Genome-Wide Association Meta-Analysis of Cortical Bone Mineral Density Unravels Allelic Heterogeneity at the Locus and Potential Pleiotropic Effects on Bone
Previous genome-wide association (GWA) studies have identified SNPs associated with areal bone mineral density (aBMD). However, this measure is influenced by several different skeletal parameters, such as periosteal expansion, cortical bone mineral density (BMDC) cortical thickness, trabecular number, and trabecular thickness, which may be under distinct biological and genetic control. We have carried out a GWA and replication study of BMDC, as measured by peripheral quantitative computed tomography (pQCT), a more homogenous and valid measure of actual volumetric bone density. After initial GWA meta-analysis of two cohorts (ALSPAC n = 999, aged ∼15 years and GOOD n = 935, aged ∼19 years), we attempted to replicate the BMDC associations that had p<1×10−5 in an independent sample of ALSPAC children (n = 2803) and in a cohort of elderly men (MrOS Sweden, n = 1052). The rs1021188 SNP (near RANKL) was associated with BMDC in all cohorts (overall p = 2×10−14, n = 5739). Each minor allele was associated with a decrease in BMDC of ∼0.14SD. There was also evidence for an interaction between this variant and sex (p = 0.01), with a stronger effect in males than females (at age 15, males −6.77mg/cm3 per C allele, p = 2×10−6; females −2.79 mg/cm3 per C allele, p = 0.004). Furthermore, in a preliminary analysis, the rs1021188 minor C allele was associated with higher circulating levels of sRANKL (p<0.005). We show this variant to be independent from the previously aBMD associated SNP (rs9594738) and possibly from a third variant in the same RANKL region, which demonstrates important allelic heterogeneity at this locus. Associations with skeletal parameters reflecting bone dimensions were either not found or were much less pronounced. This finding implicates RANKL as a locus containing variation associated with volumetric bone density and provides further insight into the mechanism by which the RANK/RANKL/OPG pathway may be involved in skeletal development.
Published in the journal:
Genome-Wide Association Meta-Analysis of Cortical Bone Mineral Density Unravels Allelic Heterogeneity at the Locus and Potential Pleiotropic Effects on Bone. PLoS Genet 6(11): e32767. doi:10.1371/journal.pgen.1001217
Category:
Research Article
doi:
https://doi.org/10.1371/journal.pgen.1001217
Summary
Previous genome-wide association (GWA) studies have identified SNPs associated with areal bone mineral density (aBMD). However, this measure is influenced by several different skeletal parameters, such as periosteal expansion, cortical bone mineral density (BMDC) cortical thickness, trabecular number, and trabecular thickness, which may be under distinct biological and genetic control. We have carried out a GWA and replication study of BMDC, as measured by peripheral quantitative computed tomography (pQCT), a more homogenous and valid measure of actual volumetric bone density. After initial GWA meta-analysis of two cohorts (ALSPAC n = 999, aged ∼15 years and GOOD n = 935, aged ∼19 years), we attempted to replicate the BMDC associations that had p<1×10−5 in an independent sample of ALSPAC children (n = 2803) and in a cohort of elderly men (MrOS Sweden, n = 1052). The rs1021188 SNP (near RANKL) was associated with BMDC in all cohorts (overall p = 2×10−14, n = 5739). Each minor allele was associated with a decrease in BMDC of ∼0.14SD. There was also evidence for an interaction between this variant and sex (p = 0.01), with a stronger effect in males than females (at age 15, males −6.77mg/cm3 per C allele, p = 2×10−6; females −2.79 mg/cm3 per C allele, p = 0.004). Furthermore, in a preliminary analysis, the rs1021188 minor C allele was associated with higher circulating levels of sRANKL (p<0.005). We show this variant to be independent from the previously aBMD associated SNP (rs9594738) and possibly from a third variant in the same RANKL region, which demonstrates important allelic heterogeneity at this locus. Associations with skeletal parameters reflecting bone dimensions were either not found or were much less pronounced. This finding implicates RANKL as a locus containing variation associated with volumetric bone density and provides further insight into the mechanism by which the RANK/RANKL/OPG pathway may be involved in skeletal development.
Introduction
Genome-wide association studies have identified reliable genetic associations with Dual x-ray absorptiometry (DXA)-derived measures related to bone mass, such as areal bone mineral density (aBMD) [1]–[4]. aBMD, is a commonly used skeletal trait measure on the basis of its ability to predict fractures at clinically important sites [5]. However, this measurement is influenced by several different skeletal parameters such as periosteal expansion, cortical BMD (BMDC), cortical thickness, trabecular number and trabecular thickness [6], all measures which may be under distinct systems of biological and genetic control. Currently, it is unclear which of these more precise bone related phenotypes identified genetic associates are associated with, whether work on aBMD is telling us more about bone size or growth [7], or what genetic variation has been missed through the use of more global bone measures.
Devices such as peripheral quantitative computed tomography (pQCT), which measure cross sections of predominantly cortical or trabecular bone, enable the different constituents of bone mass to be analysed separately (Figure 1) and may offer advantages over DXA in terms of identifying genetic correlates of specific bone phenotypes. Recently we examined whether genetic polymorphisms found to be associated with aBMD in recent GWA studies were also related to pQCT parameters, based on analysis of adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC) and young adult men from the ‘Gothenburg Osteoporosis and Obesity Determinants’ (GOOD) cohort [8]. We found that rs3018362 (near RANK), and rs4355801 and rs6993813 (near OPG), reported as being associated with aBMD in previous GWA studies [1], [3], were associated with BMDC as measured by tibial pQCT, but not with any other pQCT phenotype. This raised the possibility that BMDC is a potentially useful phenotype for detecting novel genetic influences on the skeleton, possibly reflecting the fact that this measure is entirely size independent.
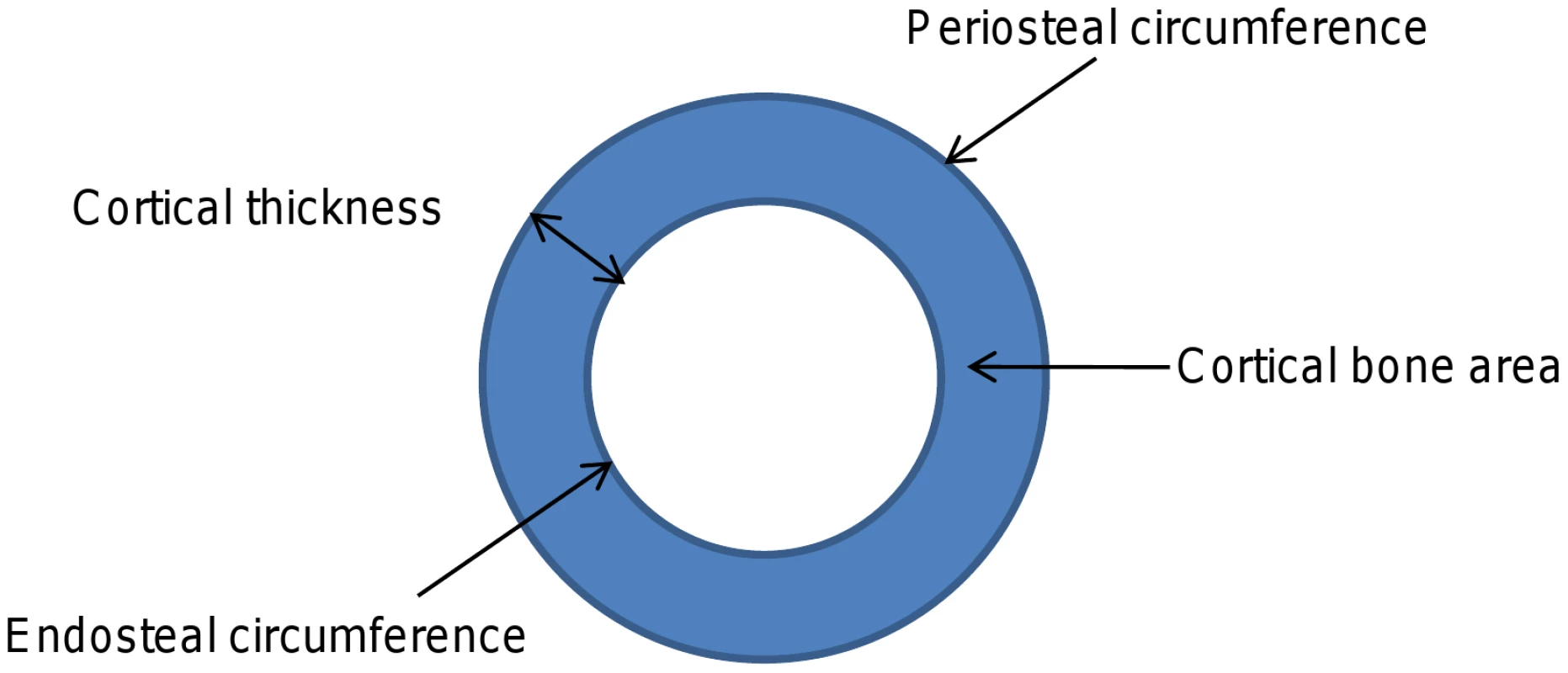
Here we perform a GWA study of BMDC, based on data from tibial pQCT scans in the ALSPAC and GOOD cohorts. We present the results of our initial GWA meta-analysis, along with those from replication studies involving a further set of individuals from ALSPAC, and the MrOS Sweden cohort of elderly men.
Results
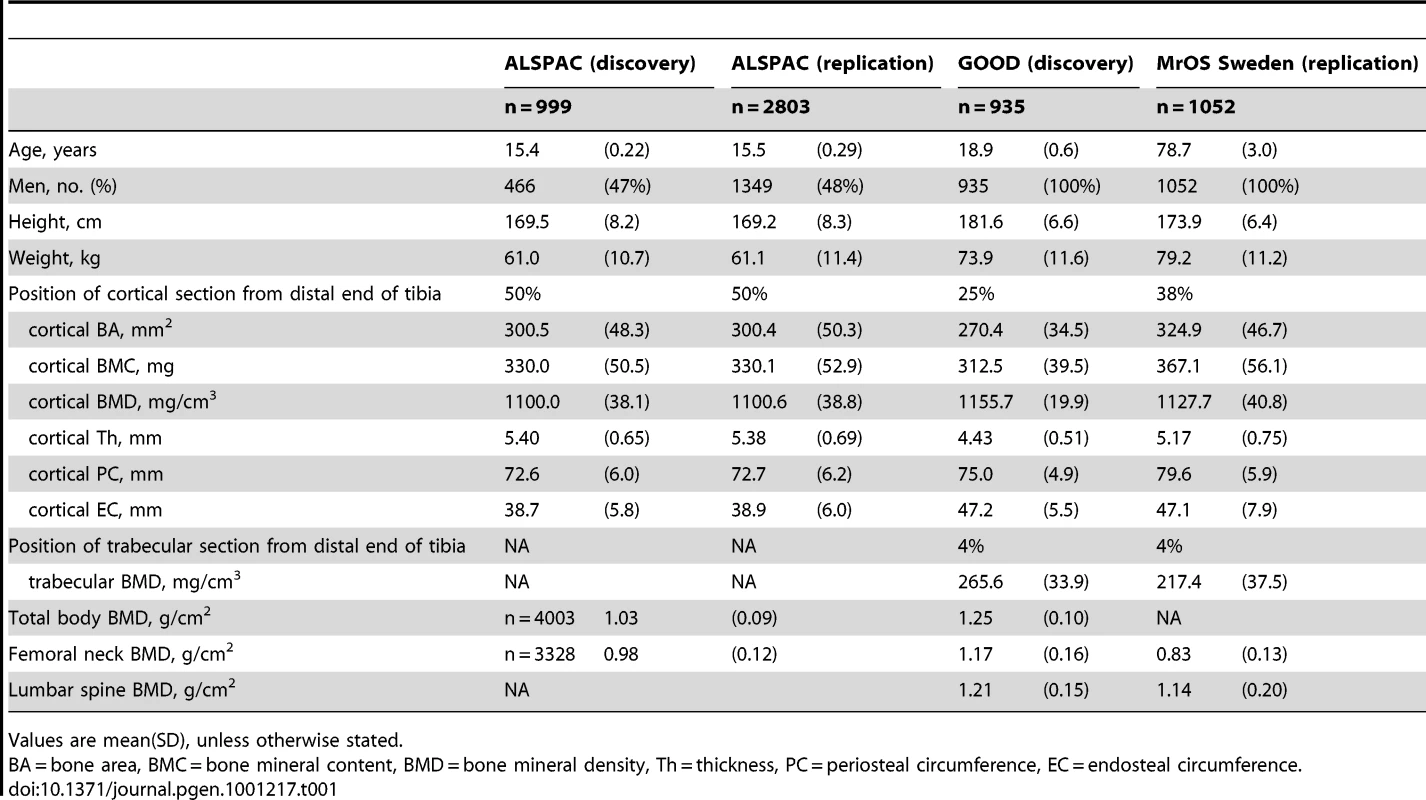
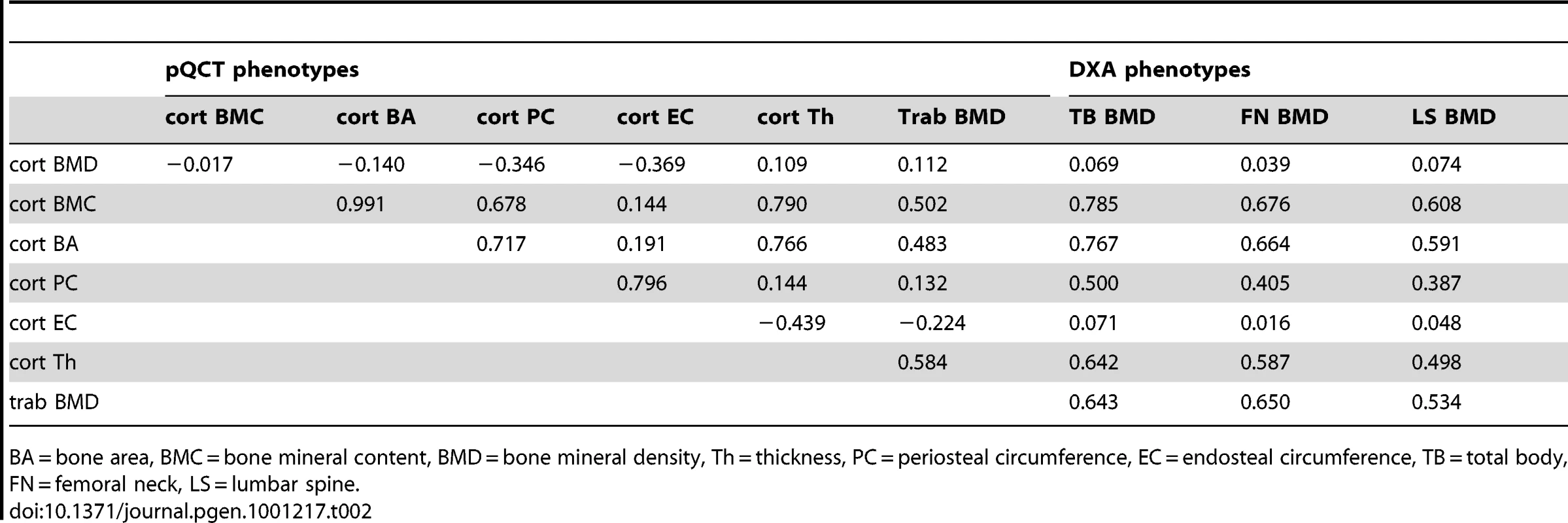
Table 1 displays the means and standard deviations of bone traits for the four cohorts (ALSPAC discovery, GOOD discovery, ALSPAC replication and MrOS Sweden replication). Overall aBMD and BMDc were higher in the young adult men in the GOOD cohort when compared to the younger subjects in the ALSPAC cohort and the older men of MrOS Sweden. BMDC is positively correlated with cortical thickness and trabecular BMD, but inversely correlated with the other pQCT traits, demonstrating the complicated interaction between these traits (Table 2). There is much lower correlation between BMDC and BMD as measured by DXA (irrespective of measurement site).
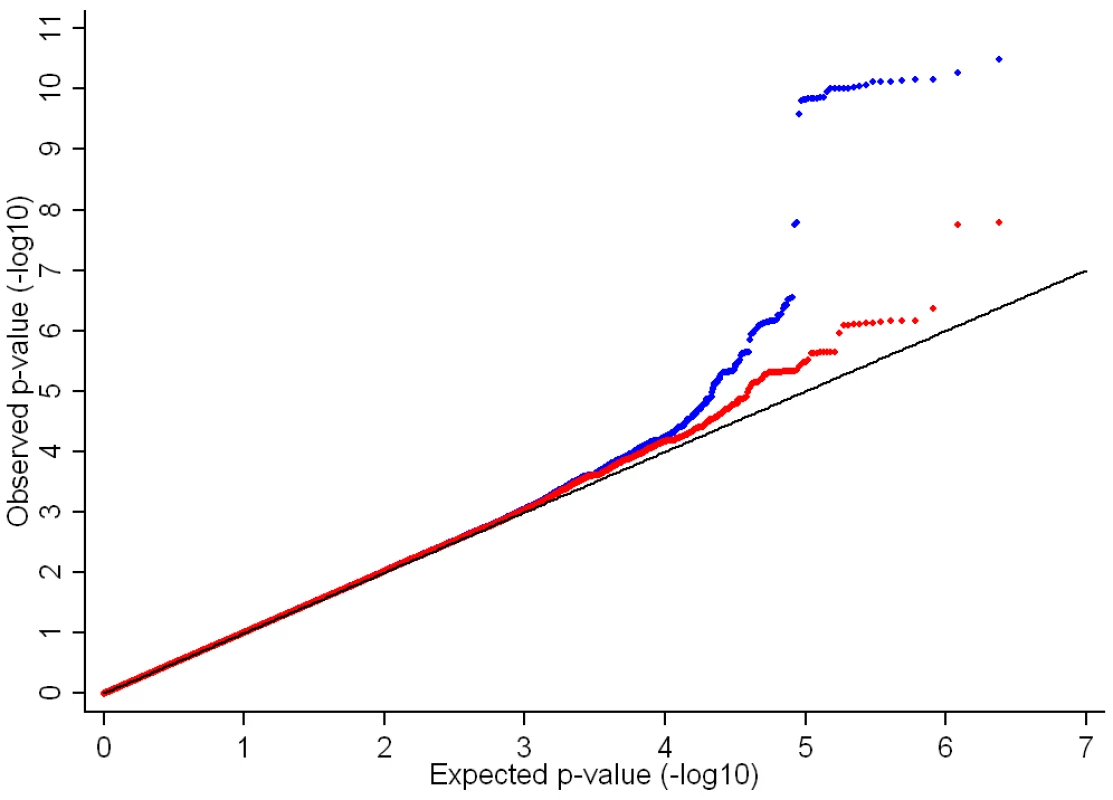
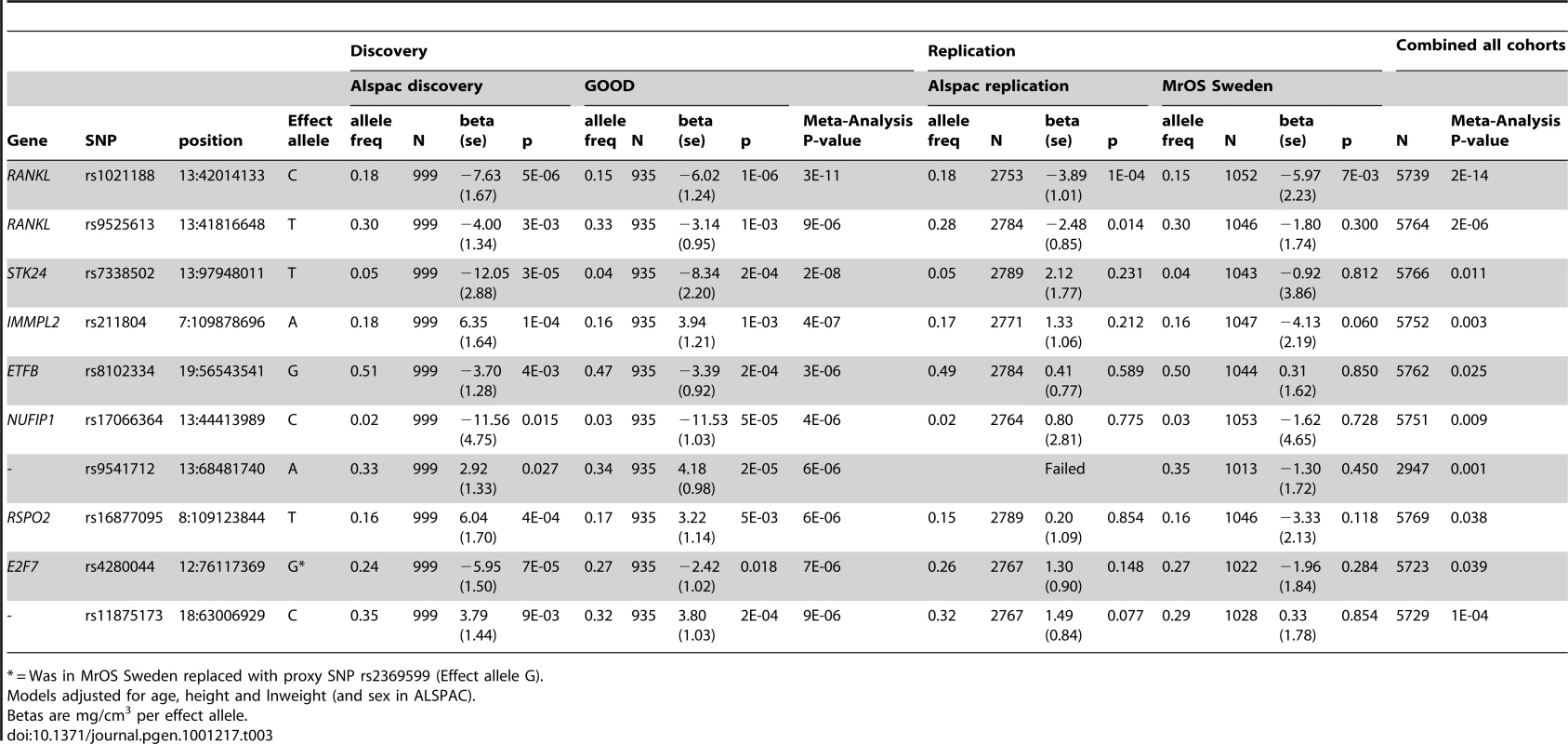
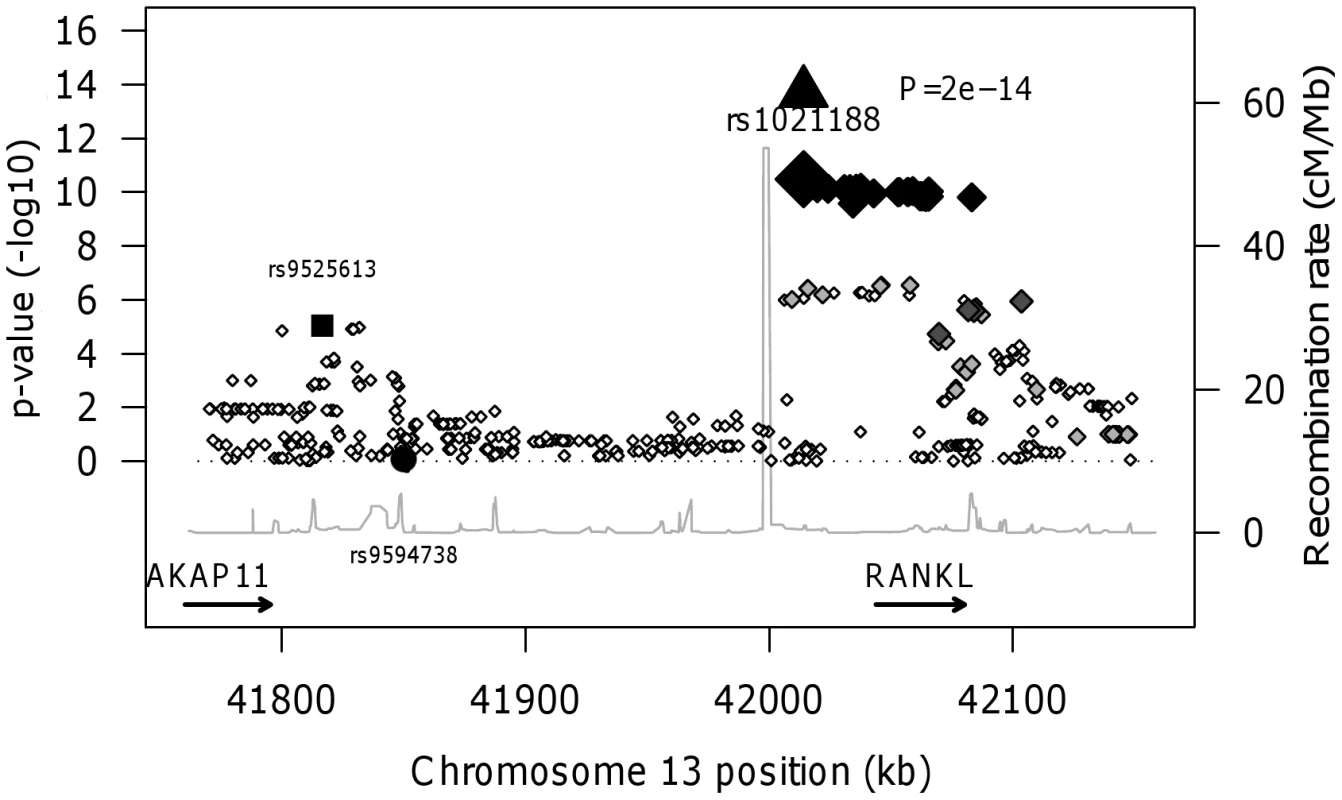
In the genome-wide association meta-analysis of the ALSPAC discovery cohort and GOOD cohort there was little systematic inflation of test statistics (λGC = 1.021 (1.0004 for ALSPAC; 1.010 for GOOD), but a marked deviation from the null amongst the lowest observed p-values (Figure 2). The greatest evidence for association between genetic variation and BMDC was seen at rs1021188 (ALSPAC β = −7.63; GOOD β = −6.02, overall p = 3×10−11 (p = 4×10−11 after applying genomic control)) on chromosome 13, slightly upstream of the RANKL gene (Figure 3). We selected the nine regions with p<1×10−5 and carried out analyses conditional on the most associated SNP in that region to check if there were multiple independently associated SNPs in each region (A full list of SNPs that exhibit nominal evidence of association (p<1×10−5) with BMDC can be found in the supplementary online material, Table S1). The RANKL region of chromosome 13 was the only region for which a second SNP (rs9525613) still showed marginal association (p = 0.008) when conditioning on the most associated SNP in this region (rs1021188). We selected both of these SNPs for replication follow-up, in addition to the top ranking SNP from each of the other regions (rs7338502, rs211804, rs8102334, rs17066364, rs9541712, rs16877095, rs4280044, rs11875173). As well as our primary analyses which adjusted for age, sex (ALSPAC only), height and weight(ln), we also carried out analyses adjusting for only age and sex and found broadly similar results.
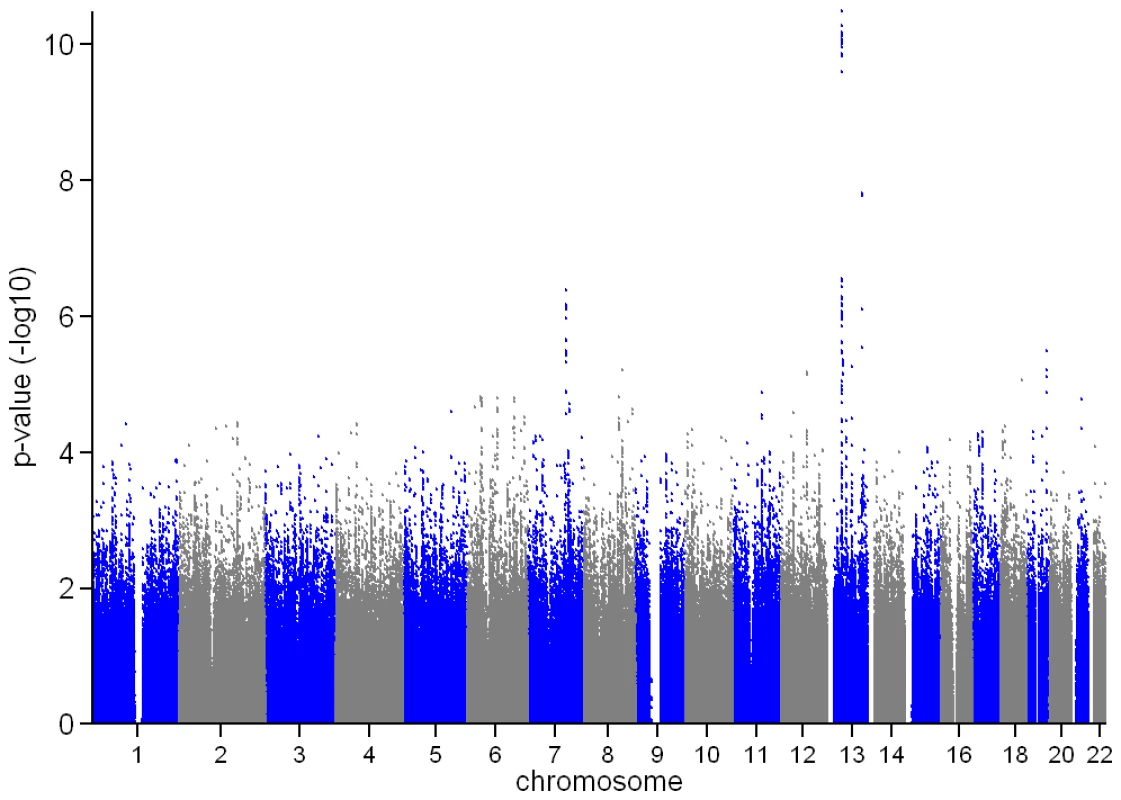
In both the replication cohorts the RANKL SNP (rs1021188) was the only variant to be consistently associated and the only SNP to reach genome-wide significance in a p-value meta-analysis of the four cohorts (p = 2×10−14, total n = 5739) (Table 3). On carrying out an inverse-variance meta-analysis of the ALSPAC, GOOD and MrOS Sweden cohorts each minor C allele was associated with a clear decrease in BMDC of ∼0.14 SD (Table 4), explaining 0.6%, 2.2% and 0.5% of the variation in the ALSPAC cohort, GOOD cohort and MrOS Sweden cohort, respectively.
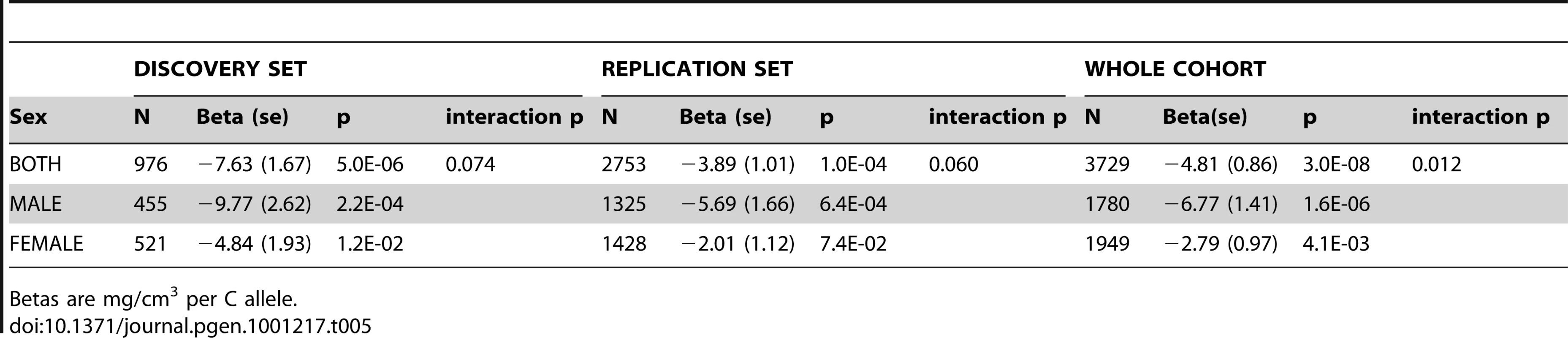
The association between rs1021188 and BMDC was stronger in males (β = −6.77, p = 2×10−6) than females (β = −2.79, p = 0.004) in the entire ALSPAC cohort (and testing for an interaction between sex and genotype gave p = 0.01) (Table 5). Both male-only cohorts showed similar effects for rs1021188 and BMDC (β = −6.02 GOOD, β = −5.97 MrOS Sweden).
After removal of the RANKL region (chr 13: 42000–42150Kb) from the main analysis, there was evidence for a marked reduction in the inflation of test statistics, however the existence of some departure from the expected null distribution suggested that further loci remain to be identified (Figure 2).
We found no association between BMDC and a RANKL variant (rs9594738) which has previously been associated with aBMD [2], [3]. We assessed the proximity of our most associated loci (rs1021188), the nearby rs9525613 (which we also attempted to replicate) and rs9594738 to the RANKL gene (Figure 4). We found that the rs1021188 SNP is much closer to the RANKL gene than the aBMD associated SNP (and rs9525613) and that there are high recombination rates between the two regions (r2 between the two SNPs is 0.01), indicating that these may be separate signals (Figure 4).
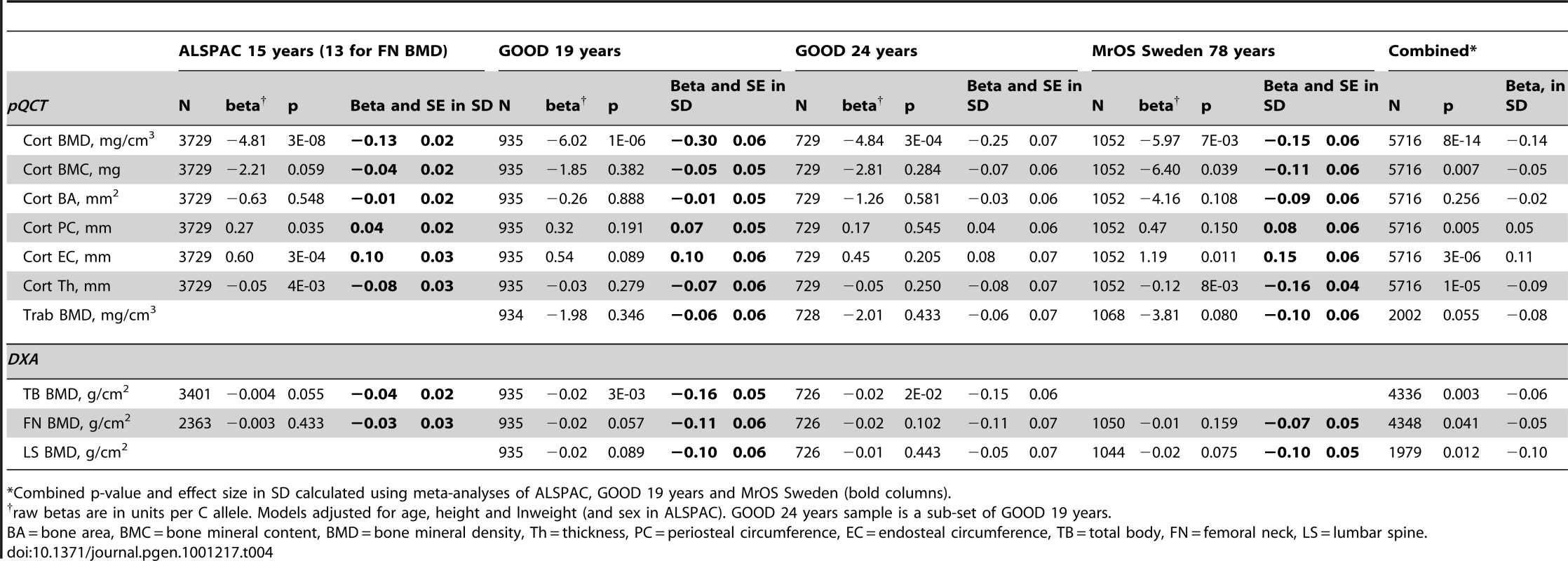
To determine if the association between rs1021188 and BMDC was specific for this bone trait, we have tested for associations between the RANKL SNP (rs1021188) and other skeletal traits in ALSPAC, GOOD and MrOS Sweden. rs1021188 shows some evidence for association with endosteal circumference and cortical thickness, but to a much lesser extent than BMDC and even less so for cortical content and periosteal circumference. Though the evidence for these associations comes mostly from the ALSPAC and MrOS cohorts, the effect sizes seen in the GOOD cohort are extremely consistent and the lack of association in this cohort is likely due to the reduced power found in this smaller study. Although there is some evidence for association between rs1021188 and total-body, femoral neck and lumbar-spine BMD (as measured by DXA), this appears to be weaker than the association seen with tibial cortical BMD, though with smaller sample sizes for these analyses these comparisons may not be robust (Table 4). In the ALSPAC cohort, the association between rs1021188 and BMDC was only slightly attenuated when the analysis was adjusted for femoral neck BMD (β = −4.49, p = 5×10−7) or total body BMD (β = −3.64, p = 3×10−5), as measured by DXA.
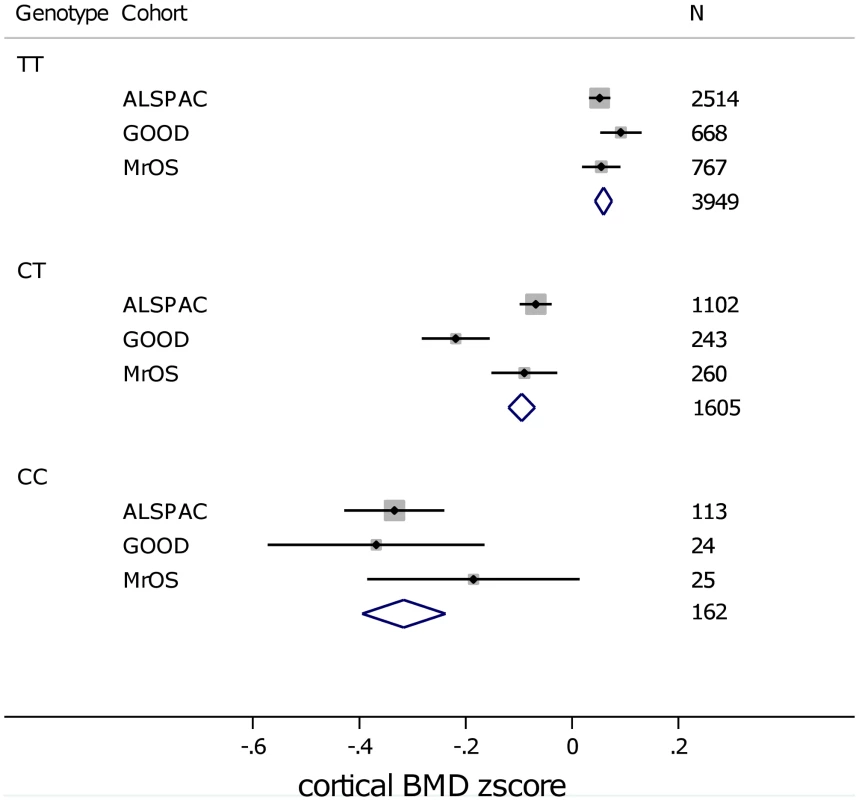
In analysis of mean BMDC z-scores per rs1021188 genotype in each of the cohorts rs1021188 had an approximately additive effect; with each C allele cortical BMD is decreased (Figure 5). This appears to be true across all ages, although the absolute BMDC values vary.
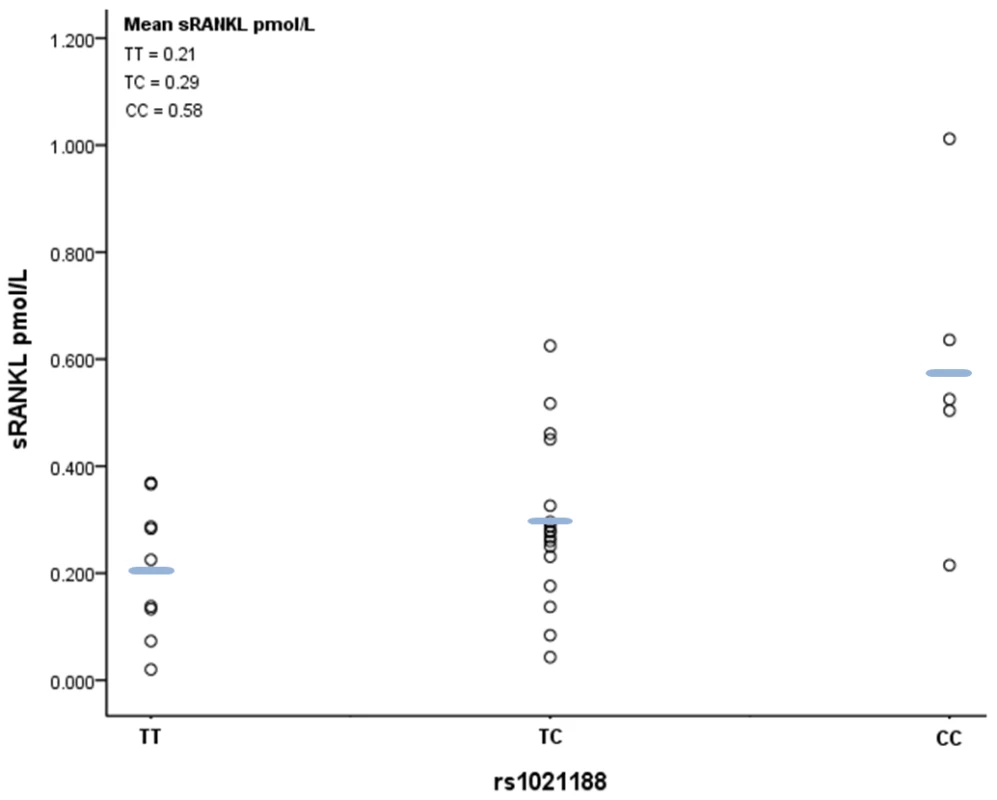
Finally, we analysed the relationship between rs1021188 genotype and plasma levels of soluble RANKL (sRANKL) in a small sample of male subjects from ALSPAC. A positive relationship was observed between number of minor rs1021188 alleles and sRANKL level (P = 0.005), such that sRANKL levels were over twice as great in those with CC versus TT genotypes (Figure 6).
Discussion
In genomewide analysis of the specific bone phenotype BMDC, we have been able to identify genetic variants different to those found from equivalent studies based on aBMD. In particular, the rs1021188 SNP was found to be reliably associated with BMDC, but has not previously been reported to be associated with any other bone phenotype to date. rs1021188 is located ∼20Kb upstream of the receptor activator of nuclear factor-κB ligand gene (RANKL) on chromosome 13. The minor C allele was associated with a decrease in BMDC (of ∼0.14SD, explaining 0.5–2.2%, of the variation - though the larger of these estimates is inflated by the more homogenous GOOD cohort having less overall variance) and there was also evidence for an interaction with sex, with larger effects being observed amongst males (though this study was not designed to test for sex-gene interactions). The effect of this marker on BMDC was similar across all cohorts, suggesting that although our discovery set comprised adolescents/young adults, this marker influences cortical density to a similar extent in the elderly.
Whereas the rs1021188 (C allele) of RANKL showed a strong inverse association with BMDC, associations with cortical bone mass were somewhat weaker. Nevertheless, the size of the latter effect was still equivalent to that of a doubling of vigorous physical activity (as measured by accelerometer) in the ALSPAC cohort at the same age (unpublished). The weaker association between rs1021188 genotype and cortical bone mass compared with that of cortical density is likely to be explained by rs1021188 being unrelated to cortical bone size (reflected by cortical bone area), which is a major determinant of cortical bone mass. Although rs1021188 was not associated with cortical bone area as a whole, a relatively strong positive association was observed with endosteal circumference, whereas there was an equivalent inverse association with cortical thickness, together suggesting that rs1021188 increases endosteal expansion. There was also a weak positive association between this marker and periosteal circumference (possibly reflecting a compensatory response), which may explain why the increased endosteal expansion associated with this marker had little impact on overall cortical bone size. Since these analyses were adjusted for height, these findings imply that RANKL influences the rate of periosteal and endosteal expansion independently of vertical growth, consistent with a local action on cortical bone (see below).
The observation that the RANKL rs1021188 is associated with cortical BMD is consistent with our recent finding from a larger-scale candidate gene study in the ALSPAC and GOOD cohorts that other genes of the RANK/RANKL/OPG pathway are also associated with BMDC [8]. Given the important role of this pathway in regulating osteoclast differentiation [9], these findings may reflect a response to enhanced net RANKL activity leading to greater osteoclast activation and hence stimulation of cortical remodeling. Our observation that the minor C allele of rs1021188 was associated with higher circulating levels of free RANKL in a preliminary analysis, as measured in a small number of ALSPAC subjects, is consistent with this interpretation. Increased bone resorption arising from higher RANKL levels is predicted to reduce BMDC both by increasing cortical porosity and reducing the available time for secondary mineralization [10]. Increased bone resorption could also explain the relationship we observed between RANKL rs1021188 and increased endosteal resorption, which is also an osteoclast-dependent process.
Although the RANKL rs1021188 marker has not been reported to be associated with any bone-related trait before, a SNP (rs9594738) ∼190kb upstream of RANKL was strongly associated (standardized beta = −0.17, p = 2.0×10−21) with lumbar spine areal bone mineral density (aBMD) in a genome-wide association and replication study in four cohorts of mostly elderly subjects (with a high proportion of postmenopausal women) [3] and a perfect proxy SNP for rs9594738 (rs9533090, r2 = 1) was also strongly associated (standardized beta = −0.12, p = 5.4×10−25) with lumbar spine aBMD in a further meta-analysis of five genome-wide association studies of a wide age range (again with a high proportion of women) [2]. These studies also found these SNPs to be associated with hip aBMD, but to a lesser extent (rs9594738: standardized beta = −0.10, p = 1.9×10−8; rs9533090: standardized beta = −0.04, p = 3.9×10−4) and not with low trauma fractures (p = 0.23), demonstrating that there may be skeletal site heterogeneity in the associations with RANKL. In contrast, we have previously shown rs9594738 to not be associated with BMDC in the ALSPAC and GOOD cohorts [8] and we show here that the two signals are likely to be independent. There are high recombination rates between the two regions where rs1021188 and rs9594738 are located, and so these two markers may well be tagging distinct functional RANKL variants which affect these different bone phenotypes individually. Alternatively, as rs9594738 is located ∼190kb upstream of RANKL and is in fact closer to another gene AKAP11, it may be that the two phenotypes are under the control of different genes entirely, although AKAP11 has not to date been implicated in skeletal regulation. In addition another SNP (rs10507508) has also been shown to be associated (p = 0.0011) with aBMD after adjusting for 59 other SNPs in the region [3], possibly representing a further independent association with BMD at this locus. This allelic heterogeneity could be important for understanding the relationship between RANKL and bone phenotypes.
The differing genetic associations between aBMD and BMDC (and site of investigation) at this RANKL locus may be explained by the genetic variants being associated with distinct properties of bone. Since analysis of lumbar spine BMD may be mostly assessing trabecular bone, previously identified RANKL variants may be particularly associated with this component. In contrast, the RANKL variant that we identify here may be more strongly associated with cortical bone, given that the tibial pQCT site (which formed the basis of this study) comprises solely of cortical bone. Here, total body BMD and femoral neck BMD showed only a weak association with the RANKL rs1021188 marker (and adjusting for these measures in the BMDC analysis only slightly attenuated the association) and the lumbar spine BMD (which largely comprises trabecular bone) showed an association very similar to that seen for tibial trabecular BMD, implying that cortical associations may be missed using DXA to assess BMD. The implication that there is little relationship between cortical BMD and BMD as measured by DXA is consistent with the weak correlations we observed between cortical BMD and DXA measured BMD (irrespective of site). This is likely to reflect the fact that BMD as measured by DXA is mainly influenced by parameters such as overall bone size and cortical thickness, as opposed to the material density of bone and/or degree of cortical porosity (as reflected by pQCT measured cortical BMD). In contrast to BMD as measured by DXA which is related to clinical end points such as fracture risk, few if any studies have analysed cortical BMD measurements in relation to fracture risk. Nevertheless, recent findings have highlighted the importance of changes in determinants of cortical density, such as cortical porosity, in the pathogenesis of osteoporotic fracture [11].
Taken together with our previous report of associations between RANK and OPG variants and BMDc, our results provide further evidence that the RANK/RANKL/OPG axis affects the skeleton at least in part by influencing material density of cortical bone. Although the precise relationship between this phenotype and clinical end points such as fracture risk remains to be established, it is clear that the availability of precise and specific measures of bone health provide great resolution in the analysis of heritable contributions to this category of traits. It is also tempting to speculate that changes in BMDC contribute to recent observations that the RANKL inhibitor denosumab reduces fracture risk [12]. Consistent with this possibility, administration of denosumab has been found to increase femoral BMDC in mice with a knock-in of humanised RANKL [13].
In summary we have performed the first GWAS with cortical BMD. We identified a SNP (rs1021188) close to the RANKL gene with strong evidence for association with BMDC in adolescent and young adult cohorts used for the discovery phase, as well as in our replication cohort of elderly men, suggesting this genetic effect acts over the whole lifetime. We also show some evidence for an interaction with sex for this variant, with the association being stronger in males. In addition, the minor C allele of rs1021188 was associated with higher circulating levels of free RANKL in a preliminary analysis. Consistent with an action of this variant via altered RANKL and hence osteoclast activity, an equivalent association was also observed with endosteal expansion as reflected by endosteal circumference. However, there was little relation between rs1021188 and overall cortical bone area, possibly reflecting compensatory changes in periosteal apposition. This signal appears to be independent of another previously reported association in this region between aBMD and rs9594738, demonstrating that there may be important allelic heterogeneity at this locus, and possibly indicating that these variants are associated with different components of bone structure. Further studies are justified to extend these findings, for example by performing further GWA studies with sufficient power to detect other important genetic influences on cortical BMD.
Methods
ALSPAC cohort
Participants
The Avon Longitudinal Study of Parents and their Children (ALSPAC) is a longitudinal population-based birth cohort that initially included over 13,000 women and their children in Avon, UK, in the early 1990s. This cohort is described in detail on the website (http://www.alspac.bris.ac.uk) and elsewhere [14]. Both mothers and children have been extensively followed from the 8th gestational week onwards using a combination of self-reported questionnaires, medical records and physical examinations. Ethical approval was obtained from the ALSPAC Law and Ethics committee and relevant local ethics committees, and written informed consent provided by all parents. Blood samples were taken and DNA extracted as previously described [15].
Bone measures
pQCT scans were performed on approximately 4500 children when they attended the age 15 research clinic at which time total body DXA scans were also performed. Results for hip DXA scans, collected at the age 13 research clinic, were also analysed. At both time points, height was measured using a Harpenden stadiometer (Holtain Limited, Wales) and weight using a Tanita Body Fat Analyser.
Cortical bone mineral content (BMCC), cortical bone mineral density (BMDC) and cortical bone area (BAC), were measured on a single slice at the mid tibia using the Stratec XCT2000L (Germany). Periosteal circumference (PC), endosteal circumference (EC) and cortical thickness (CT) were derived using a circular ring model. A threshold routine was used for defining cortical bone, which specified a voxel with a density >650 mg/cm3 as cortical bone. Of the 4500 pQCT scans obtained in ALSPAC 89 were rejected as being of insufficient quality. The coefficients of variation (CVs) based on 139 ALSPAC subjects scanned a mean of 31 days apart, were 2.7%, 1.3% and 2.9% for BMCC, BMDC, BAC, respectively.
Total body BMD and BMD of the left femoral neck were measured using a Lunar Prodigy scanner, for which CVs were 1.0% (146 subjects) and 1.7% (166 subjects) respectively.
Discovery set genotyping
1760 ALSPAC individuals were genotyped using the Illumina HumanHap317K SNP chip. Markers with <1% minor allele frequency, >5% missing genotypes or which failed an exact test of Hardy-Weinberg equilibrium (HWE) (p<1×10−7) were excluded from further analysis. We also excluded any individuals who did not cluster with the CEU individuals in multidimensional scaling analysis, who had >5% missing data, heterozygosity of >36.4% or <34.3% and a male who scored heterozygous at many X chromosome loci. After data cleaning we were left with 1518 individuals (999 with pQCT data) and 315,807 SNPs. We carried out imputation to HapMap release 22 using Mach 1.0, Markov Chain Haplotyping [16].
Replication set genotyping
Genotyping (of the SNPs with p<1×10−5 in the GWAS meta-analysis) was carried out on the entire ALSPAC cohort for whom DNA was available (10121 individuals) by KBioscience (http://www.kbioscience.co.uk), who employ a novel form of competitive allele specific PCR (KASPar) for genotyping. Those individuals who were included in the ALSPAC discovery analysis as well as those of non-white reported ethnicity, with >10% missing genotypes and with siblings in the cohort were excluded from the analysis. For each SNP there were between 2753 and 2789 individuals with both pQCT and genotype data. All but one SNP (rs9541712) were successfully genotyped. All were in HWE.
Measurement of free RANKL levels
Soluble RANKL levels (sRANKL) were measured on fasting blood samples (which were obtained according to standard protocols, collected in heparin tubes, and stored at minus 80 degrees until further use), in 37 male individuals attending the age 15 research clinic, randomly selected after stratification by RANKL rs1021188 genotype. Measurements, which were performed blind, were carried out using the ampli-sRANKL enzyme-linked immunosorbent assay (ELISA) from Biomedica (Vienna, Austria) according to manufacturer's instructions, with the exception that 90µl of plasma was used per well. Measurement ranges, intra- and inter-assay coefficients of variation (CVs) were 0.02–2pmol/l, <9% and <6% respectively. Duplicate samples with a coefficient of variation of <20% were considered for further statistical analysis. In sample(s) where the recorded concentration was below the detection limit of 0.02 pmol/l, the latter value was entered in subsequent analyses.
GOOD cohort
Participants
The Gothenburg Osteoporosis and Obesity Determinants (GOOD) study was initiated to determine both environmental and genetic factors involved in the regulation of bone and fat mass [17], [18]. Male study subjects were randomly identified in the greater Gothenburg area in Sweden using national population registers, contacted by telephone, and invited to participate. To be enrolled in the GOOD study, subjects had to be between 18 and 20 years of age. There were no other exclusion criteria, and 49% of the study candidates agreed to participate (n = 1068). The subjects of the GOOD cohort were also analysed after five years of follow-up, between 23 and 25 years of age. The GOOD study was approved by the local ethics committee at Gothenburg University. Written and oral informed consent was obtained from all study participants. Height was measured using a wall-mounted stadiometer, and weight was measured to the nearest 0.1 kg.
Bone measures
BMDC, BMDC and BAC were measured on a single tibial diaphyseal slice (at 25% of the bone length in the proximal direction of the distal end of the bone) using the Stratec XCT2000 (Germany). PC, EC and CT were derived using a circular ring model. A threshold routine was used for defining cortical bone, which specified a voxel with a density >710 mg/cm3 as cortical bone. Trabecular vBMD was measured using a scan through the distal metaphysis (at 4% of the bone length in the proximal direction of the distal end of the bone) of tibia. Tibia length was measured from the medial malleolus to the medial condyle of the tibia. The CVs were <1% for all pQCT measurements.
aBMD (g/cm2) of the whole body, femoral neck (of the left leg), and lumbar spine were assessed using the Lunar Prodigy DXA (GE Lunar, Madison, WI, USA). The CVs for the aBMD measurements ranged from 0.5% to 3%, depending on application.
Discovery set genotyping
Genotyping was performed with Illumina HumanHap610 arrays at the Genetic Laboratory, Department of Internal Medicine, Erasmus Medical Center, Rotterdam, the Netherlands. Genotypes were called using the BeadStudio calling algorithm. Genotypes from 935 individuals passed the sample quality control criteria [exclusion criteria: sample call rate <97.5%, gender discrepancy with genetic data from X-linked markers, excess autosomal heterozygosity >0.33 (∼FDR<0.1%), duplicates and/or first degree relatives identified using IBS probabilities (>97%), ethnic outliers (3 SD away from the population mean) using multi-dimensional scaling analysis with four principal components]. Across 22 duplicate samples, genotype concordance exceeded 99.9%. We carried out imputation to HapMap release 22 (after excluding SNPs with MAF<1%, SNP call rate <98% and HWE p value <1×10−6) using Mach 1.0, Markov Chain Haplotyping [16].
MrOS Sweden cohort
Participants
The Osteoporotic Fractures in Men (MrOS) study is a multicenter, prospective study including older men in Sweden (3014), Hong Kong (≅2000), and the United States (≅6000). In the present study, associations between candidate polymorphism and skeletal parameters were investigated in the Swedish cohort (Table 1), which consists of three sub-cohorts from three different Swedish cities (n = 1005 in Malmö, n = 1010 in Göteborg, and n = 999 in Uppsala). Study subjects (men aged 69–81) were randomly identified using national population registers, contacted and asked to participate. To be eligible for the study, the subjects had to be able to walk without assistance, provide self-reported data, and sign an informed consent; there were no other exclusion criteria [19]. The study was approved by the ethics committees at the Universities of Gothenburg, Lund, and Uppsala. Informed consent was obtained from all study participants.
Bone measures
Validated pQCT analyses were available for the Gothenburg and Malmö cohorts. BMCC, BMDC and BAC, were measured on a single tibial diaphyseal slice slice (at 38% of the bone length in the proximal direction of the distal end of the bone) using the Stratec XCT2000 (Germany). PC, EC and CT were derived using a circular ring model. A threshold routine was used for defining cortical bone, which specified a voxel with a density >710 mg/cm3 as cortical bone. Trabecular vBMD was measured using a scan through the distal metaphysis (at 4% of the bone length in the proximal direction of the distal end of the bone) of tibia. The CVs were <1% for all pQCT measurements. Adjustments for study centre were performed.
Total body areal BMD (aBMD, g/cm2), as well as aBMD of the femoral neck and lumbar spine (L1-L4) were assessed at baseline using the Lunar Prodigy dual energy X-ray absorptiometry (DXA) (n = 2004 from the Uppsala and Malmö cohorts; GE Lunar Corp., Madison, WI, USA) or Hologic QDR 4500/A-Delphi (n = 1010 from the Göteborg cohort; Hologic, Waltham, MA, USA). The CVs for the aBMD measurements ranged from 0.5% to 3%, depending on the application. To be able to use DXA measurements performed with equipment from two different manufacturers, a standardized BMD (sBMD) was calculated, as previously described [19]. Adjustments for study centre were performed.
Replication set genotyping
Genotyping (of the SNPs with p<1×10−5 in the GWAS meta-analysis) was carried out using matrix-assisted laser desorption ionization-time of flight mass spectrometry on the Sequenom MassARRAY platform (San Diego, CA, USA). The genotyping call rate was >97% and the SNP-s were in Hardy-Weinberg equilibrium.
Statistical methods
Genome-wide meta-analysis and replication
The ALSPAC discovery set (n = 999) and GOOD (n = 935) contributed to the genome-wide meta-analysis. We analysed only those imputed SNPs which had a minor allele frequency of >0.01 and an r2 imputation quality score of >0.3 in both sets (n = 2,417,199). We carried out genome-wide association analyses for BMDC using additive linear regression in Mach2QTL for both ALSPAC and GOOD (using GRIMP [20] for the GOOD analyses). We included age, sex, height and weight(ln) as covariates in ALSPAC, and age, height and weight(ln) as covariates in the male only GOOD cohort.
We carried out meta-analyses of the results from the two cohorts using two methods in METAL (www.sph.umich.edu/csg/abecasis/metal). In the p-value meta-analysis study-specific Z-statistics are calculated (which summarise the p-values and direction of effect) for each SNP's association. The Z-statistics are then summed across studies, using weights proportional to the square-root of each study's sample size, to provide a summary p-value for each association. In the inverse variance method standardized betas and standard errors from each study are combined using a fixed effect model which weights the studies using the inverse variance. We also carried out the meta-analyses with and without genomic control. The results using each method were very similar and the selection of SNPs was based on the p-value meta-analysis not adjusted for genomic control. Genome-wide significance was taken to be p<5×10−8.
We selected one SNP from each independent region that had a p<1×10−5 for replication in the ALSPAC replication cohort and MrOS Sweden. Additive linear regression analyses were carried out for the associations between these SNPs and BMDC in PLINK [21] (ALSPAC) or in SPSS Statistics 17.0 (MrOS Sweden) using age, sex, height and weight(ln) as covariates. We calculated the combined p-value (for all four cohorts) using METAL (method described above).
Association between the RANKL SNP and other traits
For the RANKL SNP (rs1021188) we also tested for associations with other bone traits; BAC, BMCC, PC, EC and CT using the pQCT data and TB BMD, FN BMD and LS BMD in all cohorts (where the appropriate measures were available). The ALSPAC discovery and replication cohorts were combined and we also show the association results from GOOD from two time-points (the original GWAS time-point (19 years) and data from the five year follow-up visit (24 years). We carried out association analyses using additive linear regression in PLINK for ALSPAC and in SPSS Statistics 17.0 for GOOD and MrOS Sweden. We included age, sex, height and weight(ln) as covariates in the model in ALSPAC, and age, height and weight(ln) as covariates in the male only GOOD and MrOS Sweden cohorts. Pubertal stage was also included as a covariate in the FN BMD analyses of ALSPAC.
An interaction between sex and rs1021188 in association with BMDC in the ALSPAC cohort was tested in PLINK, which tests the significance of the interaction term in the linear model.
We carried out meta-analyses using standardized betas and standard errors from each of the studies. This was carried out in METAL using the inverse-variance method described above.
sRANKL levels were transformed using the Rank-Based Inverse Normal Transformation. Linear regression was used to determine the relationship between sRANKL level and the addition of the minor rs1021188 allele.
Supporting Information
Zdroje
1. RichardsJ
RivadeneiraF
InouyeM
PastinenT
SoranzoN
2008 Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371 1505 1512 Available: http://linkinghub.elsevier.com/retrieve/pii/S0140673608605991
2. RivadeneiraF
StyrkársdottirU
EstradaK
HalldórssonBV
HsuY
2009 Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41 1199 1208 Available: http://www.nature.com/doifinder/10.1038/ng.446
3. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2008 Multiple genetic loci for bone mineral density and fractures. New Engl J Med 358 2355 2365 Available: http://www.ncbi.nlm.nih.gov/pubmed/18445777
4. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2009 New sequence variants associated with bone mineral density. Nat Genet 41 15 17 Available: http://www.ncbi.nlm.nih.gov/pubmed/19079262
5. JohnellO
KanisJA
OdenA
JohanssonH
LaetCD
2005 Predictive Value of BMD for Hip and Other Fractures. J Bone Miner Res 20 1185 1194 doi:10.1359/JBMR.050304
6. BachrachL
2001 Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrin Met 12 22 28
7. TimpsonNJ
TobiasJH
RichardsJB
SoranzoN
DuncanEL
2009 Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet 18 1510 1517 Available: http://www.ncbi.nlm.nih.gov/pubmed/19181680
8. PaternosterL
OhlssonC
SayersA
VandenputL
LorentzonM
2010 OPG and RANK polymorphisms are both associated with cortical BMD: findings from a meta-analysis of the ALSPAC and GOOD cohorts. J Clin Endocrinol Metab 95 3940 3948 Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2917784
9. BoyleWJ
SimonetWS
LaceyDL
2003 Osteoclast differentiation and activation. Nature 423 337 342 Available: http://www.nature.com/doifinder/10.1038/nature01658
10. SeemanE
DelmasP
2006 Bone Quality — The Material and Structural Basis of Bone Strength and Fragility. New Engl J Med 354 2250 2261
11. ZebazeRM
Ghasem-ZadehA
BohteA
Iuliano-BurnsS
MiramsM
2010 Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375 1729 1736 Available: http://www.ncbi.nlm.nih.gov/pubmed/20472174
12. CummingsSR
San MartinJ
McClungMR
SirisES
EastellR
2009 Denosumab for prevention of fractures in postmenopausal women with osteoporosis. New Engl J Med 361 756 765 Available: http://www.ncbi.nlm.nih.gov/pubmed/19671655
13. KostenuikPJ
NguyenHQ
McCabeJ
WarmingtonKS
KuraharaC
2009 Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res 24 182 195
14. GoldingJ
PembreyM
JonesR
2001 ALSPAC-the avon longitudinal study of parents and children. I. study methodology. Paediatr Perinat Epidemiol 15 74 87 Available: http://www3.interscience.wiley.com/journal/118982396/abstract
15. JonesRW
RingS
TyfieldL
HamvasR
SimmonsH
2000 A new human genetic resource: a DNA bank established as part of the Avon longitudinal study of pregnancy and childhood (ALSPAC). Eur J Hum Genet 8 653 660 Available: http://www.ncbi.nlm.nih.gov/pubmed/10980570
16. LiY
AbecasisGR
2006 Mach 1.0: Rapid Haplotype Reconstruction and Missing Genotype Inference. Am J Hum Genet S79 2290
17. LorentzonM
MellströmD
OhlssonC
2005 Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: the GOOD study. J Bone Miner Res 20 1936 1943
18. LorentzonM
SwansonC
AnderssonN
MellströmD
OhlssonC
2005 Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. J Bone Miner Res 20 1334 1341 Available: http://www3.interscience.wiley.com/journal/123197455/abstract
19. MellströmD
JohnellO
LjunggrenO
ErikssonA
LorentzonM
2006 Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21 529 535 Available: http://www.ncbi.nlm.nih.gov/pubmed/16598372
20. EstradaK
AbuseirisA
GrosveldFG
UitterlindenAG
KnochTA
2009 GRIMP: a web- and grid-based tool for high-speed analysis of large-scale genome-wide association using imputed data. Bioinformatics 25 2750 2752 Available: http://bioinformatics.oxfordjournals.org/cgi/content/abstract/25/20/2750
21. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet 81 559 575 Available: http://pngu.mgh.harvard.edu/purcell/plink/
Štítky
Genetika Reprodukčná medicínaČlánok vyšiel v časopise
PLOS Genetics
2010 Číslo 11
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
Najčítanejšie v tomto čísle
- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- A Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species (Green Peach Aphid)
