-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
microRNAs (miRNA) have been detected in the deeply branched protist, Giardia lamblia, and shown to repress expression of the family of variant-specific surface proteins (VSPs), only one of which is expressed in Giardia trophozoite at a given time. Three next-generation sequencing libraries of Giardia Argonaute-associated small RNAs were constructed and analyzed. Analysis of the libraries identified a total of 99 new putative miRNAs with a size primarily in the 26 nt range similar to the size previously predicted by the Giardia Dicer crystal structure and identified by our own studies. Bioinformatic analysis identified multiple putative miRNA target sites in the mRNAs of all 73 VSPs. The effect of miRNA target sites within a defined 3′-region were tested on two vsp mRNAs. All the miRNAs showed partial repression of the corresponding vsp expression and were additive when the targeting sites were separately located. But the combined repression still falls short of 100%. Two other relatively short vsp mRNAs with 15 and 11 putative miRNA target sites identified throughout their ORFs were tested with their corresponding miRNAs. The results indicate that; (1) near 100% repression of vsp mRNA expression can be achieved through the combined action of multiple miRNAs on target sites located throughout the ORF; (2) the miRNA machinery could be instrumental in repressing the expression of vsp genes in Giardia; (3) this is the first time that all the miRNA target sites in the entire ORF of a mRNA have been tested and shown to be functional.
Published in the journal: The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence. PLoS Pathog 10(2): e32767. doi:10.1371/journal.ppat.1003791
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003791Summary
microRNAs (miRNA) have been detected in the deeply branched protist, Giardia lamblia, and shown to repress expression of the family of variant-specific surface proteins (VSPs), only one of which is expressed in Giardia trophozoite at a given time. Three next-generation sequencing libraries of Giardia Argonaute-associated small RNAs were constructed and analyzed. Analysis of the libraries identified a total of 99 new putative miRNAs with a size primarily in the 26 nt range similar to the size previously predicted by the Giardia Dicer crystal structure and identified by our own studies. Bioinformatic analysis identified multiple putative miRNA target sites in the mRNAs of all 73 VSPs. The effect of miRNA target sites within a defined 3′-region were tested on two vsp mRNAs. All the miRNAs showed partial repression of the corresponding vsp expression and were additive when the targeting sites were separately located. But the combined repression still falls short of 100%. Two other relatively short vsp mRNAs with 15 and 11 putative miRNA target sites identified throughout their ORFs were tested with their corresponding miRNAs. The results indicate that; (1) near 100% repression of vsp mRNA expression can be achieved through the combined action of multiple miRNAs on target sites located throughout the ORF; (2) the miRNA machinery could be instrumental in repressing the expression of vsp genes in Giardia; (3) this is the first time that all the miRNA target sites in the entire ORF of a mRNA have been tested and shown to be functional.
Introduction
The deeply branching protozoan parasite Giardia lamblia, is responsible for the diarrheal disease giardiasis, which affects over 200 million people worldwide [1], [2]. In developing countries, multiple exposures to the pathogen result in repeated chronic infections. The persistence of infection is believed to be due to variations of the variant-specific surface proteins (VSPs) on the membrane surface of Giardia trophozoites. Only a single species of VSP is expressed on the membrane surface of a trophozoite at a given time, which is replaced by another VSP every 6 to 13 generations [3], [4], [5]. This variation likely allows Giardia to avoid the host immune response during infection [6]. VSPs are zinc finger-containing proteins containing two well-conserved structural motifs localized to the C-terminal domain of the molecule [7]. Motif 1, located upstream from the C-terminus, contains the zinc finger domain. Motif 2, located at the very C-terminus, is likely the transmembrane domain, which is followed by a 5 amino acid sequence CRGKA with a yet unknown function [8], [9]. Using these structural criteria, it was recently estimated that there are about 73 vsp genes in Giardia WB isolate [7]. Although it might not be the final number of vsps upon further investigation in the future, it is, nevertheless, the best verified number at present time. We have thus used it in the present study.
The regulatory mechanisms of VSP expression and variation have been poorly understood. All the vsp mRNAs appear to be continuously transcribed in Giardia trophozoites [10], [11]. Regulation of vsp expression is thus most likely at the posttranscription level. Our previous studies have postulated the involvement of a translational-repression mechanism involving a microRNA (miRNA) machinery in regulating VSP expression [12], [13], [14], [15], [16]. Six miRNAs have since been identified in Giardia, among which, five were found to translationally repress the expression of specific vsp mRNA subsets in Giardia, though full repression has not yet been observed (see below) [12], [13], [14], [15], [16].
miRNAs are small regulatory RNAs responsible for post-transcriptional regulation of mRNA expression among eukaryotes [17]. A primary miRNA in the nucleus is first processed by the nuclear RNaseIII, Drosha/Pasha, to generate a hairpin shaped pre-miRNA, which is then transported into the cytoplasm where another RNaseIII, Dicer, further processes it into a double-stranded miRNA [17]. The double-stranded miRNA is bound to an Argonaute protein, which releases its passenger strand and forms the miRNA-induced silencing complex (miRISC) [17]. Interaction between the miRISC-associated miRNA and its mRNA target site results in translational repression and degradation of the mRNA [18], [19]. In higher eukaryotes, the majority of miRNA target sites are found in the 3′ untranslated regions (UTR) of mRNAs [20], [21], though a few target sites have also been identified in the 3′-end of the coding region [22], [23], [24], [25], [26], [27], [28], [29]. A further search for conserved regions in coding regions also revealed that let-7 miRNA targets the Dicer within its coding sequence [30]. A recent study further suggested that the reduced frequency of functional miRNA target sites in the coding region of an mRNA could be due to ribosomal displacement of the miRISC during translation resulting in reduced miRNA-mediated translational regulation and an evolutionary bias against coding region target sites [31]. This hypothesis may explain why the majority of target sites have been primarily found in the 3′ UTR ∼15 nts away from the stop codon [20], [21]. But up to 50% of miRNA target sites have been identified in the open reading frames (ORFs) with a few hits in the 5′ UTR from HITS-CLIP analysis [32], [33]. These target sites have not yet been vigorously tested for their potential functions. Recent results from the large-scale protein mis-expression studies suggested that the target sites in upstream ORFs are actually functional, and can enhance the miRNA regulation when a 3′ UTR target site is also present [34]. The biological significance in the miRNA target sites present in the upstream portion of ORF remains thus to be further clarified.
In Giardia, 6 miRNAs (miR2, miR3, miR4, miR5, miR6 and miR10) have been identified and characterized [12], [13], [14], [16]. Since the mRNAs in Giardia have 3′-UTRs with an average length of only 30 nts [35], [36], there is in some cases no miRNA target site in the 3′-UTR or only one spanning across the stop codon and shared by both 3′-UTR and the 3′-end of ORF. A more recent report by Franzen et al. (2013) indicated that the median 3′-UTR length is 80 nts [37]. The additional length of the 3′ UTR may contain more than one miRNA target site but has not yet been tested. When a defined region of 100 nts upstream and 50 nts downstream of the stop codon in mRNAs was examined, 5 of the 6 identified miRNAs (with the exception of miR3) were shown to have their putative target sites in that region among certain vsp mRNAs. All 5 miRNAs demonstrated partial repression of either a Renilla luciferase reporter gene containing the corresponding target site in its 3′ UTR or expression of the vsp mRNA carrying the miRNA target sites in the defined 3′-region. The effects were additive when the target sites were separated from each other. This observation raised a probable scenario for a miRNA-mediated mechanism of inhibiting expression of the vsp genes in a Giardia trophozoite. The vsp mRNAs may each carry multiple target sites, allowing for multiple miRNA actions. When all the sites are saturated with their corresponding miRNAs, expression of the mRNA may be completely repressed.
In order to verify this hypothesis, the complete spectrum of miRNAs in Giardia trophozoites needs to be identified. We analyzed 3 next-generation sequencing (NGS) libraries from Giardia Argonaute (GlAgo)-associated small RNAs and identified a total of 105 putative miRNAs, including the 6 previously characterized miRNAs. All the newly identified miRNAs tested thus far were able to partially repress expression of their corresponding vsp mRNAs. Only partial repression was achieved when only target sites confined within the artificially defined 3′-region were saturated with miRNAs. When most of the target sites in the ORF of a vsp mRNA were saturated with miRNAs, however, a near total repression of expression was observed.
Results
Small RNA library construction and sequence analysis
We have previously reported co-immunoprecipitation of tagged GlAgo with a well-defined small RNA band in the 26 nt range [14]. The small RNAs in this band contained a 5′ phosphate group and a 3′ hydroxyl group and could be primarily the miRNAs [14]. To sequence these small RNAs, we constructed 3 independent libraries for NGS, using the GlAgo associated small RNAs from Giardia WB trophozoites as templates [14]. Two independent libraries (TDIP1 & TDIP2; Trophozoite Detergent GlAgoIP) were constructed from the small RNAs recovered from GlAgo immunoprecipitation after detergent lysis of Giardia trophozoites. A third library (TDSIP; Trophozoite Detergent/Sonication GlAgoIP) was constructed from cells by the same procedure except that a sonication step was added to the detergent lysis to insure a more complete trophozoite breakdown. As the subsequent results will verify (see below), the added sonication did not significantly alter the experimental outcome.
The 3 libraries thus constructed were sequenced using the Illumina HiSeq 2500 sequencer and each produced over 190 million reads (Table 1). After removing the adaptors, low quality sequences (Q<20) and sequences smaller than 20 nts or larger than 32 nts, the 3 libraries each retained over 95% of the original reads (Table 1). Over 60% of the pre-processed reads in each of the remaining 3 libraries could be aligned to the genome using the Bowtie software [38] and Giardia genome scaffolds downloaded from GiardiaDB (Table 1) [39]. About 40% of the aligned reads were derived from tRNAs and rRNAs, which was increased to 50% when sonication was included in preparing the lysate. These sequences were removed, and the three libraries each retained an average of 2 million unique reads (Table 1).
Tab. 1. Identification of unique reads from three NGS libraries. 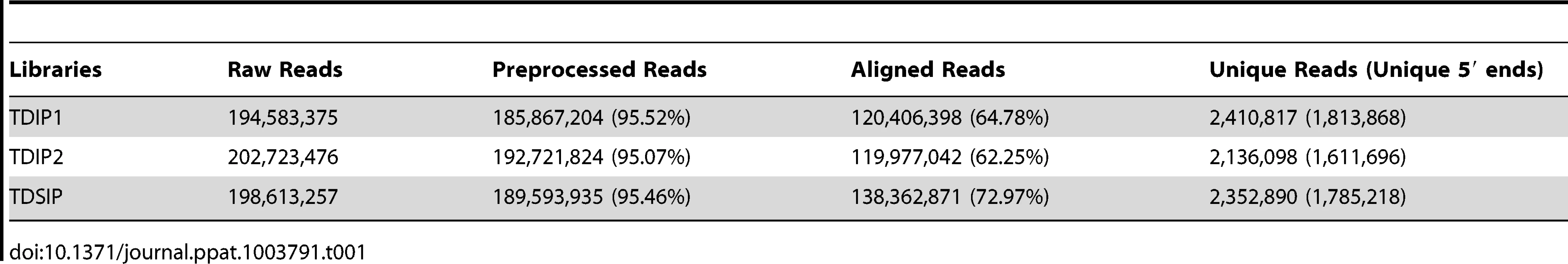
Length distributions of the unique reads among the three libraries are highly similar. There is a 26 nt peak, which agrees with the size of miRNAs anticipated from the Giardia Dicer (GlDcr) crystal structure [40] and the sizes of miRNAs identified in our previous studies [12], [13], [14], [15], [16] (Figure 1A).
Fig. 1. Analysis of the NGS libraries. 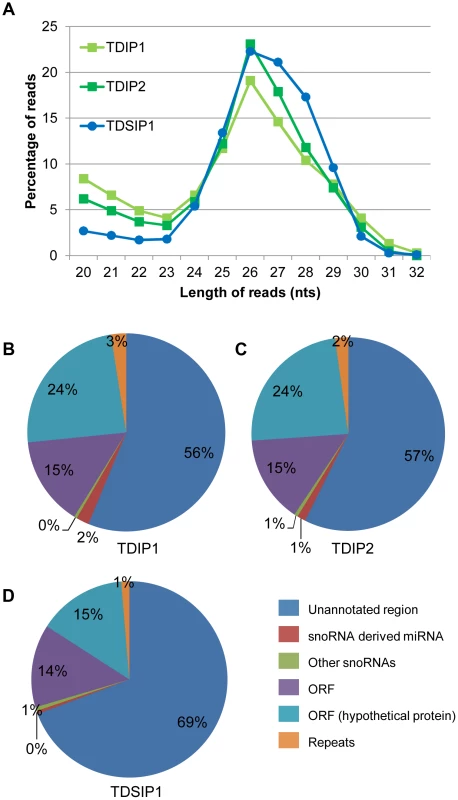
A) Size distribution of sequences remaining after alignment to the Giardia genome and removal of rRNA and tRNA. A peak at 26 nts corresponds to the expected size of miRNAs in Giardia. B) Analysis of read origins in the TDIP1 library. The majority of potential miRNAs are derived from ORFs or unannonated regions. C) Analysis of read origins in the TDIP2 library. D) Analysis of read origins in the TDSIP library shows a 12–13% increase in the reads derived from unannonated regions with a corresponding decrease in reads derived from ORFs. The majority of sequences among the unique reads are derived from unannotated regions of the Giardia genome followed by sequences derived from the ORFs (Figures 1B, C, D). They constitute about 95% of the populations in all 3 libraries. The remaining sequences are derived from snoRNAs including primarily the few snoRNA-derived miRNAs we have already identified [12], [13], [15], [16]. The sequence distributions in the 3 libraries indicate that they are of high quality with data very similar to one another.
Identification of putative miRNAs
Putative miRNA candidates in the 3 libraries were selected by initially determining the minimum read counts for the 6 miRNAs we have identified previously [12], . miR3 had the minimum read count of 235, 365, and 512 in the TDIP1, TDIP2, and TDSIP libraries, respectively. This read count was used to weed out sequences present at lower levels. Next, candidate miRNAs with overlapping sequences that do not exceed an overall length of 40 nt were compiled into a peak. The 5′ and 3′ ends with the highest read count were taken as the ends of the putative miRNA. Potential precursors (the pre-miRNA) for the putative miRNAs were generated by extracting a 100 nt sequence upstream from the 3′ end of the putative miRNA utilizing a 1 nt sliding window that shifted the sequence towards the 3′ end while maintaining a 100 nt total length. These potential pre-miRNAs were then folded using the RNAfold software to predict a hairpin structure that could be a suitable substrate for GlDcr [41], [42]. Those that failed to form a hairpin or placed the miRNA in the loop were discarded. Following this selection, 623 candidates for miRNA were identified in TDIP1, 634 in TDIP2 and 663 in TDSIP. Identical sequences, however, were found in different contigs due to overlapping sequences in GiardiaDB. Removal of duplicate sequences further reduced the number of putative miRNAs to 491, 455, and 548 for TDIP1, TDIP2, and TDSIP libraries, respectively (detailed sequence data available upon request). When the miRNA candidates from TDIP1 and TDIP2 were compared and those with the same 5′ end were chosen, the number was reduced to 208. When these sequences were compared with those from TDSIP, a pool of 105 miRNAs was identified, which included the 6 that have been already identified in our previous studies [12], [13], [14], [15], [16] (Table S1). This pool may represent the minimal number of miRNAs in Giardia [43], [44].
An analysis of the sizes of the 105 putative miRNAs indicated a primary range of 24 to 28 nts with a peak population at 26 nts (Figure 2A). The 5 previously characterized miRNAs derived from snoRNAs turned out to be the only snoRNA-derived miRNAs found in the pool, constituting only 4.8% of the total (Figure 2B). There are 55.2% of the putative miRNAs derived from ORFs and 40.0% from the unannotated genomic region (Figure 2B). The results from a detailed analysis of the ORFs, presented in Table S1, suggest that many of them could be RNAs not coding for protein. Since Drosha/Pasha is known to be absent from Giardia [16], these likely non-coding RNAs may be first processed by GlDcr to generate the pre-miRNAs [14], and then digested by GlDcr again to produce the miRNAs [14].
Fig. 2. Analysis of potential miRNAs. 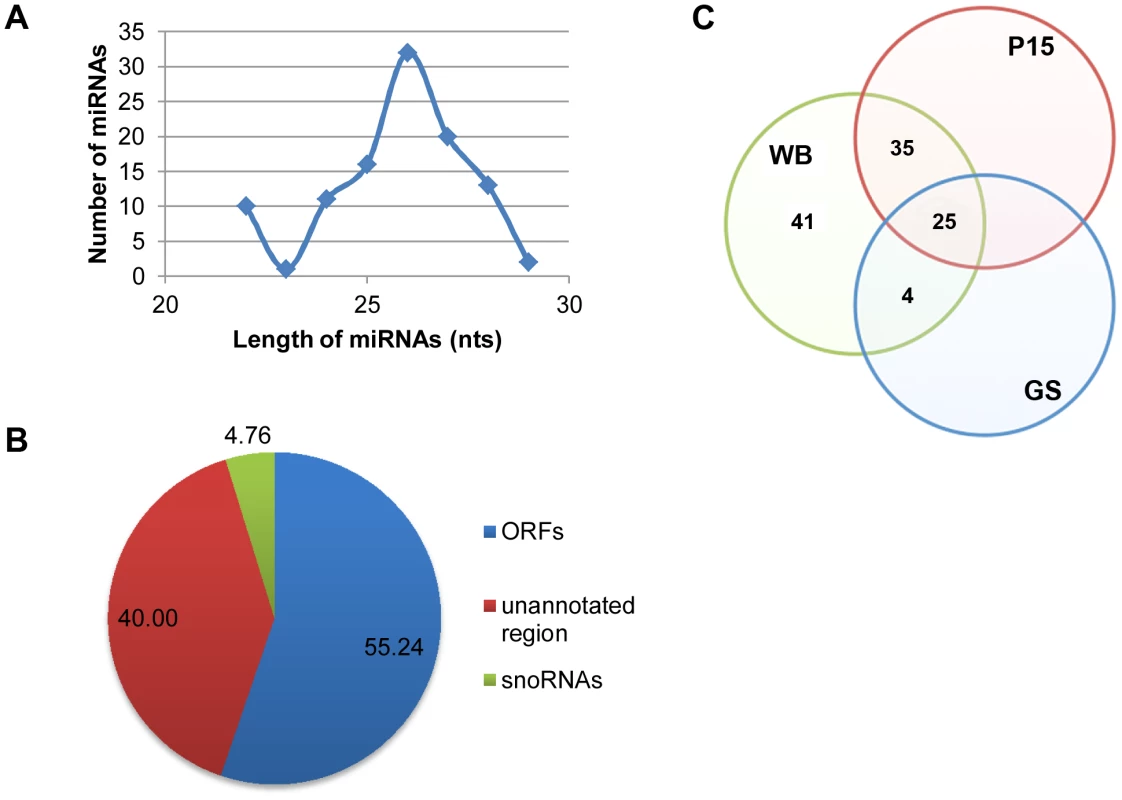
A) Size distribution of the 105 miRNAs shows a range from 22–29 nts with a peak at 26 nts. B) Origins of the 105 miRNAs show that the majority is derived from ORFs and unannotated regions. C) A Venn diagram showing the conservation of potential miRNAs among the three sequenced isolates of Giardia. A BLAST search resulted in 41 miRNAs only in WB, 4 miRNAs conserved between WB and GS whereas 35 shared between WB and P15. A total of 25 miRNAs are conserved among all three isolates. See also Table S1. In order to verify if the putative miRNAs thus identified are bona fide miRNAs, we used RT-qPCR to search for the previously well-identified miRNAs; miR2, miR4 [14], miR5 [12], miR6 and miR10 [13] in the ∼26 nt small RNA band co-immunoprecipitated with HA-GlAgo. Every one of these miRNAs was positively identified in the small RNA band, suggesting that most, if not all, of the miRNAs are associated with the immunoprecipitated ∼26 nt small RNA band.
Two isolates of Giardia other than the WB isolate, GS and P15, have also their genomes sequenced and the data incorporated into GiardiaDB. Phylogenetic studies showed that WB is more closely related to P15 than GS [45], [46]. Genomic analysis showed that average protein identity is 90% between P15 and WB but only 81% between P15 and GS [46]. We performed a BLAST search to identify putative miRNAs in GS and P15 with identical sequences to the 105 miRNAs in WB, allowing but two mismatches. The results presented in Figure 2C indicate that 25 miRNAs are conserved among all 3 isolates, whereas 41 are specific to the WB isolate. Four miRNAs are conserved between WB and GS while 35 are conserved between WB and P15 (Figure 2C). This distribution of miRNAs agrees with the results from previous phylogenetic and genetic analysis, and suggests significant diversity in the miRNA population among the three isolates.
Identification of potential miRNA target sites among the vsp genes
The miRanda program was used to analyze potential target sites for the 105 miRNAs among the newly identified 73 vsp genes in Giardia [7], [47]. The miRanda analysis was first performed within a defined 3′-region of the gene (100 nts upstream and 50 nts downstream of the stop codon). It was then used to analyze the full length ORFs plus 50 nts upstream and 50 nts downstream to cover the potential 5′-UTRs and 3′-UTRs [35], [36]. The outcome indicated that multiple miRNA targeting sites are present in the 3′ region of many vsp genes (data not shown). Much higher numbers of miRNA target sites were identified in the full-length vsp ORFs with no target site found in the 5′-UTRs (data not shown). A total of 81 miRNAs were found to have potential target sites in the 3′-region while 102 of the miRNAs had potential target sites in the entire ORFs of the 73vsp genes. Only 3 out of the entire 105 miRNAs, miR59, miR67 and miR78, do not target any vsp genes. This apparent abundance of putative miRNA target sites among the vsp genes provided an opportunity to test whether the newly identified miRNAs exert repression on corresponding VSP expression in Giardia. An even more important question addresses whether interactions between the miRNAs and their target sites confined to the defined 3′-region of the VSP mRNA are enough to totally repress its expression or if the target sites upstream from this region are also involved.
Regulation of VSP expression by targeting miRNA target sites confined to the 3′-region of mRNAs
In previous studies, we found that VSPs tagged at the C-terminal with 3xmyc can translocate to the cell membrane similar to native VSPs [7]. An N-terminal 3xmyc tag, however, inhibited VSPs localization to the cell surface [7]. Here we choose two VSPs; VSP-2 and VSP-76 for further analysis, because they carry relatively low numbers of potential target sites at their 3′-ends and are thus easier for further analysis. Both VSPs were 3xmyc-tagged at the C-terminus (VSP-2-myc and VSP-76-myc) and episomally expressed in Giardia trophozoites. Both tagged VSPs could be detected on the cell membrane surface in an immunofluorescence assay (Method S1 and Figures S1A & S1B). The full-length vsp-2 cDNA and vsp-2-myc cDNA were synthesized by RT-PCR and sequenced (Figures 3A & 3B). There is no putative target site for the 105 miRNAs within the short 3′-UTR of vsp-2 mRNA (Figure 3A) or the 3xmyc ORF and the artificial 15 nt 3′-UTR in vsp-2-myc mRNA (Figure 3B). The two mRNAs thus have an identical pattern of miRNA targeting sites. There are two overlapping potential target sites for miR24 and miR56 within the confinement of the 100 nts limit for the 3′-end of the vsp-2 ORF (Figure 3A). The addition of the 126 nt 3xmyc tag in the vsp-2-myc construct, however, increases the length of the vsp mRNA resulting in a shift of the miRNA target sites further upstream of the stop codon. Thus, by using the fusion protein, we tested target sites already beyond the original artificial limit of 100 nts upstream from the stop codon and well into the coding region.
Fig. 3. miRNA-mediated regulation of VSP-2 expression. 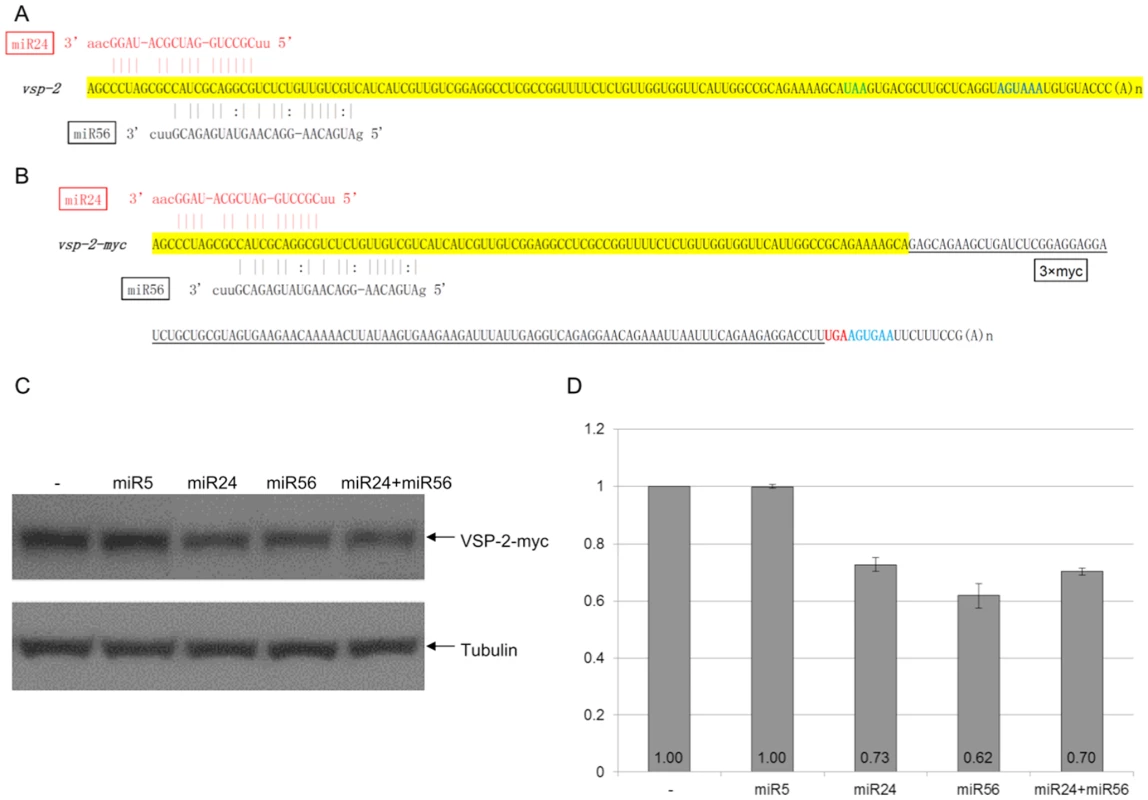
A) Schematic diagram of the vsp-2 mRNA 3′ region (100 nts upstream of the stop codon and 3′ UTR) with the binding sites for miR24 and miR56. miRNAs with fully complementary seed sequence binding sites are shown in red while miRNAs with G:U wobble pairs in the seed sequence are in black. The stop codon is shown in green and the polyadenylation signal is in blue. B) Schematic diagram of the vsp-2-myc mRNA detailing incorporation of the 3xmyc tag and elimination of the native 3′ UTR. Coloring is the same as in A). C) VSP-2-myc expression in Giardia cells transfected with the indicated miRNAs was analyzed with Western blot. Tubulin was used as a loading control. D) Densitometry analysis of three independent Western blot experiments is shown as a histograph with the error bars representing the standard deviation. To test the function of the newly identified miRNAs, 1 µg of synthetic miR24 and miR56 were each transfected into Giardia trophozoites expressing VSP-2-myc and were found to repress expression to 73% and 62% of the control, respectively (Figures 3C & 3D). When miR5, which has no target site in the vsp-2 mRNA, was tested under the same experimental conditions, it showed no detectable effect on VSP-2-myc expression (Figures 3C & 3D), thus indicating the specificity of miRNA action. When a combination of miR24 and miR56 was introduced at 0.5 µg each, for a total of 1 µg, the expression of VSP-2-myc was repressed to 70%, indicating no additive effect, which could be attributed to the overlapping locations of the two target sites (Figures 3C & 3D). More importantly, the results also indicate that miRNA target sites located upstream from the artificially defined 100 nt limited 3′-region can still function well in interacting with the corresponding miRNAs.
The 3′-UTR of vsp-76 mRNA also lacks any miRNA targeting site, but it consists of two separated target sites for miR69 and miR31 in the confined 3′-100 nt ORF region (Figures 4A & 4B). miR30, which does not have a target site in the vsp-76 mRNA, demonstrated no effect on VSP-76-myc expression and was used as a negative control (Figures 4C & 4D). miR69 and miR31 repressed the expression of VSP-76-myc to 76% and 70%, respectively (Figures 4C & 4D). In combination, they repressed the expression further down to 44%, thus indicating the additivity of miRNA repression when the target sites locate separately from each other (Figures 4C & 4D). But the combined repression still fails to completely knock down VSP-76-myc expression, indicating once again that interactions between target sites and miRNAs confined within the artificially defined 3′-region of the ORF may be inadequate for total repression.
Fig. 4. miRNA-mediated regulation of VSP-76 expression. 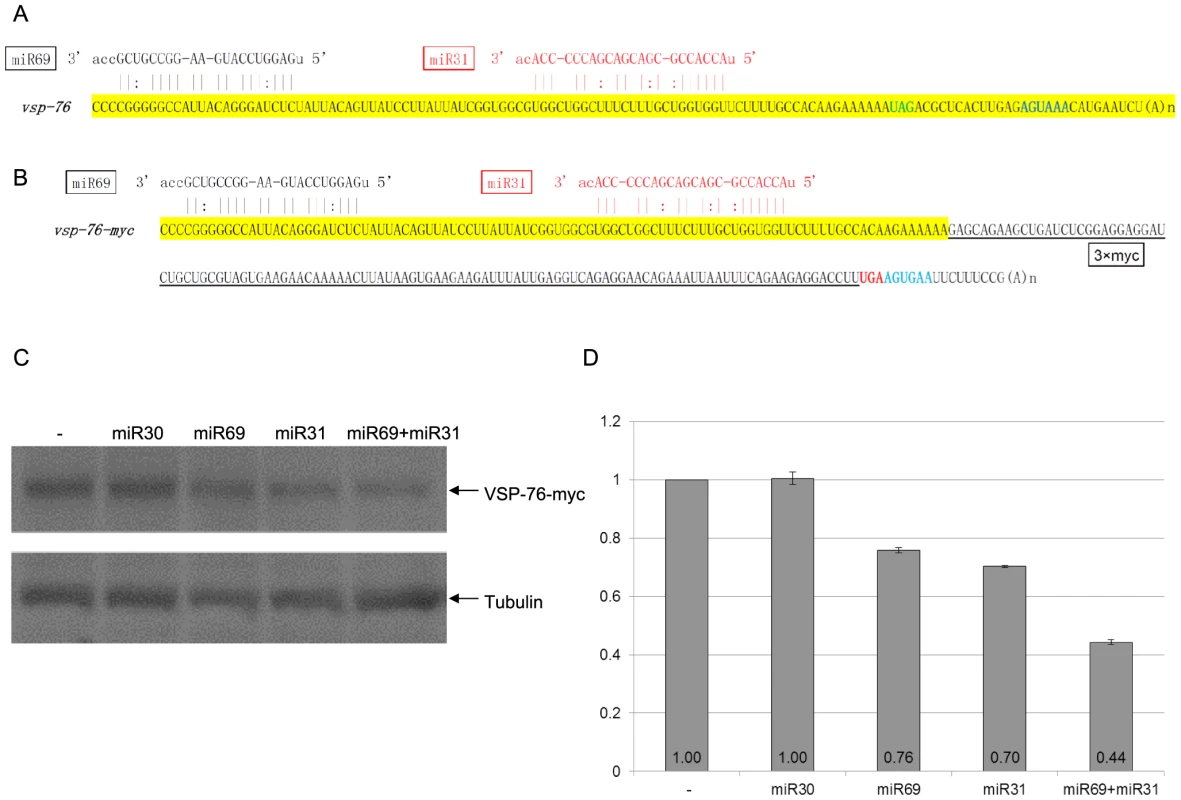
A) Schematic diagram of the vsp-2 mRNA 3′ region (100 nts upstream of the stop codon and the 3′ UTR) with the binding sites for miR24 and miR56. Coloring is as in Figure 3A. B) Schematic diagram of the vsp-76-myc mRNA detailing incorporation of the 3xmyc tag and elimination of the native 3′ UTR. C) Giardia cells expressing vsp-76-myc transfected with the indicated miRNAs was analyzed with Western blot. Tubulin was used as a loading control. D) Densitometry analysis of three independent Western blot experiments is shown as a histograph with the error bars representing the standard deviation. Expression of VSP-76-Myc is repressed in the presence of miR69 or miR31 to 76% and 70% of the control, respectively. The presence of both miRNAs represses VSP-76-Myc expression down to 44%, indicating cooperative miRNA action. miR30, which does not target vsp-76, does not affect VSP-76-myc expression. Regulation of VSP expression by targeting miRNA target sites in the entire ORF of mRNA
In order to learn if miRNA target sites further upstream from the confined 3′-region are required to interact with miRNAs in order to reach a higher degree of repression, we selected two relatively short vsp mRNAs for further analysis. One encodes a truncated version of VSP-175 (VSPA6-S) (Figure 5A). This truncated VSP (ΔVSP-175) has the N-terminal 432 amino acid residues eliminated, leaving only 20 amino acid residues upstream from motif 1 and motif 2, which are well conserved among VSPs [7]. When 3xmyc was tagged to the C-terminus and the gene (Δvps-175-myc) expressed episomally in transfected Giardia trophozoites, ΔVSP-175-myc was found localized to the cell membrane surface (Figure S1C). The cDNAs encoding ΔVSP-175 and ΔVSP-175-myc were synthesized by RT-PCR and sequenced. The results, presented in Figures 5A and 5B, indicate the absence of any miRNA target site in the 3′-UTR of Δvsp-175 mRNA. It and the Δvsp-175-myc mRNA thus consist of an identical pattern of 15 miRNA target sites throughout their respective ORFs (Figures 5A & 5B). Of the 15 target sites, two of the target sites, located far apart in the ORF, recognize the same miRNA, miR88 (Figures 5A & 5B). There are thus only 14 miRNAs acting on the two mRNAs. The 14 miRNAs were chemically synthesized and each introduced into Giardia trophozoites (1 µg) and assayed for repression of ΔVSP-175-myc expression. The outcome, presented in Figure 5C, shows that 11 out of the 14 miRNAs repress the expression of ΔVSP-175-myc down to a range of 60–85% when compared to the control. miR88 has the most effective repression down to 60%, which could be attributed to the presence of double non-overlapping target sites. The target site for miR96 is localized near the 5′ end of the ORF (Figure 5B). This miRNA exerts an expression repression down to 65% of the control, indicating that target sites near the 5′ end of mRNAs are also functional.
Fig. 5. miRNA-mediated regulation of ΔVSP-175 expression. 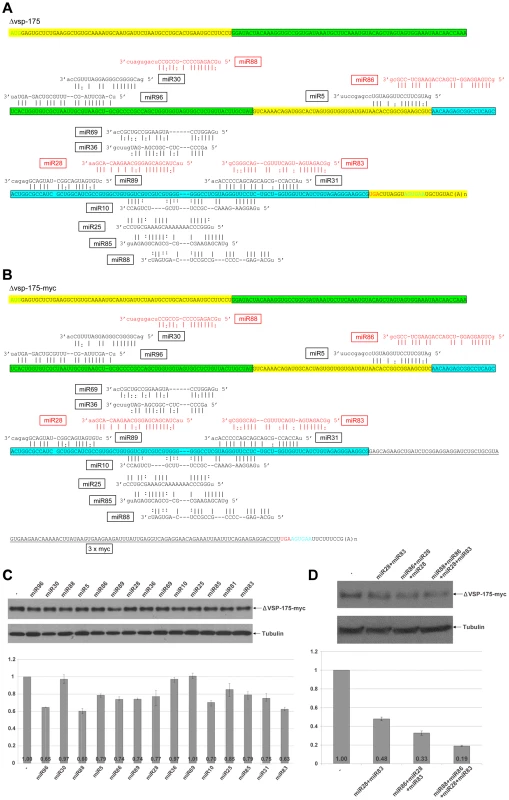
A) Schematic diagram of Δvsp-175 mRNA with all 15 potential miRNA target sites indicated. miRNAs with fully complementary seed sequence binding sites are shown in red while miRNAs with G:U wobble pairs in the seed sequence are in black. Motif 1 in the VSP is highlighted in green while motif 2 in blue. The start codon is shown in green, stop codon in red and the polyadenylation signal in blue. B) Schematic diagram of Δvsp-175-myc with miRNA target sites. Coloring is the same as in A) with the 3xmyc tag underlined and the 3′ UTR in Δvsp-175 mRNA modified. C) ΔVSP-175-myc expression in Giardia cells expressing Δvsp-175-myc transfected with the indicated miRNAs was analyzed by Western blot. Tubulin was used as a loading control. Densitometry analysis of three independent Western blot experiments is shown as a histograph with error bars representing the standard deviation. D) The ability of miRNAs with non-overlapping target sites to cooperatively repress the expression of ΔVSP-175-myc was analyzed with Western blot analysis. The 3 miRNAs, miR30, miR36 and miR69, that failed to show any appreciable effect on ΔVSP-175-myc expression (Figure 5C), however, could effectively repress expression of VSP-117-myc (see Figure 6C for miR30 and miR36) or VSP-76-myc (Figure 4D for miR69). Their failure in repressing ΔVSP-175-myc expression could be attributed to different locations of the target site in different mRNAs (see Discussion).
Fig. 6. miRNA-mediated regulation of VSP-117 expression. 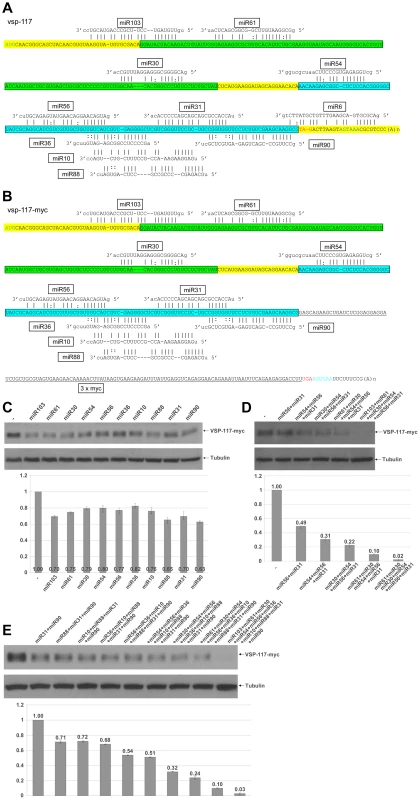
A) Schematic diagram of the vsp-117 mRNA with all 11 potential miRNA target sites. Coloring is as described in Figure 6A. B) Schematic diagram of the vsp-117-myc mRNA with 10 potential miRNA target sites. Addition of the 3xmyc tag eliminates the miR6 target site shared between the ORF and 3′-UTR. Coloring is as described above. C) Expression of vsp-117-myc transfected with the indicated miRNAs was analyzed by Western blot. Tubulin was used as a loading control. Densitometry analysis of three independent Western blot experiments is shown as a histograph with the error bars representing the standard deviation. D) The extent to which miRNA with non-overlapping target sites could repress express of VSP-117-myc was tested. The results indicate that a combined action of 6 miRNAs is sufficient to nearly totally eliminate the mRNA expression. E) Co-transfection of miRNA combinations with overlapping target sites repressed VSP-117-myc expression without cooperative effect. A combination of all 10 miRNAs progressively decreases the expression of VSP-117-myc to near zero. When combined, effects of the miRNAs on non-overlapping target sites were measured. miR28 and miR83 (0.5 µg of each) repressed ΔVSP-175-myc expression down to 48%, miR86+miR28+miR83 (0.33 µg of each) down to 33% and miR88+miR86+miR28+miR83 (0.25 µg of each) down to 19% (Figure 5D). The additivity of inhibitory effects among the miRNAs is thus applicable to the non-overlapping target sites throughout the entire ORF of the mRNA (Figure 5A). More than 80% repression of expression was achieved by a combination of the four miRNAs.
In order to verify if the miRNA repressive effect could be reversed by its corresponding 2′-O-methyl antisense oligo (ASO), the ASOs of miR5 and miR10 were each co-transfected with the corresponding miRNA at an equal concentration. The results showed a total reversal of the inhibitory effects of both miR5 [12] and miR10 [13], indicating the specificity of miRNA action.
We then sequenced another cDNA encoding VSP-117 (GL50803_97820), which consists of 309 bp and 11 putative miRNA target sites in the entire ORF and across the 3′-UTR (Figure 6A). When it is compared with the cDNA sequence of vsp-117-myc, a miR6 target site located across the stop codon in vsp-117 mRNA was elimated due to the 3xmyc tagging (Figure 6B). The miR6 target site has been found located across the stop codons of multiple vsp mRNAs. miR6 and its target site in the mRNAs of VSP-1267 and VSP-213, tagged at the N-terminus with 3xmyc, have been analyzed in our previous studies and shown to repress expression by 30% in each case [13]. VSP-117-myc was localized to the cell membrane similar to the other tagged VSPs (Figure S1D).
An ASO of miR6 was also co-transfected with the miRNA. The result indicated a total abolition of the repressive effect of miR6 [13], verifying the specificity of miR6 action.
We tested the ability of the10 miRNAs other than miR6 to independently repress the expression of VSP-117-myc as described above. The results indicate that every miRNA exerts a partial repression of expression ranging from 63 to 82% of the control (Figure 6C). We then tested combinations of miRNAs with non-overlapping target sites. The data show that when increasing numbers of miRNAs are included in the combination, a higher extent of repression is observed (Figure 6D). When six miRNAs with non-overlapping target sites, miR103+miR61+miR30+miR54+miR56+miR31, were combined at 0.17 µg each, the expression of VSP-117-myc was repressed down to 2% of the control level (Figure 6D). A near total repression was thus achieved even though not all the available target sites were occupied by miRNAs. The 6 miRNAs with non-overlapping target sites are sufficient to nearly completely eliminate mRNA expression. The most intriguing finding is perhaps that near total repression was achieved even when the miR6 target site at the 3′-UTR was eliminated and miR6 was not involved in targeting the mRNA. When additional miRNAs with target sites overlapping the target sites of the initial 6 miRNAs were added, they did not further enhance the level of repression (Figure 6E). Thus, when all 10 miRNAs that target vsp-117-myc were combined at 0.1 µg each, expression of VSP-117-myc was repressed to 3%. This is not significantly different from the extent of repression when only the 6 miRNAs with non-overlapping target sites were included. This outcome suggests that multiple miRNAs with overlapping target sites could competitively regulate expression of vsp mRNAs. The redundant miRNAs and miRNA target sites could function to increase the rate of target site occupancy.
We thus conclude that essentially all the target sites in the ORF of vsp mRNAs may be functional. They do not, however, need to be fully occupied in order to achieve total repression of the mRNA expression. Occupation of some of the non-overlapping target sites throughout the ORF should be sufficient to eliminate mRNA expression. The presence of additional target sites at overlapping positions, however, may allow different combinations of miRNAs to effectively repress expression of the vsp mRNA. This could be a means of maintaining VSP expression in a repressed state while the levels of miRNAs may fluctuate. This is also the first time, as far as we are aware, that miRNA target sites throughout the entire ORF are found to be functional and essential for full repression of mRNA expression.
Discussion
We have successfully constructed 3 libraries of the GlAgo associated small RNAs from Giardia WB trophozoites for next generation sequencing (NGS). The numbers of raw reads, preprocessed reads, aligned reads and unique reads derived from each of the 3 independent libraries appear highly similar (see Table 1). This similarity suggests high quality and reproducibility of data among the 3 libraries and a high degree of reliability in the number and identity of reads derived from them. The 105 miRNAs resulted from the final analysis could be thus not far off from the actual number of miRNAs in the WB isolate of Giardia.
About two dozens of the newly identified miRNAs have since undergone in vivo tests for their potential activities in repressing the expression of vsp mRNAs carrying the corresponding target sites. All of them demonstrated the ability to repress their target mRNAs, indicating their authenticity and suggesting that all the 105 miRNAs could be bona fide miRNAs in Giardia. The extent of vsp mRNA repression by these miRNAs is usually in the range of 20 to 40%, similar to those we have observed previously with the 5 snoRNA-derived miRNAs, suggesting that the origins of miRNAs do not significantly affect the potencies of miRNAs.
The data from analyzing miRNA actions on Δvsp-175-myc and vsp-117-myc expressions also indicate that a miRNA acting on a target site close to the 5′-end of ORF is just as effective as a target site close to the 3′-end (Figures 5C and 6C). This is in sharp contrast to the observations made in higher eukaryotes, which have the majority of miRNA target sites located in the 3′-UTR [20], [21], a few in the 3′-end of coding region [22], [25], [26], [28], [29], [30],,but none in the 5′-end of ORFs. Thus, even though up to 50% of potential miRNA target sites have been identified in the ORFs by HITS-CLIP analysis, they have not yet been extensively tested and assumed to be largely nonfunctional [32], [33]. This assumed lack of function of the miRNA target sites in the 5′ portion of ORF was attributed to active translation, which impedes miRNA-programmed miRISC association with target mRNAs [31]. Such an explanation cannot be, however, applied to the active miRNA function on the target sites throughout the ORF of vsp mRNAs in Giardia. The mechanism of translational initiation in Giardia does differ from that in the higher eukaryotes in certain aspects. There is apparently no mechanism of ribosome scanning required for searching of the initiation codon in order to start translation in Giardia [48]. The highest translational efficiency is achieved when AUG is positioned immediately in front of the ORF [48], which is also reflected by the relatively short 5′-UTRs among the mRNAs in Giardia [35], [36]. Whether this interesting feature in translational initiation in Giardia may contribute to the apparent importance of miRNA and target site interactions at the 5′-end of ORFs remains to be seen. Overall, a more thorough analysis of the miRNA target sites in the 5′-region of ORFs appears to be in order among higher eukaryotes at this time.
The 3′-UTRs in Giardia mRNAs have an average length of 30 nts [35], [36]. Thus, in most cases, they are apparently barely long enough to accommodate a single miRNA target site. To date, we have been unable to identify in Giardia a miRNA target site located completely within the 3′ UTR. Instead, 3′ UTR target sites usually straddle the stop codon with the miRNA seed sequence binding in the 3′ UTR and the remaining miRNA sequence binding to the 3′ end of ORF. A recent publication extended the median size of Giardia 3′ UTRs to 80 nts [37], which may indicate the potential presence of miRNA target sites in the 3′-UTRs of some mRNAs. In view of the partial repression exerted by the interaction between a single miRNA and a single target site and the lack of additivity between miRNA actions on overlapping target sites, it is not possible for Giardia to rely only on miRNA action in the 3′-UTR of mRNA alone for effective control of post-transcriptional gene expression. In fact, among the 4 vsp mRNAs we have analyzed in the present study, 3 do not have a target site in their 3′-UTRs at all. This may make it necessary for the target sites in the ORF of vsp mRNAs to be functional in order to achieve total repression of its expression in Giardia. There have been some recent examples of miRNA actions on target sites in the ORFs of mammalian mRNAs. They include the action of miR-183 on βTrCP1 mRNA in colorectal cancer cells [23], the action of miR-143 on mouse pleiotrophin mRNA [27] and the action of miR-34a on MDM4 mRNA in colon carcinoma cells [24]. Though the data are still too preliminary to reveal any potential significance in miRNA target sites localized to the upstream portion of ORF, further studies may throw more light on the subject.
We have also noted in the current as well as previous studies that the locations of some miRNA target sites are highly conserved among different vsp mRNAs. For example, the miR10 target site in all the vsp mRNAs is typically located at positions −62 to −32 upstream from the stop codon, whereas the miR6 target site is usually located across the stop codon [13]. Similarly, the majority of miR69 target sites are located at positions −100 to −75. When it is not located at this position in Δvsp-175-myc mRNA, it was found not functional (see Figure 5). The location of miR36 target site in Δvsp-175-myc and vsp-117 is shifted by only one nucleotide. It was found functional in vsp-117 but not in Δvsp-175-myc. Finally, the miR30 target site is located at different positions upstream from the 100 nt 3′-end region in Δvsp-175-myc and vsp-117 and showed functional differences (see Figures 5 & 6). These differences in target site functionality could be attributed to differences in mRNA secondary structure and distinctions in thermodynamic stability of miRNA binding.
Our current observations have provided a tentative explanation for how expression of vsp mRNAs is regulated in Giardia trophozoites. All 73 vsp mRNAs carrying multiple target sites in their ORFs for miRNAs are likely present in the trophozoites [7]. A variety of combinations of miRNAs from among the 102 miRNAs could effectively repress the expression of each of the vsp mRNAs. Not all the target sites in an ORF need to be saturated with miRNAs, however, and a significant extent of miRNA redundancy could maintain most of the vsps in a silent state (see Figure 6). The example presented in Figure 6 indicates that only 6 miRNAs with non-overlapping target sites in the ORF are sufficient to bring about near total repression of mRNA expression. This repression does not even require the interaction between miR6 and its target site located partly in the 3′-UTR. Thus, the mechanism of miRNA-mediated translational repression in Giardia targets apparently the entire coding region of the mRNA. This could require mechanisms of miRISC action not present or not yet been explored in higher eukaryotes.
In all, our current study has determined the pool of miRNAs in Giardia, the tentative mechanism of miRNA repression of VSP expression in Giardia, and, most of all, a likely first indication that miRNAs can act on target sites throughout the entire ORF of a mRNA.
Materials and Methods
NGS library construction
The NGS libraries of GlAgo associated small RNAs were constructed using a modified version of the Mello Lab protocol (www.lsi.umich.edu/files/SmallRNACloning.pdf). Briefly, the GlAgo associated small RNAs were isolated from Giardia WB trophozoites as previously described and resuspended in 10 µl RNase-free diH2O [14]. For the addition of a sonication step to the procedure, the cells were resuspend in RIPA buffer as described previously [14] and sonicated twice with five 1 second bursts at a 20% duty cycle. The purified small RNAs were ligated at the 3′-end to 5–10 µM gel purified miRNA Cloning Linker-1 (IDT) using T4 RNA ligase 2 and truncated with K227Q (NEB) by incubating at 25°C for 1 hr, 15°C for 2 hrs and followed by a final 4°C incubation overnight. The reaction mixture was analyzed in 15% polyacrylamide/8 M Urea gel, and the band of ligated product was excised. The gel slice was crushed, resuspended in 1× TE buffer along with 1 U/µl SUPERase-In (Life technologies), and incubated with shaking at 37°C overnight. The reaction mixture was then transferred to a Spin-X cellulose acetate filter centrifuge tubes to remove the gel fragments, RNA was precipitated using ethanol and resuspended in 5 µl RNase-free diH2O. The small RNA with 3′-adaptor was then ligated to the 5′ adaptor (TCTACrArGrUrCrCrGrArCrGrArUrC; r IDT) by incubating with 1 µl T4 RNA ligase 1 at 37°C for 15 min. This step was repeated and the reaction was incubated at 15°C for 2 hrs followed by a final incubation at 4°C overnight. The product was analyzed and extracted as described above and resuspended in 10 µl RNase-free diH2O. The resulting 3′ and 5′ adaptor linked small RNAs (2.5 µl) were reverse transcribed using an RT DNA oligo (ATT GAT GGT GCC TAC AG) with Superscript III (Life Technologies) as per the manufacturer recommended protocol. Of the 20 µl cDNA reaction, 5 µl was PCR amplified with the RT DNA oligo and DNA oligo (GTT CTA CAG TCC GAC GAT C) primers for 10 cycles (94°C, 1 min; 94°C, 20 sec; 50°C, 20 sec; 68°C, 20 sec) using Platinum Taq (Life Technologies). Following the 10 cycles, Illumina 5′ (AAT GAT ACG GCG ACC ACC GAC AGG TTC AGA GTT CTA CAG TCC GAC GAT C) and Illumina 3′ (CAA GCA GAA GAC GGC ATA CGA ATT GAT GGT GCC TAC AG) primers were added to the reaction and incubated for an additional 9 cycles to incorporate the Illumina adapter sequences. The PCR reaction mixture was analyzed in native 10% polyacrylamide gel, and the amplified band of cDNA library was purified. The library was then analyzed using the Agilent Bioanalyzer with the Agilent High Sensitivity DNA kit. The library concentration was adjusted and sequenced using the Illumina HiSeq 2500.
Reads trimming and filtering
Over 190 million raw reads were obtained for each library. They were filtered to retain only the high quality reads. First, the low quality ends (Q = 2) in the 5′ half of the read were discarded. The reads with an average quality less than Q = 20 were then discarded. The 3′ adapter sequence was removed. Reads shorter than the length of a typical miRNA (<20 nt) were then discarded. This resulted in approximately 5% of reads discarded from each library.
Aligning reads to the Giardia scaffolds
Some of the trimmed and filtered reads contained polyA tails of variable lengths, whereas some of the others had the 3′-end of tRNA ‘CCA’. A zero-mismatch alignment using Bowtie [38] against the G. lamblia scaffolds (obtained from GiardiaDBv2.5) [39] was applied:
-
The reads were aligned without mismatch to the scaffolds. Non-aligning reads that did not end with an ‘A’ nucleotide at the 3′ end were discarded.
-
For the reads ending with an ‘A’ from the previous step, the ‘A’ was removed. Any reads shorter than 20 nt were discarded. The remaining reads were then aligned without mismatch to the scaffolds. Non-aligning reads were again separated into two files based on whether the 3′ nucleotides ended with an ‘A’ or a ‘CC’. Reads not ending in these nucleotides were discarded.
-
For the reads ending with a ‘CC’ from the previous step, the ‘CC’ nucleotides were removed and the reads were aligned without mismatch to only the tRNA sequences.
-
Steps two and three were repeated until there were no reads remaining.
Identification of putative miRNAs
Putative miRNAs candidates in the 3 libraries were selected by initially determining the minimum read counts for known miRNAs. Only sequences with similar or higher read counts were retained to eliminate those from random degradations present at lower levels. When all the reads are combined, peaks with overlapping reads that do not exceed 40 nt were identified. Exceptions such as the ORF for miR4 which contains a significant number reads throughout the RNA (see below), however, do exist in the library. These regions were analyzed similarly when individual peaks could be identified. The 5′ and 3′ ends were determined by taking the read with the highest read count in the isolated peak. Potential precursors for each putative miRNA were generated by extracting a 100 nt sequence with the potential miRNA at the 3′ end. Additional potential precursors were generated by utilizing a 1 nt sliding window that shifted the sequence towards the 3′ end while maintaining a 100 nt total length. Sequences were generated until the potential miRNA was located at the 5′ end. These potential precursors were then folded using RNAfold to predict hairpin structures [41]. Potential miRNAs that did not form hairpin structures that could be GlDcr substrates were discarded. Hairpin structures where the potential miRNA is located in the loop were also discarded. An exact match of miRNA sequences among the three libraries were chosen to be the bona fide miRNAs.
Target prediction
Targets of miRNA candidates were predicted with miRanda [47] on either the 3′ region of each ORF (100 nts upstream and 50 nts downstream of the stop codon), or the full length ORF plus 50 nts upstream and 50 nts downstream of the coding region.
Cloning of 3xmyc-tagged vsp genes
The entire coding region of each chosen vsp gene was PCR amplified from Giardia genomic DNA using specific primers. The product was cloned into pGEM-T Easy (Promega), sequenced and sub-cloned into the pNlop4 vector used in our previous studies [7]. The pNlop4 vector was modified to introduce the vsp gene and the 3xmyc epitope upstream from the TAA stop codon.
Cell culture, transfection and selection
Giardia (WB clone C6, ATCC50803) trophozoites were grown anaerobically in plastic culture tubes at 37°C in the modified TYI-S-33 medium supplemented with antibiotics as described [49]. Transfections of Giardia trophozoites were carried out using electroporation as previously described [13]. Briefly, cells were harvested and washed twice in phosphate buffered saline (PBS) and once in electroporation buffer (Cytomix buffer: 10 mM K2HPO4–KH2PO4 pH 7.6, 25 mM HEPES free acid, 120 mM KCl, 0.15 mM CaCl2, 2 mM EGTA, 5 mM MgCl2, 2 mM ATP, 4 mM glutathione). A 400 µl cell suspension (containing 107 cells) was transferred to a 0.2-cm-gap electroporation cuvette and a sample of 50 µg plasmid DNA or yeast tRNA (125 µg) and synthetic 5′-phosphate-miRNA (1 µg) was added to the cell suspension. The cells were immediately subjected to electroporation using a Bio-Rad Gene Pulser Xcell (Voltage: 450 V, Capacitance: 500 mF, Resistance: ∞). The electroporated cells were incubated on ice for 10 min, added to pre-warmed culture medium, and incubated at 37°C. For selection, 200 µg/ml G418 was added to the medium 16 hrs after transfection. The selected cells were incubated with 5 µg/ml of tetracycline at 37°C for 16 hrs to induce expression of the cloned gene.
To ensure a consistent transfection efficiency, a RLuc expressing plasmid was co-transfected with or without the miRNAs in repeated control experiments. The results were always highly reproducible [12], [13], [14], [15], [16]. The miRNAs used for transfection were in the saturating level, which gave also highly reproducible outcomes.
Western blot
Giardia trophozoites containing episomally expressed vsp genes were transfected with the appropriate miRNAs and incubated for 16–20 hrs at 37°C in pre-warmed medium containing tetracycline (5 µg/ml). The cells were cooled on ice, pelleted, washed once with cold PBS and resuspended in lysis buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 1×Halt protease inhibitor cocktail (Thermo Scientific)) and incubated at 4°C for 30 min. The cell lysate was cleared by centrifugation at 16,000 g at 4°C for 20 min. For SDS-PAGE separation, 25 µg of protein from each sample was used. The protein was transferred to a PVDF membrane (Bio-Rad) for detection with the anti-c-myc-HRP antibody (Invitrogen). It was subsequently stripped using Restore Western Blot Stripping Buffer (Thermo Scientific) and re-blotted with anti-Tubulin antibody (Sigma) for loading control. Protein bands were quantified using the BioRad Quantity One software package. The results were derived from statistical analysis of three independent experiments. Margins of errors in the data from these experiments were generally quite small, which support confidence in the accuracy of the data.
Accession numbers
The pre-processed have been deposited into the Giardia database (GiardiaDB.org) a part of the EuPathDB project.
Supporting Information
Zdroje
1. BarwickRS, LevyDA, CraunGF, BeachMJ, CalderonRL (2000) Surveillance for waterborne-disease outbreaks–United States, 1997–1998. MMWR CDC Surveill Summ 49 : 1–21.
2. SavioliL, SmithH, ThompsonA (2006) Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 22 : 203–208.
3. AdamRD, NigamA, SeshadriV, MartensCA, FarnethGA, et al. (2010) The Giardia lamblia vsp gene repertoire: characteristics, genomic organization, and evolution. BMC Genomics 11 : 424.
4. NashTE, BanksSM, AllingDW, MerrittJWJr, ConradJT (1990) Frequency of variant antigens in Giardia lamblia. Exp Parasitol 71 : 415–421.
5. NashTE, LujanHT, MowattMR, ConradJT (2001) Variant-specific surface protein switching in Giardia lamblia. Infect Immun 69 : 1922–1923.
6. DeitschKW, LukehartSA, StringerJR (2009) Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat Rev Microbiol 7 : 493–503.
7. LiW, SaraiyaAA, WangCC (2013) Experimental Verification of the Identity of Variant-Specific Surface Proteins in Giardia lamblia Trophozoites. MBio 4: e00321–13.
8. MowattMR, AggarwalA, NashTE (1991) Carboxy-terminal sequence conservation among variant-specific surface proteins of Giardia lamblia. Mol Biochem Parasitol 49 : 215–227.
9. NashTE, MowattMR (1992) Characterization of a Giardia lamblia variant-specific surface protein (VSP) gene from isolate GS/M and estimation of the VSP gene repertoire size. Mol Biochem Parasitol 51 : 219–227.
10. PruccaCG, SlavinI, QuirogaR, EliasEV, RiveroFD, et al. (2008) Antigenic variation in Giardia lamblia is regulated by RNA interference. Nature 456 : 750–754.
11. FaghiriZ, WidmerG (2011) A comparison of the Giardia lamblia trophozoite and cyst transcriptome using microarrays. BMC Microbiol 11 : 91.
12. LiW, SaraiyaAA, WangCC (2011) Gene regulation in Giardia lambia involves a putative microRNA derived from a small nucleolar RNA. PLoS Negl Trop Dis 5: e1338.
13. LiW, SaraiyaAA, WangCC (2012) The profile of snoRNA-derived microRNAs that regulate expression of variant surface proteins in Giardia lamblia. Cell Microbiol 14 : 1455–73.
14. SaraiyaAA, LiW, WangCC (2011) A microRNA derived from an apparent canonical biogenesis pathway regulates variant surface protein gene expression in Giardia lamblia. RNA 17 : 2152–2164.
15. SaraiyaAA, LiW, WangCC (2013) Transition of a microRNA from repressing to activating translation depending on the extent of base pairing with the target. PLoS One 8: e55672.
16. SaraiyaAA, WangCC (2008) snoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathog 4: e1000224.
17. KrolJ, LoedigeI, FilipowiczW (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11 : 597–610.
18. BazziniAA, LeeMT, GiraldezAJ (2012) Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336 : 233–237.
19. DjuranovicS, NahviA, GreenR (2012) miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336 : 237–240.
20. BartelDP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136 : 215–233.
21. PasquinelliAE (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13 : 271–282.
22. DuursmaAM, KeddeM, SchrierM, le SageC, AgamiR (2008) miR-148 targets human DNMT3b protein coding region. RNA 14 : 872–877.
23. ElchevaI, GoswamiS, NoubissiFK, SpiegelmanVS (2009) CRD-BP protects the coding region of betaTrCP1 mRNA from miR-183-mediated degradation. Mol Cell 35 : 240–246.
24. MandkeP, WyattN, FraserJ, BatesB, BerberichSJ, et al. (2012) MicroRNA-34a modulates MDM4 expression via a target site in the open reading frame. PLoS One 7: e42034.
25. ShenWF, HuYL, UttarwarL, PassegueE, LargmanC (2008) MicroRNA-126 regulates HOXA9 by binding to the homeobox. Mol Cell Biol 28 : 4609–4619.
26. TayYM, TamWL, AngYS, GaughwinPM, YangH, et al. (2008) MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells 26 : 17–29.
27. YiC, XieWD, LiF, LvQ, HeJ, et al. (2011) MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett 585 : 3303–3309.
28. JungHM, PatelRS, PhillipsBL, WangH, CohenDM, et al. (2013) Tumor suppressor miR-375 regulates MYC expression via repression of CIP2A coding sequence through multiple miRNA-mRNA interactions. Mol Biol Cell 24 : 1638–1637, 1638-1648, S1631-1637.
29. QinW, ShiY, ZhaoB, YaoC, JinL, et al. (2010) miR-24 regulates apoptosis by targeting the open reading frame (ORF) region of FAF1 in cancer cells. PLoS One 5: e9429.
30. FormanJJ, Legesse-MillerA, CollerHA (2008) A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci U S A 105 : 14879–14884.
31. GuS, JinL, ZhangF, SarnowP, KayMA (2009) Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat Struct Mol Biol 16 : 144–150.
32. ChiSW, ZangJB, MeleA, DarnellRB (2009) Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 460 : 479–486.
33. ZisoulisDG, LovciMT, WilbertML, HuttKR, LiangTY, et al. (2010) Comprehensive discovery of endogenous Argonaute binding sites in Caenorhabditis elegans. Nat Struct Mol Biol 17 : 173–179.
34. FangZ, RajewskyN (2011) The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS One 6: e18067.
35. AdamRD (2000) The Giardia lamblia genome. Int J Parasitol 30 : 475–484.
36. AdamRD (2001) Biology of Giardia lamblia. Clin Microbiol Rev 14 : 447–475.
37. FranzenO, Jerlstrom-HultqvistJ, EinarssonE, AnkarklevJ, FerellaM, et al. (2013) Transcriptome profiling of Giardia intestinalis using strand-specific RNA-seq. PLoS Comput Biol 9: e1003000.
38. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25.
39. AurrecoecheaC, BrestelliJ, BrunkBP, CarltonJM, DommerJ, et al. (2009) GiardiaDB and TrichDB: integrated genomic resources for the eukaryotic protist pathogens Giardia lamblia and Trichomonas vaginalis. Nucleic Acids Res 37: D526–530.
40. MacraeIJ, ZhouK, LiF, RepicA, BrooksAN, et al. (2006) Structural basis for double-stranded RNA processing by Dicer. Science 311 : 195–198.
41. HofackerIL, FontanaW, StadlerPF, BonhoefferLS, TackerM, et al. (1994) Fast folding and comparison of RNA secondary structures. Monatshefte für Chemie/Chemical Monthly 125 : 167–188.
42. HofackerIL, StadlerPF (2006) Memory efficient folding algorithms for circular RNA secondary structures. Bioinformatics 22 : 1172–1176.
43. MacRaeIJ, ZhouK, DoudnaJA (2007) Structural determinants of RNA recognition and cleavage by Dicer. Nat Struct Mol Biol 14 : 934–940.
44. ParkJE, HeoI, TianY, SimanshuDK, ChangH, et al. (2011) Dicer recognizes the 5′ end of RNA for efficient and accurate processing. Nature 475 : 201–205.
45. EyPL, MansouriM, KuldaJ, NohynkovaE, MonisPT, et al. (1997) Genetic analysis of Giardia from hoofed farm animals reveals artiodactyl-specific and potentially zoonotic genotypes. J Eukaryot Microbiol 44 : 626–635.
46. Jerlstrom-HultqvistJ, FranzenO, AnkarklevJ, XuF, NohynkovaE, et al. (2010) Genome analysis and comparative genomics of a Giardia intestinalis assemblage E isolate. BMC Genomics 11 : 543.
47. JohnB, EnrightAJ, AravinA, TuschlT, SanderC, et al. (2004) Human MicroRNA targets. PLoS Biol 2: e363.
48. LiL, WangCC (2004) Capped mRNA with a single nucleotide leader is optimally translated in a primitive eukaryote, Giardia lamblia. J Biol Chem 279 : 14656–14664.
49. KeisterDB (1983) Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg 77 : 487–488.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic HelixČlánek Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron MobilizationČlánek AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for ImmunityČlánek Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden HamstersČlánek Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Viral Enhancer Mimicry of Host Innate-Immune Promoters
- The Epstein-Barr Virus-Encoded MicroRNA MiR-BART9 Promotes Tumor Metastasis by Targeting E-Cadherin in Nasopharyngeal Carcinoma
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- The Consequences of Reconfiguring the Ambisense S Genome Segment of Rift Valley Fever Virus on Viral Replication in Mammalian and Mosquito Cells and for Genome Packaging
- Substrate-Induced Unfolding of Protein Disulfide Isomerase Displaces the Cholera Toxin A1 Subunit from Its Holotoxin
- Male-Killing Induces Sex-Specific Cell Death via Host Apoptotic Pathway
- Highly Active Antiretroviral Therapies Are Effective against HIV-1 Cell-to-Cell Transmission
- The microRNAs in an Ancient Protist Repress the Variant-Specific Surface Protein Expression by Targeting the Entire Coding Sequence
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Type III Secretion Protein MxiI Is Recognized by Naip2 to Induce Nlrc4 Inflammasome Activation Independently of Pkcδ
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
- Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Strains
- Structure of the Membrane Anchor of Pestivirus Glycoprotein E, a Long Tilted Amphipathic Helix
- Foxp3 Regulatory T Cells Delay Expulsion of Intestinal Nematodes by Suppression of IL-9-Driven Mast Cell Activation in BALB/c but Not in C57BL/6 Mice
- Iron Acquisition in : The Roles of IlsA and Bacillibactin in Exogenous Ferritin Iron Mobilization
- MicroRNA Editing Facilitates Immune Elimination of HCMV Infected Cells
- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Identification of Host-Targeted Small Molecules That Restrict Intracellular Growth
- A Cyclophilin Homology Domain-Independent Role for Nup358 in HIV-1 Infection
- Engagement of NKG2D on Bystander Memory CD8 T Cells Promotes Increased Immunopathology following Infection
- Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress Expression
- CIB1 Synergizes with EphrinA2 to Regulate Kaposi's Sarcoma-Associated Herpesvirus Macropinocytic Entry in Human Microvascular Dermal Endothelial Cells
- A Gammaherpesvirus Bcl-2 Ortholog Blocks B Cell Receptor-Mediated Apoptosis and Promotes the Survival of Developing B Cells
- Metabolic Reprogramming during Purine Stress in the Protozoan Pathogen
- The Post-transcriptional Regulator / Activates T3SS by Stabilizing the 5′ UTR of , the Master Regulator of Genes, in
- Tailored Immune Responses: Novel Effector Helper T Cell Subsets in Protective Immunity
- AvrBsT Acetylates ACIP1, a Protein that Associates with Microtubules and Is Required for Immunity
- Epstein-Barr Virus Large Tegument Protein BPLF1 Contributes to Innate Immune Evasion through Interference with Toll-Like Receptor Signaling
- The Major Cellular Sterol Regulatory Pathway Is Required for Andes Virus Infection
- Insights into the Initiation of JC Virus DNA Replication Derived from the Crystal Structure of the T-Antigen Origin Binding Domain
- Domain Shuffling in a Sensor Protein Contributed to the Evolution of Insect Pathogenicity in Plant-Beneficial
- Lectin-Like Bacteriocins from spp. Utilise D-Rhamnose Containing Lipopolysaccharide as a Cellular Receptor
- A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects
- Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection
- Interleukin-33 Increases Antibacterial Defense by Activation of Inducible Nitric Oxide Synthase in Skin
- Protective Vaccination against Papillomavirus-Induced Skin Tumors under Immunocompetent and Immunosuppressive Conditions: A Preclinical Study Using a Natural Outbred Animal Model
- Gem-Induced Cytoskeleton Remodeling Increases Cellular Migration of HTLV-1-Infected Cells, Formation of Infected-to-Target T-Cell Conjugates and Viral Transmission
- Viral MicroRNA Effects on Pathogenesis of Polyomavirus SV40 Infections in Syrian Golden Hamsters
- Genome-Wide RNAi Screen Identifies Broadly-Acting Host Factors That Inhibit Arbovirus Infection
- Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung
- Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response
- Human Genome-Wide RNAi Screen Identifies an Essential Role for Inositol Pyrophosphates in Type-I Interferon Response
- The Master Regulator of the Cellular Stress Response (HSF1) Is Critical for Orthopoxvirus Infection
- Code-Assisted Discovery of TAL Effector Targets in Bacterial Leaf Streak of Rice Reveals Contrast with Bacterial Blight and a Novel Susceptibility Gene
- Competitive and Cooperative Interactions Mediate RNA Transfer from Herpesvirus Saimiri ORF57 to the Mammalian Export Adaptor ALYREF
- The Type III Secretion Chaperone Slc1 Engages Multiple Early Effectors, Including TepP, a Tyrosine-phosphorylated Protein Required for the Recruitment of CrkI-II to Nascent Inclusions and Innate Immune Signaling
- Yeasts: How Many Species Infect Humans and Animals?
- Clustering of Pattern Recognition Receptors for Fungal Detection
- Distinct Antiviral Responses in Pluripotent versus Differentiated Cells
- Igniting the Fire: Virulence Factors in the Pathogenesis of Sepsis
- Inactivation of the Host Lipin Gene Accelerates RNA Virus Replication through Viral Exploitation of the Expanded Endoplasmic Reticulum Membrane
- Inducible Deletion of CD28 Prior to Secondary Infection Impairs Worm Expulsion and Recall of Protective Memory CD4 T Cell Responses
- Clonal Expansion during Infection Dynamics Reveals the Effect of Antibiotic Intervention
- The Secreted Triose Phosphate Isomerase of Is Required to Sustain Microfilaria Production
- Unifying Viral Genetics and Human Transportation Data to Predict the Global Transmission Dynamics of Human Influenza H3N2
- ‘Death and Axes’: Unexpected Ca Entry Phenologs Predict New Anti-schistosomal Agents
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Reversible Silencing of Cytomegalovirus Genomes by Type I Interferon Governs Virus Latency
- Implication of PMLIV in Both Intrinsic and Innate Immunity
- Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention
- Lundep, a Sand Fly Salivary Endonuclease Increases Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



