High active potential antimycobacterial agents against Mycobacterium avium
Vysoce účinné potenciální antimykobakteriální látky proti Mycobacterium avium
Deriváty 3-fenyl-2H-1,3-benzoxazin-2,4(3H)-dionu jsou aktivní vůči Mycobacterium avium, pokud jsou substituovány v poloze 7 methylem. Jedna nebo dvě karbonylové skupiny musí být zaměněny za thioxo skupiny. Nejaktivnější sloučeniny nebyly substituovány na fenylu, nebo byly substituovány na fenylu v poloze 3 nebo 4 chlorem, bromem či methylem. Substituce v poloze 3 a 4 dvěma atomy chloru však aktivitu snižuje. Sloučeniny jsou aktivní vůči INH rezistentním kmenům. Připravili jsme dalších 44 derivátů s podobnou strukturou studovaným látkám, avšak s jiným substituentem v ploze 7 (chlor, brom, methoxy). Aktivita vůči M. avium byla nízká. Můžeme uzavřít, že se jedná o novou skupinu významně aktivních látek vůči Mycobacterium avium.
Klíčová slova:
benzoxazin thioxo skupina, Mycobacterium avium, antimykobacteriání aktivita
Authors:
Karel Waisser; Jiří Kuneš; Jiřina Stolaříková
Authors‘ workplace:
Regional Institute of Public Health
; Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Inorganic and Organic Chemistry
Published in:
Čes. slov. Farm., 2012; 61, 127-129
Category:
Short News
Overview
Derivatives of 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dione are active against Mycobacterium avium when substituted in position 7 with a methyl. One or two carbonyl groups have to be replaced with a thioxo group. High active derivatives are the compounds without substitution on the phenyl, or those substituted on the phenyl in position 3 or 4 with chlorine, bromine or a methyl. The substitution in position 3 and 4 with two atoms of chlorine lowers the activity. The compounds are active against INH resistant strains. We synthesized other 44 derivatives with a similar structure of the compounds as in the paper but substituted in position 7 with other substituents (chlorine, bromine methoxy). The activity against M. avium was poor. It can be concluded that a new group of compounds with an excellent activity against M. avium has been found.
Keywords:
benzoxazine, thioxo group, Mycobacterium avium, antimycobacterial activity
Introduction
The recent reappearance of tuberculosis and other infectious diseases is a serious problem worldwide. According to the World Health Organization, there were 9.4 million new tuberculosis cases in 2008 including 1.4 million cases among HIV positive people. In the year 2008, WHO also reported the highest rates of MDR-TB ever recorded1). Therefore, there is a great need to develop new antituberculotics and antibiotics. New mycobacterial diseases have occurred which have been recently considered noncontagious to humans (mycobacterioses produced by potentially pathogenic strains). The development of antimycobacterial drugs is usually focused on Mycobacterium tuberculosis and Mycobacterium leprae. Mycobacterial diseases due to multiresistant strains of Mycobacterium avium are not frequent, but often fatal in the end. The review of chemotherapy of nontuberculous mycobacterial infections is taken over from Tortoll2). The goal of the present paper is a search in the group of 7 methyl-3-phenyl-4-thioxo-2H-1,3-benzoxazine-2(3H)-ones for active compounds against M. avium.
Experimental
Chemistry
General information
The melting points were determined on a Kofler apparatus. The samples for the analyses and antimycobacterial tests were dried over P2O5 at 61 °C and 66 Pa for 24 h. The elemental analyses (C, H, N) were performed on a CHNS-O CE elemental analyzer (Fisions EA 1110, Milan) and were within ± 0.4 % of the theoretical values. The IR spectra were measured in KBr pellets on a Nicolet Impact 400 apparatus; the wavenumbers are given in cm–1. The purity of compounds was checked by TLC on silica gel plates precoated with the fluorescent indicator Silufol UV 254 + 366 (Kavalier Votice, Czech Republic), hexane-acetone (3 : 1) being used as the mobile phase. The 1H NMR and 13C NMR spectra of the new compounds were recorded in DMSO–d6 solutions at ambient temperature on a Varian Mercury-Vx BB 300 spectrometer operating at 300 MHz for 1H NMR and 75 MHz for 13C NMR.
General procedure for the preparation of 7-methyl 3 phenyl-4-thioxo-2H-1,3-benzoxazine-2(3H)-ones and 7 methyl 3-phenyl-2H-1,3-benzoxazine-2,4(3H) dithiones
The derivatives of 7-methyl-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-diones prepared in our previous paper3) (4 mmol) were melted with P4S10 (10 mmol) for 45 minutes (180—210 °C). After cooling to room temperature, a 10% potassium carbonate solution was poured into the reaction mixture; the crude product was filtered off, and dissolved in toluene (p.a., at the most 40 ml). Column chromatography on silica gel yielded substituted derivatives of 7-methyl-3-phenyl-4-thioxo-2H-1,3-benzoxazine-2(3H)-one or (1–11) and derivatives of 7-methyl-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dithione (12–22) as orange-yellow and red solids, respectively. Recrystallization from ethanol was necessary.
Microbiology
The antimycobacterial activity of the compounds was determined in the Šula semisynthetic medium (SEVAC, Prague). This medium (with bovine serum) is routinely used in the Czech Republic. Each strain was simultaneously inoculated into a Petri dish containing the Löwenstein-Jensen medium for the control of sterility of the inoculum and its growth. The compounds were added to the medium in DMSO solutions. The final concentrations were 1000, 500, 250, 125, 62.5, 32, 16, 8, 4, 2, 1 and 0.5 μmol/l. The minimum inhibitory concentration MICs were determined after incubation at 37 °C for 14 days and 21 days. The evaluation was repeated three times and the values of the minimum inhibitory concentration were the same.
Results
Chemistry
The reaction of phosphorus pentasulfide with 7-methyl-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-diones was used. The product was a mixture of 7-methyl-3-phenyl-4-thioxo-2H-1,3-benzoxazine-2(3H)-one and 7-methyl-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dithione. The mixture is easily separable by chromatography on silica gel. The structures of compounds are characterized by NMR and IR spectroscopy and their purity was checked by TLC and elemental analysis. The starting compounds are described3).
Microbiology
An INH-resistant strain of M. avium Czech Collection of Type Cultures (CNCTC) My 330/88 was used (identical with ATCC 12.478). The results are summarized in Table 1 and Table 2.
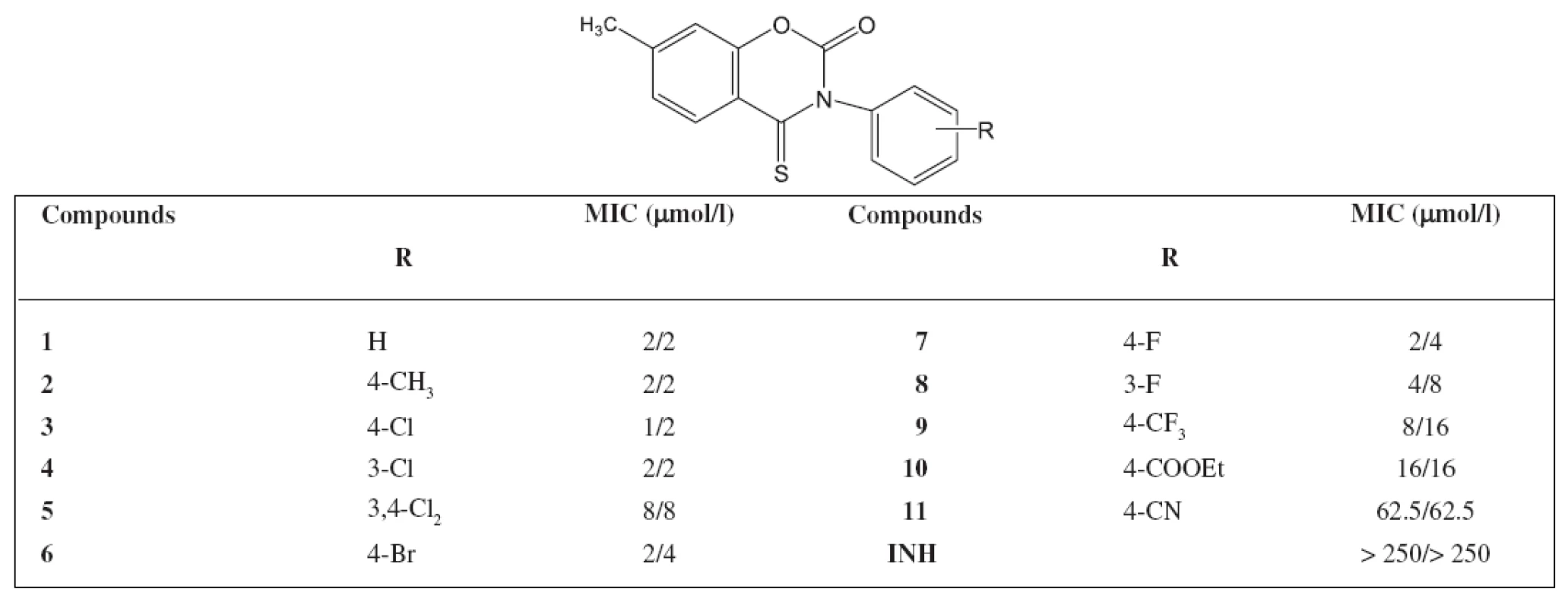
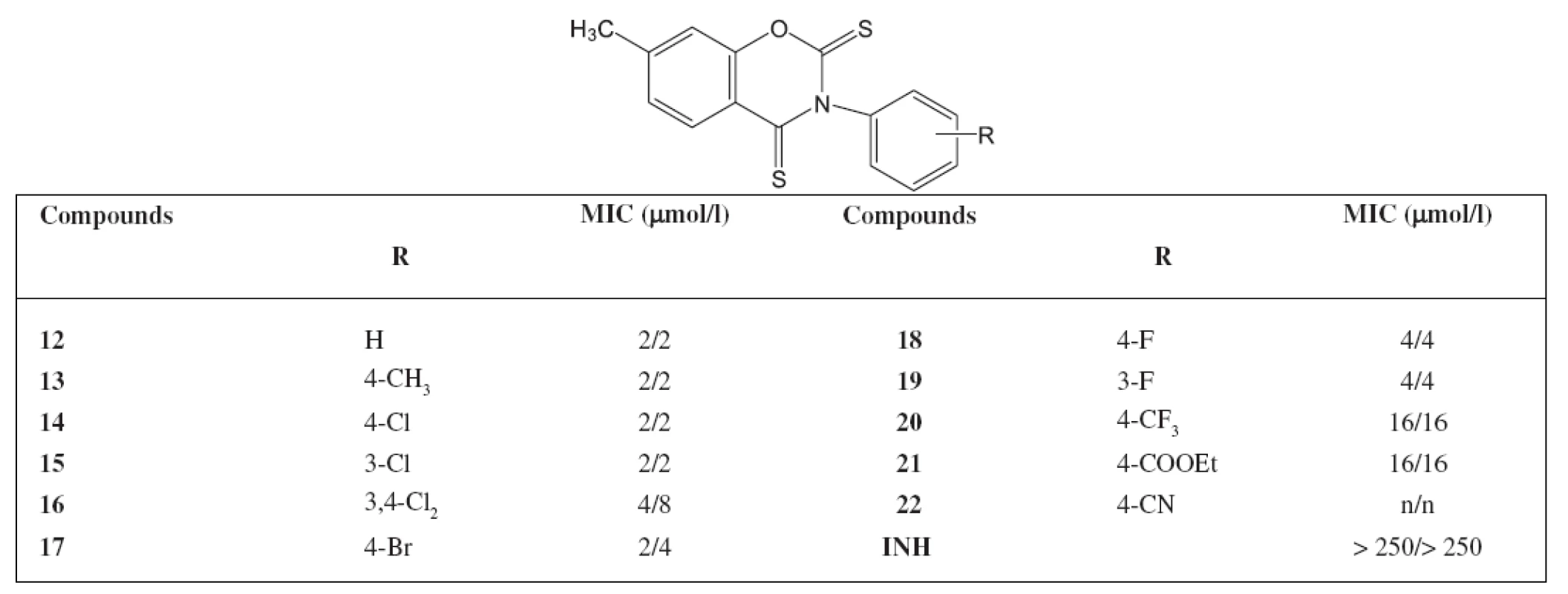
Findings
The highest antimycobacterial activity was shown by 7-methyl-3-phenyl-4-thioxo-2H-1,3-benzoxazine-2(3H)-one and its derivatives substituted on the phenol ring by chlorine or bromine. The activity of 7-methyl-3-(4-methylphenyl)-4-thioxo-2H-1,3-benzoxazine-2(3H)-one was admirable as well. Similar substituted derivatives in the group of 7-methyl-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dithione show also such an activity. The activity against M. avium of the starting 7 methyl-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dione was not significant We synthesized other 44 derivatives with a similar structure of the compounds as in the paper but substituted in position 7 with other substituents (chlorine, bromine, methoxy). The activity against M. avium was poor. It can be concluded that a new group of compounds with an excellent activity against M. avium has been found.
Conflicts of interest: none.
Received 21. March 2012 / Accepted 24. April 2012
prof. RNDr. Karel Waisser, DrSc.; J. Kuneš
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Inorganic and Organic Chemistry
Heyrovského 1203, 500 03 Hradec Králové, Czech Republic
e-mail: waisser@faf.cuni.cz
J. Stolaříková
Regional Institute of Public Health
Sources
1. WHO (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response, 2010 (cit. 2010-10-14). Available from: http://whqlibdoc.who.int/ publications/2010/9789241599191_eng.pdf.
2. Tortoll E. Clinical manifestations of nontuberculous mycobacteria infections. Journal Compilations, European Society of Clinical Microbiology and Infection Diseses 2009; 906–910.
3. Waisser K., Bureš O., Holý P., Kuneš J., Oswald R., Jirásková L., Pour M., Klimešová V., Palát K. Jr., Kaustová J., Dahse H.‑D., Möllman U. Antimycobacterial 3-aryl-2H-1,3-benzoxazine-2,4(3H)-diones. Pharmnazie 2003; 58 : 83–94.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2012 Issue 3
-
All articles in this issue
- Gamma-secretase inhibitors in Alzheimer’s disease therapy
- Modulation of leukotriene pathway – potential targets
- Microbial secondary metabolites as inhibitors of pharmaceutically important transferases and oxidoreductases
- Phenotyping of enzymes participating in drug metabolism
-
Studies of local anaesthetics – Part 197
Effect of xylitol on pharmaceutical availability of lidocaine and flow properties of hydrogels - High active potential antimycobacterial agents against Mycobacterium avium
- Molecularly imprinted polymers
- Hot-melt extrusion
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Phenotyping of enzymes participating in drug metabolism
- Hot-melt extrusion
- Modulation of leukotriene pathway – potential targets
- Microbial secondary metabolites as inhibitors of pharmaceutically important transferases and oxidoreductases
