The effect of Crataegus extract on heart mitochondrial function
Vliv hlohového extraktu na funkci mitochondrií srdce
Cílem práce bylo sledovat in vitro vliv různých koncentrací extraktu z hlohových plodů (70 ng/ml – 13,9 μg/ml fenolických sloučenin (FS)) na oxidativní fosforylaci v izolovaných mitochodriích srdce potkanů. Respirační frekvence mitochodriií byla stanovena oxygraficky pomocí elektrodového systému typu Clark s použitím různých substrátů: pyruvát + malát, sukcinát a palmitoyl-L-karnitin. Z našich výsledků vyplývá, že hlohový extrakt v dávkách obsahujících méně než 278 ng/ml, FS nemá žádný efekt na funkci mitochondrií. Při koncentracích FS 278 ng/ml – 6,95 μg/ml stimuloval extrakt respirační frekvenci v 2. metabolickém stavu o 11–27 % ve všech použitých substrátech. Nejvyšší v našem experimentu použitá koncentrace hlohového extraktu (13,9 μg/ml FS) zvyšovala respirační frekvenci v 2. metabolickém stavu o 27–34 % a výrazně snižovala (12–19 %) maximální respirační frekvenci mitochodrií stimulovanou ADP, což je způsobeno inhibicí dýchacího řetězce mitochondrií.
Klíčová slova:
extrakt z hlohových plodů – mitochodrie srdce – oxidativní fosforylace – flavonoidy
Authors:
D. Majienė 1,2; J. Bernatonienė 1; R. Masteiková 3; K. Dvořáčková 3
Authors‘ workplace:
Department of Drug Technology and Pharmaceutical Management, Faculty of Pharmacy, Kaunas University of Medicine, Lithuania
; Institute for Biomedical Research, Kaunas University of Medicine, Lithuania
2; University of Veterinary and Pharmaceutical Sciences Brno, Faculty of Pharmacy, Department of Pharmaceutics, Czech Republic
3
Published in:
Čes. slov. Farm., 2008; 57, 239-243
Category:
Original Articles
Overview
This study was aimed to investigate in vitro the influence of different concentrations of Crataegus fruit extract (70 ng/ml – 13.9 μg/ml of phenolic compounds (PC)) on oxidative phosphorylation in isolated rat heart mitochondria. The mitochondrial respiratory rates were determined oxygraphically by means of Clark-type electrode system with different substrates: pyruvate + malate, succinate and palmitoyl-L-carnitine. Our results demonstrate that Crataegus extract at doses under to 278 ng/ml of PC had no effect on mitochondrial functions. At concentrations of 278 ng/ml – 6.95 μg/ml of PC, extract stimulated the State 2 respiration rate by 11–27% with all used substrates. The highest Crataegus extract concentration used in our investigations (13.9 μg/ml of PC) increased the State 2 respiration rate by 27–34% and significantly reduced (12–19%) the maximal ADP-stimulated mitochondrial respiration rate due to the inhibition of mitochondrial respiratory chain.
Key words:
Crataegus fruit extract – heart mitochondria – oxidative phosphorylation – flavonoids
Introduction
Cardiovascular system diseases remain the principal cause of mortality in both developed and developing countries. In the cardiac muscle cells, mitochondria make up 35% of the cell volume and produce 95% of energy necessary for the action of the heart, so their role in the cell is obvious. Damage to mitochondrial structure and function and their failure to supply the cell with a necessary amount of energy can be a decisive factor in the development of myocardium function disorders. Therefore, the number of scientific research studies on the effects of various medicinal preparations on the mitochondrial structure and function are increasing in the world.
The range of effective plant products for the target phytotherapy of cardiovascular system diseases is not wide. Crataegus monogyna Jacq.(Hawthorn) extract is among the most popular herbal medicinal products that have been used around the world since ancient times 1, 2). Nowadays, extracts of Crataegus fruits, leaves and flowers are used as a potential natural remedy in the prevention and treatment of various cardiovascular diseases (hypertension, arrhythmias, congestive heart failure, coronary artery disease, myocardial weakness, etc.) due to positive inotropic and negative chronotropic effects, reduction in coronary spasm, blood pressure and total plasma cholesterol, antioxidative and antiinflammatory properties 3–5). Such wide pharmacological effects are conditioned by active substances in the extract, consisting of flavonoids (hyperoside, vitexin, rutin, quercetin, quercitrin, procyanidins), triterpenes (ursolic, oleanolic, and crataegolic acids), phenolic acids (chlorogenic, caffeic acids), amines, etc. 6).
Despite a number of preclinical and clinical studies aimed at the clarification of the positive effect, the mechanisms of the protective effects of Crataegus extract remain poorly understood. Moreover, there are no findings on its action on energetic metabolism of the cardiac muscle cells, which is essentially determined by the activity of mitochondria, ensuring the main physiological function of cardiac muscles, i.e. continuous mechanical contraction.
Therefore, the aim of this study was to investigate in vitro the influence of Crataegus fruit extract (CE) on rat heart mitochondrial oxidative phosphorylation. In addition to that, we also investigated the effects of the main biologically active compounds of this extract on rat heart mitochondrial respiration.
Experimental
Preparation of Crataegus extract and quantitative analysis
The dry fruits of Crataegus monogyna Jacq. were extracted (1 : 10) with 70% ethanol, the particle size was 2–3 mm, the production method was percolation, and the flow speed of the extract was 0.5 ml/min 7).
The content of total phenolic compounds in CE was determined spectrophotometrically (Hitachi 557) using the Folin-Ciocalteu method. Appropriate dilutions of CE were oxidized with Folin-Ciocalteu reagent and then the reaction was neutralized with sodium carbonate. The color was developed for 2 hours at room temperature, the suspension was centrifuged at 5000 rpm, and the absorption of the obtained solution was measured at 760 nm wavelength. The measurement was compared to a standard curve of prepared gallic acid solution. The results are reported at Gallic Acid Equivalent.
HPLC analysis was carried out using the Waters 2690 Alliance HPLC system (Waters Corporation, Milford, MA, USA), equipped with a Waters 2487 UV/Vis detector, an on-line degasser and an auto sampler, and a Waters XTerra RP18 150 × 3.9 mm column. UV detection was achieved at 360 nm on the detector. The chromatographic elution was accomplished by a gradient solvent system consisting of water containing 0.1% TFA (A) and acetonitrile containing 0.1% TFA (B). The gradient conditions were: 0 min, 95% A, 5% B; 45 min, 55% A, 55% B, kept to 50 min; 55 min, 95% A, 5% B at the flow rate of 400 μl/min. The injection volume was 10 μl. Buffer solutions were filtered through a 0.2 μm disposable membrane filter (Roth, Karlsruhe, Germany) and degassed prior to use. Data were collected and analyzed using the Waters Millennium 2000® chromatographic manager system. The eluted constituents were identified by comparison of the retention time. Regression of the calibration curves of the reference standards is linear, correlation coefficient (R2) of all curves > 0.9999, and the resolution (R ) of standards peaks > 1.5.
Isolation of rat heart mitochondria
Male Wistar rats weighing 250–300 g were used for the study. The animals were killed according to the rules defined by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes (License No. 0006). The hearts of rats were excised and rinsed in an ice-cold 0.9% KCl solution. Heart mitochondria were isolated in the medium containing 220 mM mannitol, 70 mM sucrose, 5 mM (N-tris[Hydroxymethyl]methyl-2-aminoethane-sulfonic acid and 0.5mM EGTA (pH 7.4, adjusted with Trizma base; 2°C) and 2 mg/ml bovine serum albumin (BSA; fraction V, A4503, Sigma). The homogenate was centrifuged at 750 × g for 5 min, then the supernatant was recentrifuged at 10 000 × g for 10 min and the pellet was washed once (10 min 10 000 × g) in the isolation medium without BSA, suspended in it and kept on ice. The mitochondrial protein concentration was determined by the biuret method using BSA as standard.
Measurement of mitochondrial respiration and membrane potential
Oxygen uptake rates were recorded at 37 oC by means of the Clark-type electrode system in a solution containing 20 mM imidazole, 20 mM taurine, 0.5 mM dithiothreitol, 1.6 mM MgCl2, 100 mM MES, 3 mM KH2PO4, 3.0 mM CaK2EGTA, and 7.1 mM K2EGTA (free Ca2+ concentration – 0.1 μM) (pH 7.1 adjusted with KOH at 37 oC) separately (1) with 6 mM pyruvate + 6 mM malate, (2) 12 mM succinate (+2 mM amytal), and (3) 9 μM palmitoyl-L-carnitine + 0.24 mM malate as substrates. The solubility of oxygen was estimated to be 422 nmolO/ml. Mitochondrial respiration rates were expressed as nmolO/min/mg protein. The final mitochondrial protein concentration in all experiments was 0.5 mg/ml.
Mitochondrial membrane potential was measured in a closed, stirred and thermostatically controlled 1.5 ml vessel fitted with a tetraphenylphosphonium (TPP+)-selective electrode using a TPP+-binding correction factor of 0.162 (μl/mg) 8). The binding correction factor was determined from the ratio of Rb+ to TPP+ accumulation as a function of mitochondrial volume. The experiments were performed at 37 °C using 6 mM pyruvate + 6 mM malate as oxidizable substrate, and 0.5mg/ml mitochondria in the same medium as mitochondrial respiration.
Statistical analysis
Data are presented as means Ī S.E.M. Nonparametric methods were applied for making inferences about the data. Differences between mean values in dependent groups were tested using Wilcoxon matched pairs test. Differences between mean values in independent groups were tested using nonparametric Kruskal-Wallis test with Dunns post-hoc evaluation. P < 0.05 was taken as the level of significance. Statistical analysis was performed by using the software package Statistica 1999, 5.5 StatSoft Inc., USA.
RESULTS
In this study we used Crataegus extract (CE) which contains 139 Ī 8 mg/100 ml of total phenolic compounds (PC). Using the HPLC analysis method, in our investigated CE we identified several classes of flavonoids: flavones (quercetin – 1.26 Ī 0.1 μg/ml, hyperoside – 29.32 Ī 0.5 μg/ml, rutin – 2.64 Ī 0.2 μg/ml, quercitrin – 0.49 Ī 0.01 μg/ml), flavonols (epicatechin – 44.92 Ī 0.9 μg/ml, procyanidin B2 – 0.71 Ī 0.06 μg/ml), and phenolic acid – chlorogenic acid – 3.46 Ī 0.03 μg/ml.
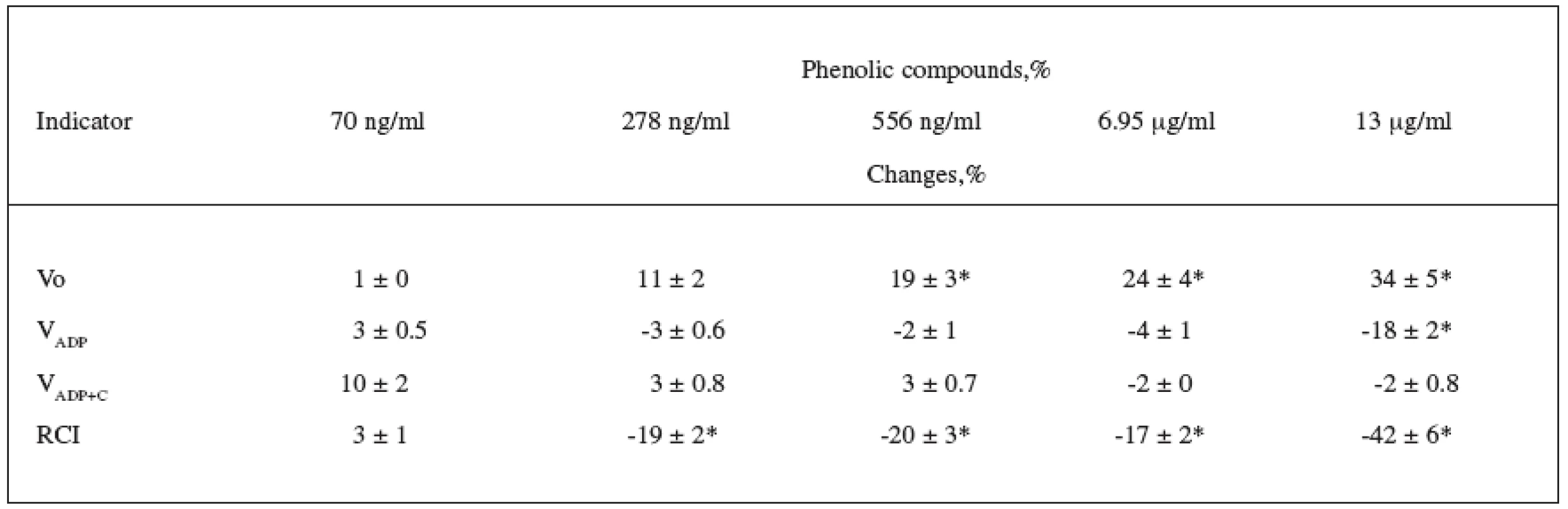
The effect of CE on mitochondrial structure and function was determined using isolated rat heart mitochondria. At the beginning we measured the mitochondrial respiration rate in State 2 (Vo). After the addition of ADP, maximal respiration in State 3 (VADP) was recorded. Cytochrome c – a component of the respiratory chain – is easily lost by mitochondria when their external membranes are damaged, and therefore the measurement of the respiration rate with cytochrome c (VADP+C) allows for determining the intactness of the outer mitochondrial membrane (OMM). ATR is ADP/ATP-translocator inhibitor; it was used for the measurement of State 4 respiration rate (VATR); in addition to that, changes in Vo and VATR show changes in the permeability of the inner mitochondrial membrane. We calculated the respiratory control index (RCI), i.e. the ratio between State 3 and State 2 respiration rates (VADP/Vo), which allows for determining the effectiveness of ADP phosphorylation in mitochondria.
In the first series of experiments, we investigated the effect of various concentrations of CE (70 ng/ml – 13.9 μg/ml of PC) on mitochondrial respiration with complex–I dependent substrates pyruvate + malate. The percentage change in the mitochondrial respiration parameters is presented in Table 1. Our findings showed that CE at the lowest investigated concentration (70 ng/ml of PC) had no effect on any mitochondrial respiratory parameters. Higher concentrations of CE (278 ng/ml – 6.95 μg/ml of PC) increased Vo (11–24%) but did not affect any other mitochondrial respiration rates (VADP, VADP+C). The highest CE concentration used in our experiments (13.9 μg/ml of PC) induced a 34% increase in State 2 respiration rate and decreased State 3 respiration rate by 18%, which was not restored by exogenous cytochrome c. This shows that the respiration rate at 13.9 μg/ml of PC decreased not due to damage of OMM but due to changes in the activity of the mitochondrial respiratory chain complexes.
In the next series of studies, we investigated the effect of CE on the oxidation of FAD-specific substrate succinate and the main respiratory substrate of the heart palmitoyl-L-carnitine (Fig. 1A,B). Vo at the concentration CE 70 ng/ml of PC did not differ from the control. At higher CE concentrations (6.95 and 13.9 μg/ml of PC), Vo increased by 27% and 30% with succinate, and by 14% and 27% – with palmitoyl-L-carnitine. At the highest used in our experiments concentration of CE (13.9 μg/ml of PC), the maximal respiration rate decreased by 12% and 19% with substrate succinate and palmitoyl-L-carnitine, respectively. All concentrations of CE used in our experiments did not change VADP+C, which indicates that the intactness of the OMM remained unchanged.

DISCUSSION
The highest CE concentration used in our investigations (13.9 μg/ml of PC) not only increased the respiration rate in State 2, but also reduced the maximal ADP-stimulated mitochondrial respiration rate and the CCCP-uncoupled respiration rate. Thus, this clearly demonstrates that CE at the concentration of 13.9 μg/ml of PC and higher suppresses mitochondrial respiratory chain. Although we did not find any literature data provided by other authors on the effect of Crataegus extracts on the activity of mitochondrial respiratory chain complexes, there have been studies on the effect of separate flavonoids of Crataegus extracts on the components of the mitochondrial respiratory chain. It has been found that calchones, flavones, and flavonols at higher investigated concentrations (100 μM) inhibit mitochondrial succinooxidase, NADH oxidase, and ATPases, and can have pro-oxidant effect 9).
One of the main functions of mitochondria is to supply a cell with energy, which is required for maintaining the regular functioning of heart. Therefore damage to these organelles may be the key factor for myocardial dysfunction. For this reason, the role of mitochondria in physiological and pathological conditions and the effect of various medical preparations on the mitochondrial processes are intensively investigated. In this study we prepared the ethanolic extract from Crataegus monogyna fruit and determined it’s effect on the isolated rat heart mitochondrial function. Our investigations showed that Crataegus extract at concentrations under to 278 ng/ml of PC had no effect on the mitochondrial respiration, but at concentrations of 278 ng/ml – 6.95 μg/ml of PC, CE stimulated the State 2 respiration rate by 11–27% with all used substrates. Thus, it indicates that Crataegus monogyna extract possesses a slight uncoupling effect in isolated cardiac mitochondria. This effect was dose-dependent; however it was practically independent of the respiratory substrate. It is interesting to note that the Crataegus monogyna induced uncoupling was clearly lower than that induced by Ginkgo biloba extract, as has been shown by our previous study. Some of biologically active compounds identified in Ginkgo biloba extract were also flavonoids like hyperoside, quercetine, rutin and quercitrin, but they had an uncoupling effect even at the lower concentrations (in ng range) 10). It is well known that “mild” uncoupling of mitochondria is involved in the cellular defense system against oxidative stress 11, 12), because CE at concentrations of 278 ng/ml – 6.95 μg/ml of PC partially separate oxidation from phosphorylation, and thus we hypothesize that this mitochondrial uncoupling could reduce the generation of free radicals within mitochondria and could play an important role in cardioprotection. The free radical-scavenging and antioxidant activity of plant flavonoids appears to be efficient due to some structural aspects (OH groups in the B ring, C2-C3 double bound, and 4-oxo group in the C ring) 13), although it may be that in vivo they also reduce ROS generation by modifie functions. Thirupurasundari et al. showed that oral pretreatment of rats with the alcoholic extract of Crataegus oxycantha at a dosage of 0.5 ml/100 g body weight/day for 30 days maintained mitochondrial antioxidant status, prevented mitochondrial lipid peroxidative damage and a decrease in Krebs cycle enzymes induced by isoproterenol in the rat heart 14).
The highest CE concentration used in our investigations (13.9 μg/ml of PC) not only increased the respiration rate in State 2, but also slightly reduced the maximal ADP-stimulated mitochondrial respiration rate with all investigated substrates. We assume that the decrease in oxidation rate may be due to the inhibition of mitochondrial respiratory chain complexes. Although we did not find any literature data provided by other authors on the effect of Crataegus extracts on the activity of mitochondrial respiratory chain complexes, there have been studies on the effect of separate flavonoids of Crataegus extracts on the components of the mitochondrial respiratory chain. It has been found that calchones, flavones, and flavonols at higher investigated concentrations (100 μM) inhibit mitochondrial succinooxidase, NADH oxidase, ATPases and can have pro-oxidant effect 9).
Thus, our results indicate that CE at concentrations (278 ng/ml – 6.95 μg/ml of PC) stimulated in a dose-dependent manner the State 2 mitochondrial respiration rate and slightly reduced the membrane potential. We hypothesize that partial mitochondrial uncoupling could reduce the generation of free radicals within mitochondria and could play an important role in cardioprotection via ROS-dependent pathway.
The authors are grateful to the KMU Research Fund for partial financing of the study.
Received 26 September
Accepted 30 October
Adresses for correspondence:
Assoc. Prof. Ruta Masteiková, Ph.D.
Department of Pharmaceutics, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno
Palackého 1/3, 612 42 Brno, Czech Republic
e-mail: masteikovar@vfu.cz
Sources
1. Fong, H. H., Bauman, J. L.: J. Cardiovasc. Nurs., 2002; 16, 1–8.
2. Kocyildiz, Z. C. et al.: Phytother. Res., 2006; 20, 66–70.
3. Chang, W. T. et al.: Am. J. Chin. Med., 2005; 33, 1–10.
4. Pittler, M. H. et al.: Am. J. Med., 2003; 114, 665–674.
5. Rigelsky, J. M., Sweet, B. V.: Am. J. Health Syst. Pharm., 2002; 59, 417–422.
6. Kirakosyan, A. et al.: Physiol. Plant., 2004; 121, 182–186.
7. Bernatoniene, J. et al.: Medicina (Kaunas), 2003; 39, 76–79.
8. Brown, G. C., Brand, M. D.: Biochem. J., 1988; 252, 473–479.
9. Hodnick, W. F. et al.: Biochem. Pharmacol., 1986; 35, 2345–2357.
10. Trumbeckaite, S. et al.: J. Ethnopharmacol., 2007; 111, 512–516.
11. Korshunov, S. S. et al.: FEBS Letters, 1997; 416, 5–18.
12. Starkov, A. A.: Biosci. Rep., 1997; 17, 273–279.
13. Middleton, R. E. et al.: Biochemistry, 2002; 41, 14734–14747.
14. Thirupurasundari, C. J. et al.: Mol. Cell. Biochem., 2006; 292, 59–67.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2008 Issue 6
-
All articles in this issue
- Consumption of hipolipidemics in the Czech Republic in 2000–2007
- Testing the effect of 2’, 3, 4’-trihydroxychalcone in experimental diabetes mellitus: a pilot study
- Determination of metoprolol and its metabolite α-hydroxymetoprolol in serum by HPLC method with fluorescence detection
- Studies of local anesthetics Part 185: Thermodynamic parameters of heptacainium chloride in the solution of NaBr
- Optimization of the extraction method for the determination of methadone and its metabolite EDDP in urine by gas chromatography
- The effect of lipophilic carrier concentration on hydrophilic-lipophilic matrix systems characteristics
- Possible effects on the liberation of alaptid from dermal semisolid preparations
- The effect of Crataegus extract on heart mitochondrial function
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Possible effects on the liberation of alaptid from dermal semisolid preparations
- Studies of local anesthetics Part 185: Thermodynamic parameters of heptacainium chloride in the solution of NaBr
- Optimization of the extraction method for the determination of methadone and its metabolite EDDP in urine by gas chromatography
- The effect of lipophilic carrier concentration on hydrophilic-lipophilic matrix systems characteristics
