Ischemic and non-ischemic causes of ST-segment elevation in patients with chest pain: a systematic review of the literature
Ischemické a neischemické příčiny elevace ST segmentu u pacientů s bolestí na hrudi: Systematický přehled literatury
Elektrokardiogram zůstává nejdůležitějším nástrojem v diagnóze infarktu myokardu s elevací ST segmentu. Je okamžitě k dispozici, je snadné jej opakovat a ekonomicky je velice výhodný. Pro trénovaného odborníka je rovněž vysoce citlivý a specifický. Časná diagnóza a následná léčba infarktu myokardu s elevací ST segmentu závisí na rychlém provedení a správné interpretaci EKG. Mimo akutního infarktu myokardu existuje u pacienta s probíhající bolestí na hrudi mnoho dalších příčin elevace ST segmentu. Lékař musí mít tyto diferenciální diagnózy na zřeteli a musí být schopen rychle potvrdit nebo vyvrátit alternativní příčiny elevace ST segmentu. Cílem tohoto článku je přezkoumání nálezů na EKG u akutního infarktu myokardu v porovnání s ostatními alternativními diagnózami, u kterých může docházet k elevaci ST segmentu. Tato diskuse je ukončena třemi kazuistickými příklady atypických příčin elevace ST segmentu u pacienta s bolestí na hrudi.
Klíčová slova:
elektrokardiogram – elevace ST segmentu – infarkt myokardu – diferenciální diagnóza
Authors:
M. Levine; Y. Kanei; M. Rachko; P. Schweitzer
Authors‘ workplace:
Beth Israel Medical Center, New York, NY, USA
Published in:
Vnitř Lék 2012; 58(7 a 8): 54-59
Category:
80th Birthday MUDr. Miroslav Mydlík, DrSc.
Overview
The electrocardiogram remains the most crucial tool in the diagnosis on ST-segment elevation myocardial infarction. It is rapidly available, easily reproducible, and highly cost effective. To the trained interpreter it is also highly sensitive and specific. Early diagnosis and subsequent treatment of ST-segment myocardial infarction relies on rapid performance and correct interpretation of the electrocardiogram. In addition to acute myocardial infarction, there are multiple other causes of ST-segment elevation in the patient with active chest pain. The clinician must be aware of these differential diagnoses, and be able to quickly confirm or exclude alternative causes of ST-segment elevation. The purpose of this article is to review the electrocardiographic findings in acute myocardial infarction in comparison to other alternative diagnoses that may present with ST-segment elevation. This discussion concludes with three case based examples of atypical causes of ST-segment elevation in the patient with chest pain.
Keywords:
electrocardiogram – ST-segment elevation – myocardial infarction – differential diagnosis
Introduction
Primary percutaneous coronary intervention (PPCI) is the preferred treatment of ST-segment elevation myocardial infarction (STEMI) [1–3]. Patients with STEMI, should undergo PPCI emergently; no later than 12 hours after the onset of chest pain [4,5]. The Task Force of American College of Cardiology (ACC) and the European Society of Cardiology (ESC) guidelines state that increased levels of troponin or other biomarkers are a main diagnostic criteria for acute myocardial infarction [6]. However, because increase troponin level is not seen earlier than 6–8 hours after an acute event, the diagnosis of STEMI must be initially confirmed or excluded based upon clinical presentation and ST-segment elevations on 12-lead electrocardiogram (ECG). It is further suggested, that ECG diagnosis of STEMI should take no longer than 10–15 minutes. This may not always be feasible, as the time between the onset of symptoms and the first contact with a patient experiencing a suspected acute coronary syndrome varies. Furthermore, first contact with the medical system is often not directly with the Emergency Department, but may vary from emergency medical services to a primary care physician. Additional causes of delay to reperfusion include patient transfer from hospitals without PPCI capability, activation of the catheterization laboratory [8,9] or intra-procedural difficulties [5]. Regardless of manner of presentation, the ECG remains the most important means of STEMI diagnosis given its rapid availability, low cost, and high sensitivity to a trained interpreter.
This report reviews the ECG diagnosis of STEMI, with the main emphasize on the differential diagnosis between ischemic and non-ischemic causes of ST-segment elevation (STE).
ECG diagnosis of STEMI
In the patient with chest pain, the diagnosis of STEMI requires new STE at the J-point in two contiguous leads of > 0.2 mV in men in leads V2–3 (> 0.15 mV for women) and/or > 0.1 mV in limb leads and V4–6 leads [6]. These ECG criteria are simple and straightforward, however, there are multiple non-ischemic causes of STE; particularly in precordial leads [7]. Important causes of STE mimicking STEMI are left ventricular hypertrophy, acute myocarditis/pericarditis, previous myocardial infarction, and numerous other non-cardiac causes [7]. The ECG diagnosis of STEMI also depends on the expertise of the initial person evaluating the patient with chest pain. As mentioned this may be a paramedic, emergency department physician or general internist, all with vastly different ECG interpretation experience and ability. There are different approaches for assessing the role and importance of the ECG in patients with suspected STEMI, and understanding how different non-ischemic etiologies may effect accurate interpretation.
False positive activation of cardiac catheterization laboratory for primary PCI
The ECG remains a highly sensitive tool for the diagnosis of STEMI. Given the seriousness of the diagnosis, most interpreters value high sensitivity over specificity. This trade-off allows for investigation of a reasonably high false positive rate of diagnosis. The incidence of false positive activation of the catheterization laboratory for PPCI varies between 6.4 to 22.7% with an average rate of 12.7% [10–12]. False positive cardiac catheterization may be defined as performance of angiography for a suspected STEMI that reveals no acute intracoronary occlusion. The strength of this approach is that all patients undergo coronary angiography. Larson et al [10] reported one of the first studies using this approach. The patient population included both transfers from community hospitals and from the emergency department of a tertiary cardiovascular center in Minnesota. 1,335 patients with suspected STEMI underwent emergent coronary angiography. 187 patients (14%) did not have a culprit coronary artery occlusion. Sixty patients without culprit coronary artery did have significant coronary artery disease requiring coronary bypass surgery, non--emergent percutaneous intervention, or conservative management. The remaining 127 patients did not have obstructive coronary artery disease. These patients were evaluated, and the etiologies of STE on ECG were determined. The most frequent causes of STE were early repolarization, previous myocardial infarction, or non--diagnostic ECG. Less common ECG abnormalities were pacemaker rhythm or right bundle branch block. It is of interest that 44% of patients with new or presumably new left bundle branch block on ECG did not have culprit coronary artery. A similar study by Gu et al [11] reported coronary angiography of 820 patients presenting with chest pain and ST-segment elevations. PPCI was performed in 767 patients. Similar to the previous study, thirty-four patients had significant coronary disease without a culprit lesion. These patients were managed either conservatively or underwent coronary bypass surgery. Nineteen patients had other structural heart diseases, vascular diseases or non-cardiac abnormalities.
Perugini et al [12] reported on false positive activation of catheterization laboratory in a cohort of 3 780 patients. The diagnosis of STEMI was confirmed in 3,447 patients, while 333 patients did not have a culprit coronary artery occlusion. Seventy-five patients with normal coronary arteries had STEMI (2%), eighty-four had chronic coronary artery disease (2.3%), and thirty-six had Takotsubo cardiomyopathy (1.0%). The remaining hundred thirty-eight patients had various non--ischemic etiology for ST-segment elevation (3.7%).
Perugini et al [12] also undertook a simulation in which two experienced cardiologists re-analyzed 369 ECGs with STE and chest pain lasting for 20 minutes. The only additional available information were age and gender. The interpreters were required to answer two questions: First, does the patient have acute myocardial infarction warranting activation of the catheterization laboratory? Second, is STEMI unlikely and activation of the catheterization laboratory is not necessary? Based on these experienced interpreters there were 240 true positive, 44 false positive, 79 true negative and 6 false negative. The sensitivity and specificity were 0.97% and 0.64%, respectively. The positive and negative predictive values were 0.71% and 0.96% respectively; showing even the most experienced of interpreters may have some difficulty in determining the etiology of ST elevations.
Nfor et al [13] analyzed false positive STEMI diagnosis in the emergency department to determine the incidence, predictors, and prognosis of false positive STEMI. There were 489 patients with possible STEMI. A culprit coronary artery was found in 89% of patients while 11% of patient did not have an infarct related artery. Independent predictors of absence of culprit coronary artery occlusion were: lack of chest pain, absence of reciprocal ST-segment changes, less than 3 risk factors for CAD, symptom duration longer than 6 hours, recent illicit drug use, stable hemodynamics, and direct transfer from other hospitals. The three most frequent causes for false positive activation of a cardiac catheterization laboratory were early repolarization (14.8%), acute myocarditis//pericarditis (14.8%) and Takotsubo cardiomyopathy (11.1%). A minority of false positives could ultimately be attributed to other abnormalities such as coronary artery spasm or NSTEMI. It is important to note that 48.1 % of patients without culprit coronary arteries had “serious acute cardiac pathology” warranting further evaluation and treatment. In this series, even non--ischemic ST-segment elevation warranted further cardiac care.
Patients with STE on ECG without culprit coronary artery occlusion can be divided into two broad categories. The first group consists of patients with coronary artery disease or other structural heart disease in which coronary angiography excludes acute myocardial infarction, but clarifies the true diagnosis and helps in patient management. The second group consists of patients with ST-segment elevation on ECG either without ischemic heart disease nor other acute cardiac pathology. These patients often have acute non-cardiac disease such as pulmonary embolization or aortic dissection. These patients gain no benefit from coronary angiography and may be harmed by potential complications of angiography as well as delay in obtaining a true diagnosis and treatment.
Errors in interpretation of the ECG and discrepancies in the emergency department
Frequently an emergency physician is the first contact with a patient complaining of chest pain. Brady et al [14] reported on ECG proficiency amongst emergency physicians. There were nine possible causes of STE, including acute myocardial infarction. Emergency physicians correctly identified STE due to acute myocardial infarction in 29 out of 31 cases (93.5%). In this study, emergency physicians misinterpreted 12 out of 202 patients with STE (5.9%). The most frequent causes of missed diagnosis were early repolarization followed by left ventricular aneurysm. Early repolarization and left ventricular hypertrophy were the incorrect diagnoses most frequently given to the two patients with a missed diagnosis of acute myocardial infarction.
The same group published a second study [15] reviewing the diagnosis of STE by six Emergency Medicine physicians. Interpreters knew only age, gender, and presence of chest pain. Each interpreter had to answer two questions. First, was there STE and second was the morphology of STE consistent with STEMI more so than any other etiology. There were 599 patients with suspected acute myocardial infarction and 211 with non-ischemic STE. Discrepancy in ECG interpretation was present in 61 patients, particularly for the diagnosis of left ventricular hypertrophy (47.5%), left bundle branch block (19.7%), early repolarization (11.5%), and less frequently acute myocardial infarction, pericarditis or left ventricular aneurysm. Similarly the most common reason for disagreement was in the interpretation of left ventricular hypertrophy (42.9%) and acute myocardial infarction (28.6%) secondary to atypical concave STE.
Discrepancies in interpretation of STE by ECG experts and invasive cardiologists
Jayroe and et al [16] asked fifteen well know ECG experts from the US, Europe, and Israel, to differentiate between ischemic and non-ischemic STE on ECG. 116 ECGs in patients with suspected STEMI were evaluated. In addition to recommending PPCI versus conservative management, they were also asked to choose between 15 possible alternative causes of STE. The patient population included Caucasians, Asians, Hispanic and African Americans. An important consideration was that only 8 out of 116 patients (6.9%) had STEMI. Recommendation for PPCI varied between 7.8 and 33%. The sensitivity of the individual reader varied between 50–100% (mean 75%) and specificity between 73–97% (mean 85%). The choices of non-acute ischemic causes of STE markedly varied. These results were unexpected and suggested need for revision of ECG criteria for STEMI [16].
In a related report of 84 patients, the authors asked 7 interventional cardiologists the same questions [17]. In contrast to the previous study, 48 % patients had STEMI. The percentage of patients in whom the intervetionalists recommended PPCI varied between 33–75%. The sensitivity for STEMI was between 55–83% (average 71%) and specificity 32–79% (average 63%). These results were similar to their first study except for a lower sensitivity.
The less than expected accuracy in diagnosis of STE etiology amongst highly experienced ECG experts is not surprising and suggests that ECG alone has important limitations in identifying patients for PPCI. Fortunately in clinical practice, ECG is always correlated with history, physical and laboratory findings.
ST-segment elevation in patients with normal coronary arteries
The incidence of normal coronary arteries in patients with suspected STEMI undergoing coronary angiography varies between 2.6% to 13% [18–20]. There are multiple reasons for normal coronary artery in a true STEMI. Likewise, there are other cardiac and non--cardiac diseases mimicking myocardial infarction (hypertrophic cardiomyopathy, myocarditis or pulmonary embolization). The incidence of ECG misinterpretation is highly variable. In Widimsky’s study [18] the incidence of ECG misinterpretation was 19% among experienced interpreters. Prasad et al [20] reported the results of 690 patients with suspected STEMI. 594 patients (86%) had culprit coronary artery occlusion, nine and eleven had severe coronary artery disease without complete arterial occlusion, or left bundle branch block respectively. Using strict STE criteria the sensitivity and specificity were 93.1 and 44.7% respectively with 79.4% positive and 59.6% negative predictive value. In comparison, expert cardiologists who re-analyzed 160 and 76 patients with STEMI and normal coronary artery confirmed STEMI in 93% in patients with culprit coronary artery but only in 55% in those with normal coronary arteries.
In patients who present with normal coronary arteries and STE, acute cardiac and non-cardiac diseases have to be ruled out. This is particularly true for pulmonary embolization, aortic dissection, coronary spasm, myocarditis or pericarditis. D-dimmer and pulmonary and aortic imaging are helpful in patients with suspected pulmonary embolization or aortic dissection [21]. More difficult is the diagnosis of myocarditis. Presently myocarditis can be accurately diagnosed by showing myocardial edema on MRI. In addition, MRI helps to confirm or exclude the diagnoses of myocardial infarction, pericarditis, Takatsubo cardiomyopathy, or valvular heart diseases. A more detailed discussion of the use MRI in patients with acute coronary syndrome, with normal coronary arteries is undertaken by Agewal et al [21].
Atypical presentations of coronary occlusions requiring urgent coronary angiography
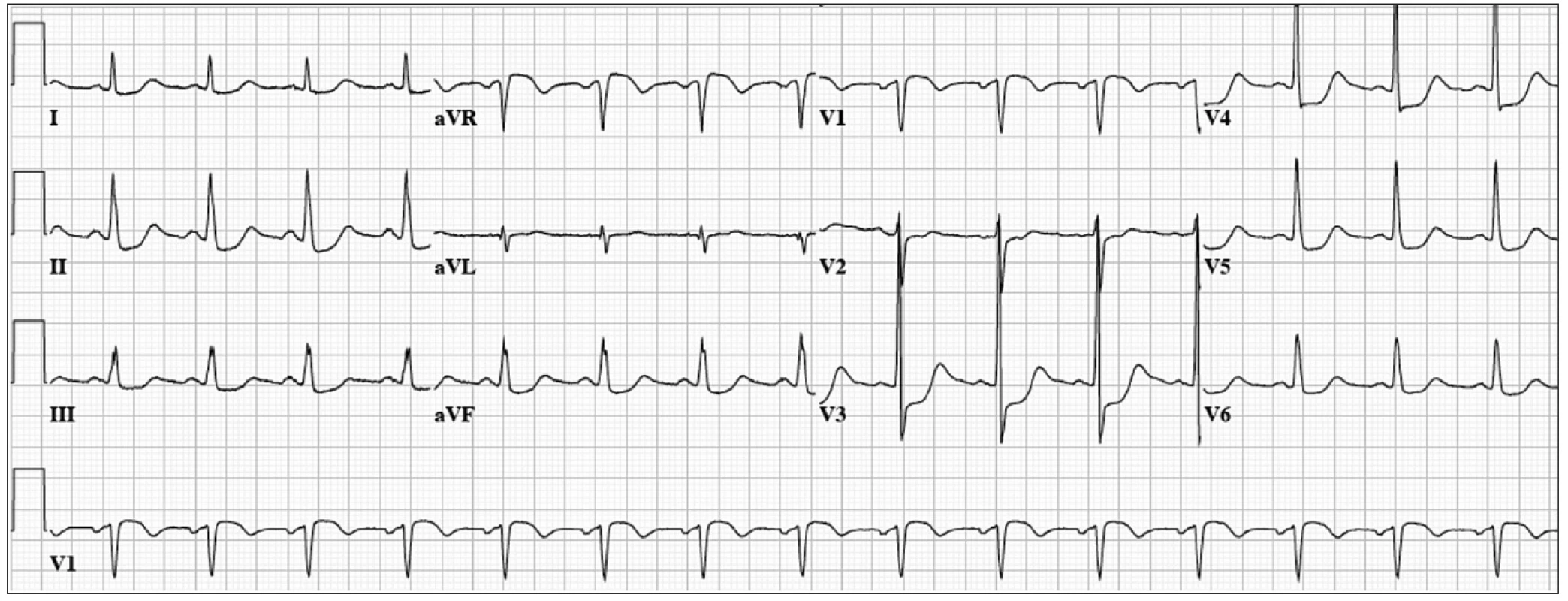
Two ischemic events requiring emergent coronary angiography are left main coronary artery disease (LMCA) [23] and unstable angina with a „Wellens” ECG pattern [24]. The most common ECG manifestations of acute occlusion of LMCA are ST-segment depression in six or more leads and ST-segment elevation in lead aVR and occasionally also in V1 [23]. Our patient was a 62 year-old woman with history of hypertension. She developed severe chest pain, hypotension and diaphoresis 24 hours after knee replacement. The ECG (Fig. 1) showed normal sinus rhythm at a rate of 94 bpm, ST segment depression in 9 leads, the most pronounced in lead V3 and V4 and ST elevation in lead aVR and V1. Coronary angiogram revealed left main coronary artery thrombosis. The patient underwent emergency coronary artery bypass and made uneventful recovery.
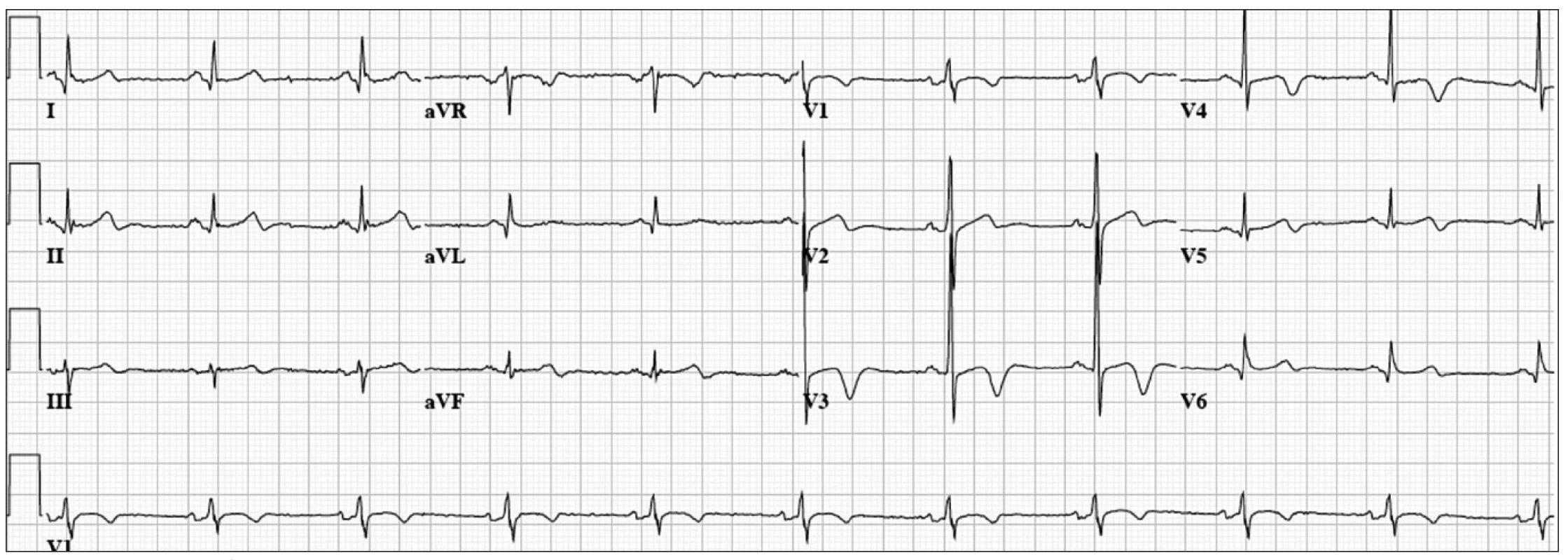
A second indication for urgent coronary angiography is unstable angina presenting with mild ST elevation followed by deep T-wave inversion in mid-precordial leads also known as the Wellens ECG pattern [24]. In these patients coronary anatomy usually shows severe mostly proximal LAD lesion. More recently, however, similar ECG patterns were reported in patients with coronary spasm, normal coronary arteries, intracerebral bleeding, myocardial bridging, and pulmonary edema [25]. Migliore et al reported 4 patients with similar ECG of different etiology in whom MRI showed myocardial edema.
Our patient was a 53 year-old man with hypertension. He developed chest pain while running. The ECG (Fig. 2) showed normal sinus rhythm at a rate of 61 bpm, mild ST-segment elevation with T wave inversion in the mid-precordial leads. Emergent coronary angiogram revealed non-obstructive coronary artery disease with significant myocardial bridging of the mid LAD. He was treated with oral calcium channel blockers and made a complete recovery.
Another atypical presentation of proximal left anterior occlusion can be prominent or normal T wave amplitude in leads V2–4 with or without ST depression lasting from 1 to 14 days. Some patients will show the normal ECG evolution of an anterior myocardial infarction. This type of acute myocardial infarction was originally reported by Sagie et al [26], and more recently with a slightly different ECG pattern by de Winter et al [27] and Verouden et al [28]. Identification of this “atypical” presentation of LAD occlusion may be difficult, but is important for early reperfusion.
Pride and et al [29] published acute coronary syndrome with isolated anterior ST-segment depression due to complete coronary artery occlusion. This was a sub-study of TRITON--TIMI 38 trial of 13 608 patients. Isolated anterior ST-segment depression was found in 1 198 patients (8.8%). Of these patients, 26% had complete occlusion of one of the main coronary arteries. The most common culprit artery was the left circumflex coronary artery. The 30-day incidence of composite death and myocardial infarction was significantly higher in patients with an occluded coronary artery.
Our final patient was a 52 year-old woman with dyslipidemia who presented with acute onset of chest pain and profound dyspnea. ECG (Fig. 3) revealed normal sinus rhythm at a rate of 78 bpm, mild ST-segment elevation in I, aVL, and V2–3, with deep T-wave inversions in the precordial leads. Emergent coronary angiogram revealed mild non-obstructive coronary artery disease, and a severely reduced ejection fraction with severe hypokinesis of the apex and distal anterior, septal, lateral, and inferior wall segments. The remaining wall segments were hyperkinetic. She was given a diagnosis of Takotsubo cardiomyopathy (previously described). She was treated for several months with beta-blockers and an ACE inhibitor with near normalization of her ejection fraction. In this case, ECG changes that are typically associated with myocardial ischemia were present without coronary artery disease.
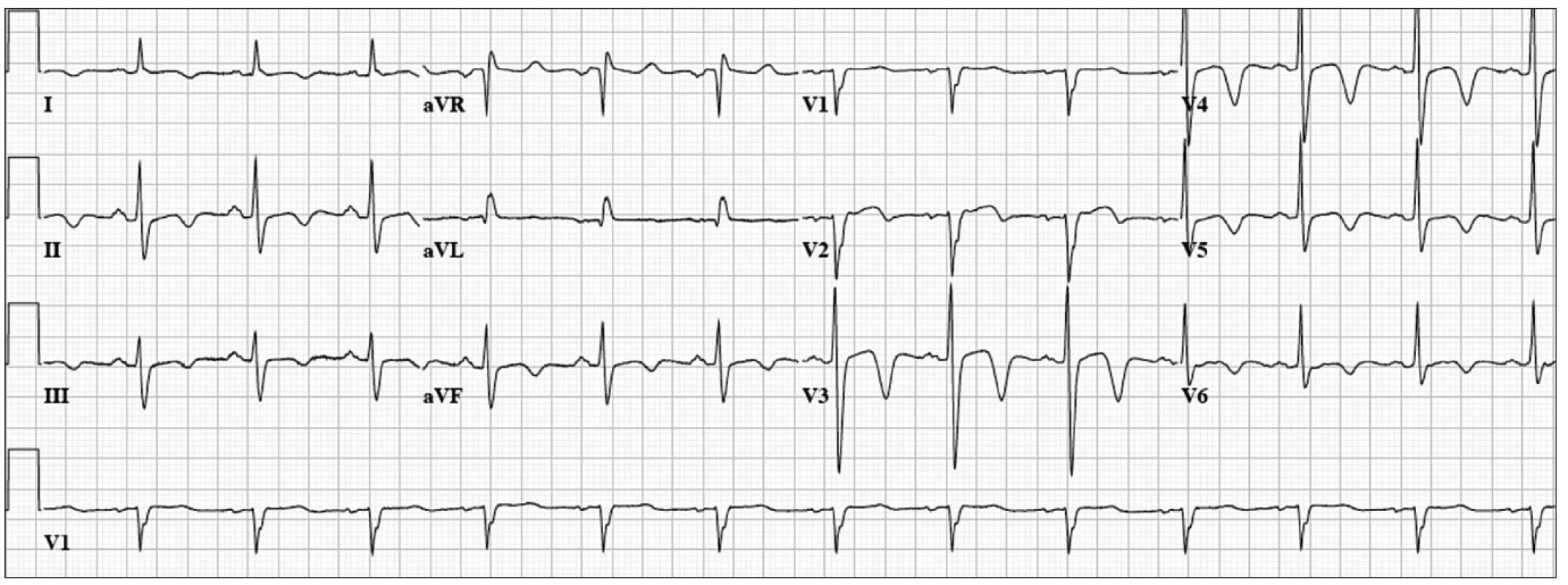
Finally, it is important to remember that acute myocardial infarction can be seen in patients with normal or non-specific ECG changes. These are most often patients with occlusion of the left circumflex artery or a branch of a main coronary artery.
Conclusions
Presently the ECG remains the initial diagnostic tool in patients with suspected STEMI. However, as discussed the ECG has important limitations. First, STE can be seen in other cardiac and non-cardiac conditions. Second, there are atypical ECG presentations of acute coronary occlusion that may not have ST-segment elevation. Third, a negative ECG does not exclude an acute coronary syndrome. Without correlating the ECG with clinical findings, and cardiac bio-markers, even experienced expert cardiologists have difficulty differentiating between ischemic and non-ischemic causes of ST-segment elevation based solely on the resting ECG. Finally, the initial diagnosis of STEMI is made by various types of physicians or paramedics, all with different ECG expertise, contributing to inaccuracies of diagnosis.
Dr. Michael Levine
www.wehealny.org
e-mail: mlevine@chpnet.org
Doručeno do redakce: 15. 5. 2012
Sources
1. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomized trials. Lancet 2003; 361 : 13–20.
2. Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous intervention for ST-elevation myocardial infarction: quantitative review of randomized trials. Lancet 2006; 364 : 579–588.
3. Andersen HR, Nielsen TT, Rasmussen K et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349 : 733–742.
4. De Luca G, Suryapranata H, Ottervagner JP et al. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109 : 1223–1225.
5. Antman EM. Time is muscle. J Am Coll Cardiol 2008; 52 : 1216–1221.
6. Thygesen K, Alpert JS, White HD. Joint ESC/ACC/AHA/WHF task force for redefinition of myocardial infarction. Universal definition of myocardial infarction. Eur Heart J 2007; 28 : 2525–2538.
7. Wang K, Asinger RW, Marriott HJL. St-segment elevation in condition other than acute myocardial infarction. N Engl J Med 2003; 349 : 2128–2135.
8. Widimsky P, Budesinsky T, Vorac D et al. Long distance transport for primary angioplasty vs immediate thrombolytic in acute myocardial infarction. Final results of the randomized national multicenter tria-Prague-2. Eur Hear J 2003; 24 : 94–104.
9. Le May MR, Dionne R, Maloney J et al. Paramedics in a primary PCI program for ST-elevation myocardial infarction. Prog Cardiovas Disease 2010; 53 : 183–187.
10. Larson DM, Menssen KM, Sharkey SW et al. “False-Positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA 2007; 298 : 2754–2760.
11. Gu YL, Svilaas T, van der Horst ICC et al. Conditions mimicking acute ST-segment elevation myocardial infarction in patients referred for primary percutaneous coronary intervention. Neth Hear J 2008; 16 : 325–331.
12. Perugini E, Di Pasquale G, Di Diodoro L et al. What is the acceptable rate of false positives for STEMI within a primary PCI network? Insight from a metropolitan system with direct ambulance-based access. Int J Cardiol 2012; 154 : 356–358.
13. Nfor T, Kostopoulos L, Hashim H et al. Identifying false-positive ST-elevation myocardial infarction in emergency department patients. J Emerg Med 2012; http://dx.doi.org/10.1016.jemermed.2011.09.027.
14. Brady WJ, Perron A, Ullman E. Error in emergency physician interpretation of ST-segment elevation in emergency department chest pain patients. Acad Emerg Med 2000; 7 : 1256–1260.
15. Erling BF, Perron AD, Brady WJ. Disagreement in the interpretation of electrocardiographic ST segment elevation: A source of error for emergency physician? Am J Emerg Med 2004; 22 : 65–70.
16. Jayroe JB, Spodick DH, Nikus K et al. Differentiating ST elevation myocardial infarction and nonischemic causes of ST elevation by analyzing the presenting electrocardiogram. Am J Cardiol 2009; 103 : 301–306.
17. Tran V, Huang HD, Diez JG et al. Differentiating ST elevation myocardial infarction from nonischemic ST elevation in patients with chest pain. Am J Cardiol 2011; 108 : 1096–1101.
18. Widimsky P, Stellova B, Groch L et al. Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial inarction: Experience from the PRAGUE study. Can J Cardiol 2006; 22 : 1147–1152.
19. Prasad SB, Richards DAB, Sadick N et al. Clinical and electrocardiographic correlates of normal coronary artery angiography in patients referred for primary precutaneous coronary intervention. Am J Cardiol 2008; 102 : 155–159.
20. Larsen AI, Galbraith D, Ghali WA et al. Characteristics and outcomes of patients with acute myocardial infarction and agiographically normal coronary arteries. Am J Cardiol 2005; 95 : 261–263.
21. Agewall S, Eurenius L, Hofman-Bang C et al. Myocardial infarction with agiographically normal coronary arteries. Atherosclerosis 2011; 219 : 10–14.
22. Stensaeth HK, Fossum E, Hoffman P et al. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int J Cardiovas Imaging 2011; 27 : 355–365.
23. Yamaji H, Iwasaki K, KusachiS et al. Prediction of acute left main Coronary artery obstruction by 12-lead electrocardiography. J Am Coll Cardiol 2001; 38 : 1348–1354.
24. de Zwaan C, Bar FW, Wellens HJJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103 : 730–764.
25. Migliore F, Zorzi A, Perazzolo M et al. Myocardial edema underlies dynamic T-wave inversion (Wellens’ ECG pattern) in patients with reversible left ventricular dysfunction. Heart Rhythm 2011; 8 : 1629–1634.
26. Sagie A, Sclarovsky S, Strasberg B et al. Acute anterior wall myocardial infarction presenting with positive T waves and without ST segment shift: Electrocardiographic features and angiographic correlation. Chest1989; 95 : 1211–1215.
27. de Winter RJ, Verouden NJW, Wellens HJJ et al. A new ECG sign of proximal LAD occlusion. N Engl J Med 2008; 359 : 2071–2073.
28. Verouden NJ, Koch KT, Peters RJ et al. Persistent precordial hyperacute T-waves signify proximal left anterior descending occlusion. Heart 2009; 95 : 1701–1706.
29. Pride YB, Tung P, Mohanavelu S et al. Angiographic and clinical outcome among patients with acute coronary syndrome presenting with isolated anterior ST-segment depression: A TRITON-TIMI 38 (Trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38) Sub study. J Am Coll Cardiol Intv 2010; 3 : 806–811.
Labels
Diabetology Endocrinology Internal medicineArticle was published in
Internal Medicine

2012 Issue 7 a 8
-
All articles in this issue
- The Restless Legs Syndrome in patients receiving hemodialysis treatment
- Aldosterone antagonists in chronic heart failure treatment
- Management of stable angina pectoris and of other chronic cardiovascular arterial diseases
- Antiatherogenic effect of HDL subpopulations in patients with newly diagnosed peripheral artery disease
- Sodium concentration in dialysate – an important but neglected parameter in haemodialysis of patients with chronic renal failure
- Oxidative stress and antioxidation systems in haemodialyzed patients
- Pharmacogenetic aspects of treatment with oral antidiabetics
- Refeeding syndrome in a young patient with the anxiety-depressive disorder
- Detecting KRAS and its mutations in biopsy of advanced colorectal carcinoma during colonoscopy
- Aortic stiffness increases central aortic pressure in patients with hypertension
- How to define people at a high risk of pancreatic cancer
- The size of LDL-lipoprotein particles among patient after acute stroke
- Chronic inflammation and the metabolic syndrome
- The prophylaxis and treatment of antiphospholipid syndrome – current options, difficulties and future perspectives
- Advancement in the area of multiple myeloma and development of connected laboratory background
- How to avoid the mistake in diagnosing incipient critical disorder of haemostasis in an out-patient clinic
- Frequencies of the new thrombophilic mutations of antithrombin (SERPINC1) (IVS +141G>A), glycoprotein GPVI (Ser219Pro) and cytochrome CYP4V2 (Lys259Gln) in healthy middle-aged people in Central Bohemia
- Megakaryopoesis and platelet genesis
- Temporary diagnostics and treatment of myeloma bone disease in clinical practice
- Changes to calcium-phosphate metabolism associated with chronic nephropathies
- End stage of chronic kidney disease and metabolic acidosis
- Chronic kidney disease and cellular calcium homeostasis
- To salt or not to salt in kidney diseases? Not more than quantum satis!
- Myocardial infarction the young – our results and experience
- An association between microalbuminuria and obesity in healthy adolescents – preliminary results from the “Respect for Health” study
- Contrast nephropathy and prevention
- Therapeutic approach to the bleeding in association with “old” and “new” anticoagulants
- Diagnosis of heparin-induced thrombocytopenia in the Czech Republic
- Acquired haemophilia A
- High dose treatments and preparatory regimens prior to haematopoietic stem cell transplantation
- Triple combination treatment of chronic hepatitis C
- An anaesthesiologist’s perspective on requirements for pre-surgery examinations
- Fixed combinations in the treatment of hypertension
- Farmacotherapy of hypertension after heart transplantation
- Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and health related quality of life: results from the SHIFT substudies
- Ischemic and non-ischemic causes of ST-segment elevation in patients with chest pain: a systematic review of the literature
- Internal Medicine
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue
- Myocardial infarction the young – our results and experience
- An anaesthesiologist’s perspective on requirements for pre-surgery examinations
- Megakaryopoesis and platelet genesis
- Ischemic and non-ischemic causes of ST-segment elevation in patients with chest pain: a systematic review of the literature
