-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Insect cuticle is composed primarily of chitin and structural proteins. To study the function of structural cuticular proteins, we focused on the proteins present in elytra (modified forewings that become highly sclerotized and pigmented covers for the hindwings) of the red flour beetle, Tribolium castaneum. We identified two highly abundant proteins, TcCPR27 (10 kDa) and TcCPR18 (20 kDa), which are also present in pronotum and ventral abdominal cuticles. Both are members of the Rebers and Riddiford family of cuticular proteins and contain RR2 motifs. Transcripts for both genes dramatically increase in abundance at the pharate adult stage and then decline quickly thereafter. Injection of specific double-stranded RNAs for each gene into penultimate or last instar larvae had no effect on larval–larval, larval–pupal, or pupal–adult molting. The elytra of the resulting adults, however, were shorter, wrinkled, warped, fenestrated, and less rigid than those from control insects. TcCPR27-deficient insects could not fold their hindwings properly and died prematurely approximately one week after eclosion, probably because of dehydration. TcCPR18-deficient insects exhibited a similar but less dramatic phenotype. Immunolocalization studies confirmed the presence of TcCPR27 in the elytral cuticle. These results demonstrate that TcCPR27 and TcCPR18 are major structural proteins in the rigid elytral, dorsal thoracic, and ventral abdominal cuticles of the red flour beetle, and that both proteins are required for morphogenesis of the beetle's elytra.
Published in the journal: Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins. PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002682
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002682Summary
Insect cuticle is composed primarily of chitin and structural proteins. To study the function of structural cuticular proteins, we focused on the proteins present in elytra (modified forewings that become highly sclerotized and pigmented covers for the hindwings) of the red flour beetle, Tribolium castaneum. We identified two highly abundant proteins, TcCPR27 (10 kDa) and TcCPR18 (20 kDa), which are also present in pronotum and ventral abdominal cuticles. Both are members of the Rebers and Riddiford family of cuticular proteins and contain RR2 motifs. Transcripts for both genes dramatically increase in abundance at the pharate adult stage and then decline quickly thereafter. Injection of specific double-stranded RNAs for each gene into penultimate or last instar larvae had no effect on larval–larval, larval–pupal, or pupal–adult molting. The elytra of the resulting adults, however, were shorter, wrinkled, warped, fenestrated, and less rigid than those from control insects. TcCPR27-deficient insects could not fold their hindwings properly and died prematurely approximately one week after eclosion, probably because of dehydration. TcCPR18-deficient insects exhibited a similar but less dramatic phenotype. Immunolocalization studies confirmed the presence of TcCPR27 in the elytral cuticle. These results demonstrate that TcCPR27 and TcCPR18 are major structural proteins in the rigid elytral, dorsal thoracic, and ventral abdominal cuticles of the red flour beetle, and that both proteins are required for morphogenesis of the beetle's elytra.
Introduction
How arthropods manufacture exoskeletons with a wide array of mechanical properties, ranging from hard and rigid to soft and flexible, is an important question in developmental biology. The insect exoskeleton, or cuticle, covers the entire body wall and attached appendages as well as the foregut, hindgut and tracheae. It is a complex extracellular biocomposite, secreted by the epidermis and consisting of several functional layers including the waterproofing envelope, the protein-rich epicuticle and the chitin-rich procuticle [1]. Cuticular proteins (CPs) and the polysaccharide chitin are the primary structural components of the exo - and endocuticular layers that comprise the procuticle. During cuticle maturation and tanning (sclerotization and pigmentation), some of the CPs are post-translationally modified and cross-linked by quinones or quinone methides produced by the laccase-mediated oxidation of N-acylcatechols [2], [3]. This process stabilizes and hardens the exoskeleton, protecting insects from microbial, physical and other environmental stresses. However, little is known about the functional importance of individual insect cuticular proteins in the morphogenesis and mechanical properties of the exoskeleton.
More than 100 genes encoding CP-like proteins have been identified in the fruit fly, Drosophila melanogaster [4], with a similar number present in the red flour beetle, Tribolium castaneum [5]. Anopheles gambiae (malaria mosquito) and Bombyx mori (oriental silkworm) have an even larger number of genes encoding CP-like proteins, each species harboring more than 200 putative CP genes [6]–[10]. Expression of specific CPs may be required to produce cuticles with a range of morphological and mechanical properties in different regions of the insect body and at different developmental stages.
Insect CPs are classified into several distinct families defined by the presence of specific sequence motifs [7], [10]. The largest of these is the CPR family, which includes proteins that have a conserved amino acid sequence known as the Rebers & Riddiford (R&R) motif [11]. The R&R motif contains a putative chitin-binding domain that may help to coordinate the interactions between chitin fibers and the proteinaceous matrix [12], [13].
A major event in the evolution and diversification of beetles was the transformation of the membranous forewings into thickened, hardened, non-flight covers (elytra) for protection of the delicate hindwings and dorsal abdomen [14]–[16]. The elytron is composed of ventral and dorsal layers of epidermal cells that secrete thin lower and thicker upper cuticular laminae [17], [18]. In the developing elytron, the space between these two layers is filled with hemolymph and supporting structures known as trabeculae that function as mechanical struts, connecting and fortifying the dorsal and ventral cuticular layers. As the elytron matures, the epidermal layers are reduced in size, possibly dying or fusing together, and the hemolymph is resorbed, leaving a cavernous interior. The dorsal layer of the elytron becomes highly tanned and rigid as a result of both pigmentation and sclerotization. The ventral layer also exhibits some pigmentation, but it remains thin and membranous in comparison to the dorsal layer. The surface of the ventral elytral cuticle is relatively smooth and makes close contact with the underlying and folded hindwings. The surface of the dorsal elytral cuticle, on the other hand, contains numerous sensory setae and rib-like structures (striae) that extend longitudinally, apparently adding rigidity to the structure [19]. Initially, the elytra are short, colorless and soft, but they expand in both length and width shortly after eclosion, and subsequently harden and darken. A similar cuticle tanning process occurs in most of the adult body wall.
In this study we have identified two highly abundant proteins that are present in rigid cuticle of the elytron, pronotum and ventral abdomen but not in the flexible cuticle of the dorsal abdomen and hindwing of T. castaneum adults, characterized their genes and expression profiles, and analyzed their roles in adult cuticle formation and stabilization. We have also determined that these two CPR proteins are essential structural components in the sclerotized dorsal cuticle of the elytron and are required for normal morphological, functional and mechanical properties.
Results
Elytra from T. castaneum contain two predominant cuticular proteins
Extracts of untanned elytra dissected from newly emerged adults contained two highly abundant proteins with apparent sizes of 10 and 20 kDa based on their electrophoretic mobilities (Figure 1). To characterize these major proteins further, each was digested with trypsin, and the resulting peptides were analyzed by MALDI-TOF/TOF mass spectrometry. Comparison of these results with conceptual trypsinization of the computed proteome of T. castaneum revealed two candidate genes, denoted as TcCPR27 (XP_971678) and TcCPR18 (XP_967633), which are members of the Rebers and Riddiford family of cuticular proteins (Figure 1, Table S1 and cuticle DB: http://biophysics.biol.uoa.gr/cuticleDB). Peptide coverages for TcCPR27 and TcCPR18 were 68.2 and 88.7%, respectively (Figure S1).
Fig. 1. Identification of two major elytral cuticle proteins from T. castaneum. 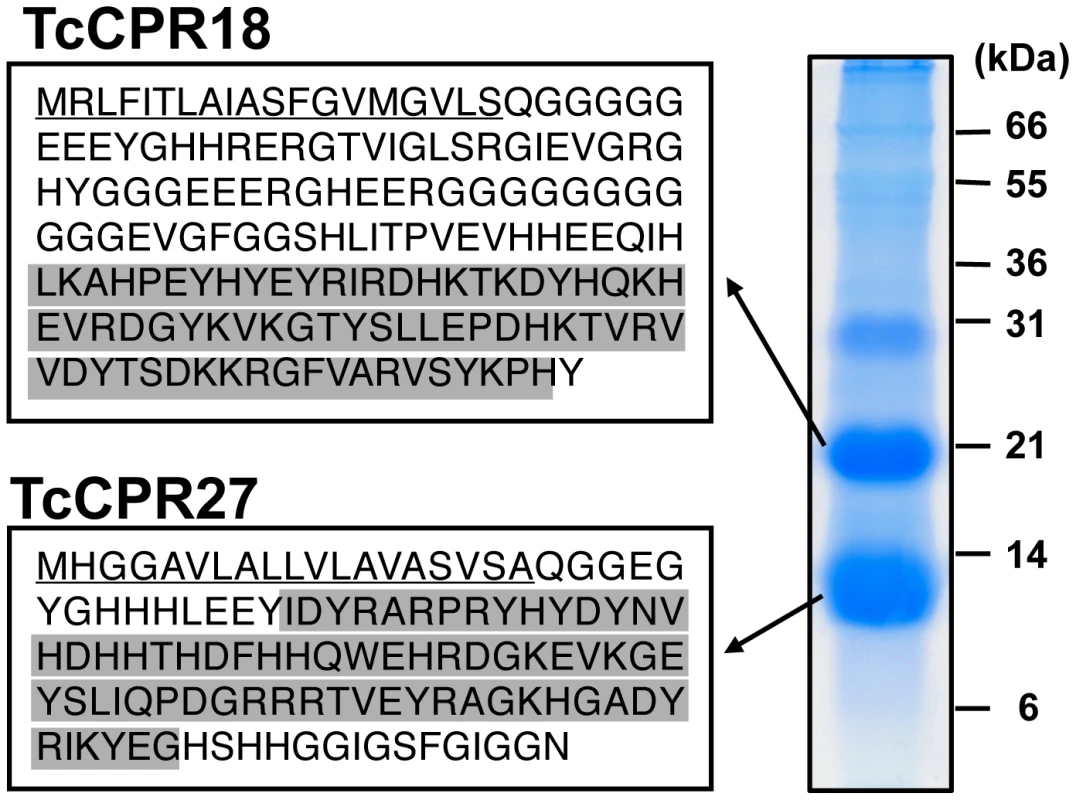
Extracts of SDS-soluble proteins from elytra dissected from newly emerged adults were analyzed by SDS-PAGE. The two major proteins (subsequently named TcCPR27 and TcCPR18), with apparent sizes of 10 and 20 kDa, respectively, were digested with trypsin, and the resulting peptides were analyzed by MALDI-TOF mass spectrometry (see Figure S1 and Table S1). Amino acid sequences deduced from cloned cDNA sequences for each protein are shown on the left. Both proteins contain an RR-2 motif (highlighted in gray). Predicted secretion signal peptides are underlined. In both TcCPR27 and TcCPR18, the amino-terminal residue of the mature forms after secretion (Gln 20) is apparently modified to pyroglutamic acid (Table S1). We cloned cDNAs corresponding to these cuticular protein genes (accession numbers HQ634478 and HQ634479). The cDNA sequence of TcCPR27 was identical to that of the NCBI RefSeq gene prediction, whereas the RefSeq prediction for TcCPR18 had one in-frame deletion of three consecutive nucleotides and a single nucleotide mismatch compared to the cDNA, resulting in a deletion of one amino acid (one of the twelve consecutive glycines at amino acid positions 65–76 in the RefSeq prediction) and a phenylalanine-to-leucine substitution at amino acid position 85 in the RefSeq prediction. TcCPR27 and TcCPR18 encode proteins containing putative secretion signal peptide sequences, with theoretical molecular masses for the mature proteins of 11.4 and 16.4 kDa, respectively.
Each mature protein contains a single RR-2 cuticular protein motif. Nearly all RR-2 proteins have a consensus region as follows: G-X(8)-G-X(6)-Y-X(6)-GF [7]. Both of these Tribolium proteins, however, have a slightly different RR2 motif. TcCPR27 contains G-X(8)-G-X(6)-Y-X(5)-GA, whereas TcCPR18 has G-X(8)-H-X(7)-Y-X(6)-GF. The former has a GA rather than the almost universal GF or GY at the end of the consensus, and the conserved Y and the third G are interrupted by five amino acids instead of the typical six. In the case of TcCPR18, the second conserved G is replaced by an H with no G residue located nearby.
TcCPR18 is rich in glycine (21.6%), whereas TcCPR27 has a high content of both glycine (16.5%) and histidine (15.5%). TcCPR18 is an apparent ortholog of the ecdysteroid-regulated, adult-specific cuticle protein acp22 of Tenebrio molitor (yellow mealworm), with 67% sequence identity (Figure S2) [20]. Both TcCPR27 and TcCPR18 map to linkage group 3 of the T. castaneum genome, but they are not tightly linked (BeetleBase: http://beetlebase.org) [21]. Elytra of three other Tribolium species, T. brevicornis, T. confusum and T. freemani, also contain predominant cuticular proteins with high amino acid sequence similarities to TcCPR27 and TcCPR18 (Figure S3 and Table S2).
TcCPR27 and TcCPR18 are abundantly expressed in rigid cuticle but not in flexible cuticle
To investigate whether TcCPR27 and TcCPR18 are present in other regions of the adult cuticle, we extracted proteins from cuticular samples dissected from the pronotum and the ventral abdomen just after adult eclosion. As in the case of the elytra, TcCPR27 and TcCPR18 proteins were also the predominant protein constituents of the pronotum, although their yields were low relative to those obtained from the elytra (Figure 2). We hypothesized that because the extent of tanning of the pronotum just after eclosion is substantially greater than that of the elytron, which tans at a later time (Figure 2), pronotum cuticular proteins were already cross-linked and much less extractable at the time of adult eclosion. To delay pronotum cuticle tanning, dsRNA for laccase-2 (dsTcLac2) was injected into 0–1 d-old pupae [2], and the pronotum cuticular proteins were subsequently extracted from samples obtained soon after adult eclosion. The yields of TcCPR27 and TcCPR18 were much higher in those extracts, indicating that the two proteins had not undergone substantial cross-linking in the absence of laccase and therefore were more readily extractable (Figure 2).
Fig. 2. TcCPR27 and TcCPR18 are abundant in cuticle of the pronotum. 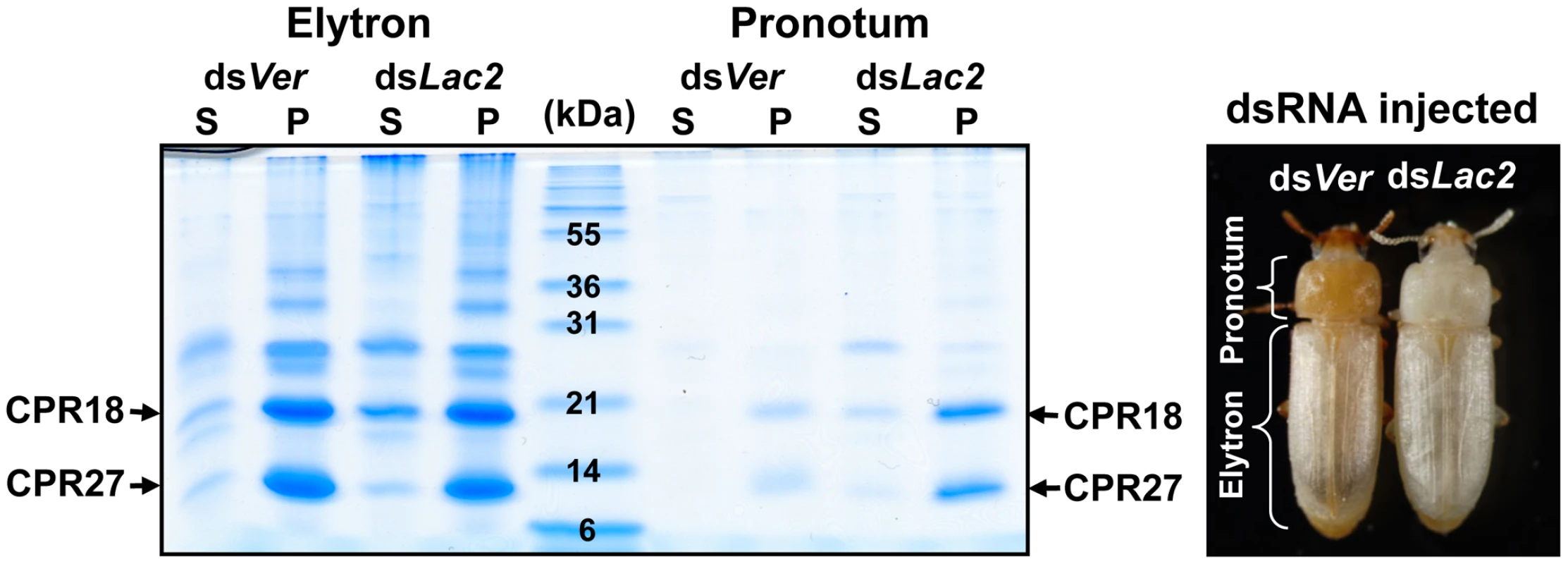
To suppress cuticle tanning, dsRNA for TcLac2 (2 ng per insect) was injected into day 0 pupae. Proteins from the pronotum and elytra were extracted from five adults at 0–30 min after eclosion, and the extracts were analyzed by SDS-PAGE (left panel). In the dsVer-treated control animals, the extent of tanning of the pronotum was substantially greater than that of the elytron (right panel). S: PBS homogenate supernatant, P: PBS homogenate pellet. Like the elytron, the adult ventral abdominal cuticle undergoes tanning and becomes hardened 3–5 d post eclosion, whereas the dorsal abdominal cuticle in the adult remains relatively untanned, flexible and transparent like the hindwing. TcCPR27 and TcCPR18 were abundant in extracts recovered from ventral abdominal cuticle of newly emerged adults, but very little or no TcCPR27 or TcCPR18 was present in extracts of the dorsal abdominal cuticle (Figure S4A). Similarly, the levels of TcCPR27 and TcCPR18 in the flexible hindwing were very low or undetectable (Figure S4A). These results show that TcCPR27 and TcCPR18 are major proteins in cuticles that become highly sclerotized and rigid, but they are absent or only very minor components of cuticles that are more flexible and membranous.
TcCPR27 and TcCPR18 genes are expressed at high levels in body regions with rigid adult cuticle
Few or no transcripts for TcCPR27 or TcCPR18 were detected during the egg, larval or early pupal stages of development. However, the transcript levels of these genes dramatically increased at the pharate adult stage 0–1 d before eclosion, declining soon thereafter (Figure 3A, 3B). Transcript levels of TcCPR27 and TcCPR18 in the elytron were approximately 1,700 - and 55-fold higher, respectively, than those in the membranous hindwing (Figure S4B). Both genes were also expressed in the pronotum and ventral abdomen, whose cuticles become highly sclerotized and hardened in mature adults. Transcript levels of TcCPR27 and TcCPR18 in the ventral abdomen were approximately 3,000 and 770 times higher, respectively, than the levels in the transparent, flexible and membranous dorsal abdomen (Figure S4B).
Fig. 3. Expression profiles of TcCPR27 and TcCPR18 genes during development. 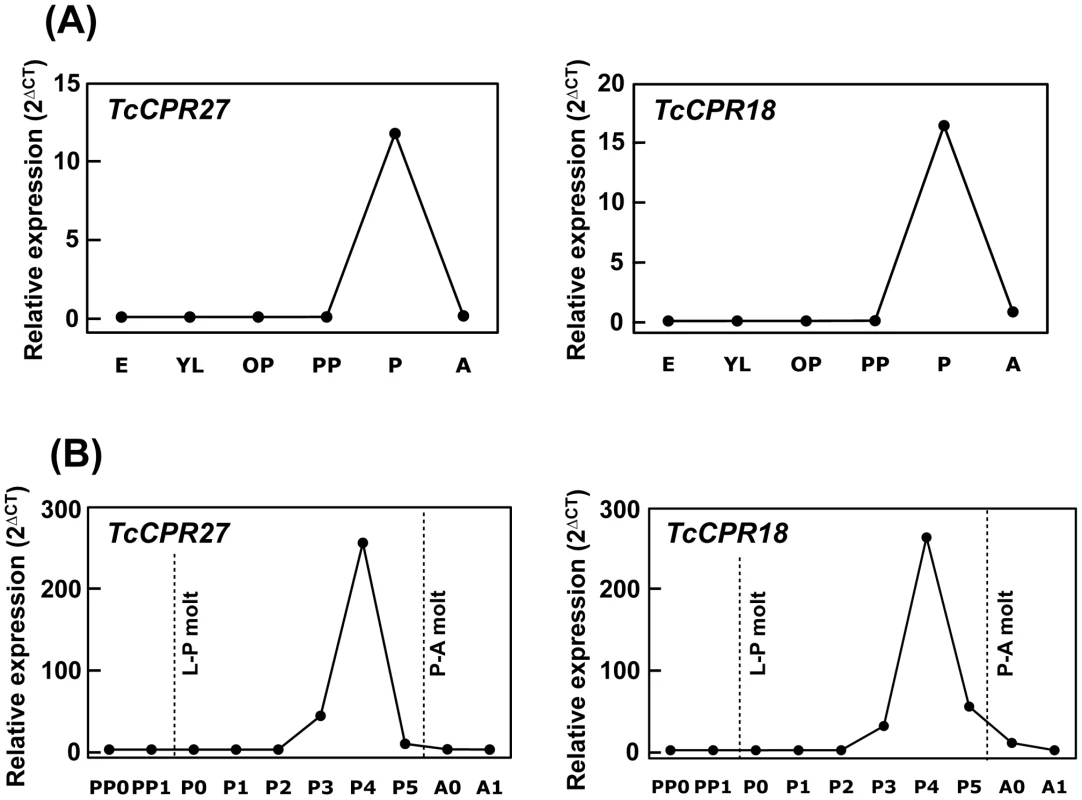
(A) To analyze the expression profiles of TcCPR27 and TcCPR18 during development, real-time PCR experiments were done using total RNA extracted from five whole insects at different developmental stages (embryo to adult). Both genes were highly expressed at the pupal stage. (B) To analyze more rigorously the expression patterns of these genes, the stages analyzed were expanded between the early pharate pupal and young adult stages. The transcript levels of both genes dramatically increased at the pharate adult stage and declined rapidly thereafter. E, embryos; YL, young larvae; ML, mature larvae; PP, pharate pupae; P, pupae; A, adults; PP0, day 0–1 pharate pupae; PP1, day 1–2 pharate pupae; P0, day 0 pupae; P1, day 1 pupae, P2, day 2 pupae; P3, day 3 pupae; P4, day 4 pupae (pharate adults); P5, day 5 pupae (pharate adults); A0, day 0 adults; A1, day 7 adults. Expression levels for TcCPR27 and TcCPR18 are presented relative to the levels of expression in embryos (E) or 0–1 d old pharate pupae (PP0). The transcript levels of the T. castaneum ribosomal protein S6 (rpS6) were measured to normalize for differences between samples in the concentrations of cDNA templates. TcCPR27 is localized in rigid cuticle of the dorsal elytra and ventral abdomen
The high histidine content of TcCPR27 and TcCPR18 (15.5% and 10.1%, respectively) allowed us to purify these proteins from elytra dissected from newly molted adults by utilizing nickel-affinity chromatography (Figure 4A). A polyclonal antibody directed against purified TcCPR27 was then generated. The CPR27 antibody specifically detected CPR27 but not CPR18 (Figure 4A). In pharate adults, TcCRP27 was co-localized with chitin in the dorsal elytral cuticle as well as in the ventral abdominal cuticle, both of which become more rigid and darker as the adult matures (Figure 4B, panels 1, 3). Little or no TcCRP27 immunoreactivity was detected in the pupal, hindwing, or ventral elytral cuticles.
Fig. 4. Immunolocalization of TcCPR27 in elytral cuticle. 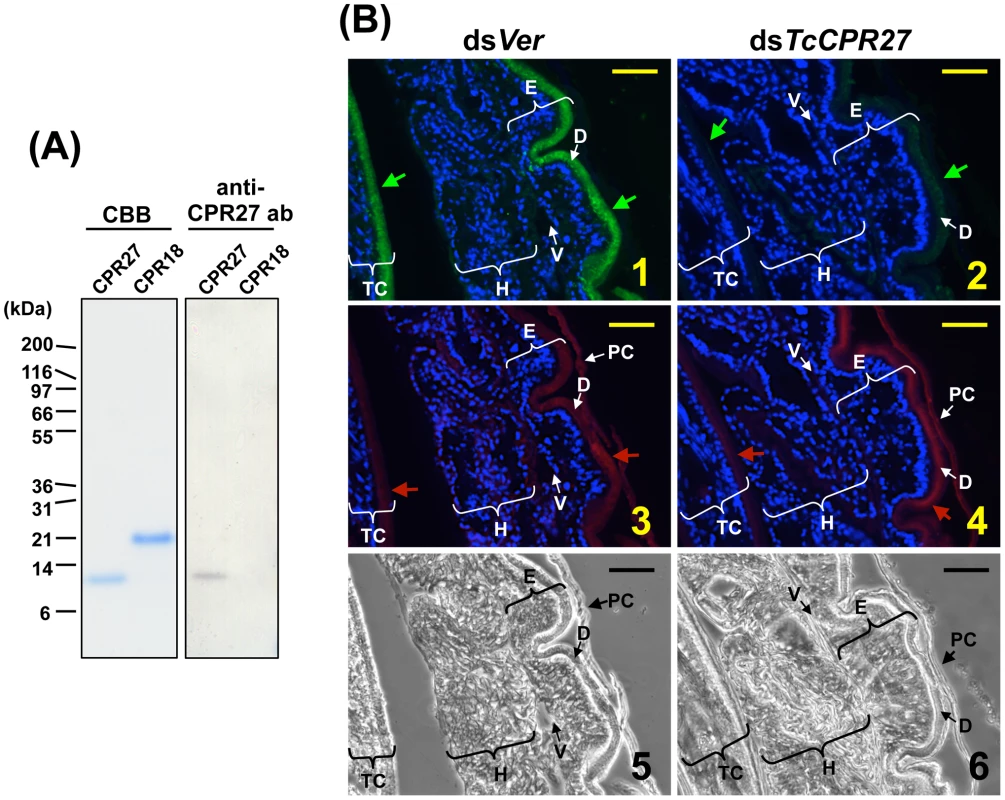
(A) Coomassie staining and immunoblot analyses of purified TcCPR27 and TcCRP18. (B) Immunolocalization of TcCPR27 in pharate adults. Cryosections (5–10 µm) of 5 d old pupae that had been injected previously with dsRNA for TcCPR27 or TcVer (T. castaneum Vermilion) in the last larval instar were incubated with the anti-TcCPR27 antibody. Anti-TcCPR27 antibody was detected by Alexa Fluor 488-conjugated anti-rabbit IgG antibody (green arrows in panels 1 and 2). The same sections were also stained with a rhodamine-conjugated chitin-binding probe (red arrows in panels 3 and 4) [41]. Nuclei were stained with DAPI (blue). E = elytron, H = hindwing, TC = thoracic cuticle, PC = pupal cuticle, D = elytral dorsal cuticle, V = elytral ventral cuticle. Scale bar = 10 µm. RNAi–mediated knockdown of TcCPR27 and TcCPR18 expression leads to malformed and weakened elytra
RNA interference (RNAi) was used to investigate the functions of TcCPR27 and TcCPR18. As a negative control we injected dsRNA for T. castaneum tryptophan oxygenase (the vermilion gene, abbreviated Ver), a gene required for normal eye pigmentation [22]. Following dsRNA injections into last instar larvae, mRNA and protein levels of TcCPR27 and TcCPR18 were analyzed by real-time PCR and SDS-PAGE. Injection of these dsRNAs led to substantial and specific down-regulation of each gene at the mRNA (Figure 5A) and protein (Figure 5B) levels. TcCPR27 immunostaining also was strongly reduced after injection of dsTcCPR27 (Figure 4B, panel 2). Chitin staining in TcCPR27-deficient insects, however, was detected at approximately the same level as in dsVer-treated control animals (Figure 4B, panel 4). These results were further supported by staining of elytral chitin with a fluorescent chitin-labeling reagent, FITC-CBD. There was no difference in chitin staining among elytra collected from dsTcCPR27-, dsTcCPR18 - and dsVer-treated insects (Figure S5).
Fig. 5. Knockdown of TcCPR27 and TcCPR18 transcripts by RNAi. 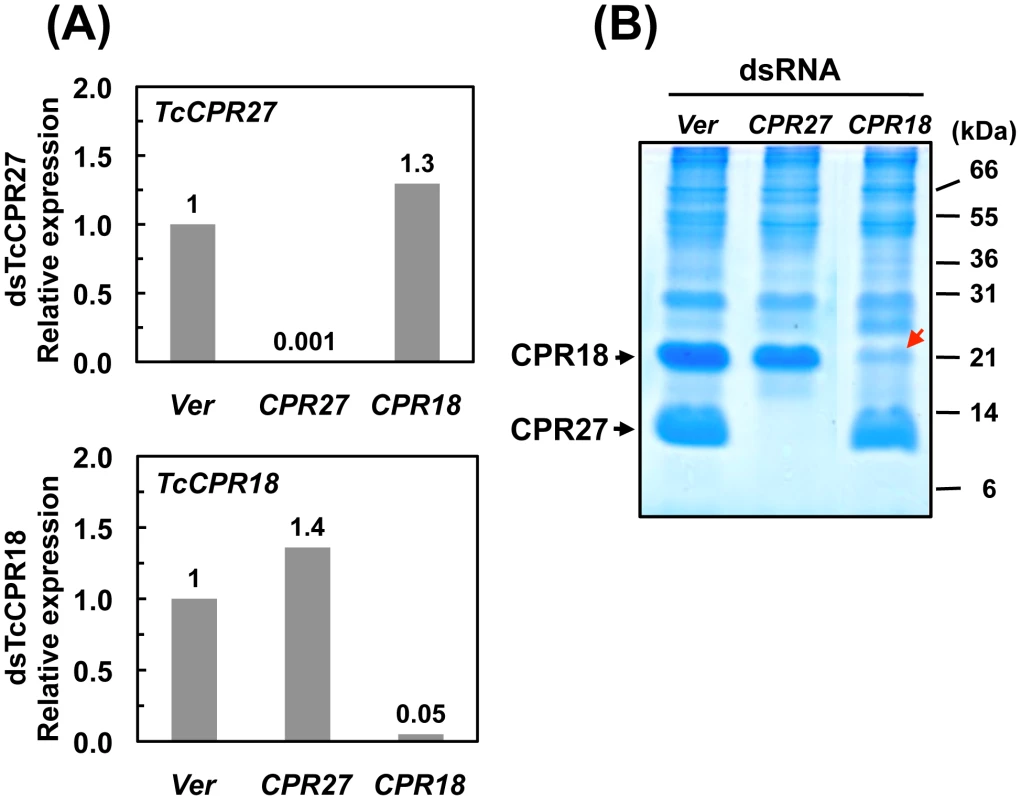
Late instar T. castaneum larvae were injected with 0.2 µg of dsRNA for TcCPR27 or TcCPR18. Following dsRNA injections, expression of TcCPR27 and TcCPR18 genes was analyzed by real-time PCR (A) and SDS-PAGE (B) to evaluate transcript and protein levels. cDNAs were prepared from total RNA isolated from five whole insects at pupal day 5 (10 d post-injection). For real-time PCR, expression levels of TcCPR27 and TcCPR18 are presented relative to the levels in Ver control insects (injected with dsRNA for T. castaneum Vermilion gene). The transcript levels of the T. castaneum ribosomal protein S6 (rpS6) were measured to normalize for differences between samples in the concentrations of cDNA templates. Proteins were extracted from elytra from five newly emerged adults for each treatment. A faint band (red arrow) with a mobility similar to that of TcCPR18 observed in extracts of whole insects injected with dsRNA for TcCPR18 was identified by mapping of tryptic peptides by MALDI-TOF to be a different CPR RR2 protein, TcCPR33, with a theoretical mass of 19.1 kDa. These data indicate that both TcCPR27, TcCPR18 were specifically down-regulated at both the mRNA and protein levels after dsRNA injections. Injection of dsTcCPR27 or dsTcCPR18 into larvae had no apparent effect on larval-pupal or pupal-adult molting or on the morphology of the pupal cuticle, as expected from the observed late pupa-specific expression of these genes. However, the elytra of the resulting adults were malformed (Figure 6 and Figure S6). The surface of the elytra of dsTcCPR18-treated adults was irregular and rough compared to those of control insects. Adults derived from dsTcCPR27-injected larvae exhibited even more severe morphological defects. Their elytra were very short, wrinkled, warped and fenestrated (Figure 6 and Figure S6) and their hindwings were unable to fold normally. Such insects died approximately one week after eclosion, apparently from dehydration that resulted from failure of the misshapen elytra to properly cover the membranous dorsal abdomen and to thereby seal it against trans-cuticular water loss. The shape of a normal elytron is “inverted boat-like” to fit snugly on top of the hindwings and abdomen in order to protect the latter structures (Figure S6). In contrast, elytra from dsTcCPR27-treated insects were flatter and/or warped and did not cover the entire abdomen. Manual excision of the distal half of the elytron from a mature adult also led to high mortality, whereas removing an entire hindwing did not cause significant mortality, as long as the elytra could adopt their normal juxtaposition (Figure S7), consistent with our observation that properly formed elytra are essential to prevent desiccation of the adult, in addition to other potentially protective functions. These results support the hypothesis that TcCPR27 and TcCPR18 are major structural proteins in rigid elytral cuticle, and are required for normal elytral morphogenesis, hindwing folding and body hydration.
Fig. 6. T. castaneum elytral defects produced by injection of dsRNAs for TcCPR27 and TcCPR18. 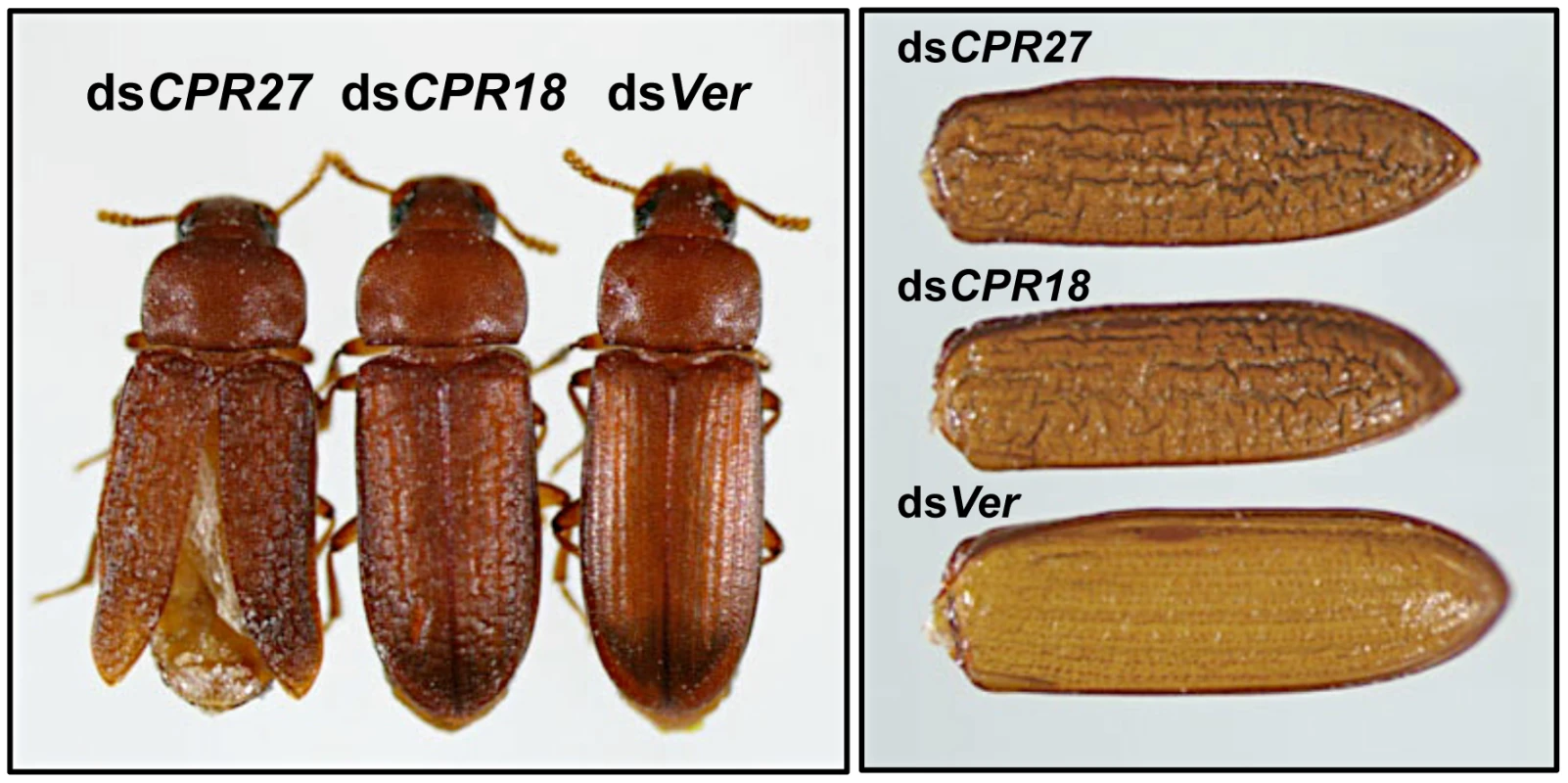
To investigate the functions of TcCPR27 and TcCPR18, specific dsRNAs for TcCPR27 (dsCPR27) or TcCPR18 (dsCPR18) (0.2 µg per insect) were injected into late instar larvae. Dorsal views of the resulting adults (1-d old) (left panel) and elytra (right panel) are shown. dsRNA for Ver (dsVer) was injected to serve as a negative control. Effect of dsRNA for TcCPR27 and TcCPR18 on the mechanical properties of elytra
We also analyzed the effects of depletion of TcCPR27 and TcCPR18 on the mechanical properties of elytra. Dynamic mechanical experiments were carried out to determine the storage modulus E′ and the loss modulus E″ of elytra as a function of oscillation frequency and strain. E′ is a measure of the elastically recoverable deformation energy, whereas E″ is a measure of viscous energy dissipation (dampening) and hence is also known as the viscous modulus. The ratio E″/E′ is known as the “loss tangent” or simply tan δ, where δ is the phase angle between sinusoidally applied stress and strain. For materials such as the elytra where E′>>E″, E′ is approximately equal to the Young's modulus obtained from the slope of simple stress-strain measurements at the same strain rate [23], [24]. Hence, E′ is a measure of the stiffness of the elytra. Elytra from animals injected with dsTcCPR27 were significantly less rigid (lower E′) than the dsVer-treated controls (Figure 7). Elytra from dsTcCPR18-injected beetles had intermediate values of strength, consistent with the less severe visible phenotype observed with TcCPR18 knockdown (Figure 6). In addition, the dsTcCPR27 elytra had reduced values for tan δ, an indication that they experienced a higher degree of cross-linking than the control. Lower E″ or tan δ in polymeric networks is typically associated with a reduction in the network of uncross-linked material, dangling chains (chains linked to the network at only one end), loops and other network imperfections [25]. It is a well-established principle in the synthesis of gels or networks by cross-linking polymers, that increasing the ratio of cross-linker molecules to polymer molecules will typically increase the overall cross-link density of a network and reduce the fraction that is not cross-linked [26]. Thus, for elytral cuticle, the deficiency of a major structural cuticular protein such as TcCPR27, while maintaining a constant concentration of quinone cross-linking molecules, would be expected to lead to a greater average number of cross-links per protein molecule. Thus, for elytral cuticle, reducing the expression level of a major structural cuticular protein such as TcCPR27 or TcCPR18, while maintaining a constant concentration of quinone cross-linking molecules, would be expected to lead to a greater average number of cross-links per protein molecule. The more severe phenotype for knockdown of TcCPR27, relative to TcCPR18 might be due to differences in the degree of knockdown of their expression in the RNAi experiments, or perhaps could be due to differences in their structural properties or cross-linking chemistry. A greater reduction in protein levels for TcCPR27 than TcCPR18 in the knockdown animals might have led to relatively more cross-linking, thus reducing tan δ, and a greater reduction in TcCPR27 protein expression would lead to a lower storage modulus. Similar observations were previously reported for elytra from insects subjected to Lac2 knockdown (reduced tan δ combined with reduced E′) [19].
Fig. 7. Mechanical properties of Tribolium dsTcCPR27 and dsTcCPR18 elytra. 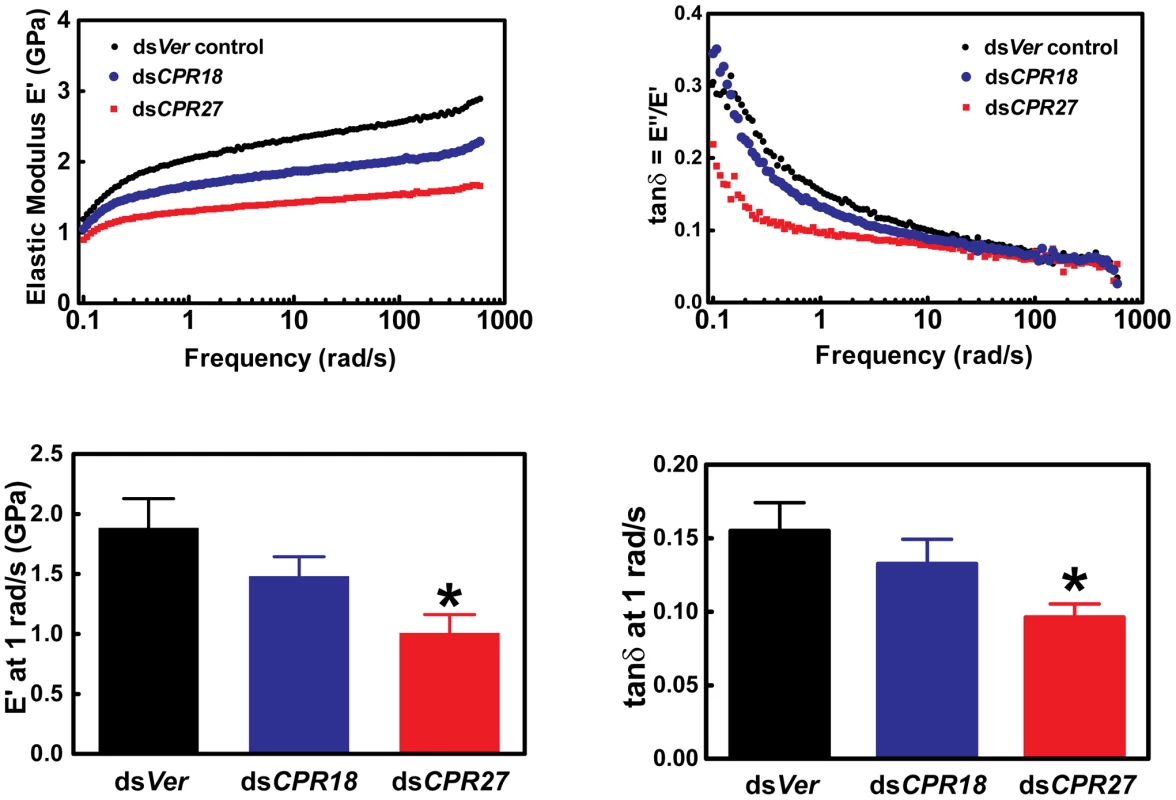
Elytra from beetles on day 2 after adult eclosion were examined by dynamic mechanical analysis over a frequency range of 0.1 to 600 rad/s. Typical scans are shown in the top panels, and mean values at 1 rad/s are presented in the lower panels. Error bars represent standard deviation (n = 3–4). The asterisk indicates significant difference from the dsVer control (p<0.05) as determined by analysis of variance and Tukey's Multiple Comparison Test. Reduced expression of the abundant CPR proteins resulted in weaker (smaller E′) yet more cross-linked (smaller tan δ) elytra. Discussion
The basic genetic patterning mechanism for dorsal appendages such as wings has been described for both D. melanogaster and T. castaneum [27]–[29]. Similar gene networks are used to pattern wings, elytra and halteres, despite the profound morphological and functional divergence of these appendages during insect evolution. Individual structural proteins are likely to substantially affect the physical properties of elytra. However, to date, there have been no detailed reports about the contributions of individual structural proteins to elytral morphogenesis.
Like other beetle species, T. castaneum adults possess elytra, modified forewings with a highly sclerotized and pigmented dorsal cuticle. Immediately after eclosion, untanned elytra have a soft white cuticle. Elytra expand shortly thereafter and then become rigid and darker during cuticle maturation. The role of structural proteins in this developmental process is the focus of this study. We identified two proteins from the RR2 family of cuticular proteins, TcCPR27 and TcCPR18, which are highly abundant in protein extracts of elytra dissected from newly emerged adults. TcCPR27 and TcCPR18 transcripts were strongly up-regulated at the developmental stage when adult cuticular proteins are expected to be synthesized (pupal day 4), and were nearly absent at earlier immature stages. Highly abundant cuticular proteins related to TcCPR27 and TcCPR18 were also present in protein extracts of elytra dissected from three other Tribolium species including T. brevicornis, T. confusum and T. freemani (Figure S3 and Table S2).
TcCPR27 and TcCPR18 were also identified in extracts of pronotum cuticle, in which tanning had been initiated before adult eclosion. The protein yields, however, were much lower than those obtained from elytral extracts unless cuticle tanning was suppressed by injection of dsRNA for the tanning enzyme TcLac2 (Figure 2). These results support the hypothesis that TcCPR27 and TcCPR18 are cross-linked by highly reactive quinones and quinone methides that are produced by the cuticle tanning phenoloxidase laccase-2, and that these proteins become inextractable after tanning has occurred. Previously, Missios et al. [30] extracted two major cuticular proteins of 10 and 20 kDa, consistent with the apparent masses of TcCPR27 and TcCPR18, respectively, from extracts of cuticle from whole bodies of newly eclosed T. castaneum adults. Neither of these proteins was extractable from 7 day-old adults, consistent with an interpretation that these proteins become cross-linked during maturation of the cuticle.
To study the functions of TcCPR27 and TcCPR18, we performed RNAi and successfully down-regulated levels of TcCPR27 and TcCPR18 mRNAs and proteins (Figure 5). These deficiencies caused several elytral defects. Although TcCPR27 and TcCPR18 are also present in other body regions such as the cuticles of the pronotum and ventral abdomen, which are heavily tanned in the mature adult, we did not observe visible phenotypic changes in those cuticles after injection of dsTcCPR27 and dsTcCPR18. The size and shape of these body regions do not change much after adult eclosion, in contrast to the elytra that are greatly expanded shortly after eclosion. The elytra of both TcCPR27 - and TcCPR18-deficient insects failed to fully expand, and their dorsal surfaces were not smooth (Figure S6). The elytra of TcCPR27-deficient insects, particularly, were very short, wrinkled and fragile. Elytra from dsTcCPR27 - and dsTcCPR18 - treated insects appear to contain more cross-linked proteins than the elytra from dsVer-treated control insects (Figure 6). Lacking these major cuticular proteins apparently increases the effective concentration of cross-linking agents (NADA and NBAD quinones), resulting in aberrant cross-linking among the remaining proteins and shortened warped elytra. The effect was seen most clearly in dsTcCPR27-treated insects, in which the modulus and tan δ were significantly reduced relative to control insects. The dsTcCPR18 - treated insects had a smaller decrease in modulus and tan δ, perhaps because of a smaller degree of protein reduction. The difference could also arise from structural differences between TcCPR27 and TcCPR18, which could have different propensities for forming intermolecular vs. intramolecular cross-links. However, the present data cannot draw that level of distinction. All of these results suggest that TcCPR27 and TcCPR18 are critical for normal elytral morphogenesis and are required to prevent dehydration and death of the adult.
In summary, we have identified in beetles two major structural proteins, TcCPR27 and TcCPR18, which account for approximately half of the extractable cuticular proteins in the elytra and also are major components of other hard cuticular structures. It is interesting to note that the proteins utilized for hard cuticles of other body regions of the beetle were apparently used to build the elytron's hard cuticle. In some saturniid moth species, proteins from the same CPR family are also used to form rigid structures such as tubercles, head capsules and hard pupal cuticle [31]. We now have biomechanical evidence on just how important these kinds of proteins are.
TcCPR27 and TcCPR18 are required not only for rigid cuticle development, but also for morphogenesis, elytral mechanical properties, and survival of the red flour beetle. In contrast, these proteins are essentially undetectable in soft cuticles. Expression of such cuticular proteins in the modified forewings appears to be a fundamental evolutionary step in transforming the flexible and thin membranous wing into a thickened and rigid elytron in the Coleoptera. In the case of TcCPR18, an orthologous gene is found in the only other beetle species examined, the lesser grain borer, Rhyzopertha dominca, in the family Bostrichidae (Schlipalius, D. and Beeman, R. W., unpublished observations) but not in any of the other sequenced arthropod genomes, including representatives of the Diptera, Hymenoptera and Lepidoptera. These structural proteins are probably cross-linked during sclerotization, via formation of histidyl-catechol adducts [32], [33]. Rigidification of the beetle forewing has likely been achieved in part through both structural protein incorporation and multiple co-options of the sclerotization pathway acting downstream of conserved wing gene network components, with the final product being primarily a rigid interpenetrating network of chitin embedded in a cross-linked protein matrix [3], [29], [34], [35]. To gain a more comprehensive understanding of the roles of cuticular proteins in defining the morphology and properties of the beetle elytron and rigid body wall cuticle, future studies are required to determine, at the ultrastructural level, the precise localization of TcCPR27, TcCPR18 and other structural proteins, and to assess the nature and extent of their covalent cross-linking during sclerotization.
Materials and Methods
Insects
The GA-1 strain of T. castaneum was used in this study. Beetles were reared at 30°C under standard conditions [36].
Protein extraction and identification
Elytra of newly emerged adults (n = 10) were homogenized in 100 µl of cold PBS containing protease inhibitor cocktail (Roche). The homogenate was centrifuged for 2 min at 4°C. The supernatant was collected as PBS soluble fraction. The pellet was homogenized in 100 µl of SDS-PAGE sample buffer, heated at 95°C for 10 min, centrifuged for 2 min. The supernatant was collected as PBS pellet fraction. Protein extracts were analyzed by 15% SDS-PAGE or 4–12% Bis-Tris gradient gel (Invitrogen). Proteins were digested with trypsin, and the resulting fragments were analyzed by MALDI-TOF mass spectrometry.
Identification of proteins by mass spectrometry
After staining gels with Coomassie G-250, the selected gel band was excised as 1–2 mm diameter pieces and transferred to a 1.5 mL Eppendorf tube. A protein-free region of the gel was also excised as background control. The control and test gel sections were destained using three 30 min washes of 60 µL 1∶1 acetonitrile: water at 30°C. Gel pieces were then dried for 10 min under vacuum. The gel sections were subjected to reduction and alkylation using 50 mM Tris (2-carboxyethyl) phosphine (TCEP) at 55°C for 10 min followed by 100 mM iodoacetamide in the dark at 30°C for 60 min. The carboxymethylated gels were thoroughly washed and re-dried in vacuo, then incubated with sequencing grade trypsin (Trypsin Gold, Promega, Madison, WI), 20 ng/µL in 40 mM ammonium bicarbonate, in 20 µL. Upon rehydration of the gels, an additional 15 µL of 40 mM ammonium bicarbonate and 10% acetonitrile was added, and gel sections were incubated at 30°C for 17 h in sealed Eppendorf tubes. The aqueous digestion solutions were transferred to clean 1.5 mL Eppendorf tubes, and tryptic fragments remaining within the gel sections were recovered by a single extraction with 50 µl of 50% acetonitrile and 2% trifluoracetic acid (TFA) at 30°C for 1 h. The acetonitrile fractions were combined with previous aqueous fractions and the liquid was removed by speed vacuum concentration. The dried samples were resuspended in 10 µL of 30 mg/mL 2,5-dihydroxylbenzonic acid (DHB) (Sigma, St. Louis, MO) dissolved in 33% acetonitrile/0.1% TFA and 2 µL of peptide/matrix solution was applied on a Bruker Massive Aluminum plate for MALDI-TOF and TOF/TOF analysis. Mass spectra and tandem mass spectra were obtained on a Bruker Ultraflex II TOF/TOF mass spectrometer. Positively charged ions were analyzed in the reflector mode. MS and MS/MS spectra were analyzed with Flex analysis 3.0 and Bio Tools 3.0 software (Bruker Daltonics). Measurements were externally calibrated with 8 different peptides ranging from 757.39 to 3147.47 (Peptide Calibration Standard I, Bruker Daltonics) and internally recalibrated with peptides from the autoproteolysis of trypsin. Peptide ion searches were performed with Beetlebase (http://www.bioinformatics.ksu.edu/BeetleBase/) (as well as Metazoa domain_201000104 in NCBInr database) using MASCOT software (Matrix Science). The following parameters were used for the database search: MS and MS/MS accuracies were set to <0.5 Da, trypsin/P as an enzyme, missed cleavages 1, carbamidomethylation of cysteine as fixed modification, and oxidation of methionine as a variable modification. Sequence motif analysis of the predicted protein sequence was searched in motifs database including PROSITE profiles and Pfam HMMs.
TcCPR27 and TcCPR18 cDNAs
The full-length coding sequences for TcCPR27 and TcCPR18 (351 bp and 504 bp, respectively) were amplified from total RNA extracted from pupae (mixture of 0 d - to 5 d-old pupae) by RT-PCR. The cDNAs for TcCPR27 and TcCPR18 were amplified using the following gene specific primers, which included predicted start and stop codons: 5′ ATG CAC GGT GGA GCA GTT C 3′ and 5′ TCA GTT GCC TCC AAT CCC G 3′ for TcCPR27, and 5′ ATG AGA TTA TTT ATT ACA TTG GCC 3′ and 5′ CTA GAT TAA TAA TGT GGT TTG TAA G 3′ for TcCRP18. PCR products were cloned into pGEMT (Promega) and sequenced.
Real-time PCR
Total RNA isolation, cDNA synthesis and real-time PCR were done as described previously [37] using the following primer sets: 5′AGG TTA CGG CCA TCA TCA CTT GGA 3′ and 5′ATT GGT GGT GGA AGT CAT GGG TGT 3′ for TcCPR27, 5′ GAA TAC CGC ATC CGT GAC CAC AAA 3′ and 5′CAG GTT CCA ACA AAC TGT AGG TTC CC 3′ for TcCPR18. Total RNA was isolated from whole insects (n = 5) to analyze developmental expression patterns and knock-down levels after RNAi of TcCPR27 and TcCPR18. Total RNA also was isolated from elytra, hindwings, ventral abdomens and dorsal abdomens of pharate adults (5 d-old pupae) (n = 10). The transcript levels of the T. castaneum ribosomal protein S6 (rpS6) were measured to normalize for differences between samples in the concentrations of cDNA templates.
Double-stranded RNA synthesis and injection
dsRNA for TcCPR27 and TcCPR18 was synthesized as described previously [38] using the primers 5′-(T7)-GAC CAC CAC ACC CAT G-3′ and 5′-(T7)-TCA GTT GCC TCC AAT C-3′ for TcCPR27, and 5′-(T7)-GGA AGA GTA CGG TCA TC -3′ and 5′-(T7)-GGT TCC CTT TAC TTT G-3′ for TcCPR18, where T7 indicates the T7 RNA polymerase recognition sequence. The sizes of dsRNAs for TcCPR27 and TcCPR18 were 204 bp and 325 bp, respectively. dsRNAs were injected into last instar larvae [39]. dsRNA for the T. castaneum vermilion gene (dsVer) was used as a negative control [40].
Purification of TcCPR27 and TcCPR18 from extracts of elytra
Proteins were extracted from 200 pairs of elytra of 5 d-old pupae as described in Materials and Methods. The homogenate was centrifuged for 2 min at 4°C. The supernatant was applied to a Ni-NTA column equilibrated with 50 mM Tris-HCl, pH 7.5 containing 0.2 M NaCl and 20 mM imidazole and washed with the same buffer. Bound proteins were eluted with a 20 to 200 mM imidazole gradient. The fractions were analyzed for protein content by SDS-PAGE. Purified TcCPR27 was used as antigen to generate rabbit antiserum by Cocalico Biologicals, Inc., PA, USA.
Mechanical analysis of elytra
Mechanical analysis of elytra was carried out using a TA Instruments RSAIII dynamic mechanical analyzer, by methods described previously [34].
Accession numbers
cDNA sequences are deposited at NCBI with accession numbers HQ634478 (TcCPR27) and HQ634479 (TcCPR18).
Supporting Information
Zdroje
1. MoussianB 2010 Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol 40 363 375
2. ArakaneYMuthukrishnanSBeemanRWKanostMRKramerKJ 2005 Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci U S A 102 11337 11342
3. HopkinsTLKramerKJ 1992 Insect cuticle sclerotization. Annual Review of Entomology 37 273 302
4. KarouzouMVSpyropoulosYIconomidouVACornmanRSHamodrakasSJ 2007 Drosophila cuticular proteins with the R&R Consensus: annotation and classification with a new tool for discriminating RR-1 and RR-2 sequences. Insect Biochem Mol Biol 37 754 760
5. RichardsSGibbsRAWeinstockGMBrownSJDenellR 2008 The genome of the model beetle and pest Tribolium castaneum. Nature 452 949 955
6. FutahashiROkamotoSKawasakiHZhongYSIwanagaM 2008 Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem Mol Biol 38 1138 1146
7. WillisJH 2010 Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem Mol Biol 40 189 204
8. CornmanRSTogawaTDunnWAHeNEmmonsAC 2008 Annotation and analysis of a large cuticular protein family with the R&R Consensus in Anopheles gambiae. BMC Genomics 9 22
9. CornmanRSWillisJH 2009 Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Mol Biol 18 607 622
10. TogawaTAugustine DunnWEmmonsACWillisJH 2007 CPF and CPFL, two related gene families encoding cuticular proteins of Anopheles gambiae and other insects. Insect Biochem Mol Biol 37 675 688
11. RebersJERiddifordLM 1988 Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J Mol Biol 203 411 423
12. TogawaTNakatoHIzumiS 2004 Analysis of the chitin recognition mechanism of cuticle proteins from the soft cuticle of the silkworm, Bombyx mori. Insect Biochem Mol Biol 34 1059 1067
13. RebersJEWillisJH 2001 A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem Mol Biol 31 1083 1093
14. HuntTBergstenJLevkanicovaZPapadopoulouAJohnOS 2007 A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 318 1913 1916
15. GrimaldiDEngelMS 2005 Evolution of the Insects. Cambridge University Press ISBN 0-521-82149-5
16. Prud'hommeBMinervinoCHocineMCandeJDAouaneA 2011 Body plan innovation in treehoppers through the evolution of an extra wing-like appendage. Nature 473 83 86
17. ChenJDaiGXuYIwamotoM 2007 Optimal composite structures in the forewings of beetles. Composite Structures 81 432 437
18. ChenJNiQQXuYIwamotoM 2007 Lightweight composite structures in the forewings of beetles. Composite Structures 79 331 337
19. LomakinJHuberPAEichlerCArakaneYKramerKJ 2011 Mechanical properties of the beetle elytron, a biological composite material. Biomacromolecules 12 321 335
20. BouhinHCharlesJPQuennedeyBDelachambreJ 1992 Developmental profiles of epidermal mRNAs during the pupal-adult molt of Tenebrio molitor and isolation of a cDNA clone encoding an adult cuticular protein: effects of a juvenile hormone analogue. Dev Biol 149 112 122
21. DittmerNTHiromasaYTomichJMLuNBeemanRW 2011 Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J Proteome Res In press
22. LorenzenMDBrownSJDenellREBeemanRW 2002 Cloning and characterization of the Tribolium castaneum eye-color genes encoding tryptophan oxygenase and kynurenine 3-monooxygenase. Genetics 160 225 234
23. FriedJR 2003 Polymer Science and Technology, 2nd ed Prentice Hall: Upper Saddle River, NJ
24. SperlingLH 2006 Introduction to Physical Polymer Science, 4th ed Wiley - Interscience, Hoboken, NJ
25. ErmanB 1997 Structures and properties of rubberlike network Oxford University Press: New York
26. GehrkeSH 2000 Synthesis and properties of hydrogels used for drug delivery. AmidonGLLeePIToppEM Transport Processes in Pharmaceutical Systems 473 546
27. HershBMNelsonCEStollSJNortonJEAlbertTJ 2007 The UBX-regulated network in the haltere imaginal disc of D. melanogaster. Dev Biol 302 717 727
28. TomoyasuYWheelerSRDenellRE 2005 Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 433 643 647
29. TomoyasuYArakaneYKramerKJDenellRE 2009 Repeated co-options of exoskeleton formation during wing-to-elytron evolution in beetles. Curr Biol 19 2057 2065
30. MissiosSDavidsonHCLinderDMortimerLOkobiAO 2000 Characterization of cuticular proteins in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 30 47 56
31. LampeDJWillisJH 1994 Characterization of a cDNA and gene encoding a cuticular protein from rigid cuticles of the giant silkmoth, Hyalophora cecropia. Insect Biochem Mol Biol 24 419 435
32. KramerKJKanostMRHopkinsTLJiangHZhuYC 2001 Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedon 57 385 392
33. MiserezARubinDWaiteJH 2010 Cross-linking chemistry of squid beak. J Biol Chem 285 38115 38124
34. LomakinJArakaneYKramerKJBeemanRWKanostMR 2010 Mechanical properties of elytra from Tribolium castaneum wild-type and body color mutant strains. J Insect Physiol 56 1901 1906
35. AndersenSO 2012 Cuticular Sclerotization and Tanning: Insect Molecular Biology and Biochemistry. GilbertLI Elsevier 167 192
36. BeemanRWStuartJJ 1990 A gene for lindane+cyclodiene resistance in the red flour beetle (Coleoptera: Tenebrionidae). J Econ Entomol 83 1745 1751
37. ArakaneYDixitRBegumKParkYSpechtCA 2009 Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem Mol Biol 39 355 365
38. ArakaneYMuthukrishnanSKramerKJSpechtCATomoyasuY 2005 The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol Biol 14 453 463
39. TomoyasuYDenellRE 2004 Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol 214 575 578
40. ArakaneYLomakinJBeemanRWMuthukrishnanSGehrkeSH 2009 Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. J Biol Chem 284 16584 16594
41. GormanMJArakaneY 2010 Tyrosine hydroxylase is required for cuticle sclerotization and pigmentation in Tribolium castaneum. Insect Biochem Mol Biol 40 267 273
Štítky
Genetika Reprodukčná medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 4- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



