A study of the properties of tablets made of directly compressible maltose
Studium vlastností tablet z přímo lisovatelné maltosy
Práce se zabývá studiem pevnosti a doby rozpadu tablet z přímo lisovatelné maltosy Advantosy 100. Je v ní studován rozdíl vlivu dvou typů mazadel stearanu hořečnatého a stearylfumarátu sodného na uvedené vlastnosti, dále jsou testovány směsi látky s mikrokrystalickou celulosou Vivapurem 102 v poměru 1 : 1 a s léčivy kyselinou askorbovou a acetylsalicylovou. Výlisky jsou získány třemi lisovacími silami, s výjimkou směsí s léčivy, kdy je použita jedna lisovací síla. U lisovacích sil 6 a 8 kN nebyl statisticky významný rozdíl v zásahu mazadel do pevnosti výlisků z Advantosy 100, pouze u lisovací síly 10 kN snížil Pruv pevnost více než stearan. Směs Advantosy 100 a Vivapuru 102 poskytovala nejpevnější tablety, přídavek Pruvu u ní snížil pevnost výlisků více než stearan. Doba rozpadu výlisků z Advantosy i směsi suchých pojiv byla delší s přídavkem Pruvu. Výlisky s kyselinou acetylsalicylovou měly vyšší pevnost a delší dobu rozpadu než s kyselinou askorbovou. Mezi hodnotami pevnosti výlisků s kyselinou acetylsalicylovou nebyl statisticky významný rozdíl v rámci typu použitého mazadla, a to v případě Advantosy 100 i její směsi s Vivapurem 102.
Key words:
Advantosa 100 – Vivapur 102 – mazadla – pevnost tablet v tahu – doba rozpadu
Authors:
J. Mužíková; J. Balhárková
Authors‘ workplace:
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Czech Republic, Department of Pharmaceutical Technology
Published in:
Čes. slov. Farm., 2008; 57, 21-27
Category:
Original Articles
Overview
The paper deals with the study of the strength and disintegration time of tablets made of directly compressible maltose Advantose 100. It studies the differences of the effects of two types of lubricants, magnesium stearate and sodium stearylfumarate, on the above-mentioned properties, and it also tests the mixtures of the substance with microcrystalline cellulose Vivapur 102 in a ratio of 1 : 1 and with ascorbic and acetylsalicylic acids. The compacts are obtained by using three compression forces, excepting mixtures with active ingredients, where one compression force is used. In the compression forces of 6 and 8 kN, no statistically significant difference was found in the intervention of the lubricants into the strength of the compacts made of Advantose 100, only in the compression force of 10 kN Pruv decreased the strength more than stearate. The mixture of Advantose 100 and Vivapur 102 yielded the strongest tablets, an addition of Pruv to it decreased the strength of compacts more than stearate. The periods of disintegration time of Advantose compacts as well as those of the mixture of dry binders were longer with an addition of Pruv. The compacts with acetylsalicylic acid possessed higher strength and a longer period of disintegration than those with ascorbic acid. There was no statistically significant difference within the type of the lubricant employed, both in the case of Advantose 100 and its mixture with Vivapur 102, between the values of strength of the compacts with acetylsalicylic acid.
Key words:
Advantose 100 – Vivapur 102 – lubricants – tensile strength of tablets – time of disintegration
Introduction
The most widely used sugars in the role of fillers in direct compression include primarily various derivatives of lactose, modified saccharose, and dextrose 1). Another more recent substance is directly compressible maltose, which is produced under the trademark Advantose™100. Maltose is a disaccharide commonly used in pharmaceutical and food industries. It exists as white crystals or crystalline powder, it is odourless, and tastes sweet. It is dissolved in water, moderately in 95% ethanol. It is produced by enzymatic cleavage of starch. Crystalline maltose serves as an auxiliary substance for direct compression of both chewing and normal tablets 2). Its spray-dried product Advantose 100 has spherical particles with excellent flow properties, it improves the compression properties of materials with lower densities, and it is nonhygroscopic. The excellent tableting properties of Advantose 100 are not markedly affected by an addition of a lubricant or a prolonged period of mixing 1,3).From the viewpoint of humidity loading, stability tests have revealed a higher stability of tablets made of Advantose 100 than of those made of microcrystalline cellulose.Another important property is the tolerance of a higher compression force without capping in the course of the ejection of the tablet from the matrix 3). Crystalline maltose is highly compressible and tablets made with this excipient tend to be non-friable and display excellent disintegration times 4).
The paper aims to study the strength and disintegration time of tablets made of Advantose 100 with various types of lubricants in dependence on compression force, and to test these properties in the presence of the model active ingredients ascorbic and acetylsalicylic acids. The mixtures of Advantose 100 with the microcrystalline cellulose Vivapur 102 in a ratio of 1 : 1 underwent the same evaluation.
EXPERIMENTAL
Materials
Advantose™ 100 – spray-dried maltose (SPI Pharma Group, France);
Vivapur®102 – microcrystalline cellulose (J. Rettenmaier & Söhne GmbH + Co, Germany);
magnesium stearate (Acros Organics, New Jersey, USA);
Pruv® – sodium stearyl fumarate (J. Rettenmaier & Söhne GmbH + Co, Germany);
Acetylsalicylic acid (Schűtz and Co. GmbH, Hamburg, Germany);
ascorbic acid (Northeast General Pharmaceutical Factory, Shenyang, China).
Preparation of tableting materials and tablets
A list of tableting materials evaluated in the study:
- Advantose 100 with 1% magnesium stearate
- Advantose 100 with 1% Pruv
- Advantose 100 and Vivapur 102 1 : 1
- Advantose 100 and Vivapur 102 1 : 1 with 1% magnesium stearate
- Advantose 100 and Vivapur 102 1 : 1 with 1% Pruv
- Advantose 100 with 50% acetylsalicylic acid and with 1% magnesium stearate
- Advantose 100 with 50% acetylsalicylic acid and with 1% Pruv
- Advantose 100 with 50% ascorbic acid and with 1% magnesium stearate
- Advantose 100 with 50% ascorbic acid and with 1% Pruv
- Advantose 100 and Vivapur 102 1 : 1 with 50% acetylsalicylic acid and with 1% magnesium stearate
- Advantose 100 and Vivapur 102 1 : 1 with 50% acetylsalicylic acid and with 1% Pruv
- Advantose 100 and Vivapur 102 1 : 1 with 50% ascorbic acid and with 1% magnesium stearate
- Advantose 100 and Vivapur 102 1 : 1 with 50% ascorbic acid and with 1% Pruv
The substances were mixed gradually always for 5 minutes in a stainless cube KB 15S (Erweka GmbH, Hausenstamm, Germany). In multicomponent mixtures, the dry binders were mixed first, then the active ingredient, and finally the lubricants sodium stearyl fumarate or magnesium stearate were added.
All tableting materials were used to produce 16 tablets compressed with the use of a special die with an upper and a lower punch on a material testing equipment T1 – FRO 50 TH.A1K Zwick/Roell (Zwick GmbH & Co, Ulm, Germany). The tablets were of a cylindrical shape without facets with a diameter of 13 mm and weight of 0.5 Ī 0.0010 g. Compression velocity was 40 mm/min and compression forces 6, 8 and 10 kN. Mixtures including active ingredients were compressed using only a compression force of 8 kN.
Measurement of tensile strength of tablets and evaluation of the lubricant sensitivity of tableting materials
Tensile strength was always evaluated in 10 tablets, first no sooner than 24 hours after compaction. Measurements were performed on a Schleuniger apparatus (Dr. Schleuniger Pharmatron AG, Solothurn, Switzerland), which measured tablet sizes accurate to 0.01 mm and destruction force in N. Tensile strength of tablets was calculated according to Eq. [1]:
where P is the tensile strength of tablets (MPa), F is the destruction force (N), d is the tablet diameter (mm), and h is the thickness of the tablet (mm) 5).
LSR (lubricant sensitivity ratio) values, which make it possible to quantify and mutually compare the lubricant sensitivity of tableting materials, were calculated according to Eq. [2]:
where Csu is the crushing strength of tablets without an added lubricant and Csl is the crushing strength with a lubricant. The more this value approaches 1, the more the dry binder is sensitive to an added lubricant from the viewpoint of decreased strength of tablets 6). In the present paper, the values of tensile strength, not those of crushing strength, are used in the equation.
Measurements of the disintegration time of tablets
Disintegration times of tablets were evaluated earliest 24 hours after compaction always in 6 tablets. Measurements were performed on an apparatus for the determination of disintegration time following the method of Ph.B. MMV in the medium of purified water tempered to 37 °C Ī 1 °C. The tablet was considered disintegrated at the moment when there was no remainder on the net 7).
The results of strengths and disintegration times were statistically processed by means of the computer programmes Excel and Qcexpert. Elementary data analysis yielded the mean values with standard deviations, which were plotted into dependences on compression force. In the cases of unclear significance of differences in the values, unpaired t-test at a level of significance of 0.05 was employed.
RESULTS AND DISCUSSION
The paper studied the tableting properties of the directly compressible maltose Advantose 100, particularly the evaluation of tensile strength and disintegration time of tablets. The factors of influence included: compression force, addition of two types of lubricants in one concentration, and addition of two types of model active ingredients. The same evaluation was carried out also in the mixtures of Advantose 100 with the microcrystalline cellulose Vivapur 102 in a ratio of 1 : 1. This mixture was compressed also without a lubricant, because it was made possible due to the lubricant effect of microcrystalline cellulose. In addition, also the sensitivity of the mixture of dried binders to the addition of the lubricant, quantitatively expressed as the LSR (lubricant sensitivity ratio) value, was calculated. In Advantose 100 alone, this value was not calculated, because the compacts made of pure maltose cannot be compressed because of high friction, and the literature reports insignificant sensitivity to the addition of the lubricant due to the mechanism of compression caused prevalently by fragmentation of particles 3). The selected compression forces were 6, 8 and 10 kN. These values were selected in order to achieve as much as possible the optimal range of tablet strength (0.56–1.11 MPa) 8) with lubricants in Advantose and to avoid extremely high values of strength in the mixtures with Vivapur. The tableting materials with active ingredients were compacted using only a compression force of 8 kN. The added lubricants were magnesium stearate and sodium stearylfumarate (Pruv) in a concentration of 1%. The tested model active ingredients were ascorbic and acetylsalicylic acid. They were selected with regard to different mechanisms of compacting and different solubility in water.
Figure 1 represents the dependence of tensile strength of tablets on compression force for Advantose 100 with the lubricants magnesium stearate and sodium stearylfumarate. With compression forces of 6 and 8 kN, there is no statistically significant difference between the values, with a compression force of 10 kN a lower value of strength was observed in the compacts with Pruv. The same dependence is represented in Figure 2, but this time it is for the mixture of Advantose 100 and Vivapur 102 in a ratio of 1 : 1. In this case, there is a lower strength in the compacts with Pruv with all compression forces, and a decrease in strength due to an addition of the lubricant is visible, because it was possible to compress also lubricant-free compacts. This decrease is due to a 50% presence of microcrystalline cellulose, which can be plastically deformed, and therefore the film from the lubricant produced on its particles decreases the strength of bonds between the particles 9). Decreased strength is quantitatively expressed by LSR values presented in Table 1. The data showed higher sensitivity of the mixture to an addition of Pruv and the highest value for both lubricants was observed with a compression force of 8 kN. The strengths of compacts from Advantose 100 and its mixtures with Vivapur 102 are summarized for the sake of comparison in Figure 3. Unambiguously the strongest tablets are those made of a mixture of Advantose 100 and Vivapur 102 in a ratio of 1 : 1 without lubricants. In the mixtures with the lubricants, it holds true that the stronger compacts are produced in the case of a mixture of dry binders than in the case of maltose alone. The differences in the values are increased with compression force, so they are the highest with a compression force of 10 kN. The only exception is the result with Pruv with a compression force of 6 kN, where the value of strength was lower in the case of the mixture of dry binders. The strengths of compacts from the individual tableting materials increase with compression force.
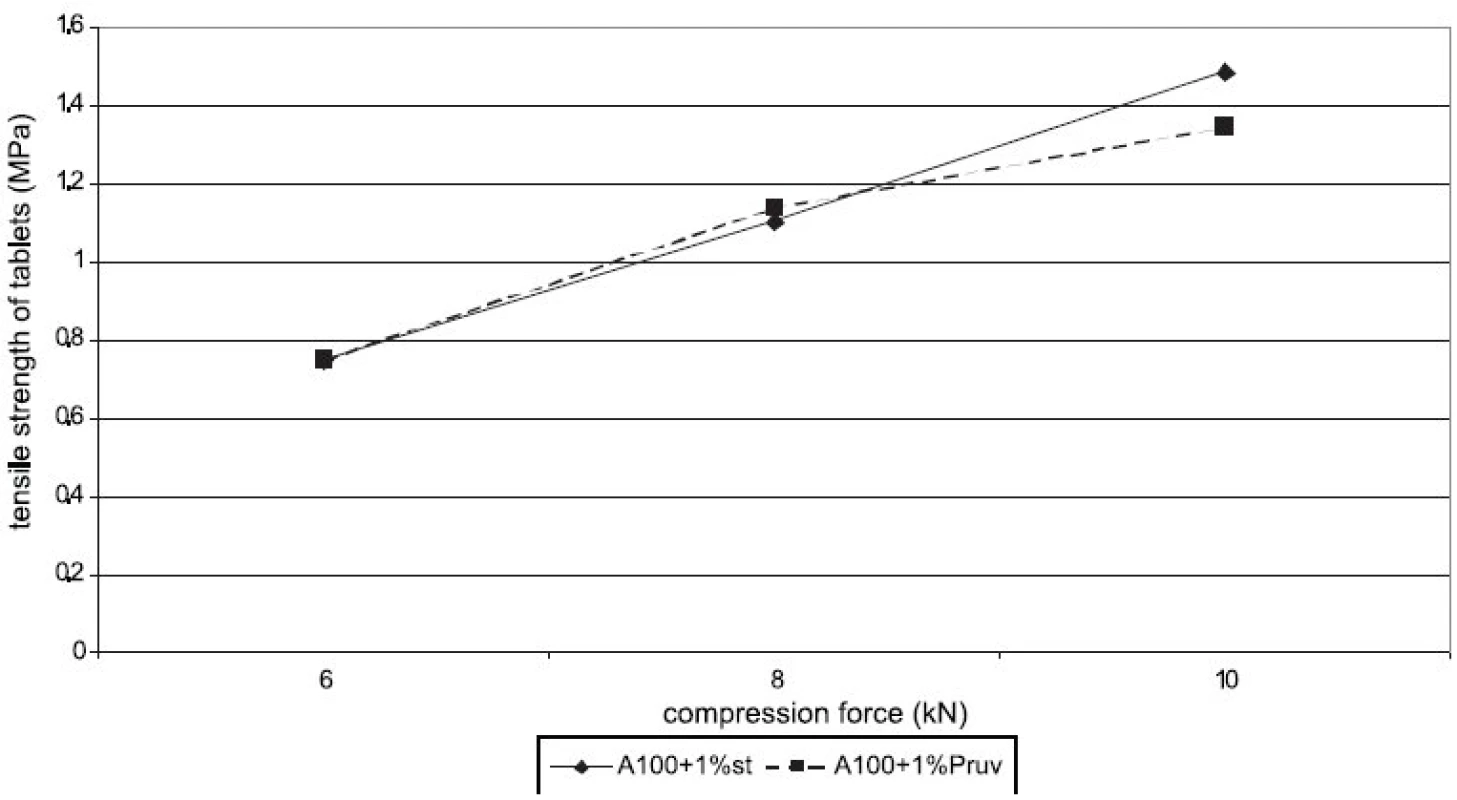
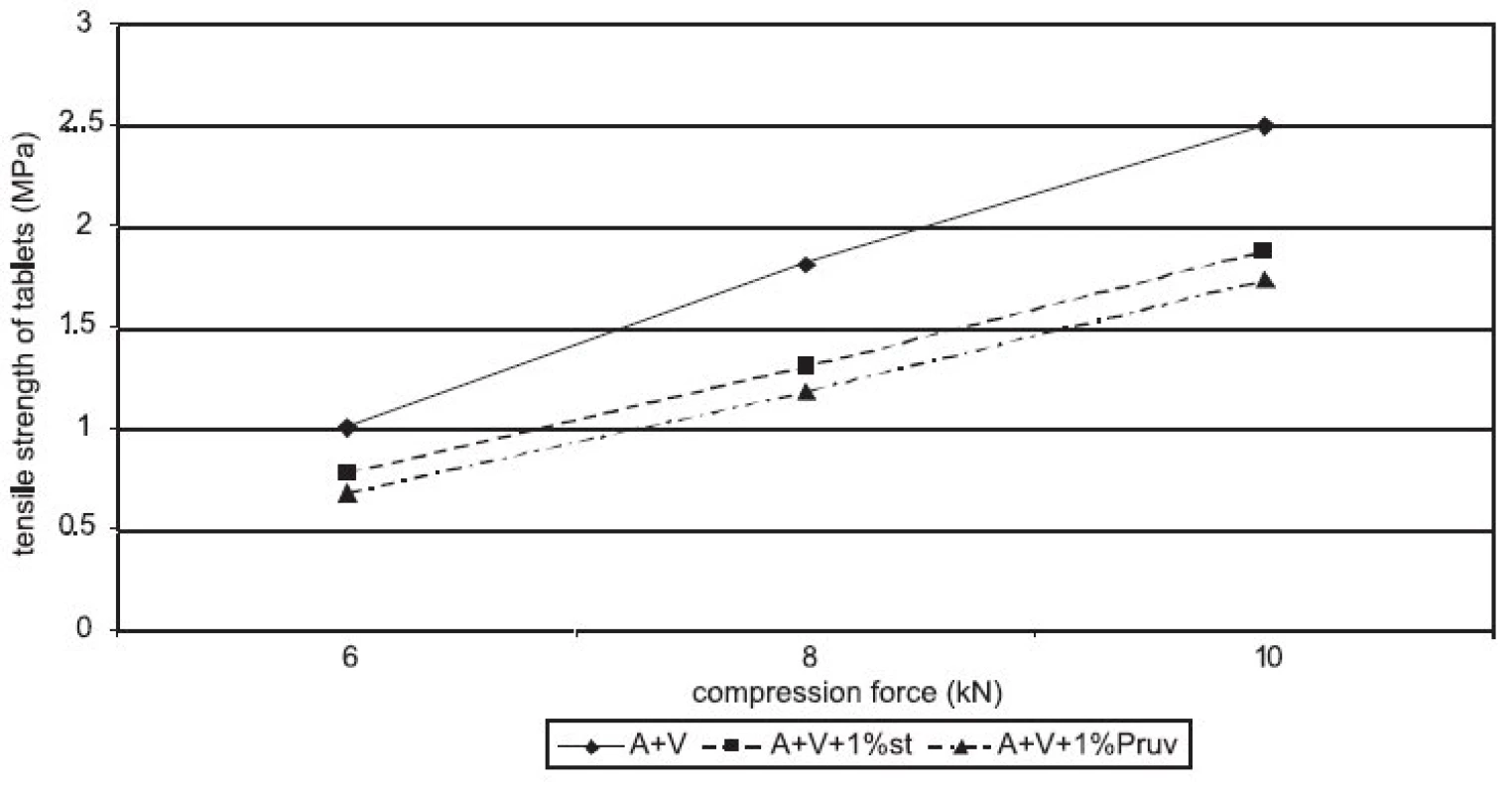

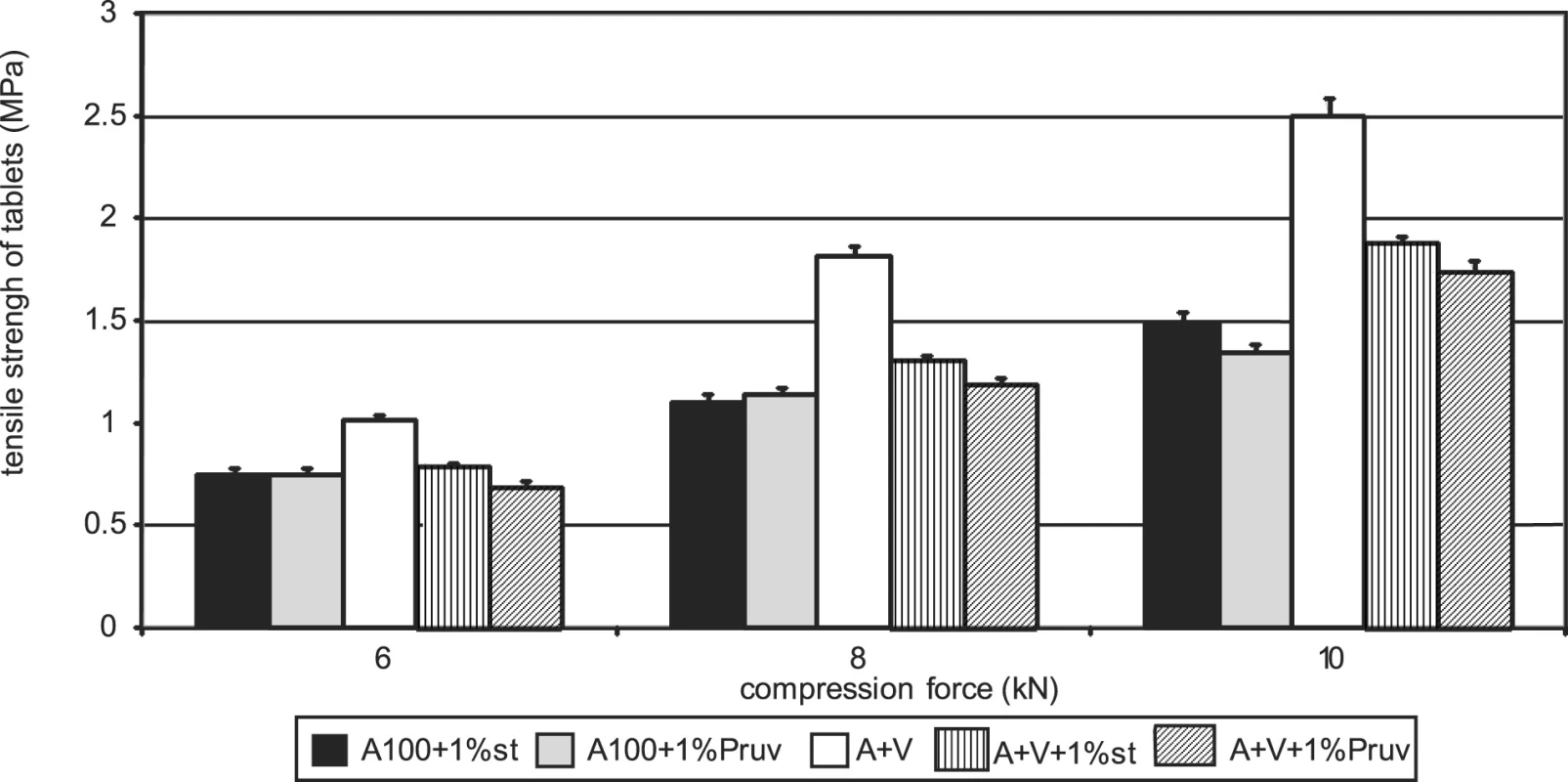
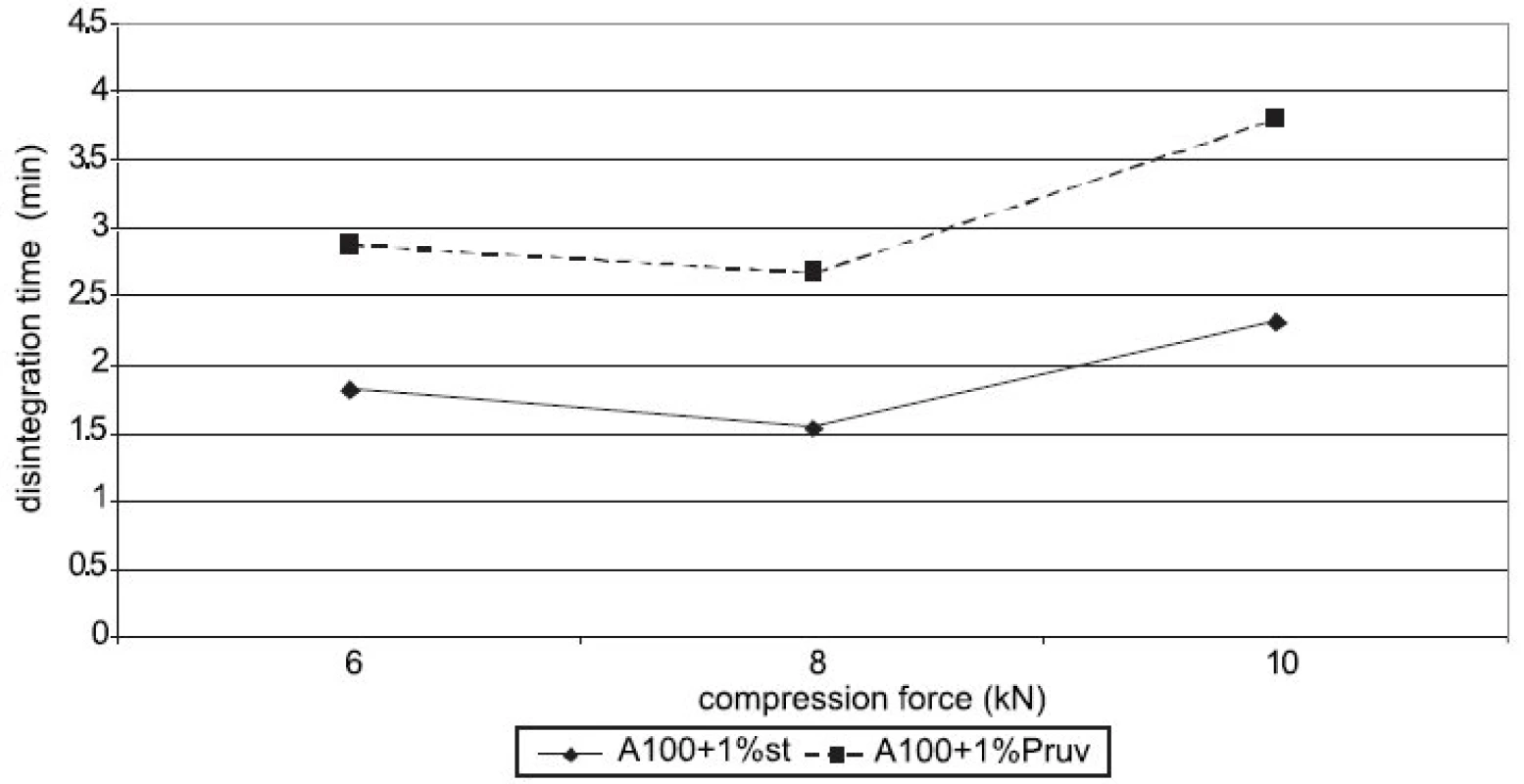
Figure 4 shows the dependence of disintegration time on compression force for Advantose 100 with lubricants. The dependences increase with compression force only between 8 a 10 kN. Disintegration time with Pruv is longer in spite of lower hydrophobicity of the lubricant. The same dependence is shown in Figure 5, but in this case for a mixture of Advantose 100 with Vivapur 102 in a ratio of 1 : 1. In this case the compacts with Pruv possess a longer disintegration time, which increases with compression force, a more marked increase being between 8 and 10 kN. In addition, a comparison is possible with the values of tablets made of a mixture without lubricants, which exert the longest disintegration time. In this case, no role is thus played by hydrophobicity of the lubricant, which would prolong disintegration time, but more probably by a lower strength of bonds between particles in the case of mixtures with lubricants. Within the type of the lubricant, a contrary result would be theoretically expected, both because of higher hydrophobicity of stearate, and because of stronger compacts with stearate. Figure 6 presents next to each other the values of disintegration time from Advantose 100 and its mixture with Vivapur 102. The data show, with compression forces of 6 a 8 kN, the longest disintegration time for Advantose 100 with 1% Pruv. With a compression force of 10 kN it is exceeded by the value of disintegration for a pure mixture of Advantose 100 and Vivapur 102 in a ratio of 1 : 1. In the case of the presence of lubricants, a longer disintegration time of compacts was observed in Advantose 100 alone. In the case of mixtures of dry powders, the disintegrating effect of the microcrystalline cellulose Vivapur 102 is evident.
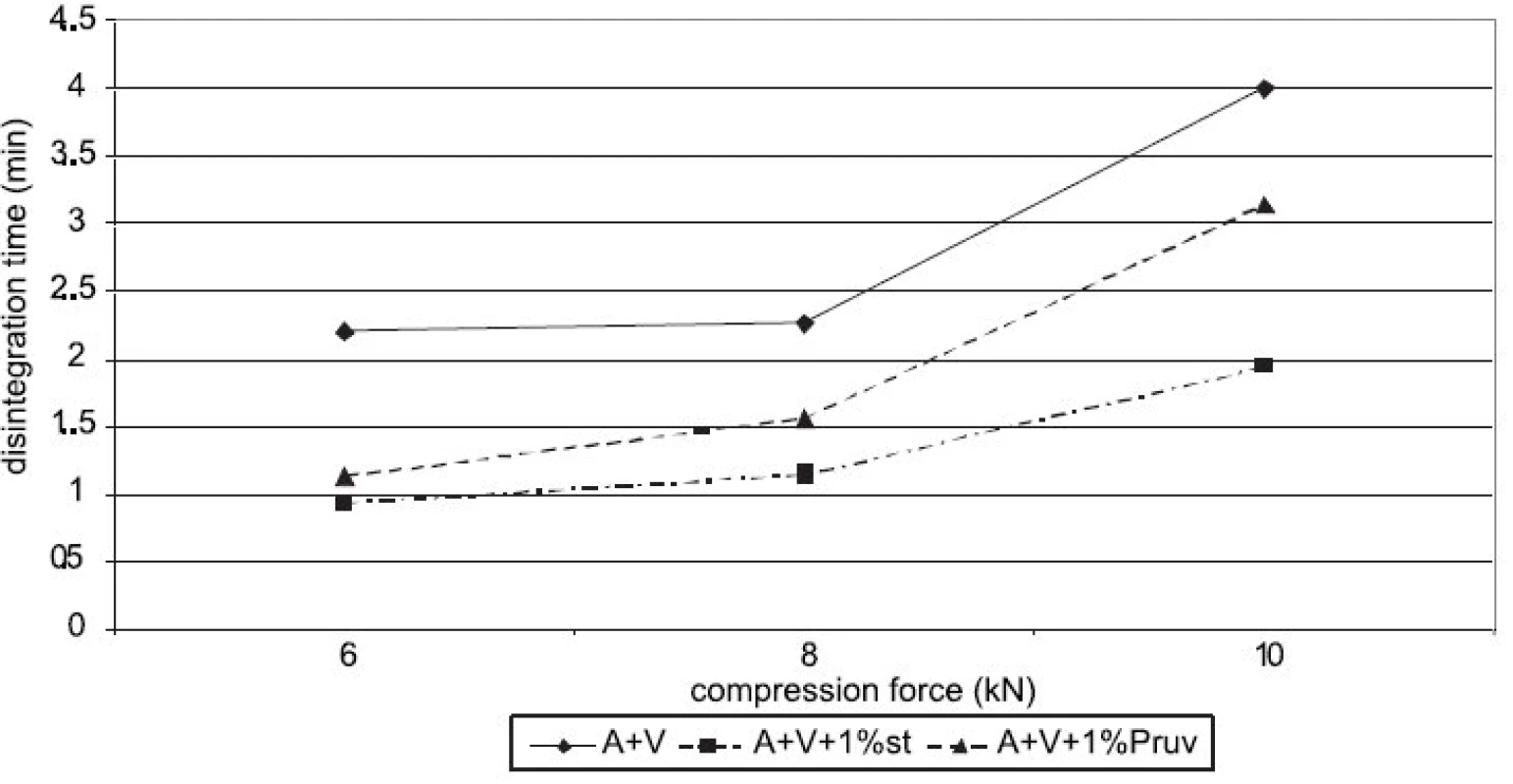
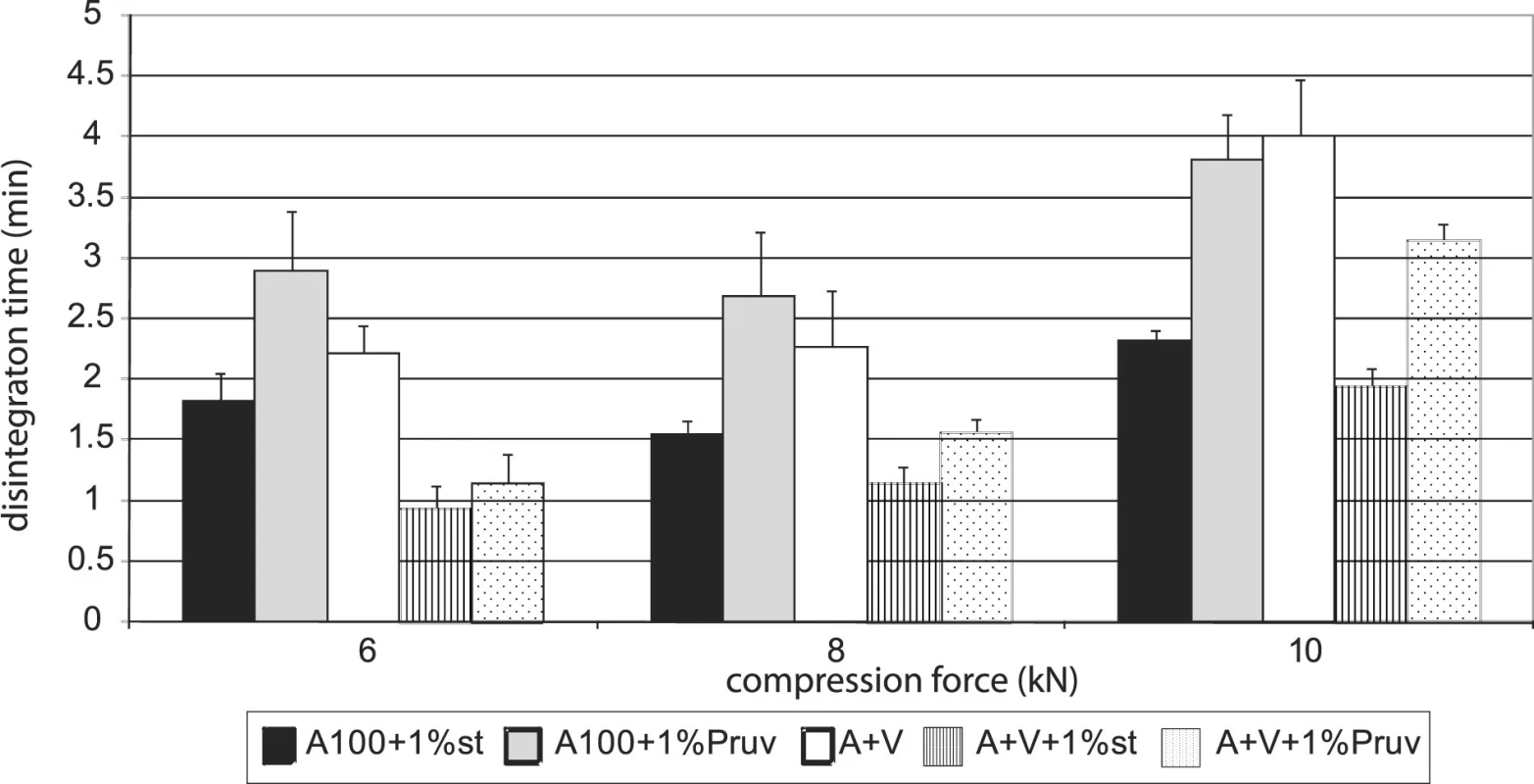
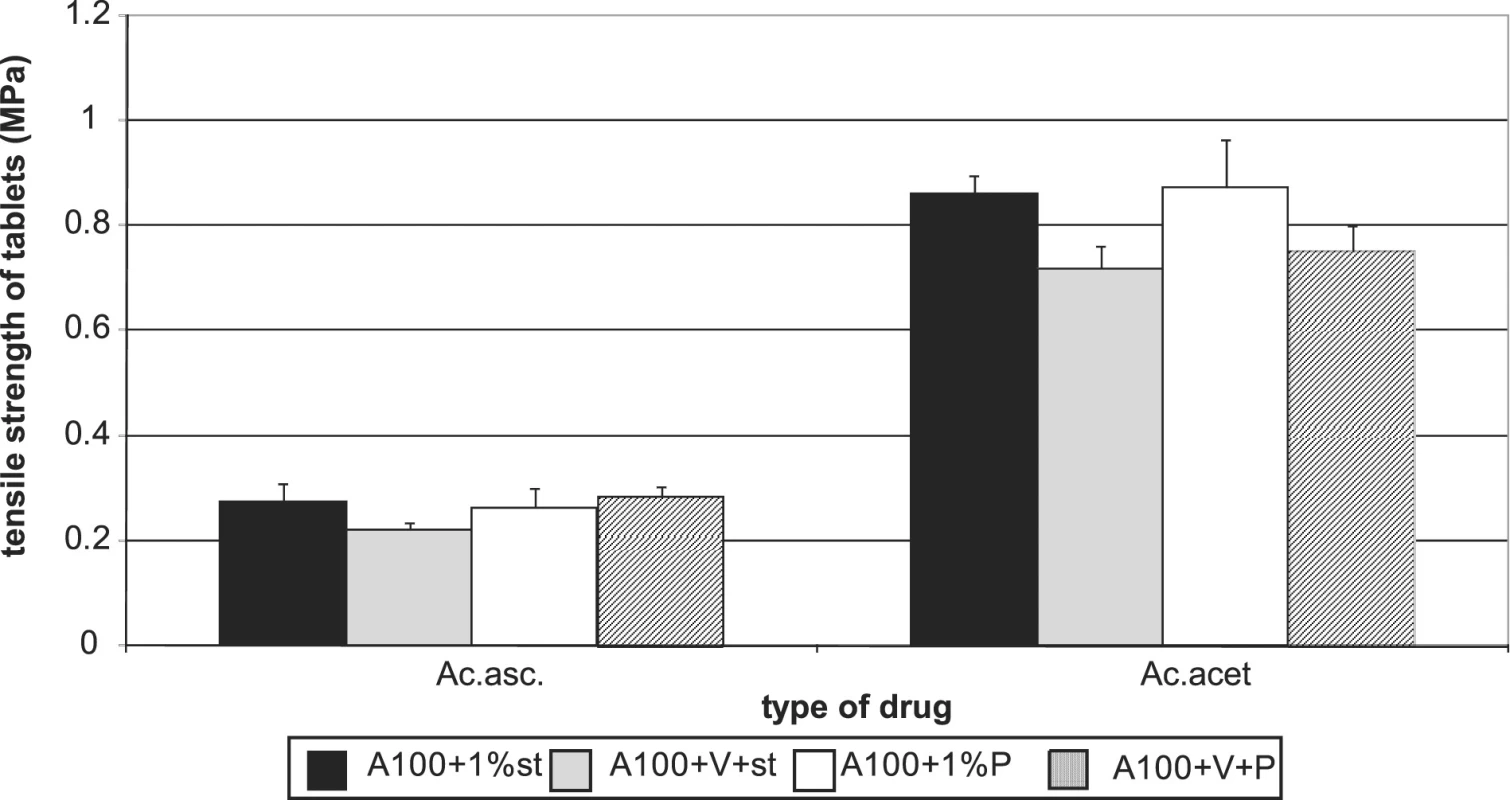
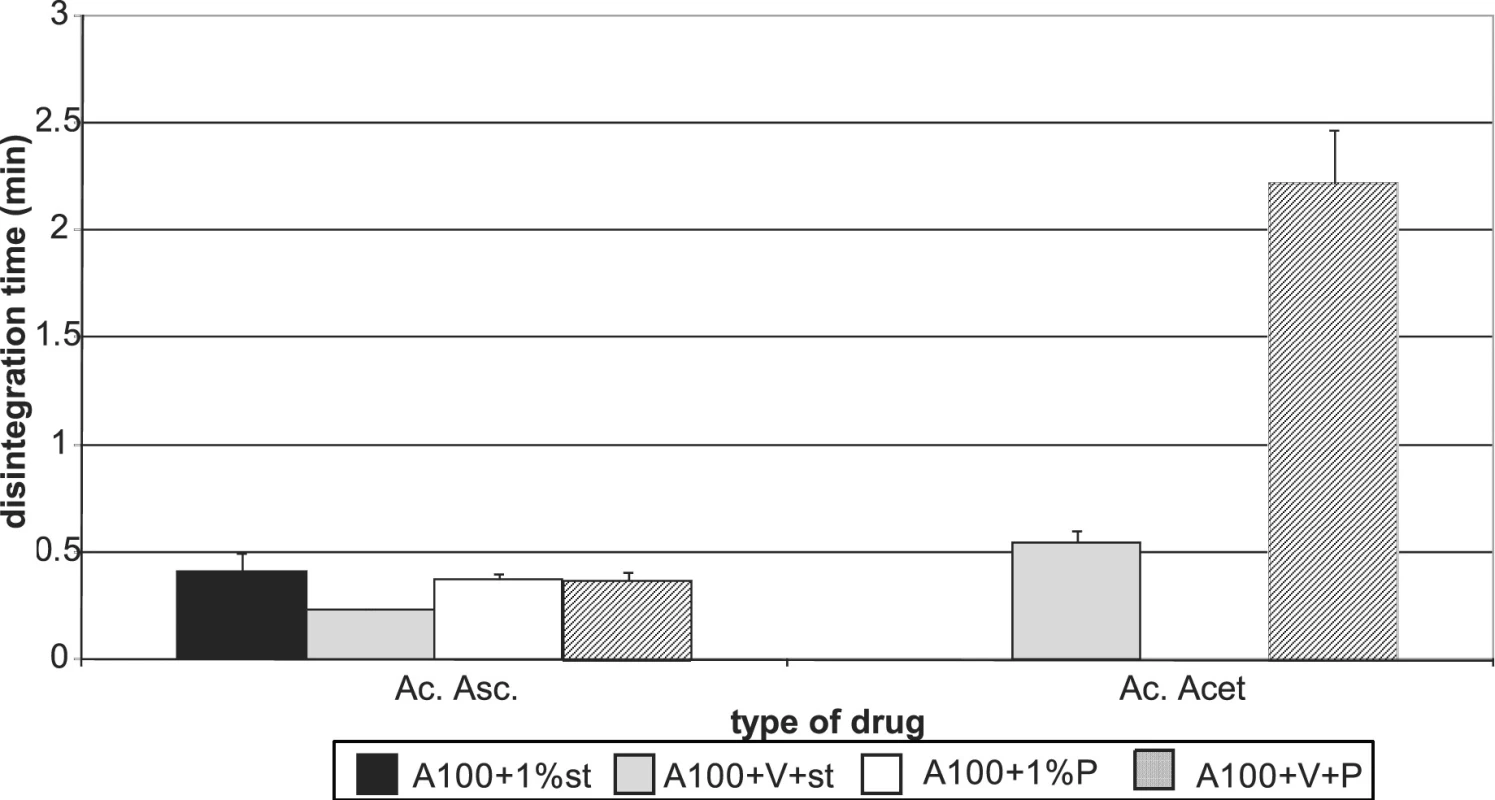
Figures 7 and 8 summarize the results for tableting materials with active ingredients. Figure 7 presents the values of tensile strengths of tablets for a compression force of 8 kN. Unambiguously a higher strength is observed in compacts containing acetylsalicylic acid. This active ingredient can be relatively well compacted and is compacted mainly by plastic deformation; in ascorbic acid the mechanism of compacting the particles prevails 10). In the case of acetylsalicylic acid, stronger compacts are only those with Advantose 100. There is no statistically significant difference within the type of the employed lubricant either, which holds true also for the mixture of dry binders Advantose 100 and Vivapur 102. The strength of compacts with acetylsalicylic acid varies within the range of the optimal strength of tablets 8). Compacts with ascorbic acid posses a much lower strength. It holds true for Advantose 100 that there is no statistically significant difference between the values of strength within the type of the employed lubricant and the strength of compacts with stearate is higher than in the mixture of dry binders. The insignificance of the difference within the type of the employed lubricant does not hold true for the mixture of dry binders, as Pruv yields stronger compacts than stearate. Fig. 8 shows the values of disintegration times of tableting materials excepting the values for Advantose 100 with acetylsalicylic acid and lubricants, as these values are extremely higher and other values would be badly recognizable. For Advantose 100 with acetylsalicylic acid, the value of disintegration times of compacts with stearate was 20.01 min and with Pruv even 43.55 min. The high values follow from bad solubility of acetylsalicylic acid in water; the higher value with Pruv is not quite clear, as Pruv is less hydrophobic than stearate and the compacts posses nearly the identical strength. In the case of the mixture of Advantose 100 with Vivapur 102, in this active ingredient there is a marked decrease in the values of disintegration times, which is most probably due to the disintegrating capacity of microcrystalline cellulose. In the case of ascorbic acid, the disintegration times are very short and there are no statistically significant differences between the values for Advantose 100 with stearate, Pruv and the mixture of dry binders with Pruv. These facts are due to good solubility of ascorbic acid in water and low strength of compacts, and there is no statistically significant difference between the values of strength of compacts for the above-mentioned tableting materials either.
The study was supported by the grant MSM 0021620822 and by the firms SPI Pharma group and J. Rettenmaier & Söhne GmbH + Co, which supplied the samples of the dry binders tested.
Received: 6. December 2007
Accepted: 20. December 2007
PharmDr. Jitka Mužíková, PhD.
Department of Pharmaceutical Technology, Charles University in Prague, Faculty of Pharmacy
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: muzikova@faf.cuni.cz
Sources
1. Bolhuis, G. K., Armstrong, N. A.: Pharm. Dev. Technol., 2006; 11, 111–124.
2. Kibbe, H. A.: Handbook of pharmaceutical excipients. Third ed., American Pharmaceutical Association and the Pharmaceutical Press, Washington, London, 2000, 175–177.
3. SPI Pharma group: Advantose™ 100 Maltose Powder for Direct Compression. Firm. Lit., http://www.spipharma. com/ProductsFolder/104Maltose/104Maltose100.html, April 2007.
4. Bowe, K. E., Bilig, J. L., Schwartz, J. B. et al.: Pharm. Technol. Eur., 1998; 10,5, 34–40.
5. Fell, J. T., Newton, J. M.: J. Pharm. Sci., 1970; 59, 5, 688–691.
6. Bos, C. E., Bolhuis, H., Van Doorne, Lerk, C. F.: Pharm. Weekbl.,1987; Sci. Ed. 9, 274–282.
7. Ph.B. MMV, Grada Publishing, a.s., Prague, 2005, vol. I, s. 269.
8. Belousov, V. A.: Khim. Farm. Zh., 1976; 10, 105–111.
9. Jarosz, P. J., Parrott, E. L.: Drug Dev. Ind. Pharm., 1984; 10(2), 259–273.
10. Bolhuis, G. K.; Chowhan, Z. T.: In: Pharmaceutical Powder Compaction Technology; (Alderborn, G.; Nyström, Ch.), New York – Basel – Hongkong, Marcel Dekker 1996, 491–493.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2008 Issue 1
-
All articles in this issue
- Antioxidant activity of tinctures prepared from hawthorn fruits and motherwort herb
- Signal pathways of cell proliferation and death as targets of potential chemotherapeutics
- Hemostatic effects of oxidized cellulose
- Substances Modifying the Activity of Caspases
- Effects of auxins on growth and scopoletin accumulation in cell suspension cultures of Angelica archangelica L.
- A study of the properties of tablets made of directly compressible maltose
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Signal pathways of cell proliferation and death as targets of potential chemotherapeutics
- Hemostatic effects of oxidized cellulose
- Substances Modifying the Activity of Caspases
- Antioxidant activity of tinctures prepared from hawthorn fruits and motherwort herb


