-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Clustering of Pattern Recognition Receptors for Fungal Detection
article has not abstract
Published in the journal: Clustering of Pattern Recognition Receptors for Fungal Detection. PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003873
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003873Summary
article has not abstract
Introduction
The innate immune system is the first line of defense against invading pathogens. Innate cells recognize microbes via pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and C-type lectin receptors (CLRs); initiate innate immune responses; and eventually trigger adaptive immunity. Association of heterologous PRRs synergistically enhances their signal intensity. Such PRR cluster formation is essential for fungal detection in generating antifungal immunity. In this review, PRR cluster formation to detect fungi and to initiate innate immune responses is discussed.
PRRs Involved in Fungal Detection
Upon fungal infection, host innate immune cells, such as macrophages, neutrophils, and dendritic cells (DCs), are the first cell types to detect the infection. During the early stages of infection, before T cells are activated, the host innate immune system initiates antifungal responses (i.e., phagocytosis; production of reactive oxygen species [ROS], cytokines, and chemokines) as sentinels. Antigen-presenting myeloid cells, such as DCs and macrophages, then activate T cells by presenting foreign antigens to trigger adaptive immune responses, along with secretion of cytokines and chemokines to instruct naïve T cells to polarize “proper” T cell subsets.
Several TLRs (including TLR1/2, TLR2/6, TLR4, and TLR9) and CLRs (including dectin-1, dectin-2, MMR [Macrophage Mannose Receptor, CD206], and DC-SIGN [Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin, CD209]) play roles as fungal sensors. Among these, TLR2 and dectin-1 are the best-characterized PRRs in detecting fungi such as Pneumocystis [1] and Candida albicans [2], [3]. TLR2 heterodimerizes with either TLR1 or TLR6 and recognizes triacylated and diacylated lipoprotein as TLR1/2 and TLR2/6, respectively. TLR4 and TLR9 recognize O-linked mannosyl residues on fungal walls and fungal DNA, respectively. TLR signaling pathways activate NFκB, which is a key transcription factor for the production of cytokines and chemokines.
Dectin-1, expressed by macrophages, neutrophils, and DCs, recognizes β-glucans in the fungal cell walls [3]. Stimulation of dectin-1 triggers phagocytosis of fungi, ROS generation, and production of cytokines and chemokines by host innate immune cells. Dectin-1 is a critical PRR in host defense against fungal infection, since TLR stimulation alone is not sufficient to induce ROS [4]. Dectin-1 signaling activates Syk (Spleen Tyrosine Kinase), and thus leads to subsequent activation of NFκB and NFAT (Nuclear Factor of Activated T-cells). NFκB is activated by a signaling adaptor complex consisting of CARD9 (CAspase Recruitment Domain-containing protein-9), BCL10 (B Cell Lymphoma/leukemia-10), and MALT1 (Mucosa-Associated Lymphoid Tissue lymphoma translocation protein-1) [5]. On the other hand, NFAT activation by dectin-1 requires PLCγ2 (Phospholipase C-gamma 2) signaling [6]. Dectin-1–mediated Syk signaling also activates the NLRP3 (NOD-Like Receptor family, Pyrin domain containing 3) inflammasome, which mediates IL-1β and IL-18 maturation, and it plays an important role in the host defense against C. albicans [7]. Other CLRs, dectin-2, dectin-3, MMR, and DC-SIGN, recognize mannan [8]. Recent study has shown that dectin-2 and dectin-3 form a heterodimer, which binds to α-mannan more effectively and elicits more potent inflammatory responses than does a dectin-2 homodimer [8]. Chitin recognition is considered to be a size-dependent process and involves a combination of dectin-1 and MMR, with or without TLR2 [9].
Synergy of PRRs
It is known that association of heterologous PRRs synergistically enhances their signal intensity. TLR2, dectin-1, and MMR play a critical role in the detection and clearance of Pneumocystis [1], [10]. In particular, TLR2 and dectin-1 physically associate each other and synergize to augment antifungal responses [4], [10]–[12]. Co-localization of TLR2 and dectin-1 in the phagocytic cap engulfing zymosan on the macrophage membrane was also reported [10], [13]. Zymosan does not activate NFκB when cells express either TLR2 or dectin-1 alone; however, co-expression of TLR2 and dectin-1 allows zymosan to activate NFκB [4], suggesting the importance of PRR synergy. Synergy of decin-1 and TLR2 was also demonstrated in human monocyte-derived macrophages via increased TNFα production with curdlan (dectin-1 ligand) and Pam3Cys (TLR2 ligand) co-stimulation [12]. Further, crosstalk between dectin-1 and TLR2 signaling pathways has been suggested from the failure to elicit collaborative receptor responses by the lack of a signaling molecule, Syk or MyD88, which are downstream of dectin-1 and TLR2, respectively [11]. In addition to TLR2, pathways of TLR4, TLR5, TLR7, and TLR9 appear to collaborate with the dectin-1/Syk signaling pathway based on the synergistic enhancement of TNF production [11], [12]. Finally, it has been reported that DC-SIGN synergizes TLR signaling through RAF1, a serine/threonine kinase, which induces phosphorylation of the p65 subunit of NFκB, followed by prolonged and enhanced transcription of Il10 by NFκB activation [14]. A sizeable number of studies thus suggest the existence of synergistic collaboration, particularly between TLR and CLR signaling pathways.
PRR Clustering in Fungal Detection
In order to achieve synergy between heterologous PRR signaling pathways, it is considered crucial to have PRRs in physical proximity to one another. Clustering PRRs on a myeloid cell surface is required for the optimum detection of fungi [10], [15]. TLR2 and dectin-1 are recruited to lipid rafts, plasma membrane microdomains rich in cholesterol and sphingolipids, after stimulation with their ligands; signals from the receptors are synergized to enhance the downstream responses [10], [16]. Disruption of lipid rafts causes decreased cytokine expression, phagocytosis, Ca2+ influx, and phosphorylation of both Syk and ERK (Extracellular signal Regulated Kinases) [16], suggesting the critical role of rafts in PRR signaling. Importance of receptor cluster formation in antifungal immunity was demonstrated by comparing soluble and particulate β-glucans immobilized on latex beads [15]. Treating bone marrow–derived macrophages with particulate β-glucans induced dectin-1 signaling but soluble β-glucans did not; the former was done through the mechanism of excluding regulatory tyrosine phosphatases CD45 and CD148 from synapse-like structures [15]. We speculate that the physical crosslink of PRRs by particulate ligands may also be critical for triggering strong receptor signaling [10]. The PRR cluster was termed “phagocytic synapse” [15], which provides a platform for fungal detection and phagocytosis (Figure 1). Such PRR clustering is reminiscent of the supramolecular activation cluster (SMAC) formed on T cell surfaces in the immunological synapse; although, PRR clusters do not appear to be as distinct and intricate as SMAC. The biology and precise mechanism of PRR clustering are clearly arenas for further research.
Fig. 1. A model example of a PRR cluster formation for fungal detection. 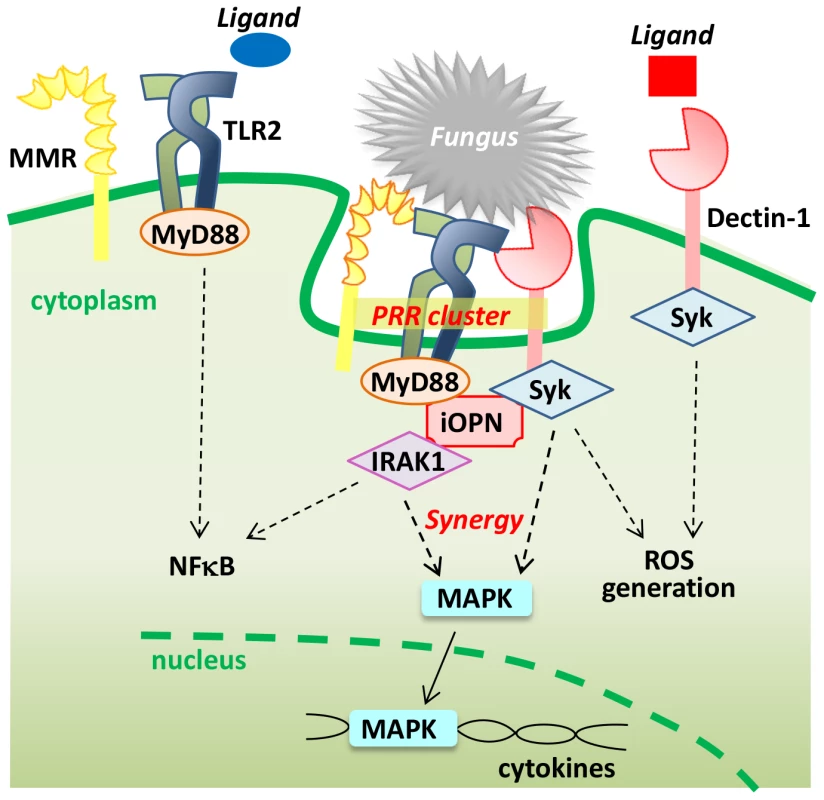
Soluble ligands do not make a PRR cluster, but a fungal spore or a particulate ligand attracts PRRs in a lipid raft on the host cell surface. In this figure, heterologous PRRs, consisting of dectin-1, TLR2, and MMR, form a PRR cluster. We reported that PRR cluster formation of TLR2 and dectin-1 is essential for antifungal innate immunity against Pneumocystis [10]. In the signaling pathway downstream of the TLR2/dectin-1 cluster, intracellular osteopontin (iOPN) [17] plays a critical role (Figure 1). Upon detection of Pneumocystis, iOPN was essential for the clustering of TLR2, dectin-1, and MMRs in macrophages [10]. iOPN simultaneously associates with IRAK1 and Syk, which are signaling molecules downstream of TLR2 and dectin-1, respectively, while enhancing MAPK (Mitogen-Activated Protein Kinases) activation [10]. OPN-deficient macrophages have shown attenuated phagocytosis of Pneumocystis and ROS generation, as well as cytokines production, suggesting that iOPN is essential for antifungal effects through PRR cluster formation. In fact, OPN-deficient immunocompromised mice are extremely susceptible to opportunistic infection by Pneumocystis [10]. iOPN is known to co-localize with actin and induces cytoskeletal rearrangement [18], [19]. It is also known that the actin cytoskeleton plays an important role in the dynamics of the clusters with transmembrane receptors [20]. Therefore, evaluating the role of iOPN in the PRR clustering in the context of cytoskeletal rearrangement upon fungal detection may be of interest.
Question to Be Asked
Depending on the fungal species to be detected, macrophages appear to form clusters comprised of a unique combination of PRRs to optimize detection of fungi and to create synergy among multiple PRRs. However, precisely how PRRs are recruited to the lipid rafts and how the synergistic signal transduction pathways crosstalk are not clear. Relative to viruses and bacteria, sizes of fungi are, in general, significantly larger. It is therefore logical for host cells to form PRR clusters in an effort to maximize the interaction between PRRs and the fungal surface. In this regard, we suggest that the mechanism of PRR cluster formation in host cells during fungal detection is an issue critical to our further understanding of detection of fungi by host cells.
Zdroje
1. ZhangC, WangSH, LasburyME, TschangD, LiaoCP, et al. (2006) Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect Immun 74 : 1857–1864.
2. VillamonE, GozalboD, RoigP, O'ConnorJE, FradeliziD, et al. (2004) Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect 6 : 1–7.
3. TaylorPR, TsoniSV, WillmentJA, DennehyKM, RosasM, et al. (2007) Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8 : 31–38.
4. GantnerBN, SimmonsRM, CanaveraSJ, AkiraS, UnderhillDM (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197 : 1107–1117.
5. GrossO, GewiesA, FingerK, SchaferM, SparwasserT, et al. (2006) Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442 : 651–656.
6. XuS, HuoJ, LeeKG, KurosakiT, LamKP (2009) Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem 284 : 7038–7046.
7. GrossO, PoeckH, BscheiderM, DostertC, HannesschlagerN, et al. (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459 : 433–436.
8. ZhuLL, ZhaoXQ, JiangC, YouY, ChenXP, et al. (2013) C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39 : 324–334.
9. MullinNP, HitchenPG, TaylorME (1997) Mechanism of Ca2+ and monosaccharide binding to a C-type carbohydrate-recognition domain of the macrophage mannose receptor. J Biol Chem 272 : 5668–5681.
10. InoueM, MoriwakiY, ArikawaT, ChenYH, OhYJ, et al. (2011) Cutting edge: critical role of intracellular osteopontin in antifungal innate immune responses. J Immunol 186 : 19–23.
11. DennehyKM, FerwerdaG, Faro-TrindadeI, PyzE, WillmentJA, et al. (2008) Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol 38 : 500–506.
12. FerwerdaG, Meyer-WentrupF, KullbergBJ, NeteaMG, AdemaGJ (2008) Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol 10 : 2058–2066.
13. BrownGD, HerreJ, WilliamsDL, WillmentJA, MarshallAS, et al. (2003) Dectin-1 mediates the biological effects of beta-glucans. J Exp Med 197 : 1119–1124.
14. GringhuisSI, den DunnenJ, LitjensM, van Het HofB, van KooykY, et al. (2007) C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 26 : 605–616.
15. GoodridgeHS, ReyesCN, BeckerCA, KatsumotoTR, MaJ, et al. (2011) Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 472 : 471–475.
16. XuS, HuoJ, GunawanM, SuIH, LamKP (2009) Activated dectin-1 localizes to lipid raft microdomains for signaling and activation of phagocytosis and cytokine production in dendritic cells. J Biol Chem 284 : 22005–22011.
17. InoueM, ShinoharaML (2011) Intracellular osteopontin (iOPN) and immunity. Immunol Res 49 : 160–172.
18. ShinoharaML, KimHJ, KimJH, GarciaVA, CantorH (2008) Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A 105 : 7235–7239.
19. SuzukiK, TakeyamaS, KikuchiT, YamadaS, SodekJ, et al. (2005) Osteoclast responses to lipopolysaccharide, parathyroid hormone and bisphosphonates in neonatal murine calvaria analyzed by laser scanning confocal microscopy. J Histochem Cytochem 53 : 1525–1537.
20. GonnordP, BlouinCM, LamazeC (2012) Membrane trafficking and signaling: two sides of the same coin. Semin Cell Dev Biol 23 : 154–164.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



