-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Essential Functions of the Histone Demethylase Lid
Drosophila Little imaginal discs (Lid) is a recently described member of the JmjC domain class of histone demethylases that specifically targets trimethylated histone H3 lysine 4 (H3K4me3). To understand its biological function, we have utilized a series of Lid deletions and point mutations to assess the role that each domain plays in histone demethylation, in animal viability, and in cell growth mediated by the transcription factor dMyc. Strikingly, we find that lid mutants are rescued to adulthood by either wildtype or enzymatically inactive Lid expressed under the control of its endogenous promoter, demonstrating that Lid's demethylase activity is not essential for development. In contrast, ubiquitous expression of UAS-Lid transgenes lacking its JmjN, C-terminal PHD domain, and C5HC2 zinc finger were unable to rescue lid homozygous mutants, indicating that these domains carry out Lid's essential developmental functions. Although Lid-dependent demethylase activity is not essential, dynamic removal of H3K4me3 may still be an important component of development, as we have observed a genetic interaction between lid and another H3K4me3 demethylase, dKDM2. We also show that Lid's essential C-terminal PHD finger binds specifically to di - and trimethylated H3K4 and that this activity is required for Lid to function in dMyc-induced cell growth. Taken together, our findings highlight the importance of Lid function in the regulated removal and recognition of H3K4me3 during development.
Published in the journal: Essential Functions of the Histone Demethylase Lid. PLoS Genet 6(11): e32767. doi:10.1371/journal.pgen.1001221
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001221Summary
Drosophila Little imaginal discs (Lid) is a recently described member of the JmjC domain class of histone demethylases that specifically targets trimethylated histone H3 lysine 4 (H3K4me3). To understand its biological function, we have utilized a series of Lid deletions and point mutations to assess the role that each domain plays in histone demethylation, in animal viability, and in cell growth mediated by the transcription factor dMyc. Strikingly, we find that lid mutants are rescued to adulthood by either wildtype or enzymatically inactive Lid expressed under the control of its endogenous promoter, demonstrating that Lid's demethylase activity is not essential for development. In contrast, ubiquitous expression of UAS-Lid transgenes lacking its JmjN, C-terminal PHD domain, and C5HC2 zinc finger were unable to rescue lid homozygous mutants, indicating that these domains carry out Lid's essential developmental functions. Although Lid-dependent demethylase activity is not essential, dynamic removal of H3K4me3 may still be an important component of development, as we have observed a genetic interaction between lid and another H3K4me3 demethylase, dKDM2. We also show that Lid's essential C-terminal PHD finger binds specifically to di - and trimethylated H3K4 and that this activity is required for Lid to function in dMyc-induced cell growth. Taken together, our findings highlight the importance of Lid function in the regulated removal and recognition of H3K4me3 during development.
Introduction
The Drosophila lid gene is essential for development and encodes a protein with multiple domains implicated in chromatin-mediated regulation of transcription, including the recently described lysine demethylase domain, Jumonji C (JmjC). Six lysine residues of histones H3 and H4 can be mono, di or trimethylated, and each modification is found in a stereotypical pattern with respect to the coding region of a gene and correlates with a different transcriptional outcome [1]–[4]. As a general rule, methylation of H3K4, K36 or K79 is found at active genes whereas H3K9, K27 and H4K20 methylation is associated with those that are repressed. We and others have shown that overexpression of Lid reduces H3K4me3 levels and that this chromatin mark is elevated in lid mutants, establishing Lid as a JmjC domain-dependent H3K4me3 demethylase [5]–[7]. The four conserved mammalian orthologs of Lid, KDM5a-d, also demethylate H3K4me3 although these proteins show broader substrate specificity than their Drosophila counterpart, also targeting H3K4me2 [8]–[10].
While there is limited data regarding the biological role of the KDM5 family of proteins in mammals, the findings that KDM5b is overexpressed in breast, bladder and prostate cancers [11]–[13] and that mutations in KDM5c are found in patients with X-linked mental retardation [14] suggest that they play important developmental roles. However, a confounding factor to the analysis of the four mammalian KDM5 paralogs is their functional redundancy, as the mouse KDM5a knock out is viable, fertile and displays no change in global H3K4me2/3 levels. In contrast, Lid is the sole KDM5 protein in Drosophila and it is essential for viability [15], providing an ideal system in which to investigate the function of this family of proteins.
Although the KDM5 family of proteins are named based on the function of their catalytic JmjC domain, metazoan KDM5 proteins have several other conserved motifs: a JmjN domain of unknown function that is present in a subset of JmjC proteins, an ARID (A/T rich interaction domain [16]) implicated in binding both A/T and G/C rich DNA sequences [17], [18], a single C5HC2 zinc finger, and two or three PHD fingers (plant homeobox domain [19]) involved in mediating protein-protein interactions [20]. Importantly, while the JmjC domain-dependent demethylase function is well defined in vitro for KDM5 family proteins, the in vivo relevance of this and other domains remains unclear.
We have previously shown that Lid is rate-limiting for cell growth induced by the Drosophila homolog of the c-Myc oncoprotein, dMyc [7]. Specifically, Lid binds directly to dMyc and is required for dMyc-dependent activation of one of its growth regulatory target genes, Nop60B. While we have demonstrated that this occurs independently of Lid's lysine demethylase activity, the molecular mechanism by which Lid functions in Myc-mediated growth is yet to be determined. Here we present an investigation of the function of Lid's domains and demonstrate that its demethylase activity is dispensable for development, however its JmjN, PHD3 and C5HC2 domains are all essential. While our observation that Lid's demethylase activity is not essential suggests that regulated removal of H3K4me3 serves primarily to modulate gene expression levels, a genetic interaction between lid and the JmjC domain-containing protein dKDM2 is consistent with these two demethylases acting redundantly on H3K4me3. We also show that the essential C-terminal PHD finger of Lid binds di - and trimethylated H3K4 and that this domain is required for lid to genetically interact with dMyc. Based on these data, we propose that Lid-dependent recognition of H3K4me2/3 facilitates dMyc binding to promoters rich in this active chromatin mark.
Results
The JmjN, ARID, PHD1, and C5HC2 zinc fingers of Lid are required for demethylase activity
To assess the contribution of each individual domain of Lid to its demethylase activity and animal development, we generated a series of deletions and point mutations that disrupt each domain of Lid to complement our previously characterized demethylase inactive version of Lid (Lid-JmjC*) that harbors two point mutations in the JmjC domain and prevents Fe2+ binding (H637 and E639 to Alanine) [7]. To enable these analyses, flies carrying UAS transgenes that specifically delete Lid's JmjN, ARID, C5HC2 zinc finger and three PHD fingers were generated to allow conditional Gal4-mediated expression in vivo (see Materials and Methods for details).
To assess the ability of our Lid mutants to demethylate, we generated clones of cells overexpressing each protein in larval fat body and examined the levels of Lid and H3K4me3 (Figure 1). Based on the intensity of the immunofluorescence signal and Western analysis, all of our transgenes expressed Lid at similar levels (data not shown; Figure 1), with the exception of LidΔPHD2, for which we were unable to detect Lid overexpression even after combining multiple transgenes (data not shown). To examine the role of Lid's second PHD finger, we created a point mutant in the first cysteine of this C4HC3 zinc finger (UAS-LidC1296A) and found that overexpression could be detected after combining two transgenes (Figure 1K). Mutating the second or deleting the third PHD domain of Lid did not affect its ability to demethylate H3K4me3 (Figure 1K–1N). In contrast, Lid's JmjN, PHD1 or C5HC2 domains were essential for enzymatic activity as overexpression of these deletion mutants resulted in no change in global levels of H3K4me3 (Figure 1D, 1H, 1J). While the role of Lid's PHD1 and C5HC2 domains in demethylation remains to be investigated, our finding that Lid's JmjN domain is required for demethylase activity is not surprising based on structural analysis of the demethylase KDM4a which shows its JmjN domain making extensive contacts within the catalytic core of its immediately adjacent JmjC domain [21]. Unlike other deletions that prevented Lid's enzymatic function, expression of LidΔARID resulted in a variable increase in H3K4me3 levels, indicating that this mutant protein can behave as a dominant negative in fat body cells (Figure 1E, 1F). We do not yet understand the mechanism by which LidΔARID increases H3K4me3 levels, but have observed a similar effect upon overexpression of Lid-JmjC* [7]. The ARID of KDM5a, b and Lid are required for demethylase activity in transient transfection assays, however a dominant interfering effect has not been reported [17], [22]. Our finding that deletion of Lid's ARID can increase H3K4 trimethylation raises the possibility that in addition or concomitant with its ability to bind DNA, this domain may cooperate with Lid's JmjC domain.
Fig. 1. Deletion of Lid's JmjN, ARID, and PHD1 domains abrogate its demethylase activity. 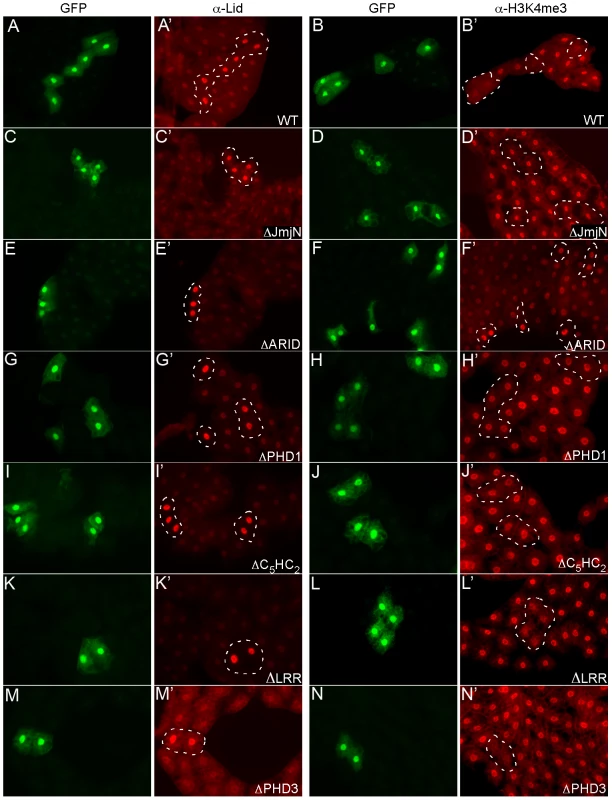
Clones of cells expressing Lid or Lid mutant transgenes in fat body were generated by crossing hs-FLP; UAS-Lid females to act>CD2>Gal4, UAS-GFP males. No heat shock was carried out since leaky FLP expression during embryogenesis leads to a small number of fat body clones. Levels of Lid (A', C', E', G', I', K', M') and trimethylated H3K4 (B', D', F', H', J', L', N') were examined. Cells expressing each transgene (as labeled on figure) are marked by co-expression of GFP and are outlined in the other panels. Lid's demethylase activity is dispensable for development
To determine the importance of Lid's conserved domains in vivo, we ubiquitously expressed our UAS-Lid transgenes in animals lacking endogenous zygotic lid expression. Because Lid is normally expressed ubiquitously throughout development [7](data not shown), we expressed our UAS-Lid (and Lid mutant) transgenes at low uniform levels in lid10424 homozygous mutants using actin-Gal4 (Figure 2E). This approximately two-fold overexpression of Lid is not sufficient to cause any change to global levels of H3K4me3 in wing discs (Figure 2E). As a control, we crossed our Lid transgenes to actin-Gal4 in a wildtype background to ensure that expression of these Lid mutants did not have any deleterious effects. In all cases, expression of our UAS transgenes in a wildtype background gave viable adults, however UAS-LidΔARID, Lid-JmjC* or LidΔPHD1 expressing adult females failed to lay eggs, so were sterile. Surprisingly, ovaries from females expressing these three mutant forms of Lid were phenotypically normal as assessed by dapi and phalloidin staining (data not shown), so the basis for their dominant interference with oviposition is not clear. This effect on egg laying is likely to be due to expression these Lid mutant transgenes in somatic cells of the ovary since germline specific expression using Nanos-Gal4 does not result in sterility (data not shown).
Fig. 2. Wildtype and demethylase inactive genomic rescue transgenes can rescue lid mutant animals. 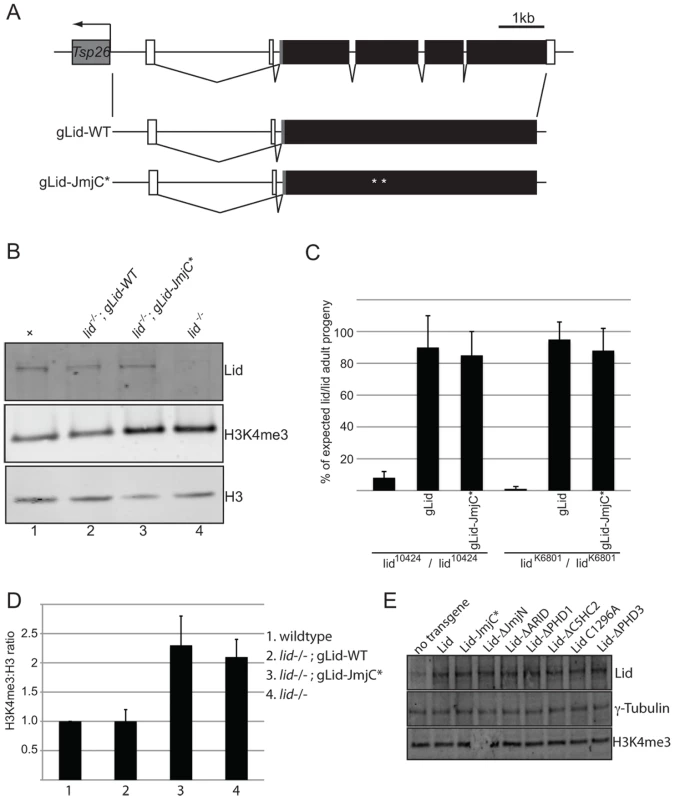
(A) Schematic representation of wildtype and mutant rescue transgenes in which 4.6 kb of upstream regulatory region fused to a Lid cDNA. (B) Western blot analysis of wing discs using anti-Lid, anti-H3K4me3 and anti-histone H3 in wildtype (lane 1), lid/lid; gLid-WT, (lane2), lid/lid; gLid-JmjC* (lane 3) and lid10424 homozygous (lid/lid, lane 4). (C) Quantitation of rescue based on the % of each genotype expected based on Mendelian genetics from the cross of lid10424/CyO (or lidK6801/CyO) females to lid10424/CyO; gLid-WT or gLid-JmjC* males in uncrowded (10 females per cross) conditions. (D) Quantitation of H3K4me3:H3 ratios from three independent experiments described in (B). (E) Western analysis of wing discs from Actin-Gal4 based rescue crosses. 12 wing discs were used per lane and the Western probed with anti-Lid, anti-H3K4me3 and the loading control γ-tubulin. As expected, actin-Gal4 driven expression of wildtype Lid rescued lid10424 mutant animals at the expected Mendelian frequency (Table 1). In contrast, expression of UAS-Lid harboring deletions of its JmjN, ARID, or PHD3 domains fail to rescue lid mutants, suggesting that these domains are essential for development. Expression of LidΔC5HC2 resulted in a small percentage (29%) of lid mutant flies eclosing, all of which died within several days indicating that this domain is essential in adults. In contrast to the third PHD, we found that the first and second PHD fingers of Lid are dispensable for development. While both sexes rescued by LidC1296A were fertile, LidΔPHD1-rescued females were sterile and, like overexpression of this transgene in a wildtype background, LidΔPHD1-rescued flies had phenotypically normal ovaries. We also tested our previously generated JmjC domain point mutant that abolishes demethylase activity for rescue of lid-associated lethality. Actin-Gal4-mediated expression of Lid-JmjC* failed to rescue lid mutants (Table 1), initially suggesting that Lid's demethylase activity is essential for development. However, since overexpression of Lid-JmjC* behaves as a dominant negative in a tissue specific manner, most notably in larval fat body cells [7], it may interfere with maternally deposited wildtype Lid in the rescue experiments described above.
Tab. 1. Domains of Lid that are essential for development. 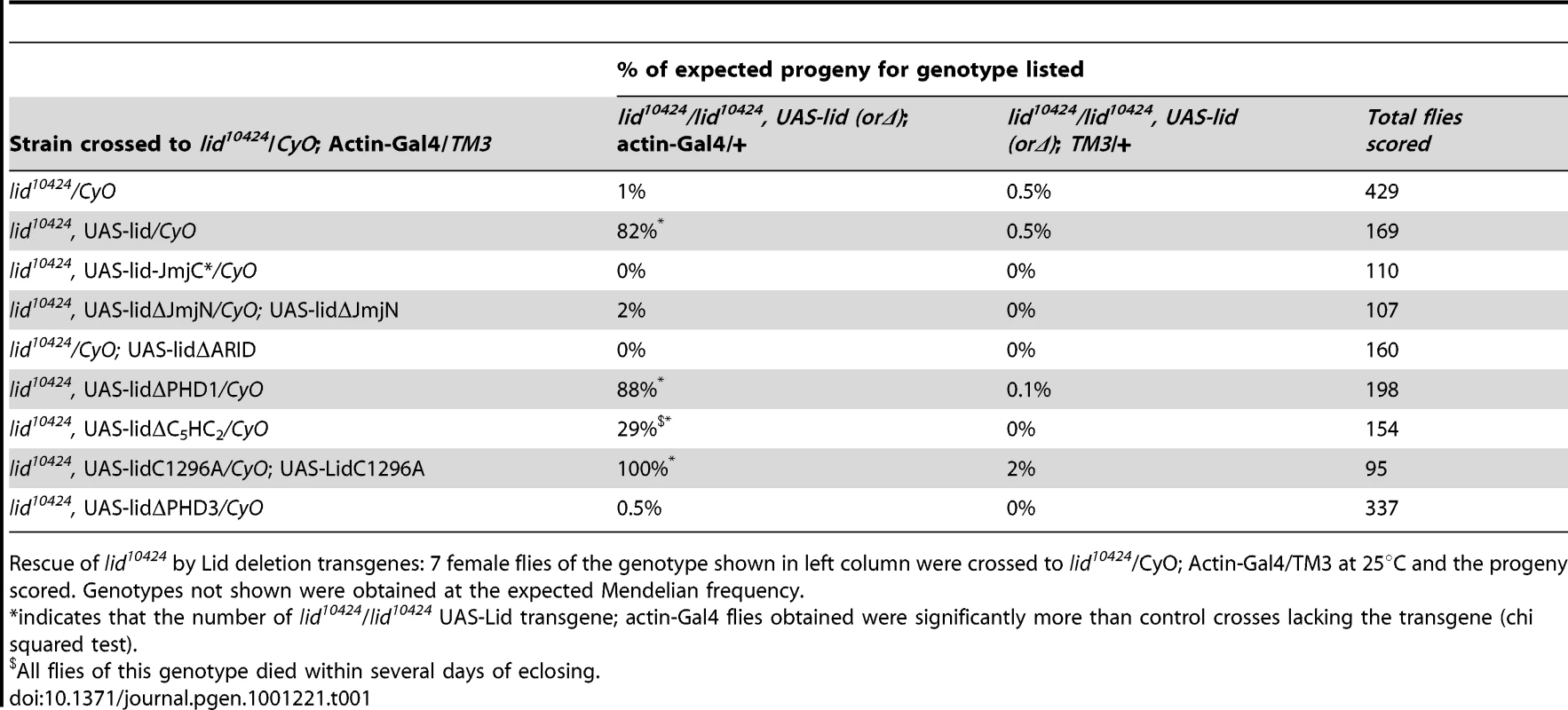
Rescue of lid10424 by Lid deletion transgenes: 7 female flies of the genotype shown in left column were crossed to lid10424/CyO; Actin-Gal4/TM3 at 25°C and the progeny scored. Genotypes not shown were obtained at the expected Mendelian frequency. To address the function of Lid's demethylase activity during development, we therefore generated genomic rescue transgenes that fused 3.9 kb of Lid's upstream regulatory region to either a wildtype or JmjC* mutant form of the lid coding region (gLid-WT and gLid-JmjC* respectively; Figure 2A). gLid-WT and gLid-JmjC* transgenes were then crossed into lid10424 and lidk6801 mutant backgrounds, the levels of transgene expression confirmed, and the number of homozygous lid mutant flies scored (Figure 2B, 2C; data not shown). Strikingly, lid mutant animals carrying one or two copies of a gLid-WT or gLid-JmjC* transgene produced phenotypically normal and fertile adult flies at the predicted frequency (Figure 2C; data not shown). Lid's demethylase activity is therefore not essential for Drosophila development. Based on the rescue of lid mutants by enzymatically inactive Lid expressed at endogenous levels, it is likely that this mutant form of Lid failed to rescue in our actin-Gal4 based rescue experiments because its overexpression interferes with maternally deposited wildtype Lid. It is therefore possible that LidΔARID also fails to rescue lid mutants due to its dominant interference with endogenous Lid, thus further examination of this domain will require generation of transgenes using lid's endogenous promoter.
Demethylase inactive animals have increased levels of H3K4me3
A majority of homozygous lid mutant animals die during pupal development and have increased global levels of H3K4me3 [5], [7], [22], [23]. While Lid can remove di and trimethylated histone H3K4 peptides in vitro, we and others have shown that it only targets H3K4me3 in vivo as only this methyl mark is altered upon Lid overexpression, in lid mutants, and in response to Lid RNAi [5], [7], [22]. Because expression of the demethylase inactive form of Lid is able to rescue lid mutants, we asked whether these animals also have increased global levels of H3K4me3. To examine this, we dissected wing discs from wildtype and lid10424 homozygous mutant larvae and compared the levels of H3K4me3 to lid mutants carrying two copies of gLid-WT or gLid-JmjC* by Western blot. As seen in Figure 2B and 2D, gLid-JmjC* animals show increased H3K4me3 indistinguishable from that observed in lid mutants, demonstrating that the increased level of H3K4me3 observed in lid mutant animals is not the cause of their lethality.
Demethylase inactive Lid flies are short-lived
Mutants and RNAi-mediated knock-down of the C. elegans Lid ortholog RBR-2 result in elevated levels of H3K4me3 and a 15–25% reduction in lifespan [24]. To determine whether this is a conserved, demethylase-specific phenotype, we assessed the lifespan of our demethylase inactive Lid flies. We find that lid mutant males rescued by gLid-JmjC* have a significantly shorter lifespan (mean of 37 days) than their wildtype (45 days) or gLid-rescued (46 days) flies (Figure 3A; data not shown). Interestingly, this effect is not observed in females, with the average lifespan of gLid-JmjC*-rescued flies not being significantly different to wildtype (48.1 and 45.1, respectively; Figure 3B). Lacking H3K4me3 demethylase activity therefore has adverse effects on processes required during male adulthood and suggests lifespan phenotypes observed in C. elegans hermaphrodites are likely to be specific to modulation of H3K4me3 levels rather than other RBR-2-dependent processes.
Fig. 3. Demethylase inactive male flies are short-lived and sensitive to paraquat. 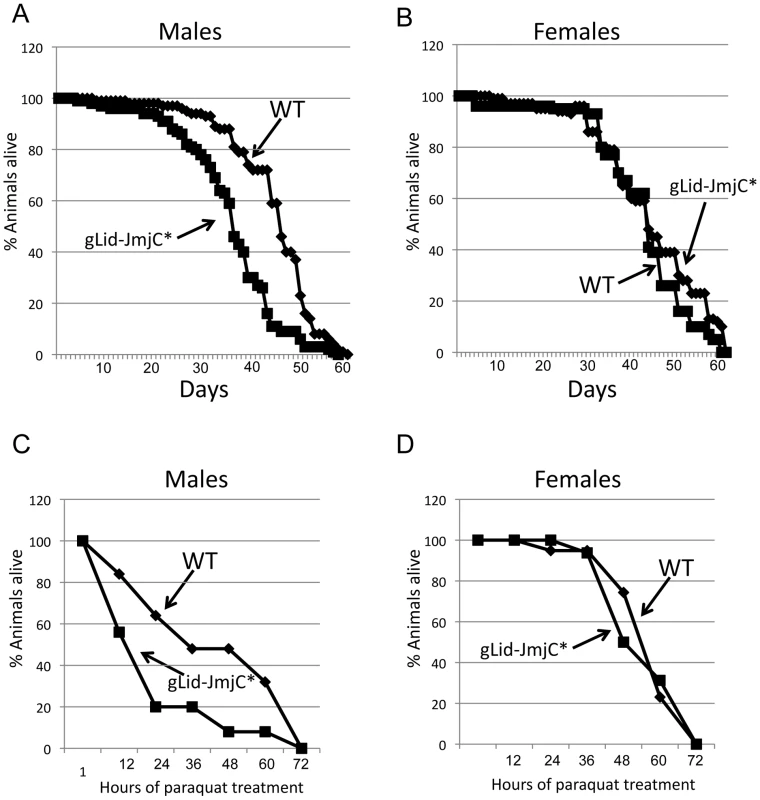
(A, B) Lifespan of wildtype and demethylase inactive Lid males and females, respectively showing that males are short-lived whereas females have a normal lifespan. (C, D). Survival in response to 20 mM paraquat of wildtype and demethylase inactive males and females, respectively. Like lifespan, males are sensitive to paraquat whereas females are not. Animals with reduced life expectancies also often show sensitivity to oxidative stressed induced by paraquat. We therefore treated wildtype and demethylase inactive flies with paraquat and found a sex-specific effect of this inducer of oxidative damage. In a similar manner to our lifespan studies in which males were more dramatically affected than females, we find that males are sensitive to paraquat whereas females are not (Figure 3C, 3D). Male Drosophila are therefore more sensitive to the loss of Lid-dependent H3K4me3 demethylation than females, although the molecular basis for this remains unclear.
The JmjC domain-containing demethylases Lid and dKDM2 genetically interact
One explanation for our finding that the loss of Lid's enzymatic activity does not adversely affect development is that its H3K4me3 demethylase activity is compensated for by another demethylase. To date, the JmjC domain-containing protein dKDM2 is the only other Drosophila protein shown to target H3K4me3, although it has also been reported to remove H3K36me2 [25], [26]. To address whether dKDM2 and Lid act in a redundant manner, we tested whether hypomorphic mutations in these two genes genetically interact. lidK6801 homozygotes survive until adulthood at a very low frequency (0.5%), but reach pupal development at 71% of the expected frequency (Table 2). The strongest dKDM2 allele, dKDM2DG18120, is semi-lethal with homozygous adults eclosing at 62% of the expected frequency (Table 2) and these adults are phenotypically normal and fertile. By combining these two mutations, we have found that the phenotype of lid, dKDM2 double mutants is significantly stronger than either single mutant (Table 2), with animals dying during the 1st and 2nd larval instar stages. To demonstrate that Lid's demethylase activity is required for this genetic interaction, we tested whether lid;dKDM2 double mutants could be rescued by our gLid-WT or gLid-JmjC* genomic rescue transgenes. As shown in Table 2, gLid-WT, but not gLid-JmjC* rescued lid;dKDM2 animals, suggesting that Lid and dKDM2 act redundantly in the regulation of H3K4me3.
Tab. 2. lid and dKDM2 genetically interact. 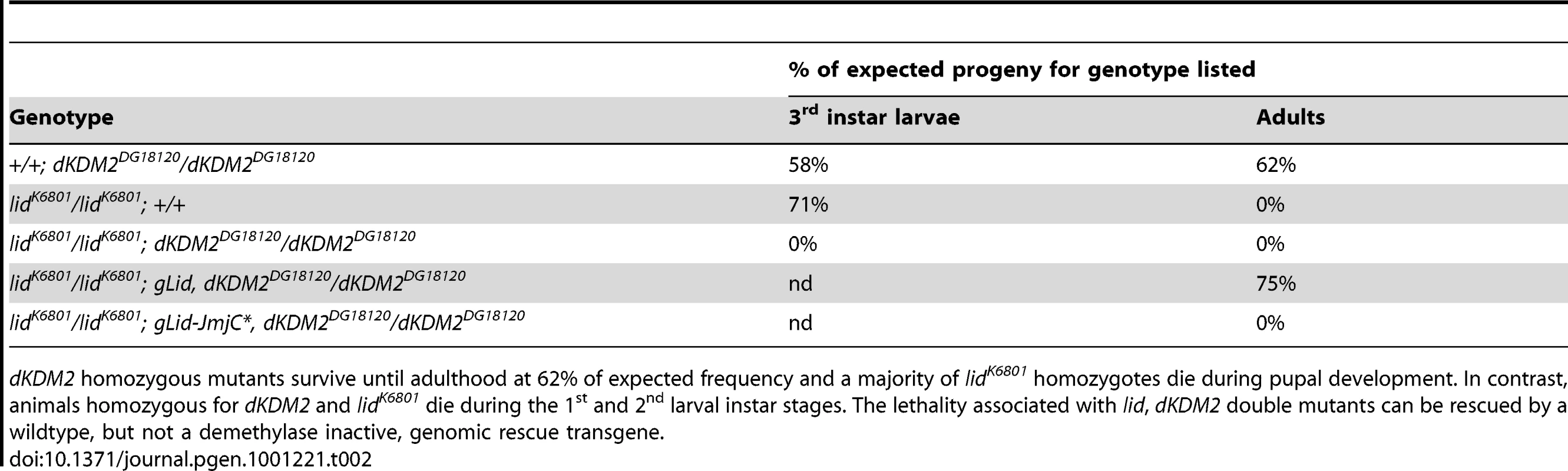
dKDM2 homozygous mutants survive until adulthood at 62% of expected frequency and a majority of lidK6801 homozygotes die during pupal development. In contrast, animals homozygous for dKDM2 and lidK6801 die during the 1st and 2nd larval instar stages. The lethality associated with lid, dKDM2 double mutants can be rescued by a wildtype, but not a demethylase inactive, genomic rescue transgene. The third PHD domain of Lid is required for it to function in Myc-dependent cell growth
We originally isolated lid in a genetic screen for regulators and mediators of dMyc-dependent cell growth based on an adult eye phenotype generated by dMyc expression in post-mitotic cells of the developing eye using GMR-Gal4 [7]. Furthermore, we showed that Lid's demethylase activity was not required for its dMyc-dependent functions. To pursue the mechanism by which Lid functions in cell growth induced by dMyc, we crossed our UAS-Lid mutant transgenes to the dMyc overexpressing fly strain and compared their ability to enhance this eye phenotype to that observed in response to expression of wildtype Lid (Figure 4A). Expression of Lid lacking its JmjN, C5HC2 or PHD3 domains failed to enhance the dMyc overexpression eye phenotype while not altering the levels of overexpressed dMyc (Figure 4B–4F; data not shown). As controls, we expressed the Lid deletion transgenes in a wildtype background and found that they resulted in no adult eye phenotype and, unlike fat body cells, Lid-JmjC* and LidΔARID do not have a dominant negative effects in post mitotic cells of the developing eye. We have previously shown that dMyc binds to two regions of Lid: its JmjC domain and its C5HC2 zinc finger [7]. To verify that all Lid deletion proteins retain their ability to bind dMyc, we carried out in vitro binding assays and found that they all bind equivalently (Figure 4G), suggesting that the JmjN, C5HC2 and PHD3 domains of Lid are likely to be required for its Myc-dependent functions in cell growth.
Fig. 4. The third PHD finger of Lid is required for it to function with dMyc. 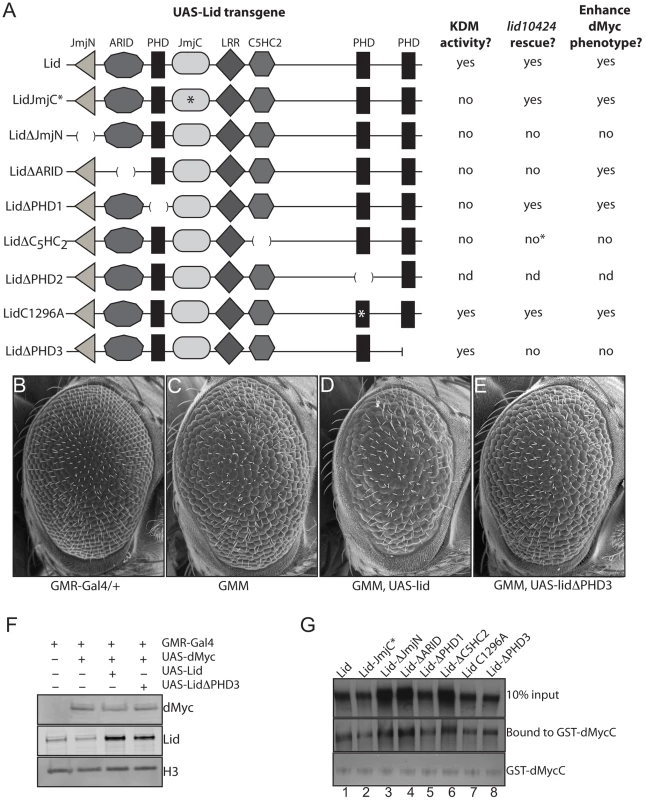
(A) Schematic representation of UAS Lid deletion and point mutant transgenes (domains are not shown to scale) summarizing their histone demethylation activity, rescue of lid mutants (lid10424), and their ability to enhance the dMyc overexpression eye phenotype. (B–E) Scanning electron micrographs of GMR-Gal4 alone (B), GMR-Gal4, UAS-dMyc (3 copies of transgene; GMM) (C), GMM, UAS-Lid (D) and GMM, UAS-LidΔPHD3 (E). B through E all contain 3 copies of the UAS-dMyc transgene. (F) Western analysis of GMR-Gal4 alone (lane 1), GMM (lane 2), GMM, UAS-Lid (lane 3) and GMM, UAS-LidΔPHD3 (lane 4). (G) In vitro binding assays demonstrating that GST-dMyc binds full-length Lid, Lid-JmjC*, LidΔJmjN, LidΔARID, LidΔPHD1, LidΔC5HC2, LidC1296A and LidΔPHD3 (lanes 1–8, respectively). The PHD fingers of Lid bind N-terminal histone tails
The primary characterized function of Lid is its histone H3 lysine 4 demethylase activity. However, since we have demonstrated that this activity is not Lid's essential function, we chose to further characterize Lid's third PHD finger as this domain is required for it to function with dMyc and is essential for development. Moreover, PHD domains have recently emerged as important interpreters the histone code that act by binding to histone tails that are unmodified, mono-, di - or tri - methylated at specific lysine residues [20]. To address whether Lid's third PHD finger is able to bind methylated histones, we incubated bacterially expressed and purified GST-PHD finger proteins with biotinylated histone peptides mono-, di - or trimethylated at K4, K9 or K27 in vitro and compared this to the binding of Lid's other two PHD fingers and the known H3K4me2/3 binding protein hING2 [27], [28](Figure 5; data not shown).
Fig. 5. The PHD fingers of Lid bind specific forms of methylated histone tails. 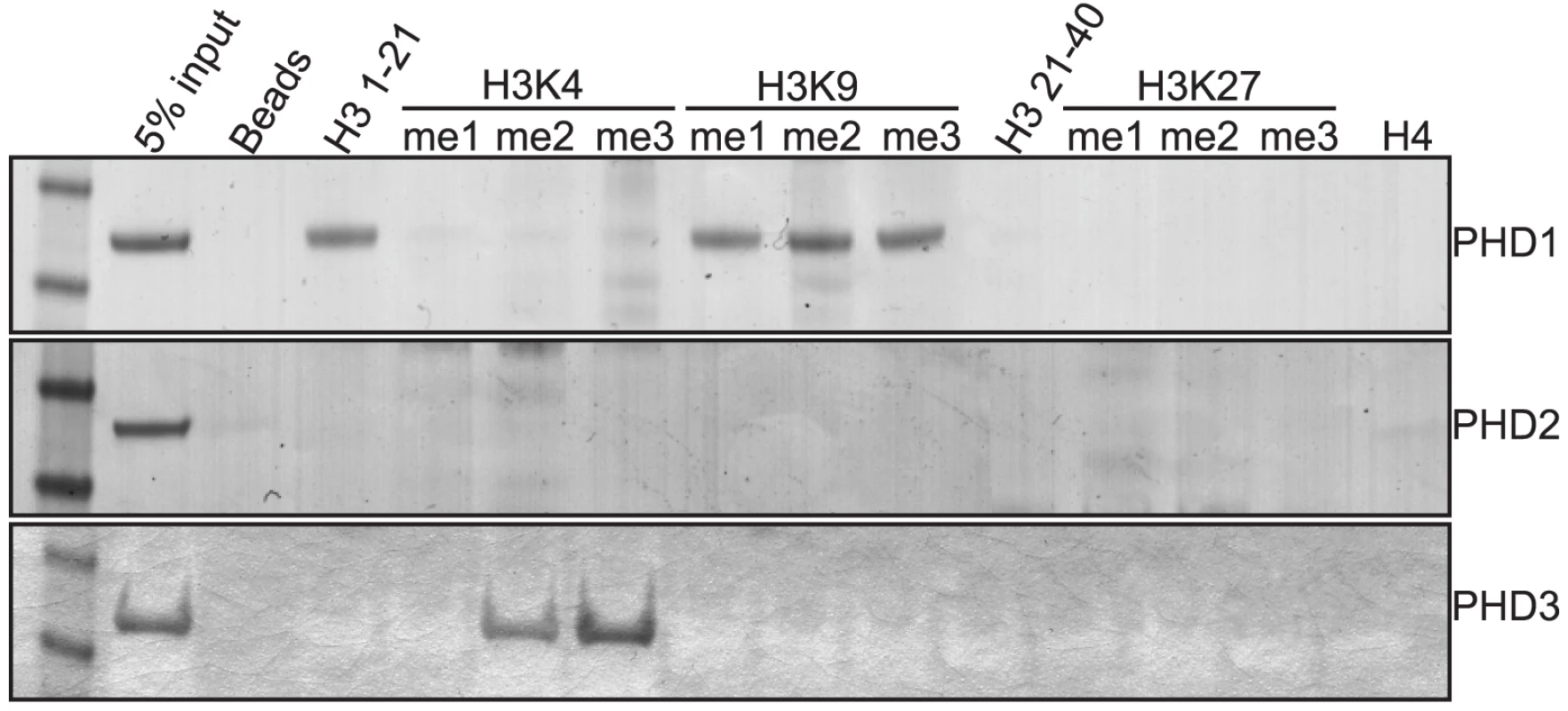
GST fusion proteins of Lid's PHD1 (top panel), PHD2 (2nd panel) and PHD3 (3rd panel) were tested for binding to biotinylated histone peptides of histone H3 amino acids 1–21, 21–44 and histone H4 1–21. In addition, peptides mono, di or tri methylated at histone H3 K4, K9 and K27 were also tested for their ability to bind these PHD fingers. As seen in Figure 5, Lid's PHD1 finger binds to amino acids 1–21 of histone H3, but not amino acids 21–40 or to histone H4. Lid's PHD1 specifically recognizes unmethylated histone H3 (H3K4me0), as binding is abrogated by mono-, di - or trimethylation of lysine 4, but not methylation of lysine 9. We were unable to detect any in vitro histone binding for Lid's PHD2 finger, however PHD3 bound to both H3K4me2 and H3K4me3, showing a consistent preference for the trimethylated form. The function of both of these PHD fingers is likely to be a highly conserved function of KDM5 proteins, as identical binding specificities have recently been reported for KDM5a [29]. Consistent with the binding of PHD3 to H3K4me2/3 being physiologically relevant, a correlation between KDM5a binding and the presence of this activating chromatin mark has been observed previously using genome-wide arrays, although its physiological relevance has remained elusive [30], [31]. Significantly, the binding of c-Myc also correlates with regions rich in H3K4me2/3 [32]. Based on our findings that Lid's third PHD finger binds H3K4me2/3 and that deleting this domain abolishes its ability to genetically interact with dMyc, we propose that Lid functions to recruit dMyc to regions with high levels of H3K4me2/3 by specifically recognizing this local chromatin context.
Discussion
Our analyses provide the first investigation of the developmental role of a JmjC domain-dependent demethylase. Five major findings come from this work: (1) Lid's JmjC domain-encoded demethylase activity is dispensable for normal development (2) Loss of Lid's demethylase activity is compensated for by dKDM2 (3) Essential functions of Lid are encoded by its JmjN, C5HC2 and C-terminal PHD zinc finger motifs (4) The N - and C-terminal PHD fingers of Lid bind specific methylated forms of histone tails (5) Lid's C-terminal H3K4me2/3 binding PHD finger is required for it to function in dMyc-mediated cell growth. Taken together, this significantly extends our knowledge of the role of regulated removal and recognition of di - and trimethylated histone H3 lysine 4 during development.
Lid-dependent regulation of H3K4me3
Our finding that Lid's lysine demethylase activity is dispensable for development demonstrates that globally increasing the levels of H3K4me3 is not generally detrimental to development. Similarly, elevating H3K4me1/2 levels by mutating the Drosophila demethylase Lsd1 does not adversely affect development, although these animals show some adult phenotypes and subtle changes to expression of the homeobox genes Ubx and Abd-A [33], [34]. Likewise, Lid's enzymatic activity may serve to fine-tune some gene expression patterns. To date, three genes, E(spl)m4, m7 and m8, have been described as direct Lid targets in Drosophila cultured S2 cells, and these show a 4-fold derepression in response to lid RNAi and a concomitant increase in promoter-proximal H3K4me3 levels [35]. Furthermore, a genetic interaction has been observed during wing development between lid and the E(spl) gene upstream regulator Notch, suggesting that this regulation is biologically important [35], [36]. In mammalian cells, MFN2 and Deltex expression are repressed upon KDM5a overexpresion, derepressed when KDM5 is knocked-down, and show changes in H3K4me3 levels in their promoters [31], [36]. We examined the levels of E(spl)m4, m7 and m8, Marf1 (the Drosophila ortholog of MFN2) and Deltex, but found that their levels were unaltered in RNA extracts from whole larvae or dissected wing imaginal discs from wildtype, lid mutant or lid mutants rescued by gLid or gLid-JmjC* (JS, unpublished). These genes may therefore be regulated by Lid in a small subset of cells in vivo, so cannot be detected using whole wing disc extracts. Effects on gene expression may also be sex-specific since male flies lacking Lid-dependent demethylase activity have a shortened lifespan and are sensitive to paraquat, whereas females are not.
While removing Lid's demethylase activity does not result in lethality, removing this function in combination with another JmjC domain-containing protein, dKDM2, does. This suggests that in the absence of Lid's demethylase activity, dKDM2 can carry out its essential functions and vice versa. RNAi-mediated knock down of dKDM2 has been found to increase H3K36me2 levels in S2 Drosophila tissue culture cells and H3K4me3 levels in adult flies [25], [26]. Surprisingly, we find that global levels of H3K4me3 and H3K36me2 are both unchanged in dKDM2DG12810, dKDM2KG04325 or dKDM2EY01336 homozygous mutant wing discs (CG and JS, unpublished). The reason for the disparity between our results obtained with dKDM2 mutants and previously published data are not clear, but may be due to the difference between the acute loss of dKDM2 mediated by RNAi and the chronic loss in dKDM2 mutants, or to off target effects of the RNAi. The most characterized function of dKDM2 and its mammalian orthologs (KDM2A, KDM2B) is its regulation of rRNA expression [25], [37]. Interestingly, repression of rRNA transcription by KDM5A correlates with changes to H3K63me2 levels, whereas H3K4me3 is unaltered [37]. Based on our genetic interaction between lid and dKDM2, this may be because Lid/KDM5a compensates for the loss of dKDM2's H3K4me3 demethylase activity. Conversely, it is likely that dKDM2 also functions outside the nucleolus and that H3K4me3 regulation by Lid and dKDM2 is essential for development. It is important to note, however, that while Lid's demethylase activity is required for the genetic interaction between lid and dKDM2, we cannot rule out the possibility that dKDM2 requires its H3K36me2 demethylase enzymatic activity not its H3K4me3 activity. Both H3K4me3 and H3K36me2 are chromatin marks associated with active transcription, and it is possible that Lid's H3K4me3 demethylase activity is functionally linked to dKDM2-mediated H3K36me2 demethylation.
Demethylase-independent functions of the JmjC domain
Among the JmjC domain-containing proteins, Lid is most structurally similar to the founding member of this class of demethylases, Jumonji (JARID2), having a JmjN, ARID and C5HC2 zinc finger in addition to a JmjC domain. In both mammals and Drosophila, Jumonji is enzymatically inactive because it lacks key residues within its JmjC domain required for Fe2+ and α-ketoglutarate binding [38]. Indeed, while Jumonji has been implicated as a regulator of transcription [30], [38]–[40], the molecular function of the JmjC domain has remained elusive. Taken in conjunction with our finding that Lid's demethylase activity is not essential for development, this raises the exciting possibility that the JmjC domain has important demethylase-independent functions. Consistent with this hypothesis, we find that a genomic rescue transgene with a deletion of the JmjC domain fails to rescue lid mutants (CG and JS, unpublished). Because more than half the known JmjC domain-containing proteins in mammals and Drosophila do not have an ascribed enzymatic activity, a demethylase-independent functions of this domain may be a common feature of this class of protein.
PHD finger-mediated histone binding by Lid
Lid has three PHD fingers and we have demonstrated that its N - and C-terminal PHDs bind specific methylated forms of the histone H3 tail. While Lid's N-terminal H3K4me0-binding PHD finger was not required for development, its third PHD finger, which binds to H3K4me2/3, is essential for viability and is required for Lid to function in dMyc-mediated cell growth. One long-standing question regarding many transcription factors is the mechanism by which they find their appropriate binding site within the genome, as many transcription factors recognize short DNA sequences that are similar or identical. This suggests that binding site specificity may additionally involve the recognition of non-DNA elements such as local chromatin environments. In mammalian cells, c-Myc shows a clear binding preference for E boxes located within a chromatin context containing highly di - and trimethylated nucleosomal histone H3K4 [32]. However, the mechanism by which Myc recognizes this chromatin landscape is unclear. We propose that Lid utilizes its H3K4me2/3 binding C-terminal PHD finger to tether Myc to its preferred chromatin context, thereby permitting selection of biologically important E boxes. Further experiments to more precisely define the role of Lid's PHD finger in Myc-mediated cell growth are ongoing.
In summary, we have demonstrated that Lid's JmjC domain-encoded demethylase activity, its histone H3K4me0-binding N-terminal PHD finger and its PHD2 of unknown function, are dispensable for development. In contrast, all other domains of Lid tested were required to rescue lid homozygous mutants, including its C-terminal, H3K4me2/3 binding, PHD finger that functions in dMyc-mediated cell growth. These findings highlight the importance of characterizing the function of individual domains of transcriptional regulators such as Lid in order to understand the mechanisms by which they regulate gene expression in a developmental context.
Materials and Methods
Fly strains and crosses
UAS-lid and UAS-lidJmjC* have been described previously [7]. All other Drosophila strains were obtained from the Bloomington stock center. Deletions within Lid were made in the pUASp vector by site directed mutagenesis and delete the following amino acids: LidΔJmjN (AA160–206), LidΔARID (AA223–314), LidΔPHD1 (AA450–499), LidΔC5HC2 (AA830–883), LidΔPHD2 (AA1296–1354), LidΔPHD3 (1749–1838 by introducing a stop codon). LidC1296A mutates the first cysteine of Lid's second PHD finger. lid genomic rescue transgenes were generated by fusing a 4.5 kb PCR-generated Xho I fragment containing the lid upstream region and a 4.8 kb Xho I/Not I fragment containing the remainder of the lid coding sequence (either wildtype or JmjC*) into the vector pCasper4. All transgenic flies were generated by The Best Gene (thebestgene.com). Lifespan studies were carried out as described by [41].
To test the ability of UAS-Lid (wildtype and deletion) transgenes to rescue the lid mutant phenotype, a UAS-Lid (or deletion) transgene was recombined onto the lid10424 chromosome. At least 2 independent P element insertions were tested for each to minimize chromosomal position effects. This lid10424, UAS-Lid (or deletion) recombinant chromosome, balanced over CyO, was then mated to the lid10424/CyO; Actin-Gal4/TM6B strain. Rescue was assessed by scoring the presence of straight winged, non-TM6B, progeny. Somatic clones overexpressing UAS transgenes marked by the co-expression of GFP were generated as described in [42]. Longevity studies were carried out as described in [41] and paraquat assays as described in [43].
In vitro binding assays
Histone binding assays: 1 µg of biotinylated histone peptides (Fisher) were incubated with 5 µg purified GST-PHD finger in 1 ml of binding buffer (50 mM Tris pH 7.5, 200 mM NaCl, 2 mM dithiothreitol, 0.5% Nonidet P-40 (v/v), 1 µM ZnSO4, 1% BSA) at 4°C overnight. Complexes were then immobilized using 10 µl Streptavidin-agarose beads (Invitrogen) for 1 hr at 4°C. Immobilized complexes were then washed three times with 1 ml of binding buffer, boiled and loaded on a 4–12% gel. Gels were stained with coomassie blue to visualize bound GST-PHD protein. GST-protein binding assays: 1 µg of purified GST-dMycC [7] was incubated with S35-labeled Lid or Lid deletion proteins made using rabbit reticulocyte lysate (Invitrogen) in 1xPBS, 1% BSA and 0.5%NP-40, washed in 1xPBS, 0.5% NP-40, boiled and loaded onto a 4–12% protein gel. GST-dMycC was visualized using coomassie brilliant blue and S35 detected via standard procedures.
Antibodies, Westerns, and immunofluorescence
The Lid rabbit and dMyc antibodies have been described previously [7], [44]. Anti-trimethylated H3K4 and H3K36me2 were obtained from Active Motif, and γ-tubulin from Sigma. Western analysis was carried out using standard protocols, infrared conjugated secondary antibodies (LiCOR) and Odyssey scanner and software. Immunofluorescence was carried out as described in [7]. Quantitation of Western blots was carried out using LiCOR odyssey v3.0 software.
Zdroje
1. BernsteinBE
HumphreyEL
ErlichRL
SchneiderR
BoumanP
2002 Methylation of histone H3 Lys 4 in coding regions of active genes. P Natl Acad Sci USA 99 8695 8700
2. BernsteinBE
KamalM
Lindblad-TohK
BekiranovS
BaileyDK
2005 Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120 169 181
3. Santos-RosaH
SchneiderR
BannisterAJ
SherriffJ
BernsteinBE
2002 Active genes are tri-methylated at K4 of histone H3. Nature 419 407 411
4. SchneiderR
BannisterAJ
MyersFA
ThorneAW
Crane-RobinsonC
2004 Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol 6 73 77
5. EissenbergJC
LeeMG
SchneiderJ
IlvarsonnA
ShiekhattarR
2007 The trithorax-group gene in Drosophila little imaginal discs encodes a trimethylated histone H3 Lys4 demethylase. Nat Struct Mol Biol 14 344 346
6. LeeN
Erdjument-BromageH
TempstP
JonesRS
ZhangY
2009 The H3K4 Demethylase Lid Associates with and Inhibits the Histone Deacetylase Rpd3. Mol Cell Biol 29 1401 1410
7. SecombeJ
LiL
CarlosLS
EisenmanRN
2007 The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Gene Dev 21 537 551
8. ChristensenJ
AggerK
CloosPA
PasiniD
RoseS
2007 RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone H3. Cell 128 1063 1076
9. IwaseS
LanF
BaylissP
de la Torre-UbietaL
HuarteM
2007 The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell 128 1077 1088
10. KloseRJ
YanQ
TothovaZ
YamaneK
Erdjument-BromageH
2007 The Retinoblastoma binding protein RBP2 is a H3K4 demethylase. Cell 128 889 900
11. HayamiS
YoshimatsuM
VeerakumarasivamA
UnokiM
IwaiY
2010 Overexpression of the JmjC histone demethylase KDM5B in human carcinogenesis: involvement in the proliferation of cancer cells through the E2F/RB pathway. Mol Cancer 9 59
12. XiangY
ZhuZ
HanG
YeX
XuB
2007 JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc Natl Acad Sci U S A 104 19226 19231
13. YamaneK
TateishiK
KloseRJ
FangJ
FabrizioLA
2007 PLU-1 is a H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell 25 801 812
14. TahilianiM
MeiPC
FangR
LeonorT
RutenbergM
2007 The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature 447 601 605
15. GildeaJJ
LopezR
ShearnA
2000 A screen for new trithorax group genes identified little imaginal discs, the Drosophila melanogaster homologue of human retinoblastoma binding protein 2. Genetics 156 645 663
16. KortschakRD
TuckerPW
SaintR
2000 ARID proteins come in from the desert. Trends Biochem Sci 25 294 299
17. TuSJ
TengYC
YuanCH
WuYT
ChanMY
2008 The ARID domain of the H3K4 demethylase RBP2 binds to a DNA CCGCCC motif. Nat Struct Mol Biol 15 419 421
18. ScibettaAG
SantangeloS
ColemanJ
HallD
ChaplinT
2007 Functional analysis of the transcription repressor PLU-1/JARID1B. Mol Cell Biol 27 7220 7235
19. AaslandR
GibsonTJ
StewartAF
1995 The Phd Finger - Implications for Chromatin-Mediated Transcriptional Regulation. Trends Biochem Sci 20 56 59
20. TavernaSD
LiH
RuthenburgAJ
AllisCD
PatelDJ
2007 How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol 14 1025 1040
21. ChenZZ
ZangJY
WhetstineJ
HongX
DavrazouF
2006 Structural insights into histone demethylation by JMJD2 family members. Cell 125 691 702
22. LeeN
ZhangJY
KloseRJ
Erdjument-BromageH
TempstP
2007 The trithorax-group protein Lid is a histone H3 trimethyl-Lys4 demethylase. Nat Struct Mol Biol 14 341 343
23. Lloret-LlinaresM
CarreC
VaqueroA
de OlanoN
AzorinF
2008 Characterization of Drosophila melanogaster JmjC+N histone demethylases. Nucleic Acids Res 36 2852 2863
24. GreerEL
MauresTJ
HauswirthAG
GreenEM
LeemanDS
2010 Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature 466 383 387
25. KaviHH
BirchlerJA
2009 Drosophila KDM2 is a H3K4me3 demethylase regulating nucleolar organization. BMC Res Notes 2 217
26. LagarouA
Mohd-SaripA
MoshkinYM
ChalkleyGE
BezstarostiK
2008 dKDM2 couples histone H2A ubiquitylation to histone H3 demethylation during Polycomb group silencing. Genes Dev 22 2799 2810
27. PenaPV
DavrazouF
ShiXB
WalterKL
VerkhushaVV
2006 Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442 100 103
28. ShiXB
HongT
WalterKL
EwaltM
MichishitaE
2006 ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442 96 99
29. WangGG
SongJ
WangZ
DormannHL
CasadioF
2009 Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature 459 847 851
30. PengJC
ValouevA
SwigutT
ZhangJ
ZhaoY
2009 Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139 1290 1302
31. Lopez-BigasN
KisielTA
DeWaalDC
HolmesKB
VolkertTL
2008 Genome-wide analysis of the H3K4 histone demethylase RBP2 reveals a transcriptional program controlling differentiation. Mol Cell 31 520 530
32. GuccioneE
MartinatoF
FinocchiaroG
LuziL
TizzoniL
2006 Myc-binding-site recognition in the human genome is determined by chromatin context. Nat Cell Biol 8 764 U225
33. Di StefanoL
JiJY
MoonNS
HerrA
DysonN
2007 Mutation of Drosophila Lsd1 disrupts H3-K4 methylation, resulting in tissue-specific defects during development. Curr Biol 17 808 812
34. RudolphT
YonezawaM
LeinS
HeidrichK
KubicekS
2007 Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol Cell 26 103 115
35. MoshkinYM
KanTW
GoodfellowH
BezstarostiK
MaedaRK
2009 Histone chaperones ASF1 and NAP1 differentially modulate removal of active histone marks by LID-RPD3 complexes during NOTCH silencing. Mol Cell 35 782 793
36. LiefkeR
OswaldF
AlvaradoC
Ferres-MarcoD
MittlerG
2010 Histone demethylase KDM5A is an integral part of the core Notch-RBP-J repressor complex. Genes Dev 24 590 601
37. TanakaY
OkamotoK
TeyeK
UmataT
YamagiwaN
2010 JmjC enzyme KDM2A is a regulator of rRNA transcription in response to starvation. Embo J 29 1510 1522
38. JungJ
MysliwiecMR
LeeY
2005 Roles of JUMONJI in mouse embryonic development. Dev Dynam 232 21 32
39. LiG
MargueronR
KuM
ChambonP
BernsteinBE
2010 Jarid2 and PRC2, partners in regulating gene expression. Genes Dev 24 368 380
40. PasiniD
CloosPA
WalfridssonJ
OlssonL
BukowskiJP
2010 JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 464 306 310
41. LooLWM
SecombeJ
LittleJT
CarlosLS
YostC
2005 The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol Cell Biol 25 7078 7091
42. JohnstonLA
ProberDA
EdgarBA
EisenmanRN
GallantP
1999 Drosophila myc regulates cellular growth during development. Cell 98 779 790
43. RadyukSN
MichalakK
KlichkoVI
BenesJ
RebrinI
2009 Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. Biochem J 419 437 445
44. ZaffranS
ChartierA
GallantP
AstierM
ArquierN
1998 A Drosophila RNA helicase gene, pitchoune, is required for cell growth and proliferation and is a potential target of d-Myc. Development 125 3571 3584
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2010 Číslo 11- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Genome-Wide Association Meta-Analysis of Cortical Bone Mineral Density Unravels Allelic Heterogeneity at the Locus and Potential Pleiotropic Effects on Bone
- Beyond QTL Cloning
- An Evolutionary Framework for Association Testing in Resequencing Studies
- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- The Functional Interplay between Protein Kinase CK2 and CCA1 Transcriptional Activity Is Essential for Clock Temperature Compensation in Arabidopsis
- Endogenous Viral Elements in Animal Genomes
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- DNA Methylation and Normal Chromosome Behavior in Neurospora Depend on Five Components of a Histone Methyltransferase Complex, DCDC
- Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes
- Genetic Basis of Growth Adaptation of after Deletion of , a Major Metabolic Gene
- Nomadic Enhancers: Tissue-Specific -Regulatory Elements of Have Divergent Genomic Positions among Species
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- CTCF-Dependent Chromatin Bias Constitutes Transient Epigenetic Memory of the Mother at the Imprinting Control Region in Prospermatogonia
- Systematic Dissection and Trajectory-Scanning Mutagenesis of the Molecular Interface That Ensures Specificity of Two-Component Signaling Pathways
- Nucleolin Is Required for DNA Methylation State and the Expression of rRNA Gene Variants in
- The Complex Genetic Architecture of the Metabolome
- ATM Limits Incorrect End Utilization during Non-Homologous End Joining of Multiple Chromosome Breaks
- Mutation Disrupts Synaptonemal Complex Formation, Recombination, and Chromosome Segregation in Mammalian Meiosis
- Mismatch Repair–Independent Increase in Spontaneous Mutagenesis in Yeast Lacking Non-Essential Subunits of DNA Polymerase ε
- The Kinesin-3 Motor UNC-104/KIF1A Is Degraded upon Loss of Specific Binding to Cargo
- Epigenetic Silencing of Spermatocyte-Specific and Neuronal Genes by SUMO Modification of the Transcription Factor Sp3
- A Coastal Cline in Sodium Accumulation in Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1
- Cyclin B3 Is Required for Multiple Mitotic Processes Including Alleviation of a Spindle Checkpoint–Dependent Block in Anaphase Chromosome Segregation
- Altered DNA Methylation in Leukocytes with Trisomy 21
- Human-Specific Evolution and Adaptation Led to Major Qualitative Differences in the Variable Receptors of Human and Chimpanzee Natural Killer Cells
- Leptotene/Zygotene Chromosome Movement Via the SUN/KASH Protein Bridge in
- RACK-1 Acts with Rac GTPase Signaling and UNC-115/abLIM in Axon Pathfinding and Cell Migration
- Genome-Wide Effects of Long-Term Divergent Selection
- Endless Forms Most Viral
- Conflict between Noise and Plasticity in Yeast
- Essential Functions of the Histone Demethylase Lid
- The Transcriptional Regulator Rok Binds A+T-Rich DNA and Is Involved in Repression of a Mobile Genetic Element in
- The Cellular Robustness by Genetic Redundancy in Budding Yeast
- Localization of a Guanylyl Cyclase to Chemosensory Cilia Requires the Novel Ciliary MYND Domain Protein DAF-25
- A Buoyancy-Based Screen of Drosophila Larvae for Fat-Storage Mutants Reveals a Role for in Coupling Fat Storage to Nutrient Availability
- A Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species (Green Peach Aphid)
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- Genome-Wide Effects of Long-Term Divergent Selection
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



