Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
Authors:
Mateusz Korzec 1; Roksana Rzycka 1; Sandra Senkała 1; Ewelina Szpaczyńska 1; Barbara Czaplińska 1; Wioletta Cieślik 1; Anna Mrozek-Wilczkiewicz 2; Marzena Rams-Baron 2; Robert Musioł 1; Jarosław Polański 1
Authors‘ workplace:
Institute of Chemistry, University of Silesia, Katowice, Poland2Silesian Center for Education and Interdisciplinary Research, Chorzow, Poland
1
Published in:
Čes. slov. Farm., 2015; 64, 298-301
Category:
44<sup>th</sup> Conference drug synthesis and analysis
Introduction
The application of fluorescence spectroscopy and imaging in biological systems has expanded tremendously over the past decades. Fluorescence spectroscopy and time-resolved fluorescence are considered to be primarily research tools in biochemistry and biophysics. Fluorescence is now a dominant methodology used in many fields of science, i. e., biotechnology, flow cytometry, medical diagnostics, DNA sequencing, forensics, genetic analysis and other1). Imaging of biological structures by fluorescence microscopy acquires special importance in the diagnosis of cancer, e. g., in photodynamic diagnosis (PDD) in urology2, 3) or brain imaging4, 5). Recently, fluorescent properties of styrylochinolines have been discovered and used, e. g., for quantification of zinc in urban runoff6), in combined therapeutics and diagnostics in protein misfolding diseases in brain cells5), as the fluorescent sensor for Fe2+ 7) demonstrates multicolor fluorescence upon addition of different metal cations8). Fluorescent properties of dyes can be dependent on perturbation of their emission by proximity to conducting particles or surfaces9, 10), as shown in the studies of DNA11, 12) and the cyanine determination oligonucleotides13, 14). Recently, metal ions interactions with fluorescent dyes have also been investigated15–19).
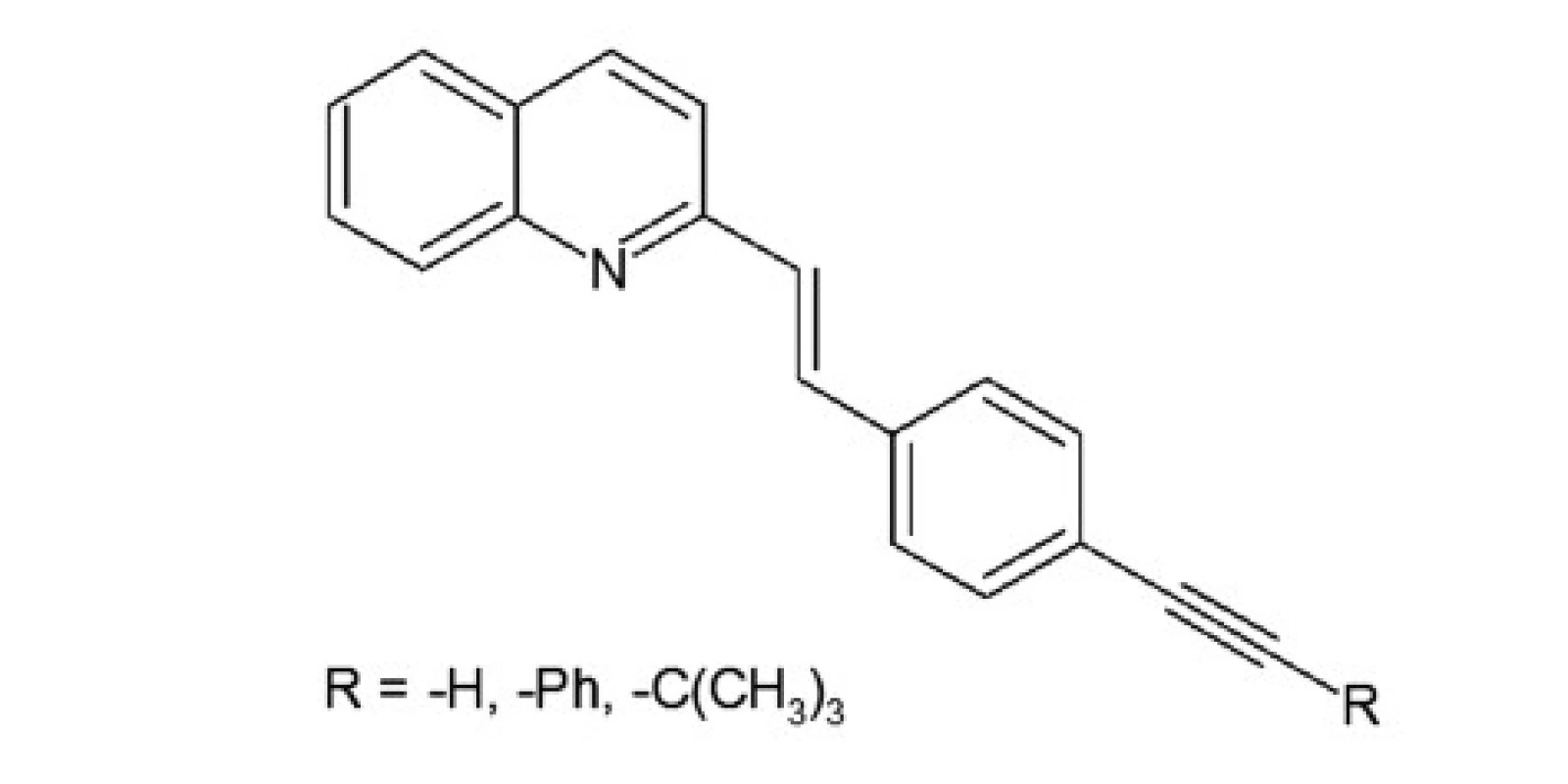
A series of quinoline derivatives were designed based on the styrylquinoline system (Fig. 1). The results indicated that representative compounds are biologically inactive (cell culture, MTS assay) but have promising physicochemical properties (Stokes shift, quantum yield) and preferentially incorporated into the plasma membrane or any other intracellular organelles20).
Experimental methods
Preparation of catalyst 5% nano-Pd/Cu
The bimetallic nano-Pd/Cu was obtained by digesting the carrier in nano-Pd/silica and transferring the nanoparticles onto electrolytic copper. Amorphous silica synthesized by sol-gel technique was used as the intermediate carrier. Nano-Pd/SiO2 and electrolytic copper were suspended in deionized water and placed in an ultrasound bath and stirred. Then, SiO2 was digested with 40% aqueous NaOH. Next, the suspension cooled to room temperature, centrifuged and washed to neutral pH with deionized water. The resulting preparation of the catalyst was dried at room temperature to constant mass.
Synthesis of styrylquinoline derivatives
Synthesis by using Pd/Cu catalyst (synthesis of aldehydes): aryl halides, Pd/Cu, PPh3 were suspended in dry triethylamine. Then, the acetylene compound was added and the mixture was stirred for 3 h in a temperature of 80 oC. Next, the mixture was cooled to room temperature and the catalyst was centrifuged, filtered and washed with ethyl acetate. The filtrate was washed three times with deionized water and then dried over magnesium sulfate, filtered and concentrated under reduced pressure to give the product.
Synthesis of styrylquinoline: quinaldine derivatives and obtained aldehyde were dissolved in acetic anhydride. The resulting mixture was stirred under argon at 130 °C for 20 h. After evaporation to dryness, the product was purified by a short SiO2 column (eluent – ethyl acetate/hexane). The solution was then cooled and concentrated under reduced pressure. Next, the mixture was washed with diethyl ether. The product was obtained as a yellow solid.
Biological tests
Biological studies of obtained compounds were carried out by using well-known methods, i. e., cell cultures, the MTS assay, cellular imaging. In cell culture, human colon adenocarcinoma cells (HCT116) and normal human fibroblast (GM 07492) were used. Cytotoxicity assay consisted of an addition of MTS reagent (CellTiter 961AQueousOne Solutions-MTS (Promega)) to the wells with cells and the test substance, and then incubation with a dye. The quantity of formazan product as measured by absorbance at 490 nm (using an Elx800 device – from BioTek Instruments, USA) is directly proportional to the amount of viable, proliferating cells. Live cell imaging involves seeding of HCT116 cells in medium and incubation under normal conditions at 37 °C in a CO2 atmosphere for 24 hours. Then the test compound is added to the cells (concentration – 25 μM) and incubated for a further 2 hours. After incubation, the cells are rinsed three times with PBS. The direct observed living cell is formed under an inverted fluorescence microscope (IX81, Olympus).
Physicochemical studies
Absorption and fluorescence spectra were measured at room temperature in a 10-mm quartz cell with a U-2900 spectrophotometer (Hitachi) and an F-7000 spectrofluorimeter (Hitachi), respectively. Styrylquinoline solutions were prepared at various concentrations in spectroscopic grade DMSO. Stokes shift (nm) were designated as the difference between positions of the band maxima of the absorption and fluorescence spectra of the same electronic transition. The fluorescence quantum yields were measured using the comparative method with anthracene in cyclohexane as a reference.
Results and discussion
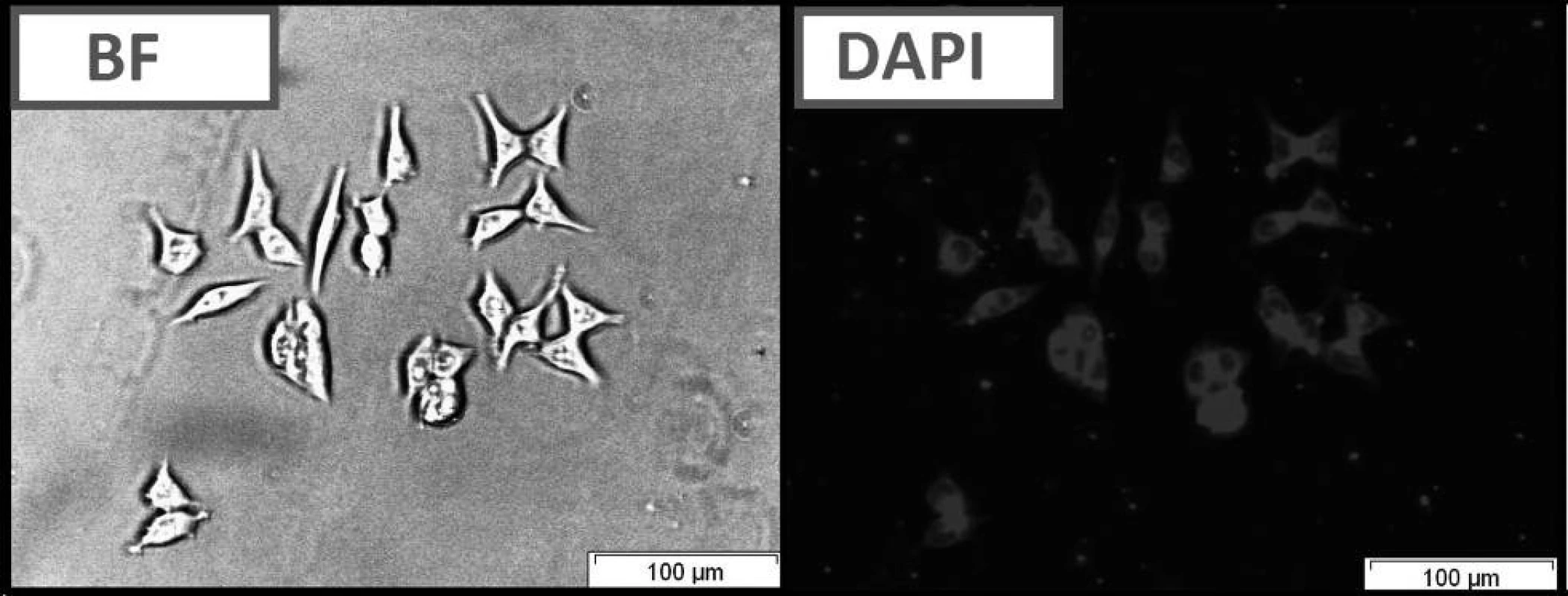
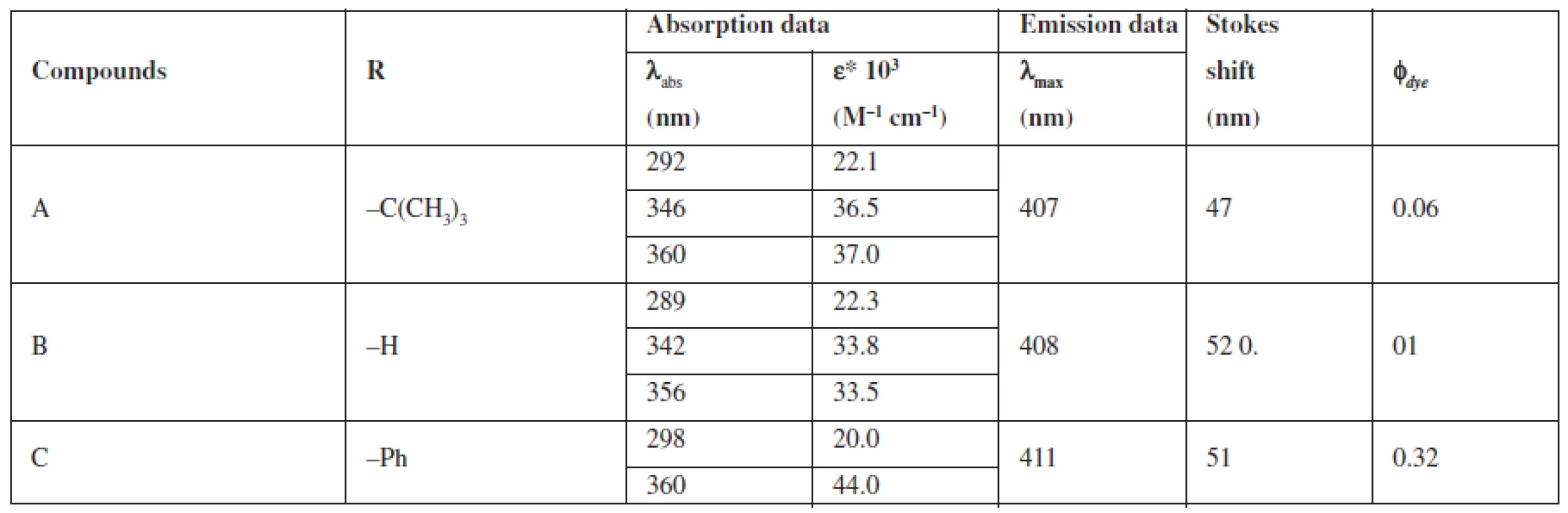
A group of styrylquinoline derivatives shown in Figure 1 was obtained and tested in our previous study. These compounds are biologically inactive but have good physicochemical properties such as Stokes shift, quantum yield (Table 1) and preferentially incorporated into the plasma membrane or any other intracellular organelles, i. e., lysosome (Fig. 5.).
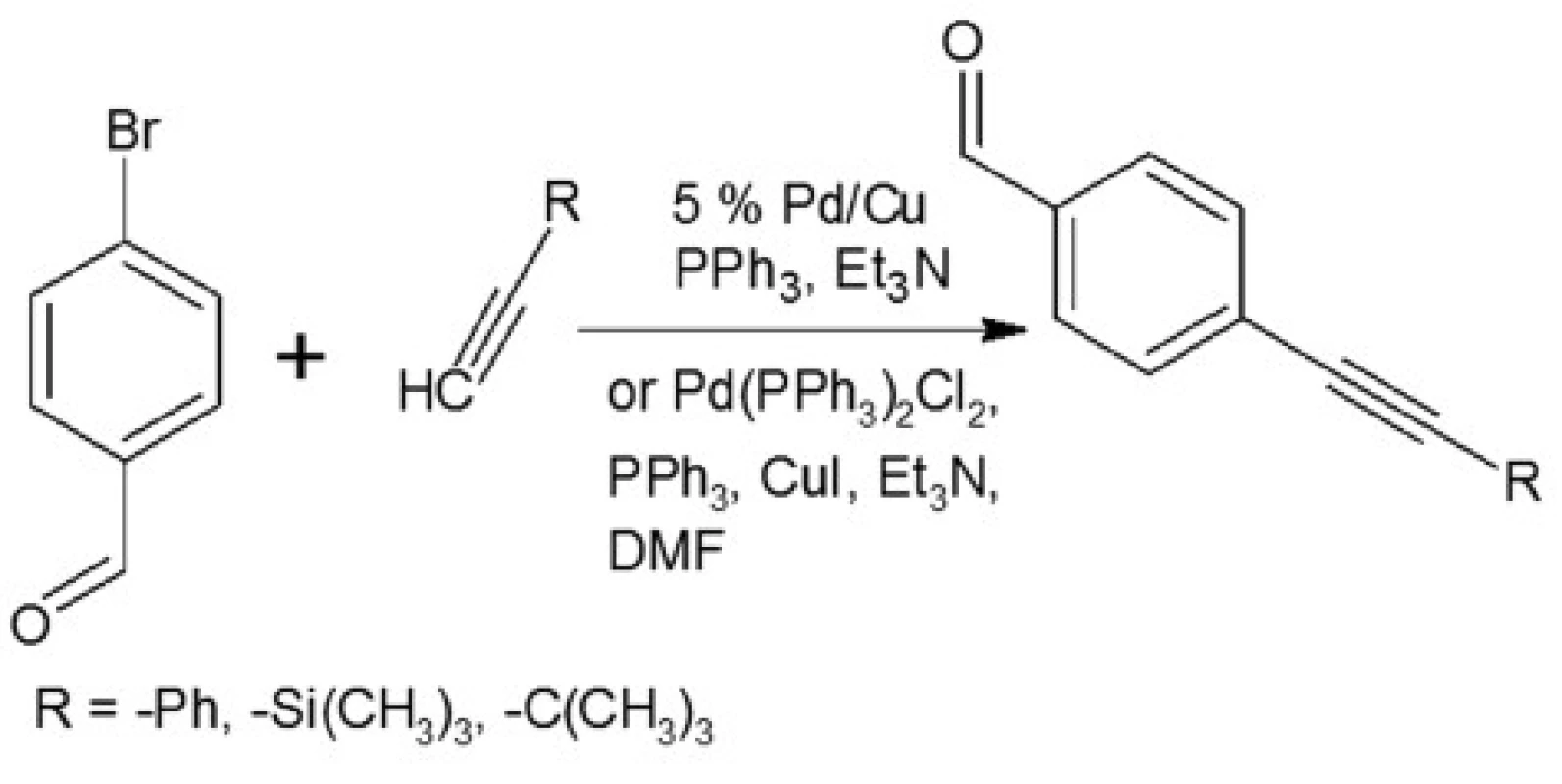
In our investigations we showed that the heterogeneous catalyst consisting of an active catalytic Pd species supported on copper is active enough to perform the Sonoghasira reaction under mild conditions. The reactions with the highly effective Pd/Cu system could provide even quantitative conversions and subsequently quantitative yields of the coupling products. Another important advantages of the use Pd/Cu catalyst are selectivity and high purity of the final products21). Synthesis of novel test compounds included: modification in preparation Pd/Cu catalyst, synthesis of building blocks – aldehydes (Fig. 2), synthesis of styrylquinoline (Fig. 3) and hydrolysis (Fig. 4). The building blocks were prepared using the Sonogashira reaction in homogeneous or heterogeneous conditions. This study employed the system of Sonogashira coupling catalysed by nano-Pd/Cu as a new approach to the synthesis of alkyne compounds having a formyl function at position 4 (Fig. 2). The next advantage of the use nano-Pd/Cu-catalyst is the lack of competitive reactions (the Glaser coupling) in the presence of air22).
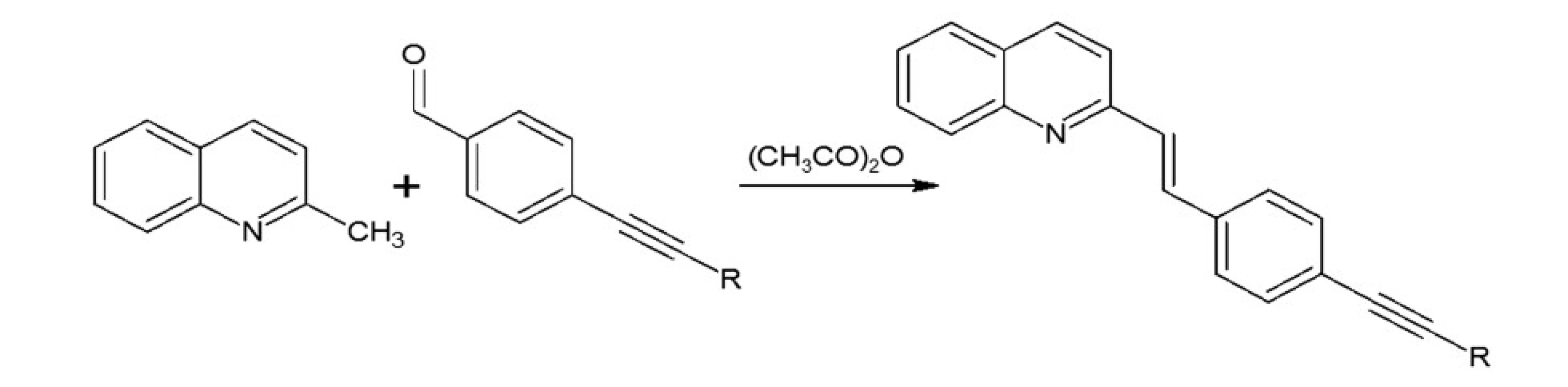
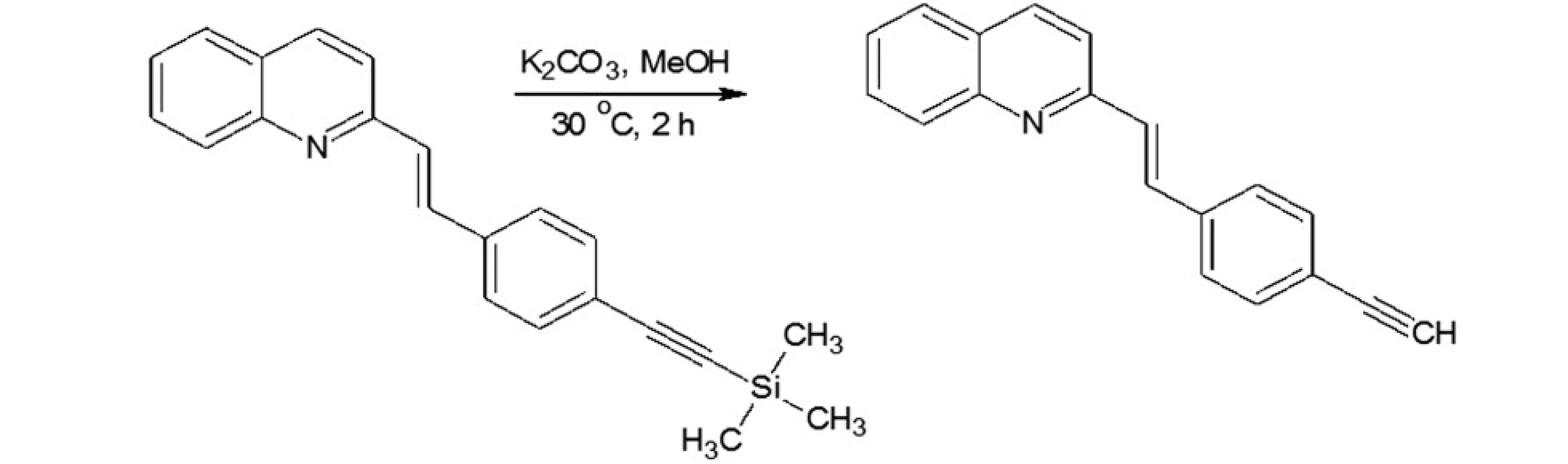
Styrylquinolines were obtained according to the standard method from quinaldine derivatives with carbonyl compounds in acetic anhydride20, 23). All new compounds were characterized by their NMR, and MS spectra elemental analysis.
The compounds that were tested appeared to be inactive against the HCT116 cell lines. The differences that were observed between excitation and emitted light for the dyes described in this report are applicable (Table 1). The quantum yields that were calculated are presented in Table 1. Among the compounds that were investigated, C had the highest quantum yield. Also for this compound we observed the highest molar absorption coefficients value (ε) in DMSO (ε = 44 000 M–1cm–1 at 360 nm). The cellular staining of compound C in the human colon carcinoma cells (HCT116) is presented in Fig. 5.
In Table 1 we showed that compounds incorporating an additional aromatic unit have better fluorescent properties. This is in agreement with the general opinion about the nature of the fluorophoric properties of the aromatic core. Polycyclic systems and scaffolds packed with π conjugated bonds are the base of the most effective fluorophores.
Conclusions
Several new quinoline derivatives were designed. These compounds were obtained according to the novel method of Sonogashira coupling on a bimetal nanocatalyst that provided us with quantitative conversions yielding the high purity target dye samples. The compounds that are presented have low toxicity and are tolerable in biological systems in concentrations that appeared effective for long time staining. The presented results suggest that the quinoline compounds that were investigated in this study may be valuable compounds for the development of new fluorescent dyes that could have biological applications. Their fluorescent properties make them potentially interesting leading structures for further development.
The financial support of the National Center for Science NCN grants 2014/13/D/NZ7/00322 (A.M.W.), 2012/07/N/NZ7/02110 (W.C.), 2013/09/B/NZ700423 (R.M.) is greatly appreciated. E.S. and M.K. appreciate the DoktoRIS studentship and W.C appreciate the CITTRFUŚ fellowship. Nano-Pd/Cu was prepared under National Research and Development Center (NCBiR) Grant ORGANOMET no: PBS2/A5/40/2014.
Conflicts of interest: none.
Mateusz Korzec, PhD. – student
Institute of Chemistry, University of Silesia
Szkolna 9, 40-006 Katowice, Poland
e-mail: korzec@autograf.pl
Sources
1. Lakowicz J. R. Principles of Fluorescence Spectroscopy. 3rd edition Boston: Springer 2006.
2. Pytel A., Schmeller N. New aspect of photodynamic diagnosis of bladder tumors: fluorescence cytology. Adult Urology 2002; 59, 216–219.
3. Jichlinski P., Jacqmin D. Photodynamic Diagnosis in Non-Muscle-Invasive Bladder Cancer. European urology supplements 2008; 7, 529–535.
4. Gomer Ch. J. Photodynamic Therapy. In: Kostron H. Photodynamic diagnosis and therapy and the brain. New York: Springer 2010. http://link.springer.com/book/10.1007/978-1-60761-697-9
5. Staderini M., Aulić S., Bartolini M., Tran H. N. A., González-Ruiz V., Pérez D. I., Cabezas N., Martínez A., Martín M. A., Andrisano V., Legname G., Menéndez J. C., Laur M. A fluorescent styrylquinoline with combined therapeutic and diagnostic activities against Alzheimer’s and prion diseases. ACS Med. Chem. Lett. 2013; 4, 225–229.
6. Hafuka A., Yoshikawa H., Yamada K., Kato T., Takahashi M., Okabe S., Satoh H. Application of fluorescence spectroscopy using a novel fluoroionophore for quantification of zinc in urban runoff. Water Res. 2014; 54, 12–20.
7. Praveen L., Reddy M. L. P., Varma R. L. Dansyl-styrylquinoline conjugate as divalent iron sensor. Tetrahedron Letters 2010; 51, 6626–6629.
8. Shiraishi Y., Ichimura C., Sumiya S., Hirai T. Multicolor fluorescence of a styrylquinoline dye tuned by metal cations. Cem. Eur. J. 2011; 17, 8324–8332.
9. Green B. Influence of pH and Metal Ions on the Fluorescence of Polycyclic Hydrocarbons in Aqueous DNA Solution. European Journal of Biochemistry 1970; 14, 567–574.
10. Lakowicz J. R., Malicka J., D’Auria S., Gryczynski I. Release of the self-quenching of fluorescence near silver metallic surfaces. Anal. Biochem. 2003; 320, 13–20.
11. Malicka J., Gryczynski I., Lakowicz J. R. DNA hybridization assays using metal-enhanced fluorescence. Biochem. Biophys. Res. Commun. 2003; 306, 213–218.
12. Malicka J., Gryczynski I., Fang J., Kusba J., Lakowicz J. R. Increased resonance energy transfer between fluorophores bound to DNA in proximity to metallic silver particles. Anal. Biochem. 2003; 315, 160–169.
13. Malicka J., Gryczynski I., Gryczynski Z., Lakowicz J. R. Effects of fluorophore-to-silver distance on the emission of cyanine-dye-labeled oligonucleotides. Anal. Biochem. 2003; 315, 57–66.
14. Malicka J., Gryczynski I., Fang J., Lakowicz J. R. Fluorescence spectral properties of cyanine dye-labeled DNA oligomers on surfaces coated with silver particles. Anal. Biochem. 2003; 317, 136–146.
15. Sankaran N. B., Banthia S., Samanta A. Fluorescence signalling of the transition metal ions: Design strategy based on the choice of the fluorophore component. Proc. Indian Acad. Sci. 2002; 114, 539–545.
16. Mastiholi B. M., Tangod V. B., Raikar U. S. Influence of metal nanoparticles on ADS560EI fluorescent laser dye. Optik 2013; 124, 261–264.
17. Sutter J. U., Birch D. J. S. Metal ion influence on eumelanin fluorescence and structure. Methods Appl. Fluoresc. 2014; 2, 1–8.
18. Elrobyab S. A. K., El-Shishtawya R. M., Makki M. S. I. Influence of the protonation, deprotonation and transition metal ions on the fluorescence of 8-hydroxyquinoline: a computational study. Molecular Simulation. 2011; 37, 940–952.
19. Asselin J., Viger M. L., Boudreau D. Metal-Enhanced Fluorescence and FRET in Multilayer Core-Shell Nanoparticles. Advances in Chemistry 2014, 1–16.
20. Rams-Baron M., Dulski M., Mrozek-Wilczkiewicz A., Korzec M., Cieslik W., Spaczyńska E., Bartczak P., Ratuszna A., Polanski J., Musiol R. Synthesis of New Styrylquinoline Cellular Dyes, Fluorescent Properties, Cellular Localization and Cytotoxic Behavior. Plos One 2015; 10, 1–17.
21. Korzec M., Bartczak P., Niemczyk A., Szade J., Kapkowski M., Zenderowska P., Balin K., Lelątko J., Polanski J. Bimetallic nano-Pd/PdO/Cu system as a highly effective catalyst for the Sonogashira reaction. Journal of Catalysis 2014; 313, 1–8.
22. Vilhelmsen M. H., Jensen J. The Glaser-Hay Reaction: Optimization and Scope Based on 13C NMR Kinetics Experiments. European Journal of Organic Chemistry 2013; 4, 701–711.
23. Cieslik W., Musiol R., Korzec M. Synthesis of Alkyne-substituted Quinolines as Analogues of Allylamines. International Bulletin of Pharmaceutical Sciences 2012; 1, 3–9.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2015 Issue 6
-
All articles in this issue
- Antibacterial activity of natural compounds – essential oils
- Cholinergic system of the heart
- Body surface area and body weight of Czech adult cancer population
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Antibacterial activity of natural compounds – essential oils
- Body surface area and body weight of Czech adult cancer population
- Cholinergic system of the heart
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid