-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
Type 2 immune responses are essential in protection against intestinal helminth infections. In this study we show that IL-22, a cytokine important in defence against bacterial infections in the intestinal tract, is also a critical mediator of anti-helminth immunity. After infection with Nippostrongylus brasiliensis, a rodent hookworm, IL-22-deficient mice showed impaired worm expulsion despite normal levels of type 2 cytokine production. The impaired worm expulsion correlated with reduced goblet cell hyperplasia and reduced expression of goblet cell markers. We further confirmed our findings in a second nematode model, the murine whipworm Trichuris muris. T.muris infected IL-22-deficient mice had a similar phenotype to that seen in N.brasiliensis infection, with impaired worm expulsion and reduced goblet cell hyperplasia. Ex vivo and in vitro analysis demonstrated that IL-22 is able to directly induce the expression of several goblet cell markers, including mucins. Taken together, our findings reveal that IL-22 plays an important role in goblet cell activation, and thus, a key role in anti-helminth immunity.
Published in the journal: IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection. PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003698
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003698Summary
Type 2 immune responses are essential in protection against intestinal helminth infections. In this study we show that IL-22, a cytokine important in defence against bacterial infections in the intestinal tract, is also a critical mediator of anti-helminth immunity. After infection with Nippostrongylus brasiliensis, a rodent hookworm, IL-22-deficient mice showed impaired worm expulsion despite normal levels of type 2 cytokine production. The impaired worm expulsion correlated with reduced goblet cell hyperplasia and reduced expression of goblet cell markers. We further confirmed our findings in a second nematode model, the murine whipworm Trichuris muris. T.muris infected IL-22-deficient mice had a similar phenotype to that seen in N.brasiliensis infection, with impaired worm expulsion and reduced goblet cell hyperplasia. Ex vivo and in vitro analysis demonstrated that IL-22 is able to directly induce the expression of several goblet cell markers, including mucins. Taken together, our findings reveal that IL-22 plays an important role in goblet cell activation, and thus, a key role in anti-helminth immunity.
Introduction
Type 2 immune responses are essential in protection against intestinal helminth infections, including the rodent hookworm Nippostrongylus brasiliensis (Nb) [1]. Type 2 immunity involves the recruitment of effector cells such as eosinophils, mast cells and production of IgE antibodies [2] and it is well established that the activation of IL-4 - and IL-13-producing CD4+ T helper 2 cells is central in the development of a successful anti-parasite response. One of the key components in the expulsion of intestinal helminths is secretion of mucus by goblet cells [2]. Intestinal goblet cells are found interspersed within the epithelial monolayer and are differentiated from epithelial progenitor cells. Goblet cells produce a number of effector molecules including a range of mucins and antimicrobial proteins, including trefoil factors and resistin-like molecules, which enable these to play a key part in innate defense mechanisms in the gut, against both bacterial and helminth infections [2], [3].
IL-22 is a member of the IL-10 cytokine family and is produced by a wide variety of innate and adaptive immune cells including CD4+ T cells, most notably Th17 and Th22 cells, CD8+ T cells, natural killer (NK) cells, lymphoid tissue inducer (LTi) cells and other group 3 innate lymphoid cells (ILCs) [4]. The heterodimeric receptor for IL-22, consisting of the IL-22R and the IL-10R2 chain, is exclusively expressed on non-hematopoietic cells, such as intestinal epithelial cells, and its signaling is mediated via Stat3 [5], [6], [7], [8], [9]. IL-22 has been shown to directly mediate epithelial defence mechanisms through the induction of IL-6, IL-8 and various antimicrobial peptides [6], [10], [11], [12], [13]. Furthermore, a protective function of IL-22 has been demonstrated in some colitis models and a model of Concanavalin A induced liver damage [14], [15], [16], [17]. Therefore it appears that the function of IL-22 is to strengthen epithelial barriers, mediate repair and wound healing mechanisms as well as participate in epithelial defence. Interestingly, IL-22 has been shown to be upregulated within the human gastrointestinal tract following infections with the whipworm Trichuris trichiuria and the hookworm Necator americanus [18], [19], but no studies have as yet demonstrated a role for IL-22 in intestinal helminth infections and the associated type 2 response.
To clarify the role for IL-22 in the defence against intestinal helminth infection, we infected wild-type (WT) and IL-22-deficient mice with Nippostrongylus brasiliensis, a rodent nematode with a life cycle resembling that of human hookworm. Our data show that IL-22-deficient mice have reduced worm clearance, despite strong induction of IL-4, IL-5 and IL-13 in the mesenteric lymph nodes and mucosal tissue. Despite this intact type 2 cytokine induction IL-22-deficient mice showed a defective goblet cell response and in vitro and ex vivo analyses revealed that IL-22 can directly regulate the expression of several goblet cell markers. Taken together, our data suggest that IL-22 plays a key role in driving intestinal goblet cell responses in vivo and thus acts as an important mediator of intestinal worm expulsion.
Materials and Methods
Ethics statement
All animal work was approved following local ethical review by MRC National Institute for Medical Research, NIMR, Animal Procedures and Ethics Committee and was performed in strict accordance with the U. K Home Office Animals (Scientific Procedures) Act 1986 (approved H.O Project License 80/2506).
Animals and infections
Six to nine week old male and female C57BL/6 and IL-22KO mice [20] were bred at the specific pathogen-free animal facility at the MRC National Institute for Medical Research (NIMR, London, UK). Age - and sex-matched experimental animals (3–8 per group) were infected with 500 infective Nippostrongylus brasiliensis (Nb) larvae by subcutaneous injection [21] or with 150 embryonated Trichuris muris (Tm) eggs by oral gavage [22].
Cell culture and cytokine analyses
Mesenteric lymph nodes were removed and single cell preparations were resuspended in RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 0.05 mM β-mercaptoethanol (Life technologies). Cells were cultured at 37°C and 5% CO2 in flat-bottomed 96-well plates at a final concentration of 5×106/ml in a final volume of 0.2 ml/well. Cells were stimulated with Nb or Tm antigen (25 µg/ml), or plate-bound anti-CD3 antibody (mAb145-2C11, 10 µg/ml, ATCC) and cell-free supernatants were harvested after 48 hours and stored at −80°C. Cytokine analyses were carried out using a multiplex cytometric bead assay (Flowcytomix, eBiosciences). Explants of small intestine were washed extensively in ice cold PBS and cultured overnight in the same medium as above, with or without the addition of recombinant IL-22 (R&D systems). LS174T cells (kindly provided by Dr AC Williams, University of Bristol, UK) were cultured in DMEM supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin.
Lamina propria cell isolation and flow cytometry
After removal of Peyer's patches the small intestine was cut into 5 mm pieces and epithelial cells and intraepithelial lymphocytes were first removed by shaking gut pieces in PBS with 10% FCS, 1 mM pyruvate, 20 µM Hepes, 10 mM EDTA, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 µg/ml Polymyxin B and 2 mM DTT for 30 min at 37 C. The remaining gut tissue was washed and digested using Collagenase D (Roche, 1 mg/ml) and DNAse1 (Sigma, 10 U/ml) for 45 minutes at 37°C, before being subjected to Percoll centrifugation (37.5%), followed by washing and resuspension of the isolated lamina propria leukocytes in medium. To identify innate lymphoid cells (ILC), isolated leukocytes were stained by using fluorochrome-coupled antibodies against CD45, Thy1.2, IL-7R (CD127) and a combination of lineage markers (Lin), including CD3, CD8, CD11b, CD11c, CD19, CD49b, TCR-β, TCR-γδ, NK1.1, GR-1 and Ter119. ILC were defined as CD45+Lin−Thy1.2+IL-7R+. For further characterization of ILC surface marker expression, antibodies against CD4 and NKp46 were used. For intracellular cytokine staining isolated leukocytes were restimulated with phorbol 12,13-dibutyrate (PdBU) and ionomycin (both at 0.5 µg/ml) in the presence of brefeldin A (1 µg/ml) for 2.5 h, fixed with formalin (3.8%), permeabilised with octylphenyl-polyethylene glycol (0.1%, Sigma), and stained with fluorochrome-coupled antibodies against IL-17A and IL-22. All samples were acquired on a LSRII flow cytometer (BD Biosciences) and analysed with the FlowJo software (Treestar Inc.).
Histopathological analyses
Consecutive lengths of small intestine taken 10 cm distal to the pyloric sphincter were fixed in neutral-buffered formalin, histologically processed using standard methods, and 5 µm sections were stained for goblet cells (Periodic Schiff). The number of cells per 20 randomly selected villus-crypt units (VCU) was determined under light microscopy from at least two sections per animal.
Real-time PCR
Tissues were harvested and stored in RNAlater (Qiagen) at −80 C until processing. RNA was purified using Trizol (Life technologies). Reverse transcription was performed using a Quantitect RT kit (Qiagen) and real time PCR was performed in an ABI 7500 sequence detection system (Applied Biosystem) using the SYBR Green PCR Master Mix (Qiagen). Results were normalised to the housekeeping gene hypoxanthine guanine phosphoribosyl transferase (HPRT).
Statistical analyses
Significant differences (P<0.05) between experimental groups were determined using Student's t-test.
Results and Discussion
N. brasiliensis infection induces IL-22 expression in Th17 cells and innate lymphoid cells in the small intestine
Upregulation of IL-22 expression in the gastrointestinal tract has been reported in human helminth infection [18], [19]. To address the question if IL-22 may play a role in anti-helminth immunity, we utilized the mouse model of Nippostrongylus brasiliensis (Nb). We first assessed IL-22 mRNA expression in the small intestine at different time points post Nb infection (p.i.) (Fig. 1A). Elevated expression of Il22 mRNA was found from day 6 p.i. and further increased at day 10 p.i. Flow cytometric analysis of lamina propria lymphocytes isolated from the small intestine of infected mice showed IL-22 cytokine staining both in the lineage-negative (Lin−) and lineage-positive (Lin+) compartments, with the majority (∼80%) of the IL-22 coming from Lin+ cells (Fig. 1B, C). Further characterization of the IL-22+Lin+ and IL-22+Lin− subsets revealed that amongst the Lin+ cells, CD4+ T cells were the predominant IL-22+ cell type, while the IL-22+Lin− population consisted predominantly of NKp46− innate lymphoid type 3 cells (ILC3) (Fig. 1D, E). Co-staining for IL-17A demonstrated that ∼70% of the T cell-derived IL-22 was produced by Th17 cells, whereas the majority of the IL-22+Lin− cells did not show co-production of IL-17A (Fig. 1F). Thus, our data show that IL-22 is produced by both T cell and non-T cell populations in the lamina propria during Nb infection. This is in agreement with a number of studies demonstrating that IL-22 can be produced by a variety of cells in the intestine, including innate cells, such as LTi, NKp46+ and NKp46− ILC3 cells, as well as conventional CD4+ T cells [23], [24], [25]. In addition, Basu et al [13] recently demonstrated that early production of IL-22 during bacterial infection is mainly derived from innate sources, shifting to CD4-derived during later stages of infection, and that both sources play a vital role in protection at different stages against enteric infection, thus demonstrating the importance of both innate and adaptive sources of IL-22 in mucosal responses.
Fig. 1. IL-22 is produced by both adaptive and innate cells in the small intestine during N.brasiliensis infection. 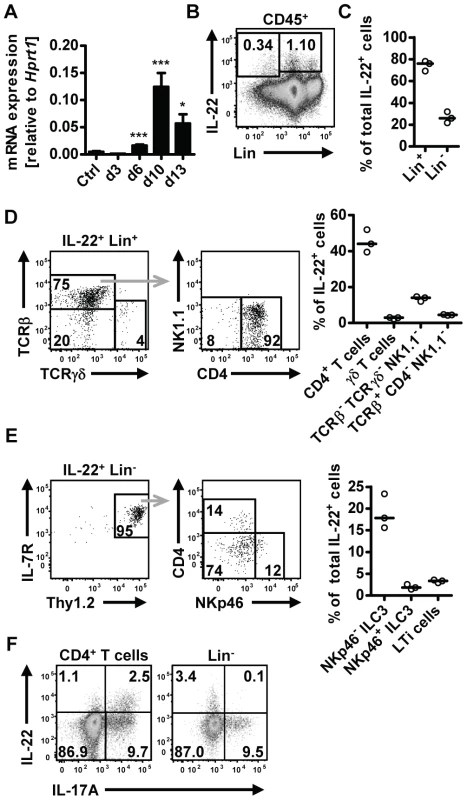
(A) C57BL/6 mice were infected with N. brasiliensis and RNA was isolated from the small intestine at various time points. IL-22mRNA expression was analysed by real-time RT-PCR. Data are pooled from two independent experiments (n = 3–7 for each time point, and shown as mean of individual animals+SEM). (B) Representative flow cytometry of leukocytes isolated from the small intestinal lamina propria of infected C57BL/6 mice after restimulation with PdBU and ionomycin at day 5 post infection. (C) Relative contribution of Lin+ and Lin− cells to total IL-22+ cells, analysed as in B. (D, E) Representative flow cytometry and relative contribution of the different cell subsets in the IL-22+Lin+ (D) and IL-22+Lin− compartment (E). Relative contribution of each subset is expressed as percentage of total IL-22+ cells, analysed as in B. (F) Representative flow cytometry for IL-22 and IL-17A after restimulation with PdBU and ionomycin gated on CD4+ T cells (left) and Lin− cells (right). Numbers in gates and quadrants represent events in percent of total cells gated. Each symbol represents one mouse. All data are representative of two to three independent experiments. *p<0.05, ***p<0.001. Il22−/− mice show delayed worm expulsion
Although the increased expression of IL-22 in human intestinal mucosa after helminth infections suggests a possible functional link between IL-22 and anti-helminth immunity [18], [19], the role of IL-22 in mouse models of gastrointestinal helminth infections has not been addressed. Therefore, we infected WT and Il22−/− mice with Nb and assessed the intestinal worm burdens at various time points after infection (Fig. 2). While similar numbers of parasites had reached the intestine by day 3 in WT and
Fig. 2. Il22−/− mice have reduced worm expulsion. 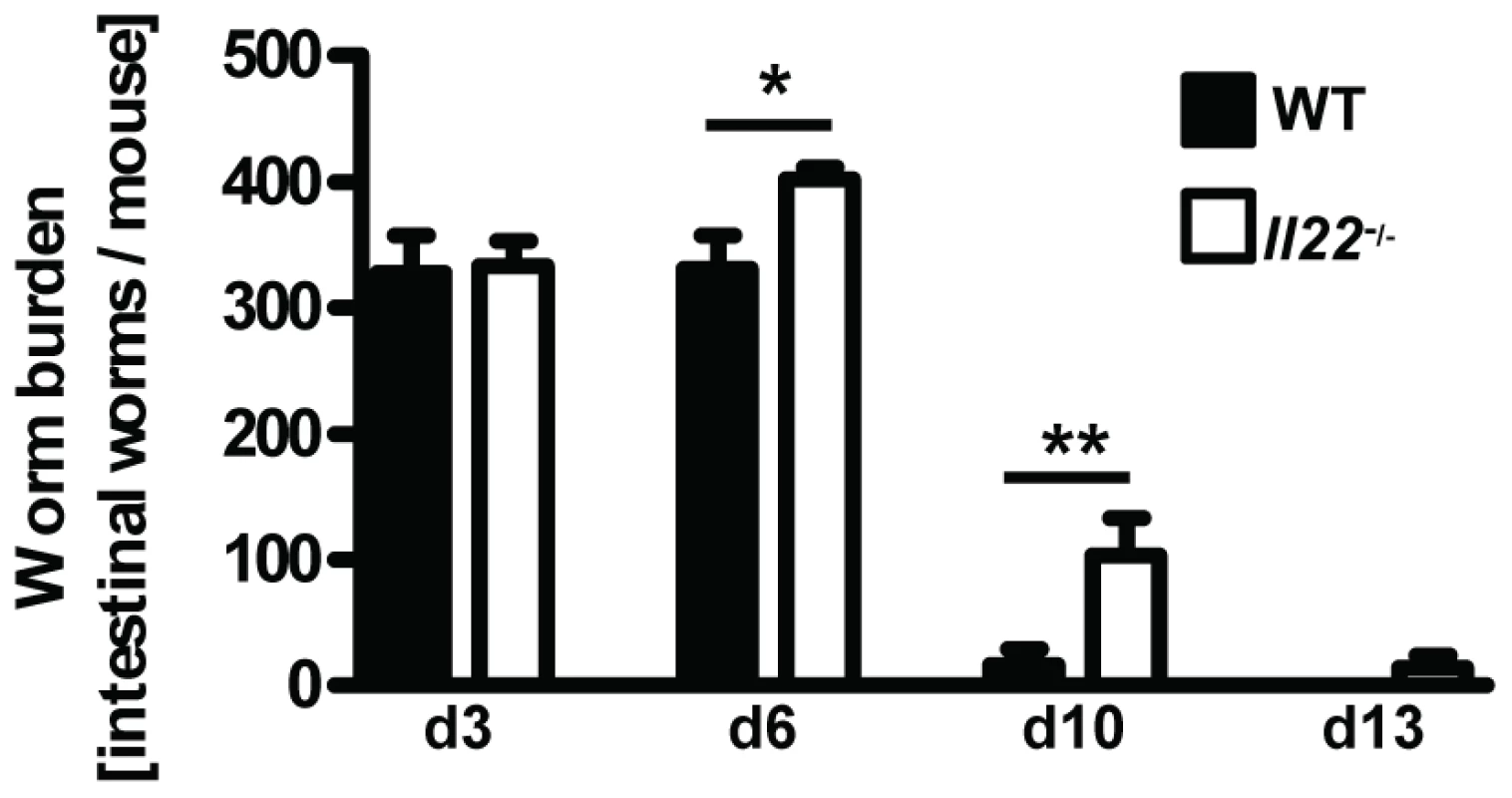
Wild-type (black bars) and Il22−/− (white bars) mice were infected with N.brasiliensis and worm burden in the small intestine was analysed at the time points indicated. Data are pooled from three independent experiments (n = 5–13 for each time point, and shown as mean of individual animals +SEM). *p<0.05, **p<0.01. Il22−/− mice, indicating an unperturbed lung passage of the Nb larvae, we observed a marked delay in worm expulsion in Il22−/− mice with significantly increased worm burdens at day 6 and day 10 compared to WT animals (Fig. 2), demonstrating a key role for IL-22 in the clearance of intestinal helminth infection.
IL-22 is required for goblet cell hyperplasia after Nb infection
Goblet cell hyperplasia in the intestinal epithelium is a hallmark of intestinal helminth infections and is crucial for worm expulsion in Nb infection [26]. Histological analysis of the small intestine of WT and Il22−/− mice at various time points after Nb infection revealed a significant reduction of goblet cell numbers in the small intestine of IL22−/− mice as compared to their WT counterparts (Fig. 3A, B). Analysis of mRNA expression of the goblet cell markers Clca3 (Gob5) [27], Retnlb (RELMβ), Tff2 (trefoil factor 2) and mucins Muc1, Muc2 and Muc3 [28], showed that their upregulation observed in Nb-infected WT mice was almost completely abolished in absence of IL-22 (Fig. 3C). Thus, the delayed worm expulsion observed in the absence of IL-22 is strongly correlated to reduced goblet cell hyperplasia and reduced expression of goblet cell mediators.
Fig. 3. Il22−/− mice have defective goblet cell responses. 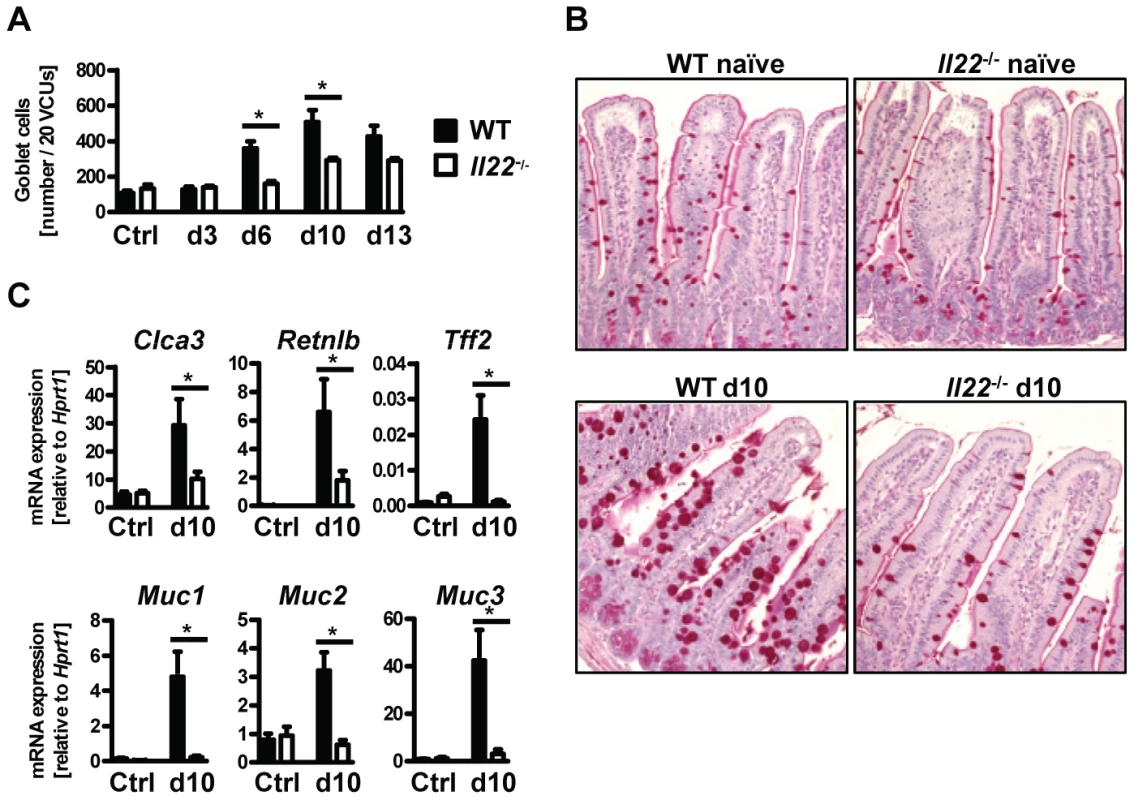
Wild-type (black bars) and Il22−/− (white bars) mice were infected with N.brasiliensis and (A) lengths of jejunum collected on various days post infection, histologically processed, sectioned, stained with Periodic acid schiff and numbers of goblet cells per twenty randomly selected villus-crypt units determined by light microscopy. (B, C) mRNA expression measured by realtime RT-PCR in the small intestine of WT (black bars) and Il22−/− (white bars) mice at day 10 post infection, compared to naïve controls. All data are pooled from three independent experiments (n = 3–7 for each time point and shown as mean of individual animals +SEM). *p<0.05. Normal type 2 cytokine production in helminth-infected Il22−/− mice
Since type 2 responses are instrumental in anti-helminth immunity and goblet cell hyperplasia, we hypothesized that impaired type 2 cytokine production may be responsible for the reduced goblet cell function and delayed worm expulsion in Il22−/− mice. However, neither type 2 cytokine mRNA expression in intestinal tissues, or protein levels in supernatants from Nb antigen-restimulated mesenteric lymph node cells at day 10 p.i. were impaired in Il22−/− mice (Fig. 4 A, B). In fact, the levels of IL-4 mRNA in the intestine and IL-5 protein from lymph nodes were even increased in Il22−/− mice, possibly as a result of overcompensation due to the increase in worm burden. Therefore, Il22−/− mice have impaired intestinal worm expulsion and reduced goblet cell function despite normal type 2 cytokine production.
Fig. 4. Il22−/− mice have normal Th2 responses. 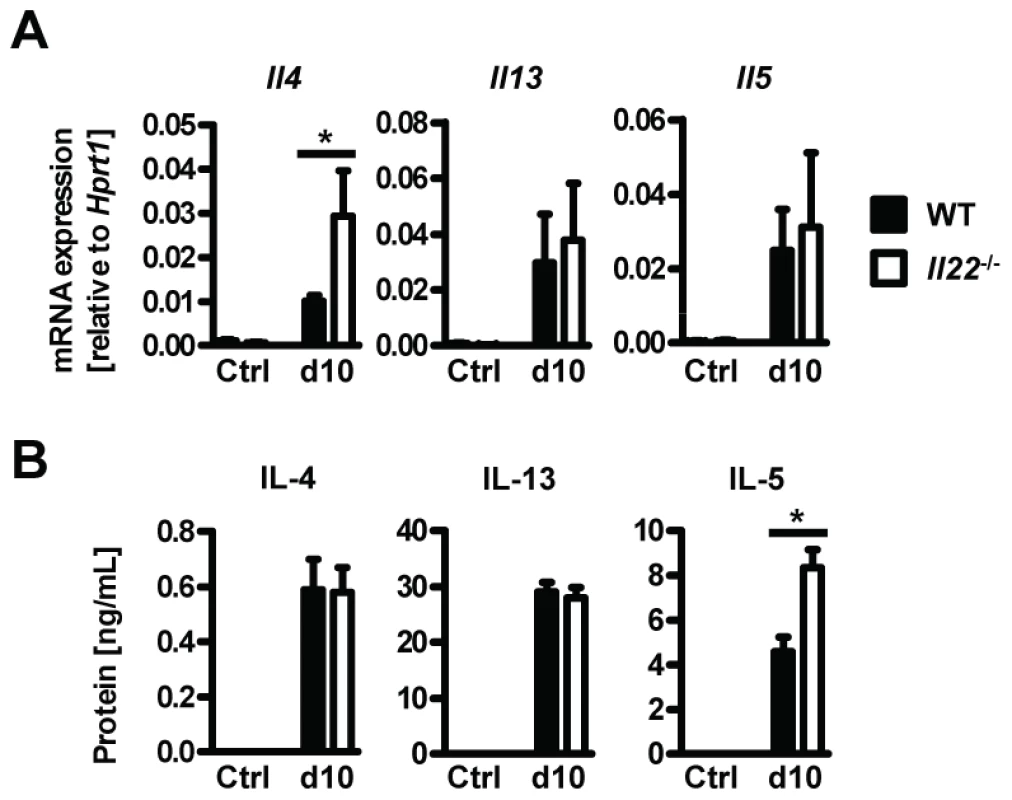
Wild-type (black bars) and Il22−/− (white bars) mice were infected with N.brasiliensis and (A) mRNA expression measured by realtime RT-PCR in the small intestine of WT (black bars) and Il22−/− (white bars) mice at day 10 post infection, compared to naïve controls. (B) Mesenteric lymph node cells from Nb-infected WT (black bars) and Il22−/− (white bars) mice, as well as from uninfected controls of both genotypes (Ctrl), were removed at day 10 p.i. and stimulated in vitro with Nb antigen. Supernatants were analysed by sandwich ELISA for the presence of IL-4, IL-13 and IL-5. All data are pooled from three independent experiments (n = 3–7 for each time point and shown as mean of individual animals +SEM). *p<0.05. IL-22 acts directly on epithelial cells to induce mucin expression
A possible explanation for the reduction in goblet cell hyperplasia detected in Il22−/− mice is a direct effect of IL-22 on the differentiation and/or activation status of goblet cells. To address this possibility, we treated small intestinal tissue ex vivo from uninfected WT mice with IL-22 and analysed expression of goblet cell markers. IL-22 treatment induced expression of Retnlb, Muc1 and Muc3, but not Clca3, Muc2 or Tff2 (Fig. 5A). In addition, we cultured LS174T cells, a human mucus-secreting intestinal adenocarcinoma cell line, in the presence of human IL-22, IL-13, or a combination of both cytokines, and observed that IL-22 alone induced expression of several mucins including MUC1, MUC3, MUC4 and MUC5b, but not MUC5AC (Fig. 5B). Similarly to our observations using mouse tissue (Fig. 5A) we did not observe induction of expression of Clca1 (the human ortholog of mouse Clca3) by IL-22, however, IL-13 alone induce expression of Clca1 and this induction was further amplified by IL-22 in a synergistic manner (Fig. 5B). Taken together, this data demonstrate that IL-22 alone is able to induce the expression of several goblet cell mediators whilst also working in synergy with other mucogenic cytokines such as IL-13, in the induction of other goblet cell mediators, such as Clca1/3. This data is in agreement with the study by Sugimoto et al, where IL-22 via STAT3 signaling, was linked to goblet cell hyperplasia and mucin expression in a model of colitis [15].
Fig. 5. IL-22 acts directly on epithelial cells to induce mucin expression. 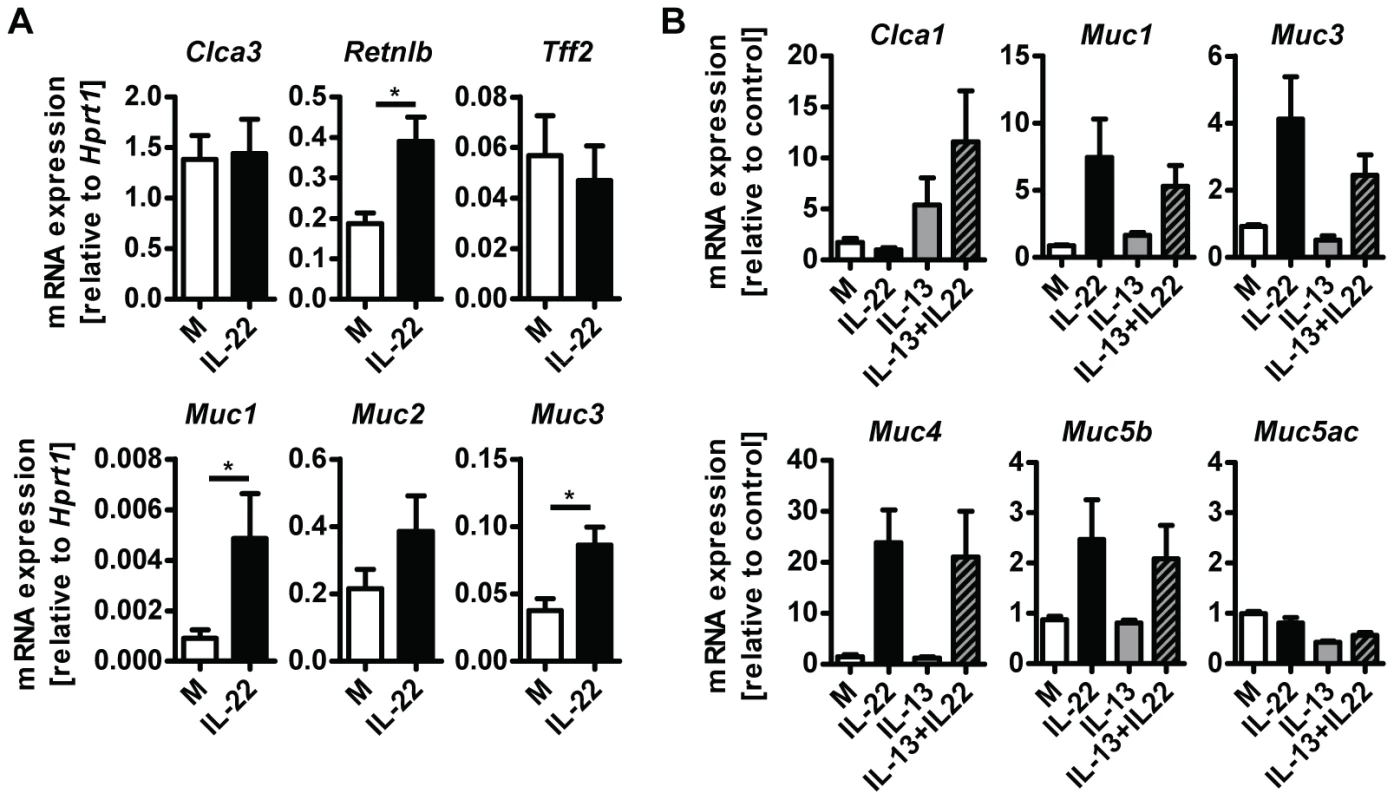
(A) Segments of small intestine from naïve C57Bl/6 mice were removed, washed in ice cold PBS and incubated at 37 C overnight in complete RPMI medium with or without the addition of 10 ng/ml recombinant murine IL-22, followed by RNA isolation and analysis by real-time RT-PCR. Data are pooled from two to three independent experiments (n = 4 for each experiment, data shown as mean of individual animals +SEM). (B) LS174T cells were grown in complete DMEM and incubated with or without 10 ng/ml recombinant human IL-22, 10 ng/ml recombinant human IL-13, or both, for 24 hours, followed by RNA isolation and analysis by real-time RT-PCR. Data are pooled from three experiments, error bars represent variation between the independent experiments. *p<0.05. Il22−/− mice have impaired worm expulsion and defective goblet cell responses to Trichuris muris infection
In order to confirm the role for IL-22 in anti-helminth immunity we infected Il22−/− and WT mice with the murine whipworm Trichuris muris, another helminth model where goblet cells and mucus production is known to play an important role in resistance to infection [29], [30], [31]. We assessed worm burden, cytokine and goblet cell responses 21 days post infection. Similarly to Nb infection we found that Tm infected Il22−/− mice had higher worm burdens despite normal type 2 cytokines responses compared to WT mice (Fig. 6). Furthermore, Tm infected Il22−/− mice had reduced goblet cell hyperplasia and reduced expression of intestinal Retnlb, Muc1, Muc3, Clca3 and Tff2, but not Muc2 (Fig. 6). Thus, our data demonstrate that IL-22 deficiency significantly impairs anti-helminth immunity and goblet cells function in two different murine nematode models.
Fig. 6. Il22−/− mice show reduced worm expulsion and goblet cell responses, but normal type 2 responses, during T.muris infection. 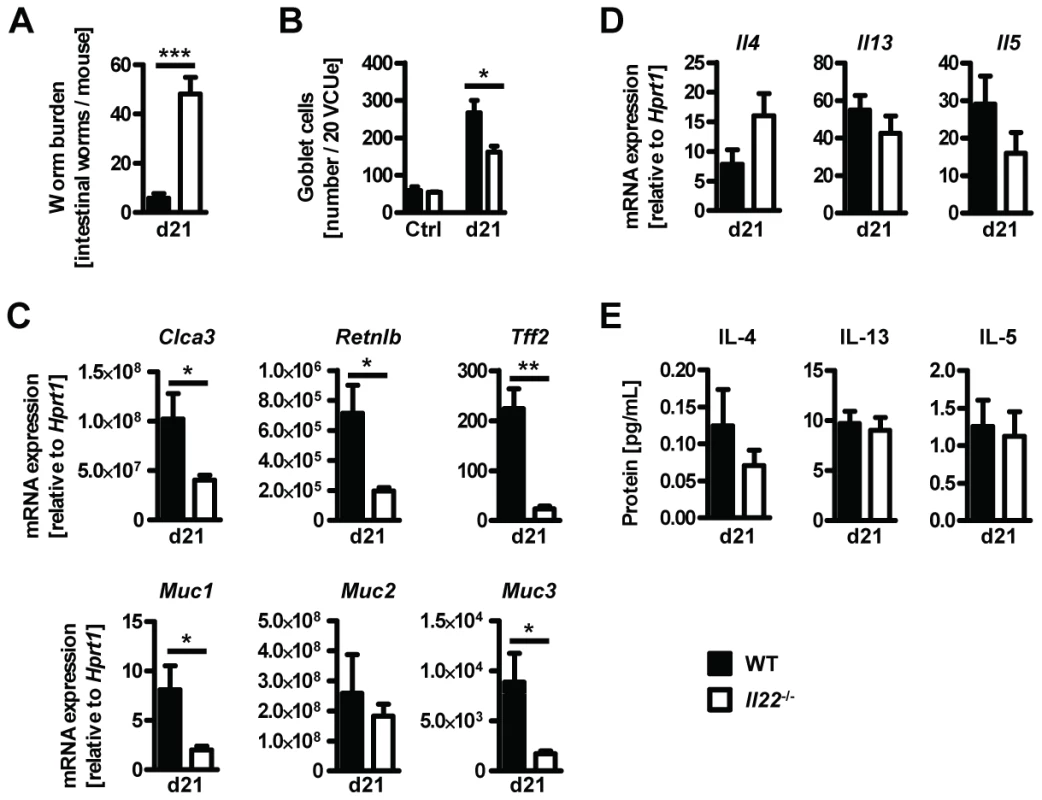
Wild-type (black bars) and Il22−/− (white bars) mice were infected with T.muris and assessed for (A) worm burden, (B) number of goblet cells, and (C, D) colonic mRNA expression of goblet cell markers and cytokines by realtime RT-PCR at 21 days p.i. (E) Mesenteric lymph node cells were stimulated in vitro with Tm antigen and supernatants analysed by sandwich ELISA for the presence of IL-4, IL-13 and IL-5. Data from one representative experiment out of three is shown (n = 5). Data is shown as mean of individual animals +SEM. *p<0.05, **p<0.01. ***p<0.001. Previous studies have shown that IL-22 is particularly important in regulating inflammatory responses within the intestine through the production of antimicrobial peptides, as well as enhancement and regulation of epithelial wound repair and regenerative mechanisms [6], [10], [11], [12], [13], [25]. In addition, IL-22 has also been shown to play a detrimental role in chronic mucosal inflammation and progression to colorectal cancer [32], [33] demonstrating a pivotal role for IL-22 in balancing tissue regeneration and tumour development in the intestinal environment.
Our data now suggest an important function for IL-22 in promoting other epithelial functions, particularly goblet cell-derived production of mucins and antimicrobial peptides, leading to anti-helminth immunity. A previous study by Wilson et al [34] found no role for IL-22 in the development of hepatic pathology during infection with the trematode helminth Schistosoma mansoni in mice, thus supporting the concept that IL-22 exerts organ and/or pathogen-specific functions. Mucus is believed to play an important part in intestinal anti-helminth mechanisms, partly through the generation of the mucus barrier, but also via other proteins in the secretions such as the antimicrobial peptide Relmβ (FIZZ2) which may impair worm movement and feeding [26], [31]. The mucins themselves are a large family of both secreted and surface bound glycoproteins, together forming the mucus layer. Quantitative and qualitative differences in mucus composition are evident during intestinal infections [35], [36] although information is still limited regarding the contribution of specific mucins to the overall physical properties of mucus and how mucus composition may change with respect to different types of infections.
Little is also known regarding the cytokine-mediated control of mucin expression, but it is clear that a number of cytokines are able to induce mucin expression, and other goblet cell products in vitro and in vivo. This includes type 2 cytokines such as IL-13 and IL-4 [37], [38], but also pro-inflammatory cytokines such as TNF and IL-1 [39], [40]. Although IL-13 and IL-4 are believed to be the key driver cytokines for the induction of goblet cell hyperplasia during helminth infections [41], [42], [43], some studies have demonstrated IL-4/13-independent goblet cell hyperplasia [44]. Furthermore, increase in intestinal Muc2 and Muc3 expression during infection with the nematode Trichinella spiralis has been reported in IL-4-deficient mice [45]. The data presented here, together with that of Sugimoto et al [15], now show that IL-22 is central to goblet cell hyperplasia and function in the intestine. Our analyses of Nb and Tm-infected IL-22-deficient mice demonstrate that IL-22 increases the abundance of goblet cells and is required for upregulation of mucins and other goblet cell products in vivo. The fact that IL-22-deficient mice mount a strong type 2 cytokine response in both draining lymph nodes and intestinal tissue provides further evidence that IL-4/13 alone are not sufficient for promoting effective goblet cell functions during intestinal helminth infection. In contrast, our ex vivo and in vitro analyses suggest that IL-22 (and not IL-13) alone might be sufficient to increase mucin production by the intestinal epithelium in certain settings. In vivo, however, a concerted action of the type 2 cytokines together with IL-22, and possibly other inflammatory mediators, is most likely needed for an efficient anti-helminth response. The reported findings of upregulated IL-22 expression following human infections with the whipworm Trichuris trichiuria and the hookworm Necator americanus [18], [19] indicate that IL-22 may play a similar role in human helminth infections.
In conclusion, our study demonstrates a key role for IL-22 in goblet cell function and, thus, for anti-helminth immunity in the intestine. In addition, our data provide additional insight into the pivotal role played by IL-22 in mucosal immunity in protection against various types of infectious pathogens.
Zdroje
1. AllenJE, MaizelsRM (2011) Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11 : 375–388.
2. ArtisD, GrencisRK (2008) The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol 1 : 252–264.
3. McGuckinMA, LindenSK, SuttonP, FlorinTH (2011) Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9 : 265–278.
4. SonnenbergGF, FouserLA, ArtisD (2011) Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol May;12 : 383–390.
5. DumoutierL, Van RoostE, ColauD, RenauldJ-C (2000) Human interleukin-10-related T cell-derived inducible factor: Molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci U S A 97 : 10144–10149.
6. WolkK, KunzS, WitteE, FriedrichM, AsadullahK, et al. (2004) IL-22 increases the innate immunity of tissues. Immunity 21 : 241–254.
7. NagalakshmiML, RascleA, ZurawskiS, MenonS, de Waal MalefytR (2004) Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol 4 : 679–691.
8. PickertG, NeufertC, LeppkesM, ZhengY, WittkopfN, et al. (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206 : 1465–1472.
9. NeufertC, PickertG, ZhengY, WittkopfN, WarntjenM, et al. (2010) Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle 9 : 652–655.
10. AndohA, ZhangZ, InatomiO, FujinoS, DeguchiY, et al. (2005) Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129 : 969–984.
11. BrandS, BeigelF, OlszakT, ZitzmannK, EichhorstSrT, et al. (2006) IL-22 is increased in active Crohns disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 290: G827–G838.
12. ZhengY, ValdezPA, DanilenkoDM, HuY, SaSM, et al. (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14 : 282–289.
13. BasuR, O'QuinnDB, SilbergerDJ, SchoebTR, FouserL, et al. (2012) Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37 : 1061–1075.
14. ZenewiczLA, YancopoulosGD, ValenzuelaDM, MurphyAJ, StevensS, et al. (2008) Innate and adaptive Interleukin-22 protects mice from inflammatory bowel disease. Immunity 29 : 947–957.
15. SugimotoK, OgawaA, MizoguchiE, ShimomuraY, AndohA, et al. (2008) IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 118 : 534–544.
16. RadaevaS, SunR, PanH-n, HongF, GaoB (2004) Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39 : 1332–1342.
17. ZenewiczLA, YancopoulosGD, ValenzuelaDM, MurphyAJ, KarowM, et al. (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity Oct; 27 : 647–659.
18. BroadhurstMJ, LeungJM, KashyapV, McCuneJM, MahadevanU, et al. (2010) IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med Dec 1;2 : 60ra88.
19. GazeS, McSorleyHJ, DavesonJ, JonesD, BethonyJM, et al. (2012) Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog Feb;8: e1002520.
20. KreymborgK, EtzenspergerR, DumoutierL, HaakS, RebolloA, et al. (2007) IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol Dec 15;179 : 8090–8104.
21. CamberisM, Le GrosG, UrbanJJ (2003) Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol Chapter 19: Unit 19.12.
22. HelmbyH, TakedaK, AkiraS, GrencisRK (2001) Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. J Exp Med 194 : 355–364.
23. SonnenbergGF, MonticelliLA, AlenghatT, FungTC, HutnickNA, et al. (2012) Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336 : 1321–1325.
24. Satoh-TakayamaN, VosshenrichCA, Lesjean-PottierS, SawaS, LochnerM, et al. (2008) Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29 : 958–970.
25. SonnenbergGF, MonticelliLA, EllosoMM, FouserLA, ArtisD (2011) CD4+ Lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34 : 122–134.
26. HerbertDR, YangJQ, HoganSP, GroschwitzK, KhodounM, et al. (2009) Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med 206 : 2947–2957.
27. KomiyaT, TanigawaY, HirohashiS (1999) Cloning and identification of the gene Gob-5, which is expressed in intestinal goblet cells in mice. Biochem Biophys Res Commun 255 : 347–351.
28. KimY, HoS (2010) Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12 : 319–330.
29. HasnainSZ, WangH, GhiaJ-E, HaqN, DengY, et al. (2010) Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138 : 1763–1771.
30. HasnainSZ, EvansCM, RoyM, GallagherAL, KindrachukKN, et al. (2011) Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 208 : 893–900.
31. ArtisD, WangML, KeilbaughSA, HeW, BrenesM, et al. (2004) RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA 101 : 13596–13600.
32. HuberS, GaglianiN, ZenewiczLA, HuberFJ, BosurgiL, et al. (2012) IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491 : 259–263.
33. KirchbergerS, RoystonDJ, BoulardO, ThorntonE, FranchiniF, et al. (2013) Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 210 : 917–931.
34. WilsonMS, FengCG, BarberDL, YarovinskyF, CheeverAW, et al. (2010) Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth Infections. J Immunol 184 : 4378–4390.
35. LindenSK, FlorinTHJ, McGuckinMA (2008) Mucin dynamics in intestinal bacterial infection. PLoS One 3: e3952.
36. HasnainSZ, GallagherAL, GrencisRK, ThorntonDJ (2013) A new role for mucins in immunity: Insights from gastrointestinal nematode infection. Int J Biochem Cell Biol 45 : 364–374.
37. Wills-KarpM, LuyimbaziJ, XuX, SchofieldB, NebenTY, et al. (1998) Interleukin-13: central mediator of allergic asthma. Science 282 : 2258–2261.
38. DabbaghK, TakeyamaK, LeeH-M, UekiIF, LausierJA, et al. (1999) IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 162 : 6233–6237.
39. JarryA, ValletteG, BrankaJE, LaboisseC (1996) Direct secretory effect of interleukin-1 via type I receptors in human colonic mucous epithelial cells (HT29-C1.16E). Gut 38 : 240–242.
40. EnssML, CornbergM, WagnerS, GebertA, HenrichsM, et al. (2000) Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res 49 : 162–169.
41. McKenzieGJ, BancroftA, GrencisRK, McKenzieANJ (1998) A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Current Biology 8 : 339–342.
42. FallonPG, JolinHE, SmithP, EmsonCL, TownsendMJ, et al. (2002) IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17 : 7–17.
43. KhanWI, BlennerhassetP, MaC, MatthaeiKI, CollinsSM (2001) Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol 23 : 39–42.
44. MarillierR, MichelsC, SmithE, FickL, LeetoM, et al. (2008) IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunology 9 : 11.
45. ShekelsLL, AnwayRE, LinJ, KennedyMW, GarsideP, et al. (2001) Coordinated Muc2 and Muc3 Mucin gene expression in Trichinella spiralis infection in wild-type and cytokine-deficient mice. Dig Dis Sci 46 : 1757–1764.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2013 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



