PAG: Možný nádorový supresor a jak to bylo na počátku. Od imunní signalizace k nádorové transformaci
PAG: A POTENTIAL TUMOUR SUPPRESSOR AND HOW IT ALL STARTED. FROM IMMUNE SIGNALLING TO NEOPLASTIC TRANSFORMATION
Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG) also known as Csk-binding protein was first fully
characterized in 2000. It was initially recognized as a ubiquitously expressed adaptor protein recruiting cytoplasmic C-terminal Src-kinase
to the close proximity of plasma membrane-anchored Src-kinases thereby allowing Csk to impose its inhibitory potential on these kinases.
A role of PAG was initially seen in negative regulation of immune reactions. Since the year 2000 other Csk-dependent and independent
interactions have been discovered and some of them showed anti-oncogenic effects in experiment. According to current opinions, these
findings place PAG in a position of tumour suppressor candidate.
Key words:
PAG – Cbp – immune signalling – oncogenesis
:
A. Švec
:
Department of Cellular Pathology, James Cook University Hospital, Middlesbrough, United Kingdom
:
Čes.-slov. Patol., 45, 2009, No. 2, p. 35-39
:
Reviews Article
Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), rovněž známý jako Csk-binding protein byl plně
charakterizován v roce 2000. V původních studiích byl PAG rozpoznán jako ubikvitní adaptorový protein, který váže cytoplazmatickou Ckoncovou
Src-kinázu (Csk) do těsné blízkosti Src-kináz zakotvených v cytoplazmatické membráně, což umožnuje, aby Csk uplatnila svůj
inhibiční vliv na tyto kinázy. Úloha PAGu byla původně spatřována v negativní regulaci imunitních reakcí. Od roku 2000 byly objeveny další
na Csk závislé i nezávislé interakce, z nichž některé měly v experimentu anti-onkogenní účinky. Podle současných názorů staví tato
pozorování PAG do role kandidáta na nádorový supresor.
Klíčová slova:
PAG – Cbp – imunní signalizace – onkogeneze
PAG – a missing adaptor protein
It was not the singularity at the ‘PAG Big Bang’ but teams of researchers determined to crack the puzzle of interactions between membrane-bound immunoreceptors and the downstream cytoplasmic signalling molecules known to translocate to lipid rafts (specialised microdomains of the plasma membrane important for cell signalling) in response to receptor stimulation. In other words the scientific teams were looking for a missing link or a particular adaptor protein(s) (16). Indeed, the verge of the second millennium turned out to be notably successful as by 2002 the quartet of so far identified lipid raft-associated transmembrane adaptor proteins (RATRAPs) of haematolymphoid cells was discovered with a substantial contribution by Czech scientists (5-7). The currently recognized RATRAPs of haematolymphoid cells are LAT, PAG, NTAL and LIME (25). The minireview presents PAG, the only ubiquitously expressed RATRAP, as a potential tumour suppressor, a feature unexpected on its discovery.
First, it should be explained that adaptor proteins are signalling molecules without their own kinase or transcriptional activity but endowed with sequences capable of interactions with other signalling molecules attracting them to the site of action at the right time (25). The next two paragraphs provide necessary background information.
A touch of cell signalling
The general principles of cell signalling delineated below are exploited by a number of cell types. These include membrane receptor activation followed by phosphorylation of first-line kinases with subsequent initiation of phosphorylation cascades with diversification and amplification of the signal before the ultimate targets are reached. The final effect is a sum of ‘molecular cross-talk’ and can differ in different cell types and under different physiological or pathological conditions (1).
The PAG story started unwinding as a research into early events in immune signalling therefore I shall use immune signalling as an example of the proximal position of PAG and its widespread effects on down-stream kinase cascades.
Immune signalling is triggered by immune receptor engagement followed by phosphorylation of repetitive sequences of immunoreceptor signalling subunits known as immunoreceptor-based activation motifs (ITAMs) executed by Src family kinases (SFK). Phosphorylated ITAMs serve as docking sites for the kinases which take up the second position in signalling cascades, namely kinases of SYK (spleen tyrosine kinase) family; SYK in B-cells and ZAP70 (ζ chain-associated protein 70) in T-cells. These are activated and in turn phosphorylate the next in line effectors and adaptors thereby setting in action several signalling pathways such as NFκB (nuclear factor κB) and MAPK (mitogen activated protein kinase) with subsequent activation transcription factors NFκB, AP1 (activator protein 1) and NFAT (nuclear factor of activated T-cells), Ca release and modification of the cytoskeleton. Ultimately, a variety of cellular functions such as proliferation, survival, motility and secretion are affected (16).
A brief insight into Src family kinase affairs
SFK are a family of protein kinases catalysing transfer of phosphate to proteins thereby modulating their activity. All nine SFK family members identified in the vertebrates show a conserved organisation of their molecule into 6 domains responsible for anchoring the molecule into the membrane, substrate recognition, kinase activity and its regulation, a function shared by several domains (9).
SFK take up a key position in proximal signalling events prior to signal amplification and diversification. The far-reaching impact of SFK activation requires a tight regulation. This is achieved by intramolecular domain-to-domain interactions and by extrinsic phosphorylation which affect a conformation of the kinase with either a close or open position of the catalytic domain – the actual substrate-binding site. A crucial event for these conformational changes is phosphorylation of C-terminal tyrosine. There are several levels of regulation corresponding to several levels of activity. The enzyme is switched on to its most active mode when C-terminal tyrosine is dephosphorylated. Turned around, keeping SFK activity on leash means keeping the C-terminal tyrosine phosphorylated and this is a task for the enzyme known as C-terminal Src-kinase (Csk). A reader should refer to reference (18) for detailed information.
PAG on the scene
Csk role in SFK inhibition was known for years but it was not understood how the cytoplasm-bound Csk can interact with the membrane-bound SFK (30, 31). The answer emerged from opposite parts of the globe in 2000 when two independent teams characterised a previously known protein of unknown function (6, 22). They determined its cDNA sequence, amino acid composition, cell localisation, tissue distribution and last but not least its interactions. Not surprisingly, two different teams gave the protein two different names - phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG) and Csk-binding protein (Cbp). (Note: The acronym CBP is also an alias for CREBBP or CREB-binding protein which is unrelated to PAG or PAG1 gene.) The International Radiation Hybrid Mapping Consortium mapped PAG encoding gene PAG1 spanning 144,258 bp to chromosome 8, band 8q21.13 (http://www.ncbi.nlm.nih.gov/genome/guide/human/).
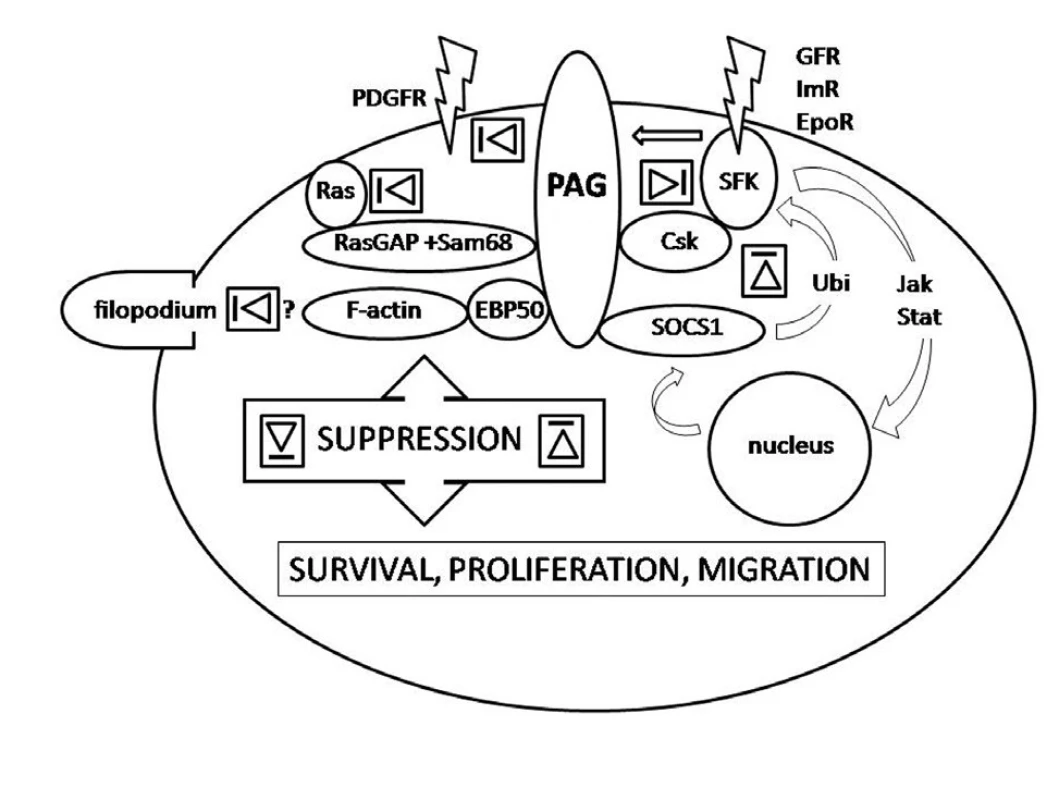
PAG was recognized as a ubiquitous adaptor protein recruiting Csk and enhancing its enzymatic activity (6, 22, 42). It brings Csk to close proximity to SFK allowing Csk to phosphorylate the SFK C-terminal negative regulatory tyrosine. Importantly, PAG itself needs to be phosphorylated to bind Csk and this is in turn executed by SFK. Obviously, this is a negative feedback curbing SFK activity. It starts by activation of SFK followed by PAG phosphorylation and Csk recruitment and ending by SFK suppression (Fig.). This regulatory loop was observed both in haematopoietic and non-haematopoietic cells (6, 10, 22, 29, 46, 49). Interestingly, two modifications of the loop have been recognized. In the first scenario T-cell receptor activation is followed by PAG dephosphorylation, release of Csk and activation of SFK Fyn and Lck. SFK are curbed in their activity again as a result of PAG phosphorylation by Fyn, the action taken by consequently recruited Csk. This loop keeps T-cells in a resting state. By contrast, upon activation of FceRI receptor PAG is phosphorylated, Csk recruited and activity of SFK suppressed. This loop is significant for setting a threshold on degranulation of mast cells (16).
As early as the original reports, the authors commented on the presence of ITAM-like repetitive sequences within the PAG cytoplasmic domain with a number of tyrosine residues and other sites rich in serine, threonin or prolin, all of which are capable of phosphorylation and interactions with yet unidentified molecules (6). This turned out to be a correct presumption as several new Csk-related or unrelated interactions were documented in the forthcoming years. Those with suggested implications for oncogenesis are reviewed in the following paragraphs. More information about PAG molecule and its interactions can be found elsewhere (39).
What was learnt from a cattle parasite?
A short trip into the realm of veterinary pathology will reveal existence of a protozoan parasite called Theileria parva, a causative agent of a serious tick born infection of the cattle known as East Coast Fever. The parasite complete a complicated life cycle both in its vector and its host where it invades lymphocytes ‘transforming” them into blasts which gain the ability to proliferate and disseminate in a way similar to human lymphomas or leukaemias (28). The parasite resides in the cytoplasm which on one hand provides a safe protection against lysosomal destruction and on the other hand it gives a good opportunity to subvert signalling pathways. Indeed, the parasite activates PI3K (phosphatidylinositol-3-kinase) and NFκB pathways thereby promoting proliferation and blocking apoptosis (12). Similarity to deregulation of signalling pathways in malignant tumours is quite obvious.
What is the major difference from malignancy is that the ‘transformed cells’ reverse to normal phenotype upon clearance of the parasite. This unique property of reversible ‘malignant transformation’ was exploited by Baumgartner et al.(3).
The authors observed that Theileria parva-induced transformation is associated with down-regulation of PAG expression, which appears to be an underlying cause of Csk release from lipid rafts followed by sustained high activity of SFK Hck. In contrast, treatment of the infection led to PAG re-expression with Csk recruitment to lipid rafts, suppression of SFK Hck activity and return to the normal phenotype. The results suggest that a lack of Csk-PAG inhibition participates in pathogenesis of the Theileria-induced lymphoproliferative disorder and that similar deregulation of SFK activity may play a role in human lymphoproliferative diseases (3).
PAG in the limelight of oncogenesis
Many signalling pathways regulating proliferation and survival start by activation of receptor tyrosine kinases (RTK) including growth factor receptors such as EGFR (epidermal growth factor receptor). These are membrane-bound receptors activated by the process of autophosphorylation as a result of dimerization or conformational changes upon ligand engagement. Activated receptors bind and phosphorylate down-stream signalling molecules thereby setting in motion several pathways including MAPK and PI3K pathways important for cell-cycle progression (34). Observations of concurrent SFK Src and EGFR over-expression in various tumours prompted research culminating in the findings that SFK Src serves as EGFR kinase, increasing its mitogenic and tumourigenic potential (45).
Not surprisingly, regulation of SFK in EGFR signalling by the Csk-PAG tandem did not escape attention of investigators. In 2004, Matsuoka documented existence of the SFK-PAG-Csk negative regulatory loop initiated by EGFR stimulation by the technique of fluorescence resonance energy transfer (27). In 2006 Jiang et al. observed that over-expression of PAG has an inhibitory effect on transformation of the NIH3T3 stable cell line and colony formation of a breast cancer cell line in agar (21). On the other hand, transformation and colony formation were enhanced in PAG mutants unable of Csk interaction or modified by PAG RNA interference silencing. Similarly to EGFR, alteration of PAG expression affected signalling pathways mediated by other RTKs such as platelet derived growth factor receptor (PDGFR), fibroblast growth factor receptor-1 and tyrosine kinase receptor A. The authors suggested that PAG has a protective effect against tumourigenesis by setting a threshold on RTK-mediated signalling (21).
This view was supported in 2008, when Veracini et al. documented a new Csk-independent regulatory mechanism based on regulation by sequestration (47). The authors showed that PAG can incite accumulation of GM1 ganglioside in caveolae (a special example of lipid rafts), which has a profound effect on localisation of PDGFR. This is pushed out of the caveolae and detached from its down-stream signalling machinery which results in suppression of mitogenic signalling via PDGFR (47).
A similar mechanism but working in the opposite direction was proposed by Oneyama et al. The authors investigated the fact that there are tumours with high activity of SFK Src despite high activity of Csk. They hypothesized that there must be deregulation(s) responsible for the failure of Csk to suppress SFK Src (32). Their results indicated that PAG directly binds SFK Src sequestrating it in lipid rafts which hampers interactions with down-stream kinase cascades. Moreover, SFK Src kept in the lipid rafts is exposed to the suppression executed by Csk (32).
Furthermore, the authors observed that mRNA levels in the colonic carcinoma was lower than in the adjacent non-neoplastic mucosa and that there was an inverse correlation between Src activity and PAG expression with nearly no PAG detectable in some cancer cell lines. Morevoer, effects of PAG over-expression in cancer cell transfectants were detected only upon stimulation. These cells inoculated into nude mice gave rise to tumours that were significantly smaller than tumours derived from non-manipulated cells, supposedly as a result of PAG activity promoted by stimulatory signals from the microenvironment (32).
The authors concluded that PAG is an important SFK Src inhibitor in vivo acting not only in tandem with Csk but also as an independent regulator whose transcriptional repression may be a significant step in Src-dependant neoplastic transformation (32).
Except for the shift towards survival and proliferation, the cardinal features of the neoplastic cells are the ability to disengage from organized tissue structure, to degrade the extracellular matrix and to migrate. Migration is a complex process requiring re-building of cell-cell and cell-extracellular matrix interactions with dynamic reshuffling of membrane-bound adhesion molecules and modification of the cytoskeleton which is reflected in morphological changes of the migrating cells. The evidence that PAG could link membrane-bound receptors with the cytoplasmic actin microfilaments was published as early as 2001 (8, 20). In 2008 Ingley supported the original results by microscopic observations of mutant cell lines (17). He investigated COS-7 cell lines modified by over-expression of wild-type PAG or PAG crippled by mutations preventing Csk or SFK Lyn binding and showed that PAG in a complex with SFK Lyn has a suppressive effect on formation of filopodia. Their formation was increased in transfectants with constitutively active Lyn whereas inactivation of Lyn resulted in lamellipodia formation (17). As filopodia represent ‘spearheads’ of migrating cells searching for signals in the extracellular environment and filopodia-like structures are rich in metastatic cells (50), the observations appear to lend support to existence of another tumour-suppressing effect mediated by PAG.
Is PAG a new tumour suppressor?
Neoplastic transformation and tumour progression are manifestations of accumulation of chromosomal aberrations that lead to ‘concerted deregulation’ of proto-oncogenes, whose products promote cell cycle progression and survival, and/or tumour-suppressor genes, whose products incite cell cycle arrest and/or apoptosis (33). (However modern may it sound, the idea of collaborative action of genes can be traced as back as 1914 (24)). Although it is a recognized fact today that cancer is both genetic and epigenetic disease, the concept of tumour suppressors as molecules preventing development of cancer by balancing actions of positive cell cycle regulators is still valid (15, 23).
The above reviewed data show that PAG lives up to the tumour suppressor nature as it has been proved that PAG has the capacity to suppress activity of SFK, counteracting their pro-proliferative and oncogenic effects by multiple mechanisms including direct suppression of a known oncoprotein Src and down regulation of PDGFR mediated signalling (2, 32, 35, 47). Two other interactions possibly significant for protection against tumourigenesis should be mentioned.
In 2006 Ingley et al. proposed a sequential functional and structural double regulatory loop mediated by PAG (19). Their experimental data indicated that PAG mediates functional suppression of SFK Lyn by Csk, hours later followed by interaction with SOCS1 (suppressor of cytokine signalling-1) responsible for Lyn ubiquitination resulting in its proteosomal degradation. In 2007 Smida et al. observed that PAG participates in down-regulation of monomeric Ras, a known oncoprotein, as a constituent of RasGAP, Sam68 and PAG complex reversing the active GTP-binding Ras into its inactive GDP-binding form (37). The models were proposed for erythroid cells and T-cells but they may be valid for other cell types as well (19, 37). All the interactions which may play a role in oncogenesis are summarised in the figure.
To keep the discussion in balance several notes should be made. PAG can be involved in positive regulatory loops (11, 38), some highly proliferative cell pools strongly express PAG (40), PAG in a complex with other molecules can prevent lymphoma cells from apoptosis (43) and some cancer cell lines have their motility diminished upon PAG RNA interference silencing (36). Last but not least, the role of PAG in Csk recruitment can be taken over by other Csk-binding molecules (11, 13, 48).
However, neither of these observations should disqualify PAG as a tumour suppressor candidate as the image of oncogenes and tumour suppressor genes is far from being black-and-white. There is accumulating evidence that products of both have the ability to bring about opposite actions (4, 14, 26).
In summary, the PAG story started as a search for a protein involved in early phases of immune signalling but along the way the investigations diversified into the field of oncogenesis with potential practical implications for cancer diagnosis and therapy (40, 41, 43, 44). Despite many questions about PAG interactions and PAG1 gene regulation yet unanswered, the published data taken together suggest that PAG is an important molecule not only for immune response but also for neoplastic transformation and progression within - and outside the haematopoietic system.
Acknowledgements:
The author is grateful to Prof. V. Horejsi for critical comments.
Alexandr Švec, MD, PhD
Department of Cellular Pathology
James Cook University Hospital
Marton Rd
Middlesbrough
TS4 3BW
Velká Británie
Telefon: +44-01642 850850
E-mail: alexandr.svec@stees.nhs.uk
Sources
1. Alberts, B., Johnson, A., Lewis, J., et al.: Biology of the Cell. 5th ed., New York: Garland Science, 2008, s. 879-964.
2. Alvarez., R.H., Kantarjian, H.M., Cortes, J.E.: The role of Src in solid and hematologic malignancies: development of new-generation Src inhibitors. Cancer, 107, 2006, s.918-29.
3. Baumgartner, M., Angelisova, P., Setterbladl, N., et al: Constitutive exclusion of Csk from Hck-positive membrane microdomains permits Src-kinase-dependent proliferation of Theileria-transformed B lymphocytes. Blood, 101, 2003, s.1874-81.
4. Besson, A., Hwang, H.C., Cicero, S., et al.: Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev., 21, 2007, s.1731-46.
5. Brdicka, T., Imrich, M., Angelisova, P. et al.: Non-T Cell Activation Linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med., 196, 2002, s.1617–1626.
6. Brdicka, T., Pavlistova, D., Leo, A. et al.: Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med., 191, 2000, s.1591-604.
7. Brdickova, T., Brdicka, P., Angelisova, O., et al.: LIME: a new membrane Raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J. Exp. Med., 198, 2003, s.1453–1462
8. Brdicková, N., Brdicka, T., Andera, L., et al.: Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Lett., 507, 2001, s. 133-6.
9. Brown, M.T., Cooper, J.A.: Regulation, substrates and functions of src. Biochim Biophys Acta., 1287, 1996 s.121-49.
10. Davidson, D., Bakinowski, M.,Thomas, M.L., et al: Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol. Cell Biol., 23, 2003, s.2017-28..
11. Davidson, D., Schraven, B., Veillette, A., et al.: PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. Mol. Cell Biol. 27, 2007, s.1960-73.
12. Dobbelaere, D.A., Küenzi, P.: The strategies of the Theileria parasite: a new twist in host-pathogen interactions. Curr. Opin. Immunol., 16, 2004, s.524-30.
13. Dobenecker, M.W., Schmedt, C., Okada, M. et al.: The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol. Cell Biol., 25, 2005, s.10533-42.
14. Engelberg, D.: Stress-activated protein kinases-tumor suppressors or tumor initiators? Semin. Cancer Biol., 14, 2004, s.271-82.
15. Esteller, M.: Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br. J. Cancer., 96, 2007, Suppl:R26-30.
16. Horejsi, V., Zhang , W., Schraven, B.: Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. Immunol., 4, 2004, s.603-16.
17. Ingley, E.: Csk-binding protein can regulate Lyn signals controlling cell morphology. Int. J. Biochem. Cell. Biol., 2008, DOI 10.1016/j.biocel.2008.12.001
18. Ingley, E.: Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochim. Biophys. Acta, 1784, 2008, s.56-65.
19. Ingley, E., Schneider, J.R. , Payne, C.J., et al: Csk-binding protein mediates sequential enzymatic down-regulation and degradation of Lyn in erythropoietin-stimulated cells. J. Biol. Chem., 281, 2006, s.31920-9.
20. Itoh, K., Sakakibara, M., Yamasaki, S., et al.: Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J. Immunol., 168, 2002, s. 541-4.
21. Jiang, L.Q., Feng, X., Zhou, W., et al.: Csk-binding protein (Cbp) negatively regulates epidermal growth factor-induced cell transformation by controlling Src activation. Oncogene, 25, 2006, s.5495-506.
22. Kawabuchi, M., Satomi, Y.,Takao, T. et al.: Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature, 404, 2000, s.999-1003.
23. Kent, O.A., Mendell, J.T.: A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene, 25, 2006, s.6188-96.
24. Knudson, A.G.: Two genetic hits (more or less) to cancer. Nat. Rev. Cancer, 1, 2001 s.157-62.
25. Lindquist, J.A., Simeoni, L., Schraven, B.: Transmembrane adapters: attractants for cytoplasmic effectors. Immunol. Rev., 191, 2003, s.165-82.
26. Lowe, S.W., Cepero, E., Evan, G.: Intrinsic tumour suppression. Nature, 7015, 2004, s.307-15.
27. Matsuoka, H., Nada, S., Okada, M. Et al: Mechanism of Csk-mediated down-regulation of Src family tyrosine kinases in epidermal growth factor signaling. J. Biol. Chem., 279, 2004, s.5975-83.
28. McKeever, D.J.: Theileria parva and the bovine CTL response: down but not out? Parasite Immunol., 28, 2006, s.339–345.
29. Ohtake, H. N., Ichikawa, M., Okada, T. Et al.: Cutting Edge: Transmembrane phosphoprotein Csk-binding protein/phosphoprotein associated with glycosphingolipid-enriched microdomains as a negative feedback regulator of mast cell signaling through the FcepsilonRI. J. Immunol. 168, 2002, s.2087-90.
30. Okada, M., Nada, S., Yamanashi, Y., et al: CSK: a protein-tyrosine kinase involved in regulation of Src family kinases. J. Biol. Chem., 25, 1991, s.24249-52.
31. Okada, M., Nakagawa, H.: Identification of a novel protein tyrosine kinase that phosphorylates pp60c-src and regulates its activity in neonatal rat brain. Biochem. Biophys. Res. Commun., 154, 1988, s.796-802.
32. Oneyama, C., Hikita, T., Enya, K. Et al: The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol. Cell, 30, 2008, s.426-36.
33. Pelengaris, S., Khan, M.: Oncogenes. Introduction. In: Pelengaris, S., Khan, M., eds. The Molecular Biology of Cancer. Oxford, UK: Blackwell Publishing Ltd., 2006, s.158-171.
34. Pollard, T.D., Earnshaw, W.C.: Integration of signals, In: Pollard, T.D., Earnshaw, W.C., Cell Biology, 1st ed., Philadelphia, Saunders, 2002, s.445-447.
35. Resh, M.D.: The ups and downs of SRC regulation: tumor suppression by Cbp. Cancer Cell, 13, 2008, s.469-71.
36. Shima, T., Nada, S., Okada, M. et al: Transmembrane phosphoprotein Cbp senses cell adhesion signaling mediated by Src family kinase in lipid rafts. Proc. Natl. Acad. Sci. U. S. A., 100, 2003, s.14897-902.
37. Smida, M., Posevitz-Fejfar, A., Horejsi, V. et al.: A novel negative regulatory function of the phosphoprotein associated with glycosphingolipid-enriched microdomains: blocking Ras activation. Blood, 2007, 110, s.596-615.
38. Solheim, S.A., Torgersen, K.M., Taskén, K. et al.: Regulation of FynT function by dual domain docking on PAG/Cbp. J. Biol. Chem., 2008, 283, s.2773-83.
39. Svec, A.: Phosphoprotein associated with glycosphingolipid-enriched microdomains/Csk-binding protein: a protein that matters. Pathol. Res. Pract., 204, 2008, s. 785-92.
40. Svec, A., Velenska, Z., Horejsi, V.: Expression pattern of adaptor protein PAG: Correlation between secondary lymphatic follicle and histogenetically related malignant lymphomas. Immunol. Lett., 100, 2005, s.94-7.
41. Svojgr, K., Burjanivova, T., Vaskova, M., et al.: Adaptor molecules expression in normallymphopoiesis and in childhood leukemia. Immunol. Lett., 2009, DOI : 10.1016/j.imlet.2008.12.008
42. Takeuchi S., Takayama, Y., Ogawa, A. et al.: Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src-kinase, Csk. J. Biol. Chem., 2000, 275 s.29183-6.
43. Tauzin, S., Ding, H., Khatib, K. et al.: Oncogenic association of the Cbp/PAG adaptor protein with the Lyn tyrosine kinase in human B-NHL rafts. Blood, 111, 2008, s.2310-20.
44. Tedoldi, S., Paterson, J.C., Hansmann, M.L. et al.: Transmembrane adaptor molecules: a new category of lymphoid-cell markers. Blood, 107, 2006, s.213-21.
45. Tice, D.A., Biscardi, J.S., Nickles, A.L. et al.: Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. U. S. A., 96, 1999, s.1415-20.
46. Torgersen, K.M., Vang, T., Abrahamsen, H. et al.: Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem., 276, 2001, s.29313-8.
47. Veracini, L., Simon, V., Richard, V. et al: The Csk-binding protein PAG regulates PDGF-induced Src mitogenic signaling via GM1. J Cell Biol. 182, 2008, s. 603-14.
48. Xu, S., Huo, J., Tan, J.E. et al.: Cbp deficiency alters Csk localization in lipid rafts but does not affect T-cell development. Mol. Cell Biol., 19, 2005, s.8486-95.
49. Yasuda, K., M. Nagafuku, T. Shima, M. Et al.: Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol., 169, 2002, s.2813–2817.
50. Yilmaz, M., Christofori, G.: EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev., 2009, DOI : 10.1007/s10555-008-9169-0.
Labels
Anatomical pathology Forensic medical examiner ToxicologyArticle was published in
Czecho-Slovak Pathology

2009 Issue 2
-
All articles in this issue
- New Trends in Diagnostics and Classification of Breast Carcinoma
- PAG: A POTENTIAL TUMOUR SUPPRESSOR AND HOW IT ALL STARTED. FROM IMMUNE SIGNALLING TO NEOPLASTIC TRANSFORMATION
- Immunohistochemical Detection of ZAP-70 Protein and its Importance in the Diagnostics of B-CLL
- Our Experience in Using Fluorescence in situ Hybridization FISH-Uro Vysion in Diagnostics of Urothelial Carcinoma
- Thyroid Carcinoma in Children and Adolescents Resulting from the Chernobyl Accident: Possible Causes of the Incidence Increase Overestimation
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- New Trends in Diagnostics and Classification of Breast Carcinoma
- Immunohistochemical Detection of ZAP-70 Protein and its Importance in the Diagnostics of B-CLL
- Our Experience in Using Fluorescence in situ Hybridization FISH-Uro Vysion in Diagnostics of Urothelial Carcinoma
- PAG: A POTENTIAL TUMOUR SUPPRESSOR AND HOW IT ALL STARTED. FROM IMMUNE SIGNALLING TO NEOPLASTIC TRANSFORMATION
