Leptin –2548 G/A Polymorphism in Endometrial Cancer
Polymorfizmus –2548 G/A v genu pro leptin u pacientek s endometriálním karcinomem
Východiska:
Polymorfizmus –2548 G/A v promotoru genu pro leptin (LEP) byl v předchozích studiích asociován s rizikem nadváhy a obezity; faktorů signifikantně asociovaných se zvýšeným rizikem endometriálního karcinomu. Leptin hraje významnou úlohu v signální transdukci buněk endometriálního karcinomu, přičemž výsledky provedených studií naznačují, že leptin podporuje růst buněk endometriálního karcinomu i jejich invazivitu a předpokládá se, že kritickými mediátory působení leptinu jsou dráhy JAK/STAT a AKT. Cílem naší studie bylo zkoumat možné asociace polymorfizmu LEP –2548 G/A s výskytem endometriálního karcinomu a s ním souvisejících znaků. Design: Do studie bylo zahrnuto 67 pacientek s endometriálním karcinomem (průměrný věk 64,3 ± 10,3 let) a 67 kontrolních žen párovaných s pacientkami podle věku, BMI a etnického původu (62,1 ± 9,8 let); pro zkoumání obecných populačních frekvencí genotypů a alel byla navíc zařazena skupina 543 zdravých žen. LEP –2548 A/G byl zkoumán pomocí PCR s následnou analýzou délky restrikčních fragmentů. Výsledky: Předkládaná studie neodhalila významné rozdíly ve frekvenci genotypů nebo alel zkoumaného polymorfizmu u endometriálního karcinomu nebo s ním souvisejících znaků (věk menarché, menopauzy, počet spontánních abortů v anamnéze nebo čas do doby propuknutí onemocnění) mezi jednotlivými skupinami, což naznačuje, že genetické varianty polymorfizmu LEP –2548 G/A nejsou relevantním markerem rizika endometriálního karcinomu v české populaci. Závěry: Nezdá se, že by polymorfizmus LEP –2548 G/A reprezentoval důležitý genetický marker rizika endometriálního karcinomu u zkoumané české populace, byl nicméně asociován s obezitou, což je v souladu s dřívějšími pracemi.
Klíčová slova:
leptin – polymorfizmus – endometriální karcinom – PCR
Authors:
Josef Chovanec 1; J. A. Bienertová-Vašků 2; Z. Dostálová 1
Authors‘ workplace:
Department of Gynecology and Obstetric, The Faculty Hospital Brno 2Institute of Pathological Physiology, Faculty of Medicine, Masaryk University, Brno
1
Published in:
Klin Onkol 2009; 22(5): 223-227
Category:
Original Articles
Overview
Background:
Previously, the polymorphism –2548 G/A within the promoter of the leptin (LEP) gene was reported to be associated with overweight and obesity, the factors significantly associated to increased endometrial cancer risk. Leptin has been described to play an important role in signal transduction in endometrial cancer cells indicating that leptin promotes endometrial cancer growth and invasiveness and implicating the JAK/STAT and AKT pathways as critical mediators of leptin action. The aim of the study was to investigate the possible associations of LEP –2548 G/A polymorphism with endometrial cancer and its related traits. Design: Using PCR with following restriction analysis, we studied 67 endometrial cancer cases (mean age 64.3 ± 10.3 years) that were enrolled in the study along with 67 controls matched for age, BMI and ethnic origin (mean age 62.1 ± 9.8 years); an additional cohort of 543 healthy individual was recruited to investigate the general population frequencies. Results: The present study revealed no significant differences between the genotypes or alleles of investigated polymorphism for endometrial cancer risk or its related traits (age of menarche, menopause, number of spontaneous abortions in personal history or waiting time till the onset of the disease) among the groups, thus indicating that the genetic variants of LEP –2548 G/A is not a relevant marker of endometrial cancer risk in this Czech population. Conclusions: To conclude, the polymorphism LEP –2548 G/A doesn’t seem to represent a major genetic marker for endometrial cancer in the studied Czech population; however, it was associated with obesity, which finding is in accordance with previous reports.
Key words:
leptin – polymorphism – endometrial cancer – PCR
Introduction
Endometrial cancer represents one of the most common gynecological malignancies in developed countries whereas the incidences of endometrial cancer in Western are up to 10-times higher than in Asia of Africa rural regions [1–2]. Obesity represents a significant risk factor for endometrial cancer and the impact of obesity on endometrial cancer development is a very interesting intersection of these major health problems. Although the progress in understanding the genetics of obesity has moved rapidly in the past few years, the role of white adipose tissue (WAT) as a crucial endocrine organ still remains to be elucidated. White adipose tissue is tightly integrated into overall metabolic control, adipocytes being actively involved in extensive cross-talk with other cell types [3–5] and controlling the energy metabolism within the body by secreting biologically active peptides named adipokines produced exclusively or substantially by white adipose tissue pre adipocytes and mature adipocytes and act by endocrine, paracrine and autocrine mechanisms [6].
The importance of the hypothalamus in control of energy homeostasis has been recognized quite long and it was expected that the brain must receive afferent inputs in proportion to current body fat level. Coleman in 1973 [7] demonstrated the existence of a circulating factor playing a great role in the regulation of body weight. By using parabiosis methodology, he observed that obesity in the ob/ob mice was due to lack of this factor, whose gene has been consequently cloned and this adiposity factor was named leptin [8].
Leptin is a multifunctional peptide hormone with numerous biological activities including appetite regulation, bone formation, reproductive functions and angiogenesis [9–11]. In the study by Sharma et al [12], leptin has been described to play an important role in signal transduction in endometrial cancer cells indicating that leptin promotes endometrial cancer growth and invasiveness and implicating the JAK/STAT and AKT pathways as critical mediators of leptin action. The promotion of endometrial cancer cell proliferation by leptin involves activation of STAT3 and ERK2 signaling pathways. Moreover, leptin induced phosphorylation of ERK2 and AKT was dependent on JAK/STAT activation.
In an association study by Petridou et al [13], leptin plasma levels were found to be strongly correlated with endometrial cancer risk. However, it remains unclear whether this association is directly causative or whether increased leptin plasma levels and endometrial cancer are both secondary consequences of obesity. In 2000, Mammes et al [14] reported an association of LEP –2548 G/A polymorphism within the promoter of the leptin gene with overweight. So far, no study focused on the possible relationship of this polymorphism with the increased risk of endometrial cancer has been published. Considering the important role of LEP-2548 G/A (dbSNP ID rs7799039) in obesity development, we hypothesize that it could also be associated with the endometrial cancer risk and the disease related traits in the Czech Caucasian population.
Material and methods
Subjects
A total of 67 endometrial cancer cases (mean age 64.3 ± 10.3 years) were enrolled in the study along with 67 controls matched for age, BMI and ethnic origin (mean age 62.1 ± 9.8 years). The diagnosis of endometrial cancer was confirmed by standard histopathological examination according to the FIGO classification. In controls, the presence of any malignancy or severe chronic diseases was excluded by standardized complex physical examination and personal history evaluation by a specialist. Moreover, additional population of 543 healthy individuals was enrolled in the study to compare the baseline allelic and genotype frequencies within the general Czech population (215 men and 328 women, mean age 48.5 ± 13.5 years). All investigated individuals were Czech Caucasians.
This study was approved by the Committee for Ethics of Medical Experiments on Human Subjects, Faculty of Medicine, Masaryk University, Brno, and was performed in adherence to the Declaration of Helsinki Guidelines. Each participant gave her written informed consent which has been archived.
Genotyping
White cell fraction from the peripheral venous blood sample (5ml) was used to extract DNA according to the standard procedure using proteinase K. SNP LEP –2548G/A (dbSNP ID rs7799039), 2 548 bp upstream to the beginning of the exon 1 was detected as described previously [15]. Briefly, each of the 12 µl reaction contained 10 ng genomic DNA, 1.5 µl 10X PCR Buffer 1.5 µl MgCl2, 200 µM dNTP, 2 pmol of each primer and 0.4 U of Taq DNA polymerase (Fermentas). The reactions were performed using XP Cycler (BIOER). PCR amplification conditions were as follows: LEP –2548G/A polymorphism: 95˚C for 5 min, 94 ˚C for 30 s, 50 ˚C for 45 s, 72 ˚C for 50 s for 35 cycles and 72 ˚C for 10 min. Genotyping reliability was assessed by double genotyping of approx. 20% samples in independent assays where no differences were found. Negative controls were included in each reaction batch to exclude possible false-positives. The genotypization success rate was approx. 99%.
Statistical analysis
Differences in genotype distributions and the consistency with Hardy-Weinberg equilibrium (HWE) were tested using the chi2 test and the differences in allele frequencies were tested by the two tailed Fisher’s exact test. Differences in parameters studied between the two groups were tested using the Mann Whitney test. Tukey-Kramer post hoc correction was used to correct for multiple comparisons where appropriate. Logistic regression analysis was used to analyze the independence of the association between quantitative variables and endometrial cancer. Cumulative hazard function plots were estimated by the Kaplan-Meier method with the log rank test in order to compare groups. The Statistica v. 8.0 (Statsoft, Tulsa, OK, USA) program package was emploeyed in all analyses.
Results
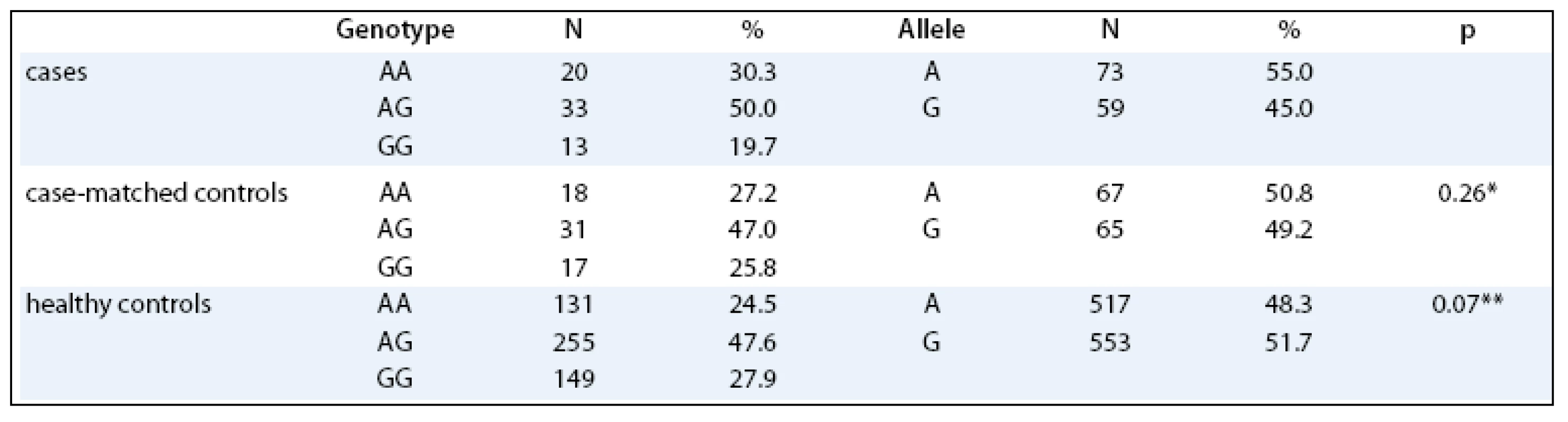
No significant differences in distribution of LEP –2548 G/A genotypes were observed between the endometrial cancer patients and the matched controls (p = 0.704). When comparing the genotype distributions of the polymorphism of the cases with the healthy Czech Caucasian population, no differences were observed as well (p = 0.248, Tab. 1). No significant differences in the age of menarche, menopause, parity and number of spontaneous abortions were observed between the cases and their precisely matched controls. Of these variables, only the number of spontaneous pregnancy losses was significantly associated with the increased risk of endometrial cancer (p = 0.003).
The associations between LEP –2548 genotype and endometrial cancer risk by BMI are summarized in Tab. 2, no significant differences between groups were observed.
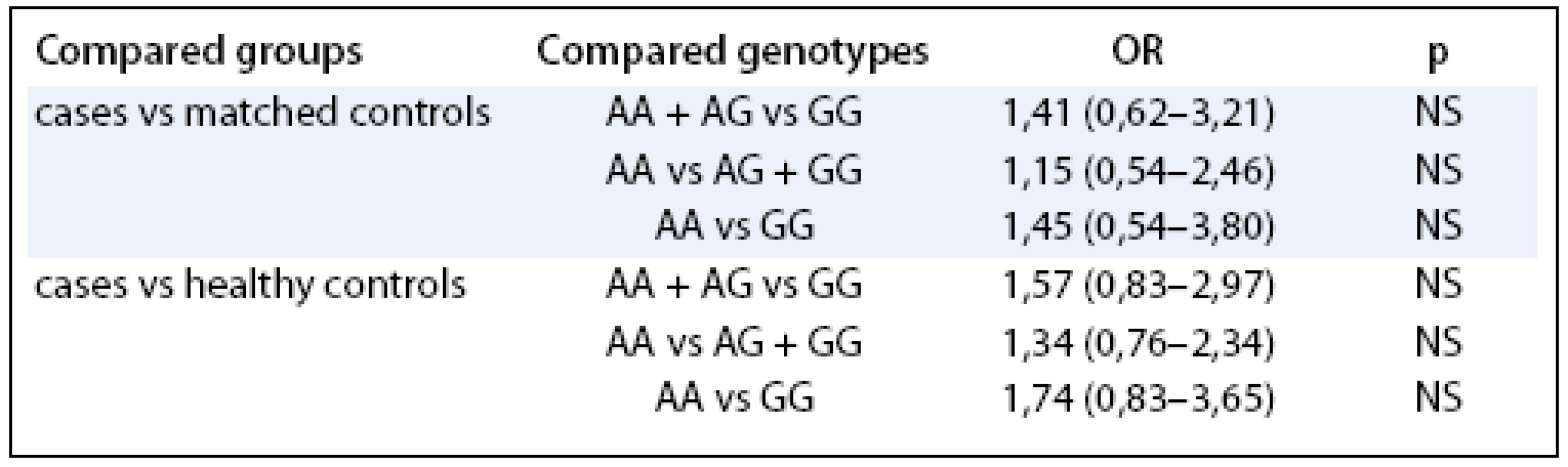
In the next step, we tested whether the LEP G/A –2548 G/A had any effect on the characteristics related to endometrial cancer independently on other known risk factors such as hormonal replacement therapy (HRT) or BMI. In the multivariate regression modeling, the LEP –2548 G/A served as an independent predictor for increased BMI (β = 1.1, p = 0.000004). Furthermore, LEP –2548 G/A was also significantly correlated with the body height (β = –0.39, p = 0.00000001). However, the examined polymorphism didn’t show independent prediction role for the endometrial cancer development.
In the following analysis, we tested, whether the LEP –2548 G/A exerts an independent prediction role on age of onset of the endometrial cancer; our data (p = 0.04) suggesting that a remarkable proportion of the gene effect could be dependent upon aging. Therefore, we consecutively tested the possible relation between age at time of endometrial cancer diagnosis and LEP –2548 G/A by subdividing the study group according to individual age (i.e. below or above 64 years, the median value of the entire cases cohort). However, these two subgroups did not differ significantly either in LEP –2548 G/A genotypes nor allele frequency (pg = 0.070, pa = 0.283).
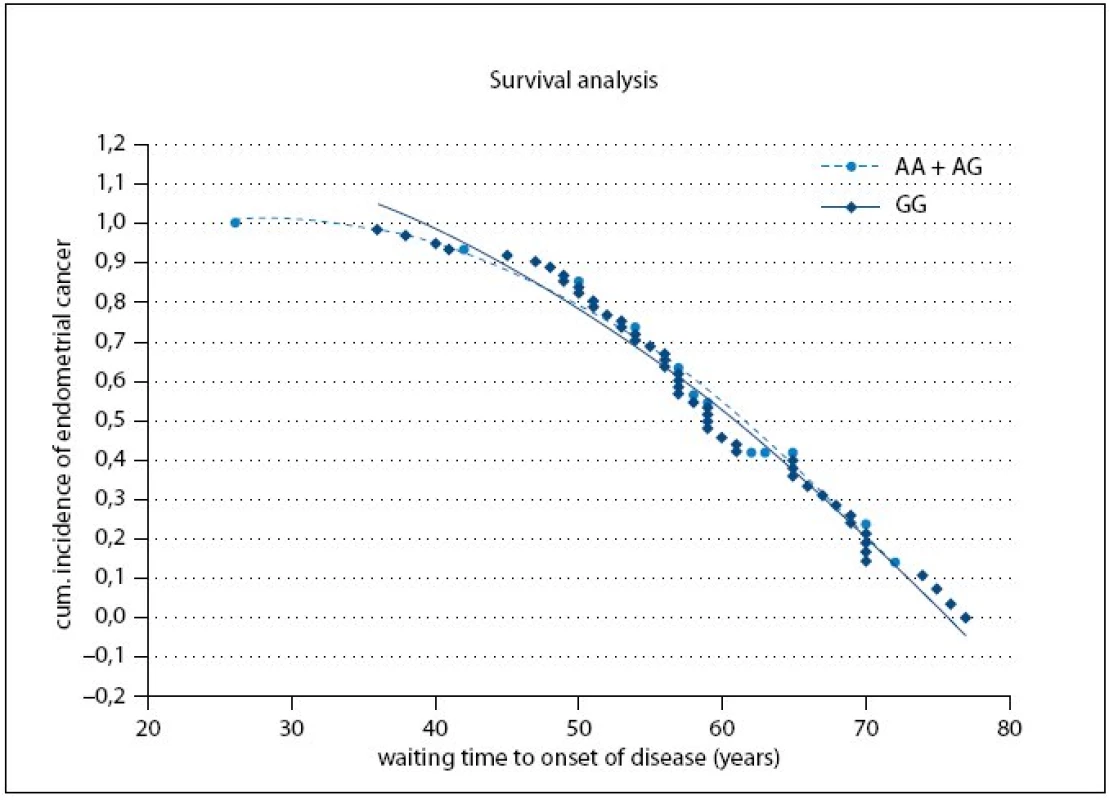
Fig. 1 shows the cumulative probabilities for endometrial cancer occurrence and waiting time to onset of disease. The time to onset of disease wasn’t significantly different between the A or G allele carriers (p = 0.79).
Discussion
Recently, leptin has been associated with cancers of the breast, ovary, prostate, small lung cancer and with non Hodgkin lymphoma and also the important role of leptin in general cancerogenesis has been proposed by observations from the previous studies [16–20].
The endometrium represents a classical hormone-dependent tissue and most of the endometrial carcinomas are hormone-dependent tumors. Leptin treated monkeys had higher mean plasma luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels than controls, demonstrating the ability of leptin to enhance gonadotropin secretion [21]. Sharma et al [12] suggested that leptin rapidly stimulates the JAK/STAT pathway and induces the phosphorylation of ERK and AKT, thus activating two key signal-transduction pathways associated with cell growth. In addition to this finding, Petridou et al [13] described significant dysregulation in serum leptin levels among cases with endometrial cancer compared to controls, however, it cannot be conclusively inferred, whether leptin levels elevation, developed in the study subjects as a consequence of obesity, played a crucial role in endometrial carcinogenesis or whether it was a simple correlate of obesity.
The common polymorphism in the promoter of the human leptin gene –2548G/A influences leptin expression, possibly at the transcriptional level, and therefore also adipose secretion levels of the hormone [22]; adipose tissue leptin mRNA levels is 60% higher in AA subjects when compared to GA/GG subjects. Taking this into account, we have hypothesized that this polymorphism could modulate the endometrial cancer susceptibility.
In this case-control study carried out in a Czech Caucasian population, we investigated the effects of –2548 G/A polymorphism in the gene encoding for leptin on endometrial cancer risk in the Czech endometrial cancer cases, BMI and age-matched controls and healthy Czech population. The present study revealed no significant differences in genotypes or alleles distribution of investigated polymorphism for endometrial cancer risk or its related traits (age of menarche, menopause, number of spontaneous abortions in anamnesis) among the groups, thus indicating that the genetic variants of LEP –2548 G/A are not a relevant marker of endometrial cancer risk in this Czech population. In the study by Mammes et al [15], the G allele of the polymorphism was associated with overweight, which is well in accordance with our results as the G allele was in our study an independent predictor for increased BMI, but not endometrial cancer, thus suggesting that the LEP –2548 G/A polymorphism is associated rather with obesity itself than endometrial cancer susceptibility.
To conclude, the polymorphism LEP –2548 G/A doesn’t seem to represent a major genetic marker for endometrial cancer in the Czech population; however, it is associated with obesity, which is well in accordance with previous reports.
Acknowledgments
The authors are grateful to Dana Polášková and Petra Přikrylová for technical assistance, and to all the study participants for participation in the project.
Study was supported by grant of Ministry of Education of the Czech Republic No. 881/2006 and by project of DANONE/2007 by Danone Institute focused on genetic variability of adipokines in obese individuals.
The
authors declare they have no potential conflicts of interest
concerning drugs, pruducts, or services
used in the study.
Autoři
deklarují, že v souvislosti s předmětem studie nemají žádné
komerční zájmy.
The Editorial Board declares that the manuscript met the ICMJE “uniform
requirements” for biomedical papers.
Redakční
rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro
publikace zasílané do biomedicínských
časopisů.
MUDr. Julie Bienertová-Vašků
Ústav
patologické fyziologie lékařské
fakulty MU
Kamenice
5
625
00 Brno
e-mail:
jbienert@med.muni.cz
Sources
1. Parkin DM. Cancer incidence in five continents. In: Parkin DM, Whelan SL, Ferlay J et al (eds). IARC Scientific. Publication. No. 143, Vol. VII, Lyon, France: International Agency for Research on Cancer 1997 : 1–1240.
2. Pisani P, Parkin DM, Ferlay J. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer 1993; 55 : 891–903.
3. Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 2005; 33(Pt 5): 1078–1081.
4. Trayhurn P. Adipose tissue in obesity-an inflammatory issue. Endocrinology 2005; 146(3): 1003–1005.
5. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92(3): 347–355.
6. Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci 1999; 892 : 146–154.
7. Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973; 9(4): 294–298.
8. Zhang Y, Proenca R, Maffei M et al. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372 : 425–432.
9. Bouloumié A, Drexler HC, Lafontan M et al. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res 1998; 83(10): 1059–1066.
10. Sierra-Honigmann MR, Nath AK, Murakami C et al. Biological action of leptin as an angiogenic factor. Science 1998; 281(5383): 1683–1686.
11. Huang L, Li C. Leptin: a multifunctional hormone. Cell Res 2000; 10(2): 81–92.
12. Sharma D, Saxena NK, Vertino PM et al. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer 2006; 13(2): 629–640.
13. Petridou E, Belechri M, Dessypris N et al. Leptin and body mass index in relation to endometrial cancer risk Ann Nutr Metab 2002; 46(3–4): 147–151.
14. Mammès O, Betoulle D, Aubert R et al. Association of the G-2548A polymorphism in the 5‘ region of the LEP gene with overweight. Ann Hum Genet 2000; 64(Pt 5): 391–394.
15. Mammès O, Betoulle D, Aubert R et al. Novel polymorphisms in the 5‘ region of the LEP gene: association with leptin levels and response to low-calorie diet in human obesity. Diabetes 1998; 47(3): 487–489.
16. Skibola CF, Holly EA, Forrest MS et al. Body mass index, leptin and leptin receptor polymorphisms, and non hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2004; 13(5): 779–86.
17. Ribeiro R, Lopes C, Medeiros R. Leptin and prostate: implications for cancer prevention – overview of genetics and molecular interactions. Eur J Cancer Prev 2004; 13 : 359–368.
18. Ribeiro R, Araújo AP, Coelho A et al. A functional polymorphism in the promoter region of leptin gene increases susceptibility for non small cell lung cancer. Eur J Cancer 2006; 42(8): 1188–1193.
19. Caldefie-Chézet F, Damez M, de Latour M et al. Leptin: a proliferative factor for breast cancer? Study on human ductal carcinoma. Biochem Biophys Res Commun 2005; 334(3): 737–741.
20. Mor G, Visintin I, Lai Y et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A 2005; 102(21): 7677–7682.
21. Finn PD, Cunningham MJ, Pau KY et al. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 1998; 139(11): 4652–4662.
22. Hoffstedt J, Eriksson P, Mottagui-Tabar S et al. A polymorphism in the leptin promoter region (–2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res 2002; 34(7): 355–339.
Labels
Paediatric clinical oncology Surgery Clinical oncologyArticle was published in
Clinical Oncology

2009 Issue 5
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- Metamizole in perioperative treatment in children under 14 years – results of a questionnaire survey from practice
-
All articles in this issue
- Proteomic Analysis of Cancer Cells
- Evaporation of Selected Cytotoxic Drugs and Permeation of Protective Gloves – Research into the Occupational Risks of Health Care Personnel Handling Hazardous Cytotoxic Drugs (CYTO Project)
- Profiles of Low-Molecular Proteome Spectrum Obtained through SELDI-TOF Mass Spectrometry in the Sera of Patients with Metastatic Malignant Melanoma: Pilot Study
- Chronic Gastrointestinal Toxicity after External-Beam Radiation Therapy for Prostate Cancer
- Reflection about the Colorectal Carcinoma Screening Evolution
- View of the Development of Oncology in the 3rd Millennium
- Rectal Neuroendocrine Tumours
- DNA and MicroRNA Microarray Technologies in Diagnostics and Prediction for Patients with Renal Cell Carcinoma
- Leptin –2548 G/A Polymorphism in Endometrial Cancer
- Clinical Oncology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Rectal Neuroendocrine Tumours
- Chronic Gastrointestinal Toxicity after External-Beam Radiation Therapy for Prostate Cancer
- Evaporation of Selected Cytotoxic Drugs and Permeation of Protective Gloves – Research into the Occupational Risks of Health Care Personnel Handling Hazardous Cytotoxic Drugs (CYTO Project)
- Proteomic Analysis of Cancer Cells
