-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
The separation of the optic neuroepithelium into future retina and retinal pigment epithelium (RPE) is a critical event in early eye development in vertebrates. Here we show in mice that the transcription factor PAX6, well-known for its retina-promoting activity, also plays a crucial role in early pigment epithelium development. This role is seen, however, only in a background genetically sensitized by mutations in the pigment cell transcription factor MITF. In fact, a reduction in Pax6 gene dose exacerbates the RPE-to-retina transdifferentiation seen in embryos homozygous for an Mitf null allele, and it induces such a transdifferentiation in embryos that are either heterozygous for the Mitf null allele or homozygous for an RPE–specific hypomorphic Mitf allele generated by targeted mutation. Conversely, an increase in Pax6 gene dose interferes with transdifferentiation even in homozygous Mitf null embryos. Gene expression analyses show that, together with MITF or its paralog TFEC, PAX6 suppresses the expression of Fgf15 and Dkk3. Explant culture experiments indicate that a combination of FGF and DKK3 promote retina formation by inhibiting canonical WNT signaling and stimulating the expression of retinogenic genes, including Six6 and Vsx2. Our results demonstrate that in conjunction with Mitf/Tfec Pax6 acts as an anti-retinogenic factor, whereas in conjunction with retinogenic genes it acts as a pro-retinogenic factor. The results suggest that careful manipulation of the Pax6 regulatory circuit may facilitate the generation of retinal and pigment epithelium cells from embryonic or induced pluripotent stem cells.
Published in the journal: A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development. PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002757
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002757Summary
The separation of the optic neuroepithelium into future retina and retinal pigment epithelium (RPE) is a critical event in early eye development in vertebrates. Here we show in mice that the transcription factor PAX6, well-known for its retina-promoting activity, also plays a crucial role in early pigment epithelium development. This role is seen, however, only in a background genetically sensitized by mutations in the pigment cell transcription factor MITF. In fact, a reduction in Pax6 gene dose exacerbates the RPE-to-retina transdifferentiation seen in embryos homozygous for an Mitf null allele, and it induces such a transdifferentiation in embryos that are either heterozygous for the Mitf null allele or homozygous for an RPE–specific hypomorphic Mitf allele generated by targeted mutation. Conversely, an increase in Pax6 gene dose interferes with transdifferentiation even in homozygous Mitf null embryos. Gene expression analyses show that, together with MITF or its paralog TFEC, PAX6 suppresses the expression of Fgf15 and Dkk3. Explant culture experiments indicate that a combination of FGF and DKK3 promote retina formation by inhibiting canonical WNT signaling and stimulating the expression of retinogenic genes, including Six6 and Vsx2. Our results demonstrate that in conjunction with Mitf/Tfec Pax6 acts as an anti-retinogenic factor, whereas in conjunction with retinogenic genes it acts as a pro-retinogenic factor. The results suggest that careful manipulation of the Pax6 regulatory circuit may facilitate the generation of retinal and pigment epithelium cells from embryonic or induced pluripotent stem cells.
Introduction
The vertebrate retinal pigment epithelium or RPE is a monolayer of melanin-containing cells that are intimately juxtaposed to the photoreceptor layer of the retina where they function as light screen and provide metabolic support for the retina's photoreceptors. Like the retina, the RPE is developmentally derived from the optic neuroepithelium and can be converted experimentally to retina, usually only during early development but in some species, such as newts, even in adults [1]–[3].
The differentiation of the optic neuroepithelium into precursors for retina and RPE is controlled by numerous growth factors and transcription factors. Among them is the microphthalmia-associated transcription factor MITF, which is a basic-helix-loop-helix-leucine zipper protein that together with the closely related proteins TFE3, TFEB and TFEC forms the Mi-T subfamily of MYC-related transcription factors [4], [5]. During mouse eye development, when the budding optic vesicle has not yet touched the surface ectoderm, low levels of MITF are found throughout the vesicle's epithelium. Mitf then is downregulated in the distal part of the vesicle, the future retina, in a pathway that involves FGF signaling and the paired-like homeodomain transcription factor VSX2 [6], [7], and it is upregulated in the proximal part, the future RPE, in a pathway that, based on work mostly in the chick, involves bone morphogenetic proteins (BMPs), several transforming growth factor-ßs including ACTIVIN, and WNT signaling [8]–[12]. If Mitf is missing, an optic cup still forms and retina development continues, but the domain that normally becomes RPE hyperproliferates, remains unpigmented, and in its dorsal part turns into a second, fully laminated retina in a process commonly referred to as “transdifferentiation” [7], [13]. Conversely, if Mitf fails to be downregulated in the distal optic vesicle, the domain that normally becomes retina hypo-proliferates and turns into a pigmented monolayer resembling the RPE [6], [7], [14].
A second transcription factor initially expressed in both distal and proximal optic vesicle is the paired domain protein PAX6. Unlike MITF, PAX6 initially remains high in both future retina and RPE but later fades away in the RPE [14]–[16]. PAX6 has widespread roles in vertebrate eye development, both in structures derived from the surface ectoderm, such as cornea and lens, and in those derived from the neuroepithelium, such as iris, ciliary body, and retina [17]. Indeed, if in mice Pax6 is missing entirely, optic vesicles fail to form properly and eye development is aborted [18]. Conditional ablation specifically in the neuroretina allows for neuroretinal development to proceed but retinal progenitor cells can only mature into amacrine interneurons instead of the many different cell types of a normal retina [19]. In the RPE, however, the roles of Pax6 are less clear as an early RPE-specific ablation is not yet available and overexpression has no major effects on RPE development [20], [21]. Nevertheless, studies involving chimeras between Pax6 wild-type and mutant mouse embryos showed that Pax6-deficient cells can contribute to the RPE although their differentiation (pigmentation) is impaired [22].
There are a number of previous studies that have suggested cross-regulations between Mitf and Pax6. Biochemical studies, for instance, have indicated that PAX6 and MITF interact at the protein level, and that this interaction results in a mutual inactivation of both proteins [23]. In vitro, PAX6 stimulates at least one of the multiple Mitf promoters, the 5′ most human A-MITF promoter, and the combined absence of PAX6 and PAX2, though not the absence of PAX6 alone, abolishes MITF expression in the optic neuroepithelium of mouse embryos as visualized by immunofluorescence [24]. Furthermore, a reduction of functional MITF in the RPE leads to an increase in PAX6 expression, most prominently in the transdifferentiating but also in the non-transdifferentiating portion [7].
Given the above findings, we reasoned that a decrease in Pax6 gene dose would alleviate RPE transdifferentiation in Mitf mutants, and upregulation further expand this transdifferentiation. Hence, we crossed mice carrying mutant Mitf alleles either with mice carrying the Pax6Sey-Neu allele, which represents a functional null allele [18], or mice overexpressing human PAX6 from a yeast artificial chromosome (YAC) transgene [20], [21]. Contrary to expectation, however, we find that a reduction of Pax6 enhances RPE transdifferentiation in Mitf mutants and overexpression suppresses it. In fact, overexpression of PAX6 increases the expression of the RPE-specific Mitf paralog Tfec which helps to compensate for the loss of the anti-proliferative, though not the pigmentary, function of Mitf. By gene expression profiling, we identified two major targets of the Pax6/Mitf/Tfec pathway that act extracellularly: Fgf15, a member of the FGF family of ligands that serves as a positive regulator of retinal development, and Dkk3, which together with FGFs inhibits RPE-promoting WNT signaling. Hence, our results show that despite the common neuroepithelial origin of retina and RPE, the two tissues differ in the way they utilize the transcription factor PAX6. These findings may shed light on the evolution of Pax6 as a major regulator of eye development and may have implications for designing optimal methods to obtain RPE or retinal cells for cell-therapeutic purposes from embryonic or induced pluripotent stem cells.
Results
Pax6 Gene Dose Regulates RPE Transdifferentiation in Mitf Mutants
As seen previously [7], [15], [16] and documented in Figure S1, PAX6 and MITF protein are co-expressed in the developing RPE of mice, suggesting that the two genes interact. In fact, although the lack of Pax6 does not alter the onset of Mitf expression in the optic neuroepithelium [24], the lack of Mitf results in increased Pax6 expression in the RPE [7], [14], prompting us to test whether manipulating Pax6 gene dose in embryos lacking Mitf would alter the development of the RPE.
As shown in Figure 1A–1F, the future RPE of embryos homozygous for the Mitf null allele Mitfmi-vga9 showed dorsal thickening and PAX6 upregulation at E13.5 while heterozygotes showed no corresponding abnormalities (for gene structures and alleles used in this study, see Table S1). When Mitfmi-vga9 heterozygous embryos were also heterozygous for the non-functional Pax6Sey-Neu allele, however, they displayed a dorsal RPE thickening reminiscent of that found in Mitfmi-vga9 homozygotes (compare Figure 1H, 1K with Figure 1C, 1F). Moreover, when Mitfmi-vga9 homozygotes were heterozygous for Pax6Sey-Neu, they displayed a massive enlargement of the RPE domain, which in its dorso-proximal part showed a morphology and expression pattern of the neuronal marker TUJ1 similar to those of the adjacent normal retina (Figure 1I, 1L). In contrast, when PAX6 was overexpressed from a homozygous human PAX6 YAC transgene (line Pax77; [21], there was no RPE thickening, not even in Mitfmi-vga9 homozygotes, and this phenotypic correction persisted even after birth (Figure 1M–1R). Similar observations were made at E11.5 (Figure S2A–S2F). That the thickening of the RPE indeed resulted from cellular hyperproliferation is based on the observation that the percentage of phosphohistone H3-positive cells was significantly increased in RPEs with dorsal thickening (Figure S2G–S2K) while no changes in cell death were found (not shown). These results indicate that in the RPE domain, Pax6 shares with Mitf an RPE-promoting function, in contrast to the retina domain where Pax6 is known to promote retina development [19]. Furthermore, because the Pax6-dependent RPE alterations were seen in the total absence of MITF protein, PAX6/MITF protein interactions as seen in vitro [23] cannot account for the observed phenotypes.
Fig. 1. Gene dose of Pax6 regulates dorsal RPE development in an Mitf mutant–lacking MITF protein. 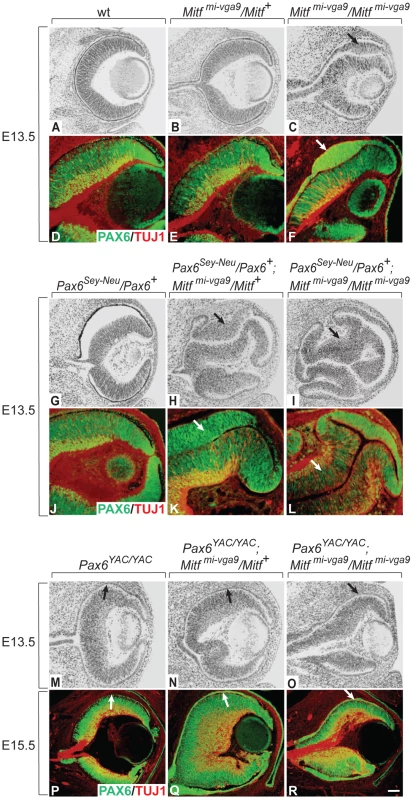
Cryostat sections of eyes of the indicated genotypes and developmental time points were stained with H&E (A–C,G–I,M–O) or labeled for the indicated markers (D–F,J–L,P–R). Note relatively mild RPE thickening in Mitfmi-vga9/Mitfmi-vga9 (C,F). In contrast, in the presence of one copy of the Pax6Sey-Neu allele, there is a massive RPE thickening (I,L) as well as staining for the neuronal marker TUJ1 (L). In the presence of the Pax6 YAC transgene (O,R), however, no such RPE abnormalities are seen. Arrows point to the thickened RPE (C,F,H,K,I,L) or to the corresponding monolayer RPE in (M–R). Scale bar (A–C,G–I,M–O): 115 µm; (D–F, J–L): 90 µm; (P–R): 60 µm. To test whether the Pax6-dependent RPE alterations were specific to the Mitfmi-vga9 null allele, we repeated the above experiments with a newly generated RPE-specific allele of Mitf. We have previously found that of the many Mitf mRNA isoforms (see Table S1), the D-Mitf isoform is the only one specific to the RPE, and that this isoform contributes up to one third of total Mitf RNA in the RPE depending on the developmental time point [14]. Hence, by specifically eliminating the D-Mitf promoter/D-Mitf exon by targeted recombination, we expected to obtain mice with an RPE-specific hypomorphic allele of Mitf. The generation and characterization of such mice, labeled Mitfmi-ΔD/Mitfmi-ΔD or, for short, D-Mitf knock-outs, is documented in Figure 2. To test for RNA expression in their developing eyes, we used microdissected embryonic or P0 RPE or retina for quantitative RT-PCR assays as previously described [14] (Figure S3). The assays showed that D-Mitf RNA was completely eliminated in the RPE fraction of D-Mitf knock-out embryos but that other isoforms, in particular H-Mitf, were compensatorily upregulated, though not before E13.5 (Figure 2C). Consequently, total Mitf mRNA, quantified separately using primers common to all isoforms, was reduced only at E11.5 but not at E13.5 and thereafter (Figure 2C, see also Figure 3N). This likely explains why D-Mitf knock out embryos show a slight reduction in the RPE expression of the MITF target gene Tyrosinase and in eye pigmentation only at E11.5 (Figure 2D–2G), but normal eye development thereafter. In fact, on visual inspection, adult D-Mitf knock-outs look completely normal (Figure 2H, 2I).
Fig. 2. Generation and analysis of mice lacking the RPE–specific D-isoform of Mitf (Mitfmi-ΔD/Mitfmi-ΔD). 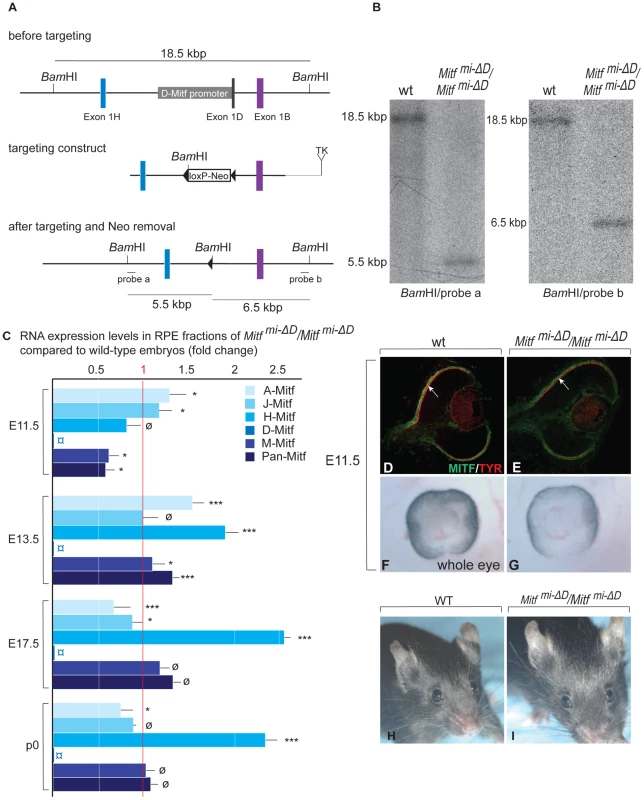
(A) Top: Schematic diagrams showing a region of the Mitf gene containing exons 1H, 1D, and 1B; Middle: the targeting construct with a novel BamHI restriction site and a floxed Neomycin cassette in place of 5.8 kbp of the D-Mitf promoter/exon 1D region; Bottom: Mitf gene portion after targeting. Probe ‘a’ recognizes a 5.5 kbp and probe ‘b’ a 6.5 kbp BamHI restriction fragment after targeting while both probes recognize the same 18.5 kbp fragment before targeting (see Materials and Methods for the details on construct design). (B) Southern hybridization of BamHI-restricted genomic DNA from wild-type and homozygous mutants shows the expected bands. (C) Mitf isoforms are upregulated in the RPE of Mitfmi-ΔD/Mitfmi-ΔD mice. Quantitative RT-PCR analysis for Mitf-isoforms from wild-type and Mitfmi-ΔD/Mitfmi-ΔD RPE fractions. Primers specific for Mitf isoforms A, J, H, D, and M were used to measure the respective RNAs. All values are normalized using the housekeeping gene Usf1. Results (mean values, S.D. and statistical significance [see Experimental Procedures] based on 3 biologically independent samples) are shown as fold change in RNA expression level in Mitfmi-ΔD/Mitfmi-ΔD compared to wild type. (D–G) Mice lacking the D-isoform of Mitf have reduced/delayed RPE pigmentation. Eye sections (D,E) from E11.5 wild type and Mitfmi-ΔD/Mitfmi-ΔD embryos show reduced MITF (green) and TYROSINASE (red) staining in the RPE (arrows in D,E). (F,G) Whole eye pictures show a mild reduction in pigmentation (F,G). (H,I) Adult Mitfmi-ΔD/Mitfmi-ΔD mice are indistinguishable on visual inspection from wild-type mice. Fig. 3. Gene dose of Pax6 regulates dorsal RPE development in a hypomorphic Mitf mutant. 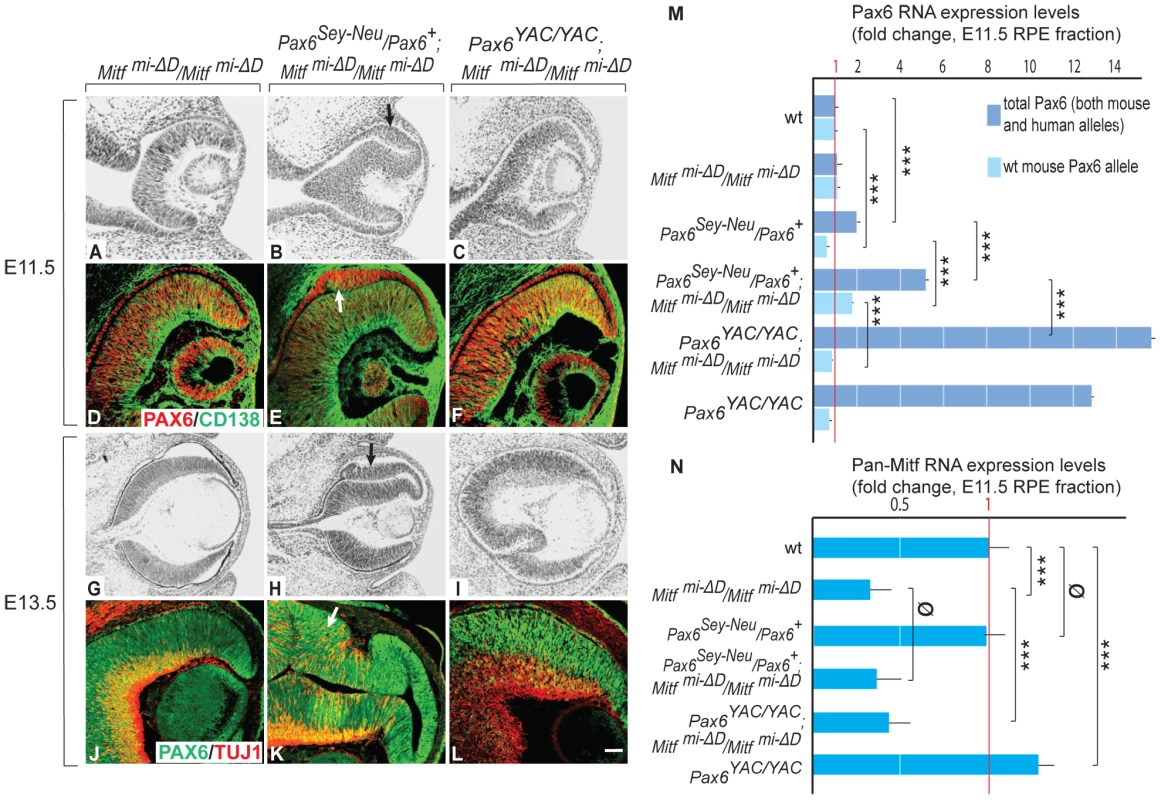
Sectioning and labeling was performed as for Figure 1. In contrast to single Mitfmi-ΔD/Mitfmi-ΔD mutants, there is dorsal RPE thickening in Pax6Sey-Neu/Pax6+; Mitfmi-ΔD/Mitfmi-ΔD mutants (arrows in B,E,H,K). Some cells in the thickened RPE are positive for CD138, a retinal progenitor marker (arrow in E), or TUJ1, a neuronal marker (arrow in K). Scale bar (A–C,G–I): 115 µm; (D–F,J–L): 90 µm. (M,N) Expression levels of the indicated RNAs in isolated RPE fractions based on quantitative RT-PCR (fold change relative to wild type). Results represent means and S.D. obtained from 3 biologically independent samples, each representing a pool of approximately 40 RPEs. Statistical significance of the results (see Experimental Procedures) is given for multiple pairwise comparisons. In contrast to homozygous D-Mitf knock-outs carrying wild-type Pax6 alleles (Figure 3A, 3D), homozygous D-Mitf knock-outs that were heterozygous for the Pax6Sey-Neu allele showed a dorsal RPE thickening at E11.5 (Figure 3B, 3E), whereby some of the cells in the thickened RPE were positive for the retinal progenitor cell marker CD138 [25] (Figure 3E, arrow). At E13.5, the dorsal thickening became prominent (Figure 3H), co-stained with TUJ1 (Figure 3K), and resembled the transdifferentiating RPE domain of Mitfmi-vga9 homozygous mutants (Figure 1C, 1F). No such RPE thickening or transdifferentiation were seen in D-Mitf knock-outs overexpressing PAX6 from the YAC transgene (Figure 3C, 3F, 3I, 3L). Quantitative RT-PCR measurements of total Pax6 RNA, wild-type mouse-specific Pax6 RNA and pan-Mitf RNA in the E11.5 RPE fraction of the different mutant combinations are shown in Figure 3M, 3N. Interestingly, the presence of the Pax6Sey-Neu allele led to a reduction in wild-type mouse Pax6 RNA when Mitf was wild type, but to an increase when D-Mitf was eliminated. This increase was likely a consequence of the increase in expression of retinogenic genes including Pax6 in the transdifferentiating portion of the RPE (Figure 3E, 3K; Figure S4). Nevertheless, the expression of just PAX6 provided by the YAC transgene interfered with dorsal thickening or transdifferentiation. This phenomenon was likely linked to the differential regulation of the Mitf paralog Tfec (see below).
As documented in detail in Figure 4 and Figures S4, S5, S6, S7, the abnormal hyperproliferation of the dorsal RPE was associated with the expression of specific retinal markers and a concomitant downregulation of RPE markers (Figure S4). The abnormal RPE re-specification seems to begin in Pax6/Mitf double mutants already at E10.0–E10.25, just when the RPE and retina become distinct domains. As shown in Figure S5, Six3, which is normally expressed in the future RPE domain at E9.5 but downregulated when the cells become committed at around E10.0, continues to be expressed at low levels in this domain in both Pax6Sey-Neu/Pax6+; Mitfmi-ΔD/Mitfmi-ΔD and Pax6Sey-Neu/Pax6+; Mitfmi-vga9/Mitfmi-vga9 mutants. Nevertheless, the retina-specific marker Vsx2 starts to be expressed at this early time point only in the RPE domain of Pax6Sey-Neu/Pax6+;Mitfmi-vga9/Mitfmi-vga9 mutants and not in that of Pax6Sey-Neu/Pax6+;mi-ΔD/Mitfmi-ΔD. Hence, the assessment of whether in the double mutants an RPE fate is never initiated or is first initiated and then reversed depends on the retinal markers used and the particular allelic combination studied. In any event, however, the abnormal specification of the RPE domain as a future retina always lags behind the specification of the normal retina. For reasons of simplicity, we therefore use the term “transdifferentiation” not only for the single Mitf mutants but also for the double mutants.
Fig. 4. Development of a differentiated laminated retina in Pax6Sey-Neu/Pax6+;Mitfmi-ΔD/Mitf mi-ΔD but not Pax6YAC/YAC;Mitf mi-ΔD/Mitf mi-ΔD mice. 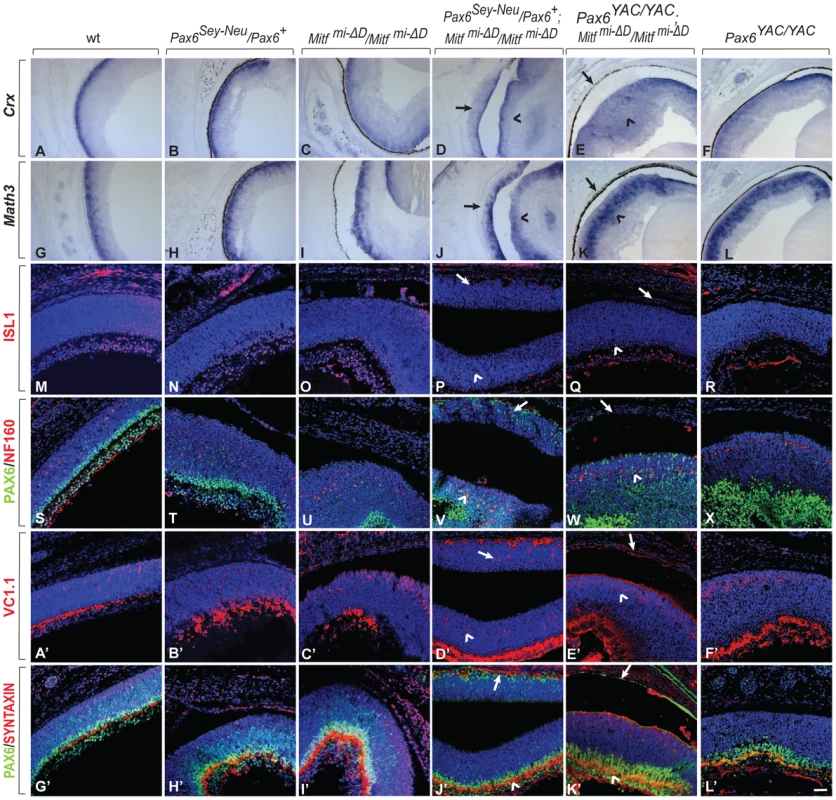
(A–L) Sections of eyes from P0 mice of the indicated genotypes were subjected to in situ hybridization for Crx, a photoreceptor marker (A–F) or Math3, an amacrine cell marker (G–L). Note that the ectopic staining is not present in the RPE of Pax6YAC/YAC;Mitf mi-ΔD/Mitf mi-ΔD mutants (compare arrows in D,J with E,K for ectopic staining; arrowheads mark normal retinal staining). (M–L′) Immunofluorescent labeling for the indicated markers on P0 eye sections of the indicated genotypes. ISL1 is a ganglion cell marker (M–R), as is PAX6 at this time point (S–X, G′–L′, green). NF160 marks horizontal cells (S–X, red); VC1.1 marks amacrine cells (A′–F′, red); and SYNTAXIN marks synapses (G′–L′, red). Arrows mark the transdifferentiating portions of the RPE in Pax6Sey-Neu/Pax6+;Mitf mi-ΔD/Mitf mi-ΔD mice (P,V,D′,J′) or the corresponding non-transdifferentiating portions in Pax6YAC/YAC;Mitf mi-ΔD/Mitf mi-ΔD mice. The normal retinas continue to express each of these markers (arrowheads in the corresponding figures). Scale bar (A–L): 115 µm; (M–X, A′–L′): 90 µm. Despite the above mentioned exacerbation of the RPE-to-retina specification, dorso-ventral polarity was maintained because neither did a dorsal marker, Tbx5, extend ventrally nor a ventral marker, Vax2, extend dorsally in E13.5 Pax6Sey-Neu/Pax6+; Mitfmi-vga9/Mitfmi-vga9 double mutants (Figure S6G–S6J). That the ventral RPE was unaffected in the Pax6/Mitf double mutants (Figure S6I, S6J) was likely due to the fact that Vax1/2 and Nr2f1/2, two genes whose alterations lead to ventral and central RPE transdifferentiation [26], [27], were wild type in these mice. Furthermore, at P0, the transdifferentiating RPE domain of Pax6Sey-Neu/Pax6+; Mitfmi-ΔD/Mitfmi-ΔD (Figure 4) and Pax6Sey-Neu/Pax6+; Mitfmi-vga9/Mitfmi-vga9 mutants (Figure S7) eventually generated differentiated retinal cells, including ISL1-positive ganglion cells, NF160-positive horizontal cells, and VC1.1-positive amacrine cells. They assumed a laminar structure similar to that seen in the normal retina, except that, as expected based on the developmental topology, the lamination was inverted relative to that of the retina [7]. In contrast, in Mitf mutants carrying the Pax6 YAC transgene, the RPE maintained its normal morphology as a monolayer. Taken together, the above results indicated that in two distinct Mitf mutants, a reduction in Pax6 gene dose promotes formation of a retina from the neuroepithelial domain that normally becomes RPE while an increase in Pax6 gene dose interferes with such a transition.
Mitf and Pax6 Regulate the Expression of the Mitf Paralog Tfec
It has previously been shown that the Mitf paralog Tfec is expressed in the developing RPE and that its expression is increased by homozygosity for Mitfmi-vga9 [28]. Tfec shares with Mitf a similar multi-promoter gene structure and encodes proteins with a basic-helix-loop-helix domain that is nearly identical with that of MITF [4], suggesting the two proteins can form heterodimers and share an overlapping set of target genes. We confirmed the increased expression of Tfec in the RPE of E11.5 Mitfmi-vga9 homozygotes by in situ hybridization (Figure 5E, 5F) and determined by qRT-PCR that this increase was 2.5-fold (data not shown). Tfec was, therefore, an excellent candidate gene that might participate in the above interplay between Mitf and Pax6 mutations. In fact, by in situ hybridization, Tfec was weakly upregulated in the RPE of E10.5 homozygous D-Mitf knock-outs (Figure 5A, 5B), downregulated when they were heterozygous for Pax6Sey-Neu (Figure 5C), and upregulated when they were transgenic for PAX6 YAC (Figure 5D). Quantitative RT-PCR confirmed that Tfec was upregulated about 1.7-fold in the E11.5 RPE of homozygous D-Mitf knock-outs, slightly, but significantly less when they carried the Pax6Sey-Neu allele, and significantly more when they carried the PAX6 YAC transgene (Figure 5I). Similar observations were made for Mitfmi-vga9/Pax6-mutant combinations by in situ hybridization even though the PAX6 YAC-mediated upregulation was not as striking as that in D-Mitf knock-outs (Figure 5E–5H). These results suggest that MITF regulates Tfec negatively in the RPE while PAX6 regulates it positively.
Fig. 5. Tfec is regulated by Pax6 and compensates an Mitf mutation in the RPE. 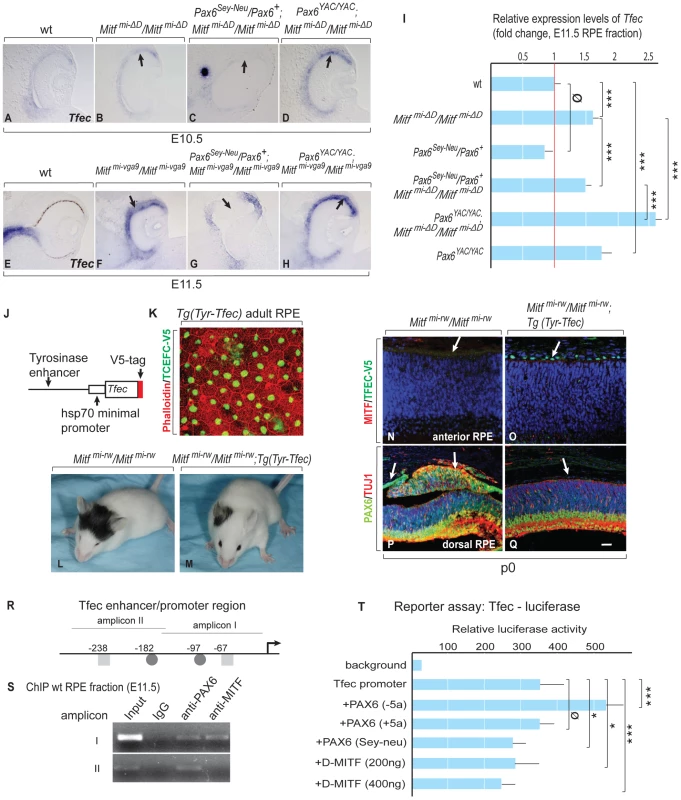
(A–D) In situ hybridization for Tfec in embryonic eyes of the indicated genotypes and developmental time points. Arrows mark areas with altered Tfec expression compared to wild type. (I) Quantitative RT-PCR analysis of Tfec mRNA levels in E11.5 RPE fractions obtained from the indicated mutants. Means and S.D. based on 3 biologically independent samples. Statistical significance shown as for Figure 3. (J) Schematic diagram of the Tyrosinase enhancer-hsp70 minimal promoter-Tfec-V5 expression cassette used for generating transgenic mice. (K) Flat mount of adult RPE from Tyr-Tfec transgenic line stained with Phalloidin (green) and anti-V5 antibody (red). (L,M) Transgenic TFEC rescues eye defects in Mitfmi-rw/Mitfmi-rw mice. The eyes of Tg (Tyr-Tfec);Mitfmi-rw/Mitfmi-rw mice (n = 18) are bigger than those of Mitfmi-rw/Mitfmi-rw mice (n = 16). (N–Q) Transgenic TFEC expression suppresses RPE-retina transdifferentiation in Mitfmi-rw/Mitfmi-rw mice. P0 mouse eye sections stained as shown, with nuclei stained with Topo3. (N,O) TFEC-V5 staining is seen only in Tg (Tyr-Tfec); Mitfmi-rw/Mitfmi-rw mice (arrows) and MITF staining is below threshold in this area of the RPE. (P,Q) Note absence of ectopic PAX6 and TUJ1 expression in the RPE of Tg (Tyr-Tfec); Mitfmi-rw/Mitfmi-rw mice. Scale bar (A–H): 110 µm; (N–Q): 90 µm. (R) Schematic diagram of the Tfec enhancer/promoter region. The positions of conserved potential binding sites for MITF (▪) and PAX6 (•) are given relative to the translation start site of Tfec isoform A [30]. (S) ChIP assays of wild-type RPE fractions dissected from E11.5 embryonic eyes, using PAX6 and MITF-specific antibodies. (T) A 700 bp Tfec enhancer/promoter region containing amplicons I and II (see R) was used for reporter assays in ARPE19 cells co-transfected with expression plasmids for the indicated transcription factors. Results represent normalized mean luciferase activity units obtained from 9 independent transfections. S.D. and statistical significance are indicated. To directly demonstrate that TFEC can rescue eye defects in an Mitf mutant, we generated lines of transgenic mice that express a V5-tagged Tfec cDNA under control of an RPE-specific enhancer of the Tyrosinase gene [29], and crossed them with microphthalmia red-eyed white (Mitfmi-rw) mice (Figure 5J–5Q). We chose this hypomorphic Mitf allele (rather than Mitfmi-vga9) because Mitfmi-rw homozygotes express small amounts of Tyrosinase and functional, though aberrant MITF protein, likely enough to stimulate the transgenic Tyrosinase enhancer, and yet they have an obvious small-eye phenotype [14]. Indeed, the Tyr-Tfec transgene was able to rescue eye size (Figure 5L, 5M) and RPE abnormalities (Figure 5N–5Q) of Mitfmi-rw homozygotes. Importantly, this rescue was not due to upregulation of the endogenous MITF (Figure 5O). Only minimal rescue in eye size was observed with transgenic lines expressing TFEC at lower levels (not shown). Hence, transgenic TFEC can replace MITF in the RPE at least with respect to its anti-proliferative functions.
To analyze whether PAX6 and MITF regulate Tfec directly, we employed chromatin immunoprecipitation (ChIP) and reporter assays, using a 5 kbp Tfec enhancer/promoter sequence. Based on expression analysis of Tfec RNA isoforms [30], this sequence corresponds to the upstream region of the only isoform we found expressed in RPE. It contains a number of conserved potential PAX6 and MITF binding sites that we arbitrarily grouped into six amplicons. ChIP analyses of wild-type RPE fractions showed PAX6 and MITF binding to amplicon I, PAX6-only binding to amplicon II (Figure 5R, 5S), and binding of neither protein to the other four amplicons (not shown). In reporter assays in the human RPE cell line ARPE19, a 700 bp promoter element containing amplicons I and II was spontaneously active. To test whether this reporter fragment could be further stimulated by PAX6, we expressed either one of two alternatively spliced Pax6 isoforms, Pax6 (+5a) and Pax6 (−5a), which differ by a 14-amino acids insertion in the paired domain and exhibit unique DNA binding properties [22], [23]. In fact, the Tfec promoter was stimulated by a vector expressing the PAX6 (−5a) but not the PAX6 (+5a) isoform. Transfection of a mutant Pax6 (−5a) cDNA that represents the Pax6Sey-Neu allele (stop codon at amino acid position 301) (18) did not stimulate the Tfec promoter. Importantly, transfection of a D-Mitf expression vector reduced the activity of the Tfec promoter in a dose-dependent manner, suggesting that Mitf can indeed repress Tfec (Figure 5T). These results suggest that in vivo, Tfec is regulated at least in part directly by PAX6/MITF and participates in the circuit that can partially compensate for the reduction or loss of MITF function, thus supporting Pax6's anti-retinogenic activities in the RPE.
Gene Expression Profiles in the Transdifferentiating RPE
To gain deeper insights into the underlying mechanisms of PAX6/MITF/TFEC-mediated RPE transdifferentiation, we performed a microarray analysis on the Affymetrix platform (Affymetrix Mouse Gene 1.0 ST). For this analysis, we used cDNAs prepared from E11.5 RPE fractions from the indicated D-Mitf knock-outs differing in their Pax6 gene dose, and from their respective controls. We centered the evaluation of up - and downregulated genes on Pax6Sey-Neu/Pax6+; Mitfmi-ΔD/Mitfmi-ΔD RPE fractions because their RPEs display the greatest extent of transdifferentiation. Table 1 and Table 2 show a selection of genes upregulated at least 1.49-fold in such RPE fractions and downregulated at least 1.43-fold when compared to wild-type RPE fractions (for full data and technical details, see Table S2). The microarray assay confirmed upregulation of retinal genes, including Vsx2, Rax, Pax6 and Six6, and downregulation of RPE genes, including Tyrp1, Silv, Tyr and Mitf, and showed the expected changes of these genes under the additional genetic configurations. Importantly, among the many genes whose expression changes in Pax6Sey-Neu/Pax6+; Mitfmi-ΔD/Mitfmi-ΔD RPEs, we found prominent upregulation of two genes that encode extracellular ligands and that are potentially involved in eye development. One, Fgf15, is a fibroblast growth factor whose paralogs have previously been shown to promote retinal at the expense of RPE development [31], [32]. The other, Dkk3, is a member of a family of genes involved in the inhibition of WNT signaling [33], which is known to promote RPE development [8], [11]. Dkk3's role in WNT signaling is not entirely clear, however, as in several cancer cell lines, WNT signaling is increased after Dkk3 downregulation [34] while in a Müller glia cell line (though not in cos7 cells), it is increased after Dkk3 upregulation [35]. This suggests that Dkk3 acts in a context-dependent way, prompting us to focus specifically on FGF15 and DKK3 in RPE transdifferentiation.
Tab. 1. Gene expression profiling in mutant E11.5 RPE fractions. 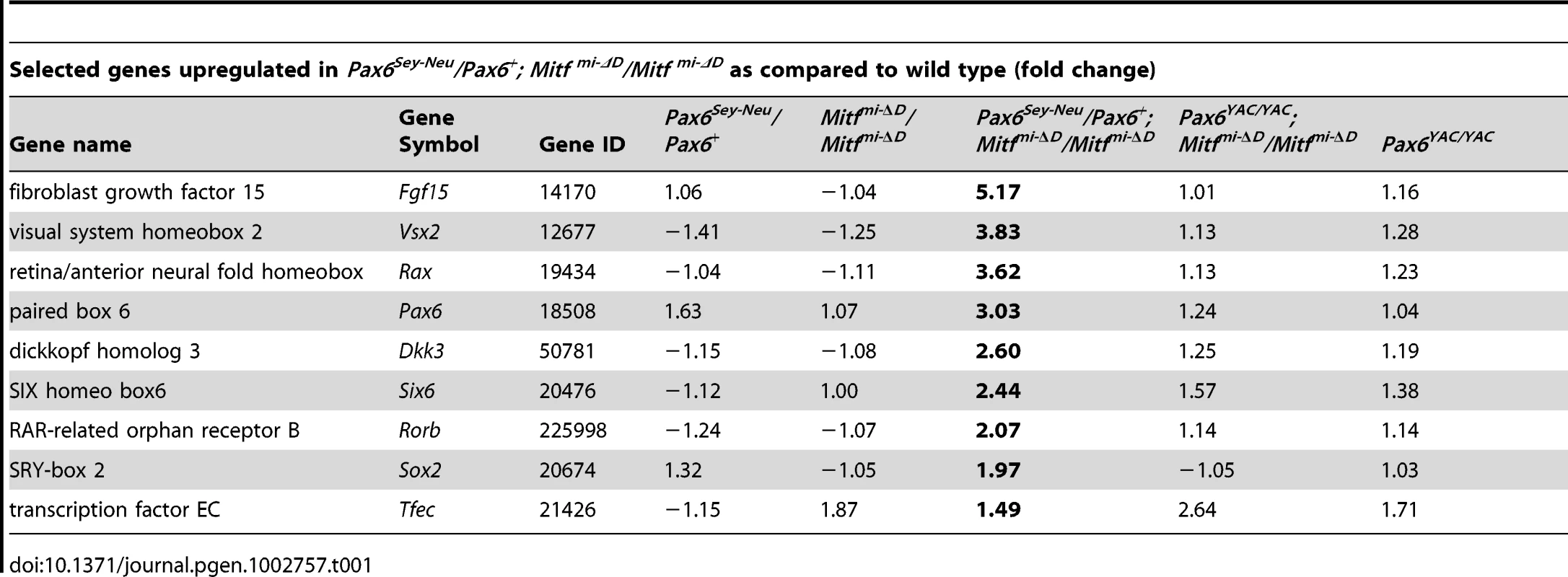
Tab. 2. Gene expression profiling in mutant E11.5 RPE fractions. 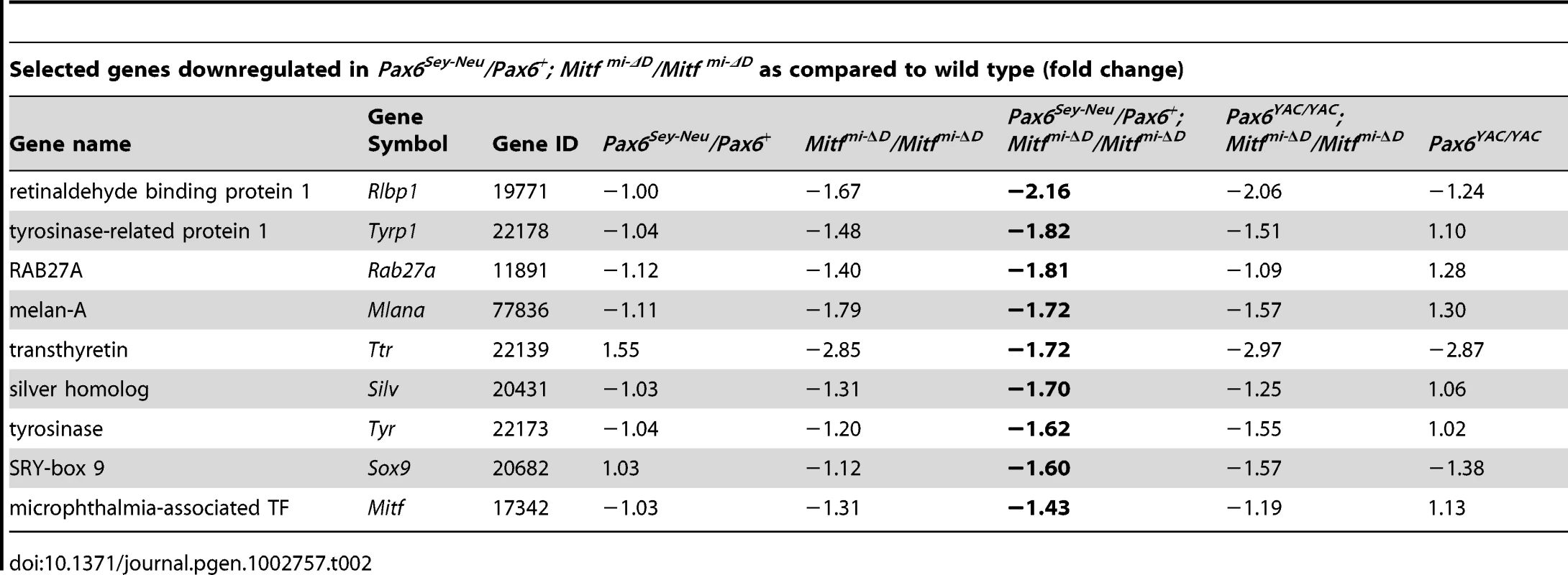
Fgf15 and Dkk3 Are Coordinately Regulated by PAX6 and MITF/TFEC
We first confirmed by qRT-PCR that Fgf15 and Dkk3 are indeed upregulated in RPE fractions of Pax6Sey-Neu/Pax6+; Mitfmi-ΔD/Mitfmi-ΔD embryos. As shown in Figure 6A, 6B, Fgf15 expression was increased approximately 15-fold and Dkk3 expression approximately four-fold compared to wild-type or Mitfmi-ΔD/Mitfmi-ΔD RPEs. Although gene expression profiling showed that another member of the Dkk family, Dkk1, was also upregulated in Pax6Sey-Neu/Pax6+;Mitfmi-ΔD/Mitfmi-ΔD RPEs (see Table S2), it did not show the prominent Pax6 gene dose-dependent difference observed for Dkk3 and was therefore not further analyzed. Strong expression of both Fgf15 and Dkk3 was also seen by in situ hybridization in the transdifferentiating RPEs of Pax6Sey-Neu/Pax6+;Mitfmi-vga9/Mitfmi-vga9 embryos at E11.5, but not in the RPEs of Pax6YAC/YAC;Mitfmi-vga9/Mitfmi-vga9 embryos (Figure 6C–6N, arrows in F,L pointing to the transdifferentiating RPE) and not at E9.5–E10 (data not shown). These results suggest that PAX6 and MITF/TFEC together normally suppress Fgf15 and Dkk3 in the developing RPE. Nevertheless, single reductions of either PAX6 or MITF alone have only mild effects on Fgf15 or Dkk3 expression, consistent with their milder phenotypes (Figure 6A–6N). The results also suggest that ectopically expressed Fgf15 and Dkk3 help to induce the RPE-to-retina transdifferentiation.
Fig. 6. PAX6 and MITF/TFEC together suppress Fgf15 and Dkk3 in the developing RPE. 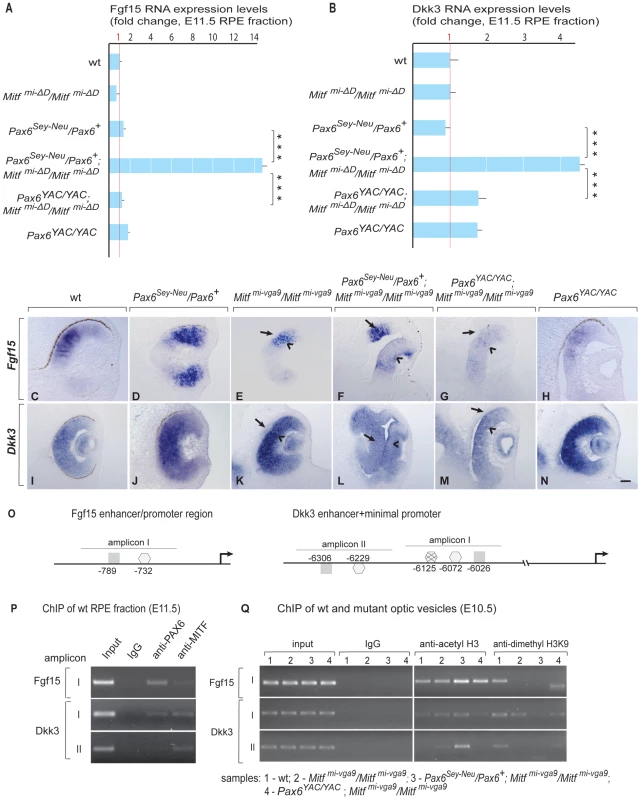
(A,B) Quantitative RT-PCR of Fgf15 and Dkk3 RNA of E11.5 RPE fractions of wild type and indicated mutants. Mean, S.D. and statistical significance based on 3 biological replicates. (C–N) In situ hybridization for Fgf15 (C–H) and Dkk3 (I–N) on eye sections of E11.5 embryos of the indicated genotypes. Arrows in E–G,K–M point to the RPE (note ectopic Fgf15 expression in the RPE of Pax6Sey-Neu/Mitfmi-vga9 double mutant in F and ectopic Dkk3 expression in the RPE of Pax6Sey-Neu/Mitfmi-vga9 double mutant in L). Scale bar (C–N):115 µm. (O) Schematic representation of Fgf15 and Dkk3 enhancer/promoter regions showing amplicons containing conserved potential binding sites for MITF (▪) and PAX6 (consensus , non-consensus ), with positions indicated relative to translation start sites. (P) ChIP assays performed with indicated antibodies on RPE fractions dissected from E11.5 wild-type embryonic eyes. Amplicons as indicated in (O). (Q) ChIP assays performed with the indicated antibodies on optic vesicle tissue dissected from E10.5 embryonic eyes of the indicated genotypes. Anti-acetyl H3 signal represents active chromatin domains and anti-dimethyl H3K9 signal inactive chromatin domains. For details see text. We next used ChIP and luciferase reporter assays similar to those shown for the Tfec promoter to test whether the regulation of Fgf15 and Dkk3 by Pax6 and Mitf/Tfec is direct or indirect. Although a 5 kbp region upstream of the Fgf15 translational start site showed various conserved potential binding sites for PAX6 and MITF, only one region, represented by amplicon I, which contains one potential PAX6 and one potential MITF binding site (Figure 6O, left side), gave ChIP signals on wild-type RPE fractions (Figure 6P and data not shown). For Dkk3, conserved potential binding sites for PAX6 or MITF, arbitrarily grouped into amplicons I and II (Figure 6O, right side) were present in a region between position −6026 and −6306 of the annotated start site of translation. ChIP assays with wild-type RPE fractions gave signals for both PAX6 and MITF with Dkk3 amplicon I, but only for MITF with amplicon II (Figure 6P). Luciferase reporter assays in RPE cells (ARPE19) showed that PAX6, MITF and TFEC negatively regulate these sequence elements, though in the case of Fgf15 only when PAX6 and MITF, or PAX6 and TFEC, were co-expressed with the reporter (Figure S8). As done for the Tfec promoter, we then evaluated the chromatin status of these elements in the mutants, again using whole optic vesicles. Although part of the ChIP signal in optic vesicles was likely contributed by the high level of expression of Fgf15 and Dkk3 in the retina, there was a substantial increase in the anti-acetyl H3 ChIP signal in Pax6Sey-Neu/Pax6+; Mitfmi-vga9/Mitfmi-vga9 for Fgf15 amplicon I and Dkk3 amplicon II (lane 3 in Figure 6Q) when compared to the respective controls. Conversely, the anti-dimethyl H3K9 ChIP signal was only seen in wild-type and not in Pax6Sey-Neu/Pax6+; Mitfmi-vga9/Mitfmi-vga9. These results suggest that the tested promoter regions are indeed subject to in vivo regulation by PAX6 and MITF and that this regulation is at least in part direct.
DKK3 and FGF Cooperate to Promote RPE Transdifferentiation
The above results show that Dkk3 and Fgf15 are major targets of the circuits that regulate RPE development. To test whether DKK3 and FGF signaling are also actively involved in RPE transdifferentiation, we employed wild-type optic vesicle explant cultures into which beads soaked in human recombinant DKK3 or FGF2 (which, like FGF15 or its human ortholog FGF19, binds the same isoforms of all four main FGF receptors, [36]) were implanted. To test if FGF and DKK3 signaling can induce transdifferentiation in RPE cells, we used explant cultures established at E10.0, when the RPE fate is just specified. If left untreated or implanted with a bead coated with bovine serum albumin, these explant cultures normally develop within 48 hours into optic cups with a clearly pigmented RPE [Figure 7A, 15/15 cultures developing a pigmented RPE; see also [7]]. The implantation of beads soaked in human recombinant DKK3 at 0.65 or 1 µg/ml had little effect on RPE development (Figure 7B, 0.65 µg/ml, 8/8 cultures with pigmented RPE). Therefore, we tested for cooperation between DKK3 and FGF signaling. Previous results showed that beads soaked in 1 µg/ml of FGF2 are capable of inducing RPE transdifferentiation on their own [6], [7]. Nevertheless, a dose response curve indicated that implanting beads soaked in 0.35 µg/ml or less of FGF2 was without effect on pigmentation (Figure 7C, 0.35 µg/ml, 10/10 cultures with pigmented RPE). Beads soaked in a mixture of 0.65 µg/ml of DKK3 and 0.35 µg/ml of FGF2, however, markedly reduced pigmentation in the vicinity of the bead (Figure 7D, 8/10 cultures showing segmental RPE depigmentation). Furthermore, in situ hybridization for Vsx2 and Six6 showed that only the double DKK3/FGF2 treatment led to expression of these retinal genes in the RPE (Figure 7E–7L). To test whether DKK3 would inhibit the canonical WNT signaling pathway, we used optic vesicle cultures from mice transgenic for the WNT reporter Tcf-LacZ [37]. As shown in Figure 7M–7P, DKK3 beads alone could not repress ßGAL expression (nor could, for that matter, FGF2 beads alone: 8/8 and 4/4 cultures, respectively, retained ßGAL staining). However, the combination of DKK3 and FGF2 at the above concentrations efficiently repressed ßGAL expression (5/7 cultures lacking ßGAL staining).
Fig. 7. FGF and DKK3 induce RPE transdifferentiation. 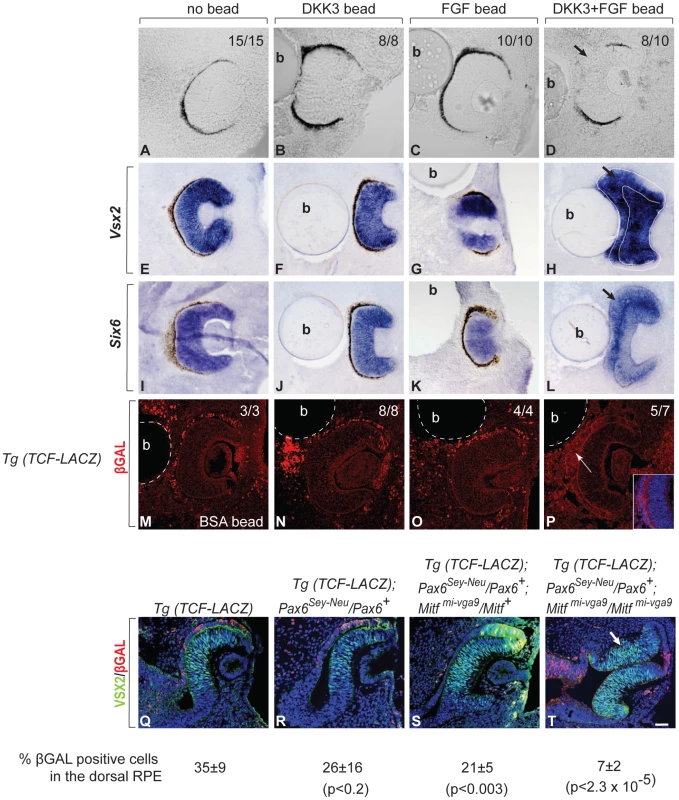
(A–P) Cultures of developing eyes explanted from E10.0 wild-type embryos. (A–D) RPE pigmentation develops within 48 hours in the absence of a bead (A) or in the presence of beads soaked in 0.65 µg/ml of recombinant DKK3 (B) or 0.35 µg/ml of recombinant FGF2 (C) but is segmentally missing in the vicinity of a bead soaked in a combination of 0.65 µg/ml of DKK3 and 0.35 µg/ml of FGF2 (D). The number of cultures with the represented results per total cultures tested is shown in the upper right corner. (E–L) Representative cultures were fixed, cryosectioned and subjected to in situ hybridization with the indicated probes. Note induction of the retinal factors Vsx2 and Six6 in the RPEs only after implantation of a DKK3/FGF2 double-coated bead (H,L). (M–P) Cultures were established from TCF-LacZ transgenic embryos and implanted with beads coated with bovine serum albumin (BSA, M) or the indicated growth factors. They were fixed, cryosectioned, and stained with antibodies to ßGAL. Note absence of ßGAL staining only in cultures implanted with double-coated beads (inset shows higher magnification of the RPE region) (P). The number of cultures with the shown results per total cultures established is shown in the right upper corner. (Q–R) VSX2/ßGAL double-labeled cryosections of E10.5 embryos of the indicated genotypes. Note absence of ßGAL labeling in the transdifferentiating portions of the RPEs in Mitfmi-vga9 heterozygous or homozygous embryos when they carry a Pax6Sey-Neu allele (S,T, arrow in T). Numbers represent % ßGAL positive cells in the dorsal RPE. P values based on Student's t test. Scale bar (A–D, M–P, Q–T): 90 µm; (E–L): 115 µm. The above results suggested that DKK3 and FGF cooperate to effect RPE transdifferentiation through the inhibition of WNT signaling and are sufficient to exert this effect. To test whether the Pax6/Mitf mutations (which, as shown above, upregulate Dkk3 and Fgf15) also inhibit WNT signaling, we crossed the Tcf-LacZ transgene into Pax6Sey-Neu/Pax6+ mice heterozygous or homozygous for Mitfmi-vga9. Double immunolabeling of E10.5 optic cups for VSX2 and ßGAL clearly showed that RPE transdifferentiation in Pax6Sey-Neu/Pax6+; Mitfmi-vga9/Mitf+ and Pax6Sey-Neu/Pax6+; Mitfmi-vga9/Mitfmi-vga9 embryos was associated with suppression of ßGAL expression, suggesting that enabling WNT signaling by inhibiting DKK3 and FGF is the common pathway through which the PAX6/MITF/TFEC regulatory circuit operates in the RPE (Figure 7Q–7T).
Discussion
Here we provide genetic evidence that the transcription factor PAX6, which is known in vertebrates to be crucial for the development of cornea, iris and retina, is also critical for early RPE development when tested in an Mitf mutant background. In fact, we find that overexpression of PAX6 in the Mitfmi-vga9 null mutant background efficiently suppresses the RPE-to-retina transdifferentiation caused by Mitf-downregulation while a reduction in Pax6 enhances this transdifferentiation. Hence, in the RPE, Pax6 shares with Mitf an anti-retinogenic effect while in the retina it is pro-retinogenic. Although Pax6 and, for that matter, many other transcription factors have evolved to play different roles in different tissues, we have to keep in mind that future retina and RPE are both derived from the same, seemingly uniform optic neuroepithelium and maintain a remarkable capacity to switch from one into the other during development and, in some vertebrates, even in adulthood [2]. As outlined below, the regulatory circuit that we here describe and schematically depict in Figure 8 may provide an explanation for the differential function of Pax6 in retina and RPE and for the easy phenotypic switch between the two.
Fig. 8. Model of the regulatory circuit involving <i>Pax6</i>, <i>Mitf</i>, and <i>Tcfec</i> during mouse RPE development. 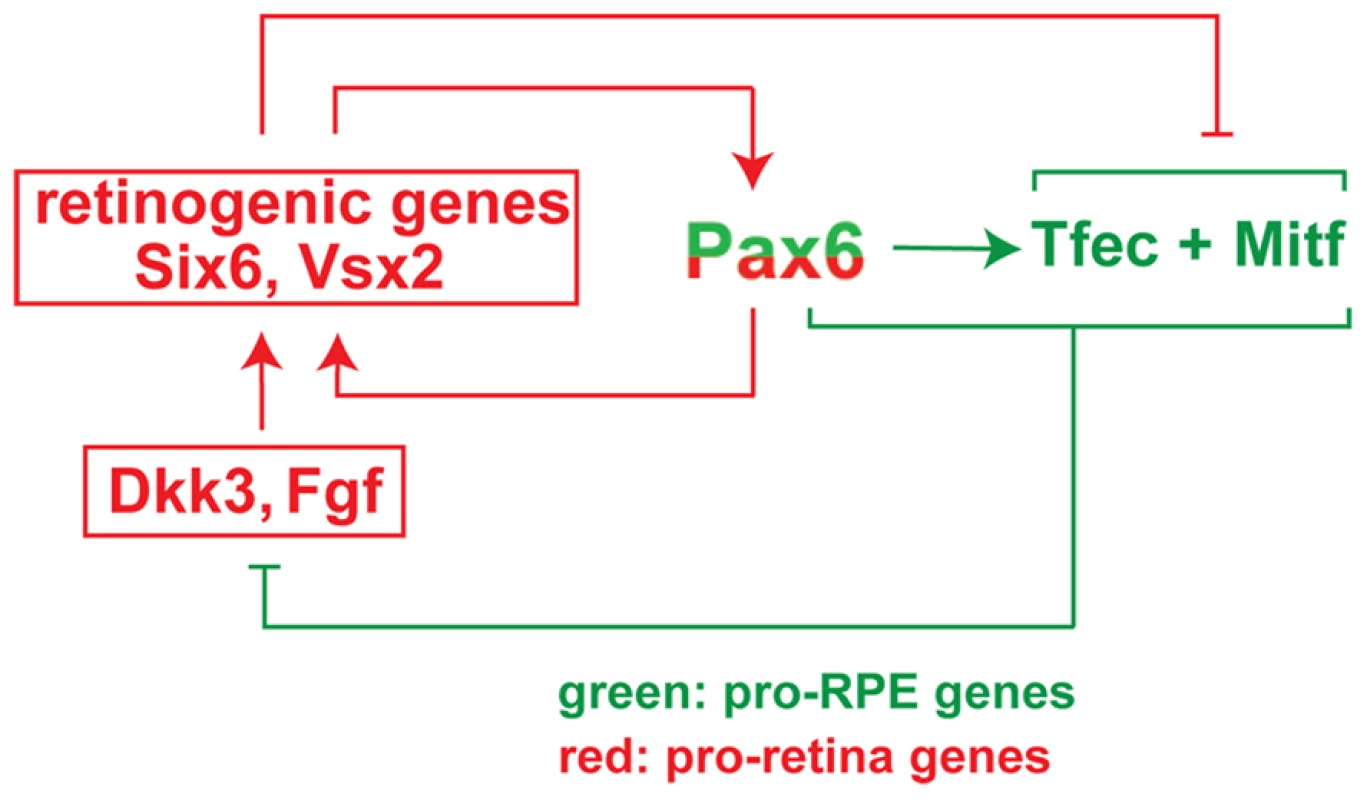
There is ample evidence that the initial separation of the optic neuroepithelium into future retina and RPE is effected by extracellular signals, predominantly FGFs, that emanate from the surface ectoderm. FGFs induce Vsx2 in the distal optic vesicle domain, which in turn downregulates Mitf and Tfec and initiates the retinal developmental program [6], [28]. In contrast, BMP, ACTIVIN and WNT signaling in or around the dorso-proximal optic vesicle may stimulate Mitf in the dorso-proximal neuroepithelium and initiate the RPE developmental program [8], [10]–[12]. As soon as a slight bias in gene expression patterns is established to support either RPE or retina differentiation, the regulatory circuit shown in Figure 8 will reinforce this bias in the following way. Coexpression of Pax6 with either Mitf or Tfec suppresses the expression of the retinogenic genes Fgf15 and Dkk3, thus enabling canonical WNT signaling, which in turn leads to upregulation of Mitf and Tfec expression. This positive feed back loop potentiates RPE differentiation and suppresses the retinal fate. If, on the other hand, PAX6, MITF and TFEC together are unable to inhibit Fgf15 and Dkk3 expression, then canonical WNT signaling is inhibited and several retinogenic genes including Six6, Lhx2, and Vsx2 are upregulated. These retinogenic genes will further stimulate the expression of Pax6 in pro-retina and anti-RPE feed-forward loops [38], and Mitf expression is suppressed. Likewise, Tfec expression is suppressed under these conditions because the repressive effect of other retinogenic genes overcomes Pax6's stimulatory effect on Tfec (for instance, based on the analysis of Vsx2 mutants, Vsx2 suppresses Tfec in the retina despite the presence of Pax6 [6], [28]. This regulatory circuit has the typical features of a bi-stable loop as it may assume only one of two states: either Mitf and/or Tfec are high and retinogenic genes (with the exception of Pax6) are low, or Mitf and/or Tfec are low, and retinogenic genes are high (Figure 8). Consequently, the neuroepithelium will develop either as a retina or as an RPE. Perturbations of the circuit, such as mutations in Mitf [14] or Vsx2 [6], [28] or gene dose changes in Pax6 could then flip the switch and lead to an inversion of cell fates rather than an indeterminate, mixed phenotype.
Experimental evidence for the above consideration is also provided by the earlier observation that physical removal of the surface ectoderm in optic vesicle cultures, and hence removal of retina-inducing signals, does not lead to the formation of two RPE-like monolayers as one might predict, but rather to a distal pigmented monolayer and a proximal retina-like tissue [7]. As this happens without deliberate addition of a source of FGFs on the proximal side, we have to assume that removal of the surface ectoderm establishes a new initial bias that is again reinforced by the Pax6/Mitf/Tfec regulatory circuit. It is conceivable that this is so because removal of the surface ectoderm removes not only FGF signals but other signals as well. In the chick, for instance, the dorsal portion of the surface ectoderm expresses bone morphogenic protein-4 and -7 which help initiate RPE development [10], and neural crest cells normally provide TGF-ß1 and ACTIVINS that stimulate WNT signaling in the dorsal ectoderm [9], [12]. Perhaps even more intriguing is the recent observation that optic neuroepithelial vesicles established entirely in vitro from mouse embryonic stem cells spontaneously self-organize into optic cups with properly oriented retina and RPE, without any overt presence of a typical surface ectoderm [39]. This phenomenon, too, could be explained if any initial developmental bias is reinforced by the proposed bi-stable nature of the circuit.
The above considerations, though, should not lead one to assume that the Pax6/Mitf/Tfec circuit is uninfluenced by other genes. A recent study showed, for instance, that in the absence of the neurogenic transcription factor SOX2, Pax6 expression in the distal optic neuroepithelium increases, leading to conversion to ciliary epithelium but not RPE [40]. Perhaps this observation is linked to the fact that in Mitf single or Pax6/Mitf double mutants, the anterior most portion of the dorsal RPE never undergoes transdifferentiation into retina, likely because of the above mentioned RPE-inducing signals from the adjacent dorsal surface ectoderm [9], [10]. Furthermore, what we observe in mice need not necessarily apply to other vertebrates. For instance, removal of the surface ectoderm from chicken optic vesicles led to a salt and pepper structure of intermingled pigmented and non-pigmented cells, not to cleanly separated pigmented monolayers and hyperproliferating retinas as seen in mice [41]. We would predict, therefore, that even though the molecular players may be the same across all vertebrates, they may not be interconnected in the same way in all vertebrates. In fact, the PAX6 binding sites that we identified in the mouse Tfec promoter, although conserved across several mammalian species, are not conserved in birds and reptiles. Such differences could well explain the differences in the developmental time frame during which RPE and retina can interconvert in different species.
Finally, we would like to draw attention to the potential importance of the role of Pax6, Mitf and Tfec and their regulatory targets for the establishment of retinal and RPE cells from embryonic or induced pluripotent stem cells. The ability to obtain such cells in vitro has recently generated much excitement for the study of the pathogenesis of blindness caused by primary retinal or RPE malfunctions, such as adult onset macular degeneration, as well as for the eventual cell-based therapy of such diseases [42]. Although the methods to generate such cells are rapidly improving, the process is still not very well controlled, and it remains to be seen whether the in vitro generated cells represent truly authentic cell types [42]. As the production of such cells has many of the hallmarks of development, we think a careful consideration of the normal developmental pathways is paramount for their successful generation. In the light of the results presented in this paper, it would seem important, therefore, that the activity levels of PAX6 and its upstream regulators and downstream targets be carefully monitored during the in vitro generation of retinal and RPE cells.
In conclusion, we have shown that positioning of PAX6 in the center of a bi-stable regulatory loop allows this single transcription factor to be bi-functional and to participate either in a pro-retinogenic or a pro-RPE developmental pathway.
Materials and Methods
Mice
Extant Pax6 and Mitf mutants and transgenics are described in Table S1 and were kept on a C57BL/6J background (Backcrosses: 20 for Pax6Sey-Neu, 2 for PAX6Yac, 7 for Mitfmi-vga9, strain of origin of Mitfmi-rw is C57BL/6J). C57BL/6J served as wild-type controls. Mitfmi-ΔD/Mitfmi-ΔD targeted mice were generated using the recombineering technology. To generate the targeting construct, the D-Mitf promoter/D-Mitf exon and its flanking regions (15,801 kbp) were cloned using plasmid rescue from BACRP23-9A13. A floxed neomycin resistance expression cassette flanked by 200 bp of sequence flanking the D-Mitf promoter/D-Mitf exon was used to replace 5.8 kbp of the D-Mitf promoter/D-Mitf exon from the above plasmid and used for standard targeting of LC3 ES cells (genotype [C57BL/6Nx129S6]F1), giving 6 correctly targeted colonies/40 colonies tested. Of several germline transmitting lines, one, officially designated Mitftm3Arnh; MGI: 5050698, was selected and crossed with C57BL/6J•129S4-Prm1-Cre deleter mice (Jackson Laboratories, stock 003328, backcrossed twice to C57BL/6J). Offspring lacking the neo-cassette (Mitftm3.1Arnh; MGI: 5050699) were backcrossed to C57BL/6J twice and then bred to homozygosity with or without the corresponding Pax6 alleles or transgenes.
Tyr-Tfec transgenic mice (strain C57BL/6N) were generated using a construct composed of (5′-3′) a 4721 bp Tyr RPE-specific enhancer, a 985 bp hsp70 minimal promoter [29], and a 1203 bp V5-tagged Tfec cDNA. Four transgenic lines were obtained and used for crosses with Mitfmi-rw mice. Genotyping of mice was performed by Southern blot and/or PCR using primers shown in Table S3. All animal experiments were covered by approved animal protocols.
Immunostaining and In Situ Hybridization
Immunostaining and in situ hybridizations were performed as described previously, using 16 µm thick coronal cryostat sections [14]. All eye sections are shown with the dorsal side up. Cell proliferation analysis was done by phosphohistone H3 staining of three different embryos. Immunostaining of adult mouse RPE flat-mounts was performed as described by [43]. A Zeiss LSM510 confocal (Zeiss, Thornwood, NY) and a Nikon E800 (Nikon, Melville, NY) microscope were used to record immunostainings. A Polyvar microscope (Reichert Jung, Depew, NY) was used for recording in situ hybridizations. For antibodies and in situ probes, see Tables S4 and S5.
Expression Analysis and Whole-Genome Expression Profiling
For RNA expression, E9.5 and E10.5 optic vesicles or RPE and retinal fractions from E11.5–E18.5 embryos and P0 mice were prepared as described. RNA was extracted from pools of 20–40 individual samples and RT-PCR and real time PCR was performed according to previously published protocols [14]. For primers, see Table S1. Statistical analysis was done using two-tailed Student's t-test. Statistical significance of the data is represented as (ø) non-significant; (*) p<0.05; (**) p<0.01; and (***) p<0.001. For microarray analysis, the Affymetrix platform was used in collaboration with the NHGRI/NINDS microarray core facility and bioinformatics core.
Chromatin Immunoprecipitation (ChIP) Assays
Pools of optic vesicles from ∼20 individual E10.5 eyes or RPE fractions from ∼30 individual E11.5 eyes were prepared as previously described [14]. ChIP assays using ChIP IT kit from Active Motif (Carlsbad, CA) was performed as described. Primers and antibodies are given in Tables S3 and S5.
Reporter Assays
Reporter assays were performed using ARPE19 (CRL-2302, ATCC, Manassas, VA) and the dual luciferase assay kit (Promega, Madison, WI). Statistical analysis was done using two-tailed Student's t-test. Details about the plasmids used for reporter assays are given in Table S3.
Optic Vesicle Culture
Cultures were established from E10.0 embryonic heads and maintained for 48–72 hours using published protocols [7]. Polyacrylamide beads used for implantation were soaked for an hour with BSA, recombinant DKK3 (R&D Systems, Minneapolis, MN), recombinant FGF2 (R&D Systems, Minneapolis, MN), or their combinations at the indicated concentrations. In situ hybridization/immunostaining of the vesicles was performed on 16 µm thick cryostat sections.
Supporting Information
Zdroje
1. BhartiKNguyenMTSkuntzSBertuzziSArnheiterH 2006 The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Research 19 380 394
2. KarlMORehTA 2010 Regenerative medicine for retinal diseases: activating endogenous repair mechanisms. Trends Mol Med 16 193 202
3. MillerSSMaminishkisALiRAdijantoJ 2010 Retinal Pigment Epithelium: Cytokine Modulation of Epithelial Physiology. DarttDA Encyclopedia of the Eye: Oxford University Academic Press 89 100 editor pp
4. HemesathTJSteingrimssonEMcGillGHansenMJVaughtJ 1994 microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev 8 2770 2780
5. HodgkinsonCAMooreKJNakayamaASteingrimssonECopelandNG 1993 Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74 395 404
6. HorsfordDJNguyenMTSellarGCKotharyRArnheiterH 2005 Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 132 177 187
7. NguyenMArnheiterH 2000 Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 127 3581 3591
8. FujimuraNTaketoMMMoriMKorinekVKozmikZ 2009 Spatial and temporal regulation of Wnt/beta-catenin signaling is essential for development of the retinal pigment epithelium. Dev Biol 334 31 45
9. GrocottTJohnsonSBaileyAPStreitA 2011 Neural crest cells organize the eye via TGF-beta and canonical Wnt signalling. Nat Commun 2 265
10. MüllerFRohrerHVogel-HopkerA 2007 Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development 134 3483 3493
11. WestenskowPPiccoloSFuhrmannS 2009 Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development 136 2505 2510
12. FuhrmannSLevineEMRehTA 2000 Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development 127 4599 4609
13. BumstedKMBarnstableCJ 2000 Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest Ophthalmol Vis Sci 41 903 908
14. BhartiKLiuWCsermelyTBertuzziSArnheiterH 2008 Alternative promoter use in eye development: the complex role and regulation of the transcription factor MITF. Development 135 1169 1178
15. GrindleyJCDavidsonDRHillRE 1995 The role of Pax-6 in eye and nasal development. Development 121 1433 1442
16. Martinez-MoralesJRRodrigoIBovolentaP 2004 Eye development: a view from the retina pigmented epithelium. Bioessays 26 766 777
17. QuinnJCWestJDHillRE 1996 Multiple functions for Pax6 in mouse eye and nasal development. Genes Dev 10 435 446
18. HillREFavorJHoganBLTonCCSaundersGF 1991 Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354 522 525
19. MarquardtTAshery-PadanRAndrejewskiNScardigliRGuillemotF 2001 Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105 43 55
20. SchedlARossALeeMEngelkampDRashbassP 1996 Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell 86 71 82
21. ManuelMPrattTLiuMJefferyGPriceDJ 2008 Overexpression of Pax6 results in microphthalmia, retinal dysplasia and defective retinal ganglion cell axon guidance. BMC Dev Biol 8 59
22. CollinsonJMQuinnJCHillREWestJD 2003 The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev Biol 255 303 312
23. PlanqueNLeconteLCoquelleFMMartinPSauleS 2001 Specific Pax-6/microphthalmia transcription factor interactions involve their DNA-binding domains and inhibit transcriptional properties of both proteins. J Biol Chem 276 29330 29337
24. BäumerNMarquardtTStoykovaASpielerDTreichelD 2003 Retinal pigmented epithelium determination requires the redundant activities of Pax2 and Pax6. Development 130 2903 2915
25. KosoHIidaATabataYBabaYSatohS 2008 CD138/syndecan-1 and SSEA-1 mark distinct populations of developing ciliary epithelium that are regulated differentially by Wnt signal. Stem Cells 26 3162 3171
26. MuiSHKimJWLemkeGBertuzziS 2005 Vax genes ventralize the embryonic eye. Genes Dev 19 1249 1259
27. TangKXieXParkJIJamrichMTsaiS 2010 COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development 137 725 734
28. RowanSChenCMYoungTLFisherDECepkoCL 2004 Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 131 5139 5152
29. MurisierFGuichardSBeermannF 2007 Distinct distal regulatory elements control tyrosinase expression in melanocytes and the retinal pigment epithelium. Dev Biol 303 838 847
30. KuiperRPSchepensMThijssenJSchoenmakersEFvan KesselAG 2004 Regulation of the MiTF/TFE bHLH-LZ transcription factors through restricted spatial expression and alternative splicing of functional domains. Nucleic Acids Res 32 2315 2322
31. GuillemotFCepkoCL 1992 Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development 114 743 754
32. ZhaoSThornquistSCBarnstableCJ 1995 In vitro transdifferentiation of embryonic rat retinal pigment epithelium to neural retina. Brain Res 677 300 310
33. NiehrsC 2006 Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25 7469 7481
34. YueWSunQDacicSLandreneauRJSiegfriedJM 2008 Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis 29 84 92
35. NakamuraREHunterDDYiHBrunkenWJHackamAS 2007 Identification of two novel activities of the Wnt signaling regulator Dickkopf 3 and characterization of its expression in the mouse retina. BMC Cell Biol 8 52
36. GuillemotFZimmerC 2011 From cradle to grave: The multiple roles of fibroblast growth factors in neural development. Neuron 71 574 588
37. MohamedOAClarkeHJDufortD 2004 Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn 231 416 424
38. ZuberMEGestriGViczianASBarsacchiGHarrisWA 2003 Specification of the vertebrate eye by a network of eye field transcription factors. Development 130 5155 5167
39. EirakuMTakataNIshibashiHKawadaMSakakuraE 2011 Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472 51 56
40. MatsushimaDHeavnerWPevnyLH 2011 Combinatorial regulation of optic cup progenitor cell fate by SOX2 and PAX6. Development 138 443 454
41. HyerJMimaTMikawaT 1998 FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development 125 869 877
42. BhartiKMillerSSArnheiterH 2011 The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res 24 21 34
43. CamposMAmaralJBecerraSPFarissRN 2006 A novel imaging technique for experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 47 5163 5170
Štítky
Genetika Reprodukčná medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



