Serum anti-laminarin antibodies in colorectal cancer: a prospective pilot study
Sérové protilátky proti laminarinu u kolorektálního karcinomu: prospektivní pilotní studie
Úvod a cíle: Laminarin je nízkomolekulární (504 Da) větvený glukózový polysacharid [ (1®3) -b-D-glukan], který je schopen modulovat humorální a buněčnou imunitní odpověď, a to nespecifickou i specifickou. Laminarin se vyznačuje anti-tumorózní aktivitou, neboť indukuje apoptózu a ovlivňuje intestinální mikrobiota. Cílem studie bylo vyšetřit anti-laminarinové protilátky u pacientů s kolorektálním karcinomem. Metody: Do prospektivní studie bylo zahrnuto 46 jedinců, z toho 14 kontrol (5 mužů, 9 žen, věk 29–80, průměr 55 ± 13) a 32 pacientů s kolorektálním karcinomem (14 mužů, 18 žen, věk 46–86, průměr 66 ± 11). Dva pacienti z CRC skupiny byli vyřazeni pro zjištěnou odlehlou hodnotu. Z 30 pacientů s CRC mělo 12 pravostranný kolorektální karcinom (12/30, 40 %). Většina pacientů s CRC měla stadium III (13/30, 43 %) nebo IV (13/30, 43 %). Pacienti měli převážně středně diferencovaný (15/30, 50 %) nebo nízce diferencovaný (11/30, 37 %) karcinom. Vzorky byly odebírány z periferní žilní krve a sérové IgG protilátky proti laminarinu byly stanoveny metodou ELISA v jednotkách U/ml. Výsledky: Sérové protilátky proti laminarinu byly signifikantně vyšší u kontrol ve srovnání s pacienty s CRC (16,23 ± 6,60; 11,41 ± 5,53; p = 0,015) a u kontrol ve srovnání s pacienty s levostranným CRC (11,38 ± 5,39; p = 0,046). Významný rozdíl byl pozorován mezi kontrolami a CRC III. stadiem (11,01 ± 3,36; p = 0,017); mezi kontrolami a CRC IV. stadiem (10,98 ± 6,06; p = 0,049). Anti-laminarinové protilátky byly signifikantně nižší u středně diferencovaného CRC ve srovnání s kontrolami (10,78 ± 5,22; p = 0,020), avšak nikoli u nízce diferencovaného CRC (12,10 ± 6,28; p = 0,755). Nebyl prokázán rozdíl mezi kontrolami a ženami s CRC (11,94 ± 6,39; p = 0,092). Byl potvrzen signifikantní rozdíl mezi kontrolami a muži s CRC (10,72 ± 4,32; p = 0,017). Závěr: Sérové protilátky proti laminarinu byly signifikantně nižší u pacientů s CRC a u podskupin osob s kolorektálním karcinomem.
Klíčová slova:
kolorektální karcinom – anti-laminarinové protilátky – nádorová biologie
Authors:
D. Kohoutová 1,2
; Drahošová M. 3; Morávková P. 1; Podhola M. 4; S. Rejchrt 1
; Bureš J. 1
Authors‘ workplace:
2nd Department of Internal Medicine – Gastroenterology, Charles University, Faculty of Medicine in Hradec Kralove and University Hospital, Hradec Kralove, Czech Republic
1; The Royal Marsden NHS Foundation Trust, London, United Kingdom
2; Institute of Clinical Immunology and Allergology, Charles University, Faculty of Medicine in Hradec Kralove and University Hospital, Hradec Kralove, Czech Republic
3; The Fingerland Department of Pathology, Charles University, Faculty of Medicine in Hradec Kralove and University Hospital Hradec Kralove, Czech Republic
4
Published in:
Gastroent Hepatol 2021; 75(4): 323-327
Category:
doi:
https://doi.org/10.48095/ccgh2021323
Overview
Introduction and objectives: Laminarin is a low molecular weight (504 Da) glucose branched polysaccharide which modulates humoral and cellular immune response, both non-specific and specific. Laminarin possesses anti-tumorous activity as it induces apoptosis and modulates the colonic microbiota. The aim of our current study was to evaluate anti-laminarin antibodies in colorectal carcinoma. Methods: A total of 46 individuals were enrolled in the prospective study including 14 controls (5 men, 9 women, age 29–80, mean 55 ± 13) and 32 patients with colorectal carcinoma, CRC (14 men, 18 women, age 46–86, mean 66 ± 11). Two outliers were identified in the CRC group. Out of 30 CRC patients, 12 individuals had right-sided CRC (12/30; 40%). Majority of the CRC patients had stage III (13/30; 43%) or IV (13/30; 43%) cancer. Most of the CRC patients had moderately (15/30; 50%) or poorly (11/30; 37%) differentiated CRC. Samples were obtained from the peripheral venous blood and investigation of the serum IgG anti-laminarin antibodies was performed by means of ELISA and measured in U/mL. Results: Serum anti-laminarin antibodies were significantly higher in controls compared to the CRC group (16.23 ± 6.60; 11.41 ± 5.53; p = 0.015) and in controls compared to left-sided carcinoma (11.38 ± 5.39; p = 0.046). A statistically significant difference was observed between controls and CRC stage III (11.01 ± 3.36; p = 0.017) and between controls and CRC stage IV (10.98 ± 6.06; p = 0.049). Anti-laminarin antibodies were significantly lower in moderately differentiated CRC compared to controls (10.78 ± 5.22; p = 0.020), but not in poorly differentiated CRC (12.10 ± 6.28; p = 0.755). No difference was identified between controls and females with CRC (11.94 ± 6.39; p = 0.092). There was a significant difference between controls and males with CRC (10.72 ± 4.32; p = 0.017). Conclusion: Serum anti-laminarin antibodies were significantly lower in the CRC group and CRC subgroups compared to controls.
Keywords:
colorectal cancer– anti-laminarin antibodies – tumorous biology
Introduction
Laminarin is a low molecular weight (504 Da) glucose branched polysaccharide. In nature, this beta-glucan polysaccharide is produced mostly by plankton through photosynthesis [1]. Laminarin exerts several biological functions; mainly it modulates humoral and cellular immune response, both non-specific (increased immune responsiveness to a wide variety of antigens) and specific (affecting a restricted type of immune response to a narrow group of antigens). Further, laminarin possesses therapeutic potential, which is related to its antigen--specific immuno-adjuvanticity [2,3]. The anti-tumorous activity of laminarin is known as well: it can induce apoptosis in human colon cancer cells [4] and it promotes anti-cancer immunity by maturation of dendritic cells [5]. Laminarin has the important capability to modulate colonic microbiota, too [6–11].
In our previous studies, we examined various antiglycan antibodies (including anti-laminaribioside ones) in inflammatory bowel disease [12,13]. Our further research focused on the evaluation of apoptotic activity in colorectal neoplasia [14] and we also investigated specific aspects of the large intestinal microbiome in colorectal cancer [15,16].
The aim of our current study was to evaluate anti-laminarin antibodies in colorectal neoplasia.
Methods
Subjects
A total of 46 individuals were enrolled into the prospective study, including 14 controls (5 men, 9 women, age 29–80, mean 55 ± 13) and 32 patients with colorectal carcinoma (CRC; 14 men, 18 women, age 46–86, mean 66 ± 11).
The control group consisted of individuals who had normal findings on colonoscopy. These subjects had a negative history of colorectal neoplasia and inflammatory bowel disease; further, they belonged to the population with an average risk for colorectal carcinoma.
The CRC group of patients had carcinoma diagnosed at the time when the serum for subsequent investigation of anti-laminarin antibodies was obtained.
Two patients with colorectal cancer were excluded from the statistical analysis, as they were outliers (an outlier is defined as a value outside the interval (Q1 – 1.5 IQR, Q3 + 1.5 IQR), where Q1 is lower quartile, Q3 is upper quartile and IQR = Q3 – Q1 is interquartile range). One outlier was a woman with right - -sided CRC, stage II; the other outlier was a man with left-sided CRC, stage IV. Statistical analysis was therefore performed on 14 controls and 30 patients with CRC.
Out of 30 CRC patients, 12 individuals had right-sided CRC (12/30, 40%) and 18 persons had left-sided CRC (18/30, 60%). Majority of the CRC patients had stage III (13/30, 43%) or IV (13/30, 43%) cancer. Most of the CRC subjects had moderately (15/30, 50%) or poorly (11/30, 37%) differentiated CRC.
The result of serum CEA was available in 24/30 (80%) patients.
Sample collection and measurement
Samples were obtained from the peripheral venous blood before the standard colonoscopy at the Endoscopy Unit, 2nd Department of Internal Medicine-Gastroenterology. Immediate blood centrifugation followed and the sera have been stored at –80°C. Investigation of serum IgG anti-laminarin antibodies was performed by means of the quantitative sandwich enzyme immunoassay technique (ELISA).
Ethical issues
All enrolled individuals were given the necessary information and provided informed consent via a signed form. The project was approved by the Joint Ethical Committee (Charles University, Faculty of Medicine in Hradec Kralove, University Hospital Hradec Kralove). For all obtained data, all personal identification information was removed in compliance with the General Data Protection Regulation (Regulation 2016/679 of the European Parliament and of the Council of 27 April 2016 and repealing Directive 95/46/EC).
Statistical analysis
The obtained data was tested statistically by means of descriptive statistics. Data with normal distribution was further analysed by a parametric unpaired t-test and data with non-normal distribution was tested by a non-parametric Mann-Whitney test. The association of variables was evaluated by a Spearman rank order correlation test. STATISTICA software, version 13, 2013, Tulsa, OK, USA and SigmaStat software, version 3.1, Jandel Corp., Erkrath, Germany, were used.
Results
Results of serum IgG anti-laminarin antibodies are summarised in Tab. 1. Statistically significant differences were found between controls and colorectal cancer (whole group of patients; p = 0.015) and between controls and left-sided carcinoma (p = 0.046). There was a trend towards a statistically significant difference observed between the controls and right-sided carcinoma (p = 0.067; type 2 error beta: 0.664). No difference was revealed between right-sided and left-sided carcinoma (p = 0.850) (Graph 1).
Tab. 1. Sérové IgG protilátky proti laminarinu u kontrol a kolorektálního karcinomu
(U/ml).
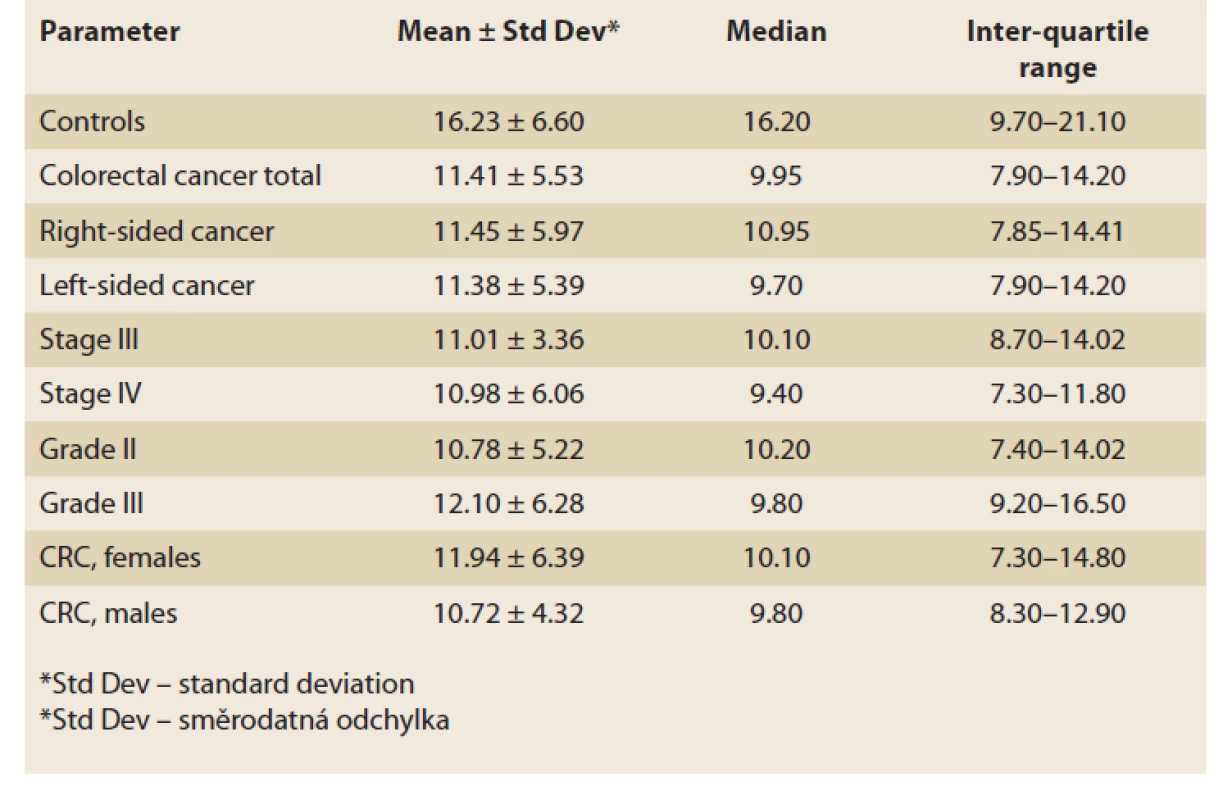
CRC: whole group of patients with colorectal carcinoma;
R-CRC: right-sided colorectal carcinoma, L-CRC: left-sided
colorectal carcinoma.
Graf 1. Sérové IgG protilátky proti laminarinu u kontrol a kolorektálního
karcinomu (U/ml).
CRC: celá skupina pacientů s kolorektálním karcinomem;
R-CRC: pravostranný kolorektální karcinom, L-CRC: levostranný
kolorektální karcinom.

A statistically sig - nificant difference was observed be - tween controls and CRC stage III (p = 0.017) and between controls and CRC stage IV (p = 0.049). No difference was confirmed between CRC stage III and CRC stage IV (p = 0.473) (Graph 2).
TNM classification used for stage of CRC.
Graf 2. Sérové IgG protilátky proti laminarinu (U/ml) u kolorektálního
karcinomu se vztahem ke stadiu onemocnění.
K určení stadia kolorektálního karcinomu byla užita TNM
klasifikace.

Serum IgG anti-laminarin antibodies were significantly lower in moderately differentiated CRC compared to controls (p = 0.020). No difference was found between poorly differentiated CRC and controls (p = 0.171), and neither between moderately and poorly differentiated CRC (p = 0.755) (Graph 3).
G2: moderately differentiated CRC; G3: poorly
differentiated CRC.
Graf 3. Sérové IgG protilátky proti laminarinu (U/ml) u kolorektálního
karcinomu se vztahem k diferenciaci nádoru.
G2: středně diferencovaný CRC; G3: nízce diferencovaný CRC.

No statistically significant difference was identified between controls and females with CRC (p = 0.092; type 2 error beta: 0.697), but there was a significant difference between controls and males with CRC (p = 0.017). No difference was revealed between females with CRC and males with CRC (p = 0.557) (Graph 4).
Graf 4. Sérové IgG protilátky proti laminarinu (U/ml) u žen
a mužů s kolorektálním karcinomem.
CRC females: ženy s kolorektálním karcinomem;
CRC males: muži s kolorektálním karcinomem.

In CRC patients, there was a significant negative correlation between age and anti-laminarin antibodies (r = –0.553; p = 0.002) and trend towards a significant negative correlation between CEA and anti-laminarin antibodies (r = –0.382; p = 0.065).
Discussion
Our prospective pilot study brought an important new insight into the possible role of laminarin in large bowel carcinogenesis. To the best of our knowledge, this is the first human study on anti-laminarin antibodies in colorectal neoplasia. Serum concentrations of these antibodies were significantly lower in left-sided carcinoma compared to the controls, while they were of borderline insignificance in right-sided cancer. In general, environmental factors are asserted more just in left-sided colorectal neoplasia [17]. We confirmed a negative correlation between anti-laminarin antibodies and the age of the investigated subjects. Antibodies were significantly lower in males compared to the controls, but not in females compared to the controls. The male gender and higher age have been clearly recognised as risk factors in colorectal carcinogenesis [17–19]. There was a borderline insignificant negative correlation between anti-laminarin antibodies and the serum concentration of carcinoembryonic antigen (CEA). CEA is an oncofetal protein that is elevated in the serum of patients with a variety of cancers, including colorectal carcinoma. Serum concentrations of CEA correlate with disease burden and have a prognostic value [20]. Serum anti-laminarin antibodies were significantly lower in the cancer stages III and IV as well as in the moderately differentiated cancers when compared to controls, but interestingly, not in poorly differentiated carcinoma in our current study.
Anti-laminarin antibodies provide indirect evidence of the possible impact of laminarin in colorectal neoplasia. These antibodies are normally present in healthy subjects [21], yet these have been studied mostly in Crohn‘s disease so far [12,22–24] as they predict a more complicated behaviour of the disease [25,26].
We can hypothesise that laminarin may be involved in colorectal carcinogenesis both on a direct and indirect basis. It is capable of stimulating apoptosis [27,28]. Laminarin slows down tumour growth and possesses anti-angiogenic activity in an experimental setting [29,30]. According to experimental studies, laminarin is able to regulate microbiota of the large bowel [6,9]. All factors, including apoptosis, anti-angiogenic status and colorectal microbiota play a crucial role in human tumorous biology [14–16,31].
We are aware of the possible limitations of our current project. This is a pilot study with a limited number of subjects. We did not investigate anti-laminarin antibodies in colorectal adenomas and serrated lesions.
Conclusions
Serum anti-laminarin antibodies were significantly lower in the CRC group and left-sided colorectal cancer when compared to healthy subjects. A significant difference was also found between controls and stages III and IV and moderately differentiated colorectal carcinoma. Further studies are needed to clarify the clinical importance and possible significance of these findings.
Submitted/Doručeno: 19. 7. 2021
Accepted/Přijato: 26. 7. 2021
Prof. Darina Kohoutova, MD, PhD
2nd Department of Internal Medicine – Gastroenterology
Charles University, Faculty of Medicine in Hradec Kralove
and University Hospital, Hradec Kralove
Sokolska 581
500 05 Hradec Kralove
Czech Republic
Conflict of Interest: The authors declare that the article/ manuscript complies with ethical standards, patient anonymity has been respected, and they state that they have no financial, advisory or other commercial interests in relation to the subject matter.
Publication Ethics: This article/ manuscript has not been published or is currently being submitted for another review. The authors agree to publish their name and e-mail in the published article/ manuscript.
Dedication: The study was supported by the academic project PROGRES Q40-15 from Charles University.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Konflikt zájmů: Autoři deklarují, že text článku odpovídá etickým standardům, byla dodržena anonymita pacientů a prohlašují, že v souvislosti s předmětem článku nemají finanční, poradenské ani jiné komerční zájmy.
Publikační etika: Příspěvek nebyl dosud publikován ani není v současnosti zaslán do jiného časopisu pro posouzení. Autoři souhlasí s uveřejněním svého jména a e-mailového kontaktu v publikovaném textu.
Dedikace: Studie byla podpořena akademickým projektem PROGRES Q40-15 Univerzity Karlovy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Sources
1. Becker S, Tebben J, Coffinet S et al. Laminarin is a major molecule in the marine carbon cycle. Proc Natl Acad Sci U S A 2020; 117 (12): 6599–6607. doi: 10.1073/pnas.1917001 117.
2. Smith AJ, Graves B, Child R et al. Immunoregulatory activity of the natural product laminarin varies widely as a result of its physical properties. J Immunol 2018; 200 (2): 788–799. doi: 10.4049/jimmunol.1701258.
3. Zargarzadeh M, Amaral AJR, Custódio CA et al. Biomedical applications of laminarin. Carbohydr Polym 2020; 232 : 115774. doi: 10.1016/j.carbpol.2019.115774.
4. Ji YB, Ji CF, Zhang H. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules 2012; 17 (8): 9947–9960. doi: 10.3390/molecules17089947.
5. Song K, Xu L, Zhang W et al. Laminarin promotes anti-cancer immunity by the maturation of dendritic cells. Oncotarget 2017; 8 (24): 38554 – –38567. doi: 10.18632/oncotarget.16170.
6. Vigors S, O‘Doherty JV, Rattigan R et al. Effect of a laminarin rich macroalgal extract on the caecal and colonic microbiota in the post-weaned pig. Mar Drugs 2020; 18 (3): 157. doi: 10.3390/md18030157.
7. Rattigan R, Sweeney T, Maher S et al. Laminarin-rich extract improves growth performance, small intestinal morphology, gene expression of nutrient transporters and the large intestinal microbial composition of piglets during the critical post-weaning period. Br J Nutr 2020; 123 (3): 255–263. doi: 10.1017/S0007114519002678.
8. Nguyen SG, Kim J, Guevarra RB et al. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet. Food Funct 2016; 7 (10): 4193–4201. doi: 10.1039/c6fo00929h.
9. Cui Y, Zhu L, Li Y et al. Structure of a laminarin-type b - (1®3) -glucan from brown algae Sargassum henslowianum and its potential on regulating gut microbiota. Carbohydr Polym 2021; 255 : 117389. doi: 10.1016/j.carbpol.2020.117389.
10. Tang C, Kamiya T, Liu Y et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing lactobacillus-mediated regulatory T-cell expansion in the intestine. Cell Host Microbe 2015; 18 (2): 183–197. doi: 10.1016/j.chom.2015. 07.003.
11. Rattigan R, Sweeney T, Vigors S et al. Effects of reducing dietary crude protein concentration and supplementation with laminarin or zinc oxide on the faecal scores and colonic microbiota in newly weaned pigs. J Anim Physiol Anim Nutr (Berlin) 2020; 104 (5): 1471–1483. doi: 10.1111/jpn.13428.
12. Rejchrt S, Drahosová M, Kopácová M et al. Antilaminaribioside and antichitobioside antibodies in inflammatory bowel disease. Folia Microbiol (Praha) 2008; 53 (4): 373–376. doi: 10.1007/s12223-008-0058-2.
13. Kohoutova D, Drahosova M, Moravkova P et al. Anti-outer membrane protein C and anti-glycoprotein 2 antibodies in inflammatory bowel disease and their association with complicated forms of Crohn‘s disease. BMC Gastroenterol 2014; 14 : 190. doi: 10.1186/s12876-014-0190-1.
14. Kohoutova D, Pejchal J, Bures J. Mitotic and apoptotic activity in colorectal neoplasia. BMC Gastroenterol 2018; 18 (1): 65. doi: 10.1186/s12876-018-0786-y.
15. Kohoutova D, Smajs D, Moravkova P et al. Escherichia coli strains of phylogenetic group B2 and D and bacteriocin production are associated with advanced colorectal neoplasia. BMC Infect Dis 2014; 14 : 733. doi: 10.1186/s12879-014-0733-7.
16. Kohoutova D, Forstlova M, Moravkova P et al. Bacteriocin production by mucosal bacteria in current and previous colorectal neoplasia. BMC Cancer 2020; 20 (1): 39. doi: 10.1186/s12885-020-6512-5.
17. Macrae FA. Colorectal cancer: epidemiology, risk factors, and protective factors. [online]. Available from URL: www.uptodate.com.
18. Kohoutova D, Rejchrt S, Cihak M et al. Importance of correct colorectal cancer screening timing in the average-risk Czech population. Abdomin Oncol 2013; 1 : 1–4.
19. Zavoral M, Suchanek S, Majek O et al. Colorectal cancer screening: 20 years of development and recent progress. World J Gastroenterol 2014; 20 (14): 3825–3834. doi: 10.3748/wjg.v20.i14.3825.
20. Moy B, Jacobson BC. Surveillance after colorectal cancer resection. [online]. Available from URL: www.uptodate.com.
21. Chiani P, Bromuro C, Cassone A et al. Anti-beta-glucan antibodies in healthy human subjects. Vaccine 2009; 27 (4): 513–519. doi: 10.1016/j.vaccine.2008.11.030.
22. Dotan I. New serologic markers for inflammatory bowel disease diagnosis. Dig Dis 2010; 28 (3): 418–423. doi: 10.1159/000320396.
23. Malickova K, Lakatos PL, Bortlik M et al. Anticarbohydrate antibodies as markers of inflammatory bowel disease in a Central European cohort. Eur J Gastroenterol Hepatol 2010; 22 (2): 144–150. doi: 10.1097/MEG.0b013e32832f5c7e.
24. Kuna AT. Serological markers of inflammatory bowel disease. Biochem Med (Zagreb) 2013; 23 (1): 28–42. doi: 10.11613/bm.2013.006.
25. Rieder F, Schleder S, Wolf A et al. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated Crohn‘s disease behavior. Inflamm Bowel Dis 2010; 16 (2): 263–274. doi: 10.1002/ibd.21 046.
26. Seow CH, Stempak JM, Xu W et al. Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype. Am J Gastroenterol 2009; 104 (6): 1426–1434. doi: 10.1038/ajg.2009.79.
27. Ji CF, Ji YB. Laminarin-induced apoptosis in human colon cancer LoVo cells. Oncol Lett 2014; 7 (5): 1728–1732. doi: 10.3892/ol.2014.1952.
28. Park HK, Kim IH, Kim J et al. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int J Mol Med 2013; 32 (2): 291–295. doi: 10.3892/ijmm.2013.1409.
29. Jolles B, Remington M, Andrews PS. Effects of sulphated degraded laminarin on experimental tumour growth. Br J Cancer 1963; 17 (1): 109 – –115. doi: 10.1038/bjc.1963.16.
30. Hoffman R, Paper DH, Donaldson J et al. Inhibition of angiogenesis and murine tumour growth by laminarin sulphate. Br J Cancer 1996; 73 (10): 1183–1186. doi: 10.1038/bjc.1996.228.
31. Kohoutova D et al. Intestinal microbiota in inflammatory bowel disease and colorectal neoplasia (in Czech). Hradec Králové: Nucleus HK 2013. ISBN 978-8087009-97-0.
Labels
Paediatric gastroenterology Gastroenterology and hepatology SurgeryArticle was published in
Gastroenterology and Hepatology
2021 Issue 4
- Possibilities of Using Metamizole in the Treatment of Acute Primary Headaches
- Metamizole at a Glance and in Practice – Effective Non-Opioid Analgesic for All Ages
- Metamizole vs. Tramadol in Postoperative Analgesia
- Spasmolytic Effect of Metamizole
- The Importance of Limosilactobacillus reuteri in Administration to Diabetics with Gingivitis
-
All articles in this issue
- Clinical and experimental gastroenterology
- Kvíz z klinické praxe
- SARS-CoV-2 infection and pancreatic disease
- Parkinson‘s disease and GIT involvement
- Heyde’s syndrome
- CMV enteritis as unusual source of bleeding to GIT
- The impact of sarcopenia and myosteatosis in liver transplant candidates on peritransplant course and patient and graft survival
- Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline and Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline
- An unusual case of systemic AA amyloidosis
- Vitamin D and non-alcoholic fatty liver disease in children
- The effect of CDED diet on the development of remission in a patient with persistent Crohn’s disease activity – a case report and workplace experience
- News from the EASL-ILC congress: advances in the knowledge and treatment of primary biliary cholangitis
- The selection from international journals
- Správná odpověď na předchozí kvíz Dlouhodobé bolesti břicha
- Kreditovaný autodidaktický test: klinická a experimentální gastroenterologie
- Serum anti-laminarin antibodies in colorectal cancer: a prospective pilot study
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Heyde’s syndrome
- SARS-CoV-2 infection and pancreatic disease
- The effect of CDED diet on the development of remission in a patient with persistent Crohn’s disease activity – a case report and workplace experience
- Vitamin D and non-alcoholic fatty liver disease in children
