Comparison of metoprolol/α-hydroxymetoprolol metabolic ratio after a single dose and in steady state
Porovnanie metabolického pomeru metoprolol/α-hydroxymetoprolol po jednorázovom a opakovanom užití
Metabolický pomer (MR) metoprololu/α-hydroxymetoprololu za 3 hod po užití jednorázovej dávky metoprololu sa používa na stanovenie fenotypu enzýmu cytochrómu P450 2D6. Cieľom tejto práce bolo porovnať metabolickú aktivitu cytochrómu P450 2D6 po prvom a opakovanom užití metoprololu. Do tejto práce bolo zahrnutých 13 pacientov s hypertenziou (7 žien), u ktorých bolo plánované nasadenie beta-blokátora metoprololu. Vek (priemer ± SD) bol 42,8 ± 12,9 rokov, telesná hmotnosť (priemer ± SD) bola 94,0 ± 25,4 kg. Dávka metoprololu bola u každého pacienta zvolená na základe klinického stavu pacienta v deň užitia prvej tablety. Krvné odbery boli urobené po užití prvej dávky metoprololu a najmenej 2 týždne od nasadenia metoprololu k zaručeniu ustáleného stavu (opakované užívanie). Na základe hodnôt MR boli všetci pacienti po jednorázovom i opakovanom užití zaradení do tej istej fenotypovej skupiny. Pozorovali sme tesnú koreláciu medzi MR po jednorázovom a opakovanom užití metoprololu (rs = 0,8418, P = 0,0003). Všetci pacienti boli v oboch odberoch zaradení do rovnakej fenotypovej skupiny, extenzivný metabolizátor i napriek signifikantnému rozdielu medzi MR metoprololu/α-hydroxymetoprololu po jednorázovom a opakovanom užití (medián 0,59 vs. 0,81, P = 0,0266), Využitie MR metoprololu/α‑hydroxymetoprololu u pacientov na dlhodobej terapii metoprololom je vhodné pre stanovenie fenotypu enzýmu cytochrómu P450 2D6.
Kľúčové slová:
cytochróm P450 2D6 – fenotyp – substrátová látka – metoprolol
Authors:
J. Ďuricová; I. Peřinová; I. Kacířová; M. Grundmann
Authors‘ workplace:
Department of Clinical Pharmacology, University Hospital Ostrava, and Faculty of Medicine, University of Ostrava, Czech Republic
Published in:
Čes. slov. Farm., 2010; 59, 222-226
Category:
Original Articles
Overview
Metoprolol/α-hydroxymetoprolol metabolic ratio (MR) 3 hours after a single metoprolol dose is used for cytochrome P450 2D6 phenotyping. The aim was to compare cytochrome P450 2D6 metabolic activity after the first metoprolol dose and in steady state. Thirteen adult hypertensive patients (7 females) in whom an introduction of the beta-blocker metoprolol was indicated were included. Age (mean ± SD) was 42.8 ± 12.9 years, weight (mean ± SD) was 94.0 ± 25.4 kg. Metoprolol dose was chosen based on clinical grounds on the day of metoprolol first ingestion. Blood samples were drawn after the first dose and at least 2 weeks since metoprolol introduction to ensure steady state. The patients were phenotyped as extensive metabolizers in both periods, after metoprolol first ingestion and in steady state. We observed a significant correlation (rs = 0.8418, P = 0.0003) between the metoprolol/α-hydroxymetoprolol MRs. All the patients were phenotyped as extensive metabolizers in both periods, despite statistically significant differences between the median MRs (0.59 versus 0.81, P = 0.0266). The differences were not of such an extent so as to assign subjects to different phenotypic groups. Metoprolol/α-hydroxymetoprolol MR in steady state is an appropriate alternative to metoprolol/α-hydroxymetoprolol MR after a single dose.
Key words:
cytochrome P450 2D6 – phenotype – probe drug – metoprolol
Introduction
The human cytochrome P450 2D6 (P450 2D6) is involved in the oxidative metabolism of about 25 % of all commonly prescribed drugs 1). The enzyme is characterized by a high range of interindividual variability 2). It is highly polymorphic with more than 100 variant alleles described so far 3). However, beside the genetic polymorphism environmental factors may also play important role in metabolic activity 4). Individual P450 2D6 activity can be affected by gender 5), liver diseases 6), pregnancy 7) and nutritional habits 8). Administration or discontinuation of an enzyme inhibitor can significantly change the metabolic activity; a phenotypic shift may even occur 9–11). Thus, the ability to measure the activity of P450 2D6 enzyme is of high significance. Genotyping alone is not sufficient to accurately predict an individual’s actual P450 2D6 activity, phenotyping on the other hand can determine the exact enzymatic activity as it also reflects non-genetic factors. Phenotyping is usually based on single administration of a probe drug, a compound that is predominately or exclusively metabolized by the enzyme. The resulting metabolic activity is determined from the metabolic ratio MR (maternal compound/metabolite) in urine or in blood 12). Bimodal or trimodal distributions of the MR are typically seen in European populations with the poor metabolizer phenotype representing a separate subgroup, which can be clearly defined by an antimode 13). Metoprolol serves as one of the probe drugs with 70–80% of its metabolism mediated by P450 2D6, of which α-hydroxylation is exclusively mediated by P450 2D6 14). The metabolic ratio of metoprolol over its metabolite α-hydroxymetoprolol in plasma 3 hours after a single metoprolol administration has been validated under standard conditions for the measurement of enzyme activity of P450 2D6 in vivo 15). A single dose of metoprolol is usually used for P450 2D6 phenotyping. Use of metoprolol MR in patients routinely treated with metoprolol would eliminate the need for administration of an additional probe drug. The fraction of metoprolol dose available systematically may increase during long-term treatment with metoprolol 16). P450 2D6 has a high affinity and a low capacity and P450 2D6 dependant metabolism could saturate during chronic exposure leading to a reduction of metabolic capacity 17). To our knowledge, no direct comparison of metoprolol/α‑hydroxymetoprolol MR after a single dose and in steady state has been performed. The aim of the present study was to compare P450 2D6 metabolic activity using metoprolol as a probe drug in patients after first metoprolol dose and in metoprolol steady state.
Materials and methods
Thirteen adult patients (7 females) attending outpatient clinic for hypertension treatment at the Department of Clinical Pharmacology, University Hospital Ostrava, were included. Age (mean ± SD) was 42.8 ± 12.9 years, body weight (mean ± SD) was 94.0 ± 25.4 kg. In all of these patients, an introduction of the beta-blocker metoprolol to their therapy was indicated due to non-satisfactory treated hypertension. Two of the patients had already been on the beta-blocker atenolol, however with persisting higher pulse, thus a shift to metoprolol was indicated. At the time of admission, one patient had been treated with 6 antihypertensive drugs, three of the patients had been treated with 5 antihypertensive agents, one patient had 3 antihypertensive drugs, three patients had been on 2 antihypertensive drugs and three patients on antihypertensive mono-therapy, two patients had been without previous antihypertensive medication. To optimize the metoprolol therapy, therapeutic drug monitoring (TDM) of metoprolol was recommended as well as P450 2D6 phenotyping.
The patients were invited to our department on the day of their first ingestion of metoprolol. They were instructed to take other “morning” medications (if they were on poly-therapy) at home on the study day. The dose of metoprolol was chosen based on clinical grounds (7 patients were prescribed 200 mg of metoprolol, 5 patients were given 100 mg of metoprolol and one patient was given 50 mg of metoprolol). Blood samples (4.5 ml each) were drawn into a neutral tube at 1, 3 and 4 hours after metoprolol intake. Baseline data including renal function test (serum creatinine, urea) and liver function test (ALT, AST, γ-GT) were obtained on the study day or were recorded if they were not older than 3 months. The patients further continued in the prescribed therapy with metoprolol. Blood samples in metoprolol steady state were drawn on the next check-up, at least 2 weeks since metoprolol introduction to ensure metoprolol steady state. The scheme of blood collections was the same as at first metoprolol ingestion including blood sample before metoprolol intake. Patients’ medical history was recorded and screened for the presence of the P450 2D6 inhibitor. In 5 patients a change in medication was reported between the two periods of blood collections. In most cases a new antihypertensive agent was added (doxazosin, rilmenidine, telmisartan, trandolapril and hydrochlorothiazide), further sibutramin, allopurinol and tamsulosin were also prescribed in one case.
Assay of metoprolol and α-hydroxymetoprolol
Serum concentrations of metoprolol and α-hydroxymetoprolol were measured by means of high-performance liquid chromatography (HPLC) with fluorescence detection at 230–300 nm, as described previously 18).
Data and statistical analysis
Data are expressed as median and range. The P450 2D6 phenotype was determined using serum metoprolol/α-hydroxymetoprolol MR 3 hours post-dose. The antimode value distinguishing between extensive metabolizers and poor metabolizers was set at MR = 10.5, in agreement with the literature 19). Values are given as median and range. Spearman’s rank correlation test was used for evaluating the relationship between MR after the first metoprolol dose and in steady state. To assess the differences between MR after the first metoprolol dose and in steady state, the Wilcoxon matched-pairs test was applied. A value of P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism for Windows version 5.0 (GraphPad Prism Software, Inc).
Results
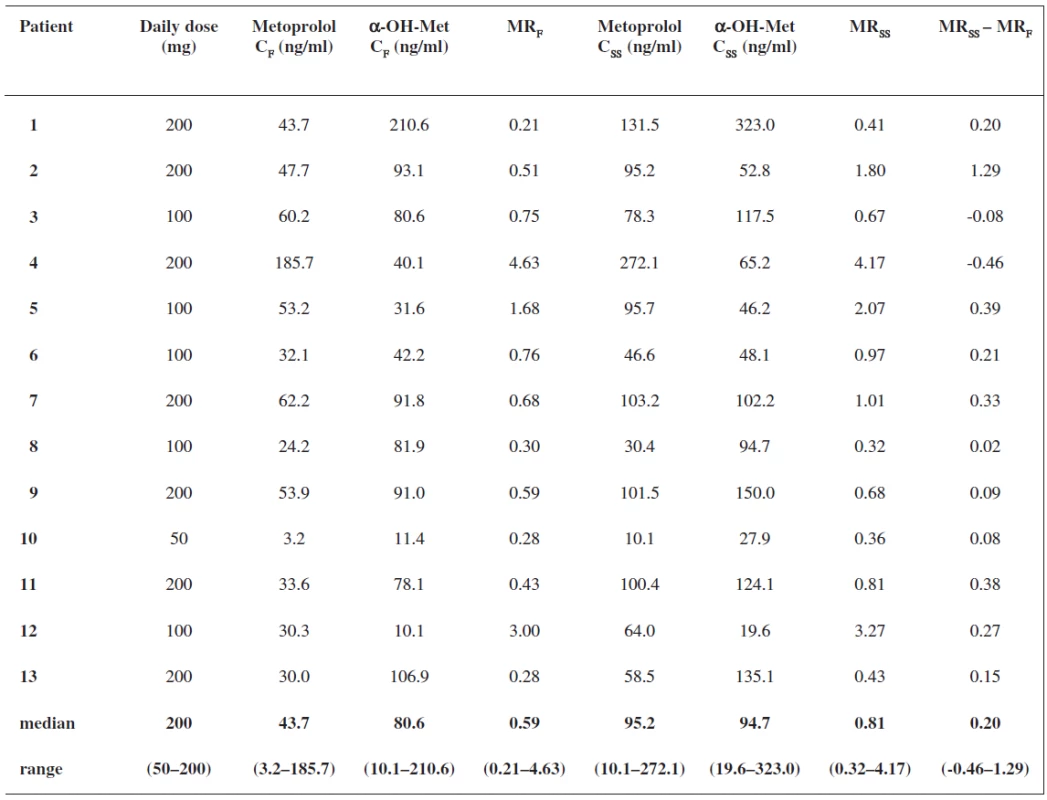
Results are summarized in Table 1. All the patients continued with the same metoprolol dose after introduction of metoprolol to their therapy. When we examined changes in patients’ medication between the two sampling periods, we did not find any drug interfering with P450 2D6 enzyme. Therefore no effect of these drugs on P450 2D6 metabolic activity between the two periods is likely to occur. We observed a significant correlation (rs = 0.8418, P = 0.0003) between the MR 3 hours after a metoprolol single dose and during repeated treatment (Fig. 1). The patients were phenotyped as extensive metabolizers in both periods, after metoprolol first ingestion and in steady state, despite statistically significant differences between the median metoprolol/α-hydroxymetoprolol MR 3 hours after the first metoprolol dose and in steady state (0.59 versus 0.81, P = 0.0266). A great variability in MR values was observed, ranging from 0.21 to 4.63 after ingestion of the first metoprolol dose and from 0.32 to 4.17 in steady state.

Discussion
Beta-blocker metoprolol is widely used in patients for the treatment of different cardiovascular diseases. Metoprolol also serves as one of the probe drugs for P450 2D6 phenotyping. However, most of the studies performed so far have used a single dose of metoprolol to determine the P450 2D6 phenotype. We compared metoprolol/α-hydroxymetoprolol MR after a single dose of metoprolol and in steady state.
First studies on P450 2D6 phenotyping were conducted by assessing the urinary MR of the probe drug derived from urine samples collections during the post-dose of 6 - to 12-h period. Thus, a more practical alternative has been sought based on only one-point sample collected at a shorter time. Sohn et al. examined the utility of the single post-dose 3-plasma metabolic ratio after an oral single dose of 100 mg metoprolol in two Asian populations. He found statistically significant (rs = 0.688 and 0,810, p < 0.0001) correlations between the post-dose urinary and plasma MR, confirming the utility of this one-point plasma MR 15). This finding was further confirmed by Tamminga et al. who observed good correlations (rp = 0.997, P = 0.049) between the metoprolol/α-hydroxymetoprolol MR of the AUC0-12 and the MR values taken from the 3-hour plasma sample after a single metoprolol dose 20). The metoprolol/ α‑hydroxymetoprolol MR 3 hours after a single metoprolol dose was further used in the study of Jonkers et al. to phenotype the subjects 19). Metoprolol MR in patients receiving the drug for several days was used in two studies. However, the MR was calculated from the area under the curve (AUC) of metoprolol to its metabolite α-hydroxymetoprolol 21, 22). Ismail et al. studied the relevance of P450 2D6 genetic polymorphism on chronic metoprolol therapy in cardiovascular patients. P450 2D6 phenotypes were determined using metoprolol as a probe drug. The distribution of the phenotypes was calculated according to the plasma ratio of metoprolol to its metabolite α‑hydroxymetoprolol at 4 hours after drug intake 23). Rau et al. studied the effect of the CYP2D6 genotype on metoprolol metabolism and the metabolic ratio during long-term therapy. The metoprolol to α‑hydroxymetoprolol ratio was determined from two blood samples drawn 1 hour apart, the first sample was drawn at a median of 3 hours after administration of the last metoprolol dose 24). Pronounced effects of multiple dosing on the bioavailability of metoprolol have been reported 16). P450 2D6 is an enzyme with a high affinity and a low capacity for its substrates and it becomes saturated at relatively low concentrations 17). Higher metoprolol systemic availability upon repeated administration of metoprolol may be the consequence of the saturation of the enzyme. Such an effect has been observed e.g. for haloperidol, where higher haloperidol doses decreased pharmacokinetic differences between P450 2D6 genotype groups 25). The clearance of metoprolol may be also decreased during chronic treatment as a result of a potential reduction in hepatic blood flow. Hence in our study we tried to compare the metoprolol/α-hydroxymetoprolol MR after a single dose and during repeated metoprolol treatment and to assess whether the two MRs may be used interchangeably. We observed a significant correlation between the MR after a metoprolol single dose and during repeated treatment. Despite statistically significant differences between the median metoprolol/α-hydroxymetoprolol MR 3 hours after first metoprolol dose and in steady state, the patients were phenotyped as extensive metabolizers in both periods, after metoprolol first ingestion and in steady state. The differences were not of such an extent so as to assign subjects to different phenotypic groups between the two sampling periods. The differences between the MRs after a single dose and in steady state might by of relevance in the case of MR values close to the antimode separating the phenotypes.
The metoprolol/α-hydroxymetoprolol MR in steady state is an appropriate alternative to metoprolol/ α‑hydroxymetoprolol MR after a single metoprolol dose. The use of metoprolol MR in patients routinely treated with metoprolol would eliminate a need for administration of an additional probe drug for P450 2D6 phenotyping.
Received 27 August 2010
Accepted 5 October 2010
Address for correspondence:
PharmDr. Jana Ďuricová
Department of Clinical Pharmacology, University Hospital Ostrava, and Faculty of Medicine, University of Ostrava
17. listopadu 1790, 708 52 Ostrava
e-mail: jankaduricova@seznam.cz, jana.duricova@fno.cz
Sources
1. Duricova, J., Grundmann, M.: CYP2D6 a jeho klinický význam. Klin. Farmakol. Farm., 2007; 21, 80–83.
2. Anzenbacher, P., Anzenbacherova, E.: Cytochrome P450 and metabolism of xenobiotics. Cell. Mol. Life Sci., 2001; 58, 737–747.
3. Home page of the human cytochrome P450 (CYP 2D6) allele nomenclature [updated 2010 July 20]. Available from: http://www.cypalleles.ki.se/cyp2d6.htm
4. Aklillu, E., Herrlin, K., Gustafsson, L. L., Bertilsson, L., Ingelman-Sundberg, M.: Evidence for environmental influence on CYP2D6-catalysed debrisoquine hydroxylation as demonstrated by phenotyping and genotyping of Ethiopians living or in Sweden. Pharmacogenetics, 2002; 12, 375–383.
5. Thürmann, P. A., Haack, S., Werner, U., Szymanski, J., Haase, G., Drewelow, B., Reimann, I. R., Hippius, M., Siegmund, W., May, K., Hasford, J.: Tolerability of ß-blockers metabolized via cytochrome P450 2D6 is sex-dependent. Clin. Pharmacol. Ther., 2006; 80, 551–553.
6. Villeneuve, J.P., Pichette, V.: Cytochrome P450 and liver diseases. Curr. Drug. Metab., 2004; 5, 273–282.
7. Wadelius, M., Darj, E., Frenne, G., Rane, A.: Induction of CYP2D6 in pregnancy. Clin. Pharmacol. Ther., 1997; 62, 400–407.
8. Nekvindova, J., Anzenbacher, P.: Interactions of food and dietary supplements with drug metabolising cytochrome P450 enzymes. Čes. slov. Farm., 2007; 56, 165–173.
9. Zourkova, A., Hadasova, E.: Paroxetine-induced conversion of cytochrome P450 2D6 phenotype and occurence of adverse effects. Gen. Physiol. Biophys., 2003; 22, 103–113.
10. Labbé, L, O’Hara, G, Lefebvre, M, Lessard, E, Gilbert, M, Adedoyin, A., Champagne, J., Hamelin, B., Turgeon, J.: Pharmacokinetic and pharmacodynamic interaction between mexiletine and propafenone in human beings. Clin. Pharmacol. Ther., 2000; 67, 44–57.
11. Grundmann, M., Duricova, J., Perinova, I., Kacirova, I., Jeziskova, I., Jurckova, N.: Influence of propafenone on enzymatic activity of P4502D6 in a patient on long therapy with metoprolol. Basic. Clin. Pharmacol. Toxicol., 2009; 105(Suppl 1), 44.
12. Frye, R. F.: Probing the world of cytochrome P450 enzymes. Mol. Interv., 2004; 4, 157–162.
13. Zanger, U. M., Raimundo, S., Eichelbaum, M.: Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch. Pharmacol., 2004; 369, 23–37.
14. Frank, D., Jaehde, U., Fuhr, U.: Evaluation of probe drugs and pharmacokinetic metrics for CYP2D6 phenotyping. Eur. J. Clin. Pharmacol., 2007; 63, 321–333.
15. Sohn, D. R., Kusaka, M., Shin, S. G., Jang, I. J., Chiba, K., Ishizaki, T.: Utility of a one-point (3-hour postdose) plasma metabolic ratio as a phenotyping test using metoprolol in two East Asian populations. Ther. Drug. Monit., 1992; 14, 184–189.
16. RegĆrdh, C. G., Johnsson, G.: Clinical pharmacokinetics of metoprolol. Clin. Pharmacokinet., 1980; 5, 557–569.
17. Brosen, K., Gram, L. F.: Clinical significance of the sparteine/debrisoquine oxidation polymorphism. Eur. J. Clin. Pharmacol., 1989; 36, 537–547.
18. Perinova, I., Duricova, J., Brozmanova, H., Kacirova, I., Grundmann, M.: Determination of metoprolol and its metabolite α-hydroxymetabolite in serum by HPLC method with fluorescence detection. Ceska. slov. Farm., 2008; 57, 254–259
19. Jonkers, R. E., Koopmans, R. P., Portier, E. J. G., van Boxtel, Ch. J.: Debrisoquine phenotype and the pharmacokinetics and beta-2-receptor pharmacodynamics of metoprolol and its enantiomers. J. Pharmacol. Experimental. Ther., 1990; 256, 959–966.
20. Tamminga, W. J., Wemer, J., Oosterhuis, B., Brakenhoff, J. P. G., Gerrits, M. G. F., de Zeeuw, R. A. de Leij, L. F. M. H., Jonkman, J. H. G.: An optimized methodology for combined phenotyping and genotyping on CYP2D6 and CYP2C19. Eur. J. Clin. Pharmacol., 2001; 57, 143–146.
21. Goryachkina, K., Burbello, A., Boldueva, S., Babak, S., Bergman, U., Bertilsson, L.: CYP2D6 is a major determinant of metoprolol disposition and effects in hospitalized Russian patients treated for acute myocardial infarction. Eur. J. Pharmacol., 2008; 64, 1163–1173.
22. Goryachkina, K., Burbello, A., Boldueva, S., Babak, S., Bergman, U, Bertilsson, L.: Inhibition of metoprolol metabolism and potentiation of its effects by paroxetine in routinely treated patients with acute myocardial infarction (AMI). Eur. J. Clin. Pharmacol., 2008; 64, 275–282.
23. Ismail, R., The, L. K.: The relevance of the CYP2D6 genetic polymorphism on chronic metoprolol therapy in cardiovascular patients. J. Clin. Pharm. Ther., 2006; 31, 99–109.
24. Rau, T., Heide, R., Bergmann, K., Wuttke, H., Werner, U., Feifel, N., Eschenhagen, T.: Effect of the CYP2D6 genotype on metoprolol metabolism persists during long-term treatment. Pharmacogenetics, 2002; 12, 165–172.
25. Roh, H. K., Chung, J. Y., Oh, D. Y., Park, Ch. S., Svensson, J. O., Dahl M. L., Bertilsson L.: Plasma concentrations of haloperidol are related to CYP2D6 genotype at low, but not high doses of haloperidol in Korean schizophrenic patients. Br. J. Clin. Pharmacol., 2001; 52, 265–271.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2010 Issue 5
-
All articles in this issue
-
Studies of local anaesthetics
Part 191 – Utilization of artificial neural networks in the study of correlations between the calculated and measured values of retention factors k in various separation systems in the group of basic esters of alkoxy-substituted phenylcarbamic acid - A study of dissolution profiles of tramadol hydrochloride from the dosage forms with controlled release of the matrix and multiple unit types
-
Obsahové látky
Philadelphus coronarius L. -
Medicinal preparations in the Renovated Prague Dispensatory of 1750
Part I – Introduction and the first nine classes of dosage forms -
Medicinal preparations in the Restored Prague Dispensatory of 1750
Part II – The examination of the remaining dosage forms - Depression relapse and antidepressants consumption in the quality of life aspect
- Comparison of metoprolol/α-hydroxymetoprolol metabolic ratio after a single dose and in steady state
-
Studies of local anaesthetics
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- A study of dissolution profiles of tramadol hydrochloride from the dosage forms with controlled release of the matrix and multiple unit types
-
Medicinal preparations in the Renovated Prague Dispensatory of 1750
Part I – Introduction and the first nine classes of dosage forms -
Obsahové látky
Philadelphus coronarius L. -
Medicinal preparations in the Restored Prague Dispensatory of 1750
Part II – The examination of the remaining dosage forms
