-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
Fragile X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurodegenerative disorder, has been recognized in older male fragile X premutation carriers and is uncoupled from fragile X syndrome. Using a Drosophila model of FXTAS, we previously showed that transcribed premutation repeats alone are sufficient to cause neurodegeneration. MiRNAs are sequence-specific regulators of post-transcriptional gene expression. To determine the role of miRNAs in rCGG repeat-mediated neurodegeneration, we profiled miRNA expression and identified selective miRNAs, including miR-277, that are altered specifically in Drosophila brains expressing rCGG repeats. We tested their genetic interactions with rCGG repeats and found that miR-277 can modulate rCGG repeat-mediated neurodegeneration. Furthermore, we identified Drep-2 and Vimar as functional targets of miR-277 that could modulate rCGG repeat-mediated neurodegeneration. Finally, we found that hnRNP A2/B1, an rCGG repeat-binding protein, can directly regulate the expression of miR-277. These results suggest that sequestration of specific rCGG repeat-binding proteins could lead to aberrant expression of selective miRNAs, which may modulate the pathogenesis of FXTAS by post-transcriptionally regulating the expression of specific mRNAs involved in FXTAS.
Published in the journal: MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats. PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002681
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002681Summary
Fragile X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurodegenerative disorder, has been recognized in older male fragile X premutation carriers and is uncoupled from fragile X syndrome. Using a Drosophila model of FXTAS, we previously showed that transcribed premutation repeats alone are sufficient to cause neurodegeneration. MiRNAs are sequence-specific regulators of post-transcriptional gene expression. To determine the role of miRNAs in rCGG repeat-mediated neurodegeneration, we profiled miRNA expression and identified selective miRNAs, including miR-277, that are altered specifically in Drosophila brains expressing rCGG repeats. We tested their genetic interactions with rCGG repeats and found that miR-277 can modulate rCGG repeat-mediated neurodegeneration. Furthermore, we identified Drep-2 and Vimar as functional targets of miR-277 that could modulate rCGG repeat-mediated neurodegeneration. Finally, we found that hnRNP A2/B1, an rCGG repeat-binding protein, can directly regulate the expression of miR-277. These results suggest that sequestration of specific rCGG repeat-binding proteins could lead to aberrant expression of selective miRNAs, which may modulate the pathogenesis of FXTAS by post-transcriptionally regulating the expression of specific mRNAs involved in FXTAS.
Introduction
Fragile X syndrome (FXS), the most common form of inherited mental retardation, is caused by expansion of the rCGG trinucleotide repeat in the 5′ untranslated region (5′ UTR) of the fragile X mental retardation 1 (FMR1) gene, which leads to silencing of its transcript and the loss of the encoded fragile X mental retardation protein (FMRP) [1]–[6]. Most affected individuals have more than 200 rCGG repeats, referred to as full mutation alleles [7]. Fragile X syndrome carriers have FMR1 alleles, called premutations, with an intermediate number of rCGG repeats between patients (>200 repeats) and normal individuals (<60 repeats) [8]. Recently, the discovery was made that male and, to a lesser degree, female premutation carriers are at greater risk of developing an age-dependent progressive intention tremor and ataxia syndrome, which is uncoupled from fragile X syndrome and known as fragile X-associated tremor/ataxia syndrome (FXTAS) [9], [10]. This is combined with cognitive decline associated with the accumulation of ubiquitin-positive intranuclear inclusions broadly distributed throughout the brain in neurons, astrocytes, and in the spinal column [11], [12].
At the molecular level, the premutation is different from either the normal or full mutation alleles. Based on the observation of significantly elevated levels of rCGG-containing FMR1 mRNA, along with either no detectable change in FMRP or slightly reduced FMRP levels in premutation carriers, an RNA-mediated gain-of-function toxicity model has been proposed for FXTAS [13]–[17]. Several lines of evidence in mouse and Drosophila models further support the notion that transcription of the CGG repeats leads to this RNA-mediated neurodegenerative disease [11], [15], [17]–[19]. The hypothesis is that specific RNA-binding proteins may be sequestered by overproduced rCGG repeats in FXTAS and become functionally limited, thereby contributing to the pathogenesis of this disorder [15], [17], [19], [20]. There are three RNA-binding proteins found to modulate rCGG-mediated neuronal toxicity: Pur α, hnRNP A2/B1, and CUGBP1, which bind rCGG repeats either directly (Pur α and hnRNP A2/B1) or indirectly (CUGBP1, through the interaction with hnRNP A2/B1) [21], [22].
MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression at the post-transcriptional level by targeting mRNAs, leading to translational inhibition, cleavage of the target mRNAs or mRNA decapping/deadenylation [23], [24]. Mounting evidence suggests that miRNAs play essential functions in multiple biological pathways and diseases, from developmental timing, fate determination, apoptosis, and metabolism to immune response and tumorigenesis [25]–[31]. Recent studies have shown that miRNAs are highly expressed in the central nervous system (CNS), and some miRNAs have been implicated in neurogenesis and brain development [32]–[34].
Interest in the functions of miRNAs in the CNS has recently expanded to encompass their roles in neurodegeneration. Investigators have begun to reveal the influence of miRNAs on both neuronal survival and the accumulation of toxic proteins that are associated with neurodegeneration, and are uncovering clues as to how these toxic proteins can influence miRNA expression [35]. For example, miR-133b is found to regulate the maturation and function of midbrain dopaminergic neurons (DNs) within a negative feedback circuit that includes the homeodomain transcription factor Pitx3 in Parkinson's disease [36]. In addition, reduced miR-29a/b-1-mediated suppression of BACE1 protein expression contributes to Aβ accumulation and Alzheimer's disease pathology [37]. Moreover, the miRNA bantam is found to be a potent modulator of poly-Q - and tau-associated degeneration in Drosophila [38]. Other specific miRNAs have also been linked to other neurodegenerative disorders, such as spinocerebellar ataxia type 1 (SCA1) and Huntington's disease (HD) [39], [40]. Therefore, miRNA-mediated gene regulation could be a novel mechanism, adding a new dimension to the pathogenesis of neurodegenerative disorders.
Here we show that fragile X premutation rCGG repeats can alter the expression of specific miRNAs, including miR-277, in a FXTAS Drosophila model. We demonstrate that miR-277 modulates rCGG-mediated neurodegeneration. Furthermore, we identified Drep-2, which is associated with the chromatin condensation and DNA fragmentation events of apoptosis, and Vimar, a modulator of mitochondrial function, as two of the mRNA targets regulated by miR-277. Functionally, Drep-2 and Vimar could modulate the rCGG-mediated neurodegeneration, as well. Finally, we show that hnRNP A2/B1, an rCGG repeat-binding protein, can directly regulate the expression of miR-277. These data suggest that hnRNP A2/B1 could be involved in the transcriptional regulation of selective miRNAs, and fragile X premutation rCGG repeats could alter the expression of specific miRNAs, potentially contributing to the molecular pathogenesis of FXTAS.
Results
Fragile X premutation rCGG repeats alter the expression of selective miRNAs
Given the important roles of miRNAs in neural development and human neurological disorders, we investigated the role of miRNAs in rCGG-mediated neurodegeneration. To determine whether fragile X premutation rCGG repeats could influence the expression of miRNAs, we profiled the expression of 72 known miRNAs using rCGG repeat transgenic flies that we generated previously [15]. In rCGG repeat transgenic flies, the severity of their phenotype depends on both dosage and length of the rCGG repeat. Moderate expression of (CGG)90 repeats exclusively in the eyes have an effect on morphology and histology; however, expression of (CGG)90 repeats in the neurons leads to lethality at the embryonic stage, preventing analysis at the adult stage [15]. Therefore, we used a shorter repeat length, r(CGG)60, which allowed us to examine the gene expression in adults. To analyze the effect of rCGG repeats in adult brains, we used RNAs isolated from the age - and sex-matched brains of control flies (elav-GAL4) and flies expressing rCGG60 repeats in neurons (elav-GAL4;UAS-CGG60-EGFP) for miRNA profiling experiments (Figure 1). We identified a subset of miRNAs that consistently displayed altered expression in rCGG repeat flies versus the control group. Seven miRNAs with a ≥two-fold increase and two miRNAs with expression decreased by ≥1.5-fold have been found in rCGG repeat flies. These results suggest that fragile X premutation rCGG repeats could lead to the dysregulation of a subset of specific miRNAs.
Fig. 1. Identification of the miRNAs with altered expression in the brains of an FXTAS Drosophila model. 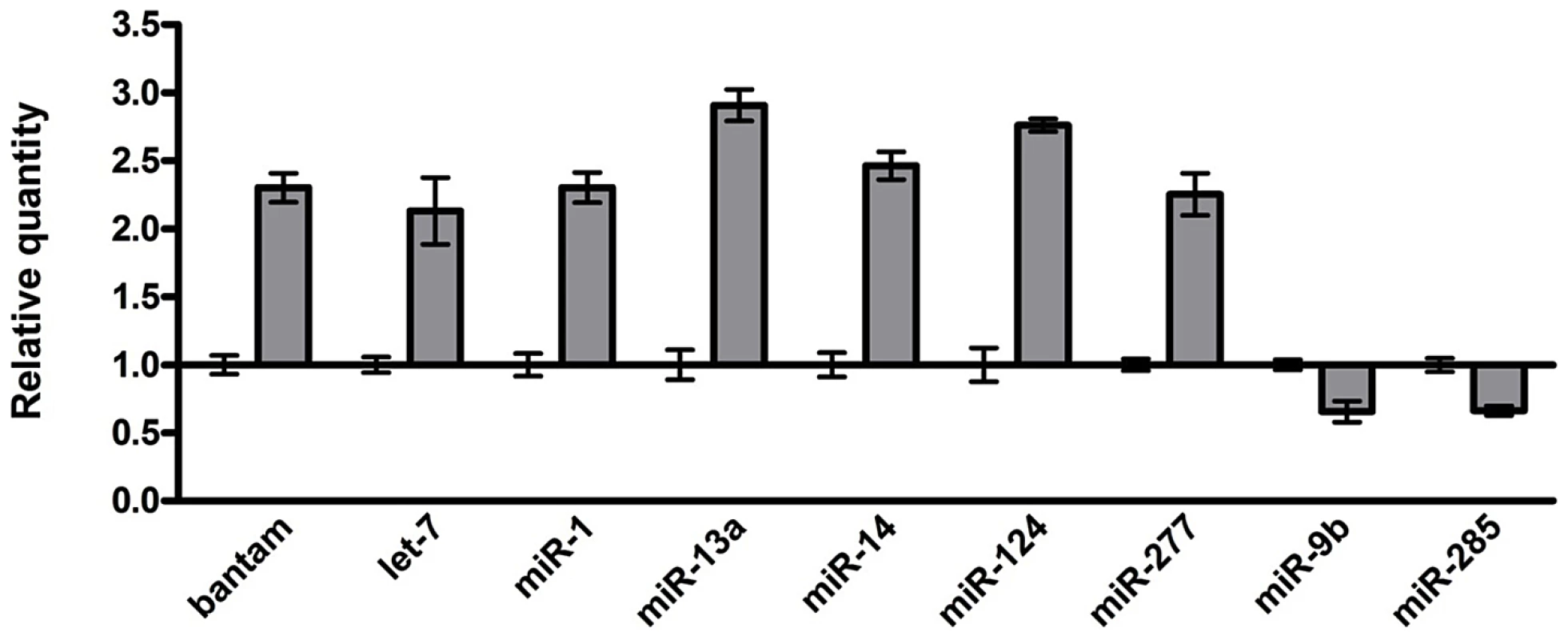
Relative quantity of miRNA with ≥two-fold change in expression shown for rCGG60 flies, calibrated to control (elav-GAL4) flies. Control relative quantity = 1. Overexpression of miR-277 enhances rCGG-mediated neurodegeneration
To assess the potential involvement of the miRNAs that showed altered expression in FXTAS fly brain, we examined the genetic interaction between specific miRNAs and rCGG-mediated neuronal toxicity based on the fragile X premutation rCGG repeat-mediated neurodegenerative eye phenotype we observed previously [15]. We generated UAS fly lines that could overexpress Drosophila miR-277, bantam, let-7, or miR-1, as well as bantam mutant lines (ban12 and ban20) that we generated previously [41]. We then crossed these transgenic lines with gmr-GAL4, UAS-(CGG)90-EGFP transgenic flies that exhibit photoreceptor neurodegeneration to determine the role of specific miRNAs in rCGG-mediated neurodegeneration. As shown in Figure 2, flies co-expressing miR-277 and rCGG90 consistently showed an aggravated eye phenotype, with enhanced disorganized, fused ommatidia compared with flies expressing rCGG90 alone (Figure 2B and 2D). Flies overexpressing miR-277 alone displayed a very mild rough eye phenotype (Figure 2C). Alterations of the levels of bantam, let-7, or miR-1 by either a gain of function or loss of function had no effect on rCGG-mediated neurodegeneration (Figure 2E–2N). These data together suggest that miR-277 could be involved in rCGG-mediated neurodegeneration. The role of miR-277 in rCGG-mediated neurodegeneration seems specific, since the other miRNAs we found with altered expression in the presence of fragile X premutation rCGG repeats, including bantam, let-7, and miR-1, had no effect on the rCGG90 eye phenotype. The rest of our work focused on the role of miR-277 and its potential mechanisms in modulating rCGG-mediated neurodegeneration.
Fig. 2. Overexpression of miR-277 enhances rCGG-mediated neurodegeneration. 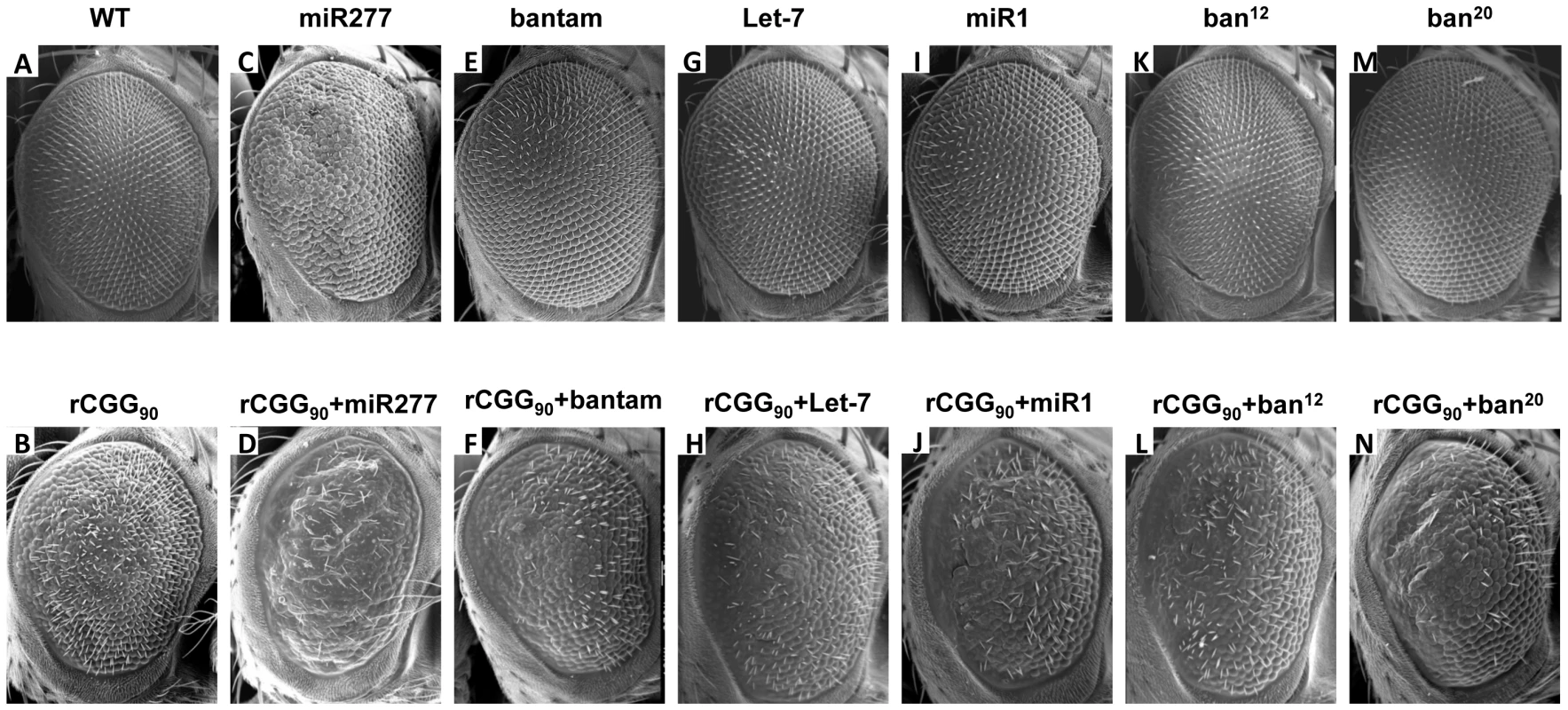
Shown are scanning electron microscope (SEM) eye images from seven-day-old flies. WT fly eyes show the normal organization of ommatidia (A). Expression of rCGG90 causes disorganized, fused ommatidia (B). Flies overexpressing miR-277 alone present with a mild rough eye phenotype (C). The rCGG90 eye phenotype is enhanced by miR-277 overexpression with aggravated disorganized, fused ommatidia (D). Alteration of bantam, let-7, or miR-1 does not modify the rCGG-induced eye phenotype (F, H, J, L, N). Alteration of bantam, let-7 or miR-1 alone does not cause an abnormal eye phenotype (E, G, I, K, M). Genotypes are B-gmr-GAL4,UAS-CGG90-EGFP/+; C-gmr-GAL4/UAS-miR-277; D-gmr-GAL4, UAS-CGG90-EGFP/UAS-miR-277; E-gmr-GAL4/+;UAS-bantam/+; F-gmr-GAL4,UAS-CGG90-EGFP/+;UAS-bantam/+; G-gmr-GAL4/+;UAS-Let-7/+; H-gmr-GAL4,UAS-CGG90-EGFP/+;UAS-Let-7/+; I-gmr-GAL4/UAS-GFP;UAS-miR-1/+; J-gmr-GAL4,UAS-CGG90-EGFP/UAS-GFP;UAS-miR-1/+; K-gmr-GAL4/+;ban12/+; L-gmr-GAL4,UAS-CGG90-EGFP/+;ban12/+; M-gmr-GAL4/+;ban20/+; N-gmr-GAL4,UAS-CGG90-EGFP/+;ban20/+. Blocking the activity of miR-277 suppresses rCGG-mediated neurodegeneration
Our miRNA profiling and genetic interaction studies indicated that an increase in miR-277 expression in rCGG repeat flies could alter the expression of specific cellular mRNAs by miR-277, resulting in the enhanced rCGG-induced eye phenotype. To further explore the potential regulatory effect of miR-277 on rCGG-mediated neurodegeneration, we generated a transgenic miR-277 sponge (miR-277SP) line, which could block the activity of miR-277, to test for any blocking effect on the rCGG-induced neurodegenerative eye phenotype. We generated the miRNA sponge transgenic construct as described previously [42], [43]. In brief, we placed 10 repetitive sequences complementary to miR-277 with mismatches at positions 9–12 into the 3′ UTR of EGFP in a pUASP expression vector (Figure 3A). We crossed miR-277SP transgenic flies with the flies expressing 90 CGG repeats and found that the expression of miR-277 sponge could consistently suppress rCGG-mediated neuronal toxicity (Figure 3B). MiR-277 sponge alone or scramble control sponge had no effect on eye morphology (Figure 3B and Data not shown). This result suggests that blocking the activity of miR-277 could mitigate the neurodegeneration caused by fragile X premutation rCGG repeats.
Fig. 3. Blocking the activity of miR-277 suppresses rCGG-mediated neurodegeneration. 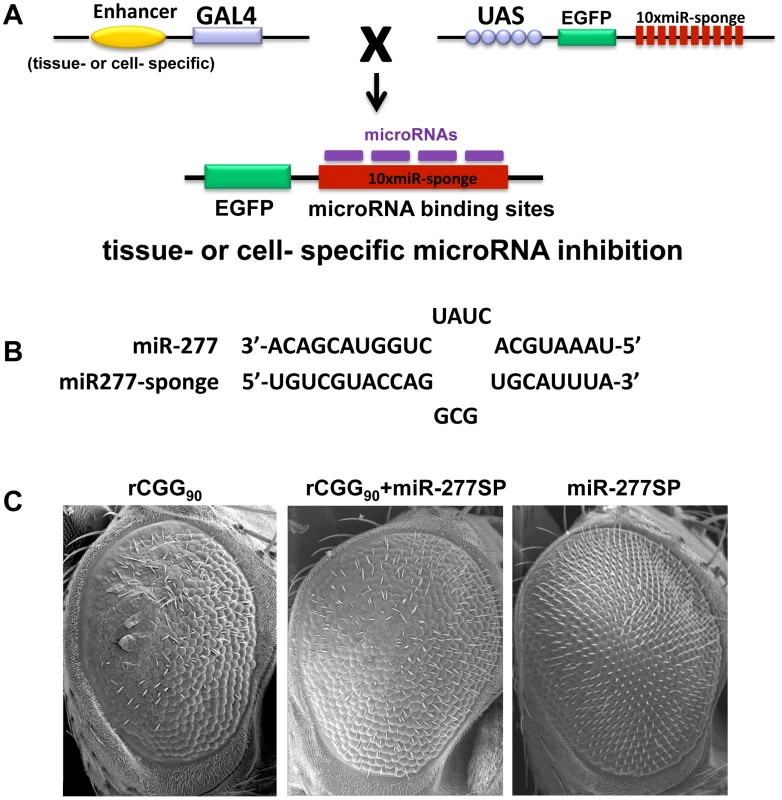
A. Shown is the diagram depicting the strategy for generation of transgenic microRNA sponge lines. The sponge construct contains 10 repetitive sequences complementary to a microRNA downstream of EGFP in a UAS-containing P-element vector. When these UAS lines are crossed to GAL4 driver lines, the F1 progeny generate tissue- or cell-specific expression of microRNA sponge to block the activity of specific miRNA in Drosophila. B. The miR-sponge contains bulged sites that are mismatched opposite microRNA at positions 9–12. The bulge is designed to protect Ago2-mediated endonucleolytic cleavage. C. Scanning electron microscope (SEM) eye images from seven-day-old flies. Block of miR-277 activity by miR-277SP could rescue the rCGG-mediated neurodegenerative eye phenotype. Knockdown of miR-277 alone does not cause an abnormal eye phenotype. Genotypes are (left)-gmr-GAL4,UAS-CGG90-EGFP/+; (middle)-gmr-GAL4,UAS-CGG90-EGFP/UAS-miR-277SP; (right)-gmr-GAL4/UAS-miR-277SP. Identification of miR-277 mRNA targets that modulate rCGG-mediated neurodegeneration
To seek the mechanisms by which miR-277 modulates rCGG-mediated neurodegeneration, we searched the RNA network and referenced TargetScanFly 5.1 to identify potential miR-277 targets [44], [45]. We selected the top candidates for miR-277 target mRNAs with the mutant alleles available for further analyses (Table 1). We then carried out a genetic screen on the rCGG90 neurodegenerative eye phenotype to identify potential miR-277 targets that could modulate rCGG-mediated neuronal toxicity. We crossed gmr-GAL4, UAS-(CGG)90-EGFP transgenic flies with fly mutants in genes coding for the top candidates for miR-277 target genes (Table 1). The progenies were then tested for potential suppression or enhancement of the disorganized eye phenotype versus flies expressing rCGG90 alone. Through this screen, we identified two modifiers of rCGG-mediated neurodegeneration, Drep-2 and Vimar. As shown in Figure 4, partial loss of Drep-2 could enhance the rCGG90 eye phenotype by increasing ommatidial disorganization (Figure 4B). Overexpression of Drep-2 could suppress the rCGG-induced eye phenotype (Figure 4C). Flies carrying the Drep-2 mutation alone or Drep-2 overexpression alone displayed normal eyes (Figure 4D and 4E). We also found a heterozygous loss-of-function mutant of Vimar that aggravated the rCGG90 eye phenotype (Figure 4F). Eyes of control flies carrying the same Vimar mutation but no rCGG90 repeats are normal (Figure 4G). These data together indicate that Drep-2 and Vimar could modulate rCGG-mediated neurodegeneration.
Fig. 4. Modification of rCGG-mediated neurodegenerative eye phenotype by Drep-2 and Vimar. 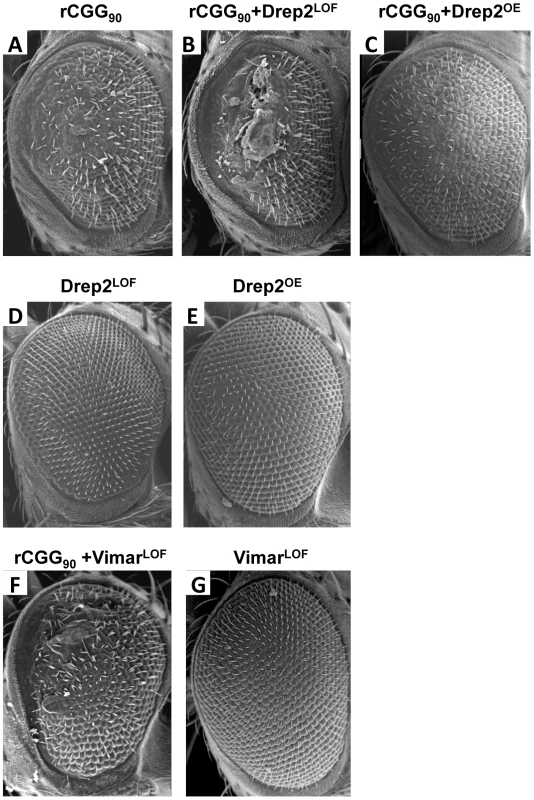
(A–C) SEM eye images from control flies expressing rCGG90, rCGG90 and a heterozygous mutation in Drep-2 (Drep-2LOF), rCGG90 and overexpression of Drep-2 (Drep-2OE), and rCGG90 and a heterozygous mutation in Vimar (VimarLOF). rCGG90 flies manifest ommatidial disorganization and fusion (A). This phenotype is enhanced in flies carrying a heterozygous loss-of-function mutation in Drep-2 (B), while the eye phenotype could be suppressed by overexpression of Drep-2 (C). Mutation of Vimar enhances the rCGG90-mediated eye phenotype (F). All the mutants alone do not cause any abnormal eye phenotype (D, E, G). Genotypes are A-gmr-GAL4,UAS-CGG90-EGFP/+; B -gmr-GAL4, UAS-CGG90-EGFP/Drep-2KG02396; C-gmr-GAL4, UAS-CGG90-EGFP/Drep-2d00223; D-gmr-GAL4/Drep-2KG02396; E-gmr-GAL4/ Drep-2d00223; F -gmr-GAL4, UAS-CGG90-EGFP/ VimarK16722; G-gmr-GAL4/ VimarK16722. Tab. 1. Mutant alleles corresponding to the mRNA targets of miR-277 used for genetic screen. 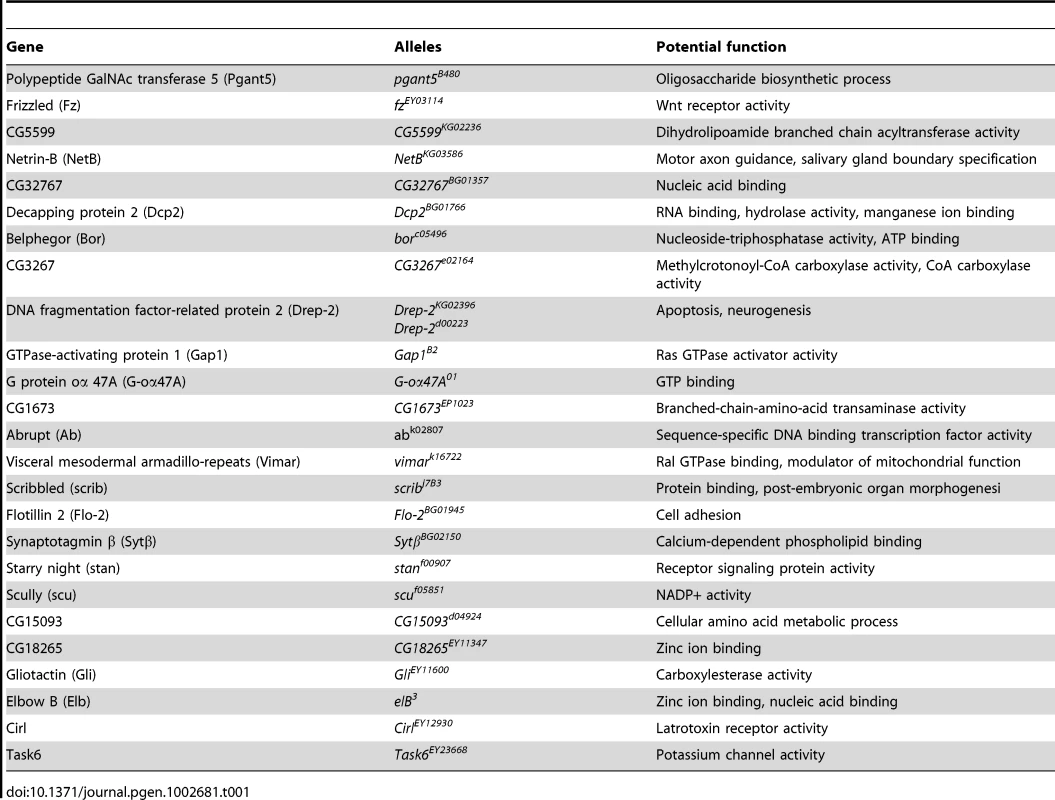
MiR-277 regulates the expression of Drep-2 and Vimar post-transcriptionally
Since both Drep-2 and Vimar were predicted to be regulated by miR-277, by introducing 3′-UTR dual luciferase assays, we tested whether miR-277 could indeed target to Drep-2 or Vimar. We cloned the 3′-UTR of Drep-2 or Vimar containing the predicted miR-137 target sites from fly cDNA into a dual luciferase (R-Luc and F-Luc) reporter construct, allowing for the assessment of protein translation of these targets regulated via their 3′-UTR (Figure 5A). These 3′-UTR dual constructs were transfected into HEK293 cells. We found that overexpression of miR-277 could suppress the R-Luc activity at 48 h post-transfection (Figure 5B and 5C). Furthermore, when we mutated the seed regions of miR-277 located within the Drep2-3′-UTR reporter and Vimar-3′-UTR reporter, we saw that the mutation alleviated the miR-277-mediated suppression of luciferase activity (Figure 5A–5C), suggesting the action of miR-277 is specific to the miR-277 seed region within the Drep-2 3′-UTR and Vimar 3′-UTR. Together, these data demonstrate that the effect of miR-277 on Drep-2 and Vimar expression is repressive and specific. Importantly, they also suggest that Drep-2 and Vimar are the functional targets of miR-277.
Fig. 5. MiR-277 regulates the expression of Drep-2 and Vimar post-transcriptionally. 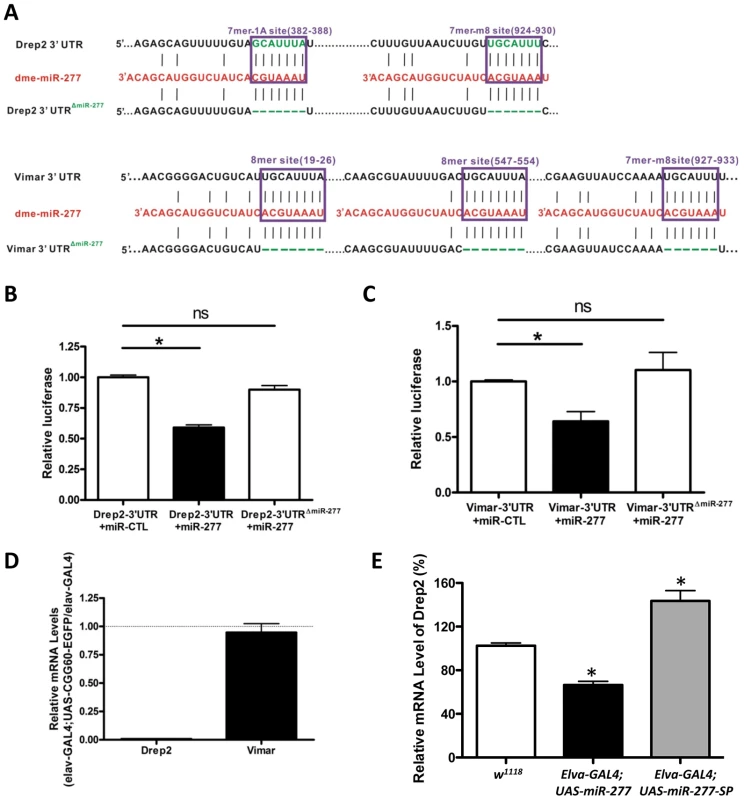
A. The two miR-277 target sites in the Drep-2 3′-UTR and three miR-277 target sites in the Vimar 3′-UTR were predicted by TargetScan. We created the mutant Drep-2 3′-UTR (Drep-2 3′-UTRΔmiR-277) with two miR-277 binding sites deleted and the mutant Vimar 3′-UTR (Vimar 3′ UTRΔmiR-277) with three miR-277 binding sites deleted, as shown. B. The expression of a luciferase reporter gene containing the Drep-2 3′-UTR was suppressed by miR-277 in HEK293FT cells. The suppression is specific to the miR-277 seed region within the Drep-2 3′-UTR, as deletion of the miR-277 target sites in the Drep-2 3′-UTR alleviated repression by miR-277. (*-P<0.05; ns-P>0.05). C. Similar miR-277-mediated suppression was observed with Vimar 3′-UTR constructs. D. Endogenous Drep-2 mRNA expression was significantly reduced in rCGG repeat flies relative to control flies (elav-GAL4), whereas no obvious change of Vimar mRNA expression was observed in the rCGG repeat flies compare to control flies. E. Endogenous Drep-2 mRNA expression was significantly altered in the flies expressing either miR-277 or miR-277 SP, *-P<0.05. Next we went on to examine the steady-state levels of Drep-2 and Vimar mRNA in rCGG repeat flies (elav-GAL4;UAS-CGG60-EGFP), in which the expression of miR-277 is increased. We saw a significant reduction of endogenous Drep-2 mRNA in rCGG repeat flies relative to control flies (elav-GAL4), whereas the Vimar mRNA expression in rCGG repeat flies remained similar to control flies (elav-GAL4) (Figure 5D). Furthermore, ectopic expression of miR-277 or miR-277 sponge could alter the endogenous mRNA level of Drep2 while have no apparent effect on Vimar mRNA level (Figure 5E). These observations suggest that miR-277 could regulate Drep-2 and Vimar mRNAs differentially, with miR-277 regulating the expression of Drep-2 mainly at the mRNA level, and Vimar via translational suppression instead.
Expression of miR-277 is regulated by the rCGG repeat-binding protein hnRNP A2/B1
Two rCGG repeat-binding proteins, Pur α and hnRNP A2/B1, were previously found to bind rCGG repeats directly and modulate rCGG-mediated neuronal toxicity [21], [22]. Intriguingly, recent studies have shown that multiple heterogeneous nuclear ribonucleoproteins (hnRNPs) could interact with heterochromatin protein 1 (HP1) to bind to genomic DNA and modulate heterochromatin formation [46]. Thus we tested whether hnRNP A2/B1 could interact directly with genomic regions proximal to miR-277. We performed hnRNP A2/B1-specific chromatin immunoprecipitation (ChIP) followed by real-time quantitative PCR across a six-kb region surrounding miR-277. Immunoprecipitation of chromatin chemically cross-linked to DNA with an hnRNP A2/B1-specific antibody demonstrated that a region 1.5 kb upstream of miR-277 was enriched ∼seven-fold relative to IgG control and adjacent regions (Figure 6A and 6B). Furthermore, the ectopic expression of hnRNP A2/B1 in fly brain could reduce the expression of miR-277 (Figure 6C). These results together suggest that hnRNP A2/B1 could directly bind to the upstream region of miR-277 and regulate its expression. In the presence of fragile X premutation rCGG repeats, hnRNP A2/B1 will be sequestered, leading to the de-repression of the miR-277 locus.
Fig. 6. Expression of miR-277 is regulated by hnRNPA2/B1. 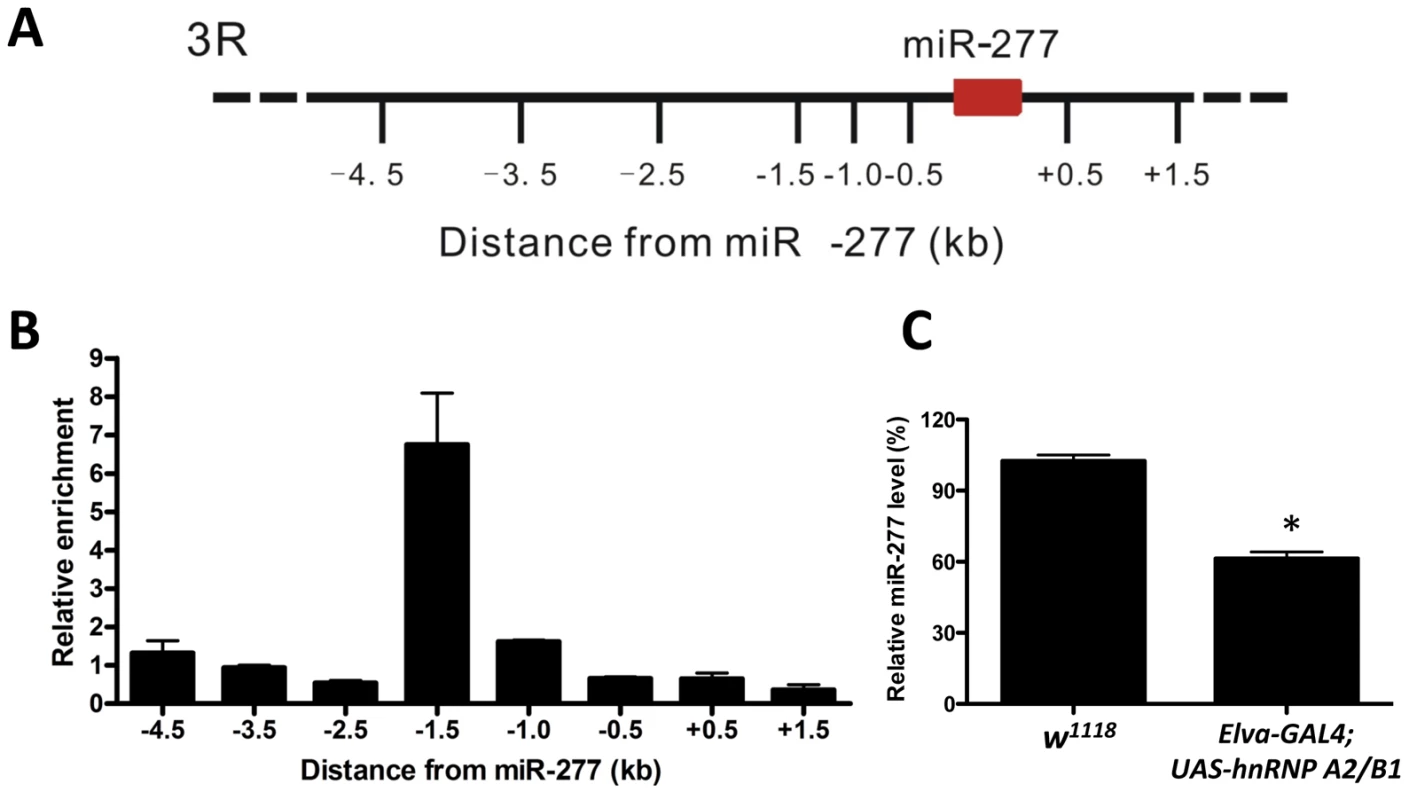
A. Schematic of the six kb proximal to miR-277 on chromosome 3R assayed in ChIP experiments. B. HnRNP A2/B1-specific ChIP assay indicates the enrichment of DNA 1.5 kb upstream of the miR-277 genomic locus. Relative enrichment is calculated relative to IgG-only nonspecific control and normalized to the empty vector. Error bars indicate mean ± SEM. C. Ectopic expression of hnRNP A2/B1 in fly brain could suppress the expression of miR-277, *-P<0.05. Discussion
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder that afflicts fragile X syndrome premutation carriers, with earlier studies pointing to FXTAS as an RNA-mediated neurodegenerative disease. Several lines of evidence suggest that rCGG premutation repeats may sequester specific RNA-binding proteins, namely Pur α, hnRNP A2/B1, and CUGBP1, and reduce their ability to perform their normal cellular functions, thereby contributing significantly to the pathology of this disorder [15], [21], [22]. The miRNA pathway has been implicated in the regulation of neuronal development and neurogenesis [32], [47]–[49]. A growing body of evidence has now revealed the role of the miRNA pathway in the molecular pathogenesis of neurodegenerative disorders [35]. Here we demonstrate that specific miRNAs can contribute to fragile X rCGG repeat-mediated neurodegeneration by post-transcriptionally regulating target mRNAs that are involved in FXTAS. We show that miR-277 plays a significant role in modulating rCGG repeat-mediated neurodegeneration. Overexpression of miR-277 enhances rCGG repeat-induced neuronal toxicity, whereas blocking miR-277 activity could suppress rCGG repeat-mediated neurodegeneration. Furthermore, we identified Drep-2 and Vimar as the functional miR-277 targets that could modulate rCGG repeat-induced neurodegeneration. Finally, we show that hnRNP A2/B1, an rCGG repeat-binding protein, can directly regulate the expression of miR-277. Our biochemical and genetic studies demonstrate a novel miRNA-mediated mechanism involving miR-277, Drep-2, and Vimar in the regulation of neuronal survival in FXTAS (Figure 7).
Fig. 7. Proposed model of miR-277 modulating fragile X premutation rCGG repeat-mediated neurodegeneration. 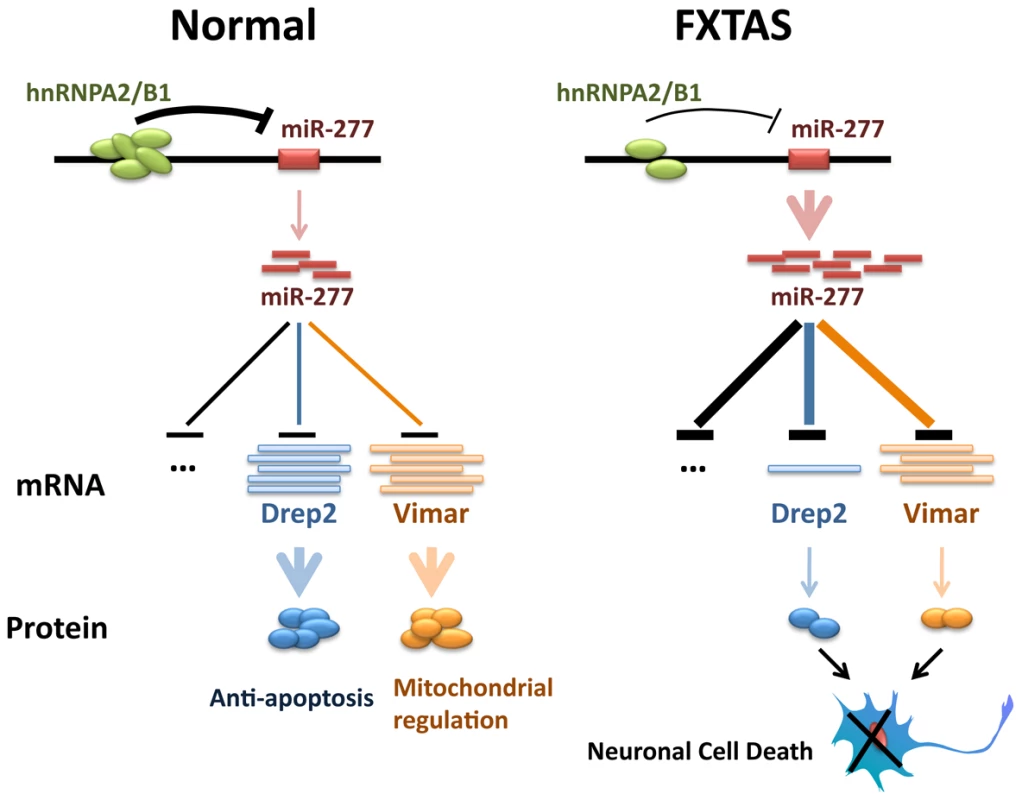
HnRNP A2/B1, an rCGG repeat-binding protein, could be sequestered by fragile X premutation rCGG repeats from their normal cellular functions. Sequestration of hnRNP A2/B1 could de-repress the expression of miR-277. MiR-277 could recognize and bind to the 3′-UTR of its mRNA targets, such as Drep-2 and Vimar, to induce their mRNA degradation or translational suppression. Misregulation of Drep-2 or Vimar, which are involved in cell apoptosis or mitochondrial regulation, could lead to the neuronal cell death associated with FXTAS. Several lines of evidence from studies in mouse and Drosophila models strongly support FXTAS as an RNA-mediated neurodegenerative disorder caused by excessive rCGG repeats [11], [15], [17]–[19]. The current working model is that specific RNA-binding proteins could be sequestered by overproduced rCGG repeats in FXTAS and become functionally limited, thereby contributing to the pathogenesis of this disorder [15], [17], [19], [20]. Three RNA-binding proteins are known to modulate rCGG-mediated neuronal toxicity: Pur α, hnRNP A2/B1, and CUGBP1, which bind rCGG repeats either directly (Pur α and hnRNP A2/B1) or indirectly (CUGBP1, through the interaction with hnRNP A2/B1) [21], [22]; how the depletion of these RNA-binding proteins could alter RNA metabolism and contribute to FXTAS pathogenesis has thus become the focus in the quest to understand the molecular pathogenesis of this disorder. Nevertheless, the data we present here suggest that the depletion of hnRNP A2/B1 could also directly impact the transcriptional regulation of specific loci, such as miR-277. We know that hnRNPs can interact with HP1 to bind to genomic DNA and modulate heterochromatin formation [46]. Our results indicate that hnRNP A2/B1 could participate in the transcriptional regulation of miR-277; however, it remains to be determined whether other loci could be directly regulated by hnRNP A2/B1, as well. Identifying those loci will be important to better understand how the depletion of rCGG repeat-binding proteins could lead to neuronal apoptosis.
In recent years, several classes of small regulatory RNAs have been identified in a range of tissues and in many species. In particular, miRNAs have been linked to a host of human diseases. Some evidence suggests the involvement of miRNAs in the emergence or progression of neurodegenerative diseases. For example, accumulation of nuclear aggregates that are toxic to neurons have been linked to many neurodegenerative diseases, and miRNAs are known to modulate the accumulation of the toxic proteins by regulating either their mRNAs or the mRNAs of proteins that affect their expression. Moreover, miRNAs might contribute to the pathogenesis of neurodegenerative disease downstream of the accumulation of toxic proteins by altering the expression of other proteins that promote or inhibit cell survival [35]. Our genetic modifier screen revealed that miR-277 could modulate rCGG repeat-mediated neurodegeneration. By combining our genetic screen and reporter assays, we identified Drep-2 and Vimar as the functional targets of miR-277 that could modulate rCGG-mediated neurodegeneration. The closest ortholog of miR-277 in human is miR-597 based on the seed sequence. It would be interesting to further examine the role of miR-597 in FXTAS using mammalian model systems.
Drep-2 is associated with the chromatin condensation and DNA fragmentation events of apoptosis [50], [51]. Drep-2 is one of four Drosophila DFF (DNA fragmentation factor)-related proteins. While Drep-1 is a Drosophila homolog of DFF45 that can inhibit CIDE-A mediated apoptosis [50]. Drep-2 has been shown to interact with Drep-1 and to regulate its anti-apoptotic activity [50]. Vimar is a Ral GTPase-binding protein that has been shown to regulate mitochondrial function via an increase in citrate synthase activity [52]. In the presence of fragile X premutation rCGG repeats, overexpression of miR-277 will suppress the expression of both Drep-2 and Vimar, thereby altering anti-apoptotic activity as well as mitochondrial functions, which have been linked to neuronal cell death associated with neurodegenerative disorders in general (Figure 7). Interestingly, we saw a significant reduction of Drep-2 mRNA in the flies expressing rCGG repeats, while Vimar mRNA levels remained similar to control flies. This observed difference may be due to the fact that miRNA could be involved in different modes of action, including mRNA cleavage, translational inhibition and mRNA decapping/deadenylation its target mRNAs [23], [24].
In summary, here we provide both biochemical and genetic evidence to support a role for miRNA and its selective mRNA targets in rCGG-mediated neurodegeneration. Our results suggest that sequestration of specific rCGG repeat-binding proteins can lead to aberrant expression of selective miRNAs that could modulate the pathogenesis of FXTAS by post-transcriptionally regulating the expression of specific mRNAs involved in this disorder. Identification of these miRNAs and their targets could reveal potential new targets for therapeutic interventions to treat FXTAS, as well as other neurodegenerative disorders.
Materials and Methods
Drosophila genetics
All flies were maintained under standard culture conditions. The rCGG repeat transgenic flies (UAS-CGG60-EGFP and UAS-CGG90-EGFP) were generated in our lab as described previously [15]. Flies mutant in genes coding for different candidate miR-277 targets were obtained from Bloomington Stock Center. The UAS-miR-277-Sponge transgenic flies were generated as described previously [43]. We introduced 10 repetitive miR-277 sponge sequences (TGTCGTACCAGGCGTGCATTTA) with a 4-nt linker between each repeat downstream of EGFP in a pUASP expression vector. Similarly a scramble control construct (GTTCACGGATAGTGCCTGTACT) was generated as well. Both constructs were confirmed by DNA sequencing and then injected in the w1118 strain by standard methods.
Relative quantification of mature miRNAs by TaqMan miRNA real-time PCR
Total RNAs were isolated from the control (elav-GAL4) and rCGG60 fly heads using Trizol. TaqMan MicroRNA Assays detecting 72 known individual Drosophila miRNAs were obtained from ABI (ABI). cDNA was prepared with High-Capacity cDNA Reverse Transcription Kits (ABI; Cat#437496). The 15-µl reverse transcription reactions consisted of 10 ng of total RNA, 5 U MultiScribe Reverse Transcriptase, 0.5 mM of each dNTP, 1× reverse transcription buffer, 4 U RNase inhibitor, and nuclease-free water. This was performed at 16°C for 30 min and at 42°C for 30 min, terminated at 85°C for 5 min and 4°C until use in TaqMan assays. For real-time PCR of TaqMan MicroRNA Assays, we used 0.5 ul 20×TaqMan MicroRNA Assay Primer, 1.33 ul undiluted cDNA, 5 ul 2×TaqMan Universal PCR Master Mix, 3.17 ul nuclease-free water. Each PCR reaction was performed in triplicate with MicroAmp optical 96-well plates using a 7500 Fast Real-Time PCR System (ABI), with reactions incubated at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. Fluorescence readings were taken during the 60°C step. RQs were calculated using the ΔΔCt method, with 2S RNA TaqMan miRNA control assay as the endogenous control, and calibrated to the control samples.
Quantitative RT–PCR
The fly heads from control (elav-Gal4) and rCGG60 flies were collected. Trizol (Invitrogen; Cat# 15596-026) was used to isolate total RNA from each genotype. RNA samples were reverse-transcribed into cDNA with oligo(dT)20 and SuperScript III (Invitrogen; Cat#18080051). Real-time PCR was performed with gene-specific primers and Power SYBR Green PCR Master Mix (Applied Biosystems; Cat# 4367660) using the 7500 Standard Real-Time PCR System (Applied Biosystems). RpL32 (Qiagen; Cat# QT00985677) was used as an endogenous control for all samples. Primers for Drep2 and Vimar transcripts were designed using Primer Express 3.0 software (Applied Biosystems) and were as follows. Drep2: forward, 5′-TGGAACGCCTCAACTCCAA-3′; and reverse, 5′-TCGGACTCGCGATCCAA-3′. Vimar: forward, 5′-GCACCCGCCGAACAGA-3′; and reverse, 5′-TGCGATCGTAGTCTTGCGTTA -3′. All real-time PCR reactions were performed in triplicate, and RQs were calculated using the ΔΔCt method, with calibration to control samples.
3′-UTR dual luciferase assays of candidate miR-277 target mRNA
Drep-2 3′-UTR and Vimar 3′-UTR sequences were PCR-amplified directly from w1118 fly brain first-strand cDNA generated from 5 ug TRIZOL-isolated total RNA using oligo-dT SuperScript III reverse transcription according to the manufacture's protocol (Invitrogen; Cat. #1808-093). The PCR products were then cloned into psiCHECK-2 dual luciferase vector (Promega; Cat# C8021). The miR-277 target sites in the Drep-2 3′-UTR and Vimar 3′-UTR were deleted using QuikChange Site-Directed Mutagenesis Kits (Stratagene; Cat. #2000518). Target sites deletions were verified by Genewiz sequencing. Briefly, 293FT cells were co-transfected by Attractene transfection reagent (Qiagen; Cat. #301005) with psiCHECK-2-3′UTR or psiCHECK-2-3′ UTRΔmiR-277 and miR-277 duplex RNA (Qiagen; Cat# MSY0000338) or control miRNA duplex (Qiagen; Cat# 1027280). All co-transfections used a total of 600 ng of plasmid DNA and 120 nmol of duplex RNA. Luciferase expression was detected using the Dual-Luciferase Reporter 1000 System (Promega; Cat# E1980) according to the manufacturer's instructions. At 48 h after transfection, R-Luc activity was normalized to F-Luc activity to account for variation in transfection efficiencies, and miR-277-mediated knockdown of R-Luc activity was calculated as the ratio of normalized R-Luc activity in the miR-277 duplex treatments to normalized R-Luc activity in the negative control duplex treatments. Luciferase experiments were repeated three times.
Chromatin immunoprecipitation (ChIP)
ChIP was performed using a ChIP Assay Kit (Millipore). S2 cells were cross-linked with 1% formaldehyde (Sigma-Aldrich) for 10 min at room temperature. Chromatin was fragmented to an average size of 500 bp by sonication (Sonicator 3000; Misonix) and immunoprecipitated with anti-Flag M2 antibody (sigma). Immunoprecipitated and purified DNA fragments were diluted to 1 ng/µl in nuclease-free water. We used 8 ng of DNA in 20-µl SYBR Green real-time PCR reactions consisting of 1× Power SYBR Green Master Mix and 0.5 µM forward and reverse primers. Reactions were run on an SDS 7500 Fast Instrument (Applied Biosystems). Primers were designed using Primer Express 3.0 software (Applied Biosystems) and were as follows. 4.5 kb upstream: forward, 5′-CAGAAAACAGGCGTGCAAAC; and reverse, 5′-GAATTTGCATTGGCTTTGGAA. 3.5 kb upstream: forward, 5′ - TTACAATTGGATGGGCTTCGT; and reverse, 5′-AAGCTGACGGCCTGACTAAAAA. 2.5 kb upstream: forward, 5′-GTTGGCTGCTGCGTCAATT; and reverse, 5′ - GCCCCAGCGGCATTTATA. 1.5 kb upstream: forward, 5′ - TTCTGGCACTGGCAGCTTT; and reverse, 5′ - CATCGTGCTGGCCAACAC. 1.0 kb upstream: forward, 5′-TGTACGGGCATGTGTATGCA; and reverse, 5′ - TCAACGAACACGCTGCGTAT. 0.5 kb upstream: forward, 5′ - GGGCATTTTCATTTCATTCCA; and reverse, 5′ - CGGGCAGCGTAATTTAAGCT. 0.5 kb downstream: forward, 5′ - CGCCCACAAGAGCTTTTGA; and reverse, 5′ - TTTCCACGGTATGCTGCTTTT. 1.5 kb downstream: forward, 5′ - CGTTTCCATTTAGTTGGATTTTTGT; and reverse, 5′ - GGCAAACCACACATTTTAACATACA. DNA relative enrichment was determined by taking the absolute quantity ratios of specific IPs to nonspecific IPs (normal mouse IgG only), IP/IgG, and normalizing to control (pUAST only). Independent chromatins were prepared for all ChIP experiments, and real-time PCR reactions were performed in triplicate for each sample on each amplicon.
Scanning electron microscopy
For scanning electron microscopy (SEM) images, whole flies were dehydrated in gradient concentration ethanol (25%, 50%, 75%, 100%), dried with hexamethyldisilazane (Sigma; Cat# 16700), and analyzed with an ISI DS-130 LaB6 SEM/STEM microscope.
Zdroje
1. FuYHKuhlDPPizzutiAPierettiMSutcliffeJS 1991 Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67 1047 1058
2. KennesonAZhangFHagedornCHWarrenST 2001 Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 10 1449 1454
3. KremerEJPritchardMLynchMYuSHolmanK 1991 Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science 252 1711 1714
4. OberleIRousseauFHeitzDKretzCDevysD 1991 Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252 1097 1102
5. PierettiMZhangFPFuYHWarrenSTOostraBA 1991 Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66 817 822
6. VerkerkAJPierettiMSutcliffeJSFuYHKuhlDP 1991 Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65 905 914
7. ShermanS 2002 Epidemiology. HagermanRJ Fragile X Syndrome: Diagnosis, Treatment and Research Baltimore, MD The Johns Hopkins University Press 136 168
8. HagermanRJHagermanPJ 2002 The fragile X premutation: into the phenotypic fold. Curr Opin Genet Dev 12 278 283
9. HagermanPJHagermanRJ 2004 The fragile-X premutation: a maturing perspective. Am J Hum Genet 74 805 816
10. HagermanPJHagermanRJ 2004 Fragile X-associated tremor/ataxia syndrome (FXTAS). Ment Retard Dev Disabil Res Rev 10 25 30
11. GrecoCMBermanRFMartinRMTassoneFSchwartzPH 2006 Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 129 243 255
12. IwahashiCKYasuiDHAnHJGrecoCMTassoneF 2006 Protein composition of the intranuclear inclusions of FXTAS. Brain 129 256 271
13. GrecoCMHagermanRJTassoneFChudleyAEDel BigioMR 2002 Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125 1760 1771
14. JacquemontSHagermanRJLeeheyMAHallDALevineRA 2004 Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. Jama 291 460 469
15. JinPZarnescuDCZhangFPearsonCELucchesiJC 2003 RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron 39 739 747
16. TassoneFHagermanRJTaylorAKGaneLWGodfreyTE 2000 Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet 66 6 15
17. WillemsenRHoogeveen-WesterveldMReisSHolstegeJSeverijnenLA 2003 The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet 12 949 959
18. HashemVGallowayJNMoriMWillemsenROostraBA 2009 Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum Mol Genet 18 2443 2451
19. ArocenaDGIwahashiCKWonNBeilinaALudwigAL 2005 Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet 14 3661 3671
20. TassoneFIwahashiCHagermanPJ 2004 FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS). RNA Biology 1 103 105
21. SofolaOAJinPQinYDuanRLiuH 2007 RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron 55 565 571
22. JinPDuanRQurashiAQinYTianD 2007 Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron 55 556 564
23. BartelDPChenCZ 2004 Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet 5 396 400
24. GuoHIngoliaNTWeissmanJSBartelDP 2010 Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466 835 840
25. BushatiNCohenSM 2008 MicroRNAs in neurodegeneration. Curr Opin Neurobiol 18 292 296
26. BaltimoreDBoldinMPO'ConnellRMRaoDSTaganovKD 2008 MicroRNAs: new regulators of immune cell development and function. Nat Immunol 9 839 845
27. HeLHeXLoweSWHannonGJ 2007 microRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat Rev Cancer 7 819 822
28. ChangTCMendellJT 2007 microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet 8 215 239
29. KarpXAmbrosV 2005 Developmental biology. Encountering microRNAs in cell fate signaling. Science 310 1288 1289
30. GiraldezAJCinalliRMGlasnerMEEnrightAJThomsonJM 2005 MicroRNAs regulate brain morphogenesis in zebrafish. Science 308 833 838
31. AbbottALAlvarez-SaavedraEMiskaEALauNCBartelDP 2005 The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell 9 403 414
32. KrichevskyAMSonntagKCIsacsonOKosikKS 2006 Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 24 857 864
33. KosikKSKrichevskyAM 2005 The Elegance of the MicroRNAs: A Neuronal Perspective. Neuron 47 779 782
34. LiXJinP Roles of small regulatory RNAs in determining neuronal identity. Nat Rev Neurosci 11 329 338
35. EackerSMDawsonTMDawsonVL 2009 Understanding microRNAs in neurodegeneration. Nat Rev Neurosci 10 837 841
36. KimJInoueKIshiiJVantiWBVoronovSV 2007 A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317 1220 1224
37. HebertSSHorreKNicolaiLPapadopoulouASMandemakersW 2008 Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A 105 6415 6420
38. BilenJLiuNBurnettBGPittmanRNBoniniNM 2006 MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell 24 157 163
39. LeeYSamacoRCGatchelJRThallerCOrrHT 2008 miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci 11 1137 1139
40. PackerANXingYHarperSQJonesLDavidsonBL 2008 The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J Neurosci 28 14341 14346
41. YangYXuSXiaLWangJWenS 2009 The bantam microRNA is associated with drosophila fragile X mental retardation protein and regulates the fate of germline stem cells. PLoS Genet 5 e1000444
42. EbertMSNeilsonJRSharpPA 2007 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4 721 726
43. LoyaCMLuCSVan VactorDFulgaTA 2009 Transgenic microRNA inhibition with spatiotemporal specificity in intact organisms. Nat Methods 6 897 903
44. RubyJGJanCHBartelDP 2007 Intronic microRNA precursors that bypass Drosha processing. Nature 448 83 86
45. StarkAKheradpourPPartsLBrenneckeJHodgesE 2007 Systematic discovery and characterization of fly microRNAs using 12 Drosophila genomes. Genome Res 17 1865 1879
46. PiacentiniLFantiLNegriRDel VescovoVFaticaA 2009 Heterochromatin protein 1 (HP1a) positively regulates euchromatic gene expression through RNA transcript association and interaction with hnRNPs in Drosophila. PLoS Genet 5 e1000670
47. PasquinelliAE 2002 MicroRNAs: deviants no longer. Trends Genet 18 171 173
48. VisvanathanJLeeSLeeBLeeJWLeeSK 2007 The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev 21 744 749
49. LiXJinP 2010 Roles of small regulatory RNAs in determining neuronal identity. Nat Rev Neurosci 11 329 338
50. InoharaNKosekiTChenSWuXNunezG 1998 CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. Embo J 17 2526 2533
51. InoharaNNunezG 1999 Genes with homology to DFF/CIDEs found in Drosophila melanogaster. Cell Death Differ 6 823 824
52. ChenJShiXPadmanabhanRWangQWuZ 2008 Identification of novel modulators of mitochondrial function by a genome-wide RNAi screen in Drosophila melanogaster. Genome Res 18 123 136
Štítky
Genetika Reprodukčná medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells inČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 5- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



