-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Synthesis and Biological Properties of Fungal Glucosylceramide
article has not abstract
Published in the journal: Synthesis and Biological Properties of Fungal Glucosylceramide. PLoS Pathog 10(1): e32767. doi:10.1371/journal.ppat.1003832
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003832Summary
article has not abstract
Introduction
Sphingolipids have recently emerged as key regulators of pathogenicity in a variety of fungi. Glucosylceramide is a sphingolipid important for fungal cell division, alkaline tolerance, hyphal formation, and spore germination and, thus, for the regulation of fungal virulence. Present in many fungi, including yeasts, molds, and in dimorphic fungi, fungal glucosylceramide exhibits a characteristic structure synthesized by fungal specific enzymes. Thus, it is envisioned as an important fungal target for compounds with specific and broad-spectrum activity.
Glucosylceramide: The Sugar-Coated Sphingolipid
Glucosylceramide (GlcCer) is a sugar sphingolipid composed of a sphingoid backbone, a fatty acid, and a glucose moiety (Figure 1). Notably, GlcCer is found in plants, fungi, and animals and absent in bacteria and in some eukaryotes such as the baker yeast Saccharomyces cerevisiae. In spite of the presence of GlcCer in most organisms, its synthetic pathway and molecular structure varies significantly [1]–[4], resulting in the occurrence of rather unique GlcCer molecular species in different organisms. The final reaction of GlcCer synthesis is catalyzed by the glucosylceramide synthase enzyme (GCS), which transfers a glucose moiety from uridine 5-diphosphate (UDP)-glucose onto the C1 hydroxyl group of ceramide via an oxygen-glycosidic bond (Figure 2).
Fig. 1. Basic structure of glycosphingolipids. 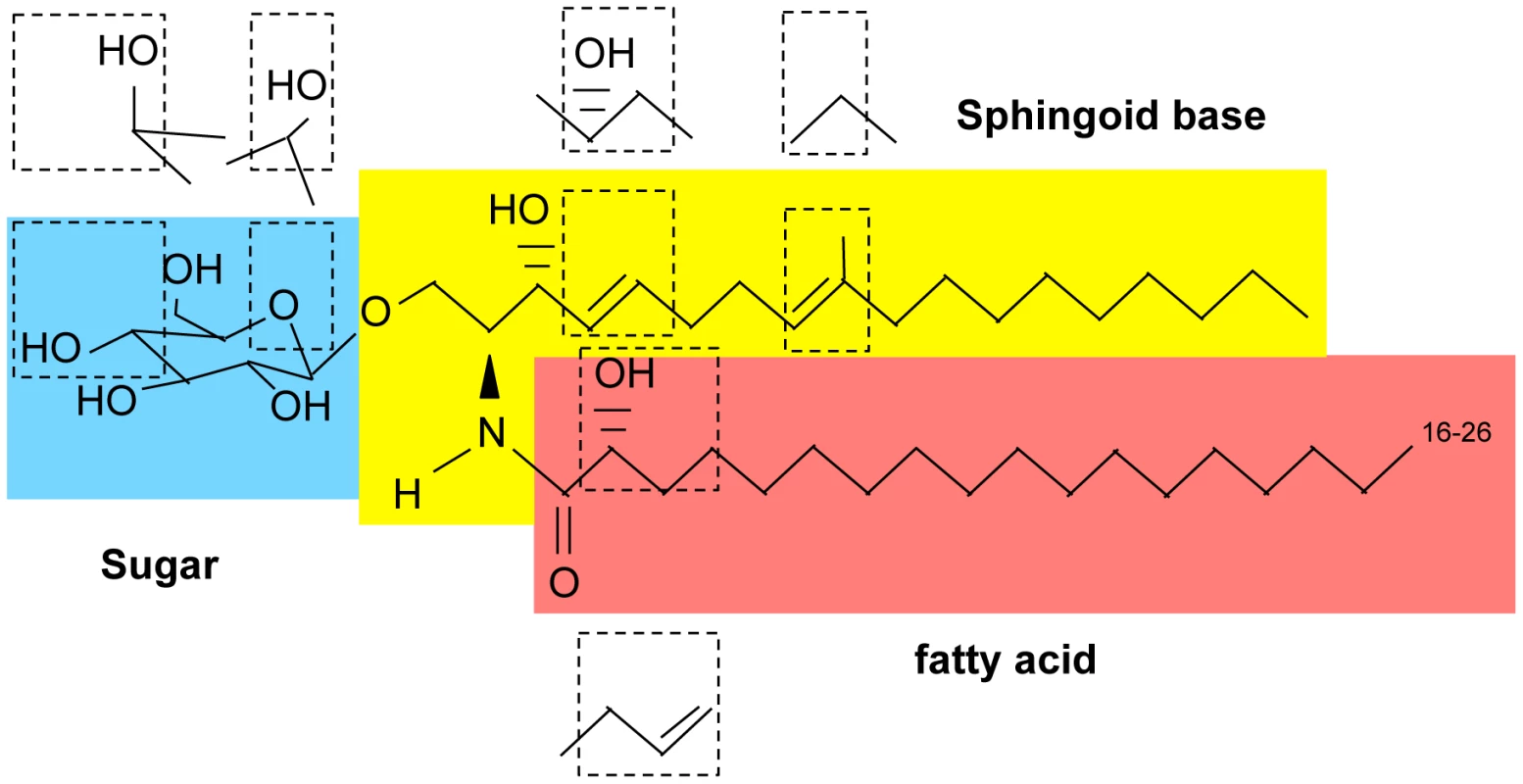
First, a long-chain sphingoid base backbone (distinguished from glycerolipids which have a glycerol backbone) is linked to a fatty acid via an amide bond with the 2-amino group and to a polar head group at the C1 position via an ester bond, forming ceramide. Second, ceramide is linked to a sugar (glucose, galactose, or inositol) via a β-glycosidic bond between the hemiacetal group of the sugar and the C1 hydroxyl group of ceramide. Fig. 2. Glycosphingolipid pathway in fungi. 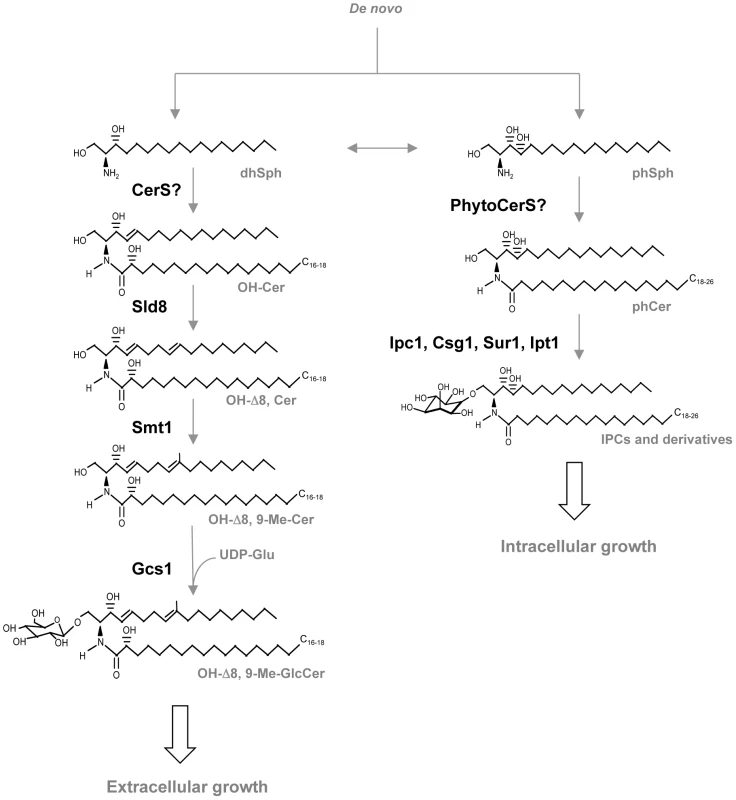
dhSph, dihydrosphingosine; CerS, ceramide synthases; OH-Cer, α-hydroxy-ceramide; Sld8, Δ8 desaturase; OH-Δ8-Cer, α-hydroxy-Δ8-ceramide; Smt1, C9-methyl transferase; OH-Δ8, 9-Me-Cer, α-hydroxy-Δ8, 9-methylceramide; Gcs1, glucosylceramide synthase 1; OH-Δ8, 9-Me-GlcCer, α-hydroxy-Δ8, 9-methyl-glucosylceramide; phSph, phytosphingosine; PhytoCerS, phytoceramide synthases; phCer, phytoceramide; Ipc1, inositol-phosphoryl ceramide synthase 1; Csg1, mannosyl phosphorylinositol ceramide synthase regulatory protein; Sur1, mannosyl phosphorylinositol ceramide synthase; Ipt1, inositol phosphotransferase 1; IPC, inositol phosphoryl ceramide. GlcCer was first isolated from the yeast Hansenula ciferri in 1971 [5]. The biochemical characterization of its structure in a variety of fungi, however, became available during the 90s, mainly by the work of two Brazilian groups: one in São Paulo coordinated by Helio Takahashi and one in Rio de Janeiro coordinated by Eliana Barreto-Bergter. During the early 2000s, the German group in Hamburg guided by Ernst Heinz and Dirk Warnecke isolated and characterized the fungal enzymes involved in its synthesis. This made possible genetic and pathobiological studies. The first observation that suggested a critical role for GlcCer in the regulation of virulence of a fungal human pathogen came in 2006, when a Cryptococcus neoformans mutant lacking the glucosylceramide synthase (Gcs1) was found to be avirulent in a mouse model [6]. C. neoformans is an environmental fungus that upon inhalation into the lung can cause a lethal meningoencephalitis, especially in immunocompromised patients. In the host environment, this fungus is a facultative intracellular pathogen and, as such, it can be found both inside and outside host cells. Once C. neoformans enters the host via the respiratory tract, it can survive and replicate in the extracellular environment (e.g., alveolar spaces, bloodstream), or it is engulfed by phagocytic cells (such as macrophages). Once inside phagocytic cells, C. neoformans can either survive and replicate in the phagolysome, or it can once again reach the extracellular space by exiting these cells without killing them [7], [8]. Since the extracellular environment is neutral/alkaline whereas the intracellular (phagolysosome) is acidic, adaptation of C. neoformans to both environments is a critical feature for its survival and virulence in the host. Intriguingly, different glycosphingolipids (sugar-complexed sphingolipids) have been found to regulate adaptation of C. neoformans to both environments [9]. In particular, glycosphingolipids carrying mannose and/or inositol have been found to regulate survival and/or replication of C. neoformans in the phagolysosome [10]–[12], whereas synthesis of GlcCer is critical for survival of the fungus in the extracellular environment [6]. Remarkably, loss of the GCS1 gene in C. neoformans produces a fungus (Δgcs1) that is not able to survive in the extracellular environment of the host and, in stark contrast to wild-type clinical isolates of C. neoformans, is completely avirulent in an immunocompetent mouse model. Indeed, while clinical isolates of C. neoformans (e.g.., wild-type H99 strain) generally cause a lethal meningoencephalitis after dissemination from the lung, mice infected with the Δgcs1 (H99-derived strain) are able to mount an effective immune response that ultimately leads to containment of the fungus in a granuloma-like structure. Importantly, these observations unequivocally underscore, first, the critical contribution of the fungal extracellular component to the pathogenicity of C. neoformans and, second, the central role played by GlcCer synthesis in regulation of this important aspect of C. neoformans virulence.
Uniqueness of the Chemical Structure of GlcCer
Fungal GlcCer has a very unique chemical structure compared to GlcCers in mammals and plants.
In plants, GlcCer is considered to be the most abundant glycosphingolipid, and its ceramide backbone has the greatest structural variety: seven different sphingoid bases can be linked to each of ten different α-hydroxy fatty acids, yielding to the production of at least 70 GlcCer species. This high variety is not present in fungi or mammals, in which only one or two sphingosine backbones can be attached to few different fatty acids. In fungi, the ceramide backbone in GlcCer is composed of a characteristic Δ8,9 methyl-sphingosine (desaturaded between carbon 8 and 9 and methylated in position 9) mainly linked to 2 hydroxy-octadecanoic acid, occasionally with a trans bond in position 3 (Figure 1 and Figure 2). GlcCer in mammals is almost exclusively made of sphingosine (double bond between carbon 4 and 5 of the sphingoid base) attached to hexadecanoic acid (Figure 1). Moreover, whereas in plants and fungi GlcCer is the end product of the synthetic pathway, in mammals GlcCer is used as a substrate to make more complex glycosphingolipids, such as lactosylceramides and gangliosides. Of note, certain fungi, such as Aspergillus fumigatus and others, produce also galactosylceramide, another type of glycosphingolipid in addition to GlcCer in which galactose is attached to ceramide [3] (Figure 1). Interestingly, galactosylceramide is absent in C. neoformans [6].
The structure of fungal GlcCer is also unique with respect to other fungal glycospingolipids such as inositol containing sphingolipids. In fact, inositol-containing sphingolipids only carry phytosphingosine as a sphingoid backbone in which carbon 4 is hydroxylated and there is no double bond between carbon 4 and 5 or carbon 8 and 9 and no methyl group on carbon 9 (Figure 1). Interestingly, whereas certain fungi, such as Candida albicans, under restrictive conditions can ultimately synthesize inositol-containing sphingolipids from methyl-ceramide, they cannot synthesize GlcCer using phytoceramide [13]. Whether this is common to other fungi is not know. The ceramide backbone of galactosylceramide in fungi is identical to GlcCer although there is a higher proportion of fatty acid with the Δ3-desaturation, but this cannot be generalized because the structure of galactosylceramide has been determined in only a few fungi [14]. On the other hand, it is interesting that its cellular level increases in the yeast phase of dimorphic fungi [14], perhaps suggesting that this glycolipid is also important for fungal growth in the host environment.
All together, these observations highlight the very specific chemical structure of fungal GlcCer compared to GlcCer in other organisms and compared to other fungal glycosphingolipids, and they raise the question of whether this unique chemical structure contributes to the functional significance of GlcCer.
GlcCer being a hydrophobic lipid, it is primarily localized in membranes, and following its synthesis in the Golgi apparatus, fungal GlcCer reaches the plasma membrane. In addition, GlcCer has also been reported in the cell wall [15], [16], especially when cell are dividing. GlcCer is also present intracellularly, and it is a main component of vesicle membranes [17]. These vesicles can be secreted, thus exposing fungal GlcCer to the extracellular environment and to host immune cells. Interestingly, the distribution of fungal GlcCer within cells changes under specific conditions. In fact, it has been shown that during cell division of C. neoformans, the level of GlcCer localized in the membranes (plasma membrane and cell wall) increases, particularly at the bud neck [15], perhaps contributing to the structural changes that lead to protrusion and/or fission of the bud. This phenomenon is particularly evident when C. neoformans cells are shifted from an acidic to a neutral/alkaline environment.
Regulation of Alkali Tolerance by GlcCer, Structural Implications, and Relevance to C. neoformans Virulence
Recent in vitro studies have uncovered a role for GlcCer/Gcs1 in the regulation of alkali tolerance of C. neoformans, providing a potential mechanism through which this lipid might affect C. neoformans virulence in mice. In fact, it was observed that the Δgcs1 strain fails to replicate and grow when the fungal cells are shifted from an acidic to a neutral/alkaline environment. Since the in vitro neutral/alkali condition mimics the host extracellular environment encountered by C. neoformans during infection (alveoli and blood stream), it is suggested that it is indeed the inability of the Δgcs1 strain to grow in these conditions that determines the failure of this strain to survive in the host extracellular environment, allowing the host to mount an effective immune response and clear the infection [6].
Moreover, the simple methylation of GlcCer seems to be essential for allowing cell division at neutral/alkaline pH, as a C. neoformans strain lacking the sphingolipid methyltransferase 1 enzyme (Smt1) responsible for this reaction [18] (Δsmt1) is mostly arrested in the stationary phase and cannot proceed through the cell cycle [6], [19]. Mechanistic studies on how methylated-GlcCer regulates cell cycle have been hampered by the fact that this glycosphingolipid is not present in the fungal model S. cerevisiae, a yeast in which, contrary to other fungi, cell cycle is very easy to synchronize. The fact that the Δgcs1 (GlcCer is absent) and the Δsmt1 (GlcCer is not methylated) C. neoformans strains share the same growth defect and cell cycle arrest phenotypes at neutral/alkaline pH supports the hypothesis that methylation of GlcCer is critical for its function in the regulation of cell cycle progression.
Similarly to the Δgcs1, the Δsmt1 mutant is avirulent in an immunocompetent mouse model [6], strongly suggesting that the loss of alkali tolerance of both Δgcs1 and Δsmt1 is the reason both strains loose pathogenicity in vivo. Of interest, Δgcs1 accumulates methylated ceramide which is undetectable in wild-type cells. However, this lipid clearly cannot compensate for the loss of methylated GlcCer in cell cycle progression and cell division, suggesting that both methylation and glycosylation of ceramide is important for cell cycle progression in fungi during alkaline tolerance.
How Does GlcCer Regulate Alkali Tolerance?
The molecular mechanism by which methylated GlcCer regulates fungal alkali tolerance is still unknown. On the other hand, the fact that in neutral/alkaline conditions GlcCer rapidly concentrates at the plasma membrane, cell wall, and bud neck from the intracellular compartment [20] and that, in its absence, there is no budding [6] suggests that, in these specific extracellular conditions, the presence of GlcCer at the bud neck and in its vicinity becomes important to allow progression through the cell cycle. The presence of methylated GlcCer might be necessary to maintain the proper curvature of the membranes at the bud neck so that the bud can initiate and grow to become the daughter cell. In addition, most of the buds present in the Δgcs1 and Δsmt1 cells exposed to alkaline pH are small, suggesting that GlcCer, and particularly methylated GlcCer, may be important for the production of bud membranes.
Also, alkali tolerance is dependent on the activity of membrane proteins that transport solutes, such as iron, hydrogen, bicarbonate, or potassium. The solutes are tightly involved in the regulation and stabilization of the membrane polarization through their movement across the membrane. An integrative model of ion regulation across the membrane [21] and the adaptation of yeast cells to alkaline pH (reviewed in [22]) has been proposed but studied in S. cerevisiae. Recent studies in Can. albicans, however, suggest that extracellular alkaline pH induces iron starvation and, as a response, the yeast up-regulates a series of transporters and pathways (e.g., Rim 101) to increase iron acquisition [23], [24]. Interestingly, this response to alkaline pH seems to be conserved also in C. neoformans [25]., Genomic studies in C. neoformans suggest that iron transporters are significantly down-regulated in the absence of methylated GlcCer (Δgcs1 and Δsmt1) and only when cells are exposed to alkaline and not acidic pH (Singh et al., in preparation). Thus, it is possible that in condition of alkaline stress, methylated GlcCer may be important for iron transporters to function properly.
The regulation of potassium (K+) channels by fungal methylated GlcCer is also an exciting hypothesis because it is well known that K+ are key modulators of transmembrane potential homeostasis and some K+ channels are stimulated by extracellular alkaline pH [26]. Interestingly, one of these K+ channels (TASK-2) is coupled with HCO−3 transport in the kidney and the highly hydrophobic region of the transmembrane 4 (TM4) is essential for its alkaline-dependence. An homolog of TASK-2 is present in C. neoformans, and it is possible that the methyl group of GlcCer is required to sustain adequate hydrophobicity of the TM4, thus regulating HCO−3 transport and transmembrane potential.
It is possible that, under alkaline conditions, the presence of methyl GlcCer in the plasma membrane ensures optimal structural organization/activity of these transporters by affecting membrane potential, organization, stability, fluidity and/or microdomain organization (rafts) of the plasma membrane. Studies in the literature are beginning to address the role and mechanisms by which GlcCer is involved in the organization and functionality of biological membranes.
Regulation of physical properties of membranes by methyl GlcCer
Glycosphingolipids, including GlcCer, have the propensity to enhance the formation of inverted micelle in the plasma membrane, a phenomenon in which the sugar moieties of the glycosphingolipids of the inner leaflet of membranes undergo self-assembly, forming a micelle with a hydrophilic instead of a hydrophobic core. These inverted micelles alter the characteristic bilayer structure and organization of plasma membranes, allowing a more flexible membrane and a massive influx or efflux of material [27] in and out of the cell during specific cellular insults. Interestingly, this phenomenon is linked to the geometrical and structural features of glycosphingolipids [28], [29] and plants have a variety of possibilities for the regulation of this phenomenon given their large number of GlcCer structures. This phenomenon may be also present in fungi where inverted micelle, by adding physical space in the inner layers of the membrane, may favor the penetration of large molecules, insertion of proteins transporters, or large lipid molecules within the membrane with the resulting effect to favor the growth and elongation of the hyphal (Can. albicans) or the growth of the membrane in daughter cells (C. neoformans) and/or the germination of spores (dimorphic fungi, molds, C. neoformans).
Since methylated GlcCer clearly adds physical space in the hydrophobic pocket of membranes, it is possible that it plays a role in allowing the proper change of orientation/movement of the membranes during cell growth and hyphal elongation, morphogenesis and polarization. As mentioned before, in C. neoformans methylated GlcCer is present at the bud neck and it may regulate the formation and the maturation of the bud. Studies in Can. albicans and Fusarium graminearum have also shown that GlcCer is concentrated at the hyphal tip and that it is important for the mechanical hyphal elongation and morphogenesis. Differently from C. neoformans, hyphal elongation is controlled by the C9-methylation of ceramide more than its glycosylation [30], [31]. In contrast, in dimorphic fungi such as Histoplasma capsulatum, Paracoccidioides brasiliensis, or Blastomyces dermatiditis, the production of GlcCer is mostly associated with the yeast-pathogenic form instead of the environmental hyphal form [14], [32]–[34]. The functions of GlcCer in these fungi are not known.
GlcCer has been also implicated in the regulation of spore germination in fungi [35]–[37], but whether the GlcCer pathway regulates these phenomena in C. neoformans is not known. However, similarly to its peculiar localization at the bud neck and its role in growth and separation of the daughter cell, GlcCer may also be important for spore growth and separation.
The structural function for GlcCer may be more evident in plants, where the lipid helps the plasma membrane to sustain climate stresses, such as drought and cold, through the synthesis and incorporation of specific molecular species of GlcCer in the plasma membrane, thus increasing hydration and resistance to low temperature. Indeed, different GlcCer molecules are found in plant membrane when exposed to different temperatures or different humidity [38]. Interestingly, importance of GlcCer in protecting against extreme temperatures/humidity may also be relevant to fungi since resistance to drought is particularly important for fungal spores in order to maintain their infectious properties.
Regulation of membrane microdomains (rafts)
Total membranes isolated from the C. neoformans Δsmt1, in which GlcCer is present but not methylated, are more leaky, and lipids extracted from these membrane do not form rafts in vitro compared to the lipids extracted from wild-type membranes [19]. In contrast, lipids extracted from membranes isolated from C. neoformans Δgcs1 strain, in which there is no GlcCer, do form more rafts in vitro than the wild-type membranes, but these rafts are very small in size and they might not be functional in transducing/regulating membrane protein receptors and/or transporters (Del Poeta, unpublished observations). Since the Δgcs1 mutant accumulates methylated ceramide, it is possible that this sphingolipid is responsible for assembly of these small lipid rafts through hydrophobic interactions of its methyl group with other membrane-embedded lipids (e.g., ergosterol); on the other hand, it does not seem sufficient for the assembly of larger microdomains. It is possible that the methyl group of GlcCer is necessary but not sufficient to produce a large, complex, lipid raft. A large lipid raft may be produced when the glucose moieties of GlcCer interacts with glucose moieties of other GlcCer molecules and/or with sugar moieties of other sphingolipid molecules (e.g., IPC and its derivatives, such as inositol and mannose). These sugar interactions mediated by intermolecular hydrogen bonds not only form a dense network among the sugar moieties of glycosphingolipids but also will likely contribute to form a compact surface membrane alignment of GlcCer/IPC/MIPC/MIP2C. The observation that 25% of raft lipids in fungus Histoplasma capsulatum are made of glycosphingolipids [39] further supports the hypothesis of a sugar network. These forces should also facilitate the entrapment of receptors and other proteins within the lipid rafts. The fact that non-methylated GlcCer (Δsmt1) is not capable of promoting raft formation like wild-type suggests that the hydrogen interactions between the sugar moieties are not sufficient, or perhaps they are much weaker if not combined with the hydrophobic interactions mediated by the methyl group of GlcCer. Thus, we propose that the hydrophobic interactions mediated by the methyl group of GlcCer are important to initiate the lipid raft formation by tightening sphingolipids and ergosterol together, whereas the hydrogen interactions between the sugar moieties are important to form large rafts and to entrap other large molecules in it (glycosphingolipids and/or proteins).
Are Other Aspects of GlcCer Regulation Relevant to Fungal Virulence?
As discussed, GlcCer is present and enriched in the membrane of vesicles that can cross the cell wall, reaching the extracellular space. The presence of these GlcCer-enriched vesicles in the extracellular space might have an important role in pathogenicity of C. neoformans and in the modulation of the host response to the fungus. Indeed, these extracellular vesicles are loaded with proteins associated with virulence of C. neoformans 40,41 and can impair macrophage functions [42]. Additionally, GlcCer is antigenic, and antibodies against GlcCer have been detected during human cryptococcal infections [15], [43]. Thus, exposure of GlcCer on the surface of the vesicles might also induce a host antibody response. Interestingly, administration of antibodies against GlcCer protects immunocompetent mice from developing a lethal meningitis [44], but their role in protecting immunocompromised hosts, such as HIV-infected patients, is less clear [43]. Even if antibodies against GlcCer would exert a protective role only in condition of immunocompetency, it is proposed that these antibodies may be useful as passive immunization against the infection caused by Cryptococcus gattii and certain dimorphic fungi, such as Histoplasma capsulatum, Blastomyces dermatiditis and Coccidioides immitis, which can also afflict immunocompetent individuals.
Can We Exploit GlcCer for Therapeutics?
All the evidence/observations discussed support a critical role for the synthesis of GlcCer in pathogenicity of C. neoformans. Furthermore, since GlcCer may influence virulence of many yeasts, molds, dimorphic fungi, and Pneumocystis spp., anti-GlcCer therapy might have a very broad applicability.
One possible therapeutic approach would be to develop small molecule inhibitors that target GlcCer synthesis. In this regard, it is essential to note that key enzymes involved in synthesis of fungal GlcCer are not present in mammalian cells, while the fungal Gcs1 is structurally different from the mammalian homologue (mammalian GCS inhibitors do not affect the activity of the fungal Gcs1 and vice versa). Thus, effort should be expended to identify and test natural or/and synthetic molecules that target these specific fungal enzymes.
Other therapeutic options should consider the use of monoclonal antibodies to fungal GlcCer since they inhibit fungal growth [15], [45] and protect mice against cryptococcal infection [44].
Another therapeutic approach to consider could be to exploit targeting of GlcCer by plants defensins. Defensins are well-known, potent antimicrobial peptides that are able to efficiently kill a variety of microbial cells. Defensins produced by plants and insects interact with fungal GlcCer and kill fungal pathogens in vitro [46]–[49] and in vivo [50].
In conclusion, fungal GlcCer is a virulence determinant with a characteristic chemical structure and synthesized by fungal specific enzymes. Since it is produced by a variety of pathogenic fungi, targeting fungal GlcCer should improve the outcome of fungal infection diseases.
Zdroje
1. LeipeltM, WarneckeD, ZahringerU, OttC, MullerF, et al. (2001) Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J Biol Chem 276 : 33621–33629.
2. RhomeR, McQuistonT, KechichianT, BielawskaA, HennigM, et al. (2007) Biosynthesis and immunogenicity of glucosylceramide in Cryptococcus neoformans and other human pathogens. Eukaryot Cell 6 : 1715–1726.
3. WarneckeD, HeinzE (2003) Recently discovered functions of glucosylceramides in plants and fungi. Cell Mol Life Sci 60 : 919–941.
4. NimrichterL, RodriguesML (2011) Fungal glucosylceramides: from structural components to biologically active targets of new antimicrobials. Front Microbiol 2 : 212.
5. KaufmanB, BasuS, RosemanS (1971) Isolation of glucosylceramides from yeast (Hansenula ciferri). J Biol Chem 246 : 4266–4271.
6. RittershausPC, KechichianTB, AllegoodJ, MerrillAHJ, HennigM, et al. (2006) Glucosylceramide is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest 116 : 1651–1659.
7. MaH, CroudaceJE, LammasDA, MayRC (2006) Expulsion of live pathogenic yeast by macrophages. Curr Biol 16 : 2156–2160.
8. AlvarezM, CasadevallA (2006) Phagosome Extrusion and Host-Cell Survival after Cryptococcus neoformans Phagocytosis by Macrophages. Curr Biol 16 : 2161–2165.
9. GarciaJ, SheaJ, Alvarez-VasquezF, QureshiA, LubertoC, et al. (2008) Mathematical modeling of pathogenicity of Cryptococcus neoformans. Mol Sys Biol 4 : 183–195.
10. FanW, KrausPR, BoilyMJ, HeitmanJ (2005) Cryptococcus neoformans Gene Expression during Murine Macrophage Infection. Eukaryot Cell 4 : 1420–1433.
11. LubertoC, ToffalettiDL, WillsEA, TuckerSC, CasadevallA, et al. (2001) Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev 15 : 201–212.
12. SheaJ, KechichianTB, LubertoC, Del PoetaM (2006) The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C (Isc1) confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun 74 : 5977–5988.
13. CheonSA, BalJ, SongY, HwangHM, KimAR, et al. (2012) Distinct roles of two ceramide synthases, CaLag1p and CaLac1p, in the morphogenesis of Candida albicans. Mol Microbiol 83 : 728–745.
14. ToledoMS, LeverySB, StrausAH, SuzukiE, MomanyM, et al. (1999) Characterization of sphingolipids from mycopathogens: factors correlating with expression of 2-hydroxy fatty acyl (E)-Delta 3-unsaturation in cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus. Biochemistry 38 : 7294–7306.
15. RodriguesML, TravassosLR, MirandaKR, FranzenAJ, RozentalS, et al. (2000) Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun 68 : 7049–7060.
16. NakaseM, TaniM, MoritaT, KitamotoHK, KashiwazakiJ, et al. (2010) Mannosylinositol phosphorylceramide is a major sphingolipid component and is required for proper localization of plasma-membrane proteins in Schizosaccharomyces pombe. J Cell Sci 123 : 1578–1587.
17. RodriguesML, NimrichterL, OliveiraDL, FrasesS, MirandaK, et al. (2007) Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6 : 48–59.
18. TernesP, SperlingP, AlbrechtS, FrankeS, CreggJM, et al. (2006) Identification of fungal sphingolipid C9-methyltransferases by phylogenetic profiling. J Biol Chem 281 : 5582–5592.
19. SinghA, NaC, SilvaLC, PrietoM, FutermanAH, et al. (2012) Membrane lipid topography controlled by sphingolipids regulates pathogenicity of Cryptococcus neoformans. Cell Microbiol 14 : 500–516.
20. RhomeR, SinghA, KechichianT, DragoM, MoraceG, et al. (2011) Surface localization of glucosylceramide during Cryptococcus neoformans infection allows targeting as a potential antifungal. PLoS One 6: e15572 doi:10.1371/journal.pone.0015572
21. KeR, IngramPJ, HaynesK (2013) An integrative model of ion regulation in yeast. PLoS Comput Biol 9: e1002879 doi:10.1371/journal.pcbi.1002879
22. ArinoJ (2010) Integrative responses to high pH stress in S. cerevisiae. OMICS 14 : 517–523.
23. BensenES, MartinSJ, LiM, BermanJ, DavisDA (2004) Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol 54 : 1335–1351.
24. MoranGP (2012) Transcript profiling reveals rewiring of iron assimilation gene expression in Candida albicans and C. dubliniensis. FEMS Yeast Res 12 : 918–923.
25. O'MearaTR, NortonD, PriceMS, HayC, ClementsMF, et al. (2010) Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog 6: e1000776 doi:10.1371/journal.ppat.1000776
26. NiemeyerMI, Gonzalez-NiloFD, ZunigaL, GonzalezW, CidLP, et al. (2007) Neutralization of a single arginine residue gates open a two-pore domain, alkali-activated K+ channel. Proc Natl Acad Sci U S A 104 : 666–671.
27. StalmansS, WynendaeleE, BrackeN, GevaertB, D'HondtM, et al. (2013) Chemical-functional diversity in cell-penetrating peptides. PLoS One 8: e71752 doi: 10.1371/journal.pone.0071752
28. MaggioB (1985) Geometric and thermodynamic restrictions for the self-assembly of glycosphingolipid-phospholipid systems. Biochim Biophys Acta 815 : 245–258.
29. PerilloMA, ScarsdaleNJ, YuRK, MaggioB (1994) Modulation by gangliosides of the lamellar-inverted micelle (hexagonal II) phase transition in mixtures containing phosphatidylethanolamine and dioleoylglycerol. Proc Natl Acad Sci U S A 91 : 10019–10023.
30. OuraT, KajiwaraS (2010) Candida albicans sphingolipid C9-methyltransferase is involved in hyphal elongation. Microbiology 156 : 1234–1243.
31. RamamoorthyV, CahoonEB, ThokalaM, KaurJ, LiJ, et al. (2009) Sphingolipid C-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot Cell 8 : 217–229.
32. BertiniS, ColomboAL, TakahashiHK, StrausAH (2007) Expression of antibodies directed to Paracoccidioides brasiliensis glycosphingolipids during the course of paracoccidioidomycosis treatment. Clin Vaccine Immunol 14 : 150–156.
33. ToledoMS, LeverySB, StrausAH, TakahashiHK (2000) Dimorphic expression of cerebrosides in the mycopathogen Sporothrix schenckii. J Lipid Res 41 : 797–806.
34. ToledoMS, SuzukiE, LeverySB, StrausAH, TakahashiHK (2001) Characterization of monoclonal antibody MEST-2 specific to glucosylceramide of fungi and plants. Glycobiology 11 : 105–112.
35. da SilvaAF, RodriguesML, FariasSE, AlmeidaIC, PintoMR, et al. (2004) Glucosylceramides in Colletotrichum gloeosporioides are involved in the differentiation of conidia into mycelial cells. FEBS Lett 561 : 137–143.
36. LeverySB, MomanyM, LindseyR, ToledoMS, ShaymanJA, et al. (2002) Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett 525 : 59–64.
37. RittenourWR, ChenM, CahoonEB, HarrisSD (2011) Control of glucosylceramide production and morphogenesis by the Bar1 ceramide synthase in Fusarium graminearum. PLoS One 6: e19385 doi:10.1371/journal.pone.0019385
38. UemuraM, JosephRA, SteponkusPL (1995) Cold Acclimation of Arabidopsis thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions). Plant Physiol 109 : 15–30.
39. TagliariL, ToledoMS, LacerdaTG, SuzukiE, StrausAH, et al. (2012) Membrane microdomain components of Histoplasma capsulatum yeast forms, and their role in alveolar macrophage infectivity. Biochim Biophys Acta 1818 : 458–466.
40. EisenmanHC, FrasesS, NicolaAM, RodriguesML, CasadevallA (2009) Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 155 : 3860–3867.
41. RodriguesML, NakayasuES, OliveiraDL, NimrichterL, NosanchukJD, et al. (2008) Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7 : 58–67.
42. OliveiraDL, Freire-de-LimaCG, NosanchukJD, CasadevallA, RodriguesML, et al. (2010) Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun 78 : 1601–1609.
43. QureshiA, WrayD, RhomeR, BarryW, Del PoetaM (2012) Detection of antibody against fungal glucosylceramide in immunocompromised patients: a potential new diagnostic approach for cryptococcosis. Mycopathologia 173 : 419–425.
44. RodriguesML, ShiL, Barreto-BergterE, NimrichterL, FariasSE, et al. (2007) Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccine Immunol 14 : 1372–1376.
45. NimrichterL, Barreto-BergterE, Mendonca-FilhoRR, KneippLF, MazziMT, et al. (2004) A monoclonal antibody to glucosylceramide inhibits the growth of Fonsecaea pedrosoi and enhances the antifungal action of mouse macrophages. Microbes Infect 6 : 657–665.
46. TavaresPM, ThevissenK, CammueBP, FrancoisIE, Barreto-BergterE, et al. (2008) In vitro activity of the antifungal plant defensin RsAFP2 against Candida isolates and its in vivo efficacy in prophylactic murine models of candidiasis. Antimicrob Agents Chemother 52 : 4522–4525.
47. ThevissenK, WarneckeDC, FrancoisIE, LeipeltM, HeinzE, et al. (2004) Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem 279 : 3900–3905.
48. AertsAM, FrancoisIE, CammueBP, ThevissenK (2008) The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci 65 : 2069–2079.
49. ThevissenK, de Mello TavaresP, XuD, BlankenshipJ, VandenboschD, et al. (2012) The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol Microbiol 84 : 166–180.
50. ThevissenK, KristensenHH, ThommaBP, CammueBP, FrancoisIE (2007) Therapeutic potential of antifungal plant and insect defensins. Drug Discov Today 12 : 966–971.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite MovementČlánek Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi ScreenČlánek IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed ChildrenČlánek Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive SubjectsČlánek Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- HIV-1 Accessory Proteins Adapt Cellular Adaptors to Facilitate Immune Evasion
- Ranaviruses: Not Just for Frogs
- Effectors and Effector Delivery in
- Plasmacytoid Dendritic Cell Dynamics Tune Interferon-Alfa Production in SIV-Infected Cynomolgus Macaques
- Lu/BCAM Adhesion Glycoprotein Is a Receptor for Cytotoxic Necrotizing Factor 1 (CNF1)
- A Substrate-Fusion Protein Is Trapped inside the Type III Secretion System Channel in
- Parvovirus-Induced Depletion of Cyclin B1 Prevents Mitotic Entry of Infected Cells
- Red Blood Cell Invasion by : Structural Basis for DBP Engagement of DARC
- NsrR, GadE, and GadX Interplay in Repressing Expression of the O157:H7 LEE Pathogenicity Island in Response to Nitric Oxide
- Loss of Circulating CD4 T Cells with B Cell Helper Function during Chronic HIV Infection
- TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- Glutamate Utilization Couples Oxidative Stress Defense and the Tricarboxylic Acid Cycle in Phagosomal Escape
- Serotonin Signaling in : A Serotonin–Activated G Protein-Coupled Receptor Controls Parasite Movement
- Recovery of an Antiviral Antibody Response following Attrition Caused by Unrelated Infection
- Regulators of Cell Cycle Progression and Differentiation Identified Using a Kinome-Wide RNAi Screen
- Absence of Intestinal PPARγ Aggravates Acute Infectious Colitis in Mice through a Lipocalin-2–Dependent Pathway
- Induction of a Stringent Metabolic Response in Intracellular Stages of Leads to Increased Dependence on Mitochondrial Metabolism
- CTCF and Rad21 Act as Host Cell Restriction Factors for Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Lytic Replication by Modulating Viral Gene Transcription
- Gammaherpesviral Gene Expression and Virion Composition Are Broadly Controlled by Accelerated mRNA Degradation
- The Arabidopsis Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes
- Inflammatory Stimuli Reprogram Macrophage Phagocytosis to Macropinocytosis for the Rapid Elimination of Pathogens
- Alphavirus Mutator Variants Present Host-Specific Defects and Attenuation in Mammalian and Insect Models
- Phosphopyruvate Carboxylase Identified as a Key Enzyme in Erythrocytic Carbon Metabolism
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Electron Tomography of HIV-1 Infection in Gut-Associated Lymphoid Tissue
- Characterisation of a Multi-ligand Binding Chemoreceptor CcmL (Tlp3) of
- Single Cell Stochastic Regulation of Pilus Phase Variation by an Attenuation-like Mechanism
- Cell Tropism Predicts Long-term Nucleotide Substitution Rates of Mammalian RNA Viruses
- Functions of CPSF6 for HIV-1 as Revealed by HIV-1 Capsid Evolution in HLA-B27-Positive Subjects
- RNA-seq Analysis of Host and Viral Gene Expression Highlights Interaction between Varicella Zoster Virus and Keratinocyte Differentiation
- Kaposi's Sarcoma Associated Herpesvirus Tegument Protein ORF75 Is Essential for Viral Lytic Replication and Plays a Critical Role in the Antagonization of ND10-Instituted Intrinsic Immunity
- DAMP Molecule S100A9 Acts as a Molecular Pattern to Enhance Inflammation during Influenza A Virus Infection: Role of DDX21-TRIF-TLR4-MyD88 Pathway
- Variable Suites of Non-effector Genes Are Co-regulated in the Type III Secretion Virulence Regulon across the Phylogeny
- Reengineering Redox Sensitive GFP to Measure Mycothiol Redox Potential of during Infection
- Preservation of Tetherin and CD4 Counter-Activities in Circulating Alleles despite Extensive Sequence Variation within HIV-1 Infected Individuals
- KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next-Generation Sequencing Reveals Novel Genomic and Functional Features
- Nutrient Limitation Governs Metabolism and Niche Adaptation in the Human Nose
- Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration
- Investigation of Acetylcholine Receptor Diversity in a Nematode Parasite Leads to Characterization of Tribendimidine- and Derquantel-Sensitive nAChRs
- Intranasal Vaccination Promotes Detrimental Th17-Mediated Immunity against Influenza Infection
- -Mediated Inhibition of Iron Export Promotes Parasite Replication in Macrophages
- Variation in RNA Virus Mutation Rates across Host Cells
- A Single Amino Acid in the Stalk Region of the H1N1pdm Influenza Virus HA Protein Affects Viral Fusion, Stability and Infectivity
- Group B Engages an Inhibitory Siglec through Sialic Acid Mimicry to Blunt Innate Immune and Inflammatory Responses
- Synthesis and Biological Properties of Fungal Glucosylceramide
- HIV Protective KIR3DL1/S1-HLA-B Genotypes Influence NK Cell-Mediated Inhibition of HIV Replication in Autologous CD4 Targets
- Recruitment of PfSET2 by RNA Polymerase II to Variant Antigen Encoding Loci Contributes to Antigenic Variation in
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Enhancing Virus-Specific Immunity by Combining Therapeutic Vaccination and PD-L1 Blockade in Chronic Hepadnaviral Infection
- Suppression of Interferon Lambda Signaling by SOCS-1 Results in Their Excessive Production during Influenza Virus Infection
- Inflammation Fuels Colicin Ib-Dependent Competition of Serovar Typhimurium and in Blooms
- Host-Specific Enzyme-Substrate Interactions in SPM-1 Metallo-β-Lactamase Are Modulated by Second Sphere Residues
- STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to
- From Scourge to Cure: Tumour-Selective Viral Pathogenesis as a New Strategy against Cancer
- Lysine Acetyltransferase GCN5b Interacts with AP2 Factors and Is Required for Proliferation
- Narrow Bottlenecks Affect Populations during Vertical Seed Transmission but not during Leaf Colonization
- Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART
- Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells
- iNKT Cell Production of GM-CSF Controls
- Malaria-Induced NLRP12/NLRP3-Dependent Caspase-1 Activation Mediates Inflammation and Hypersensitivity to Bacterial Superinfection
- Detection of Host-Derived Sphingosine by Is Important for Survival in the Murine Lung
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Lyme Disease: Call for a “Manhattan Project” to Combat the Epidemic
- Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen f.sp.
- IFNγ/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children
- Human and Plant Fungal Pathogens: The Role of Secondary Metabolites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



