Evaluation of resection margins in oral squamous cell carcinoma
Authors:
Král D. 1; Tvrdý P. 1; Šašková L. 1; Zapletalová J. 2; Michálek J. 3; Pink R. 1
Authors‘ workplace:
Department of Oral and Maxillofacial Surgery, Faculty of Medicine and Dentistry, Palacký University Olomouc and University Hospital Olomouc, Czech Republic
1; Department of Medical Biophysics, Faculty of Medicine and Dentistry, Palacky University Olomouc, Czech Republic
2; Department of Clinical and Molecular Pathology, Faculty of Medicine and Dentistry, Palacký University Olomouc and University Hospital Olomouc, Czech Republic
3
Published in:
ACTA CHIRURGIAE PLASTICAE, 64, 3-4, 2022, pp. 110-115
doi:
https://doi.org/10.48095/ccachp2022110
Introduction
Oral squamous cell carcinoma (OSCC) represents more than 90% of all oral malignant tumors [1]. Surgery is the primary treatment modality for these tumors, combined with adjuvant radiotherapy or chemotherapy, if it is indicated [2,3]. Complete removal of the tumor with sufficient surrounding healthy tissue margin in all planes is the purpose of the surgery [4]. The status of the resection margins belongs among information that are crucial for planning further treatment (reoperation, adjuvant therapy) and also for estimation of the disease prognosis. According to the distance between the invasive tumor and the edge of resected tissue itself, resection margins can be divided into positive, close and negative. There are differences in the definition of the individual resection margins between different publications and even between recommendations of individual professional societies [5,6]. The size of the resection margin is affected by a number of factors such as the anatomical conditions in the surroundings of the tumor, biological characteristics of the tumor, depth of invasion, resection technique, type of surgery, tissue shrinkage after resection and during fixation of specimens for histopathological examination [7].
Positive resection margins represent an indication for reoperation or adjuvant therapy, while negative resection margins represent an indication for clinical follow-up or adjuvant therapy if other adverse features are present [4,6,8]. However, in the case of close resection margins, no consensus regarding their true prognostic value has been reached. According to some authors, close resection margins are associated with worse treatment outcomes when it comes to disease-free survival (DFS) and overall survival (OS) [9,10]. Other studies suggest that patients with close resection margins may manifest DFS and OS outcomes similar to patients with negative resection margins [11]. The aim of this study was evaluation of the relationship between resection margins and disease recurrence, disease-free survival and overall survival.
Material and methods
The study included 98 patients who were treated for oral squamous cell carcinoma at the Department of Oral and Maxillofacial Surgery, Olomouc University Hospital between 2011 and 2016. Entry criteria consisted of: histopathological verification of the disease, localization of the tumor strictly in the oral cavity, performance of curative surgery, unambiguous pathologist's report on the resection margins and complete clinical follow-ups of patients for at least 5 years after diagnosis. Exclusion criteria consisted of: completion of neoadjuvant treatment (regional intra-arterial perfusion), ambiguous pathologist's report on the resection margins, incomplete clinical follow-ups. Patients with carcinomas of the upper and lower lip were also excluded because of the different etiology and treatment of these tumors. During surgery, all patients underwent an excision of the tumor in the oral cavity and an elective or therapeutic selective neck dissection. Further treatment strategy (clinical follow-ups, adjuvant therapy) was determined by a multidisciplinary team consisting of maxillofacial surgeons, oncologists, pathologists and radiologists. In patients who met the entry criteria, we recorded sex, age at the time of diagnosis, tumor location, T and N pathological categories, tumor grading, disease stage, type of surgery (tumor extirpation with primary suture or secondary healing of the postoperative defect, extirpation of the tumor with reconstruction of the postoperative defect with a flap), diagnosis of recurrence, overall survival, type of treatment (only surgical therapy, adjuvant therapy - radiotherapy, chemoradiotherapy). Histopathological examination of the samples collected during surgery included evaluation of the resection margins of each tumor by a pathologist.
The classification of resection margins was based on their definition according to the National Comprehensive Cancer Network (NCCN). Resection margins > 5 mm were classified as negative (N), 0–5 mm as close (C), and 0 mm as positive (P). There are also other classifications of resection margins, e.g. according to the International Collaboration on Cancer Reporting (ICCR) margins are defined as negative > 5 mm, close 1–5 mm and positive < 1 mm. As in our department the NCCN Guidelines are used to determine further treatment of patient, the NCCN classification was used in this study. Resection margins were recorded for each patient after the primary surgery. Some patients with close or positive resection margins underwent reoperation – re-excision during the second surgery, and we scored the resection margins as negative in these cases. In patients who did not undergo reoperation, the resection margins corresponded to the resection margins after the primary surgery.
The data were analyzed using the IBM SPSS Statistics statistical program, version 23 (Armonk, NY: IBM Corp.). The relationship between resection margins and disease recurrence was assessed using Fisher's exact test. Kaplan-Meier analysis with the log-rank test was used to analyze the relationship between resection margins and disease-free survival and the overall survival. Cox proportional hazard regression model, the stepwise forward method was used to find the most significant predictors of the overall survival. P-values < 0.05 were considered statistically significant.
Results
A group of 98 patients consisted of 68 men (69.4%) and 30 women (30.6%). The average age at the time of diagnosis was 60 years, with the youngest patient being a woman of 36 years and the oldest a man of 82 years. The most common tumor location was the tongue in 34 patients (34.7%), followed by the floor of the mouth in 28 patients (28.6%), the upper and lower alveolar process in 17 patients (17.3%), the buccal mucosa in 6 patients (6.1%), the palate in 8 patients (8.2%), and the retromolar region in 5 patients (5.1%). Based on histopathological examination, the tumor category was determined as pT1 in 34 patients (34.7%), pT2 in 36 patients (36.7%), pT3 in 11 patients (11.3%), and pT4a in 17 patients (17.3%). During the evaluation of metastatic involvement of the cervical lymph nodes, category pN0 was recorded in 59 patients (60.2%), category pN1 in 22 patients (22.4%), category pN2 in 17 patients (17.3%), specifically pN2a in 3 patients and pN2b in 14 patients. The disease was in stage I in 28 patients (28.6%), in stage II in 19 patients (19.4%), in stage III in 23 patients (23.5%), and in stage IVA in 28 patients (28.6%). Tumor grade was G1 in 22 patients (22.5%), G2 in 46 patients (46.9%), and G3 in 30 patients (30.5%). In 76 patients (77.6%), tumor excision was performed with primary closure of the postoperative defect or with leaving the defect for secondary healing; in 22 patients (22.4%), the defect after tumor excision was reconstructed with a flap. Surgery alone was the main treatment modality in 46 patients (46.9%), 27 patients underwent surgery and adjuvant radiotherapy (27.6%), and 25 patients underwent surgery and adjuvant chemoradiotherapy (25.5%).
In evaluation of resection margins after primary surgery, the margins were evaluated as negative in 12 patients (12.2%), as close in 53 patients (54.1%), and as positive in 33 patients (33.6%). This low number of negative resection margins may appear as a failure, but the explanation for this phenomenon follows: in other 38 patients who otherwise met the other entry criteria, the pathologist’s report stated that the tumor did not reach the edges of excision, but did not specify the distance of the tumor from the edges of excision in detail, so it was not possible to determine whether the margins were close or negative. Therefore, we could not include these patients in our sample; however, it can be assumed that the number of patients with negative resection margins would then be higher. A total of 24 patients (24.5%) underwent reoperation – re-excision during the second surgery, 13 of these were patients with close resection margins and 11 were patients with positive margins. Thus, we recorded negative resection margins (including reoperated patients) in 36 patients (36.7%), close margins in 40 patients (40.8%), and positive margins in 22 patients (22.5%).
All patients attended regular clinical follow-ups for at least 5 years after the diagnosis, and in case of death, the cause of death was recorded. During the monitored period, disease recurrence occurred in 41 patients (41.8%); the most frequent was local recurrence in 37 patients (90.2% of recurrences), 2 patients were diagnosed with regional recurrence (4.9% of recurrences), and 2 patients (4.9% of recurrences) were diagnosed with distant metastases (once in the skeleton, once in the lungs). Average time from surgery to disease progression was 19 months. Fifty-seven patients (58.2%) developed neither locoregional recurrence nor distant metastases. During the monitored period, 55 patients (56.1%) died, 33 of whom died from causes directly related to cancer (33.7%) and 22 patients died from other unspecified causes (22.5%). At the end of the monitored period, 43 patients were alive (43.9%), and all of these patients were free of evidence of cancer at the last follow-up.
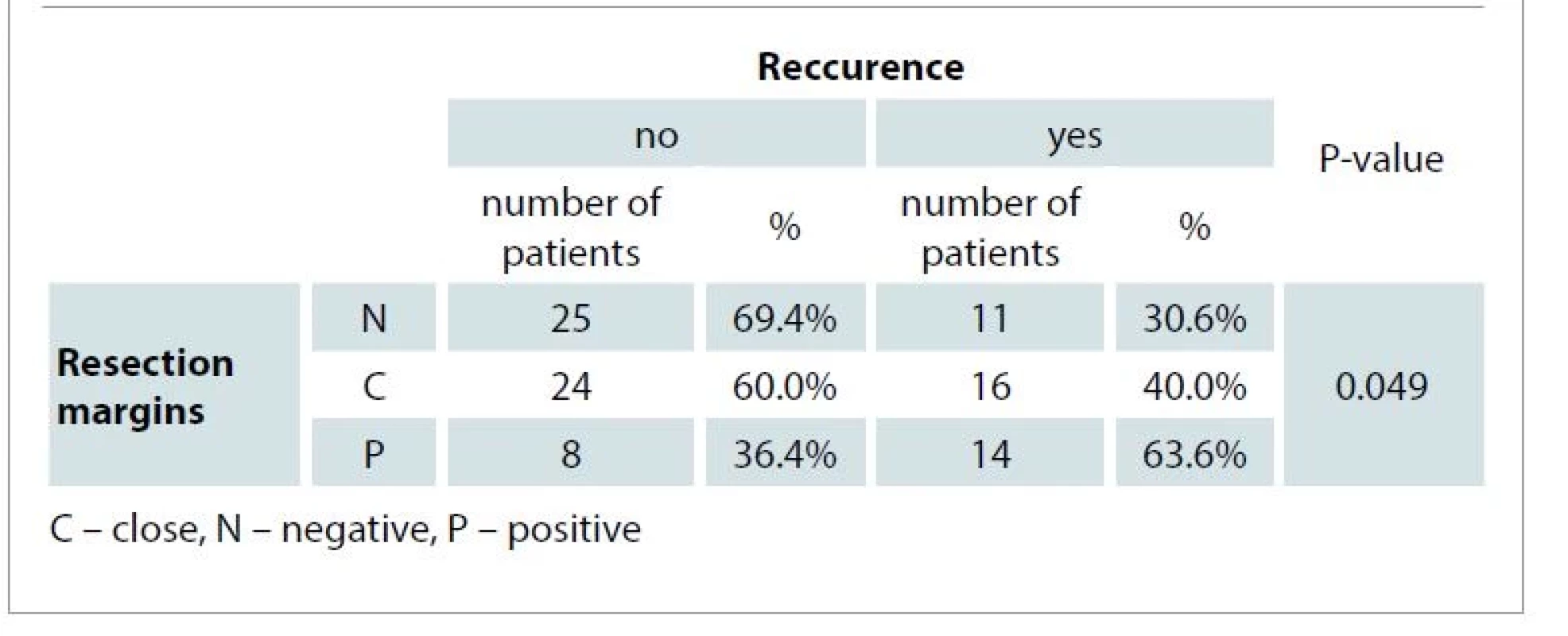
We compared the incidence of disease recurrence according to the individual resection margins. Recurrence occurred in 11 patients with negative resection margins (30.6% of all patients with negative resection margins), in 16 patients with close resection margins (40.0%), and in 14 patients with positive resection margins (63.6%) (Tab. 1). A significant correlation between resection margins and disease recurrence was proven. Patients with positive resection margins had a statistically significantly higher recurrence rate compared to patients with negative resection margins. The difference in recurrence rate in patients with close resection margins compared to patients with negative margins was not statistically significant.
The average time between surgery and disease recurrence was 12 months for patients with tumors with positive resection margins, 24 months for patients with close resection margins, and 19 months for patients with negative resection margins. A significant relationship between DFS and resection margins was proven on the basis of Kaplan-Meier analysis (Graph 1). Patients with positive resection margins had statistically significantly shorter DFS compared to patients with negative resection margins. The difference in DFS between patients with close resection margins and patients with positive resection margins was close to being statistically significant (P = 0.052). The difference in DFS between patients with negative resection margins and patients with close resection margins was not significant.

We also evaluated the overall survival of patients according to resection margins. The average survival time was 60.7 months for patients with negative resection margins, 55.7 months for patients with close margins, and only 30.6 months for patients with positive resection margins. Five-year survival rate was 63.9% for patients with negative resection margins, 57.5% with close, and only 13.6% for patients with positive resection margins. Thus, the status of resection margins correlated with patient survival. A significant relationship between resection margins and both five--year and overall survival was proven. The Kaplan-Meier analysis (Graph 2) showed that patients with positive resection margins had statistically significantly shorter overall survival compared to patients with negative and close resection margins. The difference in overall survival between patients with negative margins and patients with close margins was not statistically significant.

Based on Cox regression analysis, the Stepwise Forward model, disease recurrence, tumor grade and resection margins were evaluated as significant predictors of overall survival in our sample. Patients with disease recurrence had a 2.97times higher risk of death compared to patients without recurrence. Furthermore, patients with tumor grade G3 had a 3.94times higher risk of death compared to patients with G1. Last but not least, the risk of death was 3.27times higher in patients with positive resection margins compared to patients with negative resection margins.
Discussion
In orofacial oncology, resection margins are considered an important prognostic factor. Their correlation with patient survival has been investigated in many studies. According to Cariati et al [8], resection margins are not directly related to overall survival and other factors can also affect the outcome of treatment significantly. The authors demonstrated a correlation between overall survival and T category of the tumor, lymph node involvement, perineural invasion, and extracapsular spread. This, according to the authors, needs to be considered especially in patients with close resection margins. Based on the results of the study, the authors further conclude that in patients with close resection margins, aggressive adjuvant therapy may help achieve the same overall survival as in patients with negative resection margins. In the study by Mitchell et al [4] significant relationship between close and especially the positive resection margins and five-year survival was proven. Five-year survival was 81%, 75%, and 54% in patients with negative, close, and positive resection margins, respectively. In our study, we reached the same conclusion that five-year survival correlates with resection margins; however, in our sample, five-year survival was lower – 64% in patients with negative resection margins, 58% with close, and 14% with positive resection margins. Also Binahmed et al [10] consider resection margins to be an independent prognostic factor; in their study, positive resection margins were associated with a 1.9times higher risk of death within 5 years of diagnosis. In a study by Sutton et al [9], in which the five-year survival of patients was similar to that in our sample, the risk of death was actually 11.6times higher in patients with positive resection margins compared to those with negative margins. In our study, this risk was 3.27 times higher.
Resection margins also appear to be an independent risk factor for local recurrence of the disease [12]. In a study by Buchakjian et al [13], 22% of patients with negative resection margins, 22% with close, and 62% with positive resection margins occured tumor recurrence. Similar results were achieved in our sample as well, with recurrence occurring in 30.6% of patients with negative resection margins, 40.0% with close and 63.6% with positive resection margins.
A number of authors have opened the debate as to what distance of healthy tissue from the invasive tumor represents close, respectively negative resection margins. The generally accepted distance by which close and negative resection margins are distinguished even by the recommendations of the ICCR and NCCN, is 5 mm. In a study by Zanoni et al [11], the authors compared the incidence of locoregional recurrences in tongue carcinomas according to resection margins. Patients with resection margins of 2.3–5.0 mm had only a 1.3times higher risk of locoregional recurrence compared to patients with resection margins of 5 mm or more; this risk was 2.83times higher for margins of 0–2.2 mm and 9.03times higher for positive margins. Therefore, the authors considered the distance of 2.2 mm as the border between negative and close resection margins. Similar results were reported in the study by Nason et al [14], where the recurrence rate in oral cancer patients with resection margins of 3 mm and 4 mm was the same as in patients with margins of 5 mm or more. Furthermore, in this study, no significant difference in survival between patients with 3 mm and 5 mm margins was proven, so the authors considered resection margins of 3 mm or more to be adequate. Wong et al [15] demonstrated a significant relationship between disease-free survival and resection margins smaller than 1.6 mm; according to the authors, resection margins of 1–2 mm should be considered close. Thus, they recommend adjuvant therapy in patients with resection margins smaller than 2 mm, but, at the same time, they point out other factors such as lymph node metastases, depth of invasion and perineural tumor spread which must be taken into consideration. In contrast, however, according to a meta-analysis by Anderson et al [16], the distance of 5 mm is the prognostically relevant border between close and negative resection margins. The results of this study showed that resection margins of less than 5 mm have a significantly higher rate of local recurrence compared to resection margins of 5 mm or more. Concurrently the authors note that even in tumors with resection margins of 5 mm or more, local recurrence occurred in more than 20%, which is also consistent with the results of our study, in which recurrence occurred in 30.6% of patients with negative margins. Thus, according to the authors, further research is needed to identify patients who are at high risk of recurrence despite negative resection margins.
Tissues excised from the human body have a natural tendency to contract; this factor may contribute to the inaccuracy during evaluation of resection margins. It has been proven that there are significant differences in resection margins measured in the mouth before tumor extirpation and those measured during histopathological examination [17]. According to Mistry et al [18] and Cheng et al [19], T1 and T2 tumors shrink more. The smaller contraction that occurs in T3 and T4 tumors can be explained by a greater destruction of contractile tissue and its replacement with scar tissue of the tumor. A difference in tissue shrinkage was also observed in tumors in different regions of the oral cavity, with the greatest contraction observed in tumors of the buccal mucosa and retromolar region [19,20]. George et al [21] studied these problems in relation to surgical excision techniques. Use of a conventional scalpel resulted in the greatest contraction compared to other methods. On the other hand, minimal shrinkage was observed in resections using cutting diathermy. Other factors, such as patient age, different specimen fixation protocols, or different laboratory specimen processing methods may also contribute to the post-operative tissue shrinkage [18,19].
Evaluation of resection margins is currently based on a conventional histopathological examination. As previously mentioned, many patients occur disease recurrence even if negative resection margins are achieved during tumor excision. The field cancerization theory presumes that histologically “clear” resection margins, i.e. tumor-free margins, may harbor genetic alterations that may lead to recurrence [22]. These subcellular alterations cannot be detected by a conventional microscopic examination. This has brought the need for more accurate methods of examining resection margins, and, at the same time, it has also led to researches aimed at detection of molecular and genetic alterations in histologically negative resection margins, such as the evidence of p53 mutation [23].
Conclusion
In our study, positive resection margins were associated with a significantly higher incidence of disease recurrence, shorter disease-free survival and shorter overall survival. When comparing recurrence rates, disease-free survival, and overall survival between patients with close and negative resection margins, the differences were not statistically significant. Thus, further research with regard to the definition of close and negative resection margins is needed. Nevertheless, an unambiguous pathologist’s statement regarding resection margins and their classification into negative, close, and positive belongs among the necessary parts of a histopathological examination of any tumor of this anatomical localization.
Roles of authors
David Král: originate concept, methodology, writing – original draft;
Peter Tvrdý: supervision, methodology, formal analysis;
Lenka Šašková, Jaroslav Michálek: investigation, data curation, formal analysis;
Jana Zapletalová: methodology, statistical analysis;
Richard Pink: supervision, methodology, validation, writing – review and editing.
Conflict of interest: The authors declare that there are no conflicts of interest regarding the publication of this article. All authors state that they had no other funding sources.
Disclosure: All procedures performed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Assoc. Prof. Richard Pink, MD, DMD, PhD
Department of Oral and Maxillofacial Surgery
Teaching Hospital Olomouc
I. P. Pavlova 185/6
779 00 Olomouc
Czech Republic
e-mail: richard.pink@fnol.cz
Submitted: 14. 10. 2021
Accepted: 10. 1. 2022
Sources
1. Johnson NW., Jayasekara P., Amarasinghe AA. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontol 2000. 2011, 57(1): 19–37.
2. Shah JP., Gil Z. Current concepts in management of oral cancer – surgery. Oral Oncol. 2009, 45(4–5): 394–401.
3. Kalavrezos N., Bhandari R. Current trends and future perspectives in the surgical management of oral cancer. Oral Oncol. 2010, 46(6): 429–432.
4. Mitchell DA., Kanatas A., Murphy C., et al. Margins and survival in oral cancer. Br J Oral Maxillofac Surg. 2018, 56(9): 820–829.
5. Smits RW., Koljenović S., Hardillo JA., et al. Resection margins in oral cancer surgery: room for improvement. Head Neck. 2016, 38 (Suppl 1): E2197–2203.
6. Head and neck cancers: NCCN guidelines version 2.2022. [online]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437.
7. Huang TY., Hsu LP., Wen YH., et al. Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol. 2010, 46(1): 49–55.
8. Cariati P., Cabello Serrano A., Mosalve Iglesias F., et al. What is the real prognostic value of close margins in oral oncology? Curr Probl Cancer. 2019, 43(6): 100500.
9. Sutton DN., Brown JS., Rogers SN., et al. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003, 32(1): 30–34.
10. Binahmed A., Nason RW., Abdoh AA. The clinical significance of the positive surgical margin in oral cancer. Oral Oncol. 2007, 43(8): 780–784.
11. Zanoni DK., Migliacci JC., Xu B., et al. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg. 2017, 143(6): 555–560.
12. Buchakjian MR., Ginader T., Tasche KK., et al. Independent predictors of prognosis based on oral cavity squamous cell carcinoma surgical margins. Otolaryngol Head Neck Surg. 2018, 159(4): 675–682.
13. Buchakjian MR., Tasche KK., Robinson RA., et al. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016, 142(12): 1191–1198.
14. Nason RW., Binahmed A., Pathak KA., et al. What is the adequate margin of surgical resection in oral cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009, 107(5): 625–629.
15. Wong LS., McMahon J., Devine J., et al. Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinoma. Br J Oral Maxillofac Surg. 2012, 50(2): 102–108.
16. Anderson CR., Sisson K., Moncrieff M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol. 2015, 51(5): 464–469.
17. Johnson RE., Sigman JD., Funk GF., et al. Quantification of surgical margin shrinkage in the oral cavity. Head Neck. 1997, 19(4): 281–286.
18. Mistry RC., Qureshi SS., Kumaran C. Post-resection mucosal margin shrinkage in oral cancer: quantification and signifikance. J Surg Oncol. 2005, 91(2): 131–133.
19. Cheng A., Cox D., Schmidt BL. Oral squamous cell carcinoma margin discrepancy after resection and pathologic processing. J Oral Maxillofac Surg. 2008, 66(3): 523–529.
20. El-Fol HA., Noman SA., Beheiri MG., et al. Significance of post-resection tissue shrinkage on surgical margins of oral squamous cell carcinoma. J Craniomaxillofac Surg. 2015, 43(4): 475–482.
21. George KS., Hyde NC., Wilson P., et al. Does the method of resection affect the margins of tumours in the oral cavity? Prospective controlled study in pigs. Br J Oral Maxillofac Surg. 2013, 51(7): 600–603.
22. Feller LL., Khammissa RR., Kramer BB., et al. Oral squamous cell carcinoma in relation to field precancerisation: pathobiology. Cancer Cell Int. 2013, 13(1): 31.
23. Shah AK. Postoperative pathologic assessment of surgical margins in oral cancer: a contemporary review. J Oral Maxillofac Pathol. 2018, 22(1): 78–85.
Labels
Plastic surgery Orthopaedics Burns medicine TraumatologyArticle was published in
Acta chirurgiae plasticae

2022 Issue 3-4
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Safety and Tolerance of Metamizole in Postoperative Analgesia in Children
-
All articles in this issue
- Editorial
- Evaluation of resection margins in oral squamous cell carcinoma
- 3D color doppler ultrasound for postoperative monitoring of vascularized lymph node flaps
- Preservation of supraclavicular nerve while harvesting supraclavicular lymph node flap
- Determination of the adequate vascular perfusion time of cross-leg free latissimus dorsi myocutaneous flaps in reconstruction of complex lower extremity defects
- Wichterle hydron for breast augmentation – case reports and brief review
- The ideal timing for revision surgery following an infected cranioplasty
- Adult orbital xanthogranuloma – a case report
- Mini-invasive technique of sclerotherapy with talc in chronic seroma after abdominoplasty – a case report and literature review
- Multifarious uses of the pedicled SCIP flap – a case series
- In memoriam
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Mini-invasive technique of sclerotherapy with talc in chronic seroma after abdominoplasty – a case report and literature review
- Multifarious uses of the pedicled SCIP flap – a case series
- 3D color doppler ultrasound for postoperative monitoring of vascularized lymph node flaps
- Evaluation of resection margins in oral squamous cell carcinoma
