Apoplexy of Rathke Cleft Cyst – a Case Report
Apoplexie Rathkeho cysty – kazuistika
Symptomatické Rathkeho cysty (Rathke Cleft Cysts, RCC) jsou zřídka se vyskytující ložiska selární a supraselární oblasti, přičemž apoplexie je jednou z nejméně obvyklých prezentací těchto cyst. Dosud bylo publikováno pouze několik případů hemoragické apoplexie RCC, přičemž patogeneze stále není objasněna. Za účelem snížení výskytu mylných předoperačních diagnóz popisujeme diagnostický postup u jednoho případu RCC a předkládáme přehled dostupné literatury na toto téma. Rovněž shrnujeme klinickopatologické vztahy mezi klinickými příznaky, výsledky zobrazovacích vyšetření a operačními vizualizacemi obsahu cyst.
Klíčová slova:
Rathkeho cysta – apoplexie – hemoragie
Authors:
X. Zhao; T. Wang; G. Liu
Authors‘ workplace:
The Fourth Affiliated Hospital of China Medical University, Shengyang, Liaoning Province, P. R. China
Published in:
Cesk Slov Neurol N 2013; 76/109(3): 367-372
Category:
Case Report
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Overview
Symptomatic Rathke cleft cysts (RCCs) are rare sellar and suprasellar lesions, and apoplexy is one of the most unusual presentations. Only a few cases of hemorrhagic apoplexy of an RCC have been reported and their pathogenesis is still poorly understood. In order to present a diagnostic thread to reduce misdiagnosis rate preoperatively, we report a case of RCC apoplexy and review associated published literature. We also summarize clinicopathological relationships between clinical symptoms, imaging features and intraoperative visualization of intracystic content.
Key words:
Rathke cleft cysts – apoplexy – hemorrhage
Introduction
Rathke cleft cysts (RCCs), residue of Rathke’s pouch during embryogenesis, are sellar or suprasellar congenital cysts. These benign lesions have been described in 22% of normal autopsies [1]. Most of them are asymptomatic. They can become symptomatic when they enlarge enough to compress surrounding structures. RCCs were found in only 2% – 9% of patients undergoing transsphenoidal surgery for symptomatic sellar region lesions [2,3]. Rarely, RCCs can present in a manner similar to pituitary apoplexy, with acute ‑ onset headaches, visual field and acuity loss and oculomotor palsies. We report a case of RCC with acute blurred vision and apoplexy confirmed by surgery and histology. We also reviewed the RCC apoplexy cases reported in the literature and summarize clinical presentation, imaging characteristics and intraoperative findings.
Case report
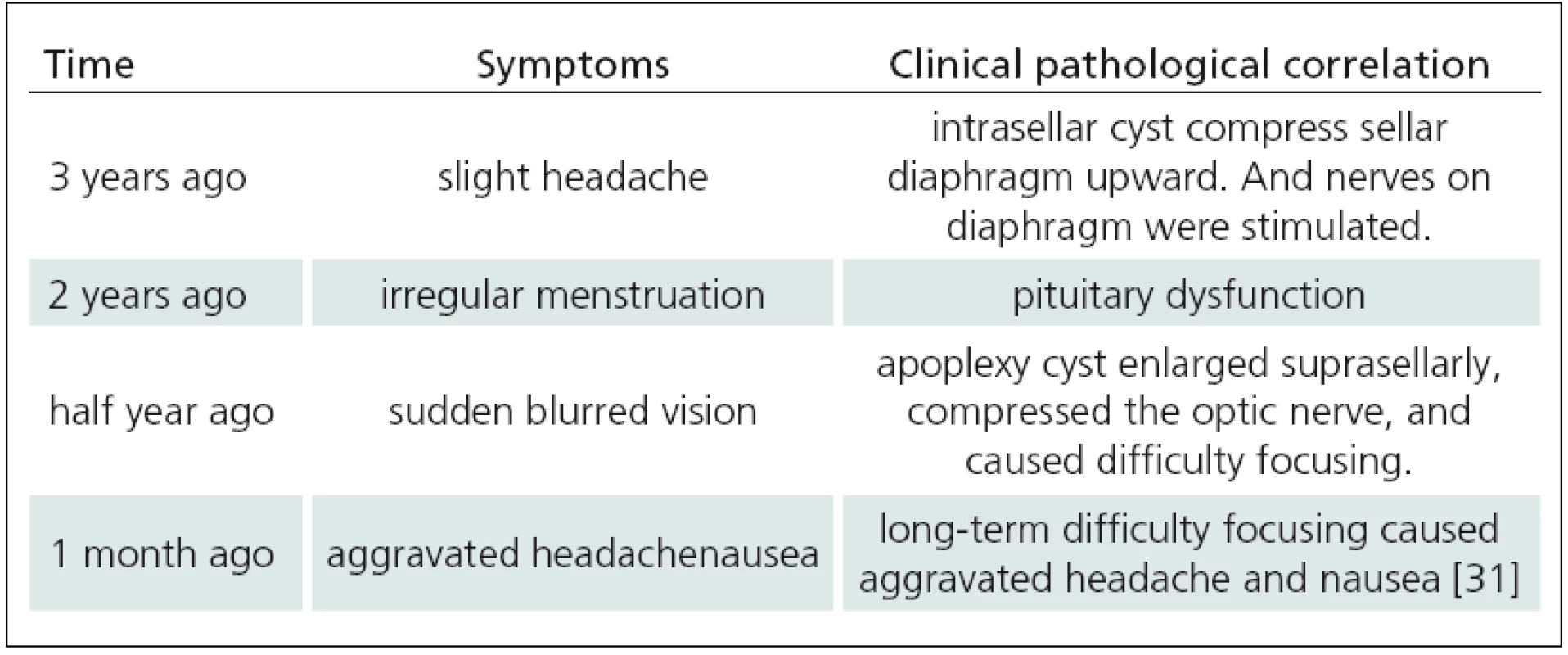
A 47‑year ‑ old female presented with 3 years of mild headache that responded to an oral analgesic. She had irregular menstruation 2 years ago and menostasis without lactation a year ago. She suffered a sudden attack of blurred vision half year ago and aggravating headache a month ago. Her serum PRL was normal. Radiological finding is shown in Fig. 1.

The sudden aggravation of this patient’scomplaints was considered to be due to expansion of the contents of the pituitary fossa. Apoplexy of pituitary adenoma is the most common cause of sudden enlargement of an intrasellar lesion. However, on T1 - weighted images, the signal of supresellar cyst was too high to correspond to a signal of half‑year ‑ old blood. High signal on T1 - weighted image could be due to lipid ‑ rich cystic content, as in Rathke cleft cyst. However, apoplexy of Rathke cleft cyst is extremely rare. Dermoid cysts principally originate at midline location, contain lipid‑like fluid and tend to suddenly enlarge or even rupture. All these characteristics were consistent with the characteristics of this case. Therefore, differential diagnosis included, in the first place, dermoid cyst, and also pituitary apoplexy and Rathke cleft cyst.
Since the content of dermoid cysts could cause severe chemical meningitis, we utilized pterional approach that provided better exposure and control of the cyst content than transsphenoidal approach. Intraoperatively, a suprasellar cyst (Fig. 2b) was seen beneath the compressed optic nerve (Fig. 2c). The thin wall of the cyst was punctured and dark red necrotic ‑ haemorragic fluid was extracted. After extraction, the cyst collapsed. The cyst wall was cut open and the cyst was entered (Fig. 2a). Yellowish mucinous content was seen. There was no septum between the “two” cysts. In fact, this was a single cyst with double‑lobular shape. No intracystic nodule was found. Histology confirmed the diagnosis of Rathke cleft cyst (Fig. 3).


Review of the published literature
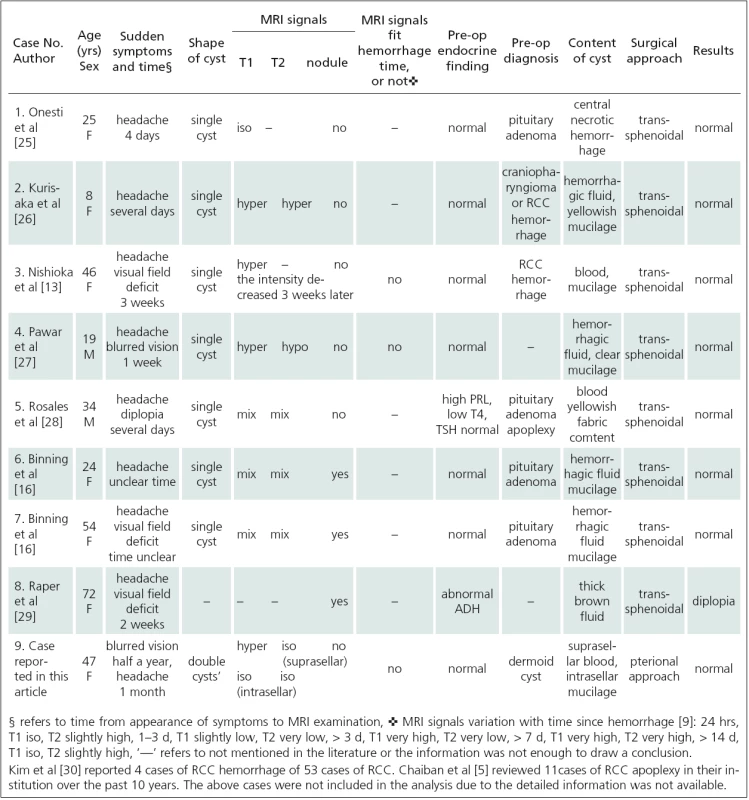
We reviewed the medical literature using PubMed to search for relevant publications on this entity. To review the literature, we used the following search terms: Rathke cyst apoplexy, Rathke cyst hemorrhage, and Rathke cyst. Case reports that documented the clinical presentation and confirmed the presence of bleeding into an RCC were selected (8 cases only) and were included in the analysis (Tab. 1).
Discussion
Clinical presentation
Symptoms of RCC apoplexy are similar with those of pituitary apoplexy but less severe [4]. Such symptoms include increasing headaches, visual changes, cranial nerve palsies, and variable degrees of hypopituitarism. They result from sudden increase in pressure on the pituitary and surrounding structures such as the sellar diaphragm, optic nerve and cavernous sinus. Most of RCC apoplexy patients have normal or slightly abnormal hormone level. Only 2 of 9 cases listed in Tab. 1 had mild endocrine abnormality. Chaiban et al [5] reported 4 cases of endocrine abnormality among 11 cases of RCC apoplexy, with 2 of them recovering immediately after the surgery and one recovering gradually. For the case in this article, we analyzed clinical pathological correlation (Tab. 2) after the diagnosis was confirmed. It would not have been possible to differentiate RCC apoplexy from pituitary apoplexy based on clinical presentation only.
MRI and cyst content
RCCs can be hypo ‑ or hyperdense on CT scan. They are usually iso ‑ or hyperintensive on MRI T1-weighted image but a minority of cases may also be hypointensive. The MRI signals of RCC on T1 - weighted image depend on content components and concentration, especially the concentration of protein, cholesterol and triglycerides [6]. The cyst content appearance is variable, including mucinous content, cholesterinic content, cytric oily liquid, similar purulent material, cerebrospinal fluid‑like fluid and necrotic ‑ haemorragic fluid etc. Among those, similar purulent material is known as concurrent infection, requires antibiotic therapy and predicts high recurrence rate [7]. Billeci et al [8] analyzed MRI signals and intraoperative appearance of RCC but no correlation was found. Similarly, we did not find any regularity on reviewed RCC apoplexy cases (Tab. 1). Therefore, intraoperative appearance of RCC content could not be estimated based on preoperative MRI signals.
MRI signals of RCCs are more complicated after intracystic hemorrhage. There are three types of the cyst according to whether or not the blood and the cyst content mix together within an RCC:
- homogeneous mixing, cyst maintains homogeneous signal (case 1, 2, 3 and 4in Tab. 1).
- heterogeneous mixing, cyst presents mixed signal (case 5, 6 and 7 in Tab. 1).
- not ‑ mixing cyst has double‑lobular shape, blood and content occupy separate lobes (case 9 in Tab. 1).
MRI signals of hemorrhage change regularly with time after hemorrhage [9]. None of the cases reviewed in Tab. 1 had corresponding MRI signal and hemorrhage time to indicate that regularity of signal change was lost after the blood and the cyst content had mixed together. Interestingly, in the present case, the blood and the cyst content occupied one lobe each but the signal of hemorrhage did not coincide with hemorrhage time. Although MRI showed clear boundary between the two lobes, no septum was found intraoperatively between them. Therefore, we assume that the blood and the cyst content could still partially mix together. Consequently, hemorrhage time of apoplexy RCC could not be judged from MRI signal.
Shape of RCCs
Most of RCCs are elliptical single cysts and remain elliptical after apoplexy. The present case is the only one described as having double‑lobular shape (Tab. 1). Russell reported 2 cases of dumbbell RCCs, with intrasellar part of the cyst being covered with simple ciliated columnar epithelium and suprasellar part being covered with squamous epithelium seen usually in craniopharyngioma. This means that these were concurrent RCCs and craniopharyngiomas. Some researchers assume that the RCC is a transitional lesion that precedes craniopharyngioma [10]. However, this theory remains controversial. Research into the reasons for intra ‑ RCC hemorrhage is lacking. RCCs constitute of simple or columnar epithelium and this makes them fragile. Oka et al [11] considered small thin‑walled vessels in the granulation tissue on the cyst wall to be the source of bleeding. The double‑lobular shape in our case was in accordance with this assumption; since the source of bleeding was located near the inner wall of the cyst, rather than at the center of the cyst, the blood did not mix with the cyst content completely and increased focal pressure on the cyst wall caused external apophysis, resulting in the observed double‑lobe shape.
Preoperative misdiagnosis
Misdiagnosis rate of RCC apoplexy is high. Of all the cases in Tab. 1, only one (case 3) was correctly diagnosed prior to the surgery. RCC apoplexy is commonly misdiagnosed as pituitary apoplexy or craniopharyngioma. Pituitary adenoma accounted for 90% of patients who underwent sellar MRI and RCCs accounted for 19% of nonadenomatous sellar masses [12]. The great disparity in the incidence was an important reason for misdiagnosis. Moreover, RCCs lack characteristic MRI presentation. They can be iso ‑ or hyper‑intensive on T1 - weighted image, and the signal on T2 - weighted image is also variable due to variable concentration of mucoitin and blood [13,14]. Byun et al [15] thought that short T1 and unenhanced nodule on the cyst wall suggested RCC diagnosis but specificity was low. Among the reviewed cases (Tab. 1), intracystic nodules were found in 3 cases only, of which case 6 and 7 were confirmed to have nodules intraoperatively and not preoperatively [16]. In some cases, the compressed normal pituitary gland presents intrasellar ring enhancement on MRI [8], likely to be mistaken for enhanced cyst wall (craniopharyngioma or cystic pituitary adenoma). RCC is preferred diagnosis if a sellar elliptical mass with smooth outline has homogeneous MRI signal without calcification or internal enhancement [17]. However, once there is an RCC apoplexy, the MRI signal is more complicated. Based on the cases reviewed in Tab. 1, when MRI is performed in a patient who has sudden symptoms of apoplexy, the main differential diagnosis includes RCC apoplexy and pituitary apoplexy. If the lesion has a mixed signal, one can hardly be differentiated from the other. If the lesion has homogeneous signal, the differential diagnosis includes RCC apoplexy and cystic pituitary apoplexy. Herein, the case with normal or slightly abnormal hormone levels suggests RCC apoplexy, while the case with hypopituitarism suggests cystic pituitary apoplexy [4]. Ring enhancement cannot be used as an evidence for the diagnosis, as this could be a compressed normal pituitary gland.
Treatment
An asymptomatic RCC should be monitored while a symptomatic RCC requires surgery [18]. RCC apoplexy requires surgery as soon as possible. It is known that transsphenoidal surgery is the best option. However, there is a controversy over the extent of resection. The focus of the controversy is in whether a total resection of the cyst wall and the content should be performed or only a partial resection (or biopsy) of the cyst wall plus cyst content drainage. The former is more aggressive with higher risk and complications but provides lower recurrence rate. The latter is safer, can improve symptoms but recurrence rate is higher. Madhok et al [19] reported 35 cases of RCC with total resection of the cyst by endoscopic transsphenoidal surgery. No complications were seen, only 2 cases recurred and did not require reoperation. Nevertheless, the current mainstream view is that it is sufficient to drain the cyst content and partially resect (or biopsy) the cyst wall. Mayo medical center analyzed 74 RCC cases and concluded that gross total resection was associated with more complications but did not reduce the overall rate of recurrence [20]. Especially for children, cyst content drainage plus cyst wall biopsy is preferable because of safety [21,22]. Transsphenoidal surgery can improve most patients’ symptoms and endocrine abnormality [23,24]. All the 8 reviewed cases of RCC apoplexy (Tab. 1) underwent transsphenoidal surgery and their symptoms were relieved, as did the 11 cases of RCC apoplexy reported by Chaiban et al [5].
Conclusion
RCCs apoplexy is a rare condition. Clinical presentation is similar to but milder than that of pituitary apoplexy. It is difficult to differentiate RCC apoplexy from pituitary apoplexy based on MRI image only. Hemorrhage time in RCC apoplexy cannot be judged from an MRI signal. Ring enhancement cannot be used as evidence either. RCC apoplexy should be considered if a patient has symptoms of apoplexy, mild endocrine abnormality and sellar mass shows homogeneous signal on MRI. Surgery and histology are required to confirm the diagnosis. Cyst content drainage plus cyst wall partial resection (or biopsy) through transsphenoidal approach is the best option to manage RCC apoplexy.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Xianlin Zhao, MD
The Fourth Affiliated Hospital of China Medical University
No. 4 Chongshan East Road of Huanggu District
Shenyang City, Liaoning Province, China
e-mail: li798242742@gmail.com
Accepted for review: 27. 9. 2012
Accepted for print: 21. 2. 2013
Sources
1. Teramoto A, Hirakawa K, Sanno N, Osamura Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology 1994; 193(1): 161 – 164.
2. Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 2007; 156(2): 203 – 216.
3. Zada G, Kelly DF, Cohan P, Wang C, Swerdloff R.Endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions. J Neurosurg 2003; 98(2): 350 – 358.
4. Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy: a review. J Intensive Care Med 2008; 23(2): 75 – 90.
5. Chaiban JT, Abdelmannan D, Cohen M, Selman WR, Arafah BM. Rathke cleft cyst apoplexy: a newly characterized distinct clinical entity. J Neurosurg 2011; 114(2): 318 – 324.
6. Hayashi Y, Tachibana O, Muramatsu N, Tsuchiya H, Tada M, Arakawa Y et al. Rathke cleft cyst: MR and biomedical analysis of cyst content. J Comput Assist Tomogr 1999; 23(1): 34 – 38.
7. Tate MC, Jahangiri A, Blevins L, Kunwar S, Aghi MK. Infected Rathke cleft cysts: distinguishing factors and factors predicting recurrence. Neurosurgery 2010; 67(3): 762 – 769.
8. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: a radiological, surgical and pathological review. Pituitary 2004; 7(3): 131 – 137.
9. Bradley WG jr. MR appearance of hemorrhage in the brain. Radiology 1993; 189(1): 15 – 26.
10. el ‑ Mahdy W, Powell M. Transsphenoidal management of 28 symptomatic Rathke‘s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery 1998; 42(1): 7 – 16.
11. Oka H, Kawano N, Suwa T, Yada K, Kan S, Kameya T. Radiological study of symptomatic Rathke’s cleft cysts. Neurosurgery 1994; 35(4): 632 – 636.
12. Famini P, Maya MM, Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: ten‑year experience in 2,598 patients. J Clin Endocrinol Metab 2011; 96(6): 1633 – 1641.
13. Nishioka H, Ito H, Miki T, Hashimoto T, Nojima H,Matsumura H. Rathke’s cleft cyst with pituitary apoplexy: case report. Neuroradiology 1999; 41(11): 832 – 834.
14. Voelker JL, Campbell RL, Muller J. Clinical, radiographic, and pathological features of symptomatic Rathke’s cleft cysts. J Neurosurg 1991; 74(4): 535 – 544.
15. Byun WM, Kim OL, Kim D. MR imaging findings of Rathke’s cleft cysts: significance of intracystic nodules. AJNR Am J Neuroradiol 2000; 21(3): 485 – 488.
16. Binning MJ, Liu JK, Gannon J, Osborn AG, Couldwell WT. Hemorrhagic and nonhemorrhagic Rathke cleft cysts mimicking pituitary apoplexy. J Neurosurg 2008; 108(1): 3 – 8.
17. Naylor MF, Scheithauer BW, Forbes GS, Tomlinson FH, Young WF. Rathke cleft cyst: CT, MR, and pathology of 23 cases. J Comput Assist Tomogr 1995; 19(6): 853 – 859.
18. Couldwella WT, Weiss MH. Surgical management of Rathke’s cleft cysts. Neuroendocrinology 2006; 13 : 351 – 355.
19. Madhok R, Prevedello DM, Gardner P, Carrau RL, Snyderman CH, Kassam AB. Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg 2010; 112(6): 1333 – 1339.
20. Higgins DM, Van Gompel JJ, Nippoldt TB, Meyer FB. Symptomatic Rathke cleft cysts: extent of resection and surgical complications. Neurosurg Focus 2011; 31(1): E2.
21. Jahangiri A, Molinaro AM, Tarapore PE, Blevins L jr, Auguste KI, Gupta N et al. Rathke cleft cysts in pediatric patients: presentation, surgical management, and postoperative outcomes. Neurosurg Focus 2011; 31(1): E3.
22. Zada G, Ditty B, McNatt SA, McComb JG, Krieger MD. Surgical treatment of rathke cleft cysts in children. Neurosurgery 2009; 64(6): 1132 – 1137.
23. Wait SD, Garrett MP, Little AS, Killory BD, White WL. Endocrinopathy, vision, headache and recurrence after transsphenoidal surgery for Rathke cleft cysts. Neurosurgery 2010; 67(3): 837 – 843.
24. Česák T, Náhlovský J, Hosszu T, Řehák S, Látr I,Němeček S et al. Longitudinal Monitoring of the Growth of Post‑Operation Non ‑ Functioning Pituitary Adenomas. Cesk Slov Neurol N 2009; 72/ 105(2): 115 – 124.
25. Onesti ST, Wisniewski T, Post KD. Pituitary hemorrhage into a Rathke’s cleft cyst. Neurosurgery 1990; 27(4): 644 – 646.
26. Kurisaka M, Fukui N, Sakamoto T, Mori K, Okada T, Sogabe K. A case of Rathke’s cleft cyst with apoplexy. Childs Nerv Syst 1998; 14(7): 343 – 347.
27. Pawar SJ, Sharma RR, Lad SD, Dev E, Devadas RV. Rathke’s cleft cyst presenting as pituitary apoplexy. J Clin Neurosci 2002; 9(1): 76 – 79.
28. Rosales MY, Smith TW, Safran M. Hemorrhagic Rathke’s cleft cyst presenting as diplopia. Endocr Pract 2004; 10(2): 129 – 134.
29. Raper DM, Besser M. Clinical features, management and recurrence of symptomatic Rathke’s cleft cyst. J Clin Neurosci 2009; 16(3): 385 – 389.
30. Kim JE, Kim JH, Kim OL, Paek SH, Kim DG, Chi JG et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg 2004; 100(1): 33 – 40.
31. Greenberg MS. Handbook of Neurosurgery. 6th ed. New York: Thieme Medical Publishers 2006.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2013 Issue 3
- Memantine Eases Daily Life for Patients and Caregivers
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Advances in the Treatment of Myasthenia Gravis on the Horizon
-
All articles in this issue
- Extracranially Metastasizing Meningiomas
- EkoSonic SVTM System for Interventional Therapy in Ischemic Stroke Patients
- The Importance of Posterior Column Signs for Differential Diagnosis of Hereditary Ataxias
- Risk Profile of Patients after Ischemic Stroke – Data Analysis from the IKTA Register
- A Comparison of Epidemiological Data on Acute Stroke in the Zlin District and the CR Analysed Using the UZIS and IKTA Methodology
- Brain Multiple Focal Processes in a HIV Positive Patient – a Case Report
- Inclusion Body Myositis with Neck Muscle Weakness and Positive Effect of Immunoglobulins – a Case Report
- Mechanisms of Spasticity and its Assessment
- Cost of Disorders of the Brain in the Czech Republic
- Multiple Sclerosis – a Role of Regulatory T Cells in the Pathogenesis and Biological Treatment of the Disease
- Human Prion Diseases in the Czech Republic – 10 Years of Experience with the Diagnosis
- Cerebral Collateral Circulation – Potential Target for Cerebral Infarction Management
- Non‑ invasive Determination of Hemispheric Language and Upper Limb Dominance in Healthy Subjects
- Apoplexy of Rathke Cleft Cyst – a Case Report
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Mechanisms of Spasticity and its Assessment
- Human Prion Diseases in the Czech Republic – 10 Years of Experience with the Diagnosis
- Inclusion Body Myositis with Neck Muscle Weakness and Positive Effect of Immunoglobulins – a Case Report
- Extracranially Metastasizing Meningiomas
