-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
THE EFFECT OF NIFEDIPINE ON THE PATENCY OF MICROVASCULAR ANASTOMOSIS IN RATS
Autoři: K. E. Panagiotopoulos; M. Koutsouris; E. Panagiotopoulos; D. Antonopoulos; V. Panagiotopoulos; A. Papalois; A. Kepenekidis
Působiště autorů: Department of Plastic and Reconstructive Surgery, KAT General Hospital, Athens, Greece
Vyšlo v časopise: ACTA CHIRURGIAE PLASTICAE, 50, 1, 2008, pp. 33-35
INTRODUCTION
Advances in reconstructive surgical procedures have led to increasing use of free flaps for closure of surgical defects. Microvascular free tissue transfer technique allows the transfer of healthy composite donor tissue to distant recipient sites and involves division of the vascular pedicle at the donor site and anastomosis of the vessels at the recipient site. The survival of the flap is hence presumed to depend significantly on the integrity of the vascular pedicle. However, despite advances in microsurgical technique, instrumentation and experience gained in clinical microvascular surgery and postoperative management, there remains the possibility of thrombosis of the vessels and subsequent necrosis of the transferred tissue. It has been established that there are two risk zones mainly associated with flap failures (7). These are thrombotic occlusion of a microvascular anastomosis (zone 1) and flow impairment in microcirculation of the transferred tissue (zone 2). Factors that may contribute to vascular pedicle thrombosis include operative trauma, pedicle malposition, kinking, hypercoagulability and arterial vasoconstriction (5, 11).
Nifedipine is a drug of calcium-channel blocking group. The main effects of calcium channel blocker drugs are decrease of vascular smooth muscle tone-vasodilation and decrease of cardiac muscle contractility. In addition, nifedipine decreases platelet aggregation and reduces the extent of cell injury of marginally viable cells by blocking the influx of intracellular calcium (10). Heparin is the most common anticoagulant agent in clinical use. The antithrombotic effect of heparin results from its catalytic effect on antithrombin III (AT III) inhibition of thrombin (1).
Therefore we conducted an experimental study in order to investigate the effect of intravenous administration of nifedipine on the patency of microvascular anastomosis of the femoral artery in rats.
MATERIALS AND METHODS
Sixty (60) Wistar rats, weighing 280-320 grams were used. The animals were cared for following the Greek and European Community guidelines regulating animal research. All animals were anesthetized with Xylazine given intramuscular. All the procedures were carried out by the same surgeon using a Zeiss OPMI II operating microscope at 10 to 24 magnification. The animals were divided randomly into three groups of 20 rats each, and a standardized experimental protocol was followed (Table 1).
Tab. 1. Experimental grouping and protocol 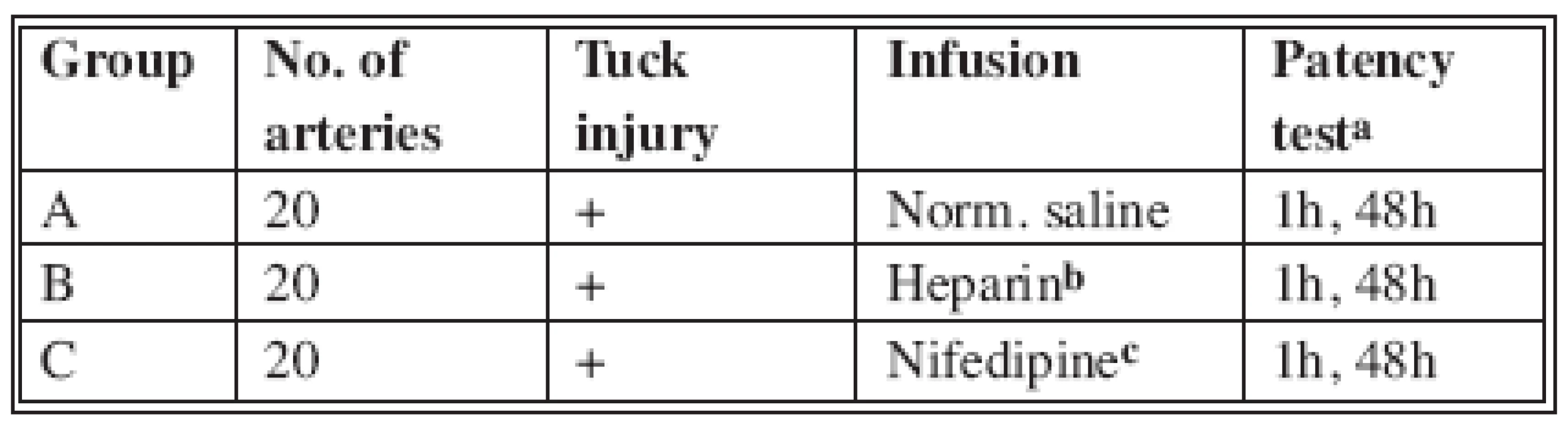
a) Distal empty-refill test b) Heparin 100 U/kg body weight c) Nifedipine 2 mg/kg body weight
The left femoral artery was exposed with a groin incision and dissected between the inguinal ligament and the superficial epigastric bifurcation. Any branches encountered were cauterized with bipolar diathermy or ligated and divided. The diameter of the artery ranged between 0.8-1.1 mm. Following application of a microvascular approximator the artery was transected. Standard end-to-end microvascular anastomosis was performed with inter-rupted stitches using 10-0 polyamide sutures (Fig. 1). After the application of the microvascular approximator and before anastomosis, the ipsilateral femoral vein was catheterized with a 29-G needle and infused with normal saline, heparin or nifedipine. Nifedipine was administered intravenous (2 mg/kg) to ensure the same dose for all the animals. No appreciable reduction in systemic blood pressure occurs at a total dose of 2 mg/kg (3). Vessel patency was assessed using the distal empty-refill test. All the arteries were patent on release of the microvascular clamps. Vessel patency was assessed 1 hour after the completion of the anastomosis and release of clamps. The wound was then closed and the animal returned to its cage. At 48 hours postoperatively, under anaesthesia, the wound was reopened, the patency of the artery was reassessed and the animal was then sacrificed.
Obr. 1. Anastomosis in nifedipine group 
There were no haematomas or wound infections in any group.
RESULTS
All variables are represented using the number of patients (n) and percent (%). Statistical analyses were performed using Fisher’s exact test. The test is two-sided with 95% significance level. Statistical analysis was done using the statistical package SPSS vr 10.00 (Statistical Package for the Social Sciences).
One hour after the completion of anastomosis and the release of the approximators in the control group 17 of the 20 arteries were patent (85%). In the heparin and the nifedipine group all 20 arteries were patent (100%). Forty eight hours after completion of anastomosis and the release of the approximators in the control group 13 of the 20 arteries were patent (65%). In the heparin and the nifedipine group 16 of the 20 arteries were patent (80%). There was no statistically significant difference of patency percentage after 1 hour and 48 hours among the three groups (p=0.231/p=0.480) (Table 2).
Tab. 2. Patency of anastomosis 1 hour and 48 hours after operation 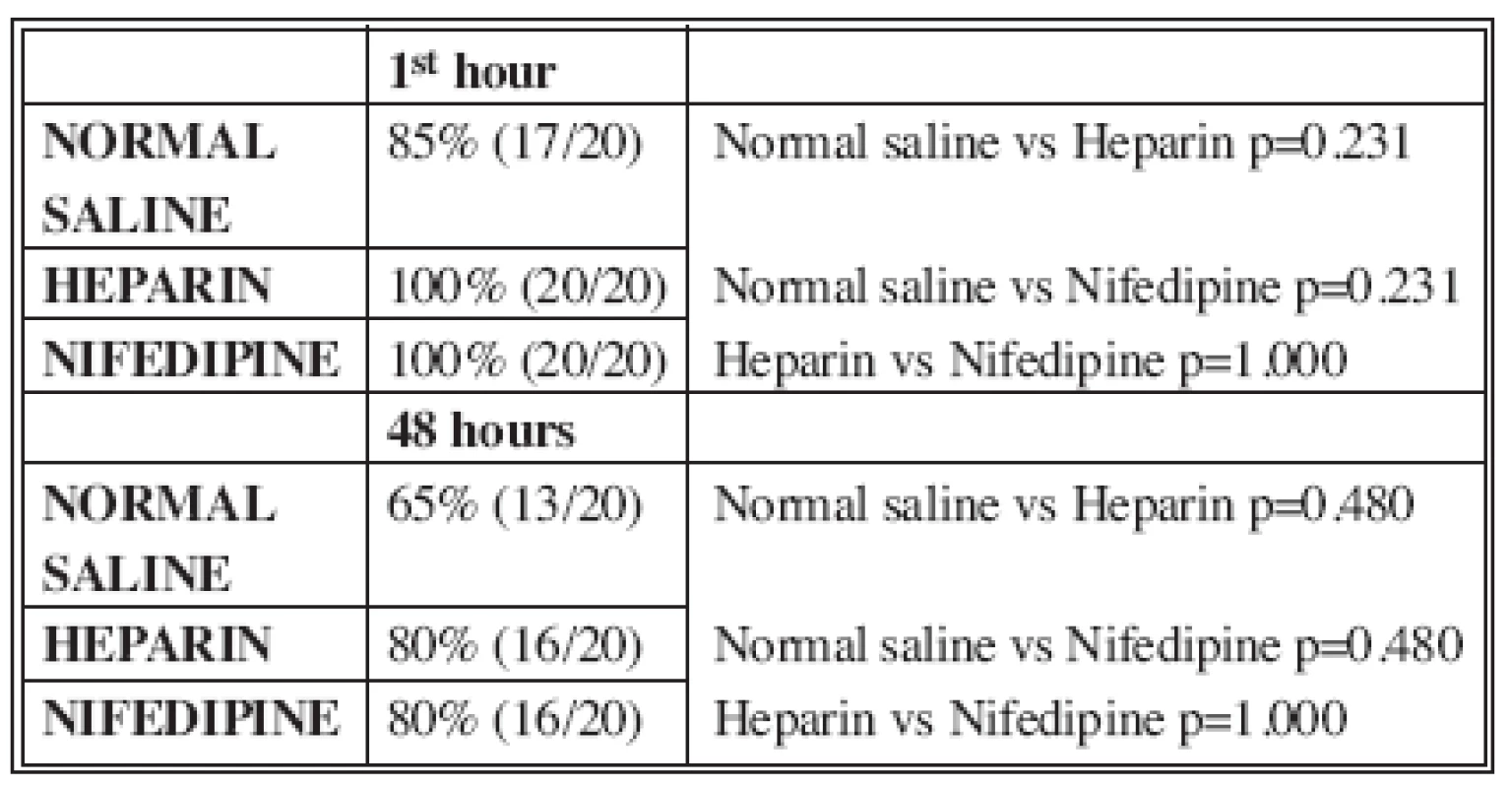
DISCUSSION
It is widely assumed that the survival of a free flap is strictly dependent on the status of microvascular anastomosis. Factors associated with the patency or failure of microvascular anastomosis are operative trauma, pedicle malposition and kinking, hypercoagulability and arterial vasoconstriction (5, 11). Arterial vasoconstriction is a problem encountered commonly in microvascular surgery (usually small diameter vessels <1cm) and usually occurs shortly after release of the vascular clamps (11). Vessel vasoconstriction appears to be mediated by α1 and α2 adrenoreceptors consisting of fast (α1, intracellular calcium dependent) and slow (α2, extracellular calcium dependent) contractile components. Nifedipine is a selective calcium channel blocker and has been shown to block completely the α2 component of vasoconstriction (9). Most types of smooth muscles are dependent on transmembrane calcium influx for normal resting tone and contractile responses. These cells are relaxed by calcium channel inhibitors (nifedipine). Vascular (arterial) smooth muscle is the most sensitive to this inhibition. In addition, vasodilation nifedipine inhibits platelet aggregation and prevents deleterious calcium influx into an ischemic cell, thereby retarding and preventing cell death (10).
Previous experimental studies used nifedipine to evaluate the effect of calcium channel blockers on skin flap survival (3, 2, 8, 4, 6). Two previous studies have not shown any beneficial effects on ischemic skin flap survival (Miller et al. 1985, Emery et al.1990). Later studies indicated that nifedipine improves survival in rats’ skin flaps (Michiya et al. 1990, Pal S. et al. 1991, Bailet et al. 1994). Nifedipine was used in the prevention and treatment of microvascular spasm (12). Weinzweig et al. (1999)suggest a potential role for intraperitoneal and topical administration of nifedipine in preventing the mechanical and ice-induced vasospasm on flap survival, as demonstrated by its temporizing effect on temperature change across anastomosis. In previous experimental models nifedipine was administrated topically, orally or intraperitoneally. The present study was designed to determine the effect of intravenously administrated nifedipine on the patency of the microvascular anastomosis of the femoral artery in rats. Intravenous administration of nifedipine ensured the same serum nifedipine level for all the animals. Another advantage of intravenous administration is the immediate effect of nifedipine on smooth muscles (vasodilation), as arterial vasoconstriction usually occurs shortly after release of vascular clamps.
To the best to our knowledge, no previous study for microvascular anastomosis with intravenous administration of nifedipine has been reported in the literature.
Using the empty-refill test we evaluated arterial patency one and forty-eight hours after the completion of anastomosis and the release of vascular approximator. The results of this study indicate that 1 hour after anastomosis in the nifedipine and heparin groups all the arteries (100%) were patent compared with 17 arteries (85%) in the control group. After 48 hours 16 arteries (80%) in the nifedipine and heparin groups were patent compared with 13 arteries (65%) in the control group. There was no statistically significant difference of patency percentage among the three groups. Despite the theoretical benefits of nifedipine, our inability to demonstrate a difference between control and treated animals suggests that the effects of nifedipine (vasodilation, inhibition of platelet aggregation and prevention of calcium influx into an ischemic cell) are not sufficient to increase patency of microvascular anastomosis.
CONCLUSIONS
Considering that microvascular anastomosis patency is still an important area of research, it is rather difficult to determine the role of pharmacologic adjunctive treatments in the overall success of microsurgical procedures. Our study suggests that careful planning, atraumatic technique and conduct of microsurgical procedures remain the most important factors for successful microvascular anastomosis.
ACKNOWLEDGMENTS
The authors thank the ELPEN AE Pharmaceutical Company (Athens, Greece) for providing laboratory facilities.
Address for correspondence:
Konstantinos E. Panagiotopoulos, M.D.
49 Autokratoros Hrakleiou str, 15122
Marousi, Athens, Greece
E-mail: konpan73@hotmail.com
Zdroje
1. Acland R., Thrombus formation in microvascular surgery: an experimental study of the effects of surgical trauma. Surgery, 73, 1973, p. 766-771.
2. Bailet JW., Hoffman LF., Trachy RE., Weymuller EA Jr. The effect of nifedipine on the skin flap survival in rats. Laryngoscope, 104(3 Pt 1), 1994, p. 253-258.
3. Emery FM., Kodey TR., Bomberger RA., McGregor DB. The effect of nifedipine on skin-flap survival. Plast. Reconstr. Surg., 85, 1990, p. 61-63.
4. Hira, M., Tajima S., Sano S. Increased survival length of experimental flap by calcium antagonist nifedipine. Ann. Plast. Surg., 24, 1990, p. 454-458.
5. Lindman D., Daniel RK. Evaluation of clinical microvascular anastomoses reasons for failure. Ann. Plast. Surg., 6, 1981, p. 215-223.
6. Miller AP., Falcone RE., Nappi J., . The lack of effect of nifedipine on failing skin flap. Dermatol. Surg. Ongol., 11, 1985, p.Ę612.
7. Effect of low dose aspirin on thrombus formation at arterial and venous microanastomoses and on the tissue microcirculation. Plast. Reconstr. Surg., 99, 1997, p. 1112-1121.
8. Pal S., Khazanchi RK., Moudgil K.ĘAn experimental study on the effect of nifedipine on ischemic flap survival in rats. Br. J. Plast. Surg., 44, 1991, p. 299-301.
9. Scarborough NL., Carrier CO. Nifedipine and Alpha Adrenoreceptors in rat aorta. Role of extracellular calcium in Alpha1 and Alpha2 Adrenoreceptor mediated vasoconstriction. J. Pharm. Exp. Ther., 231, 1984, p. 597-602.
10. Schwartz A., MatlibĘM., Balwierczak J., Lathrop DA.. Pharmacology of calcium antagonists. Am. J. Cardiol., 15, 1985, p. 3C-7C.
11. Seaber AV. Experimental vasospasm. Microsurgery, 8, 1987, p. 234-241.
12. Weinzweig N., Lukash F., Weinzweig J.. Topical and system calcium channel blockers in prevention and treatment of microvascular spasm in a rat epigastric island flap model. Ann. Plast. Surg., 42, 1999, p. 320-326.
Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článek ČESKÉ A SLOVENSKÉ SOUHRNYČlánek HOW EAST DISCOVERED WEST
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2008 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Možnosti využití metamizolu v léčbě akutních primárních bolestí hlavy
-
Všetky články tohto čísla
- MutilATing electrotrauma – case REPORT
- Award of the G. Whitaker International Burns Prize for 2007
- Specific aspects of the treatment of patients with multiple mechanical and burn injuries
- Catheter-Related Infections in Burn Patients at the Burn Centre of the University Hospital in Ostrava
- G. WHITAKER INTERNATIONAL BURNS PRIZE – PALERMO (Italy)
- Acinetobacter – serious danger FOR burn patients
- THE 25TH ANNIVERSARY OF BRNO BURN CENTRE: WHAT HAS CHANGED AND WHAT HAS NOT
- THE EFFECT OF NIFEDIPINE ON THE PATENCY OF MICROVASCULAR ANASTOMOSIS IN RATS
- Report of the 12th Congress of European Burns Association (EBA), Budapest, Hungary, September 12–15, 2007
- ČESKÉ A SLOVENSKÉ SOUHRNY
- HOW EAST DISCOVERED WEST
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Specific aspects of the treatment of patients with multiple mechanical and burn injuries
- Acinetobacter – serious danger FOR burn patients
- THE EFFECT OF NIFEDIPINE ON THE PATENCY OF MICROVASCULAR ANASTOMOSIS IN RATS
- MutilATing electrotrauma – case REPORT
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



