Improvement of diagnostic approach to Lyme neuroborreliosis in children by using recombinant antigens in detection of intrathecally produced IgM/IgG
Zlepšení diagnostického přístupu použitím rekombinantních antigenů v detekci intratekální produkce protilátek IgM/IgG u dětí s lymeskou neuroboreliózou
Cíl:
Záměrem studie bylo vyhodnocení testu 3. generace EIA Borrelia recombinant IgM and IgG (TestLine, Brno, ČR) v séru a mozkomíšním moku (mm) dětí s lymeskou neuroborreliózou (LNB).
Metodika:
Ke srovnání byly využity 3 testy: celobuněčná EIA z Borrelia garinii (EIA 1) byla srovnána s EIA testem využívajícím rekombinantní antigeny (EIA 2) a imunoblot. Celkem bylo vyšetřeno 364 vzorků sér a mm. V první skupině dětí s LNB bylo vyhodnoceno 86 párových vzorků sér a mm. Druhou skupinu tvořilo 36 dětí s pravděpodobnou LNB. Jako kontroly byly využity vzorky 66 dětí s neuroinfekcí neborreliové etiologie.
Výsledky:
V první skupině dětí s prokázanou LNB, EIA 2 vykazovala více pozitivních výsledků v séru ve třídě IgG protilátek ((p = 0.006; OR = 7,5), stejně jako v mm (p < 0,001; OR = 4,5). V pozitivních výsledcích ve třídě IgM v séru nebyl statisticky významný rozdíl mezi EIA 1 a EIA 2 (p = 0,54; OR = 0,71). V mm dětí s LNB bylo pomocí EIA 2 stanoveno signifikantně méně pozitivních vzorků ve třídě IgM (p = 0,001; OR = 0,06). Protilátkový index IgG (AI) vyhodnocený oběma metodami vykazoval obdobné výsledky (p = 0,646; OR = 1,38). Obě metody jsou srovnatelné, ale AI IgM stanovené pomocí EIA 2 prokázalo signifikantně méně pozitivních výsledků (p < 0,001; OR = 0,04). Ve skupině dětí s pravděpodobnou LNB a v kontrolní skupině nebyly rozdíly v detekci pozitivních IgM/IgG protilátek v séru a mm statisticky významné.
Závěr:
EIA 2 vykazovala více pozitivních výsledků než EIA 1 a než imunoblot v detekci pozitivních protilátek v séru a mm. Ve skupině dětí s prokázanou LNB nebyl mezi oběma metodami rozdíl ve výpočtu protilátkového indexu IgG. Po srovnání IgG a IgM protilátkových indexů počítaných z výsledků obou metod byla citlivost EIA 2 pro IgG 68 % a 26 % pro IgM. Pro oba testy je specificita 100 %.
KLÍČOVÁ SLOVA:
lymeská neuroborrelióza – rekombinantní antigeny – specifický protilátkový index proti borreliím – sérodiagnostika
Authors:
L. Krbková 1; J. Bednářová 2; Z. Čermáková 3
Authors‘ workplace:
Department of Children’s Infectious Diseases, University Hospital, Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic
1; Department of Clinical Microbiology, University Hospital, Brno, Czech Republic
2; Department of Clinical Biochemistry, University Hospital, Brno, Department of Laboratory Methods, Faculty of Medicine, Masaryk University, Brno, Czech Republic
3
Published in:
Epidemiol. Mikrobiol. Imunol. 65, 2016, č. 2, s. 112-117
Category:
Original Papers
Overview
Background:
The purpose of the study was to evaluate new 3rd-generation test kits, EIA Borrelia recombinant IgM and IgG (TestLine, Brno, Czech Republic), in serum and cerebrospinal fluid (CSF) of children with Lyme neuroborreliosis.
Methods:
Comparison of three tests was used: the whole-cell EIA from Borrelia garinii (EIA 1) was compared with the EIA using recombinant antigens (EIA 2) and immunoblot. In total, 364 samples of serum and CSF were examined. Eighty-six paired sera and CSF samples were evaluated in the first group of children with Lyme neuroborreliosis. The second group consisted of 30 children with probable Lyme neuroborreliosis. Sixty-six samples from children with neuroinfections other than borrelial etiology were used as controls.
Results:
In the first group of children with proven LNB, EIA 2 gave significantly more positive results for IgG in serum (P = 0.006; OR = 7.5) as in CSF (P < 0.001; OR = 4.5). There was no statistically significant difference in the IgM positivity of serum (P = 0.54; OR = 0.71). EIA 2 determined significantly (P = 0.001; OR = 0.06) less positive results of IgM in CSF in the LNB patients. IgG antibody index (AI) assessed by both methods revealed similar results (P = 0.646; OR = 1.38). Both methods are comparable, but IgM AI assessed by EIA 2 showed significantly less positive results (P < 0.001; OR = 0.04).
The differences in the detection of positive IgM/IgG antibodies in serum and CSF did not reach statistical significance either in the groups of children with excluded LNB or in controls.
Conclusions:
EIA 2 showed better results than EIA 1 and western blot for the detection of positive IgG antibodies in serum and CSF. The difference in the calculation of AI IgG by EIA 1 and EIA 2 was not noticeable in the group of LNB patients. Comparing IgG and IgM AIs calculated from both tests, the sensitivity for EIA 2 was 68% for IgG and 26% for IgM. The specificity is 100% for both tests.
KEYWORDS:
Lyme neuroborreliosis – recombinant antigens – specific anti-Borrelia antibody index – serodiagnosis
INTRODUCTION
Lyme borreliosis is a frequent diagnosis in the Czech Republic, where the annual incidence varies from 30 to 40 cases per 100,000. Neurological involvements occur in up to 25% of all patients. Among children, the incidence of Lyme neuroborreliosis (LNB) is five times greater than is that of tick-borne encephalitis in the region of Brno, South Moravia, where both infections are endemic. Aseptic meningitis and cranial neuritis are typical for neuroborreliosis in children, although none of these involvements are pathognomonic.
Laboratory diagnostic investigation includes assessment of antibodies in serum and cerebrospinal fluid (CSF), cytology and biochemistry of CSF, and intrathecal antibody synthesis. A reliable diagnosis can be achieved by combining clinical involvement and lymphocytic pleocytosis. Intrathecal synthesis is crucially important. According to the case definition [1] and European Federation of Neurological Societies guidelines [2], all three criteria need to be met for definite diagnosis of LNB.
The methods for direct detection of borreliae, such as cultivation and polymerase chain reaction (PCR), are not suitable for routine practice because of their long cultivation period and low sensitivity in CSF, even though they are 98–100% specific. Using a modified Kelly medium, the cultures are positive in 10–30% of early LNB cases [3]. PCR methods are not standardized, and CSF sensitivity reaches 10–35% in early LNB [4]. There is a lack of comparative studies of diagnostic specificity for detection of exclusively Borrelia burgdorferi sensu lato DNA.
Specific antibodies in serum and CSF detected by enzyme-linked immunosorbent assay (ELISA) are widely used with sonicated whole-cell antigen, recombinant antigens or the single VlsE antigen for the diagnosis of LNB [5].During early disseminated LNB, expression of antigens by borreliae occurs at variable times. Assays using only one recombinant protein with the correct sequence may not detect antibodies at each stage of infection [6].
The outer surface protein C (OspC) antigen is associated with an early dominant immune response to Lyme borreliosis. Variable major protein-like sequence, expressed (VlsE), a variable surface lipoprotein of B. burgdorferi s.l., contains variable and invariable domains. Within the VlsE variable domain there are six invariable regions (IR 1–6). IR 6 is exposed on the surface of VlsE and is immunodominant in patients with Lyme borreliosis [7–8]. Variability of the antibody response among patients with Lyme borreliosis has been described in many studies [9–11].
The purpose of our study was to evaluate new kits of the 3rd generation, EIA Borrelia recombinant IgM and IgG (TestLine, Clinical Diagnostics, Brno, Czech Republic), in serum and CSF of children with LNB and to compare the results with intrathecally produced IgG and IgM antibodies measured by ELISA using whole-cell antigen (TestLine, Clinical Diagnostics, Brno, Czech Republic) and with western blot (EUROIMMUN, Medizinische Labordiagnostika, Lübeck, Germany).
METHODS AND PATIENTS
Methods. Serum samples used for testing were routinely obtained by venipuncture and CSF samples were collected by lumbar puncture before the treatment was initiated. Albumin, IgG and IgM concentrations in serum in g/L and in CSF in mg/L were determined in addition to routine examination of CSF (cytology, protein, glucose and lactate). The concentrations of albumin and immunoglobulins in serum were measured by photometry/immunoturbidimetry using a COBAS 8000 analyzer (Roche, Basel, Switzerland) and in CSF using an immunonephelometry analyzer IMMAGE 800 (Beckman Coulter, Fullerton, CA, USA). Albumin quotient (Qalb) was calculated as the ratio of CSF to serum albumin, Qalb > 5 was evaluated as light disturbance of the blood–CSF barrier, whereas Qalb >10 was assumed to indicate severe disturbance of the blood-CSF barrier. Antibody indices (AI) for IgM and IgG were determined in all CSF samples by the method of Reiber et al. [12] , which is regarded as the gold standard for the definition of LNB. The AI expresses the ratio of pathogen-specific antibodies in the CSF to specific antibodies in blood serum in relation to condition of the blood-CSF barrier and concentration of the total immunoglobulins in the CSF and serum. The AI was calculated by using Antibody Index Software and calibration curve from AI-Standard (TestLine, Clinical Diagnostics, Brno, Czech Republic). An AI value >1.4 for IgM and IgG antibodies against recombinant antigens or against Borrelia garinii was taken as positive. AI was calculated from both tests and compared.
EIA Borrelia garinii (TestLine, Clinical Diagnostics, Brno, Czech Republic) IgM and IgG, further EIA 1, contains sonificated whole-cell antigen of B. garinii with a higher content of p83, p41 (flagellin), p39, OspA, OspC, p18 and p14.
The new ELISA (TestLine, Clinical Diagnostics, Brno, Czech Republic) IgM and IgG, further EIA 2, tests are based on the following selected antigen fragments: OspC, p39 and internal flagellin (p41i) for IgM and p17, OspC, p39, p41i, p83 and VlsE for IgG. Recombinantly produced protein fragments were selected according to the most commonly occurring borrelial subspecies in European countries, including the Czech Republic: B. garinii, B. afzelii and B. burgdorferi sensu stricto. Selected antigen fragments are typical for early and late antibody response with production of IgM/IgG antibodies.
Both EIA tests were processed by the instructions of manufacturer. The cut-off control is a component of the test kit which makes positivity index (IP) evaluation possible. Positive results are considered to be those which were indicated as borderline (IP = 0.9–1.1) or positive (IP > 1.1). Samples having IP < 0.9 were evaluated as negative. The evaluation of IP is the same for EIA 1 and EIA 2.
Western blot of B. garinii (EUROIMMUN, Medizinische Labordiagnostika, Lübeck, Germany) was used as the third comparative method for detection of anti-Borrelia specific antibodies in serum and CSF. Interpretation criteria were applied as recommended by the manufacturer. The following specific antigens are included: p19, p21, OspC, OspA, p30, p39, p83 without VlsE.
Second samples (n = 9) were obtained for clinical suspicion of LNB with the negative result from the first sample, for unsatisfactory treatment status or as control for extremely high pleocytosis and elevated CSF protein (day 19 to 21). Second samples were evaluated by standard methods, and AI was calculated from EIA 1, positive result of AI in the second sample confirmed the final diagnosis of LNB in children with the negative result from the first samples. The only exceptions were 7 stored second samples, which could be used for the detection of antibodies by EIA 2 and AI was calculated. These results are not used for statistical analysis, but only in detailed description of patients.
For viral etiology, herpetic viruses including HSV1 and 2, HHV6, EBV and enteroviruses were tested in serum and CSF by PCR while tick-borne encephalitis virus (TBEV) antibodies were tested using ELISA.
All biochemistry procedures were carried out at the Department of Clinical Biochemistry of the University Hospital in Brno, Czech Republic. Serological examinations, PCR methods and AI were performed and calculated at the Department of Clinical Microbiology of the same hospital.
Statistical analyses. McNemar’s nonparametric test, Fisher’s exact test and the odds ratio (OR) were used for statistical analysis. Statistical significance was determined at P < 0.05. Borderline results (IP = 0.9–1.1) of both EIAs and western blot were assigned to positive ones for statistical analysis due to the small number of patients with borderline values.
Results from samples obtained before treatment were included for statistical analysis.
Sensitivity and specificity calculations of the assays are based on LNB group of children and LNB excluded (n = 116) and controls (n = 66).
Patients. Three groups were created from a total of 182 children in the retrospective study. In the first group were included 86 children with proven LNB, manifested by aseptic meningitis (n = 25) and facial/abducens palsy (n = 8) or both (n = 53). CFS findings included pleocytosis, elevated protein level, blood-CSF disturbance and specific anti-Borrelia intrathecal synthesis. The classification of patients was based on AI calculation from EIA 1 test.
The second group consisted of 30 children who were admitted with suspicion of LNB. The following clinical symptoms and signs were present for this group: solitary or multiple erythema migrans (n = 7), headache (n = 22), arthralgia (n = 3), myalgia (n = 3), vertigo (n = 1), dysathria (n = 1), positive meningeal signs (n = 1) and fatigue (n = 1). Symptoms were either acute (< 3 months duration, n = 27) or long-lasting (> 3 months duration, n = 3). Used as controls were 132 samples from 66 children with neuroinfections other than LNB. These children were diagnosed as having tick-borne encephalitis (n = 3), enteroviral meningitis (n = 3), other viral meningitis (n = 10), isolated facial palsy of other than borrelial etiology (n = 22), or myelitis (n = 1) or they were children (n = 27) with negative CSF findings in which neuroinfection had been excluded.
The children had been admitted to the Department of Children’s Infectious Diseases of the University Hospital, Brno, Czech Republic, which serves an area with endemic Lyme borreliosis.
General informed consent of the University Hospital, including permission for statistical analysis and publication of clinical data and laboratory tests, had always been signed by one of the parents before lumbar puncture was performed. The study was approved by the Ethical Committee of University Hospital Brno.
RESULTS
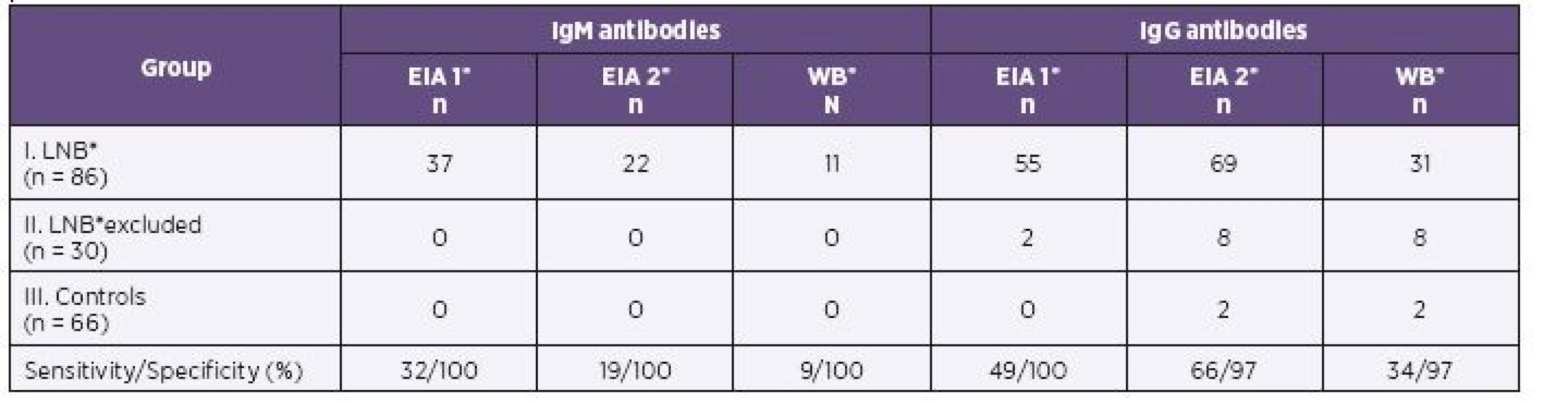
Results of EIA 1, EIA 2 and of western blot in children of all three groups are shown in Table 1 (serum) and Table 2 (CSF) for IgM and IgG antibodies.
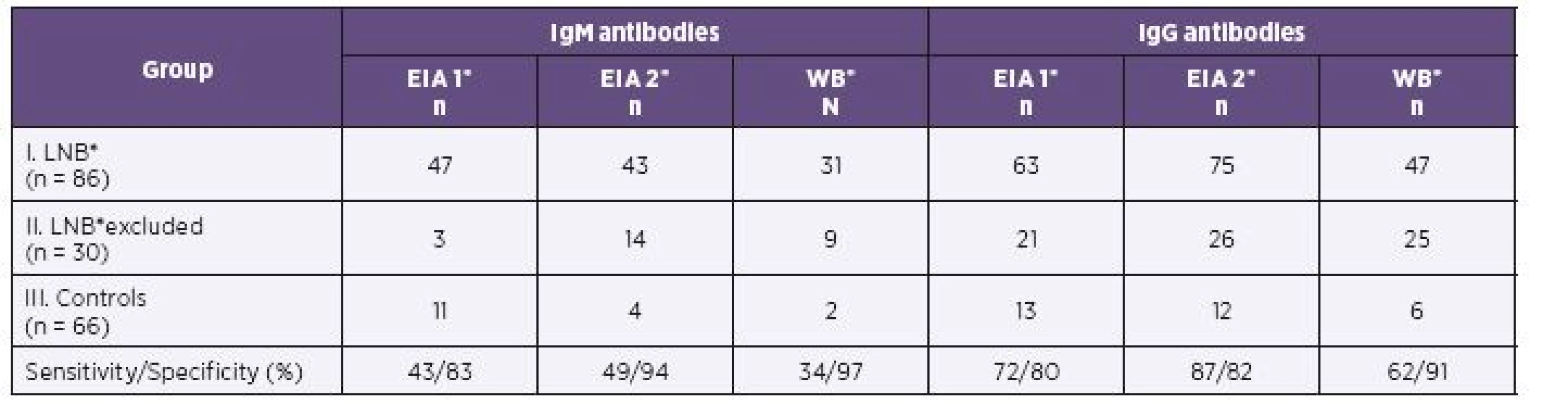
Anti-Borrelia IgM and IgG in serum and cerebrospinal fluid in LNB patients group
In the first group of children with proven LNB, 75/86 samples were identified IgG positive by EIA 2 versus 63/86 samples positive by EIA 1 in serum. CSF samples (69/86) were found IgG positive by EIA 2 and 55/86 samples were positive by EIA 1. EIA 2 gave significantly more positive results for IgG in serum (P = 0.006; OR = 7.5) as in CSF (P < 0.001; OR = 4.5).
Both assays gave similar positive IgM results in serum (47/86 by EIA 1 versus 43/86 by EIA 2). There was no statistically significant difference in the IgM positivity of serum (P = 0.54; OR = 0.71). IgM antibody reactivity seen in CSF was detected in only 22/86 samples by EIA 2 versus 37/86 samples by EIA 1. EIA 2 determined significantly (P = 0.001; OR = 0.06) less positive results of IgM in CSF in the LNB patients.
In the first group of children with proven LNB, EIA 2 showed better results in IgG than EIA 1 for both serum and CSF. For IgM positivity, both methods were comparable from statistical point of view, but positive results in CSF were less frequently detected by EIA 2.
Parallel results were obtained by western blot. The number of IgG positive samples was 47/86 in serum and 31/86 in CSF. EIA 2 gave significantly more positive results in IgG compared to western blot for both serum (P = 0.037; OR = 2.3) and CSF (P < 0.001; OR = 6.2). In serum, 31/86 IgM positive samples were found by western blot and 43/86 by EIA 2. EIA 2 gave more positive results in IgM antibodies than western blot in serum and the difference was significant (P < 0.001; OR = 0.04). The EIA 2 gave significantly more positive IgM in CSF (n = 22) than immunoblot (n = 11; see Table 2).
Comparison of AI if calculated from CSF/serum quotients for antibody reactivity in EIA 1 and EIA 2
The difference in the calculation of AI IgG by EIA 1 and EIA 2 was not noticeable in the group of LNB patients. AI IgG assessed by both methods revealed similar results (P = 0.646; OR = 1.38). Both methods are comparable, but AI IgM assessed by EIA 2 showed significantly less positive results (P < 0.001; OR = 0.04).
IgM or IgG AIs or both were calculated positive in 77/86 samples by EIA 1 and 74/86 samples by EIA 2. No sample was positive by EIA 1, but five by EIA 2 in the group of children with excluded LNB (Table 3).
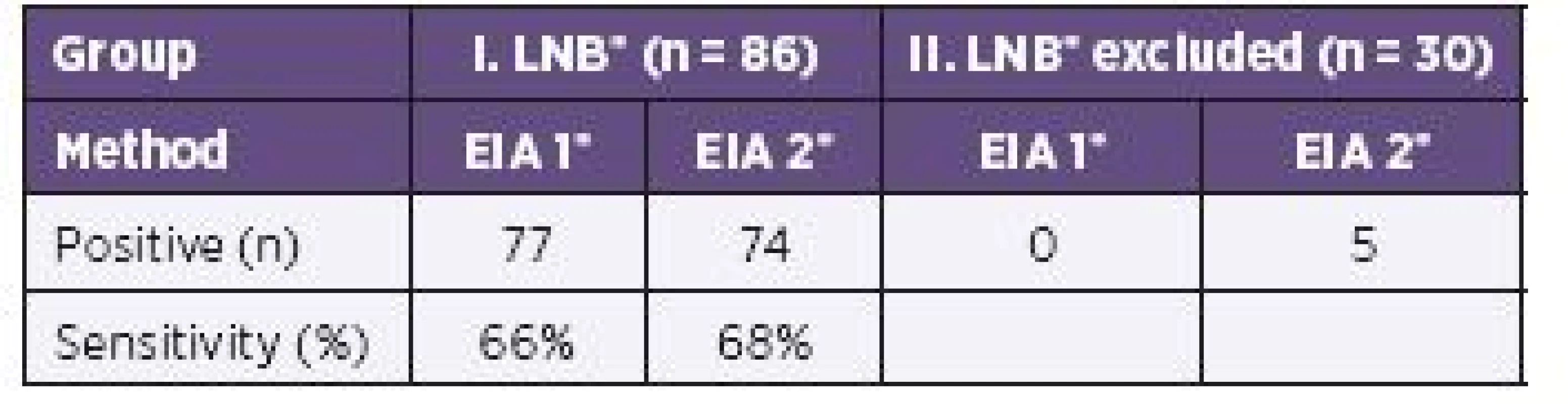
The use of the IgM/IgG calculation of AIs seems to correlate with the diagnosis of LNB when using both tests. There was no statistical significance (P = 0.606; OR = 0.67).
Negative results of EIA 2 were consistent with negative antibody indices using calculation by EIA 1 in samples of 8 children for which the serum and CSF specimens were obtained early in the infection. LNB was proven from the second specimen. Clinically, meningitis with (n = 7) or without (n = 1) facial palsy was diagnosed in 8 children altogether. Only 1 child had a negative AI in CSF (AI IgG = 0.82, AI IgM not measurable), albeit with lymphocytic pleocytosis and without blood–CSF disturbance (Qalb = 2.4). Erythema migrans and facial palsy were still present at the time of admission. The duration of symptoms before the serum and CSF samples were obtained was 3 d. Dermatological and neurological involvements disappeared after antibiotic treatment.
AI IgM positivity without AI IgG was detected in 6 CSF samples by EIA 1, 5 of which had a positive result in the EIA 2 test.
Nine (10.4%) CSF samples tested from 86 children with LNB had no IgG or IgM intrathecal synthesis if calculated from the results of EIA 1. The children fulfilled the criteria for LNB by clinical findings: facial palsy (n = 8), erythema migrans (n = 2), inflammatory changes in CSF (n = 9), and AI positive (n = 8) calculated by EIA 1 from the second CSF sample. From 9 false negative first samples, 7 were stored and 6 of these were positive, and if antibody indices were calculated from results with EIA 2 from the second sample, then IgG ranged from 1.48 to 11.5 and IgM from 1.89 to 34.47. Only 1 sample was negative in both tests for IgG and IgM antibody index.
Results of patients, where LNB was excluded and controls
No CSF sample was IgM positive with EIA 2 test in the second group of children. Eight children had positive IgG antibodies determined from EIA 2 in CSF, but no sample was positive for IgM intrathecal synthesis of specific antibodies and none of the children had lymphocytic pleocytosis in CSF. Five samples were AI IgG positive (see Table 3). These results correspond to the diagnosis of excluded LNB despite some suspicious clinical findings in favor of neurological involvement of Lyme borreliosis or skin manifestations typical for dermatoborreliosis. The differences between EIA 1 and EIA 2 in the detection of positive results in serum and CSF for IgG were P = 0.228 and P = 0.077, respectively.
For the groups of excluded LNB children and controls, the pairwise comparison of the test performance could not be determined, only negative results have occurred in one of the two groups. In the third group, there was no statistical difference between the two EIA 1 and EIA 2 tests either in serum or in CSF samples (P ~ 1.0).
Sensitivity and specificity
The sensitivity of EIA 1, EIA 2 and western blot ranged from 34% to 49% for the detection of IgM antibodies in serum, and the corresponding values for IgG antibodies were 62% to 87%. The lowest sensitivity was established by western blot. The maximum sensitivity was achieved for EIA 2 (see Table 1).
The sensitivity of EIA 2 IgG increased from 72% by EIA 1 to 87% in serum samples and from 49% by EIA 1 to 66% in CSF samples. The specificity for sera in IgG antibodies was 82% and for CSF it was 97% (see Table 1 and 2).
The sensitivity of IgM with EIA 2 is 49% for serum and 19% for CSF, but the specificity is high for both serum and CSF, at 94% and 100%, respectively (see Table 1 and 2).
IgG AI was found positive in 79/116 samples and IgM AI in 30/116 samples, if calculated by EIA 2. Comparing IgG and IgM antibody indices calculated from both tests, the sensitivity for EIA 2 reached 68% for IgG but just 26% for IgM. The specificity is 100% for both tests.
The comparison of IgM/IgG antibody indices calculated by EIA 1 and EIA 2 in samples from LNB patients revealed a similar sensitivity between EIA 1 (66%) and EIA 2 (68%) without reducing the specificity (see Table 3).
DISCUSSION
The diagnosis of LNB is based on clinical signs which are typical not only for neurological involvement in disseminated Lyme borreliosis. CSF examination is therefore needed to reach a definite diagnosis. Tests using a peptide based on an immunodominant conserved region of B. burgdorferi VlsE have been developed to improve the diagnosis of Lyme borreliosis [8–9].
Their diagnostic sensitivities range from 70% to 88% [13–15].
The humoral immune response measured in children with early LNB depends on the duration of symptoms, the use of antibiotics before serum and CSF samples were obtained, the individual host response to borrelial infection, and the method used for detecting the antibodies.
The diagnostic performance of the new enriched EIA with recombinant antigens was compared with whole-cell EIA from B. garinii in the retrospective study of 86 children with confirmed LNB. Lymphocytic pleocytosis was demonstrated in all of them. One of the first clinical involvements in children, cranial neuritis, allows early examination of serum and CSF. Pareses n. facialis and n. abducentis were present in 70.9% (61/86) of the children.
Enriched EIA 2 showed better results than EIA 1 for both serum and CSF in the detection of IgG antibodies. For the detection of IgM antibodies in serum and CSF samples, both methods were comparable but positive results in CSF were less frequently detected by EIA 2.
The difference in the calculation of AI IgG by EIA 1 and EIA 2 was not noticeable in the group of LNB patients. AI IgM assessed by EIA 2 showed significantly less positive results. The calculation of IgM/IgG AI from the results of both tests revealed similar results.
This heterogeneity of the intrathecal antibody response corresponded to the Slovenian study, albeit the number of patients was limited to 25 adults [16].
Among children with early disseminated LNB, IgM or IgG or both antibody indices calculated from results of the EIA 1 were positive in 89.5% (77/86) of CSF samples. In CSF samples of 9 children (10.5%), no intrathecal synthesis was detected with the EIA 1. AI was calculated from the second sample after antibiotic treatment in 9 children and 8 samples were positive. Our study was based on 99% positivity of AI.
There is an ongoing discussion in the literature about the usefulness of the calculation of AI IgM [1-2, 16-17]. To our opinion, an elevated AI IgM is equivalent to AI IgG in pediatric patients. In this study, we have confirmed, that positive AI IgM independently from positive AI IgG, is a reliable parameter to confirm the diagnosis of LNB especially in accordance with clinical findings and lymphocytic pleocytosis. In the group of children with suspicion for LNB, CSF was found positive AI IgM neither from EIA 1 nor from EIA 2.
In a Slovenian study of adult patients with neuroborreliosis [18], IgM and IgG antibodies positivity in CSF was detected in 61.8% and 58.8% with the Liaison test, but was lower (at 20.6% in IgM and 41.2% in IgG), and especially in IgM, when the IDEIA kit was used. These differences are explained by different sensitivities and/or specificities. The heterogeneity of borreliae in Europe must be taken into consideration in developing new diagnostic tests. Because the recombinantly produced protein fragments used in the new tests were selected according to the most commonly occurring borrelial subspecies in the Czech Republic, the sensitivity of the EIA 2 Borrelia recombinant test – at 87% for IgG and 49% for IgM – in detecting antibody response in serum in children with early disseminated neuroborreliosis is comparable with that of western blot. Sensitivity of 68% was achieved for IgM/IgG detection of intrathecal synthesis of borrelia antibodies by EIA 2. In a study [15] using sera of 36 neuroborreliosis patients, the diagnostic sensitivity (86.1%) of the recombinant western blot was increased without loss of specificity, and the improvement was mainly due to the presence of VlsE.
Western blot is technically demanding, and the scoring of reactive bands is subjective. An advantage of enriched ELISA is that it consumes one-third the amount of serum and CSF for dilution of sample compared with B. garinii, B. afzelii and B. burgdorferi sensu stricto western blots.
CONCLUSION
The EIA 2 Borrelia recombinant test with the highly specific antigen VlsE showed an increased sensitivity for IgG antibodies in samples obtained from children with proven neuroborreliosis while specificity is comparable to that of western blot. Our data suggest that analysis of IgM antibodies detected in serum by EIA 2 showed no difference compared to EIA 1, but EIA 2 determined less positive results of IgM antibodies in CSF in the LNB patients.
The similar patterns of responses were observed when AIs were detected by EIA 1 and EIA 2.
Acknowledgments
We are grafeful to Michal Kyr, MD, PhD for statistical analysis of all data and physicians Lenka Klapacova, MD and Iva Capovova, MD for contributing data for this study.
We thank Iva Stoklaskova, RNDr and Lenka Pokorna, RNDr, Ph.D. from company TestLine, Clinical Diagnostics, Brno, Czech Republic for donating the test kits of the 3rd generation and the EIA tests with whole-cell antigen of B. garinii for this study.
The study was not sponsored and there are no conflicts of interest.
Do redakce došlo dne 11. 6. 2015.
Adresa pro korespondenci:
MUDr. Lenka Krbková, CSc.
Klinika dětských infekčních nemocí
Lékařská fakulta MU Brno a
Fakultní nemocnice Brno-Bohunice
Černopolní 22a
625 00 Brno
e-mail: lkrbkova@fnbrno.cz
Sources
1. Stanek G, Fingerle V, Hunfeld K-P, et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect, 2010;17:69–79.
2. Mygland A, Ljostad U, Fingerle V, et al. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol, 2010;17:8–16.
3. Marques AR, Stock F, Gill V. Evaluation of a new culture medium for Borrelia burgdorferi. J Clin Microbiol, 2000;38:4239–4241.
4. Pícha D, Moravcová L, Žďárský E, et al. PCR in Lyme neuroborreliosis: a prospective study. Acta Neurol Scand, 2005;112:287–292.
5. Aguero-Rosenfeld ME, Wang G, Schwarz I, et al. Diagnosis of Lyme borreliosis. Clin Microbiol Rev, 2005;18:484–509.
6. Pachner AR, Dail D, Li L, et al. Humoral immunne response associated with Lyme borreliosis in nonhuman primates: analysis by immunoblotting and enzyme-linked immunosorbent assay with sonicates or recombinant proteins. Clin Diagn Lab Immunol, 2002; 9:1348–1355.
7. Zhang JR, Hardham JM, Barbour AG, et al. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell, 1997;89:275–285.
8. Liang FT, Philipp MT. Analysis of antibody response to invariable region of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect Immun, 1999;67:6702–6706.
9. Liang FT, Steere AC, Marques AR, et al. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol, 1999;37:3990–3996.
10. Peltomaa M, McHugh G, Steere AC. The VlsE (IR6) peptide ELISA in the serodiagnosis of Lyme facial paralysis. Otol Neurotol, 2004;25:838–841.
11. Skogman BH, Croner S, Forsberg P, et al. Improved laboratory diagnostics of Lyme neuroborreliosis in children by detection of antibodies to new antigens in cerebrospinal fluid. Pediatr Infect Dis J, 2008;27:605–612.
12. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci, 2001;184:101–122.
13. Panelius J, Lahdenne P, Saxén H, et al. Diagnosis of Lyme neuroborreliosis with antibodies to recombinant proteins DbpA, BBK32, and OspC, and VlsE IR6 peptide. J Neurol, 2003;250:1318–1327.
14. Heikkilä T, Saxen H, Seppälä I, et al. New antigens for serological diagnosis of neuroborreliosis in children. Pediatr Infect Dis J, 2005;24:709–712.
15. Schulte-Spechtel U, Lehnert G, Liegl G, et al. Significant improvement of the recombinant Borrelia-specific immunoglobulin G immunoblot test by addition of VlsE and DbpA homologue derived from Borrelia garinii for diagnosis of early neuroborreliosis. J Clin Microbiol, 2005;41:1299–1303.
16. Stanek G, Lusa L, Ogrinc K, et al. Intrathecally produced IgG and IgM antibodies to recombinant VlsE, VlsE peptide, recombinant OspC and whole cell extracts in the diagnosis of Lyme neuroborreliosis. Med Microbiol Immunol, 2014;203:125–132.
17. Henningsson AJ, Christiansson M, Tjernberg I, Löfgren S, Matussek A. Laboratory diagnosis of Lyme neuroborreliosis: a comparison of free CSF anti-Borrelia antibody assay. Eur J Clin Microbiol Infect Dis, 2014;33(5):797–803.
18. Cerar T, Ogrinc K, Strle F, et al. Humoral immune responses in patients with Lyme neuroborreliosis. Clin Vaccine Immunol, 2010;17:645–650.
Labels
Hygiene and epidemiology Medical virology Clinical microbiologyArticle was published in
Epidemiology, Microbiology, Immunology

2016 Issue 2
Most read in this issue
- A report of 10 cases of familial Creutzfeld-Jakob disease
- Novel approaches to control the rise in pertussis cases
-
The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic
Part I. Ixodes ricinus ticks and tick-borne encephalitis virus - The effect of oxygen on endotoxin production in bacteria of the Bacteroides fragilis group isolated from patients with colorectal carcinoma
