-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
Carriage of the genetic combination encoding a high expression inhibitory Killer Immunoglobulin-like Receptor (KIR)3DL1 with its ligand, HLA-B*57 (*h/*y+B*57) is associated with slower time to AIDS and better HIV viral load control than being a Bw6 homozygote (Bw6hmz). Natural Killer (NK) cells from *h/*y+B*57 carriers receive potent educational signals through HLA-B*57 KIR3DL1 ligation leading to high functional potential. NK cells from Bw6hmz are not educated through KIR3DL1 because Bw6 antigens do not interact with this inhibitory receptor. To better understand the impact of KIR/HLA combinations on NK cell mediated anti-viral activity we measured NK cell mediated inhibition of HIV replication in autologous infected CD4 (iCD4) cells by assessing the frequency of p24 positive CD4 targets and supernatant levels of HIV p24 longitudinally in the presence versus absence of NK cells. Forty-seven HIV uninfected subjects were studied, including carriers of *h/*y+B*57, a low expression KIR3DL1 genotype with HLA-B*57 termed *l/*x+B*57, a genotype designated 3DS1+*80I and Bw6hmz. NK cells from *h/*y+B*57 carriers, like those from 3DS1+*80I subjects, inhibited HIV replication in autologous iCD4 cells better than those from Bw6hmz and *l/*x+B*57 carriers. Cell contact between NK and iCD4 cells activated NK cells to inhibit viral replication in a non-contact dependent fashion through secretion of CC-chemokines. iCD4 stimulated NK cells from *h/*y+B*57 and 3DS1+*80I carriers produced higher levels of CC-chemokines than those from Bw6hmz or *l/*x+B*57 carriers. Higher levels of CC-chemokines were produced by KIR3DL1+ than KIR3DL1− NK cells. We conclude that NK-mediated inhibition of viral replication in autologous iCD4 cells is partially due to a block at the level of HIV entry into new targets by secreted CC-chemokines.
Published in the journal: HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets. PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003867
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003867Summary
Carriage of the genetic combination encoding a high expression inhibitory Killer Immunoglobulin-like Receptor (KIR)3DL1 with its ligand, HLA-B*57 (*h/*y+B*57) is associated with slower time to AIDS and better HIV viral load control than being a Bw6 homozygote (Bw6hmz). Natural Killer (NK) cells from *h/*y+B*57 carriers receive potent educational signals through HLA-B*57 KIR3DL1 ligation leading to high functional potential. NK cells from Bw6hmz are not educated through KIR3DL1 because Bw6 antigens do not interact with this inhibitory receptor. To better understand the impact of KIR/HLA combinations on NK cell mediated anti-viral activity we measured NK cell mediated inhibition of HIV replication in autologous infected CD4 (iCD4) cells by assessing the frequency of p24 positive CD4 targets and supernatant levels of HIV p24 longitudinally in the presence versus absence of NK cells. Forty-seven HIV uninfected subjects were studied, including carriers of *h/*y+B*57, a low expression KIR3DL1 genotype with HLA-B*57 termed *l/*x+B*57, a genotype designated 3DS1+*80I and Bw6hmz. NK cells from *h/*y+B*57 carriers, like those from 3DS1+*80I subjects, inhibited HIV replication in autologous iCD4 cells better than those from Bw6hmz and *l/*x+B*57 carriers. Cell contact between NK and iCD4 cells activated NK cells to inhibit viral replication in a non-contact dependent fashion through secretion of CC-chemokines. iCD4 stimulated NK cells from *h/*y+B*57 and 3DS1+*80I carriers produced higher levels of CC-chemokines than those from Bw6hmz or *l/*x+B*57 carriers. Higher levels of CC-chemokines were produced by KIR3DL1+ than KIR3DL1− NK cells. We conclude that NK-mediated inhibition of viral replication in autologous iCD4 cells is partially due to a block at the level of HIV entry into new targets by secreted CC-chemokines.
Introduction
NK cells function in innate immune responses to transformed and virally infected cells. They can exert their anti-viral effects soon after encountering infected targets without prior sensitization [1]. NK cell function is determined by signals from activating and inhibitory cell surface receptors, which include Killer Immunoglobulin-like Receptors (KIR) [2]. Among these are inhibitory KIR3DL1 (3DL1) and activating KIR3DS1 (3DS1) receptors, which are encoded by alleles at the same KIR3DL1/S1 locus [3]. 3DL1 receptors can be classified into those expressed on NK cell surfaces at high levels (*h) low levels (*l) or *004, which is only transiently expressed [4]–[7]. 3DL1 homozygous genotypes can be dichotomized into *h/*y and *l/*x groups where *h/*y genotypes encode receptors expressed on the NK cell surface at higher levels than those encoded by *l/*x genotypes [6].
Epidemiological studies have found that several 3DL1 homozygous genotypes co-carried with a subset of HLA-B and –A alleles belonging to the HLA-Bw4 group are associated with slower time to AIDS and viral load (VL) control [7]. HLA-Bw4 antigens differ from the remaining HLA-Bw6 (Bw6) antigens by amino acids at positions 77–83 [8]. The genotype combinations that confers the highest degree of protection in terms of time to AIDS and VL control is 3DL1*h/*y co-carried with HLA-B*57 (*h/*y+B*57) [7]. Subjects with this combined genotype are more frequent among HIV Exposed Seronegative (HESN) than HIV susceptible individuals, implicating carriage of this genotype combination in reducing HIV infection risk [9]. NK cells from carriers of *h/*y+B*57 have more potent NK cell functional potential as defined by HLA-null cell induced secretion of IFN-γ and TNF-α and expression of CD107a, a marker for degranulation, than those from carriers of the receptor or ligand alone, including those from carriers of the *l/*x+B*57 KIR/HLA genotype and Bw6 homozygotes (Bw6hmz) [9], [10]. Bw6 antigens do not interact with 3DL1 receptors and are thus unable to educate NK cells through this inhibitory NK receptor [11], [12]. NK cell education is an ontological process that depends on the interaction of inhibitory NK receptors, such as 3DL1, with their MHC class I (MHC-1) ligands. The strength of educational signals received during NK cell development determines NK cell functional potential [11], [13], [14]. Thus, NK cells from *l/*x+B*57 carriers may be less functional than those from *h/*y+B*57 positive subjects since the former express less 3DL1 than the later and thus receive lower level educational signals upon interaction with the same ligand [6], [10], [15].
The KIR/HLA combination 3DS1 co-expressed with a Bw4 antigen having an isoleucine at position 80 of the HLA heavy chain (3DS1+*80I) is also associated with slower time to AIDS and VL control [16], [17]. NK cells from carriers of the 3DS1+*80I genotype inhibit viral replication in autologous HIV-infected CD4 (iCD4) T cells more potently than those from individuals carrying the receptor or ligand alone, or neither [18]. Together, these functional studies suggest that the association of certain KIR/HLA genotypes with either protection from HIV infection in HESN subjects or slow time to AIDS and VL control in those who are HIV infected, is linked to NK cell function.
How NK cells inhibit viral replication in autologous CD4 T cells is not completely understood.
One possibility is through the secretion of the CC-chemokines CCL3, CCL4, and CCL5 upon activation following recognition of autologous HIV iCD4 cells. These chemokines can suppress HIV replication by competing with the virus for binding the CCR5 co-receptor and blocking HIV entry into CD4 cells [19], [20]. In this report we investigated whether NK cells from individuals carrying *h/*y+B*57 inhibited HIV replication in autologous HIV iCD4 cells better than those from *l/*x+B*57 carriers and Bw6hmz. The cell contact requirement for inhibition of viral replication was assessed. We also measured the production of CC-chemokines by NK cells stimulated with autologous HIV iCD4 cells. We determined whether CC-chemokine secretion levels differed based on the KIR/HLA genotype and evaluated the effect of CC-chemokine neutralization on NK cell mediated inhibition of HIV replication.
Results
NK cells inhibit HIV viral replication in autologous iCD4 T cells
CD4 cells from 17 individuals were infected with HIV and co-cultured with or without autologous NK cells at an NK∶iCD4 ratio of 10∶1 and 1∶1. Fig. S1 shows that NK cells inhibited viral replication, at all times tested and at both NK∶iCD4 ratios. For the 10∶1 and 1∶1 NK∶iCD4 cell ratios there were no significant between time point differences in viral inhibition (p = 0.15 and p = 0.42, Friedman test). Viral inhibition was significantly higher at days 7 and 10 for the 10∶1 versus 1∶1 NK∶iCD4 ratio (p = 0.51, 0.002 and 0.008 for days 3, 7 and 10, respectively, Mann-Whitney test). The higher viral inhibition levels seen in wells containing NK and iCD4 cells at a 10∶1 ratio compared to a 1∶1 ratio or for iCD4 cells cultured alone could have been due to differences in the number of cells in these culture conditions. For example, it was possible that higher cell numbers limited cell survival and this is what led to inhibition of HIV replication. To rule out this possibility we compared the number of live CD4 cells present at days 7 and 10 of culture between conditions where NK and iCD4 were cultured at 10∶1 with those where iCD4 were cultured alone. No significant differences in CD4 numbers were found (not shown). Based on the 10∶1 NK∶iCD4 T cell ratio showing more potent inhibition of viral replication than the 1∶1 ratio, we used the 10∶1 ratio for subsequent experiments.
NK-iCD4 cell contact contributes to NK cell mediated inhibition of HIV replication
NK cell mediated inhibition of viral replication was assessed by measuring the frequency of intracellular HIV-Gag-p24 positive CD4 cells using anti-p24 specific KC57 monoclonal antibody (mAb). Fig. S2 shows the gating strategy used to assess the percent of p24 positive CD4 cells. Fig. 1A depicts flow cytometry plots showing the frequency of p24 positive CD4 cells at day 7 for several culture conditions for a single individual. Fig. 1B shows longitudinal results for up to 12 subjects, 5 on day 3, 10 on day 7 and 12 on day 10. In the presence of NK cells (NK+iCD4) the frequency of p24 positive CD4 cells was lower than that in cultures of iCD4 cells alone (p = 0.18, p = 0.002 and p<0.001 for day 3, 7 and 10, respectively, Wilcoxon matched-pairs test). When NK and iCD4 cells were cultured in different transwell chambers (NK/iCD4 TW), which prevents NK and iCD4 cell contact, the frequency of p24 positive CD4 cells was significantly higher than in conditions where NK and iCD4 were cultured together either in regular wells or the same chamber of a transwell (NK+iCD4 or NK+iCD4 TW versus NK/iCD4 TW, p<0.05 for all comparisons at days 7 and 10, Wilcoxon). However, the frequency of p24+ CD4 cells in the NK/iCD4 TW condition remained below that observed in iCD4 cells (p≤0.002 for comparisons at days 7 and 10). These results implicate NK iCD4 cell contact as a contributing factor in suppression of virus spread. However, since abrogating NK and iCD4 contact does not return the percent of p24 positive CD4 cells to levels seen in iCD4 cells cultured alone, non-contact dependent mechanisms are also likely involved in NK cell mediated inhibition of HIV replication.
Fig. 1. NK cells inhibit HIV replication in autologous HIV infected CD4 T cells in a contact dependent manner. 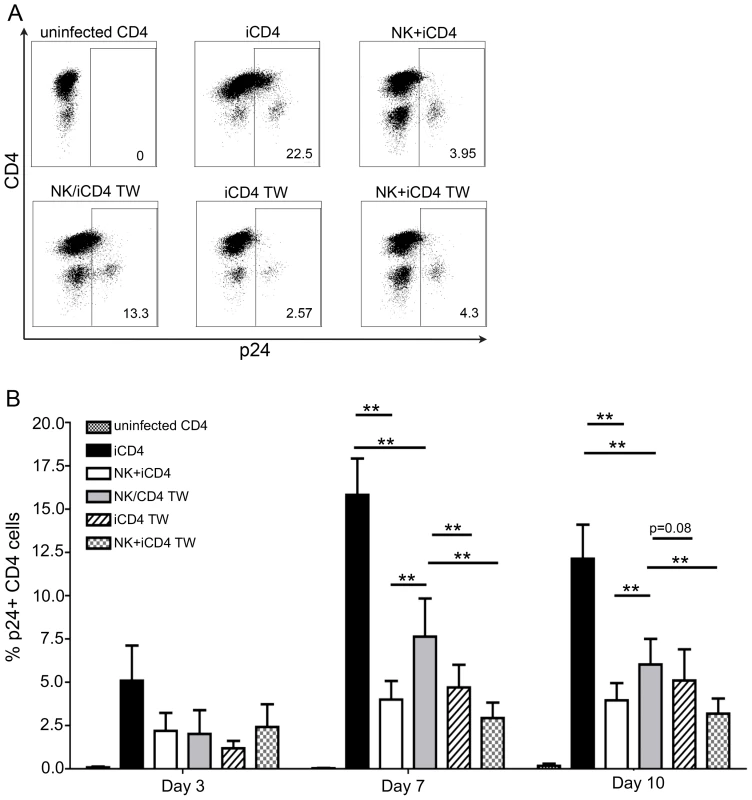
(A) Flow plots show the frequency of p24 positive CD4 cells from a single individual cultured for 7 days under the following conditions: uninfected CD4 T cells cultured alone, infected CD4 (iCD4) cells cultured alone, iCD4 cells cultured with autologous NK cells in the same well at a 10∶1 NK∶iCD4 ratio (NK+iCD4), iCD4 cells and NK cells cultured in separate transwell chambers at a 10∶1 NK∶iCD4 ratio (NK/iCD4 TW), iCD4 cells cultured alone in the upper chamber of a transwell with NK cells and iCD4 cells cultured together in the lower transwell chamber at a 10∶1 NK∶iCD4 ratio (iCD4 TW), iCD4 cells cultured with NK cells in the same transwell chamber at a 10∶1 NK∶iCD4 cell ratio (NK+iCD4 TW). (B) Bar graphs show the frequency of HIV infected cells on days 3, 7 and 10 under the same culture conditions as described in (A) for up to 12 individuals. One subject was positive for *h/*y+B*57, 7 were 3DS1+*80I, 2 were Bw6hmz, 1 was 3DS1+Bw4 not *80I and 1 was 3DL1hmz+*80I (not B*57). Bar height and error bars represent the mean and the standard error of the mean for each group. Lines linking bars indicate comparisons where means are significantly different. “*” = a p-value<0.05, “**” = a p-value of <0.01. If iCD4 cells and co-cultures of NK and iCD4 cells are incubated in upper and lower transwell chambers, respectively, the frequency of p24 positive CD4 cells in the upper chamber (iCD4 TW) is lower than that seen when only NK cells are present in the lower chamber (NK/iCD4 TW) (p = 0.007 and p = 0.08 for days 7 and 10, Wilcoxon). These results suggest that contact between NK and autologous HIV iCD4 cells produces soluble factors that can then suppress HIV spread in the same well or cross a transwell membrane to suppress the spread of HIV in iCD4 cells physically separated from NK cells.
NK cells produce CC-chemokines in response to stimulation with autologous HIV iCD4 cells
We questioned whether autologous iCD4 cells could activate NK cells to secrete CC-chemokines. We reasoned that if this were the case, these soluble factors could be responsible for inhibiting HIV replication under conditions where iCD4 are either co-cultured with NK cells or in a separate transwell chamber from NK+iCD4 cultures.
We assessed CC-chemokine secretion under several conditions at days 1, 2 and 3 of culture. Fig. 2A–C show that PHA stimulated HIV iCD4 cells co-cultured with NK cells and recombinant human IL-2 (IL-2) (NK+iCD4+IL-2) produced CCL3, CCL4 and CCL5 at higher levels than do either NK cells alone with IL-2 (NK+IL-2), NK cells cultured with uninfected CD4 cells and IL-2 (NK+CD4+IL-2) or iCD4 cells with IL-2 (iCD4+IL-2) (p<0.05 for all CC-chemokines on each day tested, Kruskal-Wallis test). All pair-wise comparisons between CC-chemokine levels secreted in the NK+iCD4+IL-2 condition and those in each of the other 3 conditions were statistically significant, except for those between NK+iCD4+IL-2 and NK+CD4+IL-2 for CCL5 at days 1, 2 and 3 (p = 0.07, 0.25 and 0.34, respectively, Dunn's post-test comparisons). NK cells cultured without IL-2 and CD4 cells, whether PHA stimulated or not, HIV infected or not and cultured with or without IL-2 produced low levels of the 3 CC-chemokines in the range of 200 pg/ml or lower (data not shown). Thus, NK cells stimulated by autologous HIV iCD4 cells and IL-2 are a source of secreted CC-chemokines and produce more CC-chemokines than NK cells or iCD4 cells alone culture media containing IL-2.
Fig. 2. Infected CD4 T cells stimulate autologous NK cell to produce CC-chemokines. 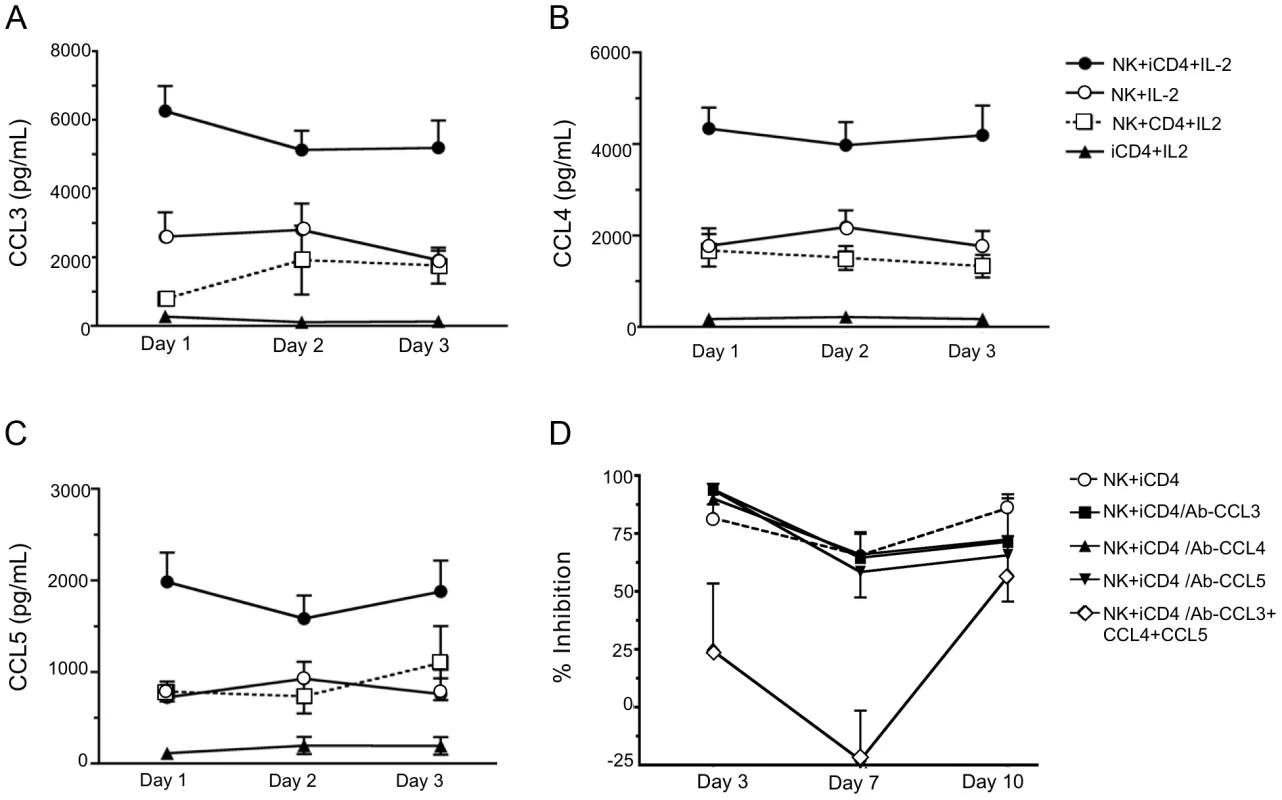
Levels of CCL3 (A), CCL4 (B) and CCL5 (C) secreted into supernatants after days 1, 2 and 3 under various culture conditions were assessed by ELISA. Shown are results for NK cells cultured with infected CD4 cells (iCD4) at a 10∶1 ratio with 100 international units (IU)/ml of human recombinant IL-2 (IL-2) (NK+iCD4+IL-2, n = 33 observations), NK cells cultured with 100 IU/ml IL-2 (NK+IL-2, n = 24 observations), NK cells cultured with uninfected CD4 and 100 IU/ml of IL-2 (NK+CD4+IL-2, n = 9 observations) and iCD4 with 100 IU/ml IL-2 (iCD4+IL-2, n = 11 observations). Data points and error bars represent the mean and the standard error of the mean for the groups. Results were generated using subjects with the following KIR/HLA genotypes; *h/*y+B*57 (n = 4), 3DS1+*80I (n = 5), Bw6hmz (n = 4), *l/*x+B*57 (n = 4) and other KIR/HLA (n = 5). Samples were tested on up to 4 occasions in separate experiments. (D) Percent inhibition of viral replication on days 3, 7 and 10 of an NK cell autologous iCD4 cell co-culture (10∶1 ratio) in the absence of anti-CC-chemokine neutralizing antibodies (nAbs), in the presence of anti-CCL3, anti-CCL4 or anti-CCL5 nAbs, individually, or together. Results were generated using subjects with the following KIR/HLA gentotypes; *h/*y+B*57 (n = 2) and 3DS1+*80I (n = 5). Data points and error bars represent the mean and standard error of the mean of values for the groups. Inhibition of HIV replication by NK cells can be reversed by neutralizing anti-CCL3, CCL4 and CCL5 antibodies (Abs)
To confirm that CC-chemokines contribute to inhibition of HIV replication, neutralizing Abs to each CC-chemokine were added to iCD4 cells at the same time as NK cells. As seen in Fig. 2D for percent inhibition of viral replication compared to iCD4 cells alone, the addition of neutralizing Abs to individual CC-chemokines had no effect on percent inhibition of HIV replication mediated by NK cells (p>0.05 for all comparisons, Wilcoxon matched pairs test). Addition of Abs to all 3 chemokines reduced NK-mediated HIV suppression. Comparisons of percent inhibition of HIV replication between NK+iCD4+neutralizing Abs to all 3 CC-chemokines and NK+iCD4 with either no Abs or antibodies to single CC-chemokines were significant for all comparisons except one at days 3 and 7 (p<0.05, Wilcoxon). The exception was the comparison of percent inhibition between NK+iCD4+neutralizing Abs to the 3 CC-chemokines and NK+iCD4 with no Abs (p = 0.23, Wilcoxon). None of the comparisons for percent inhibition at day 10 achieved statistical significance. These results indicate that iCD4 stimulated NK cell secretion of CC-chemokines contributes to inhibition on HIV replication.
NK cells from individuals carrying protective KIR/HLA genotypes inhibit HIV replication more potently than those from Bw6hmz
We next questioned whether NK cells from carriers of *h/*y+B*57, a genotype combination that confers protection from HIV disease progression, VL control and lowered infection risk, inhibits viral replication better than NK cells from Bw6hmz [7], [9]. Fig. 3 shows results for inhibition of HIV replication by NK cells from subjects positive for *h/*y+B*57 (n = 7), 3DS1+*80I (n = 9), *l/*x+B*57 (n = 4) and Bw6hmz (n = 11). In this experiment NK cells from 3DS1+*80I carriers are used as a positive control since Alter et al. had previously shown their capacity to inhibit HIV replication in autologous iCD4 cells [18]. NK cells from *h/*y+B*57 carriers inhibited HIV replication better than those from Bw6hmz and this was significant at all times tested (p = 0.01, 0.007, and 0.05 for days 3, 7, and 10, respectively, Mann-Whitney test). They also inhibited HIV replication better than those from *l/*x+B*57 carriers (p<0.05 for days 7 and 10). We confirmed that NK cells from 3DS1+*80I carriers inhibit HIV replication better than those from Bw6hmz and *l/*x+B*57 carriers (p<0.05 for all comparisons at days 7 and 10). NK cells from carriers of 3DS1+*80I and *h/*y+B*57 inhibit viral replication in autologous iCD4 cells with a similar potency at the times tested. We verified that these results are not due to a differential ability of HIV to replicate in CD4 cells from subjects carrying these 4 genotypes (Fig. S3). HIV p24 levels in culture supernatants of iCD4 cells from carriers of the 4 genotypes was equivalent at all times tested (p>0.05, Kruskal-Wallis test). Together these results show that NK cells from carriers of the *h/*y+B*57 genotype inhibit HIV replication in autologous CD4 cells better than those from Bw6hmz or carriers of the *l/*x+B*57 genotype.
Fig. 3. NK cells from subjects carrying *h/*y+B*57 and 3DS1+*80I suppress viral replication better than those from Bw6hmz and *l/*x+B*57 carriers. 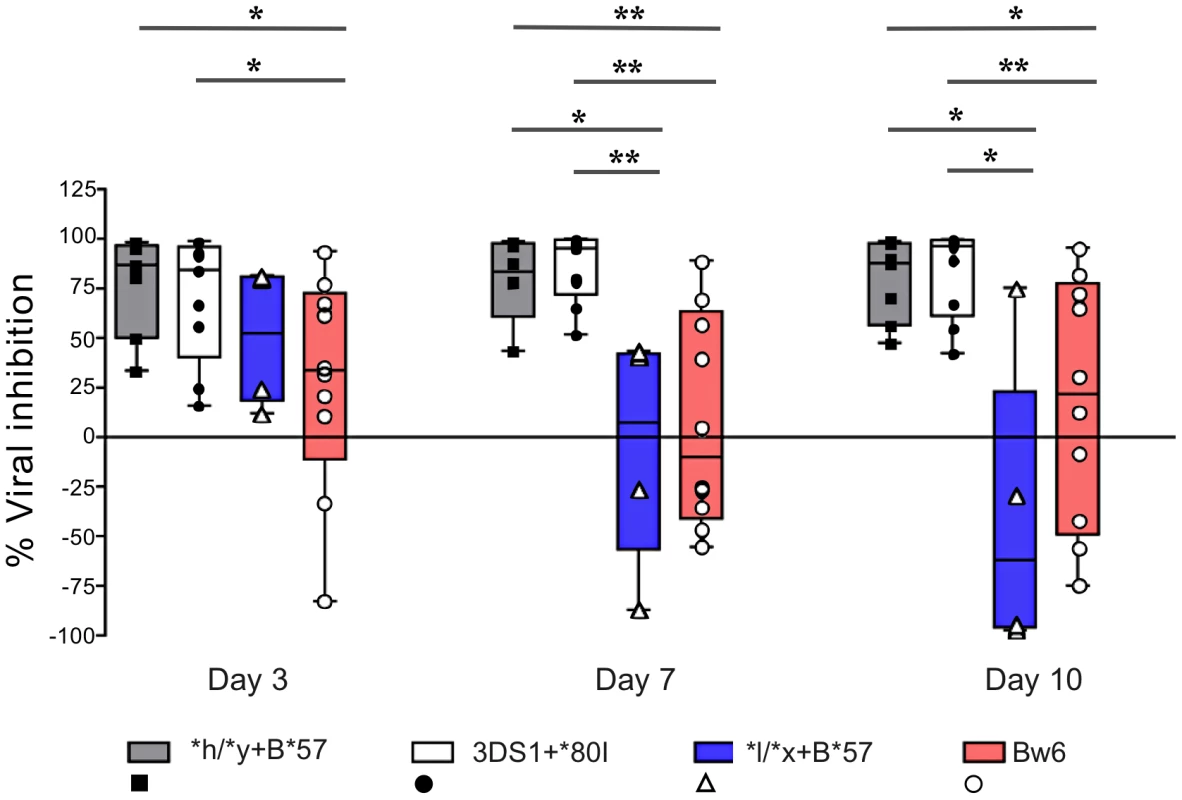
The box and whisker plots show the percent viral inhibition observed when NK cells from subjects positive for *h/*y+B*57 (n = 7) 3DS1+*80I (n = 9), Bw6hmz (n = 10) and *l/*x+B*57 (n = 4) are cultured with autologous HIV infected CD4 (iCD4) cells at a ratio of 10∶1 for up to 10 days. The line in each box represents the median value, the lower and upper limits of the boxes the 25% and 75% quartiles and the whiskers the minimum and maximum values for each group; each point is the percent viral inhibition value for a single individual. Lines linking groups indicate comparisons where medians were significantly different. “*” = p<0.05, “**” = p<0.01. NK cells from individuals carrying protective KIR/HLA genotypes secrete higher levels of CC-chemokines than those from Bw6hmz
We next asked whether NK cells from individuals carrying protective KIR/HLA genotype combinations and Bw6hmz differed from each other in the amount of CC-chemokines they secreted upon stimulation with autologous iCD4 cells. We assessed the amount of CC-chemokines secreted over 3 days by NK cells from 7 *h/*y+B*57, 12 3DS1+*80I and 5 *l/*x+B*57 carriers and 10 Bw6hmz. Stimulated NK cells from *h/*y+B*57 and 3DS1 +*80I carriers secreted similar levels of CCL3, CCL4 and CCL5 to each other and more than those from Bw6hmz (Figs. 4, S4, S5 and Table S1). CC-chemokine secretion by stimulated NK cells from *l/*x+B*57 carriers was similar to that from Bw6hmz for CCL3 and CCL5 and higher than that from Bw6hmz for CCL4 (Fig. S5 and Table S1). In general, iCD4 stimulated NK cells from *h/*y+B*57 and 3DS1+*80I carriers secreted higher CC-chemokine levels compared to those from *l/*x+B*57 carriers, though several of these comparisons did not achieve statistical significance (Fig. S5 and Table S1).
Fig. 4. NK cells from subjects positive for *h/*y+B*57 secrete more CC-chemokines in response to autologous HIV infected CD4 (iCD4) cells than those from Bw6hmz. 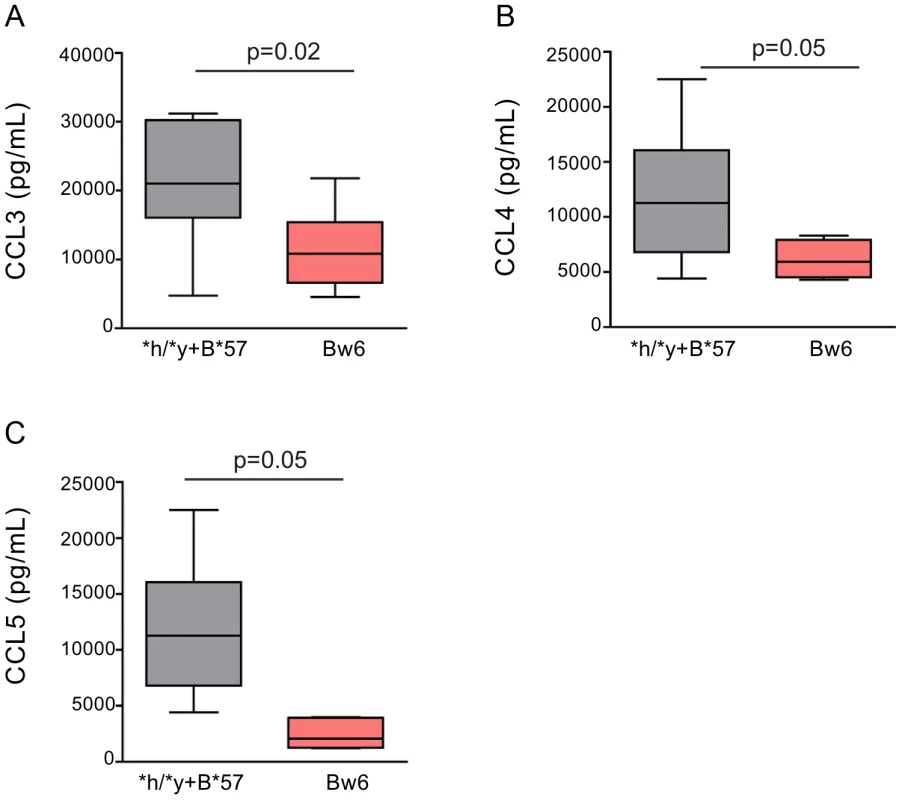
Box and whisker plots show the levels of CCL3 (A), CCL4 (B) and CCL5 (C) secreted over 3 days into the supernatant of cultures of NK cells and autologous iCD4 cells at a 10∶1 ratio from individuals positive for *h/*y+B*57 (n = 7) or from Bw6hmz (n = 10). The line in each box represents the median value, the lower and upper limits of the boxes the 25% and 75% quartiles and the whiskers the minimum and maximum values for each group. Lines linking groups indicate comparisons where medians were significantly different. We also stimulated NK cells overnight with autologous 7 day iCD4 and assessed intracellular CCL3, CCL4, IFN-γ and CD107a expression by total NK cells as well as by 3DL1+ and 3DL1− NK cell subsets using the gating strategy shown in Fig. S6. Fig. 5 shows for CCL3 in the upper and CCL4 in the lower panels that a higher frequency of NK cells from *h/*y+B*57 carriers secrete these chemokines upon stimulations with autologous iCD4 than those from Bw6hmz. A similar but non-significant trend is when 3DL1+ NK cells are gated on that is absent in the 3DL1− population (Fig. 5). We also compared the frequency of 3DL1+ and 3DL1− cells within individuals secreting CCL3, CCL4 and IFN-γ and expressing CD107a (Figs. S7, S8, S9, S10). In general, a higher frequency of functional 3DL1+ than 3DL1− NK cells was observed in *h/*y+B*57 carriers (p = 0.15, 0.02, 0.05 for CCL3, CCL4 and IFN-γ secretion, respectively), but not in *l/*x+B*57 carriers and Bw6hmz. It would have been desirable to compare the frequency of intracellular CCL3, CCL4 and IFN-γ positive cell in 3DL1+ *h versus *l allele expressing NK cell subsets following iCD4 stimulation of *l/*x+B*57 NK cells. Unfortunately, only 2 *l/*x+B*57 subjects carried both an *h and *l allele. The others were either homozygous for *l alleles or carried an *l and an *004 allele. The composition of the *l/*x+B*57 group precluded making firm conclusions regarding CC-chemokine or IFN-γ expression in these 3DL1+ NK subsets. Together, the intracellular cytokine staining results show that KIR/HLA genotype is a determinant of iCD4 stimulated NK cell functionality with regard to CC-chemokine secretion. The higher functionality of 3DL1+ NK cells in *h/*y+B*57 compared to *l/*x+B*57 carriers and Bw6hmz implicates this KIR/HLA combination in potent NK cell licensing for functional potential.
Fig. 5. Percent of CCL3+ and CCL4+ NK cells and NK cell subsets following stimulation with autologous HIV infected CD4 (iCD4) cells. 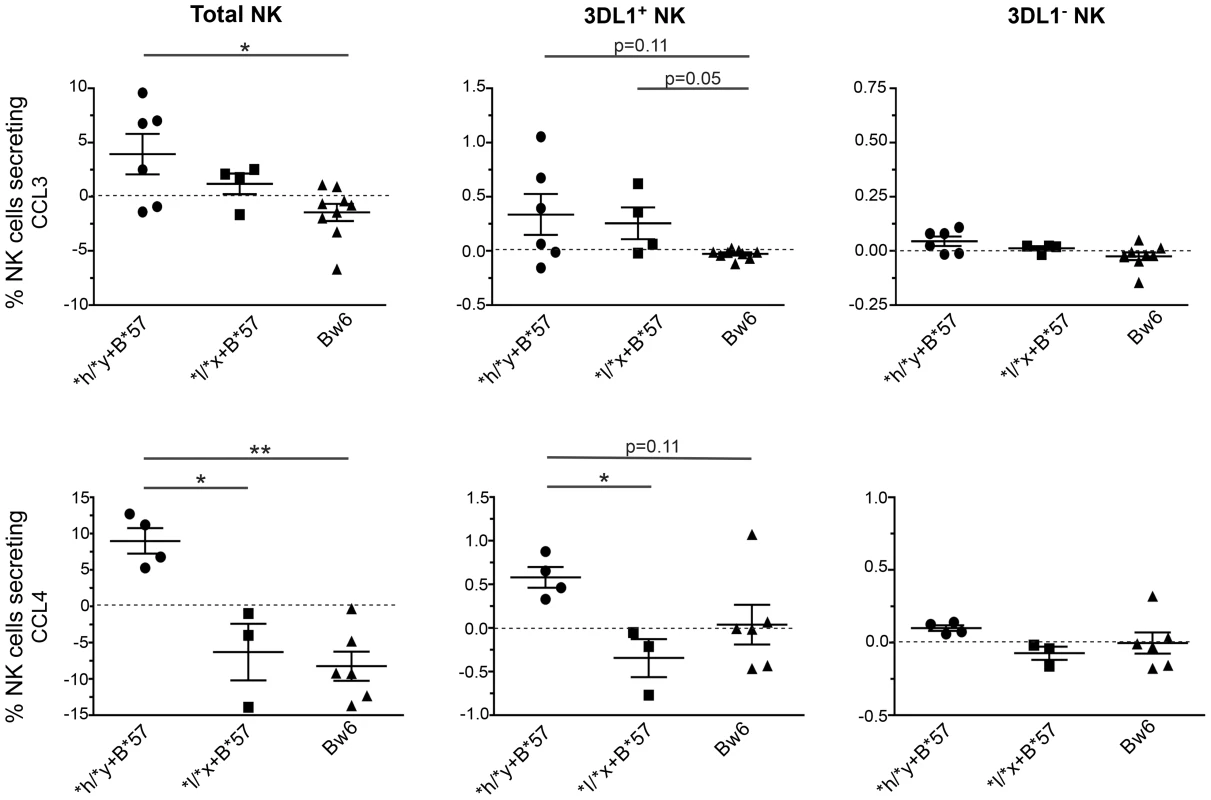
CD4 cells infected with HIV and cultured for 7 days were used to stimulate autologous NK cells for 24+ (upper panels) and CCL4+ (lower panels) total NK cells (left), KIR3DL1+ (middle) and KIR3DL1− (right) NK cell subsets in subjects positive for *h/*y+B*57 (n = 7) *l/*x+B*57 (n = 4) and Bw6hmz (n = 9). Each point represents the value for a single individual, the line and error bars through each group show the mean and the standard error of the mean for each data set. Lines linking groups indicate between-group comparisons. “*” = a p-value<0.05, “**” = a p-value of <0.01. Discussion
In this report we showed that NK cells cultured with autologous iCD4 cells limit the spread of HIV resulting in a lower frequency of HIV iCD4 cells and lower levels of viral replication compared to iCD4 cells cultured alone. Contact between NK and iCD4 cells stimulates NK cells to produce soluble factors, which suppress HIV replication in a non-contact dependent fashion. NK cells activated by autologous iCD4 cells in the presence of IL-2 secrete CC-chemokines at higher levels than when only IL-2 is present. CC-chemokine secretion is responsible, at least in part, for the inhibitory effect of NK cells on viral replication. KIR/HLA genotype influences the potency of inhibition of viral replication. We showed that NK cells from *h/*y+B*57 and 3DS1+*80I carriers, genotypes associated slower time to AIDS and VL control, inhibited HIV replication more potently than did those from Bw6hmz and carriers of the *l/*x+B*57genotype. NK cells, and in particular the 3DL1+ subset of NK cells, from carriers of the *h/*y+B*57 genotype secrete higher levels of CC-chemokines than those from Bw6hmz and *l/*x+B*57 subjects.
The superior control of HIV replication in autologous iCD4 cells by NK cells from carriers of *h/*y+B*57 versus those from *l/*x+B*57 and Bw6hmz subjects implicates NK cell education as a determinant of this anti-viral NK function. NK cell education is important for the development of self-tolerant NK cells and for endowing NK cells with the capacity to mediate cytokine/chemokine secretion and cytolysis upon encountering cells with reduced MHC-I cell surface expression such as occurs in the context of HIV infected targets [11], [21], [22]. The ligation of inhibitory NK receptors such as 3DL1 is required for NK education but the process is tuned by the set of signals received from all the NK cell surface activating and inhibitory receptors interacting with their ligands on neighboring target cells [23]–[25]. The stronger the inhibitory signals received during NK cell education the broader and more potent the effector functions that NK cells will have against appropriate targets [23]. The *h/*y+B*57 KIR/HLA combination appears to be a particularly potent one for NK cell education, since NK cells from *h/*y+B*57 carriers showed higher functionality when stimulated with HLA-null cells than those from carriers of 3DL1*h/*y genotypes co-carried with other Bw4 or *80I alleles, 3DL1*l/*x genotypes co-carried with B*57 or those from Bw6hmz [10], [15]. The difference in functional potential between NK cells from carriers of *h/*y+B*57 versus those from 3DL1hmz who carry other Bw4 alleles may reflect differences in the impact of HLA-B*57 versus other Bw4 antigens in providing educational signals to NK cells during development. Transgenic mice expressing single MHC-I alleles have been used to show that MHC-I antigens differ in their impact on NK cell education [24]. The strength of the inhibitory input during education, as determined by the strength of the interaction between inhibitory NK receptors and their ligands, is directly related to the functional responsiveness of individual NK cells [23], [24]. Thus, it appears that B*57 differs from most other Bw4 molecules in the strength with which it interacts with 3DL1 to educate NK cells. NK cells from 3DL1*h/*y positive subjects express higher levels of 3DL1 inhibitory receptors than those from 3DL1*l/*x positive individuals [6] The observation that NK cells from *l/*x+B*57 carriers secrete less CC-chemokines and inhibit HIV replication more poorly than those from *h/*y+B*57 carriers may be related to less potent NK education due to lower levels of cell surface 3DL1 mediating lower inhibitory signals for NK cell education, even in the presence of the potent B*57 3DL1 ligand. A caveat to this interpretation is that while there is experimental evidence that B*57 binds 3DL1 it has not been demonstrated that the affinity of the interaction between these 2 molecules is greater than that between 3DL1 and other Bw4 molecules because different peptides influence 3DL1 Bw4 binding [26], [27]. In the presence of the same epitope and 3DL1 receptor HLA-Bw4*80T variants bind with about 60% of the affinity of B*57 [27].
The impact of *h/*y+B*57 on NK cell education and the relationship between NK education and NK cell responsiveness may underlie epidemiological findings that carriers of this genotype have a lower risk of HIV infection and in those who become infected have a slower time to AIDS and lower VL than carriers of other 3DL1 hmz Bw4 genotypes, including *l/*x+B*57 carriers [7], [9]. The influence of *h/*y+B*57 on NK cell education may also play a role in the superior ability of NK cells from carriers of this KIR/HLA genotype to inhibit viral replication in autologous HIV infected cells compared to those from Bw6hmz.
It is notable that the frequency of p24 positive CD4 cells in conditions where NK and iCD4 cells are in separate transwells is lower than that of iCD4 cells cultured alone but higher than that of iCD4 cells and NK cells cultured together. This implies that NK-CD4 cell contact contributes to NK cell activation and secretion of soluble factors that can inhibit HIV replication in a non-contact dependent manner. IL-2 by itself can also activate NK cells to secrete soluble factors such as CC-chemokines, though at lower levels than when iCD4 cells are also present. This may be why the percent of p24+ CD4 cells in conditions where iCD4 and NK cells are in separate transwell chambers is not as high as when iCD4 are cultured alone. It is not known whether these soluble factors are limited to CC-chemokines. Simultaneous neutralization of the CCL3, CCL4 and CCL5 restored HIV replication measured at 3 and 7 days of culture to levels that were significantly higher than when NK and iCD4 cells were co-cultured in the absence of CC-chemokine neutralization. Neutralization of all 3 CC-chemokines was not sufficient to reduce NK cell mediated inhibition of HIV replication at day 10 of culture. The reason for this is unclear but may be due to the continued production of chemokines over and above the amounts that anti-CC-chemokine Abs are able to neutralize. High inter-subject variability precludes making a clear determination as to whether CC-chemokine neutralization is sufficient to reverse NK cell mediated inhibition. It is possible that iCD4 stimulate NK cells to inhibit HIV replication by other mechanisms in addition to CC-chemokine secretion. These activities could target other stages of the HIV replication cycle and may or may not be dependent on contact between NK and iCD4 cells.
Previous studies have shown that NK cells secrete CC-chemokines following stimulation through CD16 cross-linking and co-culture with iCD4 cells in the presence of IL-2 [20]. Here we report for the first time that a KIR/HLA genotype combination that influences the potency of NK cell education also determines the level of CC-chemokines that NK cells secrete in response to autologous iCD4 cells. CC-chemokines can bind CCR5, the HIV co-receptor, and prevent HIV from interacting with this receptor thus reducing HIV entry [19], [20]. Transwell experiments implicate cell contact as a factor in NK cell stimulation leading to CC-chemokine secretion.
Pelak et al. reported that in carriers of 3DS1+*80I, the copy number of 3DL1 alleles influenced NK cell mediated inhibition of HIV replication in autologous iCD4 T cells [28]. Copy number variation (CNV) is common at the 3DL1/S1 locus. Screening for CNV at this locus revealed no duplications or deletions at this locus among subjects having the 4 genotypes focused on in this study. Therefore, CNV at the 3DL1/S1 locus can be excluded as a factor influencing the experimental findings reported here.
In summary, we show that NK cells from carriers of *h/*y+B*57 inhibit HIV viral replication in autologous iCD4 cells more effectively than those from *l/*x+B*57 carriers and Bw6hmz. The level of anti-viral function of NK cells from carriers of this genotype is likely related to NK cell education arising from B*57 interactions with high expression inhibitory 3DL1 receptors. Anti-viral function is mediated at least in part by CC-chemokine secretion levels able to block HIV entry into CD4 cell targets. The higher level of CC-chemokine secretion by NK cells from carriers of protective versus non-protective KIR/HLA genotypes may underlie their superior ability to inhibit HIV replication in infected targets.
Materials and Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. It was approved by the Institutional Review Boards of the Comité d'Éthique de la Recheche du Centre Hospitalier de l'Université de Montréal and the Research Ethics Committee of the McGill University Health Centre - Montreal General Hospital. All subjects provided written informed consent for the collection of samples and subsequent analysis.
Study population
We studied 47 HIV seronegative individuals, including 7 who were positive for *h/*y+B*57, 12 for 3DS1+*80I, 11 who were 3DL1hmz and Bw6hmz, 4 who were *l/*x+B*57 positive and 13 with other KIR/HLA genotypes (Table 1). Informed consent was obtained from all study subjects, and the research conformed to all ethical guidelines of all the authors' institutions.
Tab. 1. Characteristics of study subjects. 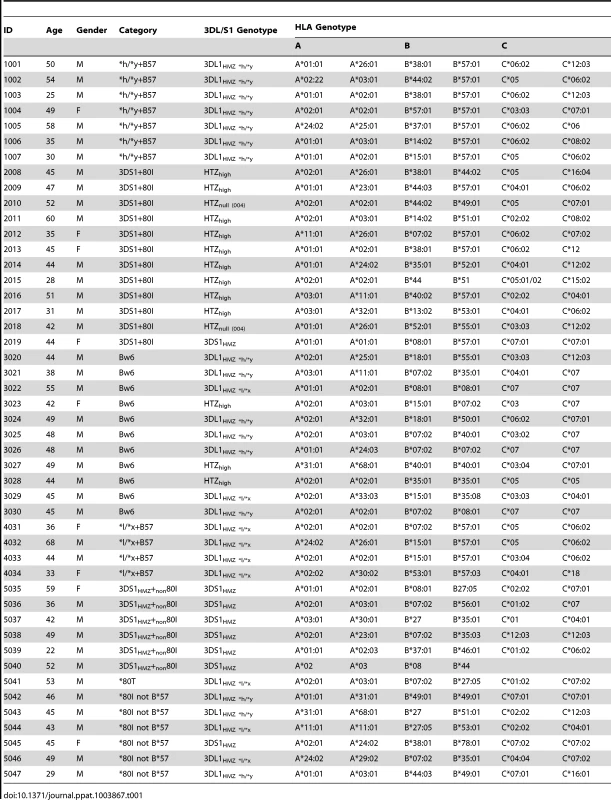
Genotyping
All subjects were typed for MHC-I alleles by sequence based typing using kits from Atria Genetics, Inc. (South San Francisco, CA). Assign 3.5+ software was used to interpret sequence information for allele assignment (Conexio Genetics, Perth, Australia). KIR3DL1/S1 generic genotyping was performed by PCR using 2 pairs of primers specific for either 3DL1 or 3DS1 alleles as previously described [29]. 3DL1 allotyping was done by sequencing 3DL1 exons as previously described [9]. Single nucleotide polymorphisms (SNP) corresponding to the 3DL1 alleles were identified by aligning the sequenced DNA to a reference consensus sequence consisting of 3DL1 cDNA sequences. The *h/*y genotype refers to a 3DL1 homozygous genotype with no *l alleles. Bw6hmz lacked Bw4 alleles at the HLA-A and -B loci.
Cell purification
Peripheral blood mononuclear cells (PBMC) were isolated from blood by density gradient centrifugation (Ficoll-Paque; Pharmacia, Uppsala, Sweden) and cryopreserved in 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) with 90% fetal bovine serum (FBS; Wisent, Inc. St. Bruno, QC, Canada). CD4 T cells were isolated from thawed PBMC by positive selection using immunomagnetic beads (STEMCELL Technologies, Inc. Vancouver, BC, Canada). The purity of the CD4 cell population was verified by flow cytometry (average 95.3%). NK cells were isolated from thawed PBMC by negative selection (STEMCELL Technologies, Inc.) and yielded an average purity of 97.2%.
Inhibition of viral replication assay
Purified CD4 cells (106/ml) were stimulated with 1 ug/ml PHA-P (MP Biomedicals, Santa Ana, CA) and 100 international units (IU)/ml of IL-2 (Chiron Corp., Emeryville, CA) overnight in RPMI medium containing 2 mM L-glutamine, 100 IU/ml Penicillin, 100 µg/ml Streptomycin (cRPMI) (all from Wisent) supplemented with 10% FBS, (Wisent, [R10]) at 37°C in a 5% CO2 humidified incubator. Stimulated CD4 cells were then washed three times with cRPMI supplemented with 2% FBS (R2), and cultured in R10 with 100 IU IL-2 for 3 days. On day 4, CD4 cells were infected at a multiplicity of infection of 0.01 with HIV-1JR CSF in R10 for 4 hrs and washed three times with R2. Equal numbers (3.0 to 4.0×104) of these iCD4 cells were plated at NK∶iCD4 ratios of 10∶1, 1∶1 or alone for 10 days in 300 ul of R10; 100 IU/ml IL-2. Supernatants were collected by removing supernatants and replenishing wells with 300 ul of fresh R10; 100 IU/ml IL-2 on days 3, 7 and 10 for assessment of p24 levels and on days 1, 2 and 3 for assessment of CC-chemokine levels.
For some experiments CD4 cells were collected on days 3, 7 and 10 for intracellular Gag p24 staining. Cells were stained with an Aqua amine reactive fluorescent dye (Invitrogen, Burlington, ON, Canada) to identify viable cells. Cell surface staining with anti-CD3 APC-eFluor 780 (eBioscience, San Diego, CA) and anti-CD4 PE (BD Biosciences, Mississauga, ON, Canada) were used to detect CD4 T cells. After fixation and permeabilization intracellular HIV Gag p24 positive cells were detected using the mAb KC57 (Beckman-Coulter, Mississauga, ON, Canada). Acquisition was done on a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). Between 50,000 and 200,000 events were acquired per sample. Flow cytometry results were analyzed with Flowjo software Mac 9.4 (Treestar, Ashland, OR). The gating strategy used for intracellular p24 positive cells is shown in Fig. S2.
To ascertain the requirement for NK-CD4 cell contact for NK cell–mediated inhibition of HIV replication, autologous NK cells were physically separated from iCD4 cells in transwell plates (Corning, Tewksbury MA). iCD4 cells (105/well) were cultured in the upper chamber with either 106 NK cells alone or 106 NK cells with 105 iCD4 T cells in the lower chamber. Cells in wells containing iCD4 cells were collected on days 3, 7 and 10 to quantitate the frequency of HIV Gag p24 positive CD4 cells.
NK cell stimulation
iCD4 or uninfected CD4 cells were prepared as above and cultured in R10; 100 IU/ml IL-2. Purified NK cells, isolated as described above were plated at a 10∶1 ratio with either iCD4 or CD4 cells for 24 hrs in R10; 100 IU/ml IL-2. Brefeldin (6 ug/ml, Sigma-Aldrich) and monensin (5 ug/ml, Golgi Stop, BD Biosciences) were added 5 hrs before the end of the culture period. Cells were stained for viability using an amine reactive dye (Invitrogen) and their Fc receptors were blocked using the TruStain FcX reagent (BioLegend, San Diego, CA) according to manufacturer's directions. Cells were then stained for surface markers with CD3-BV605 (OKT3), CD56-BV711 (NCAM), 3DL1-BV421 (DX9) (all from BioLegend) and anti-CD107a-PE-CF594 (BD), for 30 min. Samples were washed with phosphate buffered saline (PBS) containing 1% FBS (Wisent), fixed and permeabilized using a cell fixation and permeabilization kit (Invitrogen) and stained for intracellular cytokines/chemokines using anti-CCL3-APC (93342), anti-IFN-γ-Alexa700 (B27) (BD) and anti-CCL4-FITC (24006) (R&D Systems, Minneapolis, MN), for 15 min. After washing, samples were fixed with a solution of 1% paraformaldehyde (Fisher Scientific, Ottawa, ON, Canada) and kept in the dark at 4°C until acquisition.
Between 400,000 and 1,500,000 total events were acquired for each sample on an LSRFortessa flow cytometer (BD).The gating strategy used to analyze the functional profile of NK cells stimulated with iCD4 or uninfected CD4 cells is shown in Fig. S6. For all analyses, NK cells were defined as CD3−CD56+. The percent of CCL3, CCL4, IFN-γ and CD107a positive total, 3DL1+ and 3DL1− NK cells was determined. Flow cytometry analysis for NK cell activation following stimulation was performed using FlowJo software. The results for the percent of functional NK cells stimulated with iCD4 reported were background corrected for NK cells stimulated with uninfected CD4 cells. Statistical analyses were performed using background corrected results.
HIV p24 ELISA
An Ab-sandwich ELISA was used to detect HIV Gag p24 in culture supernatants as described previously [30]. Briefly, 96-well ELISA plates were coated with anti-p24 Ab clone 183 H12-5C at 2.5 ug/ml overnight at RT. Culture supernatants (100 ul/well) were added for 1 hr at 37°C. The following sequential additions were made with 3 washes between steps using PBS; 0.05% Tween 20 (Sigma-Aldrich): 1) biotinylated anti-p24 Ab clone 31-90-25 at 0.5 ug/ml for 1 hr at 37°C, 2) 0.067 ug/ml horseradish peroxidase conjugated-steptavidin (Fitzgerald Industries International, Acton, MA) for 30 min at RT and 3) 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate (Sigma-Aldrich) for 20 min at RT. Color development was stopped with 50 ul of 1 M H2SO4. ELISA plates were read at OD450 on an ELISA plate reader (PerkinElmer, Montreal, QC, Canada). The p24 concentration in test supernatants was determined by comparison with a p24 standard curve included on each plate. Percent viral inhibition was calculated using the equation [(p24 levels in iCD4 wells – p24 levels in NK+iCD4 wells)/(p24 levels in iCD4 wells) *100].
CC-chemokine ELISA
Culture supernatant levels of CC-chemokines was measured using Ab sandwich ELISAs detecting CCL3, CCL4 and CCL5 (R&D Systems) according to directions provided by the manufacturer.
Statistical analysis
Statistical analyses and graphical presentations were performed using GraphPad InStat 3.05 and GraphPad Prism 5. Mann-Whitney and Kruskal-Wallis tests with Dunn's post tests were used to compare the significance of mean differences between 2 and more than 2 groups, respectively. Wilcoxon matched pairs and Friedman tests were used to compare the effect of a condition on 2 or more than 2 matched groups, respectively. P-values less than 0.05 were considered significant.
Supporting Information
Zdroje
1. StetsonDB, MohrsM, ReinhardtRL, BaronJL, WangZE, et al. (2003) Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med 198 : 1069–1076.
2. LanierLL (2005) NK cell recognition. Annu Rev Immunol 23 : 225–74.: 225–274.
3. BashirovaAA, ThomasR, CarringtonM (2011) HLA/KIR restraint of HIV: surviving the fittest. Annu Rev Immunol 29 : 295–317 10.1146/annurev-immunol-031210-101332 [doi].
4. PandoMJ, GardinerCM, GleimerM, McQueenKL, ParhamP (2003) The protein made from a common allele of KIR3DL1 (3DL1*004) is poorly expressed at cell surfaces due to substitution at positions 86 in Ig domain 0 and 182 in Ig domain 1. J Immunol 171 : 6640–6649.
5. TanerSB, PandoMJ, RobertsA, SchellekensJ, MarshSG, et al. (2011) Interactions of NK cell receptor KIR3DL1*004 with chaperones and conformation-specific antibody reveal a functional folded state as well as predominant intracellular retention. J Immunol 186 : 62–72 jimmunol.0903657 [pii]; 10.4049/jimmunol.0903657 [doi].
6. YawataM, YawataN, DraghiM, LittleAM, PartheniouF, et al. (2006) Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med 203 : 633–645.
7. MartinMP, QiY, GaoX, YamadaE, MartinJN, et al. (2007) Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 39 : 733–740.
8. WanAM, EnnisP, ParhamP, HolmesN (1986) The primary structure of HLA-A32 suggests a region involved in formation of the Bw4/Bw6 epitopes. J Immunol 137 : 3671–3674.
9. BouletS, KleymanM, KimJY, KamyaP, SharafiS, et al. (2008) A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS 22 : 1487–1491.
10. KamyaP, BouletS, TsoukasCM, RoutyJP, ThomasR, et al. (2011) Receptor-ligand requirements for increased NK cell poly-functional potential in *h/*y+B57 HIV-1 infected Slow progressors. J Virol JVI.02652-10 [pii]; 10.1128/JVI.02652-10 [doi].
11. KimS, SunwooJB, YangL, ChoiT, SongYJ, et al. (2008) HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A 105 : 3053–3058 0712229105 [pii]; 10.1073/pnas.0712229105 [doi].
12. GumperzJE, LitwinV, PhillipsJH, LanierLL, ParhamP (1995) The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med 181 : 1133–1144.
13. KimS, Poursine-LaurentJ, TruscottSM, LybargerL, SongYJ, et al. (2005) Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436 : 709–713 nature03847 [pii];10.1038/nature03847 [doi].
14. AnfossiN, AndreP, GuiaS, FalkCS, RoetynckS, et al. (2006) Human NK cell education by inhibitory receptors for MHC class I. Immunity 25 : 331–342.
15. BouletS, SongR, KamyaP, BruneauJ, ShoukryNH, et al. (2010) HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J Immunol 184 : 2057–2064 jimmunol.0902621 [pii];10.4049/jimmunol.0902621 [doi].
16. MartinMP, GaoX, LeeJH, NelsonGW, DetelsR, et al. (2002) Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31 : 429–434.
17. QiY, MartinMP, GaoX, JacobsonL, GoedertJJ, et al. (2006) KIR/HLA Pleiotropism: Protection against Both HIV and Opportunistic Infections. PLoS Pathog 2: e79.
18. AlterG, MartinMP, TeigenN, CarrWH, SuscovichTJ, et al. (2007) Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med 204 : 3027–3036.
19. CocchiF, DeVicoAL, Garzino-DemoA, AryaSK, GalloRC, et al. (1995) Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270 : 1811–1815.
20. OlivaA, KinterAL, VaccarezzaM, RubbertA, CatanzaroA, et al. (1998) Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest 102 : 223–231 10.1172/JCI2323 [doi].
21. CollinsKL, ChenBK, KalamsSA, WalkerBD, BaltimoreD (1998) HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391 : 397–401.
22. CohenGB, GandhiRT, DavisDM, MandelboimO, ChenBK, et al. (1999) The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10 : 661–671.
23. BrodinP, LakshmikanthT, JohanssonS, KarreK, HoglundP (2009) The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood 113 : 2434–2441 blood-2008-05-156836 [pii];10.1182/blood-2008-05-156836 [doi].
24. JohanssonS, JohanssonM, RosmarakiE, VahlneG, MehrR, et al. (2005) Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med 201 : 1145–1155 jem.20050167 [pii];10.1084/jem.20050167 [doi].
25. FauriatC, IvarssonMA, LjunggrenHG, MalmbergKJ, MichaelssonJ (2010) Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood 115 : 1166–1174 blood-2009-09-245746 [pii];10.1182/blood-2009-09-245746 [doi].
26. FaddaL, O'ConnorGM, KumarS, Piechocka-TrochaA, GardinerCM, et al. (2011) Common HIV-1 peptide variants mediate differential binding of KIR3DL1 to HLA-Bw4 molecules. J Virol 85 : 5970–5974 JVI.00412-11 [pii];10.1128/JVI.00412-11 [doi].
27. VivianJP, DuncanRC, BerryR, O'ConnorGM, ReidHH, et al. (2011) Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature 479 : 401–405 nature10517 [pii];10.1038/nature10517 [doi].
28. PelakK, NeedAC, FellayJ, ShiannaKV, FengS, et al. (2011) Copy number variation of KIR genes influences HIV-1 control. PLoS Biol 9: e1001208 10.1371/journal.pbio.1001208 [doi]; PBIOLOGY-D-11-02515 [pii].
29. BouletS, SharafiS, SimicN, BruneauJ, RoutyJP, et al. (2008) Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS 22 : 595–599.
30. BounouS, LeclercJE, TremblayMJ (2002) Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J Virol 76 : 1004–1014.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed ChildrenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



