoters after curdlan stimulation of control-silenced DCs, albeit in different ratios (Figure 2C). Malt1 silencing completed abrogated binding of c-Rel to the Il1b, Il23, Il6 and Il12a promoters after dectin-1 triggering (Figure 2C), consistent with a pivotal role for Malt1-mediated signaling in c-Rel activation. The absence of c-Rel activation allowed binding of p65 to the promoters as is evident from the higher p65 association with the different promoters (Figure 2C). Notably, c-Rel binding to the Il1b promoter was completely replaced by p65 binding after Malt1 silencing (Figure 2C). This suggests that the Il1b promoter is preferentially bound by c-Rel and that c-Rel is a stronger activator of Il1b transcription than p65, since c-Rel replacement by p65 after Malt1 silencing resulted in significantly reduced IL-1β expression (Figure 1B). While Malt1 silencing abolished c-Rel binding to both the Il23 and Il12a promoter after dectin-1 triggering, loss of c-Rel activation had opposite effects on Il23 and Il12a transcription as IL-23p19 mRNA levels were severely decreased, while IL-12p35 mRNA was enhanced (Figure 1B). These results are consistent with our previous findings showing that c-Rel is a stronger transactivator of Il23 but a weaker transactivator of Il12a transcripton than p65 [10]. IL-6 expression was enhanced after Malt1 silencing (Figure 1B), suggesting that c-Rel functions as an inhibitory factor when bound to the Il6 promoter. The Il12b promoter was not bound by c-Rel in either control - or Malt1-silenced cells after curdlan stimulation (Figure 2C), consistent with the similar IL-12p40 mRNA levels in both control - and Malt1-silenced cells after dectin-1 triggering (Figure 1B).
In order to further demonstrate the importance of c-Rel in the transcriptional regulation of the Il1b, Il23, Il6 and Il12a genes, we measured cytokine expression in c-Rel-silenced DCs (Figure S1) after curdlan stimulation (Figure 2D). Similar to Malt1 silencing, c-Rel silencing strongly decreased IL-1β and IL-23p19 mRNA, while enhancing IL-6 and IL-12p35 mRNA levels compared to control-silenced cells after dectin-1 triggering (Figure 2D). IL-12p40 mRNA expression was independent of c-Rel activation (Figure 2D), as was LPS-induced cytokine expression (Figure S2). These results strongly suggest that Malt1-mediated c-Rel activation leads to the expression of IL-1β and IL-23, key cytokines in TH-17 differentiation.
Malt1 proteolytic activity is required for dectin-1-induced c-Rel-dependent cytokine expression
Since Malt1 has paracaspase activity [22], [23], we next investigated whether the adaptor or protease function of Malt1 is involved in the selective activation of c-Rel after dectin-1 triggering. We used z-VRPR-FMK, a compound which blocks the proteolytic activity of Malt1 [22]. Inhibition of Malt1 proteolytic activity completely abolished activation of c-Rel without affecting the other NF-κB subunits (Figure 3A), which is similar to Malt1 silencing (Figure 2A), Immunofluoresence stainings confirmed that Malt1 inhibition specifically interferes with nuclear translocation of c-Rel after curdlan stimulation (Figure S3). Malt1 paracaspase inhibition also markedly reduced both IL-1β and IL-23p19 mRNA levels and slightly enhanced IL-6 and IL-12p35 mRNA production after curdlan stimulation (Figure 3B), similarly to Malt1 silencing (Figure 1B). IL-12p40 mRNA production was neither dependent on Malt1 expression nor activation (Figure 3B). We next measured cytokine production and found that Malt1 inhibition severely reduced IL-1β and IL-23 protein expression, without affecting IL-12p70 expression and only slightly enhancing IL-6 expression (Figure 3C), indicating that dectin-1-induced cytokine expression is primarily regulated at the transcriptional level. These results show that Malt1 protease activity is required for specific c-Rel activation and plays a central role in the induction of TH-17-polarizing cytokines by dectin-1.
Fig. 3. Malt1 paracaspase activity is required for c-Rel activation and cytokine induction by dectin-1. 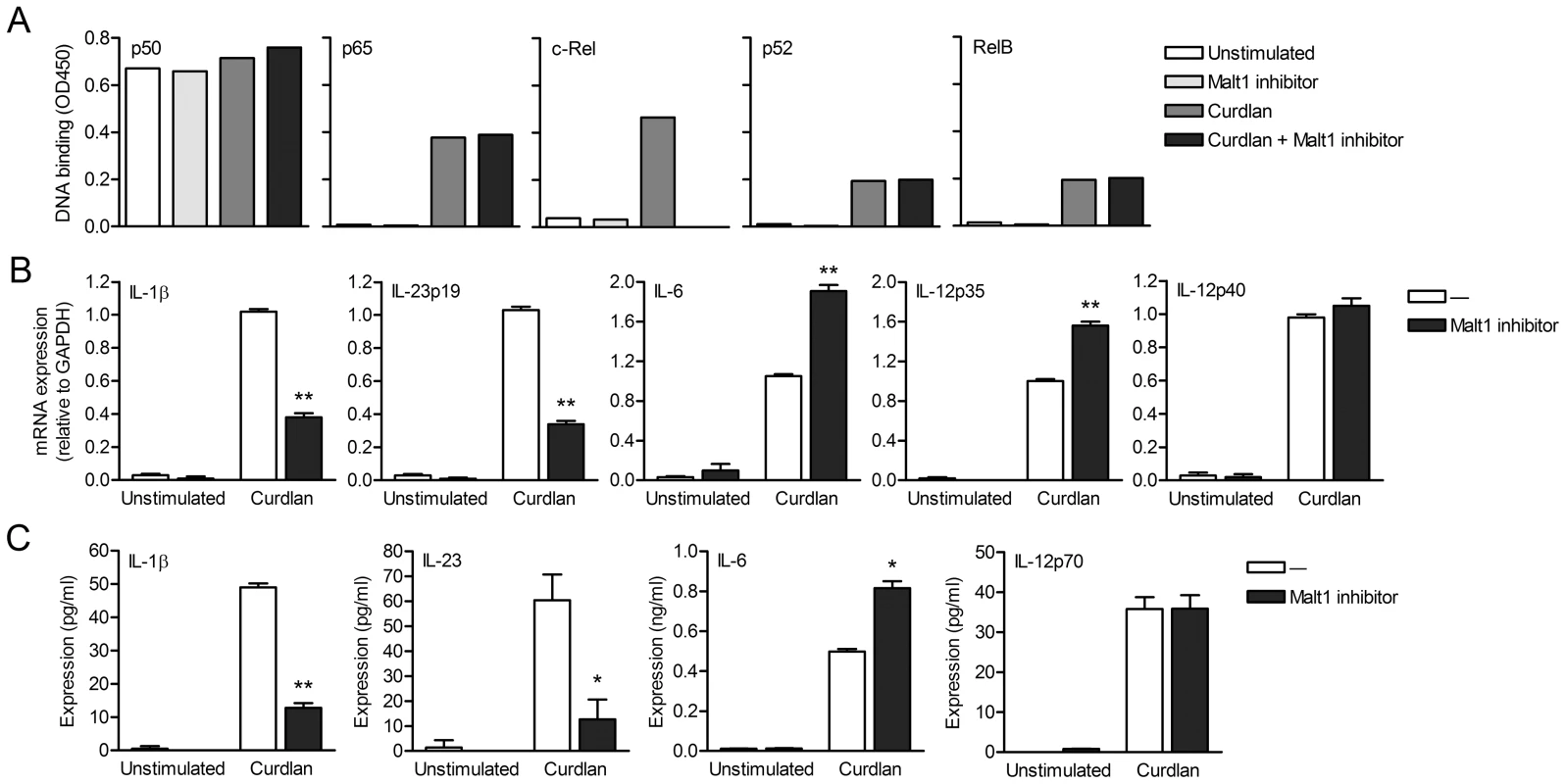
(A) DNA binding of NF-κB subunits in nuclear extracts of curdlan-stimulated DCs after inhibition of Malt1 paracaspase activity by z-VRPR-FMK. Data are representative of three independent experiments. (B) Quantitative real-time PCR for indicated mRNAs in curdlan-stimulated DCs after Malt1 paracaspase inhibition. Expression is normalized to GAPDH and set at 1 in curdlan-stimulated cells. Data are mean ± s.d. of three independent experiments, **p<0.01 (Student's t-test). (C) Cytokine production was determined by ELISA in supernatants of DCs stimulated with curdlan in the absence or presence of Malt1 paracaspase inhibitor. Data are mean ± s.d. of duplicate cultures, and are representative of five independent experiments, *p<0.05 and **p<0.01 (Student's t-test). Malt1 directs expression of TH-17-polarizing cytokines during Candida spp. infection
Dectin-1 plays an important role in anti-fungal immunity through the induction of TH1 and TH-17 differentiation [10], [14]. Since Candida albicans infections are amongst the most common causes of invasive fungal infections in immunocompromised patients [24], [25], we used two different C. albicans strains to investigate the importance of Malt1 signaling in anti-fungal immune responses. Consistent with its function in the induction of TH-17-polarizing cytokines, Malt1 activation is required for expression of IL-1β and IL-23 by DCs in response to both C. albicans strain CBS8781 and CBS2712 (Figure 4A). As observed with curdlan stimulation, the expression of IL-6 was slightly upregulated as a result of Malt1 protease inhibition, whereas IL-12p70 production was unaffected by Malt1 signaling after C. albicans stimulation (Figure 4A). To elucidate the contribution of dectin-1 signaling to the Malt1-dependent cytokines responses, we treated DCs with C. albicans in the presence of blocking dectin-1 antibodies. Notably, we observed that C. albicans CBS8781-induced cytokine expression was completely abrogated after blocking dectin-1, whereas cytokine production after C. albicans CBS2712 stimulation was only partially inhibited by dectin-1 antibodies (Figure 4A). Malt1 inhibition decreased C. albicans CBS2712-induced IL-1β and IL-23 expression more strongly than dectin-1 inhibition (Figure 4A). These results suggest that fungal infections trigger not only dectin-1 but also other receptors to induce anti-fungal TH-17 responses via Malt1. To further investigate this, we used two different Candida species, C. lusitaniae and C. nivarienis, both emerging pathogenic fungi causing opportunistic infections in transplant and immunocompromised patients [24], [26]. C. lusitaniae CBS4413 induced cytokine production in a dectin-1-dependent manner, while C. nivariensis CBS9983 was only partially dependent on dectin-1 signaling for the production of IL-1β, IL-23, IL-6 and IL-12p70 (Figure 4B). Both IL-1β and IL-23 production by C. lusitaniae and C. nivariensis was largely dependent on Malt1 protease activity (Figure 4B). Noteworthy, Candida spp. that trigger Malt1 activation via dectin-1 in combination with other unidentified receptor(s) induce higher levels of IL-1β and IL-23 in DCs than those that trigger only dectin-1 (Figure 4A and 4B). Similar to the C. albicans strains, C. lusitaniae and C. nivariensis stimulation resulted in slightly enhanced IL-6 expression after Malt1 inhibition, while IL-12p70 was unaffected (Figure 4B). These data suggest that c-Rel activation by Malt1 signaling controls anti-fungal TH-17 immunity to Candida spp.
Fig. 4. Malt activation controls IL-1β and IL-23 production in response to Candida spp. 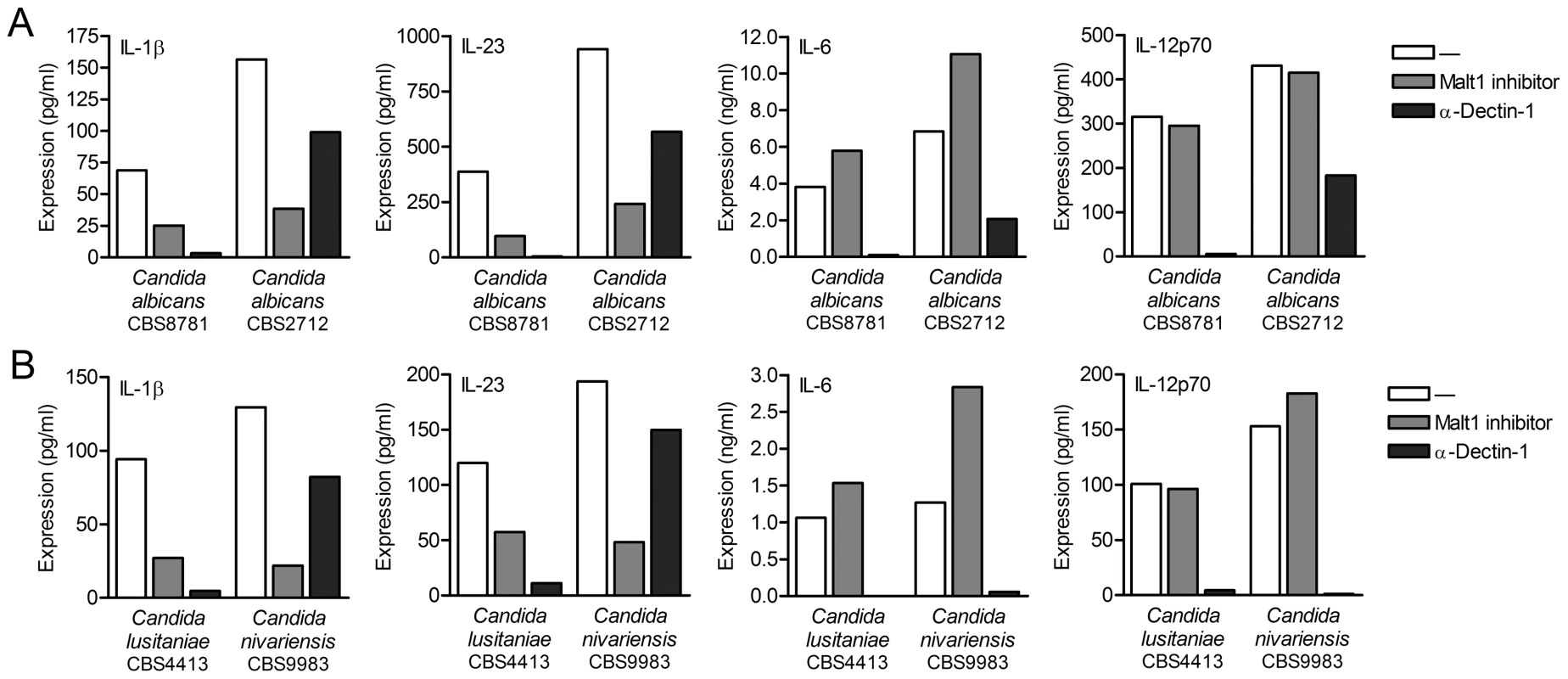
(A and B) Cytokine production was determined by ELISA in supernatants of DCs stimulated with Candida albicans spp. (A), C. nivariensis or C. lusitaniae (B) in the absence or presence of Malt1 paracaspase inhibitor z-VRPR-FMK or blocking dectin-1 antibodies. Data are representative of three independent experiments. Dectin-2 contributes to cytokine response during Candida spp. infection
We next set out to identify the fungal PRR(s) on DCs that are triggered by C. albicans CBS2712 and C. nivariensis to induce Malt1 activation independently of dectin-1. The C-type lectin dectin-2 has been shown to participate in fungal TH-17 immunity in mice [19], [20]. We explored a role for dectin-2 in cytokine responses to Candida spp. by using blocking antibodies against dectin-2. Notably, induction of IL-1β and IL-23p19 mRNA by both C. albicans CBS2712 and C. nivariensis was partially abolished by dectin-2 antibodies, while blocking both dectin-1 and dectin-2 completely abrogated expression of IL-1β and IL-23p19 (Figure 5). These data strongly suggest that dectin-2 signaling contributes together with dectin-1 to the induction of these TH-17-polarizing cytokines by C. albicans CBS2712 and C. nivariensis. Blocking dectin-2 triggering by C. albicans CBS2712 or C. nivariensis slightly increased IL-6 but greatly enhanced IL-12p35 mRNA expression (Figure 5), most likely reflecting the negative influence of c-Rel binding to the respective promoters on Il6 and Il12a transcription. C. nivariensis-induced IL-6 and IL-12p35 mRNA expression was dependent on both dectin-1 and dectin-2, while C. albicans CBS2712 induced IL-6 and IL-12p35 expression via dectin-1, as blocking both dectin-1 and dectin-2 had no additional effects compared to blocking dectin-1 alone (Figure 5). IL-12p40 mRNA expression by C. albicans CBS2712 and C. nivariensis was independent of dectin-2 triggering (Figure 5). These data suggest that dectin-2 signaling through Malt1 controls only c-Rel-dependent gene expression without affecting c-Rel-independent transcription. The residual expression of IL-6, IL-12p35 and IL-12p40 induced by C. albicans CBS2712 after either blocking dectin-1 or dectin-1 plus dectin-2 suggests additional involvement of other receptors, such as TLRs (Figure 5A). As expected, dectin-2 antibodies did not interfere with cytokine expression induced by C. albicans CBS8781 and C. lusitaniae, since cytokine induction was completely inhibited by blocking dectin-1 antibodies (Figure 4 and 5). Cytokine protein levels confirmed the mRNA expression data (Figure S4). Our data demonstrate that both dectin-1 and dectin-2 contribute to anti-fungal TH-17-polarizing cytokine responses to various Candida spp.
Fig. 5. Dectin-1 and dectin-2 contribute to Candida spp.-induced cytokine expression. 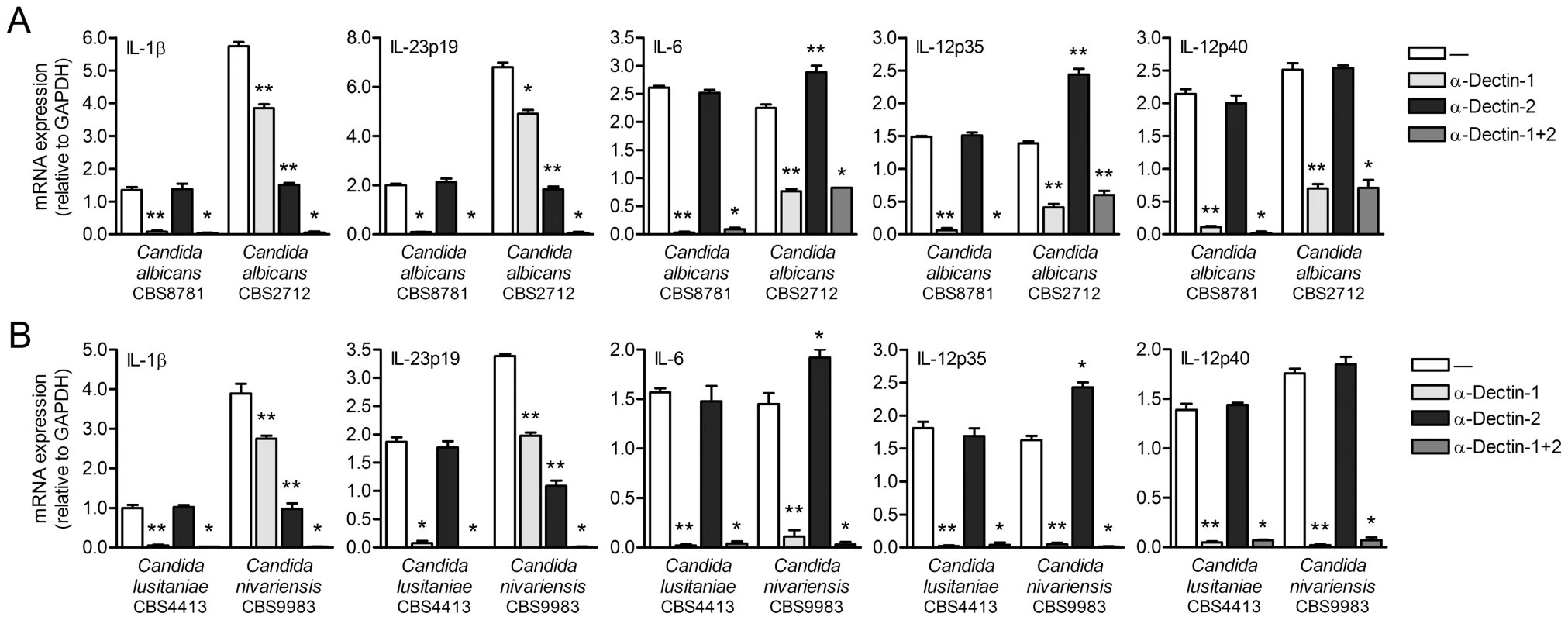
(A and B) Quantitative real-time PCR for indicated mRNAs in DCs stimulated with Candida albicans spp. (A), C. nivariensis or C. lusitaniae (B) in the absence or presence of blocking antibodies against dectin-1 and/or dectin-2. Expression is normalized to GAPDH and set at 1 in curdlan-stimulated cells. Data are mean ± s.d. of four independent experiments, *p<0.05 and **p<0.01 (Student's t-test). Malt1 relays dectin-2 signals to induce c-Rel-dependent cytokine expression
We next triggered dectin-2-FcRγ signaling by crosslinking dectin-2 with antibodies and investigated cytokine expression induced by human DCs. Dectin-2 crosslinking induced high levels of IL-1β and IL-23p19 mRNA expression (Figure 6A). Remarkably, dectin-2 crosslinking did neither induce IL-6, IL-12p35 nor IL-12p40 mRNA expression (Figure 6A), consistent with our observations when blocking dectin-2 binding by Candida spp. (Figure 5) and strongly suggesting that dectin-2 triggering specifically induces IL-1β and IL-23p19. These results confirm that dectin-1 and dectin-2 signaling converge to boost the expression of TH-17-polarizing cytokines, as we observed after Candida spp. stimulation.
Fig. 6. Dectin-2 signaling induces Malt1- and c-Rel-dependent IL-1β and IL-23p19 expression. 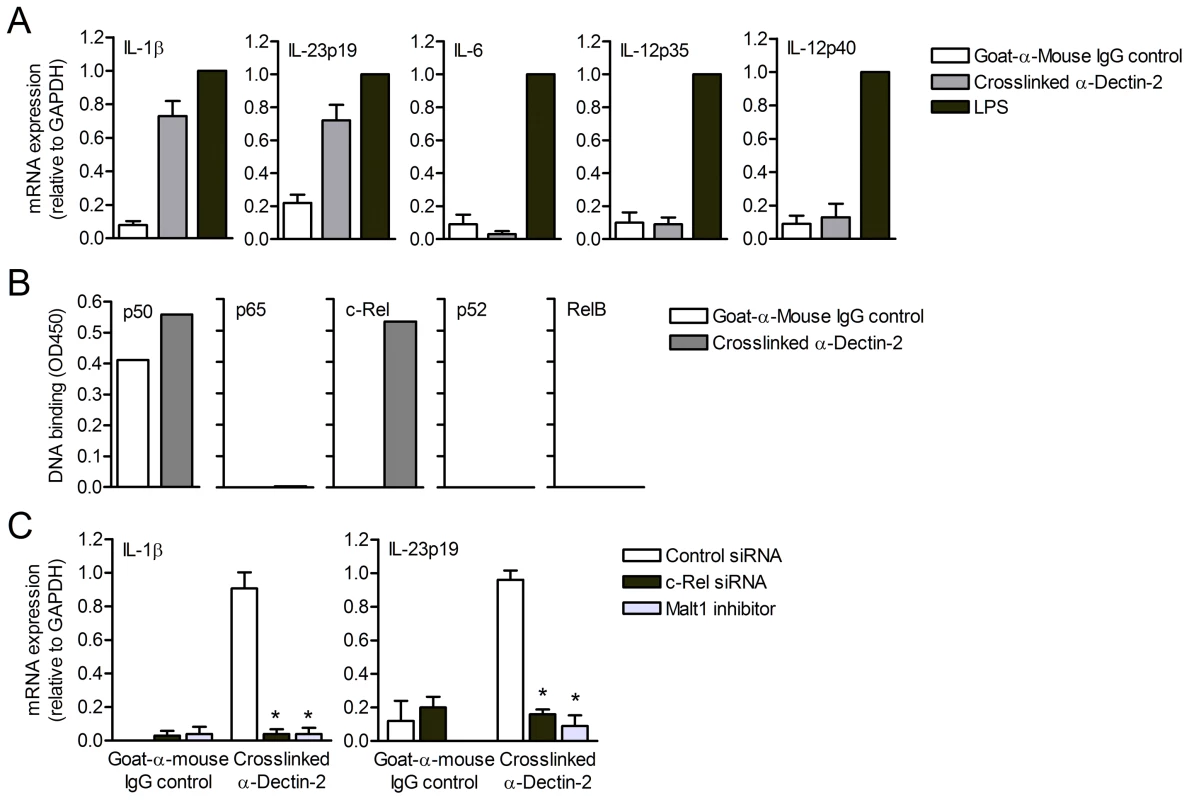
(A) Quantitative real-time PCR for indicated mRNAs in DCs stimulated with crosslinked dectin-2 antibodies or LPS. In (A–C), goat-anti-mouse IgG was coated as a control for aspecific activation. Expression is normalized to GAPDH and set at 1 in LPS-stimulated cells. Data are mean ± s.d. of at least four independent experiments. (B) DNA binding of NF-κB subunits in nuclear extracts of dectin-2-triggered DCs. Data are representative of two independent experiments. (C) Quantitative real-time PCR for indicated mRNAs in DCs stimulated with crosslinked dectin-2 antibodies after c-Rel silencing or Malt1 inhibition with paracaspase inhibitor z-VRPR-FMK. Goat-anti-mouse IgG was coated as a control for non-specific activation. Expression is normalized to GAPDH and set at 1 in LPS-stimulated cells. Data are mean ± s.d. of two independent experiments, *p<0.05 (Student's t-test). We next investigated whether dectin-2 crosslinking induces NF-κB activation. Notably, dectin-2 triggering resulted in the specific activation of c-Rel, whereas the other NF-κB subunits p65, RelB and p52 were not activated (Figure 6B). Consistently, c-Rel-silenced DCs exhibited a defect in the induction of IL-1β and IL-23p19 mRNA expression after dectin-2 crosslinking (Figure 6C). We next silenced Malt1 expression to investigate whether dectin-2-FcRγ signaling, like dectin-1, employs Malt1 to specifically activate c-Rel and induce c-Rel-dependent cytokine expression. Similar to c-Rel silencing, Malt1 silencing completely abolished IL-1β and IL-23p19 mRNA production in response to dectin-2 crosslinking (Figure 6C). These data demonstrate that dectin-2 has a specialized function in adaptive immunity and specifically contributes to the induction of IL-1β and IL-23p19, emphasizing the importance of the Malt1-c-Rel activation axis in TH-17 immunity.
Malt1 signaling skews T helper cell polarization towards TH-17
Since Malt1 links dectin-1 and dectin-2 to the expression of the TH-17-polarizing cytokines IL-1β and IL-23 via the activation of c-Rel, we investigated whether Malt1 activation affects adaptive immunity to Candida spp. We first co-cultured curdlan-primed DCs with CD4+ T cells and measured IL-17 secretion after 5–12 days of co-culture [7]. Malt1 inhibition markedly reduced the capacity of curdlan-primed DCs to induce IL-17 expression in CD4+ T cells (Figure 7A, 7B and 7C). Thus, Malt1 activity is essential for the induction of TH-17-polarizing cytokines in DCs via dectin-1 triggering and subsequent TH-17 skewing. The ability of DCs primed by the different Candida spp. to promote IL-17 expression in CD4+ T cells was completely blocked when Malt1 activation was inhibited in the DCs (Figure 7D and 7E). This effect of Malt1 inhibition on the ability of Candida-primed DCs to induce TH-17 polarization was irrespective of the involvement of either dectin-1 alone (C. albicans CBS8781 and C. lusitaniae) or combined dectin-1 and dectin-2 triggering (C. albicans CBS2712 and C. nivariensis), consistent with a general role for Malt1 in inducing the TH-17-polarizing cytokines IL-1β and IL-23. Thus, the marked impact of Malt1 inhibition on IL-1β and IL-23p19 expression in response to fungal infections translates to a block in TH-17 immunity. Our data demonstrate that Malt1 signaling to c-Rel activation drives anti-fungal TH-17 responses.
Fig. 7. Malt1 signaling skews T helper cell polarization towards TH-17. 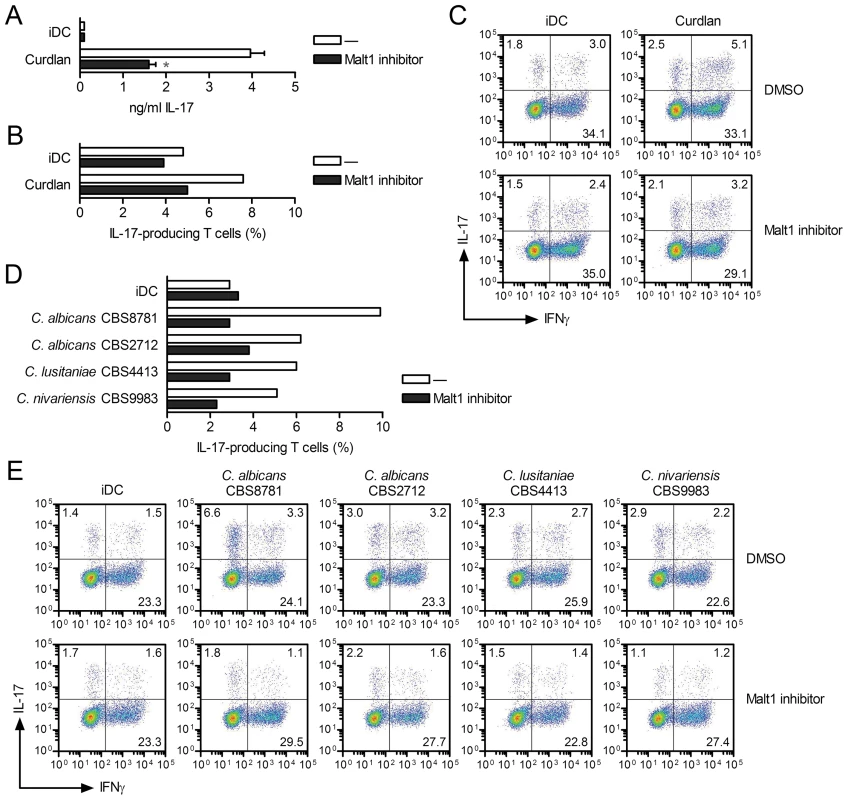
(A–E) T helper cell polarization was assessed by ELISA by measuring IL-17 production in supernatants at day 5 (A) or by flow cytometry by staining for intracellular IL-17 or IFNγ expression at day 12 after PMA plus ionomycin restimulation (B–E), after co-culture of memory CD4+ T cells with DCs left unstimulated (iDC) or primed with curdlan (A–C) or Candida spp. (D and E) in the absence or presence of Malt1 paracaspase inhibitor z-VRPR-FMK. In (B) and (D) the percentage of IL-17-producing T cells are shown, corresponding to the upper left and right quadrants of (C) and (E), respectively. Data are mean ± s.d. of duplicate cultures, *p<0.05 (Student's t-test), and are representative of three (A) or two (B–E) independent experiments. Discussion
C-type lectins are amongst the most important innate receptors on DCs to induce anti-fungal TH-17 immunity [5], [11], [14]. Expression of cytokines upon dectin-1 triggering by fungi requires NF-κB activation through Syk-dependent CARD9-Bcl10-Malt1 signaling [13], [15]. Here we demonstrate that Malt1 activation by dectin-1 and dectin-2 on human DCs induces the expression of TH-17-polarizing cytokines IL-1β and IL-23 through selective activation of the NF-κB subunit c-Rel. c-Rel is crucial for optimal transcription of the Il1b and Il23p19 genes. Dectin-1-induced activation of p65, RelB and c-Rel is completely dependent on the recruitment of CARD9 and Bcl10. Notably, Malt1 through its proteolytic paracaspase activity is specifically involved in activation of c-Rel, but dispensable for p65 and RelB activation. Malt1 activation of c-Rel is similarly essential in the induction of TH-17-polarizing cytokines by dectin-2. Strikingly, dectin-2 signaling, unlike dectin-1, only induces strong c-Rel, but not p65 and RelB activation, strongly suggestive of a specific TH-17 polarizing function of dectin-2. Furthermore, the involvement of dectin-1 and detcin-2 in anti-fungal immunity by human DCs depends on the Candida species. Our data strongly suggest that dectin-2 is crucial in recognition of some pathogenic Candida species to boost dectin-1-induced TH-17 responses via Malt1. Thus, Malt1-dependent activation of c-Rel dictates adaptive TH-17 immunity to fungi by dectin-1 and dectin-2.
Protective immunity against fungal infections via TH-17 cellular responses requires the expression of IL-1β, IL-23 and IL-6 by DCs. Here we demonstrated that selective activation of NF-κB family member c-Rel by dectin-1 and dectin-2 signaling in response to fungi was essential to expression of IL-1β and IL-23 and consequently TH-17 immunity. Our data showed that loss of c-Rel binding to the Il1b and Il23p19 promoters strongly decreased IL-1β and IL-23p19 expression even though p65 bound to the NF-κB binding sites in the absence of c-Rel activation. These data further showed that c-Rel was the stronger activator of Il1b and Il23 transcription. Furthermore, c-Rel had an inhibitory effect on Il6 transcription, although p65-driven IL-6 expression allows for sufficient IL-6 to direct TH-17 polarization.
Both dectin-1 and dectin-2 triggering resulted in c-Rel activation and c-Rel-dependent IL-1β and IL-23 expression. Engagement of dectin-1 by fungal ligands leads to phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM)-like sequence within its cytoplasmic domain [15], [27] and subsequent association of the spleen tyrosine kinase Syk. Syk activation by dectin-1 is required for NF-κB activation via the assembly of the CARD9-Bcl10-Malt1 module [13], [14]. Unlike dectin-1, dectin-2 requires pairing with the adaptor molecule FcRγ to induce signaling [12], [20]. Dectin-2 triggering results in phosphorylation of the ITAM of FcRγ and activation of Syk signaling, which induces cytokine expression [20]. Dectin-2 signaling is CARD9-dependent, however a role for Bcl10 and Malt1 remains to be established [20]. In antigen receptor signaling, oligomerization of CARD11 (CARMA1) triggers the formation of a scaffold that physically bridges the CARD11-Bcl10-Malt1 complex with downstream signaling effectors, such as TRAFs and TAK1, to activate the NF-κB-regulating IKK complex [28]. Here we demonstrated that c-Rel activation by dectin-1 and dectin-2 is completely dependent on Malt1 activation. Malt1 is an unique protein as it is the only human paracaspase known [22], [23] and our data showed that its paracaspase activity was essential to the activation of c-Rel by dectin-1 and dectin-2. Malt1 has a distinctive function within the CARD9-Bcl10-Malt1 complex induced upon dectin-1 and dectin-2 triggering since silencing of CARD9 and Bcl10 by RNA interference completely abrogated the activation of all NF-κB subunits, while Malt1 silencing selectively abrogated c-Rel activation. It is unclear how Malt1 specifically activates c-Rel. A similar observation has been reported for B cell receptor signaling, which uses the CARD11-Bcl10-Malt1 complex for NF-κB activation [29], while Malt1 is involved in RelB activation after BAFF stimulation in specific B cell subsets [30]. In T cell receptor signaling, the paracaspase activity of Malt1 partially accounts for the amount of NF-κB activation [22], which might reflect the c-Rel-dependency in T cell receptor responses. Only two substrates for Malt1 are known, its binding partner Bcl10 and A20 that functions as an inhibitor of NF-κB activation [22], [23], but it remains to be determined if they have any role in the selective activation of c-Rel via Malt1. We showed that dectin-2 signaling only induced strong c-Rel activation, while dectin-1 triggering activated all NF-κB subunits; possibly the differential use of downstream molecules like TRAFs by dectin-1 and dectin-2 might underlie these differences in NF-κB activation.
Crosstalk between signaling pathways triggered by recognition of different PAMPs by various PRRs is essential to the induction of immune responses [5], [6], [11]. Here we demonstrated that dectin-1 and dectin-2 play distinct roles in immunity to fungi. While dectin-1 triggering induced cytokines involved in promoting both TH1 and TH-17 polarization, dectin-2 triggering resulted specifically in IL-1β and IL-23p19 expression, which enhanced IL-1β and IL-23 expression in response to different pathogenic Candida spp. This suggests that dectin-1 functions more broadly as an anti-fungal receptor inducing protective immunity, while dectin-2 is more specialized in boosting TH-17 cellular responses. Our data also demonstrated that even related pathogenic fungi triggered different sets of PRRs, likely contributing to tailoring of pathogen-specific immunity. C. albicans strain CBS8781 and C. lusitaniae induced cytokine expression in a dectin-1-dependent manner. In contrast, C. albicans strain CBS2712 and C. nivariensis triggered both dectin-1 and dectin-2 and showed higher IL-1β and IL-23 responses, strongly suggesting that dectin-1 and dectin-2 signaling pathways converge to enhance TH-17 immunity. Other Candida species might preferentially trigger dectin-2 but not dectin-1 for IL-1β and IL-23p19 protein expression as shown in the study of Saijo et al. [19]. Notably, C. albicans CBS2712 also induced dectin-1 - and dectin-2-independent expression of IL-6, IL-12p35 and IL-12p40. The contribution of TLR signaling, especially via TLR2, and collaboration with dectin-1 signaling has previously been recognized in cytokine responses in Candida infections [11], [31]. However, C-type lectin triggering seems to be more specialized in IL-23p19 and IL-1β induction. The situation in mice might be more complex as murine TLRs seem to induce c-Rel activation [32], while human TLRs do not [33]. Our data emphasize that immune responses are tailored not only to pathogens from different species but even within species. Thus, interpretation of data obtained with a single pathogen should be done with caution. Research into the role of dectin-1 in fungal infections using knock-out mice has resulted in conflicting data [34], [35] and the use of different yet related fungi might underlie these differences. Genetic variation within the Candida clade might not only account for differences in pathogenicity [36] but also for the differential recognition by innate receptors. We have demonstrated here that even closely related C. albicans strains trigger different sets of PRRs to activate adaptive immune responses.
Malt1-mediated c-Rel activation might be a general mechanism for induction of protective TH-17 immunity against fungi and other microbes, since the Card9-Bcl10-Malt1 complex might couple other C-type lectins besides dectin-1 and dectin-2 to NF-κB activation. Furthermore, the carbohydrate specificities of dectin-1 and dectin-2 for β-glucans and high mannoses, respectively, signify their importance in more general anti-fungal immunity against species from the phylum Ascomycota that contain mannan, chitin and glucan structures in their cell-wall [37], [38]. Many pathogenic ascomycetes such as Candida spp., Aspergillus spp., Coccidiodides spp., Pneumocystis jirovecii (previously known as Pneumocystis carinii), Histoplasma capsulatum, Trichophyton rubrum and Microsporum audouinni have been identified as dectin-1 and/or dectin-2 ligands [12], [20], [34], [35], [39]–[41]. In contrast, the cell-wall of fungi from the phylum Basidiomycota, such as Cryptococcus and Malassezia spp, differs from that of ascomycetes, as it is enfolded in a glucuronic acid-rich carbohydrate capsule or consists of lipophilic structures, respectively [42], [43]. TH-17 responses to Cr. neoformans have been reported [44] but are not mediated by dectin-1 [45].
Thus, dectin-1 and dectin-2 control c-Rel activation distinctively via Malt1 activation to induce IL-1β and IL-23 expression and as such tailor TH-17 immune responses against fungal pathogens. Given the pivotal role of TH-17 responses not only in protective immunity against fungi but also in the pathology of human autoimmune diseases like Crohn's disease, ulcerative colitis, psoriasis and in vaccine development against tuberculosis [3], [46], our results might benefit therapeutic developments as Malt1 presents a rational target for immunomodulatory drugs.
Materials and Methods
Cells, stimulation, inhibition and RNA interference
This study was performed in accordance with the ethical guidelines of the Academic Medical Center. Immature DCs (iDC, day 6 and 7) were generated as described previously [10]. DCs were stimulated with 10 µg/ml curdlan (Sigma), heat-killed Candida spp. [47] (multiplicity of infection (MOI) 10) and 10 ng/ml LPS from Salmonella typhosa (Sigma). Dectin-2 triggering was induced by pre-incubating DCs for 2 h at room temperature with 5 µg/ml anti-dectin-2 (MAB3114; R&D Systems), followed by crosslinking on goat-anti-mouse IgG (115-006-0710; Jackson)-coated culture plates. Cells were preincubated with blocking antibodies or inhibitor for 2 h with 20 µg/ml anti-dectin-1 (MAB1859; R&D Systems), 20 µg/ml anti-dectin-2 (MAB3114; R&D Systems) or 75 µM z-VRPR-FMK (Malt1 inhibitor [22]; Alexis). DCs were transfected with 25 nM siRNA using transfection reagent DF4 (Dharmacon), and used for experiments 72 h after transfection. ‘SMARTpool’ siRNAs used were: Syk (M-003176-03), CARD9 (M-004400-01), Bcl10 (M-004381-02), Malt1 (M-005936-02), c-Rel (M-004768-01) and non-targeting siRNA (D-001206-13) as a control (Dharmacon). This protocol resulted in nearly 100% transfection efficiency as determined by flow cytometry of cells transfected with siGLO-RISC free-siRNA (D-001600-01) and did not induce IFN responses as determined by quantitative real-time PCR analysis [10]. Silencing of expression was verified by real-time PCR and flow cytometry (Figure S1).
Quantitative real-time PCR
mRNA isolation, cDNA synthesis and PCR amplification with the SYBR green method in an ABI 7500 Fast PCR detection system (Applied Biosystems) were performed as described [10]. Specific primers were designed using Primer Express 2.0 (Applied Biosystems; Table S1). The Ct value is defined as the number of PCR cycles where the fluorescence signal exceeds the detection threshold value. For each sample, the normalized amount of target mRNA was calculated from the obtained Ct values for both target and GAPDH mRNA with Nt = 2Ct(GAPDH)−Ct(target). The relative mRNA expression was obtained by setting Nt in curdlan - or LPS-stimulated samples at 1 within one experiment and for each donor.
Cytokine production
Cell culture supernatants were harvested after 28 h of stimulation and concentrations of IL-1β, IL-23, IL-6 (Invitrogen) and IL-12p70 (eBioscience) were determined by ELISA.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using the ChIP-IT Express Enzymatic kit (Active Motif) to determine occupancy of the regulatory regions of the Il1b, Il23p19, Il6, Il12a and Il12b promoters by NF-κB as described by the manufacturer. Protein/DNA complexes were immunoprecipitated using anti-p65 (3034; Cell Signaling), anti-c-Rel (4727; Cell Signaling), anti-RelB (4954; Cell Signaling) or negative control IgG (53010; Active Motif), and protein G-coated magnetic beads. DNA was purified after reversal of crosslinks and real-time PCR reactions were then performed with primer sets spanning the NF-κB binding sites (Table S1). Primers spanning genomic DNA at cytogenetic location 12 p13.3 (Active Motif) were used as a negative control. To normalize for DNA input, a sample for each condition was taken along which had not undergone immunoprecipitation with a specific antibody (‘input DNA’); the results are expressed as the % input DNA.
Immunofluorescence staining
Stainings were performed as described previously [10] with with anti-p65, anti-c-Rel or anti-RelB (all from Cell Signaling) followed by Alexa Fluor 594-conjugated goat anti-rabbit (A11072; Molecular Probes).
NF-κB DNA binding
Nuclear extracts of DCs were prepared using NucBuster protein extraction kit (Novagen) and NF-κB DNA binding determined using TransAM NF-κB family kit (Active Motif).
TH-17 polarization assay
Memory CD4+ T cells were isolated as described previously [7]. iDCs were preincubated for 2 h with inhibitors, activated for 16 h with curdlan or heat-killed Candida spp. and subsequently co-cultured with memory CD4+ T cells as described (20,000 T cells/2000 DCs in the presence of 10 pg/ml Staphylococcus aureus enterotoxin B (Sigma) [7]. After 5 days of co-culture, supernatants were harvested and analyzed for IL-17 production by ELISA (Biosource). Cells were further cultured in the presence of 10 U/ml IL-2 (Chiron) and resting cells were restimulated after 12 days with 100 ng/ml PMA (Sigma) and 1 µg/ml ionomycin (Sigma) for 6 h, the last 5 h in the presence of 10 µg/ml brefeldin A (Sigma), and analyzed for intracellular cytokine expression by staining with biotinylated mouse anti-IL-17 (ebio64DE; eBioscience), followed by incubation with streptavidin-PE (BD Pharmingen) and FITC-conjugated mouse anti-IFN-γ (25723.11; BD).
Statistical analysis
Student's t-test for paired observations was used for statistical analyses. Statistical significance was set at a P values of less than 0.05.
Supporting Information
Zdroje
1. RomaniL
2008 Cell mediated immunity to fungi: a reassessment. Med Mycol 46 515 529
2. OuyangW
KollsJK
ZhengY
2008 The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28 454 467
3. LoutenJ
BonifaceK
de Waal MalefytR
2009 Development and function of TH17 cells in health and disease. J Allergy Clin Immunol 123 1004 1011
4. RomaniL
2004 Immunity to fungal infections. Nat Rev Immunol 4 11 23
5. GeijtenbeekTBH
GringhuisSI
2009 Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 9 465 479
6. MedzhitovR
2007 Recognition of microorganisms and activation of the immune response. Nature 449 819 826
7. van BeelenAJ
ZelinkovaZ
Taanman-KueterEW
MullerFJ
HommesDW
2007 Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27 660 669
8. WeaverCT
HattonRD
2009 Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol 9 883 889
9. MurphyKM
ReinerSL
2002 The lineage decisions of helper T cells. Nat Rev Immunol 2 933 944
10. GringhuisSI
den DunnenJ
LitjensM
van der VlistM
WeversB
2009 Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-[kappa]B activation through Raf-1 and Syk. Nat Immunol 10 203 213
11. NeteaMG
BrownGD
KullbergBJ
GowNAR
2008 An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Micro 6 67 78
12. SatoK
YangXL
YudateT
ChungJS
WuJ
2006 Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem 281 38854 38866
13. GrossO
GewiesA
FingerK
SchaferM
SparwasserT
2006 Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442 651 656
14. LeibundGut-LandmannS
GrossO
RobinsonMJ
OsorioF
SlackEC
2007 Syk - and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8 630 638
15. RogersNC
SlackEC
EdwardsAD
NolteMA
SchulzO
2005 Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22 507 517
16. GlockerEO
HennigsA
NabaviM
SchafferAA
WoellnerC
2009 A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361 1727 1735
17. FerwerdaB
FerwerdaG
PlantingaTS
WillmentJA
van SprielAB
2009 Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361 1760 1767
18. McGrealEP
RosasM
BrownGD
ZamzeS
WongSY
2006 The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16 422 430
19. SaijoS
IkedaS
YamabeK
KakutaS
IshigameH
2010 Dectin-2 Recognition of [alpha]-Mannans and Induction of Th17 Cell Differentiation Is Essential for Host Defense against Candida albicans. Immunity 32 681 691
20. RobinsonMJ
OsorioF
RosasM
FreitasRP
SchweighofferE
2009 Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 206 2037 2051
21. HaydenMS
GhoshS
2008 Shared principles in NF-[kappa]B signaling. Cell 132 344 362
22. RebeaudF
HailfingerS
Posevitz-FejfarA
TapernouxM
MoserR
2008 The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat Immunol 9 272 281
23. CoornaertB
BaensM
HeyninckK
BekaertT
HaegmanM
2008 T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-[kappa]B inhibitor A20. Nat Immunol 9 263 271
24. ButlerG
RasmussenMD
LinMF
SantosMA
SakthikumarS
2009 Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459 657 662
25. PfallerMA
DiekemaDJ
2007 Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20 133 163
26. BormanAM
PetchR
LintonCJ
PalmerMD
BridgePD
2008 Candida nivariensis, an emerging pathogenic fungus with multidrug resistance to antifungal agents. J Clin Microbiol 46 933 938
27. UnderhillDM
RossnagleE
LowellCA
SimmonsRM
2005 Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106 2543 2550
28. RawlingsDJ
SommerK
Moreno-GarciaME
2006 The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat Rev Immunol 6 799 812
29. FerchU
BuschenfeldeCMz
GewiesA
WegenerE
RauserS
2007 MALT1 directs B cell receptor-induced canonical nuclear factor-[kappa]B signaling selectively to the c-Rel subunit. Nat Immunol 8 984 991
30. TuscheMW
WardLA
VuF
McCarthyD
Quintela-FandinoM
2009 Differential requirement of MALT1 for BAFF-induced outcomes in B cell subsets. J Exp Med 206 2671 2683
31. GantnerBN
SimmonsRM
CanaveraSJ
AkiraS
UnderhillDM
2003 Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197 1107 1117
32. SaccaniS
PantanoS
NatoliG
2003 Modulation of NF-kappaB activity by exchange of dimers. Mol Cell 11 1563 74
33. GringhuisSI
den DunnenJ
LitjensM
HofBV
van KooykY
2007 C-type lectin DC-SIGN modulates toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappa B. Immunity 26 605 616
34. SaijoS
FujikadoN
FurutaT
ChungSH
KotakiH
2007 Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 8 39 46
35. TaylorPR
TsoniSV
WillmentJA
DennehyKM
RosasM
2007 Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8 31 38
36. Hertz-FowlerC
PainA
2009 Unity in diversity: lessons from Candida. Nat Rev Micro 7 763
37. ChaffinWL
Lopez-RibotJL
CasanovaM
GozalboD
MartinezJP
1998 Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev 62 130 180
38. BernardM
LatgeJP
2001 Aspergillus fumigatus cell wall: composition and biosynthesis. Med Mycol 39 Suppl 1 9 17
39. HohlTM
Van EppsHL
RiveraA
MorganLA
ChenPL
2005 Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog 1 e30
40. SorgiCA
SecattoA
FontanariC
TuratoWM
BelangerC
2009 Histoplasma capsulatum cell wall {beta}-glucan induces lipid body formation through CD18, TLR2, and Dectin-1 receptors: correlation with leukotriene B4 generation and Role in HIV-1 Infection. J Immunol 182 4025 4035
41. ViriyakosolS
FiererJ
BrownGD
KirklandTN
2005 Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and dectin-1. Infect Immun 73 1553 1560
42. AshbeeHR
EvansEG
2002 Immunology of diseases associated with Malassezia species. Clin Microbiol Rev 15 21 57
43. BoseI
ReeseAJ
OryJJ
JanbonG
DoeringTL
2003 A Yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot Cell 2 655 663
44. KleinschekMA
MullerU
BrodieSJ
StenzelW
KohlerG
2006 IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol 176 1098 1106
45. NakamuraK
KinjoT
SaijoS
MiyazatoA
AdachiY
2007 Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol Immunol 51 1115 1119
46. CooperAM
KhaderSA
2008 The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev 226 191 204
47. de JongMA
VriendLE
TheelenB
TaylorME
FluitsmaD
2010 C-type lectin Langerin is a beta-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol Immunol 47 1216 1225
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Salivary Gland NK Cells Are Phenotypically and Functionally Unique
- Genetic Epidemiology of Tuberculosis Susceptibility: Impact of Study Design
- Early Target Cells of Measles Virus after Aerosol Infection of Non-Human Primates
- Multiple Plant Surface Signals are Sensed by Different Mechanisms in the Rice Blast Fungus for Appressorium Formation
- Biofilm Development on by Is Facilitated by Quorum Sensing-Dependent Repression of Type III Secretion
- Distinct Patterns of IFITM-Mediated Restriction of Filoviruses, SARS Coronavirus, and Influenza A Virus
- Molecular Basis of Increased Serum Resistance among Pulmonary Isolates of Non-typeable
- A Helminth Immunomodulator Exploits Host Signaling Events to Regulate Cytokine Production in Macrophages
- HCMV Spread and Cell Tropism are Determined by Distinct Virus Populations
- Characteristics of the Earliest Cross-Neutralizing Antibody Response to HIV-1
- Induction of a Peptide with Activity against a Broad Spectrum of Pathogens in the Salivary Gland, following Infection with Dengue Virus
- Structural Basis for the Recognition of Cellular mRNA Export Factor REF by Herpes Viral Proteins HSV-1 ICP27 and HVS ORF57
- Identification and Characterization of the Host Protein DNAJC14 as a Broadly Active Flavivirus Replication Modulator
- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of
- Selective C-Rel Activation via Malt1 Controls Anti-Fungal T-17 Immunity by Dectin-1 and Dectin-2
- Imaging Single Retrovirus Entry through Alternative Receptor Isoforms and Intermediates of Virus-Endosome Fusion
- Aerosols Transmit Prions to Immunocompetent and Immunodeficient Mice
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



