The frequency of silent brain infarcts in polycythaemia vera and essential thrombocytosis
Frekvence tichého mozkového infarktu při pravé polycytémii a esenciální trombocytóze
Cíle: U pacientů s myeloproliferativními neoplaziemi negativními na Ph chromozom je často hodnocena CMPnebo tranzitorní ischemická ataka, ale výzkum týkající se tichých mozkových infarktů je omezený. V naší studii byla zkoumána frekvence tichých mozkových infarktů ve skupině pacientů s pravou polycytémií (polycytemia vera; PV) a esenciální trombocytózou (ET) a její souvislost s faktory souvisejícími s onemocněním. Metody: Jednalo se o retrospektivní studii. Pacienti s PV a ET, kteří byli sledováni v letech 2015–2021, byli znovu vyšetřeni na výskyt tichých mozkových infarktů. U 66 pacientů s PV a ET byla provedena MR mozku. Devět pacientů bylo vyloučeno z důvodu symptomatického cerebrovaskulárního onemocnění a 25 pacientů bylo vyloučeno z důvodu jednoho či více z následujících onemocnění či stavů: arteriální hypertenze, diabetes mellitus 2. typu, migréna, kouření, stenóza karotid. Výsledky: Mutace JAK-2 (Janusova kináza 2) byla pozorována u 23 pacientů (67,6 %) a tichá cerebrální ischemie byla zjištěna u 22 pacientů (35,3 %). Míra výskytu mutace JAK-2 u pacientů s tichou cerebrální ischemií byla statisticky významně vyšší než u pacientů bez ní (p = 0,027). Dále bylo shledáno, že existuje statisticky významný vztah také mezi remisí a tichou cerebrální ischemií. U pacientů v remisi bylo pozorováno méně tichých cerebrálních ischemií (p = 0,036). Závěr: Poznatky týkající se průměrného věku a četnosti tichých cerebrálních infarktů ukazují, že PV a ET jsou rizikovými faktory tichého mozkového infarktu a v průběhu dlouhodobého sledování je třeba provádět jejich screening.
Klíčová slova:
tichá cerebrální ischemie – CMP – pravá polycytemie – esenciální trombocytóza – mutace JAK-2
Authors:
E. Karaçay 1; U. Cenikli 2; A. Özşimşek 1; İ. Atalay Karaçay 3; Y. Yüksel 4; V. Karakuş 5; G. Kutlu 6
Authors‘ workplace:
Neurology Department, ALKU Medicine, Faculty, Alanya, Turkey
1; Neurology Department, Training and, Research Hospital, Muğla, Turkey
2; Pathology Department, ALKU Training, and Research Hospital, Alanya, Turkey
3; Radiology Department, ALKU Medicine, Faculty, Alanya, Turkey
4; Hematology Department, ALKU Medicine, Faculty, Alanya, Turkey
5; Neurology Department, Sıtkı Koçman, University Medicine Faculty, Muğla, Turkey
6
Published in:
Cesk Slov Neurol N 2021; 84/117(4): 388-392
Category:
Original Paper
doi:
https://doi.org/10.48095/cccsnn2021388
Overview
Aim: Stroke or transient ischaemic attack in Ph chromosome-negative myeloproliferative neoplasms patients have been investigated many times, whereas there is limited research on silent brain infarcts. In our study, we explored the frequency of silent brain infarcts in a polycythaemia vera (PV) and essential thrombocytosis (ET) patient group and its relationship to disease-related factors. Methods: The study was designed retrospectively. PV and ET patients who were followed up between 2015–2021 were re-examined in terms of silent brain infarcts. There were 66 PV and ET patients who had an MRI scan of the brain. Nine patients were excluded due to symptomatic cerebrovascular disease, 25 were excluded due to having one or more of the following diseases or conditions: arterial hypertension, diabetes mellitus type 2, migraine, smoking, carotid stenosis. Results: JAK-2 (Janus Kinase-2) mutation was observed in 23 patients (67.6%) and silent cerebral ischaemia was detected in 22 patients (35.3%). The rate of JAK-2 mutation in patients with silent cerebral ischaemia was found to be statistically higher than those without (P = 0.027). The relationship between remission status and silent cerebral ischaemia was also found to be statistically significant, patients in the remission period having a lower rate of silent cerebral ischaemia (P = 0.036). Conclusion: The findings of mean age and silent cerebral infarction frequency support the theory that PV and ET are also risk factors of silent brain infarction and need to be screened during the follow-up term.
Keywords:
silent cerebral ischaemia – stroke – polycythaemia vera – essential thrombocytosis – JAK-2 mutation
Introduction
Myeloproliferative neoplasms (MPNs) are clonal stem cell-derived diseases [1]. In this study, we included patients who suffer from polycythaemia vera (PV) and essential thrombocytosis (ET), which are all subcategorized as Philadelphia (Ph) chromosome-negative classical MPNs. We evaluated silent brain infarcts in this patient group.
Thrombosis in this patient group is widespread. Detected thrombosis at or prior to the diagnosis of a MPN was estimated to be 34–38.6% in PV, 9.7–29.4% in ET [2]. The cumulative rate of thrombosis after diagnosis of MPN is estimated to be around 3% per patient-year in PV and ET [3]. Thrombotic events in MPNs patients may vary from mild microcirculatory disturbances to severe complications, myocardial infarction, ischaemic stroke, transient ischaemic attack (TIA), cerebral venous thrombosis or deep venous thrombosis [2].
Stroke and (TIA) are considered to be cerebrovascular diseases that often present with specific symptoms, nevertheless, neuropathological and imaging studies also show asymptomatic ischaemia as a kind of cerebrovascular disease [4]. Advancements in imaging techniques and widespread use of imaging tools have added a new dimension to the definition of cerebrovascular disease. These asymptomatic ischaemic areas are also defined as silent ischaemia or silent brain infarcts [5].
There are many studies on stroke or TIA in Ph chromosome-negative MPNs patients which have been investigated many times, whereas silent brain infarcts are under-researched. In our study we explored the frequency of silent brain infarcts in the PV and ET patient groups and its relationship to disease-related factors.
Methods
The study was designed retrospectively. Ph chromosome-negative MPNs patients (PV and ET) who were followed up at Muğla Sıtkı Kocman University Medical Faculty Hematology Clinic and Alanya Training and Research Hospital Hematology Clinic, Turkey between 2015–2021 were re-examined in terms of silent brain infarcts.
The World Health Organization (WHO) revised version of MPN diagnostic criteria from 2016 was used for the diagnostics of Ph chromosome-negative MPNs patients, the diagnoses of the patients diagnosed before 2015 were checked and updated using 2016 diagnostic criteria. In PV, remission was defined as a haemoglobin value lower than 16 g/dL in women and 16.5 g/dL in men. ET remission was defined as a platelet value lower than 450×103/µL. These are the basic remission criteria defined in WHO’s revised MPN diagnostic criteria guideline.
There were 66 PV and ET patients who had a MRI scan of the brain. Nine patients were excluded due to symptomatic cerebrovascular disease, 25 were excluded due to having one or more of these diseases or conditions; arterial hypertension, diabetes mellitus type 2, migraine, smoking, carotid stenosis. These are the main risk factors of silent brain infarcts. 34 out of 66 patients were included in the study.
T2-weighted MRI scans are used to identify silent brain infarcts and were supported with other sequences.
The data were analysed with the IBM SPSS 23.0 package program (IBM Corp., Armonk, NY, USA). Descriptive statistics are presented with N (%) and mean ± standard deviation (min-max) and median (min-max) values. The Pearson chi-square test and Fisher‘s Exact test were used in the analysis of the relationships between the categorical variables. The Shapiro-Wilk test was used in the analysis of normality assumption. For the analysis of the difference between the measurement values of the two groups, the Mann-Whitney U test was used when the normal distribution was not fit, and Student‘s t test was used when it was fit. P values lower than 0.05 were considered statistically significant.
Results
General characteristics of the patients is presented in Tab. 1.
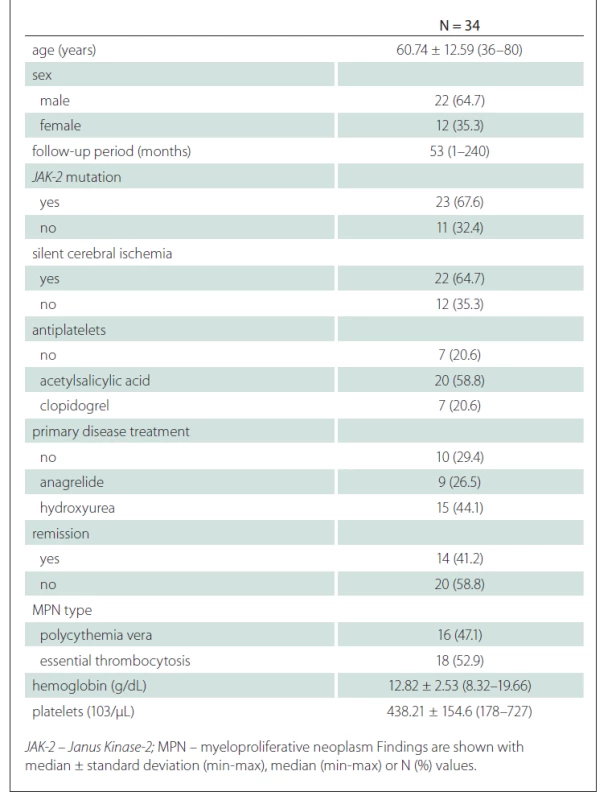
When the general characteristics of the patients were compared according to the presence of silent cerebral ischaemia, no difference was found in terms of the average age and sex distribution of the patients according to the presence of silent cerebral ischaemia. There was no significant difference between the mean follow-up periods of the group with silent cerebral ischaemia and the group without (P = 0.631) (Tab. 2).
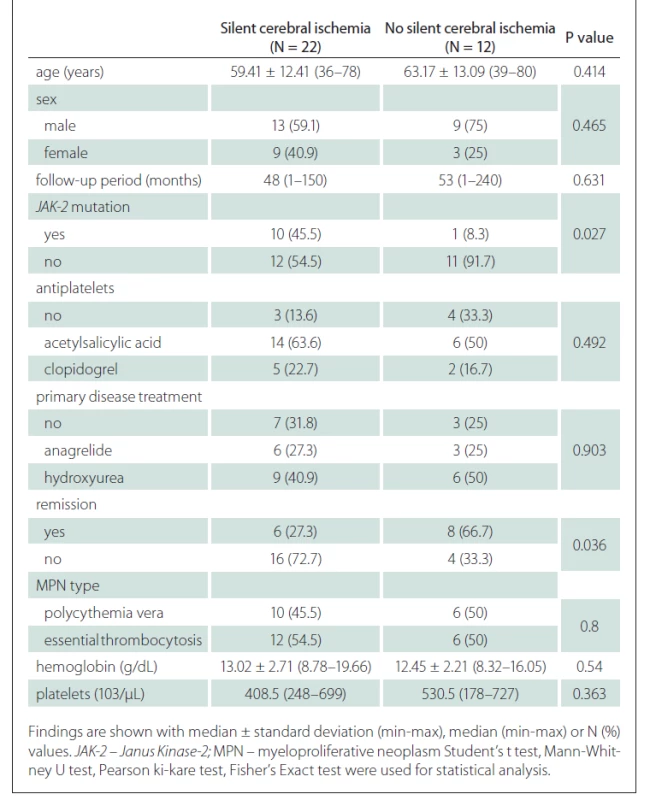
The rate of JAK-2 mutation was statistically significantly higher in patients with silent cerebral ischaemia than in patients without silent cerebral ischaemia (Tab. 2).
The relationship between remission status and silent cerebral ischaemia was found to be statistically significant. Accordingly, patients in the remission period had a lower rate of silent cerebral ischaemia (P = 0.036) (Tab. 2).
No significant difference was found in terms of antiplatelets usage, primer disease treatments, primary disease type, haemoglobin and platelet values according to the presence of silent cerebral ischaemia (Tab. 2).
Discussion
Thrombosis is a common complication of Ph chromosome-negative MPNs (PV and ET), especially in older patients [6]. Stroke is a well-known complication of Ph chromosome-negative MPNs. However, silent brain infarcts as a complication of Ph chromosome-negative MPNs have not been studied before. In our study, we estimated the frequency of silent brain infarcts in the Ph chromosome-negative MPNs patient group (PV and ET), and its relationship to disease-related factors. Silent brain infarcts are premises of disabling strokes, and screening these asymptomatic changes can reduce the risk of stroke and dementia with preventive therapies [5].
The prevalence of MRI-defined silent brain infarcts varies within the general population. The Helsinki Aging Brain Study (HABS) found the prevalence of silent infarction was 16% at the ages between 55 and 85 [7]. The Cardiovascular Health Study reported a 28% prevalence of silent brain infarction [8]. In the Austrian Stroke Prevention Study, their prevalence was 8% [9]. According to these studies, the prevalence of silent cerebral infarction in the general population is 8–28%. When we examined the selected groups with risk factors of stroke and silent ischaemia, the prevalence of silent cerebral infarction was found to be higher. In the review study by Vermeer et al, the frequency of silent cerebral infarction in patients with a mean age of 60 years and with coronary artery disease was 32%. Likewise, the frequency in patients with a mean age of 68 years and with atrial fibrillation was 32% [5]. According to Mathiesen et al, in patients with a mean age of 68 years with asymptomatic carotid stenosis the silent cerebral infarction prevalence was 23% [10]. Studies on the silent cerebral infarction frequency in patients with arterial hypertension reported silent cerebral infarction frequency at around 43% [11–16]. Vermeer et al also report the MRI-defined silent cerebral infarction frequency as 38% in diabetes mellitus patients with a mean age of 62 years [5]. In the same review, the prevalence of MRI-defined silent cerebral infarction in a sickle cell patient group with a median age of 24 years was found to be 30%. In our study, we found a 35.3% prevalence of MRI-based silent cerebral ischaemia. The patients’ mean age was 60.74 years (36–80). Age is one of the main risk factors of silent cerebral infarction [17–19]. The mean age of the patients in our study was mostly similar to the risk groups mentioned above. The findings of mean age and silent cerebral infarction frequency support the theory that Ph chromosome-negative classical MPNs (PV and ET) are also risk factors of silent brain infarction and need to be screened during the follow-up term.
The presence of the JAK-2 mutation in Ph chromosome-negative MPNs diseases is associated with thrombosis [20]. Weston et al examined thrombosis in ET patients and stated that there was a trend toward increased rates of prior thrombosis among JAK2-positive patients (27 vs. 5%) [21]. Larsen et al evaluated an ET cohort for possible clinical correlations to the JAK2 mutation status including a history of previous thrombosis and reported a significant increase in the prevalence of arterial thrombosis in JAK2-positive ET patients (P = 0.001) [22]. In our study, the JAK-2 mutation was observed in 67.6% of the patients and the rate of JAK-2 mutation in patients with silent cerebral ischaemia was statistically significantly higher than those without it (P = 0.027).
Increased blood viscosity and increased platelet activation are risk factors of thromboembolism in PV and ET [23,24]. In our study, remission status was also statistically significantly related to the occurrence of silent cerebral ischaemia, patients in the remission period had a lower rate of silent cerebral ischaemia (P = 0.036). We assessed remission with parameters of complete blood count parameters but better assessment of JAK-2 gene positive patients’ remission is performing molecular genetic detection of JAK-2 gene.
In our cohort, 7 patients (20.6%) were not using antiplatelets, 20 patients (58.8%) were using acetylsalicylic acid and 7 patients (20.6%) were using clopidogrel. No significant difference was found in terms of antiplatelets usage in patients with versus those without silent cerebral ischaemia. Low rates of silent brain ischaemia are expected in patients using antiplatelets according to stroke prevention procedures [25]
Hasson et al reported that hydroxurea prevented silent stroke in sickle cell disease [26]. In our study, 29.4% of patients did not receive treatment for their primary disease, 26.5% received anagrelide and 44.1% received hydroxyurea treatment. 20 patients (58.8%) were in remission. No significant difference was found in terms of primary disease treatments and silent brain ischaemia. We assume that this is related to the low number of patients using disease treatments.
Our study has some limitations. First of all, it is a retrospective study. Future studies can be designed prospectively. Second, we evaluated mainly ET and PV patients. Future work could include primary myelofibrosis patients. Third, the sample size of the study should be increased. A larger sample size can capture the disease related factors and silent cerebral ischaemia more accurately.
Conclusion
Early diagnosis of silent cerebral ischaemia helps to prevent symptomatic strokes, even against large vessel occlusion which may cause serious disabilities, and dementia. It gives us time to take precautions. Ph chromosome-negative MPNs (PV and ET) are also a group of diseases that can be evaluated as a risk factor for cerebral silent ischaemia as shown in this study. We need prospective and larger sample sized studies to gain more detailed information about the relationship between silent cerebral ischaemia and Ph chromosome-negative MPNs.
Ethical principles
The entire study was conducted in accordance with the Helsinki Declaration of 1975 (as revised in 2004 and 2008). Ethics committee approval was obtained from the Alanya Alaattin Keykubat University Ethics Committee.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Ertan Karaçay, MD
Neurology Department
Alanya ALKU Medicine Faculty
Oba Mahallesi Fidanlik Caddesi
07400 Alanya/Antalya
Turkey
e-mail: ertan.karacay1@hotmail.com
Accepted for review: 18. 3. 2021
Accepted for print: 12. 8. 2021
Sources
1. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013; 22 (13): 2176–2184. doi: 10.1182/blood-2013-03-460154.
2. Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost 2007; 33 (4): 313–320. doi: 10.1055/s-2007-976165.
3. McMahon B, Stein BL. Thrombotic and bleeding complications in classical myeloproliferative neoplasms. Semin Thromb Hemost 2013; 39 (1): 101–111. doi: 10.1055/s-0032-1331153.
4. Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology 1965; 15 : 774–784. doi: 10.1212/wnl.15.8. 774.
5. Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007; 6 (7): 611–619. doi: 10.1016/S1474-4422 (07) 70 170-9.
6. Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol. 128 (3): 275–290. doi: 10.1111/j.1365-2141.2004.05277.x.
7. Ylikoski A, Erkinjuntti T, Raininko R et al. White matter hyperintensities on MRI in the neurologically nondiseased elderly: analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995; 26 (7): 1171–1177. doi: 10.1161/01.str.26.7.1171.
8. Price TR, Manolio TA, Kronmal RA et al. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. Stroke 1997; 28 (6): 1158–1164. doi: 10.1161/01.str.28.6.1 158.
9. Schmidt R, Schmidt H, Pichler M et al. C-reactive protein, carotid atherosclerosis, and cerebral small-vessel disease. Results of the Austrian Stroke Prevention Study. Stroke 2006; 37 (12): 2910–2916. doi: 10.1161/01.STR.0000248768.40043.f9.
10. Mathiesen EB, Waterloo K, Joakimsen O et al. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromsø study. Neurology 2004; 62 (5): 695–701. doi: 10.1212/01.wnl.0000113759.80 877.1f.
11. Hougaku H, Matsumoto M, Kitagawa K et al. Silent cerebral infarction as a form of hypertensive target organ damage in the brain. Hypertension 1992; 20 (6): 816–820. doi: 10.1161/01.hyp.20.6.816.
12. Kamide K, Raguki H, Nakano N et al. Insulin re-sistance is related to silent cerebral infarction in pa - tients with essential hypertension. Am J Hypertens 1997; 10 (11): 1245–1249. doi: 10.1016/s0895-7061 (97) 00 282-3.
13. Sugiyama T, Lee JD, Shimizu H et al. Influence of treated blood pressure on progression of silent cerebral infarction. J Hypertens 1999; 17 (5): 679–684. doi: 10.1097/00004872-199917050-00012.
14. Kario K, Pickering TG, Hoshide S et al. Morning blood pressure surge and hypertensive cerebrovascular disease: role of the a-adrenergic sympathetic nervous system. Am J Hypertens 2004; 17 (8): 668–675. doi: 10.1016/j.amjhyper.2004.04.001.
15. Kario K, Pickering TG, Umeda Y et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107 (10): 1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa.
16. Kato T, Inoue T, Yamagishi S et al. Low-density lipoprotein subfractions and the prevalence of silent lacunar infarction in subjects with essential hypertension. Hypertens Res 2006; 29 (5): 303–307. doi: 10.1291/hypres.29.303.
17. Longstreth WT Jr, Bernick C, Manolio TA et al. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 1998; 55 (9): 1217–1225. doi: 10.1001/archneur.55.9.1217.
18. Vermeer SE, den Heijer T, Koudstaal PJ et al. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan study. Stroke 2003; 34 (2): 392–396. doi: 10.1161/01.str.0000052631.98 405.15.
19. Morrison AC, Fornage M, Liao D et al. Parental history of stroke predicts subclinical but not clinical stroke: the Atherosclerosis Risk in Communities Study. Stroke 2000; 31 (9): 2098–2102. doi: 10.1161/01.str.31.9. 2098.
20. Zerjavic K, Zagradisnik B, Stangler Herodez S et al. Is the JAK2 V617F mutation a hallmark for different forms of thrombosis? Acta Haematol 2010; 124 (1): 49–56. doi: 10.1159/000314645.
21. Weston H, Bird R, Griffiths V et al. JAK2 V617F Mutation status predicts thrombotic complications, including arterial thrombosis, in patients with essential thrombocytosis (ET). Blood 2007; 110 (11): 2551. doi: 10.1182/blood.V110.11.2551.2551.
22. Larsen TS, Pallisgaard N, Mølller MB et al. High prevalence of arterial thrombosis in JAK2 mutated essential thrombocythaemia: Independence of the V617F allele burden. Hematology 2008; 13 (2): 71–76. doi: 10.1179/102453308X315960.
23. Lowe GD, Fowkes FG, Dawes J et al. Blood viscosity, fibrinogen, and activation of coagulation and leukocytes in peripheral arterial disease and the normal population in the Edinburgh Artery Study. Circulation 1993; 87 (6): 1915–1920. doi: 10.1161/01.cir.87.6. 1915.
24. Gori T. Viscosity, platelet activation, and hematocrit: progress in understanding their relationship with clinical and subclinical vascular disease. Clin Hemorheol Microcirc 2011; 49 (1–4): 37–42. doi: 10.3233/CH-2011-1455.
25. Ezekowitz MD. Prevention of stroke. Am J Geriatr Cardiol 1998; 7 (5): 42–46.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2021 Issue 4
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Editorial
- Why do the nerve tracts decussate? Basic principles of the vertebrate brain organization
- The role of microRNAs in pathogenesis of spinal muscular atrophy
- New possibilities of laboratory diagnostics of diseases associated with amyloid formation
- Use of corneal confocal microscopy in neurological disorders
- COVID-19 related olfactory impairment – diagnostics, significance and treatment
- Study protocol – robot-assisted gait therapy using Lokomat Pro FreeD in patients in the subacute phase of ischemic stroke
- Validation of the Multiple Sclerosis Walking Scale-12 – Czech version
- COVID-19 in patients with myasthenia gravis
- CANVAS – a newly identified genetic cause of late-onset ataxia. Description of the first cases in the Czech Republic
- COVID-19 associated myelitis – a case report of rare complication of severe SARS-CoV-2 infection
- Intramedullary abscess
- Informace vedoucího redaktora
- Prof. MUDr. Hana Krejčová, DrSc. 90letá
- Aktualita z kongresu EAN 2021
- Kappa free light chains in multiple sclerosis – diagnostic accuracy and comparison with other markers
- Characterization of swallowing disorders in myasthenia gravis through a fibre-optic endoscopic evaluation
- Standardisation of the Slovenian version of the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-Cog)
- The frequency of silent brain infarcts in polycythaemia vera and essential thrombocytosis
- Benefits of 18F-FET PET in preoperative assessment of glioma heterogeneity demonstrated in two case reports
- Successful usage of rituximab in a patient with overlapping myelin oligodendrocyte glycoprotein encephalomyelitis and systemic lupus erythematosus
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- COVID-19 related olfactory impairment – diagnostics, significance and treatment
- CANVAS – a newly identified genetic cause of late-onset ataxia. Description of the first cases in the Czech Republic
- Why do the nerve tracts decussate? Basic principles of the vertebrate brain organization
- COVID-19 associated myelitis – a case report of rare complication of severe SARS-CoV-2 infection
