Endoscopic and medical therapy of gastric antral vascular ectasia: case series and review of available methods
Endoskopická a medikamentózní terapie cévních ektázií žaludečního antra (GAVE): série kazuistik a přehled metod
Cévní ektázie žaludečního antra (GAVE – gastric antral vascular ectasia) je vzácná diagnóza, která může vést ke chronické sideropenické anemii v důsledku okultního nebo zjevného krvácení do gastrointestinálního traktu. Za základ léčby GAVE jsou považovány endoskopické termické metody – z nichž nejužívanější je argon-plazma koagulace, dále radiofrekvenční ablace nebo kryoterapie. Příznivé výsledky jsou publikovány také v souvislosti s používáním endoskopické ligace. V případě GAVE refrakterní na předchozí endoskopickou léčbu lze použít farmakoterapii, jejíž podstatou je redukce tvorby cévních malformací a krevního průtoku v nich. Prezentujeme přehled dostupných endoskopických a neendoskopických metod pro léčbu GAVE a naše zkušenosti s léčbou refrakterní GAVE kombinací endoskopických metod a thalidomidu formou kazuistik. Navrhujeme možný algoritmus pro terapii GAVE.
Klíčová slova:
cévní ektázie žaludečního antra – argon-plazma koagulace – endoskopická ligace – thalidomid – krvácení z trávicího traktu
Authors:
Šramková M.; Kroupa R.; Dastych M.; Konečný Š.; Kunovsky L.; Dolina J.
Authors‘ workplace:
Department of Gastroenterology and Internal Medicine, Universtity Hospital Brno, Faculty of Medicine, Masaryk University, Brno, Czech Republic
Published in:
Gastroent Hepatol 2021; 75(3): 221-228
Category:
doi:
https://doi.org/10.48095/ccgh2021221
Overview
Gastric antral vascular ectasia (GAVE) is an uncommon diagnosis leading to chronic iron deficiency anaemia caused by occult or overt gastrointestinal bleeding. The mainstay of the therapy is based on endoscopic therapy using endoscopic thermal methods – particularly argon plasma coagulation and others – radiofrequency ablation, coagulation or cryotherapy. Endoscopic band ligation presents satisfying results as well. Pharmacotherapy aiming at reduction of the development of new vascular lesions and reduction of blood flow in them could be considered in patients refractory to endoscopic treatment. We present a review of available endoscopic and non-endoscopic methods for GAVE therapy and our experience with the therapy of refractory GAVE using a combination of endoscopic methods and thalidomide on case series. The algorithm for GAVE therapy is proposed.
Keywords:
gastric antral vascular ectasia – argon plasma coagulation – endoscopic band ligation – thalidomide – gastrointestinal bleeding
Background
Gastric antral vascular ectasia (GAVE) is an uncommon source of upper gastrointestinal (GI) bleeding and can result in chronic iron-deficiency anaemia. It accounts for 4% of non-variceal upper gastrointestinal bleeding [1]. It was first described by Rider et al in 1953 [2]. It is often described as watermelon stomach due to the typical macroscopic appearance of red spots aggregated in linear stripes radiating from the pylorus. A diffuse form of GAVE with haemorrhagic lesions extending to the stomach body can also be encountered. If the endoscopic image is unclear, histopathology analysis can be provided. Characteristic dilatated, ectatic vessels in the mucosa and submucosa, fibromuscular hyperplasia, microvascular thrombi and minimal inflammation changes will be revealed [3].
GAVE is almost entirely observed in the elderly population, two out of three patients are women [4].
The pathophysiology is still not clear, however mechanical stress, hypermotility, gastrin and pepsinogen level imbalance and increased levels of 5-hydroxytryptamine and vasoactive intestinal polypeptide are considered to be important factors in the development of GAVE.
Most patients suffer from severe medical conditions and systemic diseases, especially from cirrhosis of the liver [5], renal failure, connective tissue disease or ischaemic or valvular heart disease [6].
According to several trials, 30% of patients with GAVE suffer from cirrhosis of the liver [7].
However, unlike portal hypertension gastropathy, a decrease of HVPG (hepatic venous pressure gradient) does not lead to a regression of GAVE [8].
The primary outcomes of GAVE therapy include cessation of bleeding, increase in mean haemoglobin level, decreased need for blood transfusion, decreased number of treatment sessions required and fewer admissions to hospital.
In managing GAVE, an endoscopic approach is preferred. If endoscopic methods are revealed to be ineffective, pharmacotherapy is considered. In rare, truly refractory cases, surgical therapy could be the definitive solution. Treating underlying conditions may support the effect of other therapeutic modalities.
Endoscopic therapy
Argon plasma coagulation (APC)
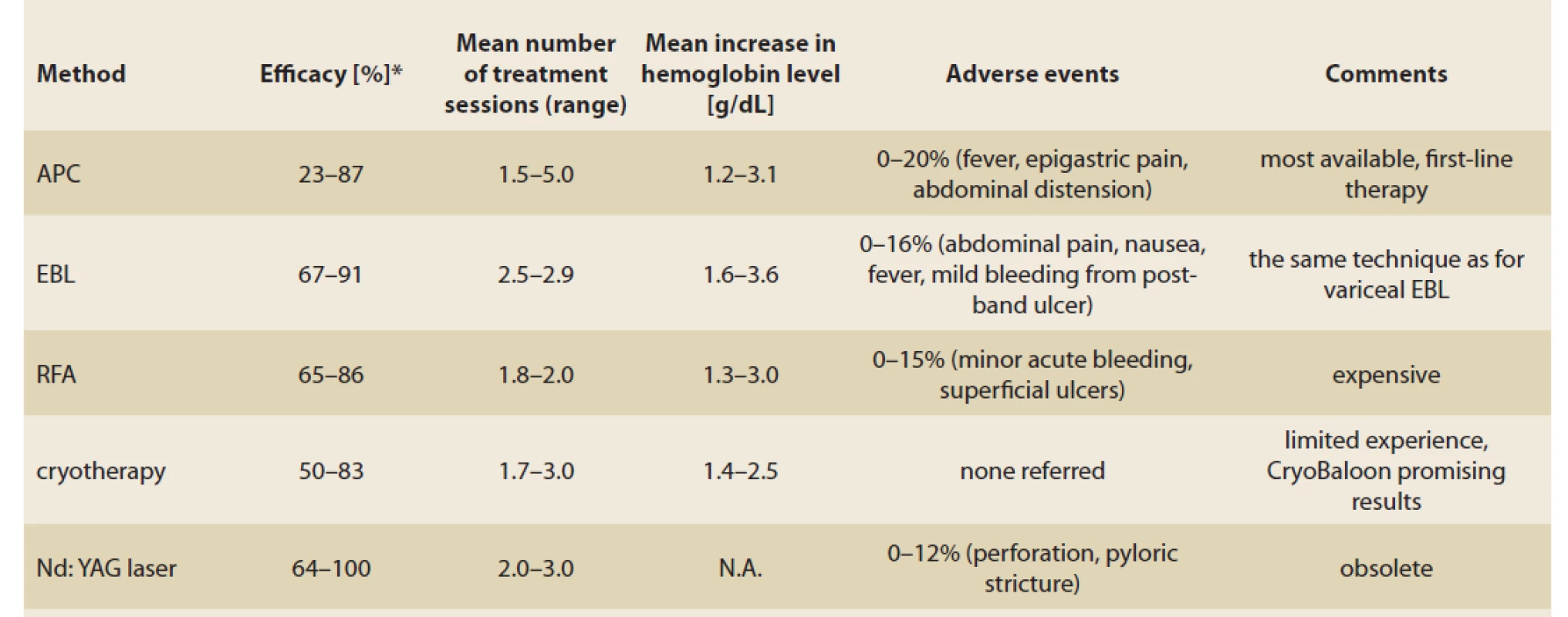
APC is a non-contact endoscopic thermoablative method widely used in GAVE therapy. Its principle lies in thermocoagulation of the tissue surface caused by a high-frequency electrical current passing through argon gas, the depth of penetration is 1–3 mm on average. This allows the eradication of ectatic vessels in superficial layers of the mucosa, which is associated with a lower risk of complications caused by perforation, however several treatment sessions are required to achieve the cessation of bleeding [9].
Adverse events such as sepsis, superficial ulceration, perforation, strictures and hyperplastic polyp formations were reported [10].
The APC is a simple, relatively cheap and widely available endoscopic method with favourable results and an acceptable risk of complications. For these reasons, it is considered to be first-line therapy in the management of symptomatic GAVE.
Endoscopic band ligation (EBL)
EBL is routinely used for the therapy of oesophageal varices. Unlike the APC, EBL achieves a deeper submucosal effect thanks to the obliteration of ectatic vessels in the mucosa and in the submucosal layer. Multiple studies comparing EBL to endoscopic thermic methods, mainly APC, have been published. According to these studies, EBL requires fewer treatment sessions with favourable results in haemoglobin increase, blood transfusion and need for hospitalisation, compared to APC [11–14]. Recent meta-analysis compared EBL to ETT (endoscopic thermic therapy APC + electrocautery) in a group of 207 patients with a follow-up period of at least 6 months [13]. In comparison with ETT, patients treated with EBL manifested a significantly higher change in mean haemoglobin level (mean difference MD 0.92 g/ dL) and required fewer post-procedural blood transfusions (MD 2.10) and fewer treatment sessions (MD 1.15). No difference in adverse events was reported.
Radiofrequency ablation (RFA)
RFA is an endoscopic method based on an application of high-energy coaptive coagulation in a short period in order to destroy ectatic vessels in superficial layers of the mucosa, followed by the formation of a normal capillary structure. The depth of the tissue ablation is uniform and allows treating a broad surface area. A recent study on twenty patients treated with RFA with a TTS (through-the-scope) catheter due to GAVE refractory to prior therapy with APC, Nd:YAG-laser or EBL was reported. An increase in haemoglobin level + 1.26 g/ dL was achieved. After six months, 3 out of 14 patients (21%) needed ongoing transfusions, 5 out of 17 patients (29%) needed ongoing iron therapy. Three out of 14 patients who continued in a 12-month follow-up required RFA retreatment (21%). No severe complications such as perforation, acute bleeding or stricture formation were reported [15].
Neodymium-yttrium-aluminium garnet laser coagulation (Nd:YAG laser)
(Nd:YAG laser) is a non-contact thermal method using the absorption of laser light. It causes tissue destruction up to a depth of 4–6 mm and leads to vessel coagulation in the superficial and submucosal layers. A retrospective study from 2004 reported results of 24 transfusion-dependent patients with a follow-up median of 55 months. After a median of two Nd:YAG laser sessions, cessation of bleeding was achieved in 20 out of 24 patients (83%). The patients remained transfusion-free for a median of 16 months [16]. The disadvantages of this approach include higher cost and risk of serious complications such as perforation, antral narrowing and mortality.
After the extension of APC, EBL and RFA in GAVE management, the number of new clinical reports regarding the use of the Nd:YAG laser decreased rapidly.
Cryotherapy
Cryotherapy is a thermic method using nitrous oxide to achieve an extremely cold temperature to cause mucosal destruction. Promising results in the treatment of GAVE were presented.
A recent retrospective study with a CryoBaloon from 2018 analysed 23 patients’ refractory to initial treatment with APC. After six months of follow-up, 87% of the patients manifested endoscopic regression and 83% of the patients manifested clinical improvement in no more than two treatment sessions [17].
Experience with other methods such as heater probe, sclerotherapy, hot forceps and endoscopic mucosal resection was demonstrated. No significant superiority of these methods over the current modalities was proven (Tab. 1).
Tab. 1. Přehled endoskopických metod v terapii GAVE.
Surgical therapy
This therapy is considered to be the last option for patients with GAVE refractory to other treatment modalities or combinations thereof. The risk of complications is not negligible, especially in patients suffering from cirrhosis of the liver and portal hypertension. Because GAVE involves predominantly the stomach antrum, antrectomy can represent a successful and effective method [9,18]. The patients’ preoperative condition should be assessed carefully because of the significant morbidity and mortality of this approach.
Pharmacotherapy
The drug therapy in GAVE aims to reduce blood flow and reduce the development of angiectasias in the stomach mucosa.
Oestrogen-progesterone
Hormonal therapy with oestrogen-progesterone was reported as a gastrointestinal angiodysplasia treatment option in multiple case reports. According to a randomised multicentric trial performed on 72 patients, no difference in the number of bleeding episodes and blood transfusion requirement was proven between the group that received the hormonal therapy and the placebo group [19]. Additionally, the long-term side effects of therapy are non-negligible.
Octreotide
Octreotide, a long-acting somatostatin analogue was used in several clinical conditions presented with gastrointestinal bleeding. The efficacy of octreotide therapy in patients with gastrointestinal angiodysplasia including GAVE was reviewed, however a small number of patients was enrolled. Most studies proclaim promising results in bleeding recurrence, however the form of application, dose and dosage interval vary [20]. In 2012, a trial evaluating 15 patients with GI angiodysplasia treated with octreotide was reported. The patients required either octreotide 20 mg/ month intramuscularly, or lanreotide (another analogue of somatostatin) 90 mg/ 28 days subcutaneously. Significant improvement in haemoglobin level and blood transfusion requirement was achieved, no adverse events occurred [21].
Thalidomide
Thalidomide is an angiogenesis inhibitor suppressing the vascular endothelial growth factor (VEGF) [22]. Multiple studies investigating the efficacy of thalidomide in gastrointestinal angiodysplasia bleeding have been published, predominantly in the form of case reports. A recent retrospective study presents 15 patients with gastrointestinal vascular malformation, including 5 patients with GAVE refractory to prior pharmacotherapy. It reported positive results in bleeding recurrence, blood transfusion requirement and hospitalisation. During the 6 months of follow-up, 5 patients (38.5%) had no recurrence of bleeding. The need for endoscopic treatment decreased in 11 patients (84.6%). In 6 patients (46%) adverse events such as fatigue, neuropathy, constipation and dizziness were noticed [23].
The interesting prospective study reported high efficacy (71.4%) of thalidomide in the therapy of refractory gastrointestinal bleeding from vascular malformations, however only three patients with GAVE were included [24].
A large retrospective study from 2016 investigated 80 patients treated with thalidomide for refractory gastrointestinal bleeding secondary to gastrointestinal vascular malformation, including GAVE [25]. The overall response rate during a one-year follow-up was 79.5%. Serious adverse effects (such as limb numbness, leukopenia and rash) were reported in 31.3%. However, many comorbidities such as severe renal, heart, pulmonary and liver disease were considered criteria for exclusion. The application of these experiences in the broad population is, therefore debatable.
Bevacizumab
Bevacizumab is a humanised anti-VEGF monoclonal IgG1 antibody. By binding to circulating VEGF, inhibits the binding VEGF to its receptors and blocks the pro-angiogenic cascade. The bevacizumab is being investigated especially in patients with bleeding resulting from hereditary haemorrhagic teleangiectasia [26]. The first trial evaluating the efficacy of intravenous bevacizumab in the treatment of refractory GI bleeding was published in 2020. In one year following the administration of bevacizumab, a significant reduction in endoscopic procedures and RBC (red blood cells) unit requirement was observed [27].
The administration of corticosteroids and tranexamic acid in GAVE therapy were also documented. In comparison to other methods no significant advantages were proven and such therapy may be associated with adverse events (Tab. 2).
Tab. 2. Přehled farmakoterapeutických možností pro GAVE.
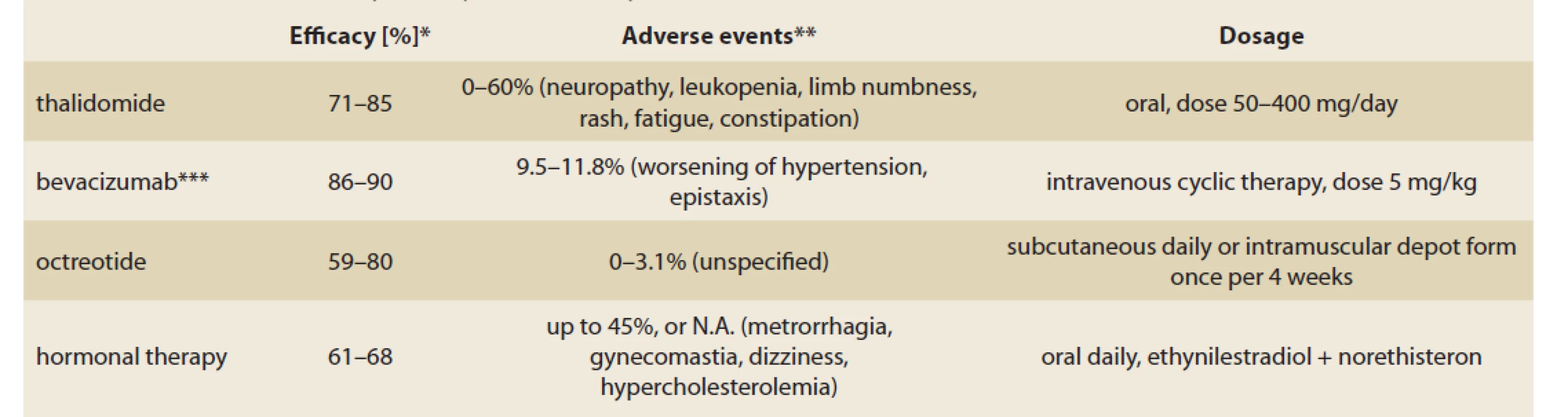
*in most studies, the results are presented together with other angiectasias (not only for GAVE)
**the assessment of adverse events was various, mild and severe adverse events included
***including patients with epistaxis secondary to hereditary hemorrhagic teleangiectasia
Case series
We present our experience with the therapy of refractory GAVE using a combination of endoscopic methods and thalidomide on case series. Informed consent was obtained from every patient for the publication of anonymised data.
Case report 1
In July 2018, a 69-year-old woman was admitted to our gastroenterology department complaining of fatigue and dyspnoea. A previous history of cirrhosis of the liver secondary to primary biliary cirrhosis, aortic stenosis and adenocarcinoma of the rectosigmoid colon successfully treated with surgical resection was noted. She had no history of oesophageal varices.
The initial haemoglobin level was 43 g/ l. An oesophagogastroduodenoscopy confirmed the presence of linear-type GAVE with signs of recent bleeding. Endoscopic treatment using APC was initiated and 2 sessions were performed at about a one-month interval.
In the following 6 months, five bleeding recurrences occurred and every episode required an admission to hospital. Ongoing endoscopic therapy was provided and a total of 6 sessions of APC and 2 sessions of EBL were performed. A reduction of the involved areas in the stomach was observed during endoscopy. The mean haemoglobin level was 74.9 g/ l and 5 RBC units/ month on average were required.
Due to the insufficient efficacy of the endoscopic approach, therapy with thalidomide at a dose of 100 mg/ day was started in December 2018.
In the next 6 months of follow-up, repeated APC and EBL therapy continued. The patient underwent another 6 sessions of APC and 2 sessions of EBL, while the dose of thalidomide was unaltered. Four admissions to hospital were reported, the mean haemoglobin level increased to 85.1 g/ l while the blood transfusion requirement decreased to 2.17 RBC units/ month on average.
During the next 21 months of the follow-up, regular oesophagogastroduodenoscopies were performed and only 3 treatment sessions were needed (2 sessions of APC and 1 session of EBL). Regression of new angiectasias in endoscopic appearance was observed. The mean haemoglobin level increased to 94 g/ l while no blood transfusion due to blood loss secondary to GAVE was reported. No adverse events in association with thalidomide were observed, except for mild dizziness, which was corrected by administration of the drug at bedtime. After 33 months of total follow-up, the patient died from an unrelated cause – pulmonary infection caused by limited mobility due to severe spine and lower limb joints degenerative changes (Fig 1, 2).
Obr. 1a) GAVE typické zarudlé oblasti s angiektáziemi a krvácením
v žaludečním antru.


Obr. 2. GAVE bezprostředně po ošetření APC.

Case report 2
A 70-year-old woman with diabetes was treated in our gastroenterology department starting in November 2012 for cryptogenic cirrhosis of the liver with a history of recurrent variceal bleeding definitely treated with transjugular intrahepatic portosystemic shunt (TIPS).
In 2014, haemorrhagic gastropathy was observed during oesophagoduodenoscopy and a diagnosis of watermelon stomach was established in July 2014. Due to anaemia progression, endoscopic treatment with APC was started in December 2014. In the next year of follow-up, ongoing endoscopic treatment was provided. The mean haemoglobin level was 90.6 g/ l and the average blood transfusion requirement constituted 0.42 RBC unit/ month.
In the fourth year of follow-up, one episode of severe bleeding from jejunal angiodysplasia occurred and it was successfully treated with one session of APC in combination with pharmacotherapy (PPI, somatostatin). In the fourth year, the mean haemoglobin level decreased to 75.3 g/ l and the average requirement of blood transfusion reached 1.9 RBC units/ month. Despite the ongoing APC therapy, episodes of severe GI bleeding recurred and no significant increase in the haemoglobin level was noticed. In 2019 (the fifth year of follow-up) the current endoscopic approach was pronounced to be inefficient and systemic antiangiogenic therapy in combination with EBL was started. In September 2019, treatment with thalidomide in a dose of 100 mg/ day was administered. In the following six months, 2 sessions of EBL were performed and regression in endoscopic appearance was noticed, however no improvement in the haemoglobin level or blood transfusion requirement was observed. Satisfying progress was registered during the following months. Within the period of 18 months following the administration of thalidomide, decreases in the average blood transfusion requirement to 0.89 units of RBC/ month and in the endoscopic treatment sessions (1 session of APC, 2 sessions of EBL) were reported.
Although there was no significant difference in mean haemoglobin level before and after thalidomide administration – 73.3 g/ l vs. 72.8 g/ l, the need for RBC unit transfusion and endoscopic treatment sessions was reduced.
No adverse event in association with thalidomide use was noticed (Fig. 3).
Obr. 3a) Slizniční uzly po endoskopické ligaci GAVE.

Obr. 3b) Mnohočetné ulcerace jako výsledek ligační terapie
GAVE po 3 týdnech.

Case report 3
A 67-year-old man with multiple severe comorbidities – end stage kidney disease, ischaemic heart disease, chronic limb ischaemia and diabetes was referred to our gastroenterology department due to progression of chronic anaemia. An oesophagoduodenoscopy was performed and moderate antral gastropathy type GAVE, confirmed with histopathology was detected. Owing to persisting severe iron-deficiency anaemia despite regular erytropoetin and iron supplementation, endoscopic management of GAVE was initiated. In November 2018, the first session of APC was provided, followed by EBL due to granular organisation of GAVE in the antrum. In the fifteen months of endoscopic treatment, 4 sessions of APC and 3 sessions of EBL were performed. After the first phase of the therapy, there was no difference in blood transfusion requirement. The previous therapy was evaluated to be ineffective and in February 2020, therapy with a thalidomide dose of 100 mg/ day was initiated. In the 10 days following the thalidomide administration, pancytopenia occurred and the thalidomide was suspended. No haematological disease was discovered. In this 4-month period, severe GI bleeding requiring RBC transfusion secondary to GAVE recurred, and repeated endoscopic treatment was required (2 sessions of APC and 1 session of EBL). One more attempt to use a reduced dose of thalidomide (50 mg) was performed, but in one month, intolerance with dizziness developed and the pharmacotherapy was withdrawn permanently. The patient continues with endoscopic interventions and the need for transfusions has partially improved.
Discussion
We reported three cases of different clinical manifestation of refractory GAVE and the use of endoscopic and non-endoscopic methods in the management. All the patients were limited by multiple comorbidities and the failure of standard endoscopic therapy with APC required consideration for other methods.
Our experience supports the use of the EBL method in the treatment of GAVE refractory to initial APC intervention. The early change in the endoscopic strategy may gain more favourable results in the transfusion requirements. The performance of EBL in the gastric antrum was technically identical to the use in oesophageal varices. The authors recommend using a multiple band ligator with at least 6 bands (or combining 6+4 bands) in the first procedure and systematically applying bands in strips proximally from the pylorus. An interval of 2–4 weeks between bandings seemed to be sufficient for healing induced ulcers [11,13]. Although the procedure-related ulcers were relatively large and with haematin, we did not observe serious bleeding from them. If fresh blood in the stomach was observed during subsequent endoscopy, it came from persistent angiectasias, a fragility of the mucosa beyond the previously banded areas.
The decision of indication of a systemic drug in the management was based on the persisting need for blood transfusion and low effectivity of initial endoscopic interventions and development of new angiectasias observed during endoscopies. No standardised medical therapy for GAVE is available. The use of any drug in this off-label indication is limited to the specific approval of the health insurance company and should be referred to the state authorities according to regulatory requirements (State Institute for Drug Control in the Czech Republic). The use of thalidomide in combination with endoscopic therapy led to a stabilisation of the red blood count with the gradual withdrawal of endoscopic interventions. The main side effects were fatigue and dizziness, without severe adverse events. No symptoms of neuropathy as the main limiting factor of long-term thalidomide use were observed [23]. Nevertheless, our patients were of rather advanced age and suffered from many comorbidities, and some symptoms induced by thalidomide might be hidden by other symptoms and causes. However, an intolerance was observed in one patient and continued endoscopic interventions were the only option.
From the practical point of view, it may be important that the insertion of a transjugular intrahepatic portosystemic shunt in one of our patients did not influence the anaemia related to GAVE in patients with cirrhosis of the liver and portal hypertension. In accordance with the published facts [8,28], the reduction of portal hypertension only had a limited effect on the bleeding from GAVE, and the background of the GAVE development even in patients with cirrhosis can probably be explained by metabolic changes and the effect of local and systemic mediators rather than only by the portal pressure. This is also the rationale for the use of systemic drugs in the management of GAVE.
The role of proton pump inhibitors in the management of GAVE has not been analysed in any clinical study. Given the pathophysiology of GAVE development, we can assume that the decreasing acidity in the stomach juice will not prevent ectatic vessel formation, however it could improve the coagulation in case of bleeding, similarly to the effect in non-variceal upper gastrointestinal bleeding [29].
The algorithm for the therapy of GAVE is proposed (Tab. 3).
Tab. 3. Navrhovaný algoritmus postupu v terapii GAVE.

Conclusion
The treatment of GAVE is often challenging. It could be the source of severe and refractory GI bleeding. The treatment options are primarily endoscopic and if inefficient, combination with non-endoscopic methods could be considered. Systemic therapy aims to affect angiogenesis and reduce the appearance of GAVE in the mucosa. Nevertheless, prospective studies including enough GAVE patients treated by anti-VEGF acting drugs are still missing.
Submitted/ Doručeno: 19. 5. 2021
Accepted/ Přijato: 8. 6. 2021
Radek Kroupa, MD, PhD.
Department of Gastroenterology and Internal Medicine
Universtity Hospital Brno
Faculty of Medicine
Masaryk University, Brno,
Jihlavská 20
625 00 Brno
Czech Republic
kroupa.radek@fnbrno.cz
Conflict of Interest: The authors declare that the article/manuscript complies with ethical standards, patient anonymity has been respected, and they state that they have no financial, advisory or other commercial interests in relation to the subject matter.
Publication Ethics: This article/manuscript has not been published or is currently being submitted for another review. The authors agree to publish their name and e-mail in the published article/ manuscript.
Dedication: This work was supported by MH CZ – DRO (FNBr, 65269705).
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Konflikt zájmů: Autoři deklarují, že text článku odpovídá etickým standardům, byla dodržena anonymita pacientů a prohlašují, že v souvislosti s předmětem článku nemají finanční, poradenské ani jiné komerční zájmy.
Publikační etika: Příspěvek nebyl dosud publikován ani není v současnosti zaslán do jiného časopisu pro posouzení. Autoři souhlasí s uveřejněním svého jména a e-mailového kontaktu v publikovaném textu.
Dedikace: Tato práce byla podpořena projektem MZ ČR – RVO (FNBr, 65269705).
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Sources
1. Dulai GS, Jensen DM. Treatment of watermelon stomach. Curr Treat Options Gastroenterol 2006; 9(2): 175–180. doi: 10.1007/ s11938-006-0036-1.
2. Rider JA, Klotz AP, Kirsner JB. Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology 1953; 24(1): 118–123.
3. Gilliam JH 3rd, Geisinger KR, Wu WC et al. Endoscopic biopsy is diagnostic in gastric antral vascular ectasia. The „watermelon stomach“. Dig Dis Sci 1989; 34(6): 885–888. doi: 10.1007/ BF01540274.
4. Gostout CJ, Viggiano TR, Ahlquist DA et al. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol 1992; 15(3): 256–263. doi: 10.1097/ 000048 36-1992100 00-00019.
5. Kukulska M, Smoła I, Gromny I et al. Watermelon stomach as the first symptom of liver cirrhosis. Gastroenterol Hepatol 2016; 70(5): 422–425. doi: 10.14735/ amgh2016422.
6. Becq A, Rahmi G, Perrod G et al. Hemorrhagic angiodysplasia of the digestive tract: pathogenesis, diagnosis, and management. Gastrointest Endosc 2017; 86(5): 792–806. doi: 10.1016/ j.gie.2017.05.018.
7. Ward EM, Raimondo M, Rosser BG et al. Prevalence and natural history of gastric antral vascular ectasia in patients undergoing orthotopic liver transplantation. J Clin Gastroenterol 2004; 38(10): 898–900. doi: 10.1097/ 00004836-200411000-00013.
8. Spahr L, Villeneuve JP, Dufresne MP et al. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut 1999; 44(5): 739–742. doi: 10.1136/ gut.44.5.739.
9. Hsu WH, Wang YK, Hsieh MS et al. Insights into the management of gastric antral vascular ectasia (watermelon stomach). Therap Adv Gastroenterol 2018; 11 : 1756283X17747471. doi: 10.1177/ 1756283X17747471.
10. Naidu H, Huang Q, Mashimo H. Gastric antral vascular ectasia: the evolution of therapeutic modalities. Endosc Int Open 2014; 2(2): E67––E73. doi: 10.1055/ s-0034-1365525.
11. Wells CD, Harrison ME, Gurudu SR et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc 2008; 68(2): 231–236. doi: 10.1016/ j.gie.2008.02.021.
12. Swanson E, Mahgoub A, MacDonald R et al. Medical and endoscopic therapies for angiodysplasia and gastric antral vascular ectasia: a systematic review. Clin Gastroenterol Hepatol 2014; 12(4): 571–582. doi: 10.1016/ j.cgh.2013.08.038.
13. Elhendawy M, Mosaad S, Alkhalawany W et al. Randomized controlled study of endoscopic band ligation and argon plasma coagulation in the treatment of gastric antral and fundal vascular ectasia. United European Gastroenterol J 2016; 4(3): 423–428. doi: 10.1177/ 205 0640615619837.
14. Chalhoub JM, Umar J, Groudan K et al. Endoscopic band ligation compared to thermal therapy for gastric antral vascular ectasia: A systematic review and meta-analysis. United European Gastroenterol J 2021; 9(2): 150–158. doi: 10.1177/ 2050640620975243.
15. Magee C, Lipman G, Alzoubaidi D et al. Radiofrequency ablation for patients with refractory symptomatic anaemia secondary to gastric antral vascular ectasia. United European Gastroenterol J 2019; 7(2): 217–224. doi: 10.1177/ 2050640618814659.
16. Mathou NG, Lovat LB, Thorpe SM et al. Nd:YAG laser induces long-term remission in transfusion-dependent patients with watermelon stomach. Lasers Med Sci 2004; 18(4): 213–218. doi: 10.1007/ s10103-003-0284-4.
17. Patel AA, Trindade AJ, Diehl DL et al. Nitrous oxide cryotherapy ablation for refractory gastric antral vascular ectasia. United European Gastroenterol J 2018; 6(8): 1155–1160. doi: 10.1177/ 2050640618783537.
18. Novitsky YW, Kercher KW, Czerniach DR et al. Watermelon stomach: pathophysiology, diagnosis, and management. J Gastrointest Surg 2003; 7(5): 652–661. doi: 10.1016/ s1091-255x(02)00435-3.
19. Junquera F, Feu F, Papo M et al. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology 2001; 121(5): 1073–1079. doi: 10.1053/ gast.2001.28650.
20. Junquera F, Saperas E, Videla S et al. Long-term efficacy of octreotide in the prevention of recurrent bleeding from gastrointestinal angiodysplasia. Am J Gastroenterol 2007; 102(2): 254–260. doi: 10.1111/ j.1572-0241.2007.01053.x.
21. Bon C, Aparicio T, Vincent M et al. Long-acting somatostatin analogues decrease blood transfusion requirements in patients with refractory gastrointestinal bleeding associated with angiodysplasia. Aliment Pharmacol Ther 2012; 36(6): 587–593. doi: 10.1111/ apt.12000.
22. McFarlane M, O‘Flynn L, Ventre R et al. Emerging role of thalidomide in the treatment of gastrointestinal bleeding. Frontline Gastroenterol 2018; 9(2): 98–104. doi: 10.1136/ flgastro-2017-100870.
23. Bayudan AM, Chen CH. Thalidomide for refractory gastrointestinal bleeding from vascular malformations in patients with significant comorbidities. World J Clin Cases 2020; 8(15): 3218–3229. doi: 10.12998/ wjcc.v8.i15.3218.
24. Ge ZZ, Chen HM, Gao YJ et al. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology 2011; 141(5): 1629–1637.e1-4. doi: 10.1053/ j.gastro.2011.07.018.
25. Chen H, Fu S, Feng N et al. Bleeding recurrence in patients with gastrointestinal vascular malformation after thalidomide. Medicine (Baltimore) 2016; 95(33): e4606. doi: 10.1097/ MD.0000000000004606.
26. Iyer VN, Apala DR, Pannu BS et al. Intravenous Bevacizumab for Refractory Hereditary Hemorrhagic Telangiectasia-Related Epistaxis and Gastrointestinal Bleeding. Mayo Clin Proc 2018; 93(2): 155–166. doi: 10.1016/ j.mayocp.2017.11.013.
27. Albitar HAH, Almodallal Y, Gallo De Moraes A et al. Intravenous Bevacizumab in Hereditary Hemorrhagic Telangiectasia-Related Bleeding and High-Output Cardiac Failure: Significant Inter-Individual Variability in the Need for Maintenance Therapy. Mayo Clin Proc 2020; 95(8): 1604–1612. doi: 10.1016/ j.mayocp.2020.03.001.
28. Kamath PS, Lacerda M, Ahlquist DA et al. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology 2000; 118(5): 905–911. doi: 10.1016/ s0016-5085(00)70176-4.
29. Barkun AN, Laine L, Leontiadis GI et al. Management of Nonvariceal Upper Gastrointestinal Bleeding. Ann Intern Med 2020; 172(8): 573. doi: 10.7326/ L20-0014.
Labels
Paediatric gastroenterology Gastroenterology and hepatology SurgeryArticle was published in
Gastroenterology and Hepatology
2021 Issue 3
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- The Importance of Limosilactobacillus reuteri in Administration to Diabetics with Gingivitis
-
All articles in this issue
- Editorial
- Kvíz z klinické praxe
- Current status of endoscopic full-thickness resection for treatment of colorectal neoplastic lesions
- Hybrid short-wire ERCP technique, five years of experience
- Severe bleeding as a complication of endoscopic choledocho-duodenal drainage using LAMS (Hot-Axios) in the treatment of distal stenosis of the common bile duct due to pancreatic head adenocarcinoma
- Endoscopic bariatric therapies
- Endoscopic and medical therapy of gastric antral vascular ectasia: case series and review of available methods
- Anal intraepithelial neoplasia and HD anoscopy
- A rare cause of dysphagia in adult
- Late tension pneumoperitoneum via the abdominal stoma after PEG-J
- Switch from original to biosimilar adalimumab SB-5 in patients with Crohn‘s disease – long-term results
- Teduglutide in the treatment of short bowel syndrome in Crohn’s disease
- Laparoscopic repair of congenital paraesophageal hernia in an 18-month-old child – a clinical case and literature review
- Telemedicine is more than a „doctor on the phone“
- Endoscopy and endotherapy in IBD, ISCARE 26.2.2021
- The selection from international journals
- Správná odpověď na předchozí kvíz Neklasifikovatelný idiopatický střevní zánět postihující tlusté střevo (IBD-unclassified)
- Erratum
- Kreditovaný autodidaktický test: digestivní endoskopie
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Anal intraepithelial neoplasia and HD anoscopy
- Endoscopic bariatric therapies
- Teduglutide in the treatment of short bowel syndrome in Crohn’s disease
- A rare cause of dysphagia in adult
