-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Communal Bacterial Adhesin Anchors Biofilm and Bystander Cells to Surfaces
While the exopolysaccharide component of the biofilm matrix has been intensively studied, much less is known about matrix-associated proteins. To better understand the role of these proteins, we undertook a proteomic analysis of the V. cholerae biofilm matrix. Here we show that the two matrix-associated proteins, Bap1 and RbmA, perform distinct roles in the biofilm matrix. RbmA strengthens intercellular attachments. In contrast, Bap1 is concentrated on surfaces where it serves to anchor the biofilm and recruit cells not yet committed to the sessile lifestyle. This is the first example of a biofilm-derived, communally synthesized conditioning film that stabilizes the association of multilayer biofilms with a surface and facilitates recruitment of planktonic bystanders to the substratum. These studies define a novel paradigm for spatial and functional differentiation of proteins in the biofilm matrix and provide evidence for bacterial cooperation in maintenance and expansion of the multilayer biofilm.
Published in the journal: A Communal Bacterial Adhesin Anchors Biofilm and Bystander Cells to Surfaces. PLoS Pathog 7(8): e32767. doi:10.1371/journal.ppat.1002210
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002210Summary
While the exopolysaccharide component of the biofilm matrix has been intensively studied, much less is known about matrix-associated proteins. To better understand the role of these proteins, we undertook a proteomic analysis of the V. cholerae biofilm matrix. Here we show that the two matrix-associated proteins, Bap1 and RbmA, perform distinct roles in the biofilm matrix. RbmA strengthens intercellular attachments. In contrast, Bap1 is concentrated on surfaces where it serves to anchor the biofilm and recruit cells not yet committed to the sessile lifestyle. This is the first example of a biofilm-derived, communally synthesized conditioning film that stabilizes the association of multilayer biofilms with a surface and facilitates recruitment of planktonic bystanders to the substratum. These studies define a novel paradigm for spatial and functional differentiation of proteins in the biofilm matrix and provide evidence for bacterial cooperation in maintenance and expansion of the multilayer biofilm.
Introduction
Bacterial biofilm formation is the process by which bacteria attach to abiotic surfaces, the surfaces of other unicellular organisms, the epithelia of multicellular organisms, and interfaces such as that between air and water. Surface adhesion enables bacteria to arrange themselves favorably in their environment and, therefore, is critical to environmental adaptation and survival.
Surface-attached bacteria may form either a single layered structure, known as a monolayer, or a multilayer biofilm [1]. Bacterial cells join monolayer and multilayer biofilms in response to distinct environmental signals, use distinct structures for adhesion in these two biofilms, and develop distinct transcriptional profiles within these two structures [2], [3]. However, the critical difference between these two types of biofilms is the extracellular matrix that surrounds cells in the multilayer biofilm. This matrix is comprised of biological polymers such as exopolysaccharide, protein, and DNA [4]. The matrix not only mediates bacterial aggregation and surface attachment but may also serve as a reservoir for extracellular, degradative enzymes and the nutrients released by their function. Therefore, the multilayer biofilm affords the bacterium advantages that monolayer biofilm does not.
Vibrio cholerae is a halophilic Gram-negative bacterium that causes the severe diarrheal disease cholera. V. cholerae makes two types of multilayer biofilms. One is dependent on environmental Ca2+ concentrations comparable to those found in seawater, while the other is dependent on the synthesis of an exopolysaccharide termed VPS [2], [5], [6], [7]. The genes required to synthesize VPS are primarily found in two large operons within the VPS island, one of which encodes the proteins VpsA through VpsK and the other of which encodes VpsL through VpsQ [8]. Transcription of these operons is controlled by a complex regulatory network, suggesting that the ability to limit biofilm matrix synthesis to a highly specific environmental niche confers a survival advantage [9], [10], [11], [12], [13], [14], [15], [16], [17].
While most studies suggest that the VPS-dependent V. cholerae biofilm is not important for colonization of the human intestine [18], [19], [20], this biofilm may be important for environmental persistence. Surface-attached V. cholerae predominate in the environment [21], [22]. Multiple avenues of evidence suggest that the chitinaceous surfaces of arthropods are an important substratum for V. cholerae biofilm formation [23], [24], [25], [26], [27]. Furthermore, V. cholerae is especially well adapted to life on chitin because of its many chitinolytic enzymes, the marked modulation of its transcriptome by the degradation products of chitin [28], and activation of its natural competence by chitin [28], [29], [30], [31].
Our laboratory and others have identified several environmental signals that activate VPS-dependent V. cholerae biofilm formation [2], [5], [12], [32], [33], [34], [35]. Among these are sugars transported by the phosphoenolpyruvate phosphotransferase system or PTS. Chitobiose and N-acetylglucosamine, which are degradation products of chitinaceous surfaces of arthropods, are transported exclusively by the PTS [36], [37]. Therefore, in the aquatic environment, association with arthropods is likely correlated with formation of a VPS-dependent biofilm.
To gain insight into the role of biofilm matrix-associated proteins in V. cholerae surface attachment, we set out to define the proteome of the V. cholerae biofilm matrix. Here, we present evidence that the biofilm matrix selectively retains secreted proteins. Furthermore, we show that RbmA and Bap1, two proteins of previously unknown function [3], [38], [39], [40], are present in the biofilm matrix. While RbmA functions similarly to previously identified biofilm matrix proteins in that it strengthens intercellular interactions [41], [42], [43], Bap1, which is jointly synthesized by biofilm-associated bacteria, is concentrated at the base of the biofilm where it reinforces the association of the biofilm with the surface and accelerates attachment of bystander bacteria not yet primed for biofilm matrix synthesis. These studies present evidence for specialization of proteins in the bacterial biofilm matrix and for bacterial cooperation in maintaining and expanding surface-associated biofilms.
Results
Identification of biofilm matrix-associated proteins
Biofilm matrix proteins were isolated by a variety of methods. Briefly, biofilms were disrupted by vortexing in the presence or absence of 1 mm glass beads. Furthermore, biotinylation of extracellular proteins prior to biofilm disruption and subsequent neutravidin affinity purification were used to enrich for extracellular proteins. The protein mixtures prepared by these methods were analyzed by MS/MS. We then used in silico methods (Genome Atlas) to predict the subcellular localization of identified proteins. As shown in Figure S1, the proportion of recovered proteins that were predicted to be extracytoplasmic increased with biotinylation. Gentler methods of biofilm disruption also resulted in isolation of a larger proportion of predicted extracytoplasmic proteins. However, the most gentle disruption methods yielded fewer proteins overall and, therefore, a smaller number of secreted proteins.
The 74 predicted extracytoplasmic proteins identified by these methods are listed in Table S1. Based on either known function or bioinformatics, we predicted that 10 of these proteins were secreted and, therefore, were candidate biofilm matrix-associated proteins (Table 1). In addition, 17 of these proteins were located in the outer membrane (OM), and 26 of these proteins were located in the periplasm. The location of 18 proteins could not be predicted with certainty (Table 2). Citrate synthase (VC2092) and a putative acetyl CoA synthase homolog (VCA0139), which were predicted to have transmembrane domains, are most likely in the inner membrane. No additional inner membrane proteins were identified. NusA (VC0642), a transcription elongation factor we identified in the proteomic analysis, was predicted to be secreted. However, because of its function, we hypothesize that it is cytoplasmic.
Tab. 1. Secreted proteins identified in preparations of biofilm matrix. 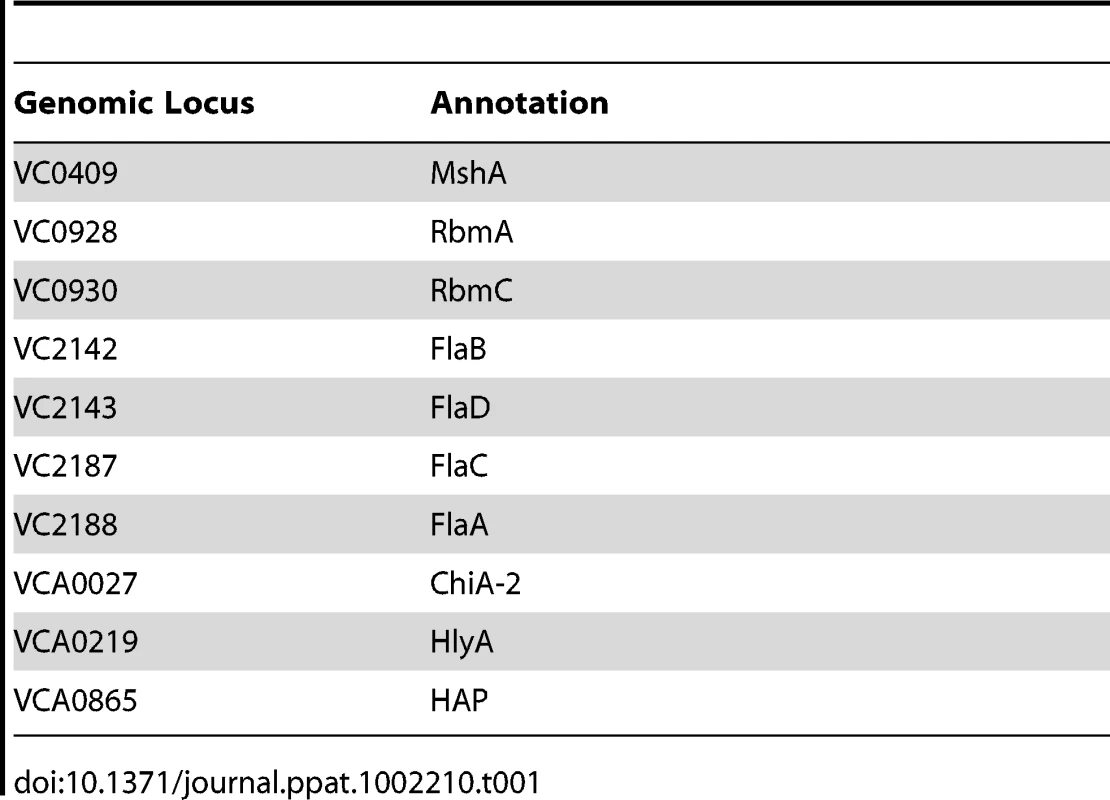
Tab. 2. Extracytoplasmic proteins of unknown location. 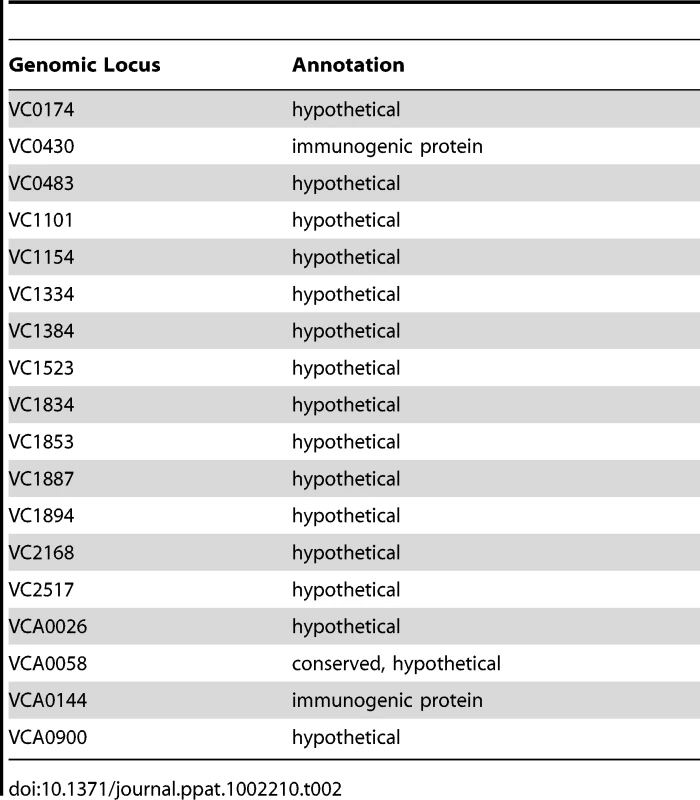
Secreted proteins identified in our analysis included those forming bacterial appendages such as the mannose-sensitive hemagglutinin type IV pilus (MshA) and the flagellum as well as RbmA and RbmC, two proteins of unknown function that alter biofilm formation and are co-regulated with the VPS synthesis genes [3], [38], [39], [40]. Three proteins not previously associated with biofilms were also identified, namely a hemolysin (HlyA, VCA0219), a chitinase (VCA0027), and the hemagglutinin/protease (HAP; VCA0865).
We hypothesized that cell-associated proteins should be similarly represented in our analyses if they represented residual cellular material contaminating the biofilm matrix preparation. In fact, while 35% of the periplasmic and OM proteins identified were found in three or more biofilm matrix preparations, only 7% of all predicted cytoplasmic proteins were identified in 3 or more preparations. Furthermore, only two putative inner membrane proteins were identified. One possibility is that a common step in the purification process resulted in formation of spheroplasts, releasing outer membrane and periplasmic proteins into the supernatant during the purification process. Another possibility is that these OM and periplasmic proteins signify the presence of outer membrane vesicles in the biofilm matrix.
Bap1 and RbmC perform redundant functions in V. cholerae biofilm formation
RbmC (957 aa), which was identified in our proteomic analysis, and its homolog Bap1 (691 aa) play uncharacterized and redundant roles in the observed colony morphology and biofilm phenotype of rugose V. cholerae variants [39]. The central portions of these proteins are 54% identical and 70% similar and include an EF hand domain, which is predicted to bind Ca2+, and a β-prism lectin-like domain surrounded by six FG-GAP domains (Figure 1A). RbmC is longer than Bap1 due to two N-terminal domains of unknown function that are also found in the E. coli mucinase StcE and a second C-terminal β-prism domain [44], [45].
We first confirmed that these proteins also serve redundant roles in biofilm formation in our V. cholerae strain MO10, which has a smooth rather than rugose colony morphology. As shown in Figure 1B, Δbap1 and ΔrbmC mutants formed a biofilm, while the double mutant did not. The biofilm defect of the Δbap1ΔrbmC mutant could be rescued by a plasmid encoding a wild-type allele of either Bap1 or RbmC (Figure S2). An rbmC allele with a truncation of the C-terminal β-prism domain not found in Bap1 (RbmC-C140) also rescued the biofilm defect of the Δbap1ΔrbmC mutant (Figure S2). These results suggest that, as previously noted for a rugose variant of V. cholerae, Bap1 and RbmC perform redundant functions in the V. cholerae biofilm. Furthermore, Bap1 represents the minimal protein required to rescue the Δbap1ΔrbmC mutant phenotype.
Fig. 1. Bap1 and RbmC perform redundant functions in biofilm formation. 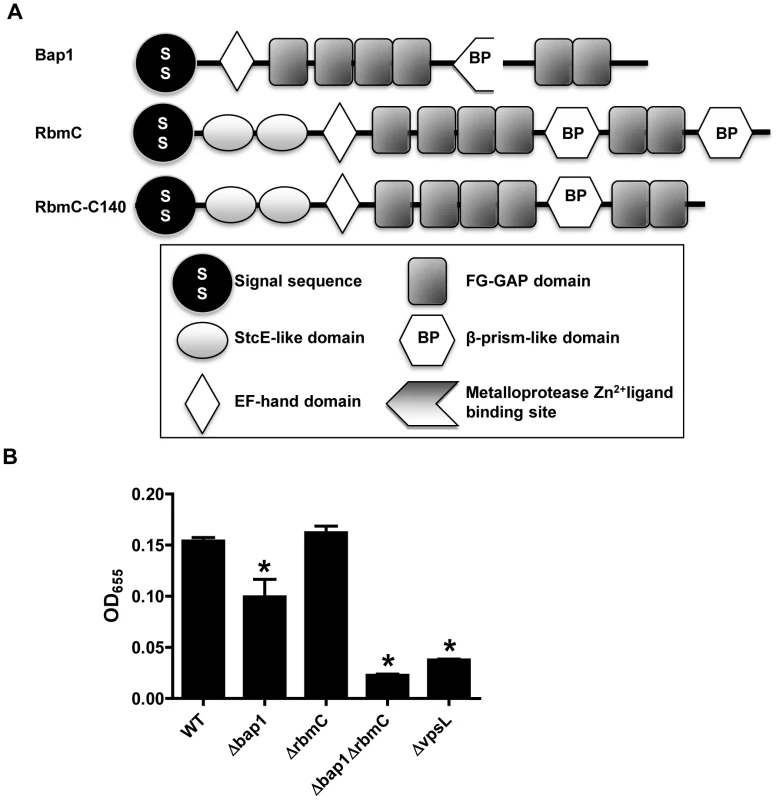
(A) Domain analysis of Bap1 and RbmC. Bap1 consists of a signal sequence, an EF hand domain, and a β-prism lectin domain surrounded by six FG-GAP domains. RbmC has two additional StcE-like domains at the N-terminus and an additional β-prism domain at the C-terminus. (B) Quantification of biofilms formed by wild-type V. cholerae (WT), a ΔvpsL mutant, a Δbap1 mutant, a ΔrbmC mutant, and a Δbap1ΔrbmC mutant. * indicate values that are statistically significantly different from wild-type. A subset of candidate biofilm matrix-associated proteins are visualized in the biofilm matrix
Our proteomic analysis identified ten candidate matrix-associated proteins (Table 1). ChiA-2, MshA, Bap1, RbmA, and the hemolysin HlyA were selected for further study. To determine whether these proteins were secreted by V. cholerae, the gene encoding each of these proteins was cloned between an inducible promoter and a C-terminal FLAG tag. As negative controls, we also cloned EIIAGlc (VC0964), a cytoplasmic component of the phosphoenolpyruvate phosphotransferase system, as well as Escherichia coli alkaline phosphatase (AP) and TcpG (VC0034), two periplasmic proteins. Each of these plasmids was introduced into V. cholerae. After culture in LB broth, the cells and supernatant were separated by centrifugation, and the presence of the tagged protein in each fraction was assessed by Western analysis (Figure 2A). The negative controls EllAGlc, TcpG, and AP were found in the cell pellet only. The secreted proteins chosen for further study were all found in the supernatant to varying degrees.
Fig. 2. Secreted proteins identified in proteomic analyses are retained in the biofilm matrix. 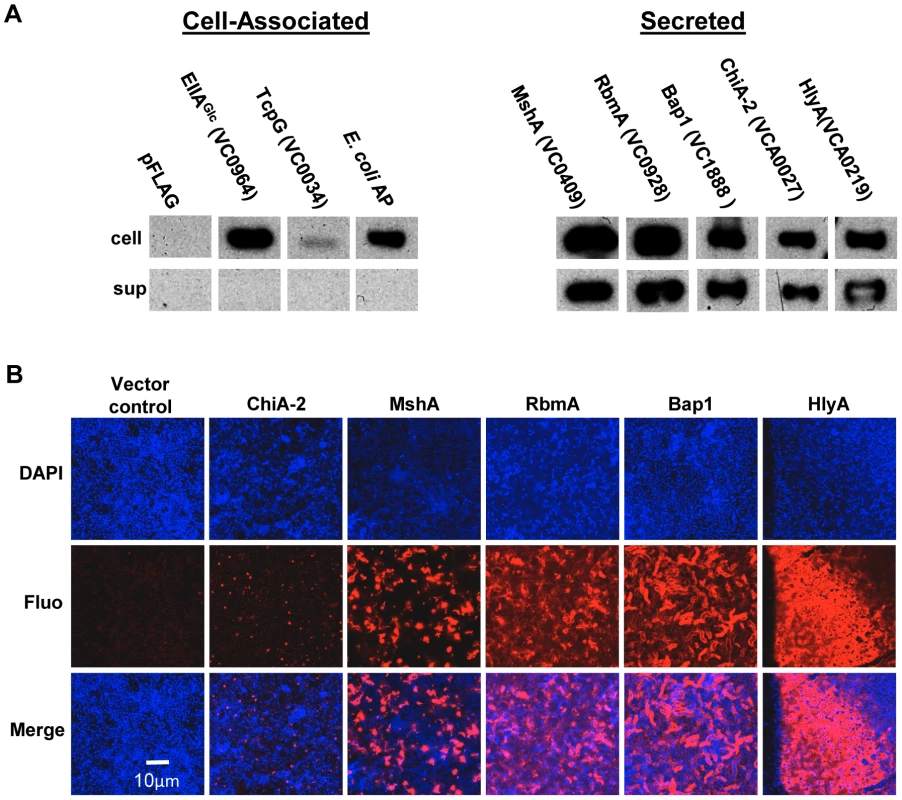
(A) Western blot of cell pellet and supernatant fractions for wild-type V. cholerae with an empty vector or a vector encoding a FLAG-tagged protein as labeled. (B) Transverse sections at the level of the substratum through biofilms containing FLAG-tagged proteins as noted. Proteins were visualized by immunofluorescent staining. To determine if these secreted proteins were retained in the biofilm matrix, we formed biofilms with wild-type V. cholerae constitutively expressing affinity-tagged versions of each of these proteins. Biofilms were rinsed, and immunofluorescence was used to visualize the affinity-tagged proteins in the biofilm matrix. No fluorescence was observed for biofilms formed by strains carrying plasmids encoding the proteins EllAGlc, TcpG, or AP (data not shown). As expected, the pilus-forming protein MshA was visualized in the biofilm matrix. In addition, RbmA, Bap1, and HlyA were observed in the biofilm matrix. Although comparable amounts of ChiA-2 were secreted, much less was observed in the biofilm matrix (Figure 2B). This suggests that RbmA, Bap1, and HlyA are selectively retained in the biofilm, while ChiA-2 does not associate strongly with the biofilm matrix.
Bap1 and RbmA have distinct distributions in the biofilm matrix
Bap1 and RbmA were previously found to alter biofilm formation [3], [38], [40]. Therefore, we hypothesized that their role in biofilm formation might be a structural one. To compare the native distributions of Bap1 and RbmA in the biofilm, we fused a FLAG tag to the C-terminal end of Bap1 and RbmA on the chromosome and visualized these tagged proteins in the biofilm by immunofluorescence. As shown in Figure 3A, RbmA was evenly distributed in the vertical dimension, while Bap1 was concentrated at the base of the biofilm. To objectively assess this difference, we measured the total fluorescence intensity in each transverse section. For each biofilm, this measurement was normalized to the transverse section with maximum fluorescence intensity and plotted as a function of distance from the substratum. As shown in Figure 3B, these measurements confirmed that Bap1 was concentrated at the biofilm-surface interface.
Fig. 3. Vertical distribution of Bap1 and RbmA in a native V. cholerae biofilm. 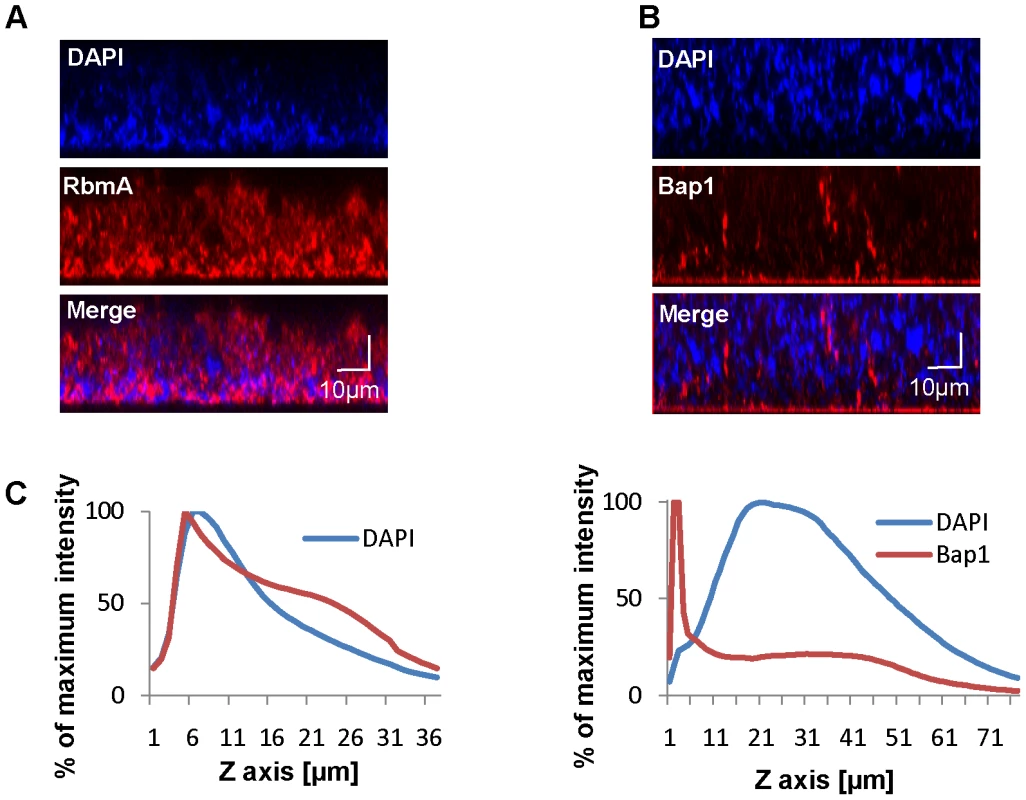
Immunofluorescent imaging of the vertical distribution of (A) Bap1-FLAG and (B) RbmA-FLAG in a native V. cholerae biofilm. Strains harbored a FLAG tag fused to the protein of interest at its chromosomal location. Bacterial DNA was stained with DAPI. (C) Quantification of the fluorescent intensity reflecting Bap1 and RbmA abundance as a function of distance from the substratum. To determine whether the distinct vertical distributions of Bap1 and RbmA in the biofilm were the result of spatially heterogeneous transcription of bap1 and rbmA, we formed a biofilm with wild-type V. cholerae constitutively expressing Bap1-FLAG or RbmA-6XHis from a plasmid. As shown in Figure 4, the vertical distribution of Bap1 and RbmA in these biofilms was similar to that in biofilms expressing Bap1 or RbmA from their respective native promoters. However, with constitutive expression, more Bap1 was observed within the biofilm, most likely due to increased levels of protein. Taken together, our data suggest that the vertical distributions of Bap1 and RbmA in the biofilm are not the result of heterogeneous transcription of bap1 and rbmA within the biofilm. Rather, we hypothesize that Bap1 migrates to the biofilm-substratum interface after secretion from the cell.
Fig. 4. Vertical distribution of constitutively expressed Bap1 and RbmA in the V. cholerae biofilm. 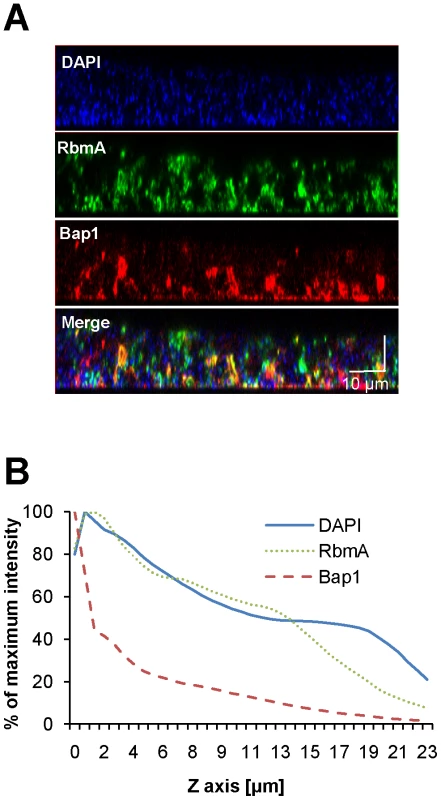
(A) Vertical section through a biofilm made by co-culturing wild-type V. cholerae carrying either a plasmid encoding Bap1-FLAG or a plasmid encoding RbmA-6X-His. Bacterial DNA was stained with DAPI, and Bap1-FLAG and RbmA-6X-His were visualized with FLAG specific and 6X-His specific antibodies, respectively. (B) Quantification of the total fluorescence due to DAPI, Bap1-FLAG or RbmA-6X-His as a function of biofilm height. Fluorescence in each section was normalized to the maximum fluorescence intensity for that biofilm. Vertical sections and quantifications of fluorescence are representative of the three experimental replicates that were performed in parallel. To assess the transverse distribution of Bap1 and RbmA in the biofilm and the extent of co-localization of these two proteins, we combined equal numbers of a Δbap1 mutant expressing Bap1-FLAG from a plasmid and wild-type V. cholerae expressing RbmA-His from a plasmid. As shown in Figure 5, in transverse sections close to the substratum, Bap1 and RbmA were both distributed around the perimeter of cells, and some co-localization was observed. However, RbmA was more evenly distributed, while foci of increased intensity were observed for Bap1. Similar transverse distributions of each protein were observed in biofilms formed by a Δbap1 mutant expressing Bap1-FLAG from a plasmid alone and by a ΔrbmA mutant expressing RbmA-FLAG from a plasmid alone (data not shown). Based on these observations, we hypothesized that Bap1 might play a different role than RbmA in biofilm formation.
Fig. 5. Transverse distribution of constitutively expressed Bap1 and RbmA in the V. cholerae biofilm. 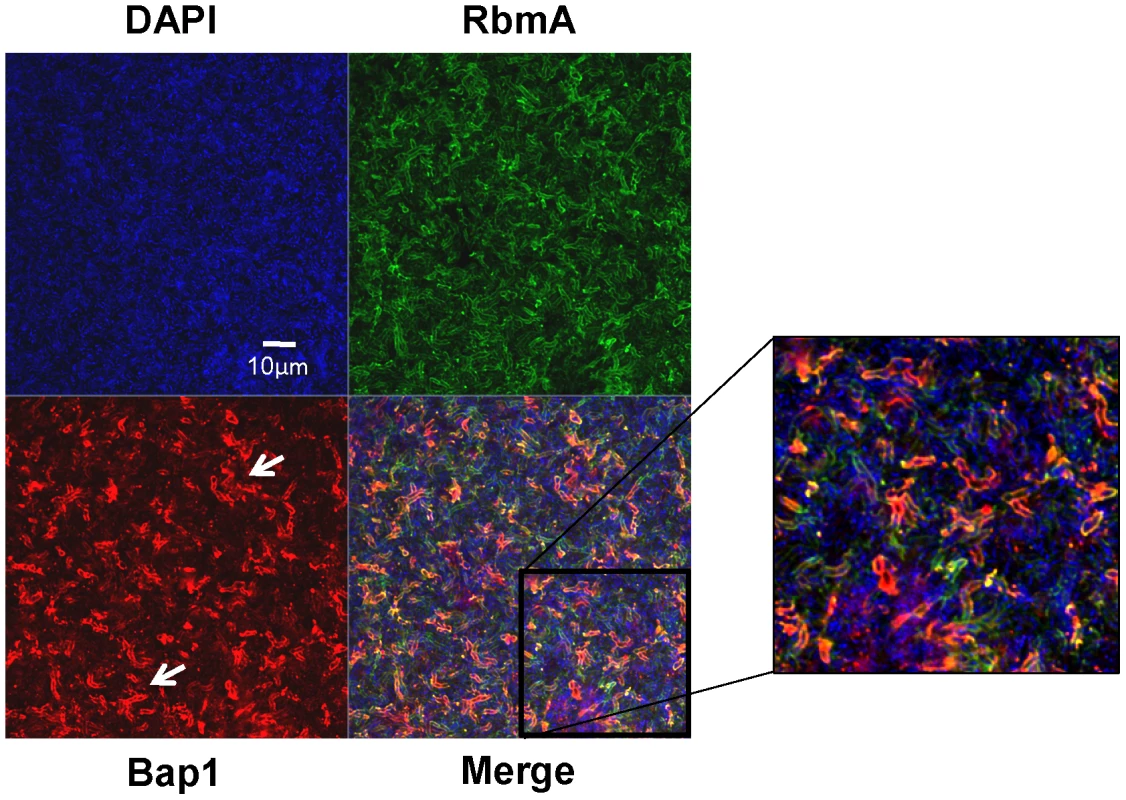
A transverse section at the level of the substratum of a biofilm made by co-culture of a Δbap1 mutant carrying a plasmid encoding Bap1-FLAG and wild-type V. cholerae carrying a plasmid encoding RbmA-6X-His. Bacterial DNA was stained with DAPI, and Bap1-FLAG and RbmA-6X-His were visualized by immunofluorescence. Horizontal sections are representative of the three experimental replicates that were performed in parallel. White arrows denote foci of Bap1 staining. Exogenously provided RbmA enhances intercellular interactions
RbmA alters biofilm stability but not overall biofilm accumulation of rugose variants of V. cholerae [38]. In V. cholerae O139 strain MO10, we observed that deletion of rbmA had a small, statistically insignificant effect on biofilm formation. Rescue of a ΔrbmA mutant with a wild-type rbmA allele produced a biofilm that was similar to that of wild-type V. cholerae but significantly increased as compared with the biofilm of the unrescued mutant (Figure 6A). Vortexing completely dispersed the ΔrbmA mutant biofilm, while larger biofilm fragments were observed after similar treatment of the wild-type V. cholerae biofilm (Figure 6C). This ΔrbmA mutant phenotype could be complemented by expression of a wild-type rbmA allele in trans.
Fig. 6. Purified RbmA-FLAG rescues a ΔrbmA mutant. 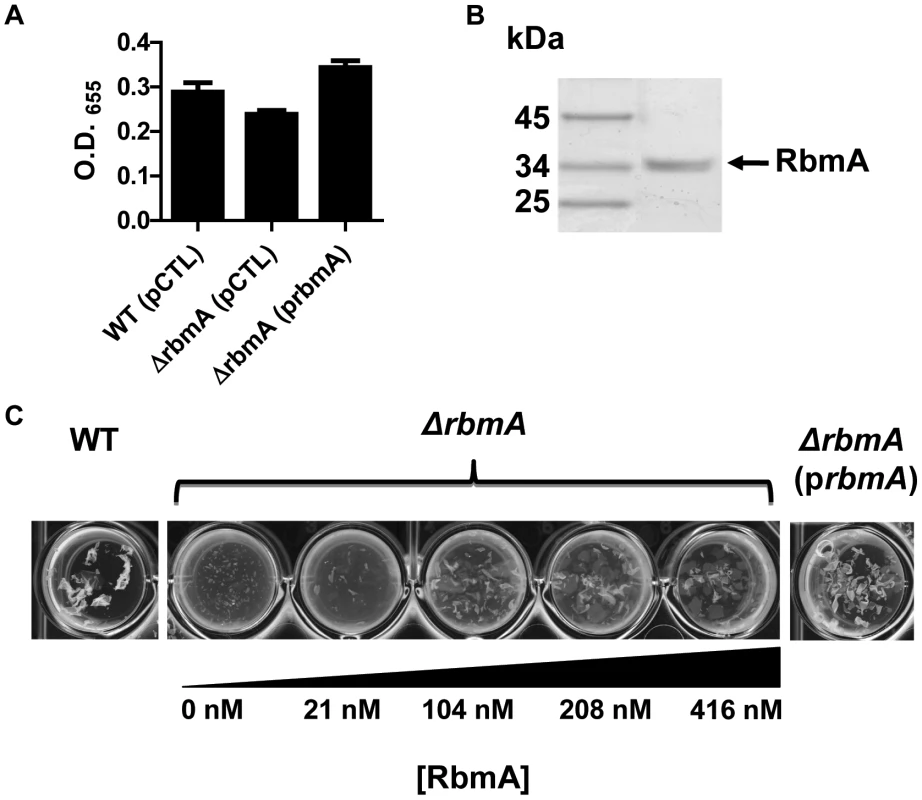
(A) Quantification of biofilms formed by wild-type V. cholerae or a ΔrbmA mutant carrying either an empty vector (pCTL) or a vector encoding a wild-type rbmA allele (prbmA). The means and standard deviations were calculated from three experimental replicates. While the biofilms formed by the ΔrbmA (pCTL) and the ΔrbmA (prbmA) strains were not significantly different from that formed by wild-type V. cholerae, the ΔrbmA(prbmA) biofilm was significantly greater than the ΔrbmA(pCTL) biofilm (p = 0.004). (B) SDS-PAGE analysis of purified RbmA-FLAG. Protein was visualized with Imperial stain. (C) Biofilms formed by a ΔrbmA mutant rescued with increasing amounts of purified RbmA-FLAG. Biofilms have been vortexed to illustrate fragmentation of the ΔrbmA mutant biofilm. We hypothesized that, if secreted RbmA were essential for biofilm integrity, exogenous RbmA should rescue the biofilm defect of a ΔrbmA mutant. To test this, we first affinity purified RbmA (Figure 6B). We then allowed the ΔrbmA mutant to form a biofilm in the presence of increasing amounts of purified RbmA. Purified RbmA was able to rescue the biofilm defect of the ΔrbmA mutant (Figure 6C). We determined that rescue required an RbmA concentration of approximately 416 nM. Assuming all molecules of RbmA are functional, this corresponds to approximately 260,000 molecules per mutant cell.
Bap1 is involved in surface adhesion
In a standard assay, the biofilm formed by a Δbap1ΔrbmC mutant was indistinguishable from that formed by a ΔvpsL mutant (Figure 1B). However, we noticed that, unlike the ΔvpsL mutant, the Δbap1ΔrbmC mutant formed a pellicle on the liquid surface after 24 hours of static growth. Interestingly, mutation of bap1 and rbmC in a rugose variant of V. cholerae was not noted to preserve pellicle formation [39]. One possible explanation for this discrepancy is that, due to a difference in the surface chemistries of smooth and rugose variants, rugose variants do not interact as strongly with the air-water interface in the absence of Bap1 and RbmC. As shown in Figure 7, the pellicle formed by the Δbap1ΔrbmC mutant was loosely associated with the glass surface. Gentle shaking dislodged the Δbap1ΔrbmC mutant pellicle from the substratum sending it to the bottom of the tube, while the wild-type pellicle remained attached. Furthermore, vortexing of the Δbap1ΔrbmC mutant pellicle caused it to fragment into many small pieces. However, these pieces were larger than those observed when a ΔrbmA biofilm was vortexed. These defects were rescued by a wild-type bap1 allele provided in trans but not by rbmA (Figure 7), again indicating that Bap1 and RbmA have distinct roles in biofilm formation.
Fig. 7. The Δbap1ΔrbmC mutant biofilm is loosely adherent to the substratum. 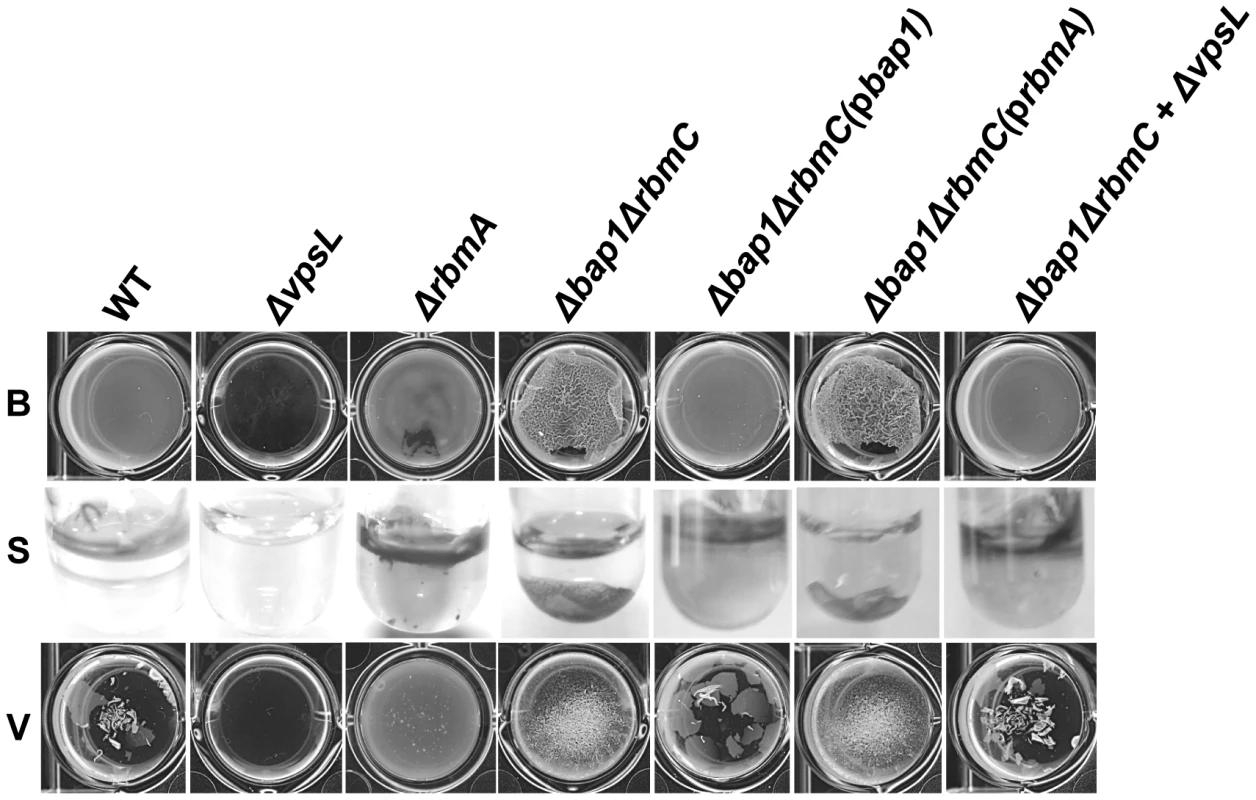
Pellicles formed by wild-type V. cholerae, a ΔvpsL mutant, a ΔrbmA mutant, a Δbap1ΔrbmC mutant, a Δbap1ΔrbmC mutant rescued with Bap1-FLAG or RbmA-FLAG expressed from a plasmid, and a Δbap1ΔrbmC mutant co-cultured with a ΔvpsL mutant. Pellicles were photographed without agitation (B), after gentle shaking (S), or after vortexing (V). We hypothesized that if secreted Bap1 were responsible for adhesion of the biofilm to the surface, exogenously provided Bap1 should also rescue the Δbap1ΔrbmC mutant biofilm defect. To test this prediction, we used affinity chromatography to purify Bap1-FLAG as shown in Figure 8A. A Δbap1ΔrbmC mutant incubated in the presence of purified Bap1 formed a biofilm that was comparable to that of a Δbap1ΔrbmC mutant rescued by Bap1 expressed from a plasmid (Figure 8B). To determine the concentration of Bap1 required to restore biofilm formation to the Δbap1ΔrbmC mutant, we titrated purified Bap1-FLAG into a Δbap1ΔrbmC mutant culture and measured biofilm formation after 24 hours. As shown in Figure 8C, an 8.8 nM solution of Bap1-FLAG was sufficient to restore surface attachment. Assuming all Bap1 molecules are functional, this corresponds to approximately 5,500 Bap1 molecules per bacterial cell. Therefore, approximately 47 times less Bap1 was required than RbmA to form a biofilm with properties similar to that of wild-type V. cholerae.
Fig. 8. Purified Bap1-FLAG restores biofilm formation to a Δbap1ΔrbmC mutant. 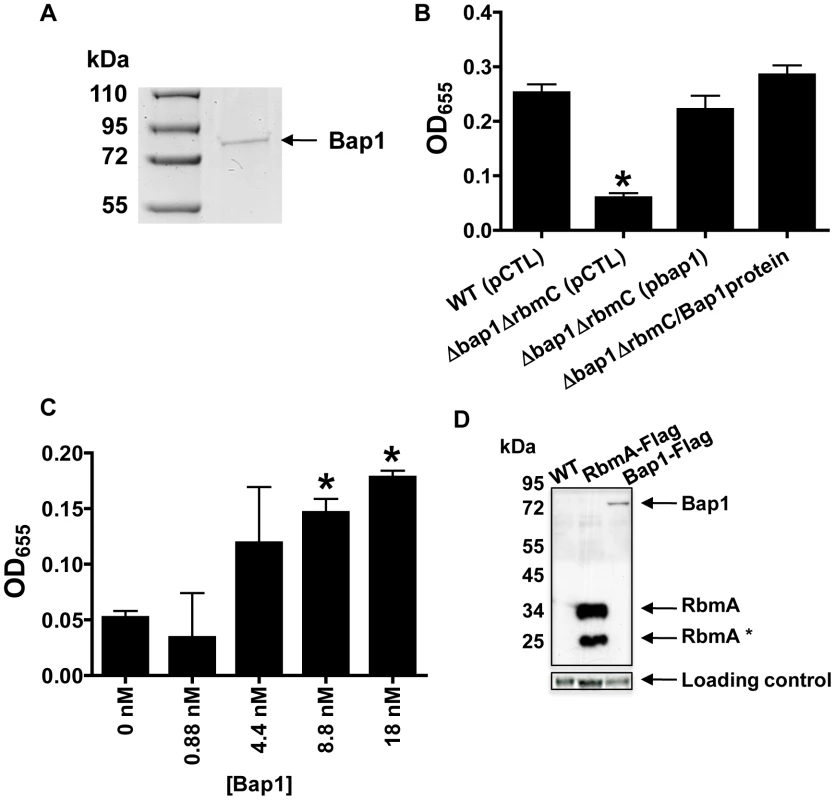
(A) SDS-PAGE of affinity purified Bap1-FLAG. A single band is seen at the predicted size of 76 kDA. Protein was visualized with Imperial stain. (B) Quantification of biofilms formed by wild-type V. cholerae (WT), a Δ bap1ΔrbmC mutant rescued with either a control plasmid (pCTL) or a plasmid expressing Bap1-FLAG, and Δ bap1ΔrbmC mutant rescued with purified Bap1 in an 18 nM final concentration. Average measurements and standard deviations were calculated from the results of three experimental replicates. * indicates values that are statistically significantly different from wild-type. (C) Quantification of biofilms made by a Δ bap1ΔrbmC mutant in the presence of increasing amounts of purified Bap1-FLAG. Average measurements and standard deviations were calculated from the results of three experimental replicates. * indicates values that are statistically significantly different from a biofilm formed by a Δbap1ΔrbmC mutant in the absence of purified Bap1. (D) Western analysis of cell extracts prepared from a wild-type biofilm (WT), a biofilm formed with a strain that expresses RbmA-FLAG from the native chromosomal location, and a biofilm formed with a strain that expresses Bap1-FLAG from the native chromosomal location. Blots were probed with an anti-FLAG antibody. Below, the α-subunit of V. cholerae RNA polymerase was visualized with an antibody to the E. coli protein for use as a loading control. Densitometry showed that approximately 16 times less Bap1 is made in biofilm cells as compared with RbmA. To validate these quantifications in a native biofilm, we used Western analysis to estimate the relative quantities of Bap1-FLAG and RbmA-FLAG synthesized in biofilms formed with V. cholerae strains expressing either Bap1-FLAG or RbmA-FLAG from the native chromosomal location (Figure 8D). Two bands were always observed for biofilm-associated RbmA, suggesting that RbmA undergoes proteolysis in the biofilm. Including both RbmA bands in the calculation, we determined that there was approximately 16 times less Bap1 in biofilm preparations as compared with RbmA, recapitulating our results with purified protein. We hypothesize that less Bap1 is required in the biofilm because it principally associates with the base of the biofilm, whereas RbmA is distributed evenly throughout.
Bap1 is a communal resource, while VPS is not
Because exogenously provided Bap1 restored biofilm surface adhesion to a Δbap1ΔrbmC mutant, we questioned whether Bap1 synthesis could be a joint venture in the biofilm community. To test this, we co-cultured a Δbap1ΔrbmC mutant with a ΔvpsL mutant. As shown in Figure 7, this produced a biofilm that was comparable to that of wild-type V. cholerae. We rationalized that (i) this biofilm might be comprised chiefly of ΔvpsL mutant cells because the Δbap1ΔrbmC mutant was providing it with the requisite biofilm exopolysaccharide, (ii) the Δbap1ΔrbmC mutant might predominate because the ΔvpsL mutant was providing it with the requisite Bap1 and/or RbmC, or (iii) approximately equal numbers of these two mutants might be found in the biofilm because each was providing the other with the requisite materials for biofilm formation. To determine whether Bap1, VPS, or both were shared resources within the biofilm, we performed a series of co-culture biofilm experiments using lacZ as a marker and determined the relative amounts of each species in the biofilm by plating on media containing 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal). As shown in Figure 9A, although the lacZ+ strain always had a slight advantage in the biofilm, cells lacking Bap1 and RbmC were always found in the biofilm when co-cultured with cells that were able to produce these proteins. In contrast, cells that were unable to synthesize VPS were always excluded from the biofilm in spite of co-culture with cells that were able to synthesize VPS. Based on these findings, we hypothesize that Bap1 is a shared biofilm resource, but VPS is not.
Fig. 9. Bap1 is a shared resource; VPS exopolysaccharide is not. 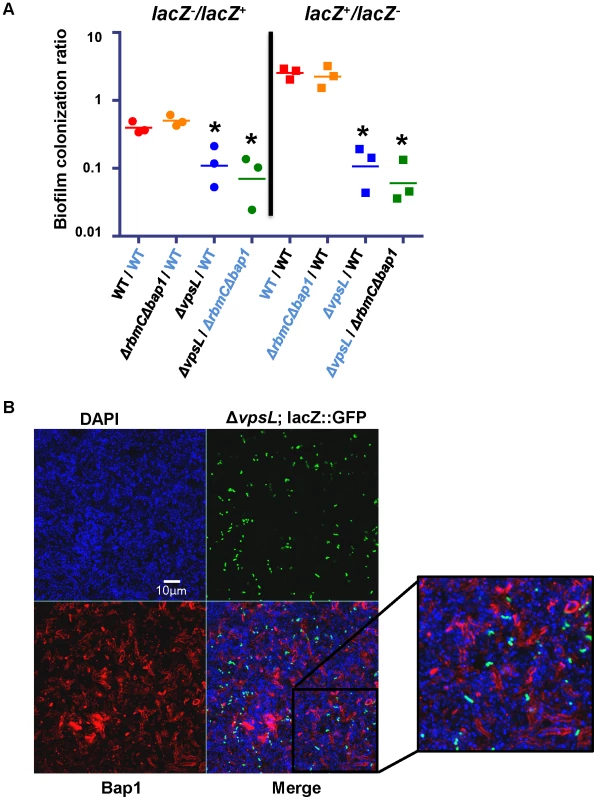
(A) Ratio of biofilm-associated CFU for a panel of co-cultured strains including wild-type V. cholerae (WT), a ΔvpsL mutant, and a Δ bap1ΔrbmC mutant. Strains were labeled by inactivation of the lacZ gene. Label-swapping experiments are shown on right. Black labels indicate a lacZ− strain, while blue labels indicate a lacZ+ strain. Three experimental replicates are shown for each condition. * indicates ratios that are statistically significantly different from that calculated for the competition of lacZ+ wild-type V. cholerae against lacZ− wild-type V. cholerae. (B) Transverse section at the level of the substratum through a biofilm formed by co-culture of a Δbap1ΔrbmC mutant with a ΔvpsL mutant carrying chromosomally encoded GFP and a plasmid encoding Bap1-FLAG. The biofilm is comprised primarily of Δbap1ΔrbmC mutant cells surrounded by Bap1-FLAG donated by the ΔvpsL mutant. ΔvpsL mutants are excluded from the biofilm. To document communal Bap1 in the V. cholerae biofilm, we then co-cultured a Δbap1ΔrbmC mutant with a ΔvpsL mutant expressing GFP from a chromosomal location and Bap1-FLAG from a plasmid. The biofilms harvested from these co-culture experiments were visualized by microscopy after immunofluorescent staining of Bap1-FLAG and DAPI staining of bacterial DNA. As expected, approximately one GFP-labeled ΔvpsL mutant cell was observed in the biofilm for every GFP-negative Δbap1ΔrbmC mutant cell (Figure 9B). However, the perimeter of many Δbap1ΔrbmC mutant cells exhibited Bap1-FLAG-based immunofluorescence. To confirm that this observation was not the result of transfer of the plasmid from the ΔvpsL mutant to the Δbap1ΔrbmC mutant, we documented that all Δbap1ΔrbmC mutant cells in the biofilm remained sensitive to ampicillin (data not shown).
Our results confirm that Bap1-FLAG provided by a ΔvpsL mutant can be incorporated into the Δbap1ΔrbmC mutant biofilm. These findings indicate that Bap1 is a communal resource. In contrast, because ΔvpsL mutant cells were excluded from both Δbap1ΔrbmC mutant and wild-type V. cholerae biofilms, we conclude that VPS produced by neighboring cells is not available to the ΔvpsL mutant and, therefore, that unlike Bap1, the biofilm exopolysaccharide VPS is not a communal resource but instead tightly associated with the cell of origin.
Bap1 mediates adhesion of bystander cells
We questioned whether Bap1 could also increase surface adhesion of bystander cells not yet committed to the sessile life style, as this would have implications for the role of Bap1 in biofilm expansion. We previously identified a medium in which V. cholerae does not synthesize enough of the biofilm matrix components to proceed past the monolayer stage of biofilm development [2]. We cultured wild-type V. cholerae, a Δbap1ΔrbmC mutant, or a ΔvpsL mutant in monolayer minimal medium with supplemented with purified Bap1. As shown in Figure 10A and quantified in Figure 10B, Bap1 increased surface adhesion of wild-type V. cholerae, a Δbap1ΔrbmC mutant, and a ΔvpsL mutant in monolayer minimal medium, while a control protein, bovine serum albumin (BSA), had no effect. This suggests that communal Bap1 secreted by nearby biofilm cells may also increase surface adhesion of bystanders that have not yet been reprogrammed for biofilm matrix synthesis.
Fig. 10. Purified Bap1-FLAG can mediate surface adhesion in the absence of VPS. 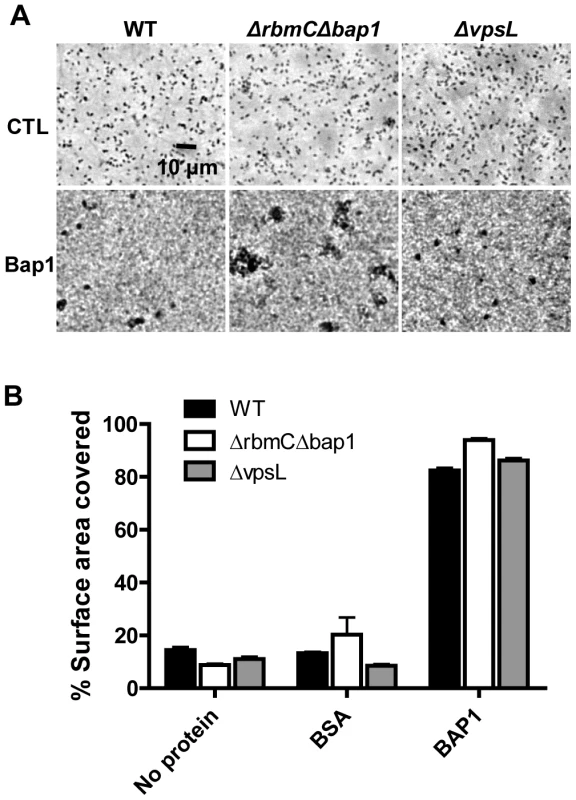
(A) Surface adhesion by wild-type V. cholerae, a Δ bap1ΔrbmC mutant, or a ΔvpsL mutant in monolayer minimal medium either alone (no protein), supplemented with purified Bap1-FLAG protein (Bap1), or supplemented with BSA. Phase contrast microscopy was used to obtain images. (B) Surface area coverage of monolayers illustrated in (A). Average measurements and standard deviations were calculated from six microscope fields derived from three experimental replicates. Discussion
The exopolysaccharide component of the bacterial biofilm matrix has been studied intensively [8], [46], [47], [48], [49], [50], [51]. More recently, components such as DNA and protein have been identified in the matrices of some bacterial biofilms. Here we provide the first proteomic analysis of a Gram-negative biofilm matrix. Our analysis revealed 10 secreted proteins, 43 periplasmic and outer membrane proteins, and 18 putative extracytoplasmic proteins whose location could not be predicted.
OM and periplasmic proteins were much more likely to be identified in multiple matrix preparations than inner membrane and cytoplasmic proteins, suggesting that these proteins may not be artifacts caused by cell lysis but rather the contents of biofilm-associated OM vesicles. Outer membrane vesicles are retained in the biofilms of Pseudomonas aeruginosa and Helicobacter pylori, and these vesicles appear to play a role in biofilm formation [52], [53], [54]. Furthermore, there is evidence that the compositions of membrane vesicles derived from the biofilm and from culture supernatants are distinct [52], [55]. V. cholerae has been reported to release outer membrane vesicles [56], [57], [58]. However, additional investigations are required to confirm the presence of these vesicles in the biofilm matrix and to determine their role in biofilm formation.
We studied four secreted proteins identified in our preliminary analysis in addition to MshA. The chitinase, ChiA-2, showed minimal retention in the biofilm matrix. However, three proteins of unknown function, Bap1, RbmA, and HlyA showed extensive association with the matrix. RbmA has no conserved domains of known function. Bap1, its homolog RbmC, and HlyA, which all contain at least one β-prism lectin domain, form a paralogous family in V. cholerae. We hypothesize that these secreted proteins are selectively retained in the biofilm, perhaps by binding to specific moieties in the polysaccharide scaffold.
Bap1 and RbmA were previously shown to play an undefined role in V. cholerae biofilm formation [3], [38], [39]. Here we show that RbmA and Bap1 have distinct distributions in the biofilm matrix. RbmA surrounds biofilm-associated cells throughout the biofilm and reinforces intercellular contacts from this location. In contrast, Bap1 concentrates around cells that form the biofilm-surface interface and stabilizes adhesion of the biofilm to surfaces. The distinct distribution of these proteins is not the result of heterogeneous expression within the biofilm. Rather, we hypothesize that it is the result of self-segregation after secretion from the cell. This is the first example of spatial and functional differentiation of secreted structural proteins in a Gram-negative biofilm matrix.
Biofilm matrix polysaccharide is considered to be a jointly synthesized, shared resource. We show here that this is not the case for the V. cholerae biofilm matrix. While the biofilm matrix protein Bap1 is a communal resource, VPS benefits only cells from which it is synthesized. Therefore, the V. cholerae biofilm exopolysaccharide is not freely secreted and available to the entire community.
Lastly, our results suggest that matrix-associated proteins may play an important role in expansion of existing bacterial biofilms on surfaces. Exogenous Bap1 increases surface adhesion of planktonic bystanders as well. Because nutritional signals and surface attachment are strong activators of the biofilm matrix synthesis genes, in aquatic environments, it is unlikely that planktonic Bap1 and RbmC would be synthesized by planktonic cells in quantities sufficient to increase surface attachment. Rather, we envision that Bap1 and RbmC secreted from an existing biofilm would condition surrounding surfaces, increasing the probability of bystander cell attachment.
These studies reveal a new paradigm for the bacterial biofilm matrix in which the biofilm exopolysaccharide forms a cell-associated scaffold to which communal biofilm matrix proteins adhere, possibly through carbohydrate-binding domains. These proteins may fulfill specialized structural roles or enable cooperative augmentation of the biofilm.
Materials and Methods
Bacterial strains, plasmids, and media
The bacterial strains and plasmids used in this study are listed in Table S2. Vectors used for protein expression included either an IPTG inducible promoter and a FLAG-tag (pFLAG-CTC, Sigma-Aldrich) or an arabinose inducible promoter and a 6X-His tag (pBAD-Topo, Invitrogen). Bacteria were cultivated either in Luria-Bertani broth (LB) or monolayer minimal media [2]. Where indicated, streptomycin (100 µg/ml), ampicillin (50 or 100 µg/ml), arabinose (0.04% wt/vol), and Isopropyl β-D-1-thiogalactopyranoside (1 mM) (IPTG) were added to the growth medium. A 0.1 M phosphate-buffered saline solution (PBS) (pH 7.0) was used in initial biofilm washes, and a 0.1 M Tris-buffered saline solution (TBS) (ph 7.0) was used to wash biofilms after biotin labeling.
Identification of biofilm matrix-associated proteins
10 mls of LB broth supplemented with streptomycin was added to a Petri dish and inoculated with V. cholerae. A biofilm including a pellicle formed over 48 hours of static incubation at 27°C. After incubation, the associated planktonic cells were removed. The remaining biofilm was washed by addition of PBS, agitation on a rotary shaker for 5 minutes with PBS, removal of PBS and non-attached cells, and addition of fresh PBS. This procedure was repeated twice. Matrix proteins were then prepared using each of the following four protocols (Figure S1). In preparation (i), the biofilm was disrupted in the presence of 1.0 mm glass beads (Biospec) and centrifuged to remove particulates. For preparations (ii), (iii), and (iv), a cell surface biotinylation kit (Pierce) was used to biotinylate extracytoplasmic proteins in the washed biofilm according to the manufacturer's instructions. After biotinylation, the biofilm was transferred to a 50 ml conical tube containing 2 mls of PBS. Disruption of the pellicle was carried out by ten sonication cycles of 10 sec (iii) or by vortexing in the presence (ii) or absence (iv) of 1.0 mm glass beads (Biospec) for one minute. The mixtures were then centrifuged at 20, 000× g for 30 min in the cold to remove particulates, the supernatants were applied to Neutravidin-agarose resin (Pierce), and the resin was washed several times with PBS. Biotinylated proteins were eluted from the resin by incubation with PBS to which 50 mM DTT had been added. This disrupts the disulfide bonds bridging biotin residues to extracellular proteins.
The four mixtures of proteins were precipitated with trichloroacetic acid, resuspended in SDS-PAGE loading buffer, run into a 4–20% gradient SDS-polyacrylamide gel, and then sent to the Taplin Mass Spectrometry Facility where the gel was cut into pieces and subjected to an in-gel trypsin digestion procedure. Peptides were extracted from the gel, dried in a speed-vac, and reconstituted in 5–10 µl of HPLC solvent A (2.5% acetonitrile, 0.1% formic acid). Each sample was loaded via a Famos auto sampler (LC Packings) onto a nano-scale reverse-phase HPLC capillary. Eluted peptides were subjected to electrospray ionization and then entered into an LTQ linear ion-trap mass spectrometer (ThermoFisher). Peptide sequences were determined by matching protein databases with the acquired fragmentation pattern by the software program, Sequest (ThermoFisher).
Protein overexpression
The ORFs of interest were amplified by PCR using primers including the start and stop codons of each gene of interest. For cloning into pBAD-Topo, PCR products were inserted into the expression vector according to the manufacturer's protocol (Invitrogen). For cloning into pFLAG-CTC, either NdeI and KpnI or NdeI and EcoRI restriction sites were included in the PCR primer pairs. The PCR products were then digested and ligated into the expression vector. The ligation products were transformed into E. coli TOP10 competent cells and selected on LB agar plates supplemented with ampicillin (100 µg/ml). The presence of the correct insert was confirmed by colony PCR and sequence analysis. Confirmed plasmids were electroporated into V. cholerae. V. cholerae strains harbouring a pBAD-Topo plasmid were grown in 0.02% arabinose
Generation of V. cholerae strains encoding FLAG-tagged Bap1 and RbmA on the chromosome
C-terminal fragments of bap1 and rbmA were amplified from the pFLAG-bap1 and pFLAG-rbmA plasmids, respectively, by the polymerase chain reaction with the following primers: Bap1 A: ATCGTCTAGAGTGTACGCGGGTTACTACGC and B: GACTGCATGCCAGACCGCTTCTGCGTTCTG and RbmA: A: AGTCTCTAGAGCCAGTGATTGAAGCAAATC and B: GACTGCATGCCAGACCGCTTCTGCGTTCTG. The resulting PCR products were digested with XbaI and SphI and ligated into the multiple cloning site of the suicide plasmid pGP704, and the sequence was confirmed. This plasmid was then integrated into the chromosome by single homologous recombination as previously described [37].
Mutant construction
The ΔrbmC in-frame deletion mutant was constructed as previously described [12]. Briefly, the following primer pairs: Pair 1 A: TGGCGCCATATTCTATGACA and B: TTACGAGCGGCCGCATACACCCTTCGGCTTCATTC and Pair 2 A: TGCGGCCGCTCGTAATATTGGGCTCAACCCACTATG and B: GGCAGTTTAATGGCGATCAT were used to amplify two genome sequences spanning an in-frame deletion in the gene of interest. These DNA fragments were joined by the SOE technique [59], cloned into pCR2.1-TOPO and then subcloned into the suicide vector pWM91 by ligation after digestion with XhoI and SpeI. This suicide plasmid was used to generate an in-frame deletion in rbmC by double homologous recombination [12]. A similar procedure was used for generation of the ΔrbmA in-frame deletion mutant using the following primer pairs: Pair 1 A: CGTACTCGAGCACCCACAATTAGTGATCGCT and B: TAACGAGCGGCCGCACAACCATTTGTTTTTACAACTGG and Pair 2 A: TGCGGCCGCTCGTTATAAATTTACCTAGTCACTTAGTCGT and B: TCGACACTAGTCAAACTCTAGAACGGAACAAAA.
Biofilm assays
Biofilm quantification assays were performed as described previously with the following modifications [13]. Briefly, a single colony of V. cholerae was inoculated into 1 ml of LB broth and allowed to grow to mid exponential phase. The culture was then diluted in LB broth to yield an OD655 of 0.05 and divided into three disposable glass culture tubes (10 mm ×75 mm). These tubes were incubated statically at 27°C. After 24 hrs, planktonic cells were removed, and the OD655 of the cells was measured. Remaining biofilms were washed with PBS and then disrupted by vortexing in the presence of 1 mm beads. The OD655 of the resulting cell suspension was measured. For assays of biofilm integrity, biofilms were formed as described above and then either gently shaken or vortexed. All assays were performed in triplicate and statistical significance was determined by a student's t-test.
Western blot analysis
To evaluate protein secretion, V. cholerae was inoculated into 2 mls of LB broth supplemented with IPTG and ampicillin and grown for 6 hours at 37°C with shaking at 200 rpm. The OD655 of the final culture was measured, and then the cells were centrifuged at 4°C for 15 minutes at 4500 rpm. The supernatants and cell pellets were separated. Cell pellets were resuspended in the volume of PBS required to yield a final OD655 of 1. Five µl of this cell suspension were diluted in 20 µl 1x Laemmli buffer solution and boiled for 5 min. Supernatants were collected and filtered through a 0.25 µm filter. 10 µl of the supernatants were added to 2 µl 5x Laemmli buffer and boiled for 5 min. The protein mixtures in the cell pellets and supernatants were separated by electrophoresis on a 4–20 % precast SDS-PAGE gel (Pierce) and transferred onto a PVDF membrane (Millipore) with a semi-dry transfer apparatus using the Fast Semi-Dry Transfer Buffer (Pierce). The affinity tagged proteins were visualized as follows. Membranes were incubated overnight in a blocking solution consisting of PBS with 0.05% Tween 20 (PBS-T) and 5% skim milk-PBS. The membranes were then incubated with a 1∶10,000 dilution of Anti-FLAG M2-Peroxidase antibody in PBS-T for 1 hour on a rotary shaker. Membranes were washed once for 15 minutes and twice for 5 minutes in PBS-T and then developed using the ECL Plus Western Blotting Detection Reagent (GE Healthcare) according to the manufacturer's instructions.
To evaluate Bap1 and RbmA in the biofilm at native levels, a similar protocol was used with the following modifications: strains carrying either Bap1-FLAG or RbmA-FLAG on the chromosome were allowed to form biofilms for 24 hours in 2 ml of LB. After removal of planktonic cells and spent medium, biofilms were washed with PBS and resuspended in 500 µl of PBS. Biofilm cell extracts were prepared by sonication, and the protein concentrations of the extracts were determined by Bradford assay. 20 µg of each extract was diluted in 20 µL of MilliQ water and 5X Laemmli buffer and separated by SDS-PAGE. As a loading control, the RNA polymerase α-subunit was detected with an antibody raised against the α-subunit of E. coli RNA polymerase (Neoclone). Relative amounts of Bap1 and RbmA in the gel were approximated by densitometry analysis using ImageQuant 5.2 (Molecular Dynamics).
Immunofluorescence
Wells of a 12 well microtiter dish were filled with 2 mls of LB broth supplemented with ampicillin and arabinose, where noted, and a tilted 18 mm ×18 mm glass cover slip was placed in each well. After 24 hours of static culture, the cover slips were placed in 6 well microtiter dishes and washed twice for 5 minutes with 2 mls of PBS on a rotary shaker. The cover slips were then incubated on a rotary shaker for one hour in a blocking solution consisting of PBS supplemented with 3% BSA. This solution was replaced with blocking solution containing Anti-6X His (1∶1, 000 dilution) (Abcam) and/or Anti-FLAG M2 (1∶1, 000 dilution) (Sigma-Aldrich), and the coverslips were then incubated for an additional hour. After this incubation, the cover slips were washed with PBS three times for 5 minutes each time. For labeling of FLAG-tagged proteins with DyLight549, biofilms exposed to the unlabeled Anti-FLAG M2 antibody underwent an additional 45 min incubation with DyLight 549 AffiniPure Rabbit Anti-Mouse IgG H+L (1∶500 dilution) (Jackson ImmunoResearch). For His-tagged proteins, the same procedure was used with an Alexa Fluor 488 Goat Anti-Rabbit Antibody (Invitrogen). The cover slips were then washed in PBS three times, for 5 minutes each time. Where indicated, the cover slips were also incubated with a 1 mg/ml DAPI solution for 5 min. Cover slips were mounted on concave glass slides filled with PBS and then sealed with nail polish. Confocal images were acquired at the Children's Hospital, Boston Imaging Core with a LSM700 microscope (Zeiss) equipped with a 63X objective and 405, 488, and 555 nm laser lines. A computer equipped with ZEN 2009 software was used to acquire and process images. As a control, a DAPI-stained biofilm was imaged before and after immunofluorescence manipulations. Very little change was observed after manipulation, demonstrating that the biofilm was not noticeably degraded by the immunofluorescence staining procedure (data not shown).
Purification of RbmA-FLAG and Bap1-FLAG
Wild-type V. cholerae carrying either a RbmA-FLAG or a Bap1-FLAG expression plasmid were grown overnight on an LB agar plate containing ampicillin. Several of the resulting colonies were inoculated into 100 mls of LB broth supplemented with ampicillin. When the culture reached mid-log phase, IPTG was added to a final concentration of 1 mM. After 4 hours of additional growth, the cells were pelleted at 5,000 rpm at 4°C (Sorvall, rotor SLA-600TC), and the recovered supernatant was distributed into two 50 ml conical tubes. 200 µl of Anti-FLAG M2 Affinity Gel prepared according to the manufacturer's instructions (Sigma-Aldrich) was added to each tube, and the tubes were agitated for 1 hour at room temperature to allow the protein to adhere to the resin. The resin was collected in 10 ml chromatography columns (Bio-Rad) and washed with 2×10 ml PBS. Proteins bound to the resin were eluted with 300 µl of 0.1 M glycine, pH 2.5 and instantly brought to pH 8 by addition of 10 mM Tris, pH 8. Protein concentration was determined by absorbance at 280 nm, and the eluate was analysed by SDS-PAGE using a 12% pre-cast gel (Pierce). After separation, the gel was stained with Imperial Stain (Pierce).
V. cholerae biofilm co-culture experiments
For quantification, equal numbers of lacZ+ and lacZ− V. cholerae strains were inoculated into LB-filled wells of a microtiter dish, and biofilms were allowed to form at 27°C over 24 hours. Biofilms were then disrupted with 1 mm glass beads, and serial dilutions of the resulting cell suspensions were plated for isolation on LB agar plates containing X-GAL. In the morning, numbers of blue and white colonies were recorded.
For microscopy, equal numbers of a ΔrbmCΔbap1 mutant and a ΔvpsL mutant carrying a chromosomally-encoded, constitutively expressed gfp allele and a plasmid-encoded bap1-FLAG allele were inoculated into LB-filled wells of a microtiter dish with a coverslip. Biofilms were allowed to form as described above. Biofilms formed on coverslips were subsequently removed and prepared for immunofluorescence as as described above. These biofilms were examined by confocal microscopy using the LSM700 microscope (Zeiss).
Evaluation of monolayer formation
Cells were grown in a 24 well microtiter dish filled with minimal medium (MM) alone or supplemented with purified Bap1 or BSA. An Eclipse TE-2000-E phase contrast microscope (Nikon) equipped with a 20X objective and an Orca digital CCD camera (Hamamatsu) was used to obtain images. Surface area coverage was calculated using IP Lab software (Nikon). Two randomly selected fields were measured in each of three biological replicates.
Accession numbers
Proteins listed in Tables 1 and 2 have the following Swiss Prot accession numbers. Table 1: MshA (Q60074), RbmA (Q9KTH4), RbmC (Q9KTH2), FlaB (P0C6C4), FlaD (P0C6C6), FlaC (P0C6C5), FlaA (P0C6C3), ChiA-2 (Q9KND8), HlyA (P09545), and HAP (P24153). Table 2: VC0174 (Q9KVH2), VC0430 (Q9KUT5), VC0483 (Q9KUN2), VC1101 (Q9KT04), VC1154 (Q9KSV2), VC1334 (Q9KSC4), VC1384 (Q9KS75), VC1523 (Q9KRW1), VC1834 (Q9KR13), VC1853 (Q9KQZ4), VC1887 (Q9KQW1), VC1894 (Q9KQV4), VC2168 (Q9KQ36), VC2517 (Q9KP59), VCA0026 (Y2826), VCA0058 (Q9KNA7), VCA0144 (Q9KN22), and VCA0900 (Q9KL48).
Supporting Information
Zdroje
1. KaratanEWatnickP 2009 Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73 310 347
2. MoorthySWatnickPI 2004 Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol Microbiol 52 573 587
3. MoorthySWatnickPI 2005 Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol Microbiol 57 1623 1635
4. FlemmingHCWingenderJ The biofilm matrix. Nat Rev Microbiol 8 623 633
5. KierekKWatnickPI 2003 Environmental Determinants of Vibrio cholerae Biofilm Development. Appl Environ Microbiol 69 5079 5088
6. KierekKWatnickPI 2003 The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2+-dependent biofilm development in sea water. Proc Natl Acad Sci U S A: 100 14357 14362
7. WatnickPIKolterR 1999 Steps in the development of a Vibrio cholerae biofilm. Mol Microbiol 34 586 595
8. YildizFH Schoolnik GK 1999 Vibrio cholerae O1 El Tor: Identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA 96 4028 4033
9. YildizFHDolganovNASchoolnikGK 2001 VpsR, a Member of the Response Regulators of the Two-Component Regulatory Systems, Is Required for Expression of vps Biosynthesis Genes and EPS(ETr)-Associated Phenotypes in Vibrio cholerae O1 El Tor. J Bacteriol 183 1716 1726
10. Casper-LindleyCYildizFH 2004 VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J Bacteriol 186 1574 1578
11. YildizFH 2008 Cyclic dimeric GMP signaling and regulation of surface-associated developmental programs. J Bacteriol 190 781 783
12. HaugoAJWatnickPI 2002 Vibrio cholerae CytR is a repressor of biofilm development. Mol Microbiol 45 471 483
13. HouotLChangSPickeringBSAbsalonCWatnickPI 2010 The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways. J Bacteriol 192 3055 3067
14. HammerBKBasslerBL 2003 Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol 50 101 104
15. SilvaAJBenitezJA 2006 A Vibrio cholerae relaxed (relA) mutant expresses major virulence factors, exhibits biofilm formation and motility, and colonizes the suckling mouse intestine. J Bacteriol 188 794 800
16. LimBBeyhanSYildizFH 2007 Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J Bacteriol 189 717 729
17. TamayoRTischlerADCamilliA 2005 The EAL domain protein VieA is a cyclic diguanylate phosphodiesterase. J Biol Chem 280 33324 33330
18. TischlerADCamilliA 2005 Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun 73 5873 5882
19. LaurianoCMGhoshCCorreaNEKloseKE 2004 The sodium-driven flagellar motor controls exopolysaccharide expression in Vibrio cholerae. J Bacteriol 186 4864 4874
20. WatnickPILaurianoCMKloseKECroalLKolterR 2001 Absence of a flagellum leads to altered colony morphology, biofilm development, and virulence in Vibrio cholerae O139. Mol Microbiol 39 223 235
21. HuoAXuBChowdhuryMAIslamMSMontillaR 1996 A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl Environ Microbiol 62 2508 2512
22. ColwellRRHuqAIslamMSAzizKMYunusM 2003 Reduction of cholera in Bangladeshi villages by simple filtration. Proc Natl Acad Sci U S A 100 1051 1055
23. ColwellRRHuqA 1994 Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann N Y Acad Sci 740 44 54
24. HuqASmallEBWestPAHuqMIRahmanR 1983 Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45 275 283
25. HuqAHuqSAGrimesDJO'BrienMChuKH 1986 Colonization of the gut of the blue crab (Callinectes sapidus) by Vibrio cholerae. Appl Environ Microbiol 52 586 588
26. TamplinMLGauzensALHuqASackDAColwellRR 1990 Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol 56 1977 1980
27. ShuklaBNSinghDVSanyalSC 1995 Attachment of non-culturable toxigenic Vibrio cholerae O1 and non-O1 and Aeromonas spp. to the aquatic arthropod Gerris spinolae and plants in the River Ganga, Varanasi. FEMS Immunol Med Microbiol 12 113 120
28. MeibomKLLiXBNielsenATWuCYRosemanS 2004 The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci U S A 101 2524 2529
29. LiXRosemanS 2004 The chitinolytic cascade in Vibrios is regulated by chitin oligosaccharides and a two-component chitin catabolic sensor/kinase. Proc Natl Acad Sci U S A 101 627 631
30. KeyhaniNORosemanS 1999 Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta 1473 108 122
31. MeibomKLBlokeschMDolganovNAWuCYSchoolnikGK 2005 Chitin induces natural competence in Vibrio cholerae. Science 310 1824 1827
32. MuellerRSBeyhanSSainiSGYildizFHBartlettDH 2009 Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J Bacteriol 191 3504 3516
33. KaratanEDuncanTRWatnickPI 2005 NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J Bacteriol 187 7434 7443
34. HouotLWatnickPI 2008 A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J Bacteriol 190 311 320
35. McGinnisMWParkerZMWalterNERutkovskyACCartaya-MarinC 2009 Spermidine regulates Vibrio cholerae biofilm formation via transport and signaling pathways. FEMS Microbiol Lett 299 166 174
36. BergTSchildSReidlJ 2007 Regulation of the chitobiose-phosphotransferase system in Vibrio cholerae. Arch Microbiol 187 433 439
37. HouotLChangSAbsalonCWatnickPI 2010 Vibrio cholerae PTS control of carbohydrate transport, biofilm formation, and colonization of the germ-free mouse intestine. Infect Immun 78 1482 1494
38. FongJCKarplusKSchoolnikGKYildizFH 2006 Identification and characterization of RbmA, a novel protein required for the development of rugose colony morphology and biofilm structure in Vibrio cholerae. J Bacteriol 188 1049 1059
39. FongJCYildizFH 2007 The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J Bacteriol 189 2319 2330
40. HungDTZhuJSturtevantDMekalanosJJ 2006 Bile acids stimulate biofilm formation in Vibrio cholerae. Mol Microbiol 59 193 201
41. BorleeBRGoldmanADMurakamiKSamudralaRWozniakDJ 2010 Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol Microbiol 75 827 842
42. Martinez-GilMYousef-CoronadoFEspinosa-UrgelM 2010 LapF, the second largest Pseudomonas putida protein, contributes to plant root colonization and determines biofilm architecture. Mol Microbiol 77 549 561
43. BrandaSSChuFKearnsDBLosickRKolterR 2006 A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol 59 1229 1238
44. LathemWWGrysTEWitowskiSETorresAGKaperJB 2002 StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol Microbiol 45 277 288
45. SzabadyRLLokutaMAWaltersKBHuttenlocherAWelchRA 2009 Modulation of neutrophil function by a secreted mucinase of Escherichia coli O157:H7. PLoS Pathog 5 e1000320
46. BaselgaRAlbizuIDe La CruzMDel CachoEBarberanM 1993 Phase variation of slime production in Staphylococcus aureus: implications in colonization and virulence. Infect Immun 61 4857 4862
47. HentzerMTeitzelGMBalzerGJHeydornAMolinS 2001 Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183 5395 5401
48. VuongCKocianovaSVoyichJMYaoYFischerER 2004 A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem 279 54881 54886
49. JacksonKDStarkeyMKremerSParsekMRWozniakDJ 2004 Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J Bacteriol 186 4466 4475
50. SmithCSHinzABodenmillerDLarsonDEBrunYV 2003 Identification of genes required for synthesis of the adhesive holdfast in Caulobacter crescentus. J Bacteriol 185 1432 1442
51. ShemeshMTamASteinbergD 2007 Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J Med Microbiol 56 1528 1535
52. SchoolingSRBeveridgeTJ 2006 Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol 188 5945 5957
53. YonezawaHOsakiTKurataSFukudaMKawakamiH 2009 Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol 9 197
54. NakamuraSHigashiyamaYIzumikawaKSekiMKakeyaH 2008 The roles of the quorum-sensing system in the release of extracellular DNA, lipopolysaccharide, and membrane vesicles from Pseudomonas aeruginosa. Jpn J Infect Dis 61 375 378
55. HauratMFAduse-OpokuJRangarajanMDorobantuLGrayMR 2011 Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem 286 1269 76
56. BishopALSchildSPatimallaBKleinBCamilliA 2010 Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect Immun 78 4402 4420
57. SchildSNelsonEJBishopALCamilliA 2009 Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun 77 472 484
58. SchildSNelsonEJCamilliA 2008 Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun 76 4554 4563
59. HortonRMHuntHDHoSNPullenJKPeaseLR 1989 Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77 61 68
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated OligosaccharidesČlánek A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes inČlánek Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Phenotypic Screens, Chemical Genomics, and Antimalarial Lead Discovery
- Characterisation of Regulatory T Cells in Nasal Associated Lymphoid Tissue in Children: Relationships with Pneumococcal Colonization
- Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated Oligosaccharides
- Absence of Cross-Presenting Cells in the Salivary Gland and Viral Immune Evasion Confine Cytomegalovirus Immune Control to Effector CD4 T Cells
- Transcriptomic Analysis of Host Immune and Cell Death Responses Associated with the Influenza A Virus PB1-F2 Protein
- A Quorum Sensing Regulated Small Volatile Molecule Reduces Acute Virulence and Promotes Chronic Infection Phenotypes
- Autocrine Regulation of Pulmonary Inflammation by Effector T-Cell Derived IL-10 during Infection with Respiratory Syncytial Virus
- A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes in
- Association of Human TLR1 and TLR6 Deficiency with Altered Immune Responses to BCG Vaccination in South African Infants
- Histo-Blood Group Antigens Act as Attachment Factors of Rabbit Hemorrhagic Disease Virus Infection in a Virus Strain-Dependent Manner
- MrkH, a Novel c-di-GMP-Dependent Transcriptional Activator, Controls Biofilm Formation by Regulating Type 3 Fimbriae Expression
- Beta-HPV 5 and 8 E6 Promote p300 Degradation by Blocking AKT/p300 Association
- Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
- Transportin 3 Promotes a Nuclear Maturation Step Required for Efficient HIV-1 Integration
- Coordination of KSHV Latent and Lytic Gene Control by CTCF-Cohesin Mediated Chromosome Conformation
- A Novel Persistence Associated EBV miRNA Expression Profile Is Disrupted in Neoplasia
- The Plant Pathogen pv. Is Genetically Monomorphic and under Strong Selection to Evade Tomato Immunity
- IL-10 Blocks the Development of Resistance to Re-Infection with
- Anti-Apoptotic Machinery Protects the Necrotrophic Fungus from Host-Induced Apoptotic-Like Cell Death during Plant Infection
- Crystal Structure of PrgI-SipD: Insight into a Secretion Competent State of the Type Three Secretion System Needle Tip and its Interaction with Host Ligands
- Evades Immune Recognition of Flagellin in Both Mammals and Plants
- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Provides Insights into the Evolution of the Salmonellae
- B Cell Repertoire Analysis Identifies New Antigenic Domains on Glycoprotein B of Human Cytomegalovirus which Are Target of Neutralizing Antibodies
- Thy1 Nk Cells from Vaccinia Virus-Primed Mice Confer Protection against Vaccinia Virus Challenge in the Absence of Adaptive Lymphocytes
- The Cytokine Network of Acute HIV Infection: A Promising Target for Vaccines and Therapy to Reduce Viral Set-Point?
- Dendritic Cell Status Modulates the Outcome of HIV-Related B Cell Disease Progression
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- A Communal Bacterial Adhesin Anchors Biofilm and Bystander Cells to Surfaces
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Activation of HIV Transcription by the Viral Tat Protein Requires a Demethylation Step Mediated by Lysine-specific Demethylase 1 (LSD1/KDM1)
- Unique Evolution of the UPR Pathway with a Novel bZIP Transcription Factor, Hxl1, for Controlling Pathogenicity of
- Disruption of PML Nuclear Bodies Is Mediated by ORF61 SUMO-Interacting Motifs and Required for Varicella-Zoster Virus Pathogenesis in Skin
- Flagellar Motility Is Not Directly Required to Maintain Attachment to Surfaces
- Viral Infection Induces Expression of Novel Phased MicroRNAs from Conserved Cellular MicroRNA Precursors
- Functional Cure of SIVagm Infection in Rhesus Macaques Results in Complete Recovery of CD4 T Cells and Is Reverted by CD8 Cell Depletion
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
- The Steroid Catabolic Pathway of the Intracellular Pathogen Is Important for Pathogenesis and a Target for Vaccine Development
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



