Effects of auxins on growth and scopoletin accumulation in cell suspension cultures of Angelica archangelica L.
Vliv auxinů na růst a akumulaci skopoletinu v suspenzní kultuře Angelica archangelica L.
Skopoletin je kumarin s mnoha zajímavými biologickými účinky, např. spazmolytickým, protizánětlivým, antimutagenním, antioxidačním, antifungálním, indukujícím apoptózu, antiproliferativním, inhibujícím acetylcholinesterázu, hypourikemickým. Rostlinné explantátové kultury představují nadějný alternativní zdroj cenných rostlinných látek. Buněčný růst a biosyntézu sekundárních metabolitů v rostlinných explantátových kulturách ovlivňuje řada fyzikálních a chemických faktorů. Mechanismus jejich působení není zcela znám. Rostlinné růstové regulátory a světelné podmínky hrají, vedle dalších faktorů, důležitou roli. Byl testován účinek čtyř auxinů (kyselina 2,4-dichlorfenoxyoctová, 2,4-D, α-naftyloctová, NAA, β‑indolyloctová, IAA nebo β-indolylmáselná, IBA) ve čtyřech koncentracích (0,2; 2; 10 nebo 20 mg/l) na růst kultury a akumulaci skopoletinu v médiu v suspenzních kulturách Angelica archangelica kultivovaných za stálého osvětlení nebo ve tmě. Nejvyšší růst kultury byl dosažen s 2 mg/l 2,4-D a 10 mg/l IAA. Nejvyšší hladiny skopoletinu byly získány s 0,2 mg/l 2,4‑D, 2 mg/l 2,4-D, 10 mg/l NAA a 20 mg/l IAA. Růst i akumulace scopoletinu v suspenzních kulturách Angelica archangelica byly světelnými podmínkami ovlivněny méně výrazně než působením auxinů a jejich koncentrací. Změny vyvolané auxiny byly modifikovány světelnými podmínkami.
Klíčová slova:
Angelica archangelica L. – suspenzní kultura – růst – skopoletin – kumariny – auxiny – světelné podmínky – průtoková injekční analýza
Authors:
T. Siatka; M. Kašparová
Authors‘ workplace:
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Czech Republic, Department of Pharmacognosy
Published in:
Čes. slov. Farm., 2008; 57, 17-20
Category:
Original Articles
Overview
Scopoletin is a coumarin possessing many interesting biological effects, e.g., spasmolytic, anti-inflammatory, antimutagenic, antioxidant, antifungal, apoptosis-inducing, antiproliferative, acetylcholinesterase-inhibitory, and hypouricemic activities. Plant tissue cultures represent a promising alternative source of valuable plant-derived substances. A number of physical and chemical factors influence the cell growth and secondary metabolite biosynthesis in plant tissue cultures. The mechanism of their action is not completely understood. Besides other factors, plant growth regulators and light conditions play an important role. Effects of four auxins (2,4-dichlorophenoxyacetic acid, 2,4-D, α‑naphthaleneacetic acid, NAA, β-indoleacetic acid, IAA or β-indolebutyric acid, IBA) at four concentrations (0.2, 2, 10 or 20 mg/l) on the culture growth and accumulation of scopoletin in the medium were tested in Angelica archangelica cell suspension cultures cultured under continuous light or in the dark. The highest culture growth was achieved with 2 mg/l 2,4-D, and 10 mg/l IAA. The best scopoletin levels were obtained with 0.2 mg/l 2,4‑D, 2 mg/l 2,4-D, 10 mg/l NAA, and 20 mg/l IAA. The effects of light conditions were less marked than those of auxins and their concentrations in influencing both the cell growth and scopoletin accumulation in Angelica archangelica cell suspension cultures. The changes brought about by auxins were modified by light conditions.
Key words:
Angelica archangelica L. – cell suspension cultures – growth – scopoletin – coumarins – auxins – light conditions – flow injection analysis
Introduction
Plants are a source of many commercially important secondary metabolites, which are usually obtained by direct extraction from plants grown in natural habitats. Plant tissue cultures represent a promising alternative to agricultural processes for producing valuable plant-derived substances 1). From the industrial point of view, the production of natural products by culturing plant cells in vitro, instead of from crude drugs, would offer several advantages, such as independence of climatic factors and political circumstances, continuous supply of material for extraction, and the possibility of regulating the procedure so that the desired compound is obtained as the principal metabolite. Plant cell cultures, to few exceptions, have not yet become of importance for the production of pharmacologically active natural products. This is mainly due to low yields of the desired substances. The reason for the low concentrations of secondary metabolites in cultures, compared with the concentrations of the same compounds in fully developed plants, is not completely understood. 2, 3) The optimum conditions for the growth and metabolite production differ for each plant culture. Therefore, it is necessary to optimize them in each one experimentally.
Scopoletin is a coumarin showing many interesting biological effects. It possesses, for example, spasmolytic 4), anti-inflammatory 5), antimutagenic 6), antioxidant 7, 8), antifungal 9, 10), apoptosis-inducing 11), antiproliferative 12), acetyl cholinesterase-inhibitory 13), and hypouricemic 14) activities.
The authors investigated the effects of auxins in Angelica archangelica cell suspension cultures. We have previously reported on the influence of auxins on the contents of coumarins in cultured cells (they were determined as the sum of fluorescent compounds which are three: scopoletin, scopolin, and the third substance, preliminarily identified as another glycoside of scopoletin) 15). The present communication deals with the cell growth and accumulation of scopoletin in the culture medium (where scopoletin is the only coumarin) in the same angelica cell cultures.
Material and methods
Chemicals
2,4-dichlorophenoxyacetic acid, α-naphthaleneacetic acid, β-indoleacetic acid, β-indolebutyric acid and 6 benzylaminopurine (Sigma, St. Louis, U.S.A.); scopoletin (Fluka, Buchs, Germany); sodium phosphate dibasic and potassium phosphate monobasic (Lachema, Brno, Czech Republic).
Instruments
A PS 20A autoclave (Chirana, Brno, Czech Republic); a roller (Vývojové dílny, Academy of Sciences of the Czech Republic, Praha, CR); a LGA 05 lyophilizer (Janetzki, Leipzig, Germany); a 200S analytical scale (Sartorius, Göttingen, Germany); a single-channel plunger LPC 3001 micropump, a PP05 peristaltic pump for sample delivery, and a TZ 4620 chart recorder (Laboratorní přístroje, Praha, CR); a home-made PTFE valve with exchangeable sample loops; and a Schoeffel FS 970 fluorescence detector (McPherson, Chelmsford, U.S.A.).
Cell suspension cultures
Tissue cultures of Angelica archangelica were derived from a bud of an in spring sprouting one-year old plant grown in the Botanical Garden of Faculty of Pharmacy in Hradec Králové. Callus cultures were established from bud meristem and maintained by subculturing every five weeks on Murashige and Skoog medium 16) supplemented with 2 mg/l 2,4-dichlorophenoxyacetic acid, 0.4 mg/l benzylaminopurine, 30 g/l sucrose, and 8 g/l agar. The medium pH was adjusted to 5.7 before autoclaving at 121 °C for 15 min. Cell suspension cultures were initiated from friable calluses in the same medium devoid of agar. They were agitated in 250 ml flasks containing 30 ml of the medium on a roller apparatus at 8 rpm, incubated at 25 °C under continuous light (3.500 lux) or in darkness, and subcultured every two weeks. The culture used in this work was kept in vitro for two years.
For testing the effects of various auxins, the cell suspension cultures were cultured in Murashige and Skoog media supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D), α-naphthaleneacetic acid (NAA), β-indoleacetic acid (IAA) or β-indolebutyric acid (IBA) at the concentrations of 0.2, 2, 10 or 20 mg/l. After 14 days, the growth of cultures and accumulation of scopoletin in the medium were evaluated.
Analytical methods
Cultures were harvested 14 days after inoculation into the tested media. Cells were separated from the culture medium by filtration under reduced pressure using a Buchner funnel with filter paper.
Filtered cells were weighed for fresh weight determination, and then freeze-dried to obtain dry weight.
Scopoletin in the culture medium was quantified fluorometrically by flow injection analysis as described in detail previously 17).
All data presented are the mean values of three replicates. Student’s t-test was used for statistical analysis of data, and differences with P < 0.05 were considered as statistically significant.
Results and discussion
A number of physical and chemical factors influence the growth and biosynthesis of secondary metabolites in plant tissue cultures 3). Mechanisms of their action are still far from clear. The optimum conditions are different for each plant culture. As for constituents of the medium, growth regulators, especially auxins, exert a great influence on the culture growth as well as the production of secondary metabolites 18 – 20). It is important to select the most appropriate auxins and to determine their optimal concentrations. Light conditions may also play an important role 21 – 23).
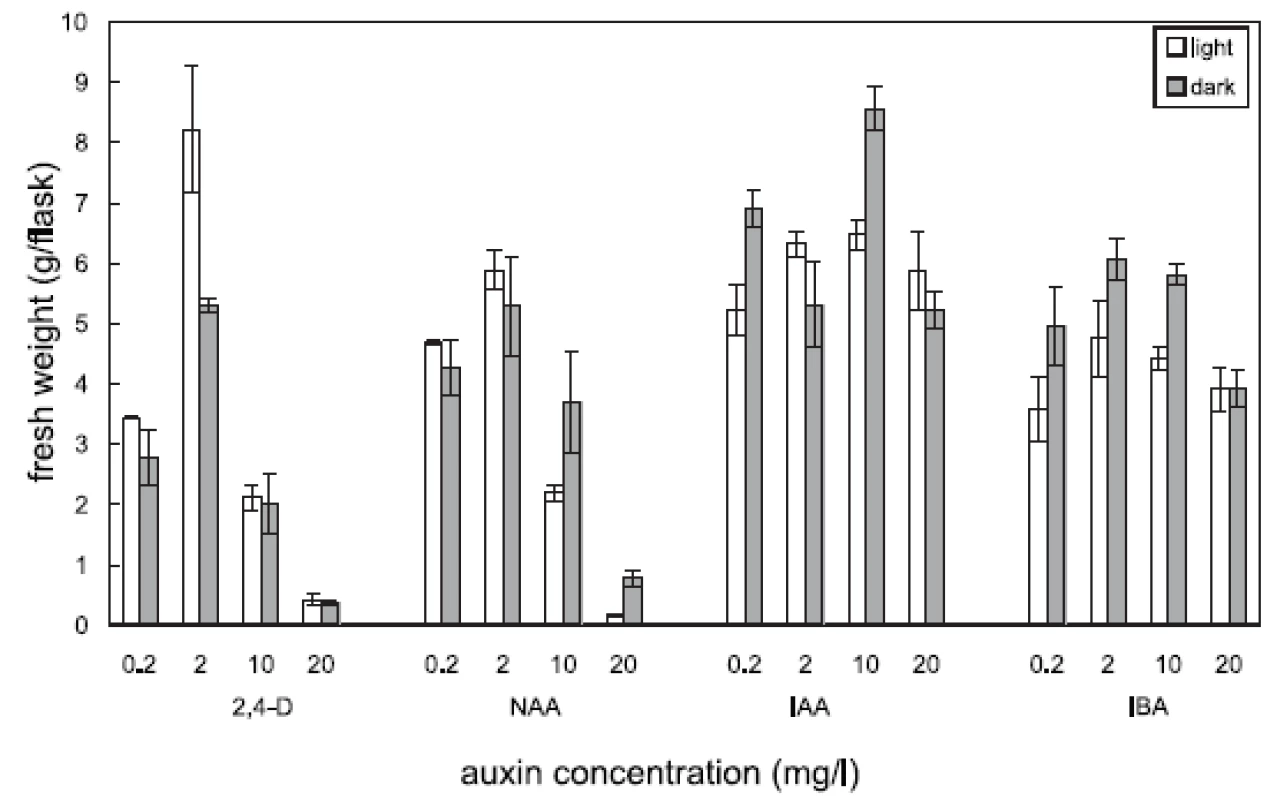
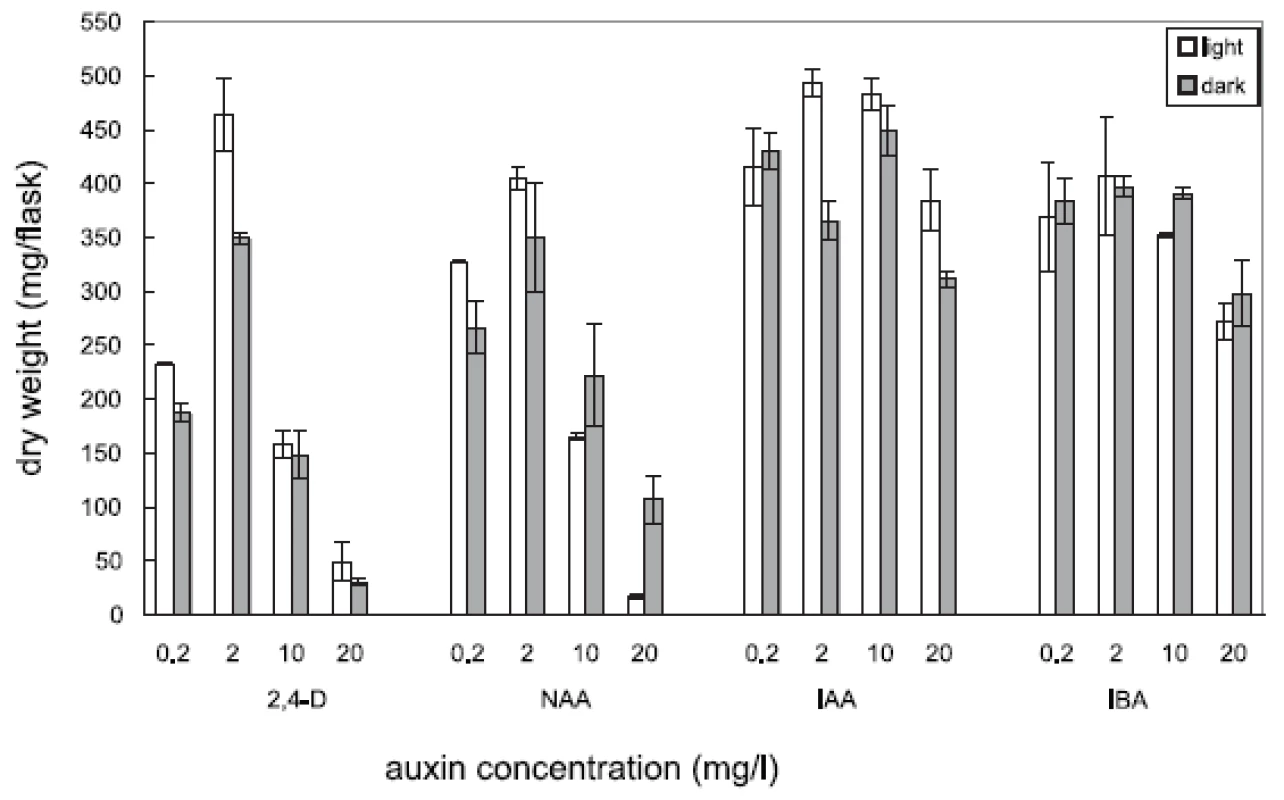
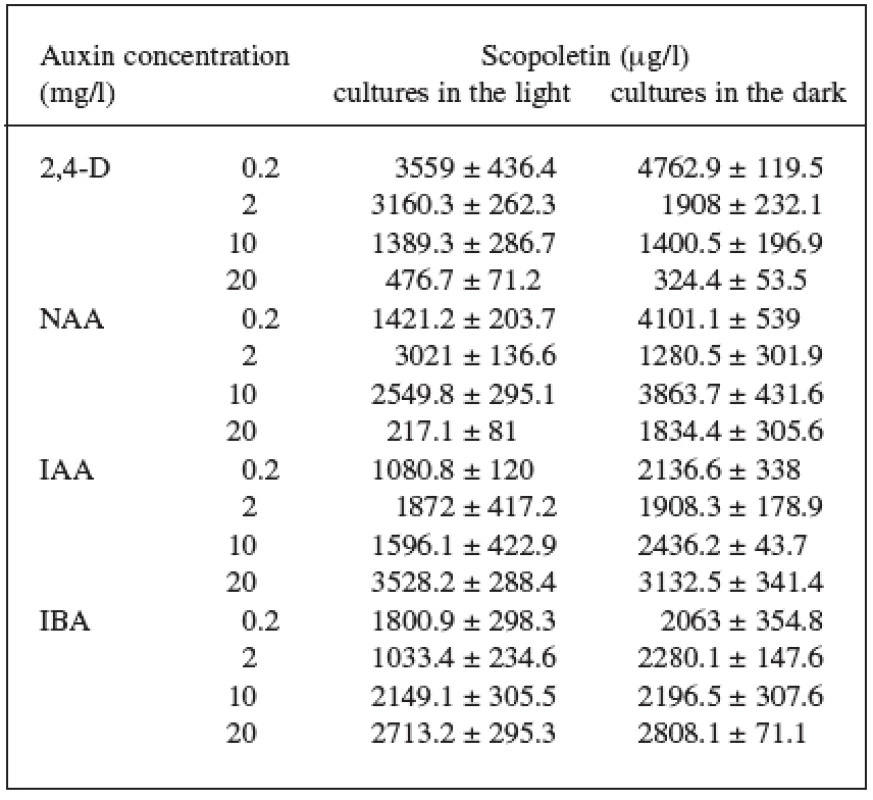
Four auxins (2,4-dichlorophenoxyacetic acid, 2,4-D, α-naphthaleneacetic acid, NAA, β-indoleacetic acid, IAA, or β-indolebutyric acid, IBA) at four different concentrations were tested in Angelica archangelica cell suspension cultures. The cultures were cultured under continuous light or in the dark. The effects of auxins on the growth of angelica suspension cultures are shown in Figure 1 (fresh biomass) and Figure 2 (dry biomass). The highest culture growth was achieved with 2 mg/l 2,4-D and 10 mg/l IAA. The inhibition of the growth was observed in cultures with 2,4-D and NAA at a concentration of 20 mg/l. IAA and IBA were favourable for the cell proliferation in all the concentrations used. The results of scopoletin accumulation in the medium are shown in the Table 1. The increasing concentrations of 2,4-D lowered scopoletin amounts, whereas those of IAA or IBA enhanced accumulation of scopoletin. Similar findings regarding effects of 2,4-D on scopoletin accumulation in the medium have been reported in tobacco cell suspension cultures 24, 25). The best scopoletin levels in angelica cultures were obtained with 0.2 mg/l 2,4-D, 2 mg/l 2,4-D, 10 mg/l NAA, and 20 mg/l IAA. In addition, some differences in the culture growth as well as scopoletin amounts were observed between cultures in the light and in the dark. However, the choice of light conditions seems to be less important than that of an auxin and its concentration for influencing both the cell growth and scopoletin accumulation in cell suspension cultures of Angelica archangelica.
This work was supported by grant No MSM 0021620822 of the Ministry of Education, Youth and Sports of the Czech Republic.
Received: 5. December 2007
Accepted: 17. December 2007
Corresponding author:
PharmDr. Tomáš Siatka, CSc.
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmacognosy
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: siatka@faf.cuni.cz
Sources
1. Srivastava, S., Srivastava, A. K.: Crit. Rev. Biotechnol., 2007; 27, 29.
2. Roberts, S. C.: Nat. Chem. Biol., 2007; 3, 387.
3. Samuelsson, G.: Drugs of natural origin, 4th ed., Swedish Pharmaceutical Press, Stockholm 1999, p. 61.
4. Oliveira, E. J. et al.: Planta Medica, 2001; 67, 605.
5. Moon, P.-D. et al.: Eur. J. Pharmacol., 2007; 555, 218.
6. Schimmer, O., Eschelbach, H.: Pharmazie, 1997; 52, 476.
7. Ng, T. B. et al.: Compar. Biochem. Physiol C: Toxicol. Pharmacol., 2003; 136, 109.
8. Thuong, P. T. et al.: Biol. Pharm. Bull., 2005; 28, 1095.
9. Prats, E. et al.: Euphytica, 2006; 147, 451.
10. Carpinella, M. C, Ferrayoli, C. G., Palacios, S. M.: J. Agric. Food Chem., 2005; 53, 2922.
11. Kim, E.-K. et al.: Life Sci., 2005; 77, 824.
12. Arcos, M. L. B. et al.: Phytother. Res., 2006; 20, 34.
13. Rollinger, J. M. et al.: J. Med. Chem., 2004; 47, 6248.
14. Ding, Z., Dai, Y., Wang, Z.: Planta Medica, 2005; 71, 183.
15. Siatka, T., Kašparová, M.: Čes. slov. Farm., 2003; 52, 186.
16. Murashige, T., Skoog, F.: Physiol. Plant., 1962; 15, 473.
17. Siatka, T., Solich, P., Kotyk, R.: Pharmazie, 1998; 53, 273.
18. Wu, C.-H. et al.: J. Plant Biol., 2006; 49, 193.
19. Jeong, G.-T, Woo, J-C., Park, D.-H.: Biotechnol. Bioprocess Eng., 2007; 12, 86.
20. Biondi, S. et al.: Plant Biosyst., 2004; 138, 117.
21. Jacques, P. et al.: Acta Bot. Gall., 2007; 154, 21.
22. Walker, T. S., Bais, H. P., Vivanco, J. M.: Phytochemistry, 2002; 60, 289.
23. Pasqua, G. et al.: Plant Physiol. Biochem., 2005; 43, 293.
24. Taguchi, G et al.: Plant Sci, 2000; 151, 153.
25. Taguchi, G et al.: Plant Sci, 2001; 160, 905.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2008 Issue 1
-
All articles in this issue
- Antioxidant activity of tinctures prepared from hawthorn fruits and motherwort herb
- Signal pathways of cell proliferation and death as targets of potential chemotherapeutics
- Hemostatic effects of oxidized cellulose
- Substances Modifying the Activity of Caspases
- Effects of auxins on growth and scopoletin accumulation in cell suspension cultures of Angelica archangelica L.
- A study of the properties of tablets made of directly compressible maltose
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Signal pathways of cell proliferation and death as targets of potential chemotherapeutics
- Hemostatic effects of oxidized cellulose
- Substances Modifying the Activity of Caspases
- Antioxidant activity of tinctures prepared from hawthorn fruits and motherwort herb
