Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
Authors:
Judit Süli 1; Linda Kolčáková 1; Jarmila Harvanová 1; Anna Ondrejková 2
Authors‘ workplace:
University of Veterinary Medicine and Pharmacy, Košice, Slovak Republic
; Department of Chemistry, Biochemistry and Biophysics
1; Department of Epizootiology and Parasitology
2
Published in:
Čes. slov. Farm., 2015; 64, 284-286
Category:
44<sup>th</sup> Conference drug synthesis and analysis
Introduction
When applying modern inactivated vaccines, it is necessary to use an adjuvant for the induction of a sufficient immune response. The oldest and still most widely used adjuvants are aluminium compounds. Alum is the principal vaccine adjuvant for clinical applications but it is a poor inducer of cellular immunity and is not an optimal adjuvant for vaccines where Th1 responses are required for protection1). The security of alum adjuvants2) is questionable. The Freund’s complete and incomplete adjuvants used in the past were in the present replaced by more effective squalene emulsions, which are also easier to handle. Squalene adjuvants are characterized by a high adjuvant effect and due to their biocompatibility they are safe3). Squalene emulsions are generally relatively stable, but squalene, an effective antioxidant alone, can be easily oxidized. The oxidation processes may disturb the stability of the adjuvant emulsion and also affect its efficacy. The greatest risk of squalene oxidation arises in time of the preparation, particularly for homogenization and during the prolonged storage of emulsion. The appropriate solution to eliminate this problem is suggested addition of antioxidants to the squalene adjuvant. To some commercially produced adjuvant formulations α-tocopherol is added in order to prevent oxidation of squalene. However, according to our findings, α-tocopherol appeared under in vitro conditions as a pro-oxidant and squalene was effectively protected by β-carotene4).
Aim of this study was to find whether β-carotene affects the particle size of the emulsion (emulsion stability indicator) and how it affects the antioxidant protection of squalene adjuvant rabies vaccine.
Experimental methods
In the preparation of an inactivated rabies vaccine with a squalene adjuvant was observed the effect of the homogenizing time prolongation from 8 to 10 min on the particle size as an indicator of stability and on the peroxide value change in the squalene emulsions. Also the effect of β-carotene on the oxidation changes in the adjuvant vaccine emulsion during prolonged homogenization and during of the vaccine storage was examined.
Preparation of the adjuvant vaccines
The vaccines with an adjuvant were prepared in one step by Süli et al.5).
Composition of adjuvant vaccines
- Oil phase: squalene (Merck-Schuchardt, Germany), final content 2.5% (w/w).
- Emulsifiers:
- Poloxamer 105 (ICI, Great Britain), HLB ≈ 18.5; final content 4% (w/w);
- Abil-Care 85 (Evonik Industries AG, Germany) HLB ≈ 10; final content 2% (w/w), available for emulsion preparing at decreased temperature (up to 37 °C).
- Antioxidant: β-carotene (Sigma, USA), final content 0.5%.
- Water phase: suspension of inactivated rabies virus from the strain Vnukovo-32 at 107th serial passage, titre 106,2 MICLD50· 0.03 cm–3 (MICLD50 – mice intracerebral 50% lethal dose)6). The final content of the water phase in the vaccine without β-carotene was 91.5% (w/w), in the vaccine with β-carotene, 91% (w/w).
Preparation of the adjuvant vaccines
The viral suspension was sterilized by filtration through a membrane filter 45 microns Minisart Plus (Sartorius, France). The other components (except for β-carotene) were sterilized in an autoclave for 1 h at 120 °C and a pressure of 200 kPa. The vaccine was prepared under sterile conditions by homogenizing using an ultrasonic disintegrator Soniprep 150 MSE (MSE, Great Britain) with a probe ULT-210-515D at a frequency of 23 kHz. The vaccine emulsions were prepared by 8-min and 10-min homogenization.
Determination of particle size in vaccine emulsions
The particle size analysis was performed on the instrument Mastersizer 3000 (Malvern Instruments Ltd., Great Britain).
Determination of the peroxide value of the adjuvant vaccines
Lipids containing unsaturated bonds are sensitive to oxidation. Oxidation processes originate primarily lipid hydroperoxides. Lipid peroxides react in an acidic pH with potassium iodide to form iodine. Iodine is determined by titration with sodium thiosulphate:
R-O-OH + 2KI + 2CH3COOH → R-OH + I2 +
+ 2CH3COOK + H2O
I2 + 2Na2S2O3 → 2NaI + Na2S4O6
The peroxide value was examined immediately after the preparation and during the 35 days storage period at weekly interval Each sample was titrated 5 time.
Storage of vaccines: in polyethylene containers at 4 °C.
Statistical evaluation of results: Student’s paired t-test.
Results and discussion
The particle size analysis of adjuvant vaccines with β-carotene and without β-carotene showed that over 90% of the particles had a diameter of below 0.561 microns, more than 50% below 0.423 microns and more than 10% less than 0.316 microns. These data indicate a good stability of emulsions prepared. At the prolonged homogenization the values were very similar. Prolongation of the homogenization period already did not have an impact on the reduction of particle size, so in terms of particle size it is irrelevant to homogenize for more than 8 min. About the prolongation of homogenization it is necessary to decide on the results of squalene oxidation, because homogenization leads to aeration of samples, or potentiation of oxidative processes.
Determination of peroxide value depending on the time of homogenization
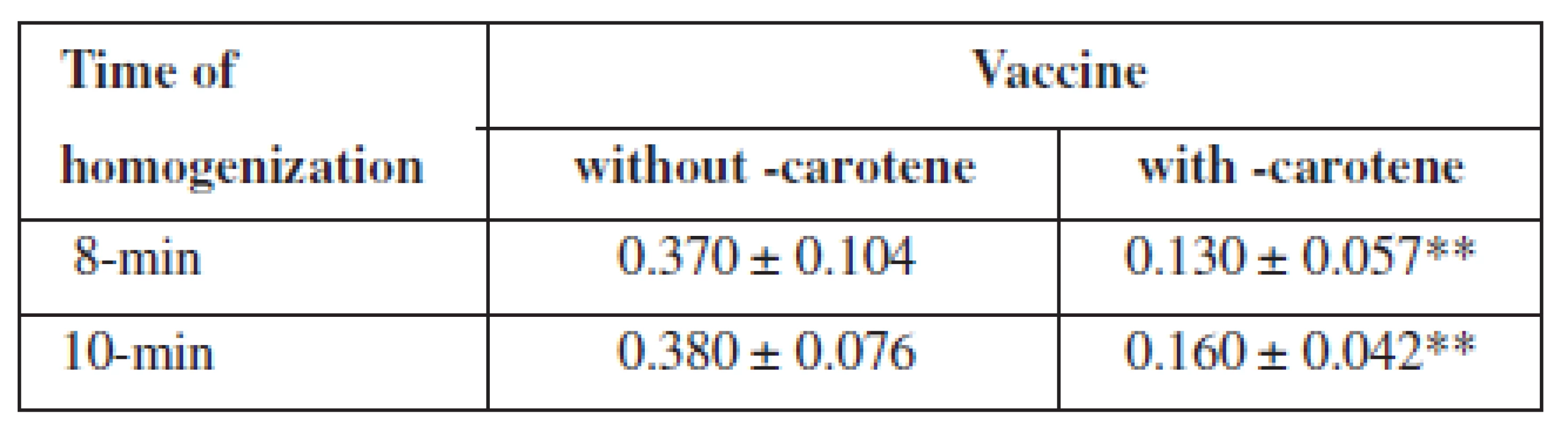
The peroxide value of the vaccine with β-carotene was significantly lower than of the vaccine without β-carotene (p < 0.01), both in 8-min, and in 10-min homogenization (Table 1). The slight increase in the peroxide value of the vaccines shows that the 8-min homogenization is sufficient.
Determination of the peroxide value during the vaccines storage
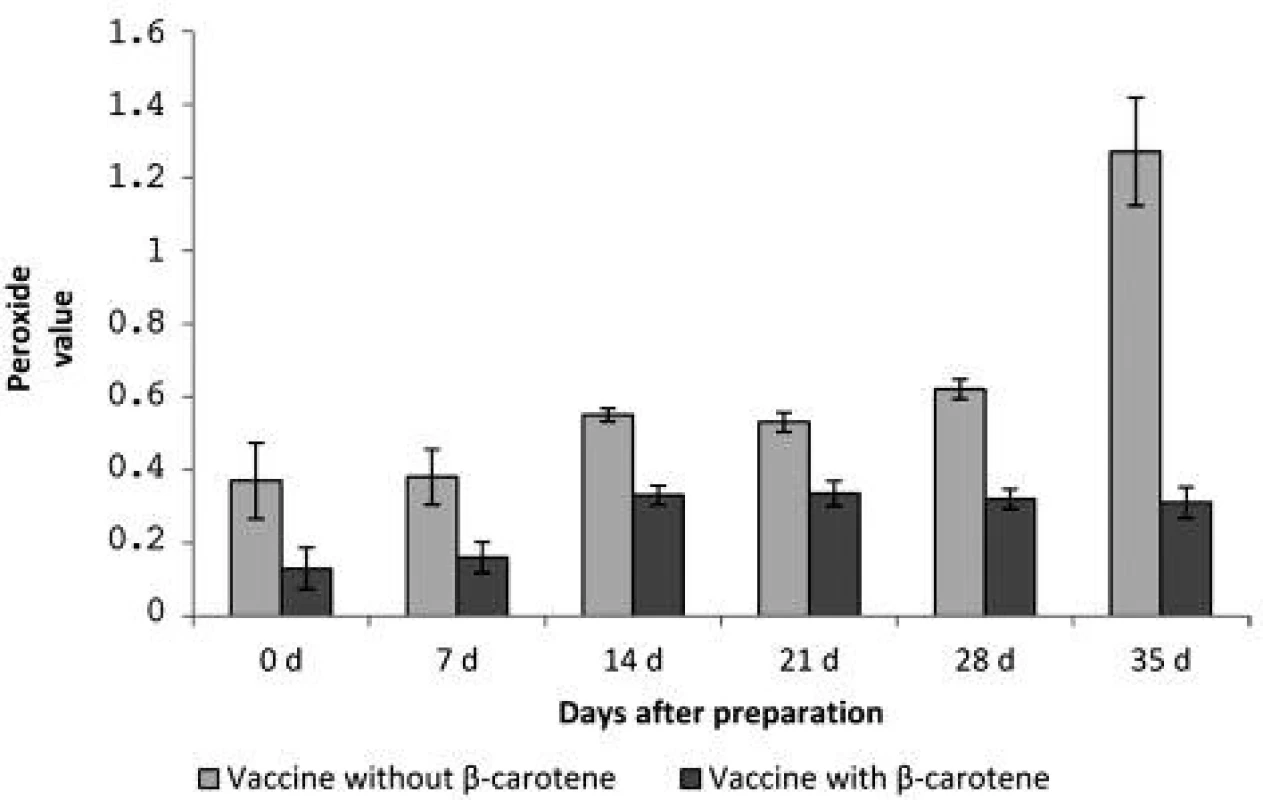
In each interval the peroxide values between the vaccine without β-carotene and the vaccine with β-carotene were compared. Not just after preparation but also during the storage the values were significantly lower in the vaccine containing β-carotene. It appears that, despite the anti-oxidative protection to day 14 of the vaccine storage, the peroxide value of the vaccine with β-carotene increased approximately twice in comparison with the values after preparation. After day 28, the values were slightly reduced, which can be explained by progressing of the oxidation processes, when unstable hydroperoxides have changed to other oxidation products. However, this phenomenon was not observed in the vaccine without β-carotene. During 35 days of storage, the amount of the peroxide value steadily increased, between days 28 and 35 the peroxide value was doubled, and compared with the value after preparation it was nearly 3.5-times higher (Figure 1).
For manufacturers of drugs and vaccines the emulsion stability is paramount. The stability of our adjuvant rabies vaccine is sufficient, particularly if the vaccine is stored in polyethylene containers instead of glass vials7). Ostwald ripening in the case of squalene emulsions is unlikely since the squalene molecules would be unlikely to diffuse through the aqueous medium3). On the other side, the squalene molecule contains six unsaturated bonds that can be easily oxidized. Oxidation processes primarily result in peroxides which may change to secondary oxidation products8). Although most researchers involved in the research of oxidation of squalene share this view, someone has argued that squalene is not very sensitive to peroxidation9). In the oxidation processes the properties of the squalene adjuvant emulsions may change. First, this may influence the emulsion stability characteristics, and on the other hand, the oxidation products are harmful8). Just for easy oxidability, squalene itself is considered an antioxidant10). Therefore, before an automatic addition of other antioxidants, it is appropriate to perform studies of their suitability for a concrete system3). Such a study has accorded to us surprising results of the pro-oxidant properties of α-tocopherol in the in vitro system with squalene, while β-carotene has been shown to be a suitable compound to reduce oxidation reactions4).
Conclusions
The authors found that homogenization during a prolonged period from 8 to 10 minutes does not change the particle size as an important indicator of stability of the vaccine emulsions. However, prolonged homogenization may lead to increased oxidation of squalene. The oxidation of the adjuvant emulsion of squalene was effectively prevented by β-carotene, when the peroxide value of the rabies adjuvant vaccine was significantly (p < 0.01) reduced not only in the preparation process, but also during a 35-day storage. The peroxide value of the vaccine without β-carotene was after the preparation 0.37 ± 0.10 and after 35 days 1.27 ± 0.15, while the vaccine with β-carotene had the peroxide value after preparation 0.13 ± 0.06 and after 35 days, 0.31 ± 0.04.
This study was financed by the scientific project APVV-0605-12 (Slovak Republic).
Conflicts of interest: none.
RNDr. Judit Süli, PhD.
UVLF, Ústav lekárskej chémie
Komenského 73, 041 81 Košice, Slovak Republic
e-mail: judit.suli@uvlf.sk
Sources
1. Mori A., Oleszycka E., Sharp F. A., Coleman M., Ozasa Y., Singh M., O’Hagan D. T., Tajber L. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur. J. Immunol. 2012; 42, 2709–2719.
2. Tomljenovic L., Shaw C. A. Aluminium vaccine adjuvants: are they safe? Curr. Med. Chem. 2011; 18, 2630–2637.
3. Fox C.B. Squalene emulsions for parenteral vaccine and drug delivery. Molecules 2009; 14, 3286–3312.
4. Süli J., Lovásová M., Sobeková A. Antioxidative protection of squalene vaccination adjuvants. Folia Vet. 2012; 56(Suppl II), 56–58.
5. Süli J., Beníšek Z., Eliáš D., Švrček Š., Ondrejková A., Ondrejka R., Bajová V. Experimental squalene adjuvant. I. Preparation and testing of its effectiveness. Vaccine 2004; 22, 3464–3469.
6. Ondrejková A., Süli J., Kolčáková L., Ondrejka R., Čechvala P., Benkö Z., Prokeš M. Influence of β-carotene on antigenic effectiveness of inactivated rabies vaccine with squalene adjuvant. In: Sborník 44. Konference Syntéza a analýza liečiv. Brno: 2015.
7. Slepecká E., Süli J., Radová J., Ondrejková A., Ondrejka R., Prokeš M., Korytár Ľ., Čechvala P., Harvanová J. Stanovenie fyzikálno-chemických vlastností a sledovanie stability skvalénových emulzií. Chem. Listy 2013; 107, 659–664.
8. Nakagawa K., Ibusuki D., Suzuki Y., Yamashita S., Higuchi O., Oikawa S., Miyazawa T. Ion-trap tandem mass spectrometric analysis of squalene monohydroperoxide isomers in sunlight-exposed human skin. J. Lipid Res. 2007; 48, 2779–2787.
9. Popa I., Băăbeanu N. E., Niţă S., Popa O., Dinu-Pârvu C. E. Squalene – natural resources and applications. Farmacia 2014; 62, 840–862.
10. Huang Z. R., Lin Y. K., Fang J. Y. Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules 2009; 14, 540–554.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2015 Issue 6
-
All articles in this issue
- Antibacterial activity of natural compounds – essential oils
- Cholinergic system of the heart
- Body surface area and body weight of Czech adult cancer population
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Antibacterial activity of natural compounds – essential oils
- Body surface area and body weight of Czech adult cancer population
- Cholinergic system of the heart
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
