-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
Many genetic and physiological treatments that extend lifespan also confer resistance to a variety of stressors, suggesting that cytoprotective mechanisms underpin the regulation of longevity. It has not been established, however, whether the induction of cytoprotective pathways is essential for lifespan extension or merely correlated. Using a panel of GFP-fused stress response genes, we identified the suites of cytoprotective pathways upregulated by 160 gene inactivations known to increase Caenorhabditis elegans longevity, including the mitochondrial UPR (hsp-6, hsp-60), the ER UPR (hsp-4), ROS response (sod-3, gst-4), and xenobiotic detoxification (gst-4). We then screened for other gene inactivations that disrupt the induction of these responses by xenobiotic or genetic triggers, identifying 29 gene inactivations required for cytoprotective gene expression. If cytoprotective responses contribute directly to lifespan extension, inactivation of these genes would be expected to compromise the extension of lifespan conferred by decreased insulin/IGF-1 signaling, caloric restriction, or the inhibition of mitochondrial function. We find that inactivation of 25 of 29 cytoprotection-regulatory genes shortens the extension of longevity normally induced by decreased insulin/IGF-1 signaling, disruption of mitochondrial function, or caloric restriction, without disrupting normal longevity nearly as dramatically. These data demonstrate that induction of cytoprotective pathways is central to longevity extension and identify a large set of new genetic components of the pathways that detect cellular damage and couple that detection to downstream cytoprotective effectors.
Published in the journal: Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways. PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002792
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002792Summary
Many genetic and physiological treatments that extend lifespan also confer resistance to a variety of stressors, suggesting that cytoprotective mechanisms underpin the regulation of longevity. It has not been established, however, whether the induction of cytoprotective pathways is essential for lifespan extension or merely correlated. Using a panel of GFP-fused stress response genes, we identified the suites of cytoprotective pathways upregulated by 160 gene inactivations known to increase Caenorhabditis elegans longevity, including the mitochondrial UPR (hsp-6, hsp-60), the ER UPR (hsp-4), ROS response (sod-3, gst-4), and xenobiotic detoxification (gst-4). We then screened for other gene inactivations that disrupt the induction of these responses by xenobiotic or genetic triggers, identifying 29 gene inactivations required for cytoprotective gene expression. If cytoprotective responses contribute directly to lifespan extension, inactivation of these genes would be expected to compromise the extension of lifespan conferred by decreased insulin/IGF-1 signaling, caloric restriction, or the inhibition of mitochondrial function. We find that inactivation of 25 of 29 cytoprotection-regulatory genes shortens the extension of longevity normally induced by decreased insulin/IGF-1 signaling, disruption of mitochondrial function, or caloric restriction, without disrupting normal longevity nearly as dramatically. These data demonstrate that induction of cytoprotective pathways is central to longevity extension and identify a large set of new genetic components of the pathways that detect cellular damage and couple that detection to downstream cytoprotective effectors.
Introduction
Lifespan can be extended in C. elegans and other organisms by a variety of ostensibly deleterious interventions: disruption of mitochondrial function, disruption of translation, disruption of insulin/IGF-1 signaling, caloric restriction, exposure to xenobiotics and others. The counterintuitive benefits of these stressful stimuli suggest a hormetic mechanism rooted in the beneficial induction of cytoprotective pathways that respond to environmental challenges, such as starvation, heat, or exposure to xenobiotics. These cytoprotective pathways may represent the mechanisms that drive lifespan extension. While the correlation of stress tolerance and longevity is well established, the underlying cytoprotective pathways have not been fully explored.
Many of the gene inactivations that extend lifespan encode core, conserved components of cells, such as translation factors or mitochondrial proteins, many of which are the molecular targets of known xenobiotics [1]. Lifespan-extending inactivation of cytochrome C reductase, ATP synthase, F59C6.5 in electron transport chain (ETC) complex I, or cytochrome C oxidase may induce the same cytoprotective responses as the xenobiotics that target them, which include antimycin, oligomycin, rotenone and sodium azide, respectively. Similarly, a wide variety of xenobiotics disrupt translation, including hygromycin, genetecin and emetine. Disruption of endoplasmic reticulum (ER) function also extends longevity and may induce cytoprotective mechanisms effective against ER-targeted xenobiotics such as tunicamycin or thapsigargin. The parity of essential cell components targeted by xenobiotics and those that extend longevity upon inactivation suggests that long-lived animals engage cytoprotective mechanisms that evolved as cellular homeostatic and detoxification responses to xenobiotics and virulence factors produced by other organisms.
Cytoprotective responses, including chaperones, antioxidants and pathogen response genes, as well as xenobiotic detoxification mechanisms, can protect extant components of the cell and may contribute to lifespan extension. Genetic studies have identified over 50 mutations that extend the lifespan of C. elegans, and each is resistant to one or more stressors, such as oxidative damage, heat stress or irradiation [2], [3]. The oxidative stress theory of aging has driven extensive analysis of oxidative damage in particular, and while long-lived animals are resistant to compounds that generate ROS, such as paraquat, identification of underlying mechanisms has proven challenging [4], [5], [6], [7], [8], [9], [10], [11], [12]. Longevity is also correlated with thermotolerance, and expression of the heat shock response gene hsp-16.2 predicts longevity in C.elegans [13], [14], [15], [16], [17], [18]. In a genetic screen for enhanced thermotolerance, the majority of isolated mutants were long-lived by at least 15% [19]. Other protein folding mechanisms, such as the ER and mitochondrial unfolded protein responses, contribute to longevity as well [20], [21], [22], [23]. Cellular damage may result from the production of toxic metabolic byproducts or exposure to xenobiotics, consistent with the extension of lifespan by overexpression of the detoxification transcription factor skn-1, a gene that is also required for lifespan extension in daf-2 mutants [24], [25], [26], [27], [28], [29], [30], [31]. The potential influence of diverse cytoprotective functions on longevity is underscored by the heat, ROS and toxin resistance of long-lived animals.
Consistent with the stress-tolerant phenotypes of long-lived animals, cytoprotective mechanisms are activated in long-lived mutants. Disruption of insulin/IGF-1 signaling induces heat shock (hsp-16.49, hsp-16.11, hsp-16.1, hsp-16.2 and hsp-12.6), antioxidant (ctl-1, ctl-2 and sod-3), and pathogen response (lys-7, lys-8 and spp-1) genes [32]. The long-lived mitochondrial mutants isp-1, clk-1 and cyc-1 induce the mitochondrial unfolded protein response [33]. Inactivation of the translation initiation factor ifg-1 induces the transcription of 51 stress responsive genes, including daf-16 and skn-1 [34]. In each of these long-lived mutants, evidence suggests concurrent induction of detoxification mechanisms. The detoxification of xenobiotics in many systems, including C. elegans, involves the upregulation of cytochrome P450's (CYPs), UDP-glucuronosyltransferases (UGTs), and glutathione S-transferases (GSTs). Transcriptional profiling of the long-lived daf-2 insulin/IGF-1 signaling mutant reveals daf-16-dependent upregulation of these functions [32], [35]. A xenobiotic response is similarly induced in long-lived mitochondrial mutants and lifespan extension by disruption of translation requires skn-1, which participates in xenobiotic stress tolerance [36], [37]. While the response to xenobiotics is, in part, the upregulation of detoxification, other cytoprotective mechanisms, such as chaperones, mitigate cellular damage; detoxification and cytoprotection may both be components of a xenobiotic response apparatus mobilized by various aging interventions.
Mechanistic evidence supports the causality of cytoprotective gene activation in lifespan extension. Loss of chaperone expression through inactivation of hsf-1, the transcriptional regulator of the heat shock response genes, abrogates lifespan extension in a daf-2 mutant, while overexpression extends the lifespan of wild-type animals [38]. The ER unfolded protein response (UPR) underlies lifespan extension in daf-2 mutants and in response to caloric restriction [22], [39]. The mitochondrial UPR is required for lifespan extension in the mitochondrial mutants isp-1 and clk-1 [21]. Lifespan regulatory factors, including daf-2, hif-1, skn-1 and hsf-1 are required for pathogen defense, further suggesting that these pathways coordinate critical elements of cytoprotection [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]. These findings highlight the potential contributions of a range of cytoprotective pathways to lifespan extension, but a systematic genetic analysis of the regulation of cytoprotective mechanisms in diverse models of lifespan extension has not been conducted.
We tested the hypothesis that the regulation of cytoprotective gene expression in long-lived animals underlies lifespan extension across mechanistically diverse models of this phenotype. We utilized xenobiotic and genetic stimuli in an RNAi screen to identify regulatory genes required for the appropriate induction of hsp-6, hsp-4, gst-4 and sod-3 in long-lived animals. We directly addressed the activity of these cytoprotection regulatory genes in lifespan extension by inactivation and subsequent lifespan analysis in three functionally diverse long-lived mutants, isp-1, eat-2 and daf-2 (overview in Figure S1). We find that these cytoprotective regulatory genes are critical to lifespan extension in all three longevity backgrounds. These results provide mechanistic support for the hypothesis that lifespan extension occurs through the activation of cytoprotective pathways triggered by xenobiotic or genetic means.
Results
Identification of Regulators of Cytoprotective Gene Induction
Long-lived mutants express cytoprotective genes at elevated levels. To identify the cytoprotective pathways induced by the diverse conditions that confer lifespan extension, we analyzed the induction of 13 stress-responsive GFP fusion genes functioning in the response to heat, ER stress, mitochondrial stress, oxidative damage, pathogenesis, osmotic stress, xenobiotics, and decreased insulin/IGF-1 signaling (Table S1) by each of 160 gene inactivations found to increase longevity in high-throughput RNAi screens (Table S2) [1], [20], [23], [32], [50], [51], [52], [53], [54], [55], [56], [57]. Clustering the expression of phsp-6::gfp (Mt UPR), phsp-60::gfp (Mt UPR), phsp-4::gfp (ER UPR), pgst-4::gfp (detoxification), psod-3::gfp (ROS), pF55G11.7::gfp (pathogenesis) and pgpdh-1::gfp (osmotic stress) generates distinct groups of longevity gene inactivations (Figure S2). Overall, cytoprotective gene activation is a hallmark of the most potent lifespan extension mechanisms. The average mean lifespan extension amongst the 88 gene inactivations that induce at least one fusion gene (Figure S2) is 27.3%, while the 72 gene inactivations that do not activate a single fusion gene exhibit an average extension of 12.5% (t-test p = 7.7E−12) (Figure S2, Table S2) [1], [50], [51].
To discern how interventions that extend longevity couple to the activation of cytoprotective pathways, we sought to identify the genes required for the activation of hsp-6 (Mt UPR), hsp-4 (ER UPR), sod-3 (ROS response) and gst-4 (detoxification) by drugs or genetic triggers. Activation of cytoprotective genes requires the capacity to detect the disruption of an essential cell function, such as translation or mitochondrial function, and to generate signals that activate downstream responses. The mechanisms by which these events occur remain largely unknown. We reasoned that components of longevity signaling might emerge from an RNAi screen to identify gene inactivations that disrupt the induction of cytoprotective pathways. Because many gene inactivations that confer longevity extension encode targets of naturally occurring xenobiotics, analysis of toxin response may explore the same cytoprotective pathways activated in long-lived mutant animals. We raised C. elegans to young adulthood before treating the animals with toxins to induce the expression of longevity-correlated cytoprotective genes. Tunicamycin, an antibiotic that disrupts N-linked glycosylation in the ER, was employed for the activation of the ER UPR reporter phsp-4::gfp [20]. Antimycin disrupts complex III of the electron transport chain, activating the mitochondrial UPR reporter phsp-6::gfp. Sodium azide treatment activates the skn-1 target pgst-4::gfp, and the activity of the daf-16 target psod-3::gfp was modulated via a temperature sensitive allele of daf-2 [53], [58]. We used RNAi to screen for gene inactivations that blocked the expected cytoprotective response to each drug or genetic stimulus.
The screen encompassed ∼1500 gene-inactivating RNAi constructs, assayed for inhibition of each of the four cytoprotective responses. This library was composed of 395 kinases and 610 transcription factors, as well as two cherry-picked small RNA and longevity sublibraries. Because small RNA pathways have been implicated in stress responses, we screened 317 gene inactivations that have emerged from screens for defects in microRNA or RNAi functions [59], [60]. Gene inactivations that abrogate the increase in longevity conferred by low insulin/IGF-1 signaling have also been identified and potentially regulate cytoprotective functions. We therefore included this set of 179 gene inactivations as well [61].
The primary screen identified 73 gene inactivations required for appropriate activation of cytoprotective responses (Table 1, Table S3). Known stress response regulatory factors (ire-1, skn-1, daf-16) score strongly and each is highly specific to its known function (Table 1). Quantification of fluorescence intensity for 32 gene inactivations that scored most strongly in the primary optical screen confirmed the role of 29 genes in cytoprotective gene induction (Table 1). Induction of phsp-16.2::gfp following heat shock and the expression of a non-stress-induced fusion gene, psur-5::gfp, were quantified to control for generic transgene silencing phenotypes; none of these gene inactivations were potent transgene silencers (Table S4). Expression of the chromosomal loci corresponding to the screened fusion genes was analyzed by quantitative PCR to distinguish transgene dysregulation from regulation of the endogenous loci (Table S5). Results confirmed that the majority of these gene inactivations decouple the chromosomal cytoprotective loci from activation by toxins. While results were largely consistent, measured decreases in fluorescence from the GFP fusion genes were more dramatic than those detected by quantitative PCR for the corresponding genetic loci. The use of multi-copy transgenic constructs may contribute to this observation. Tissue specificity may do so as well, since quantitative PCR averages gene induction over all C. elegans cells while cytoprotective gene induction may be isolated to particular tissues. In addition, the efficacy of RNAi is reduced in neurons and other excitable cells.
Tab. 1. Gene inactivations that inhibit cytoprotective responses. 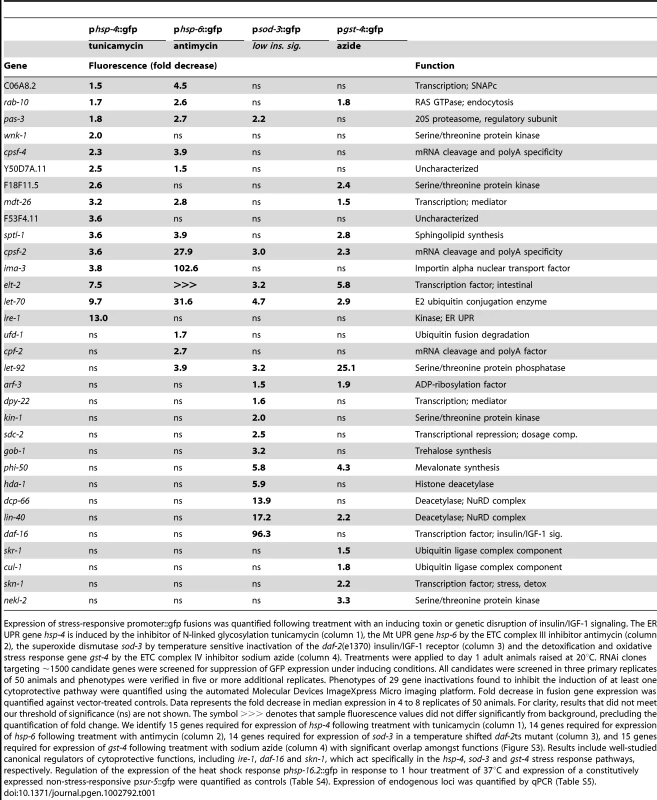
Expression of stress-responsive promoter::gfp fusions was quantified following treatment with an inducing toxin or genetic disruption of insulin/IGF-1 signaling. The ER UPR gene hsp-4 is induced by the inhibitor of N-linked glycosylation tunicamycin (column 1), the Mt UPR gene hsp-6 by the ETC complex III inhibitor antimycin (column 2), the superoxide dismutase sod-3 by temperature sensitive inactivation of the daf-2(e1370) insulin/IGF-1 receptor (column 3) and the detoxification and oxidative stress response gene gst-4 by the ETC complex IV inhibitor sodium azide (column 4). Treatments were applied to day 1 adult animals raised at 20°C. RNAi clones targeting ∼1500 candidate genes were screened for suppression of GFP expression under inducing conditions. All candidates were screened in three primary replicates of 50 animals and phenotypes were verified in five or more additional replicates. Phenotypes of 29 gene inactivations found to inhibit the induction of at least one cytoprotective pathway were quantified using the automated Molecular Devices ImageXpress Micro imaging platform. Fold decrease in fusion gene expression was quantified against vector-treated controls. Data represents the fold decrease in median expression in 4 to 8 replicates of 50 animals. For clarity, results that did not meet our threshold of significance (ns) are not shown. The symbol >>> denotes that sample fluorescence values did not differ significantly from background, precluding the quantification of fold change. We identify 15 genes required for expression of hsp-4 following treatment with tunicamycin (column 1), 14 genes required for expression of hsp-6 following treatment with antimycin (column 2), 14 genes required for expression of sod-3 in a temperature shifted daf-2ts mutant (column 3), and 15 genes required for expression of gst-4 following treatment with sodium azide (column 4) with significant overlap amongst functions (Figure S3). Results include well-studied canonical regulators of cytoprotective functions, including ire-1, daf-16 and skn-1, which act specifically in the hsp-4, sod-3 and gst-4 stress response pathways, respectively. Regulation of the expression of the heat shock response phsp-16.2::gfp in response to 1 hour treatment of 37°C and expression of a constitutively expressed non-stress-responsive psur-5::gfp were quantified as controls (Table S4). Expression of endogenous loci was quantified by qPCR (Table S5). The 29 regulators of cytoprotection identified are annotated to function in RNA processing (cpsf-2, cpsf-4, cpf-2), protein degradation (pas-3, let-70, ufd-1, skr-1, cul-1), deacetylation (sdc-2, hda-1, dcp-66, lin-40), phosphorylation (wnk-1, F18F11.5, let-92, kin-1, nekl-2), transcription (mdt-26, dpy-22, elt-2) and other activities (Table 1). Fourteen have been annotated as candidate cofactors for miRNA function, four as cofactors of RNAi and eight as positive regulators of lifespan extension in the long-lived daf-2 mutant [59], [60], [61]. Of the eight putative insulin/IGF-1 signaling factors, five were found to potently regulate the transcription of sod-3 downstream of daf-2 [61].
Sixteen gene inactivations that disrupt the coupling of cellular dysfunction to cytoprotective gene activation demonstrate specificity to one of our four tested cytoprotective gene fusions, including the canonical stress response regulatory factors daf-16, skn-1, and ire-1 (Table 1, Figure 1, Figure S3). The canonical factors score most strongly amongst these, with the exception of nekl-2, which regulates the expression of gst-4::gfp 50% more potently than skn-1. F53F4.11, cpf-2 and dcp-66 are also noteworthy as the most potent previously unidentified pathway-specific gene inactivations, regulating the expression of hsp-4, hsp-6 and sod-3 fusion genes, respectively. We observe the greatest degree of regulation, however, amongst the 16 regulators of cytoprotective gene expression that regulate 2 or more of the tested cytoprotective pathways. lin-40 gene inactivation disrupts psod-3::gfp induction 17-fold, and pgst-4::gfp 2-fold. let-92 gene inactivation results in the most potent disruption of pgst-4::gfp induction, decreasing expression 25-fold, and inhibiting induction of psod-3::gfp and phsp-6::gfp by 3 - and 4-fold, respectively. ima-3 and elt-2 gene inactivations both dramatically decrease induction of phsp-6::gfp by antimycin. ima-3 gene inactivation additionally inhibits the induction of pgst-4::gfp (4-fold). elt-2, like let-70 and cpsf-2, is required for the appropriate regulation of all four tested cytoprotective pathways (Table 1, Figure 1). We conclude that the cytoprotective pathways upregulated by conditions that increase longevity are regulated by both distinct and shared genetic components (Table 1, Figure S3). We speculate that shared regulatory genes may be upstream of pathway-specific factors, though the complexity of this regulatory network remains unexplored.
Fig. 1. Xenobiotic response regulatory factors are specific to one or more cytoprotective pathways. 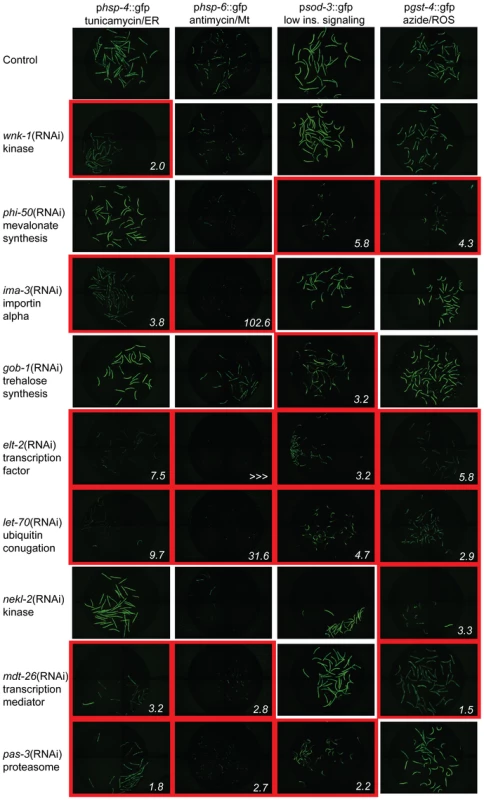
Animals carrying promoter::gfp fusions to genes in key cytoprotective responses were exposed to stimuli that normally induce the expression of these constructs. These include hsp-4, an ER UPR gene induced by treatment with tunicamycin (column 1), hsp-6, an Mt UPR gene induced by treatment with antimycin (column 2), sod-3, an oxidative stress response gene induced in a temperature-sensitive daf-2 mutant background (column 3), and gst-4, an oxidative stress response and detoxification gene induced by treatment with sodium azide (column 4). Gene inactivations found to inhibit the expression of one or more of these gfp fusions include RNAi of wnk-1 (row 2, suppresses hsp-4 response), phi-50 (row 3, suppresses sod-3 and gst-4 responses), ima-3 (row 4, suppresses hsp-4 and hsp-6 responses), gob-1 (row 5, suppresses sod-3 response), elt-2 (row 6, suppresses hsp-4, hsp-6, sod-3 and gst-4 responses), let-70 (row 7, suppresses hsp-4, hsp-6, sod-3 and gst-4 responses), nekl-2 (row 8, suppresses gst-4 response), mdt-26 (row 9, suppresses hsp-4, hsp-6 and gst-4 responses) and pas-3 (row 10, suppresses hsp-4, hsp-6 and sod-3 responses). Representative images of conditions with decreased cytoprotective gene expression are outlined in red, and the fold reduction in GFP expression, quantified in Table 1, is shown in the lower right. None regulate the expression of the constitutively expressed gene fusion psur-5::gfp and only elt-2 regulates expression of the heat shock responsive gene fusion phsp-16.2::gfp, suggesting specificity to stress functions (Table S4). Endogenous gene expression was measured by qPCR (Table S5). Lifespan Extension Requires Cytoprotective Gene Induction
If the cytoprotective pathways normally induced by conditions that confer increased longevity are essential for that increase, decoupling their induction might shorten the lifespan of long-lived mutants more than that of wild-type animals. To test this hypothesis, we asked whether the 29 gene inactivations that disrupt cytoprotective gene induction also abrogated the increase in lifespan conferred by mitochondrial dysfunction (isp-1;ctb-1), reduced feeding (eat-2) or disruption of insulin/IGF-1 signaling (daf-2).
Inactivation of an idealized lifespan regulatory gene would reduce the lifespan of a long-lived strain to that of the control strain (N2) without perturbing wild-type lifespan. In these experiments, 12 of 29 tested gene inactivations abrogate 2/3 or more of the lifespan extension observed in eat-2, isp-1 and/or daf-2 mutants (Table 2). These gene inactivations shorten wild type lifespan much less dramatically, differentiating these lifespan-regulatory gene inactivations from generalized sickness. While dcp-66, pas-3 and arf-3 exert their largest suppression of lifespan in isp-1, inactivation of cpf-2, wnk-1 and nekl-2 are most potent in the eat-2 mutant (Table 2, Figure 2). Of these, however, only dcp-66, which reduces lifespan extension in a mitochondrial mutant (isp-1) by 87%, does not significantly influence lifespan extension in at least one additional mutant. The remaining 6 gene inactivations, including phi-50, ima-3, gob-1, ufd-1, let-70, and elt-2, are critical to lifespan extension in both the isp-1 and eat-2 mutants (Table 2, Figure 2). Two of these, phi-50 and ima-3, also reduce the lifespan of daf-2 mutants by more than 2/3 (Table 2, Figure 2). These phenotypes represent the most potent inhibitions of lifespan extension. Applying a less conservative standard of 15% reduction in lifespan extension, 25 of 29 regulators of cytoprotective gene induction suppress the extension of lifespan in at least one long-lived strain (Table 2). Cumulatively, we find that genes required for the appropriate transcriptional response to xenobiotic stress and disruption of insulin/IGF-1 signaling contribute to diverse axes of lifespan extension, and in some cases, in particular phi-50 and ima-3, to all three studied lifespan extension axes (Table 2, Figure 2).
Fig. 2. Loss of cytoprotective gene activation dramatically reduces lifespan extension in long-lived mutants but not controls. 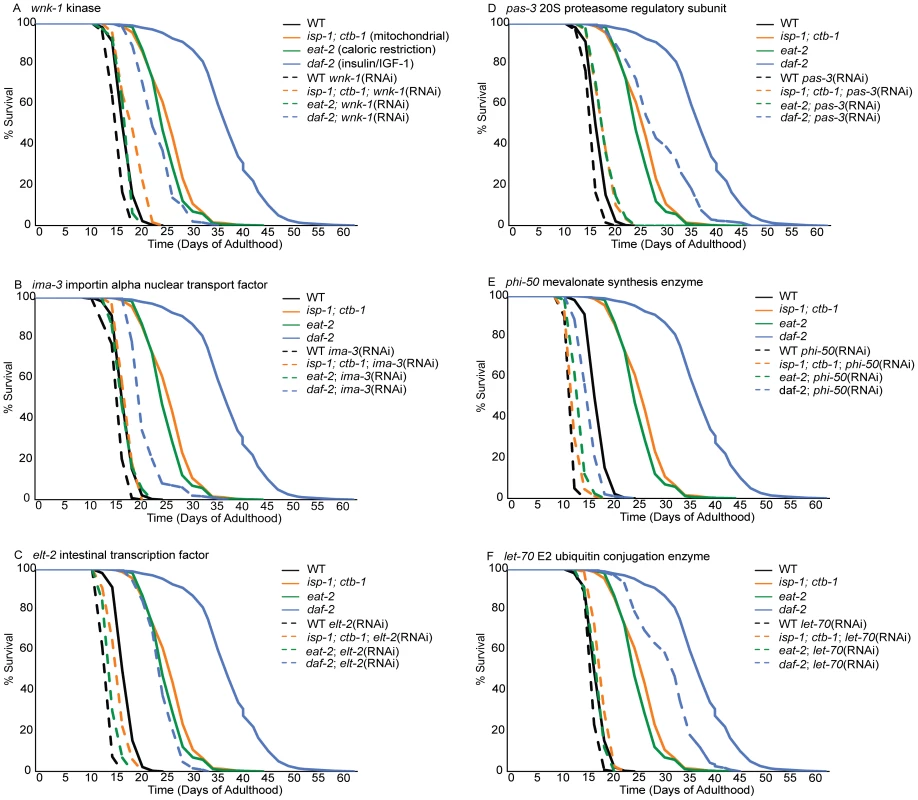
Lifespans of animals treated with empty-vector RNAi or with inactivation of wnk-1 (A), ima-3 (B), elt-2 (C), pas-3 (D), phi-50 (E) and let-70 (F) were determined from three replicates comprising, an average of 103 worms per condition. Lifespan was measured in wild-type N2 controls (black lines) and three long-lived mutants, including a mitochondrial mutant (isp-1;ctb-1, orange lines), an insulin/IGF-1 signaling mutant (daf-2, blue lines) and a feeding mutant that models caloric restriction (eat-2, green lines). Data for control (empty vector-fed) animals of each strain are represented by solid lines and RNAi-treated animals by dashed lines. Inactivation of these cytoprotective response regulatory genes dramatically reduces lifespan extension in at least one long-lived mutant background, but not in wild-type controls. Tab. 2. Cytoprotective gene regulation is essential to lifespan extension by diverse mechanisms. 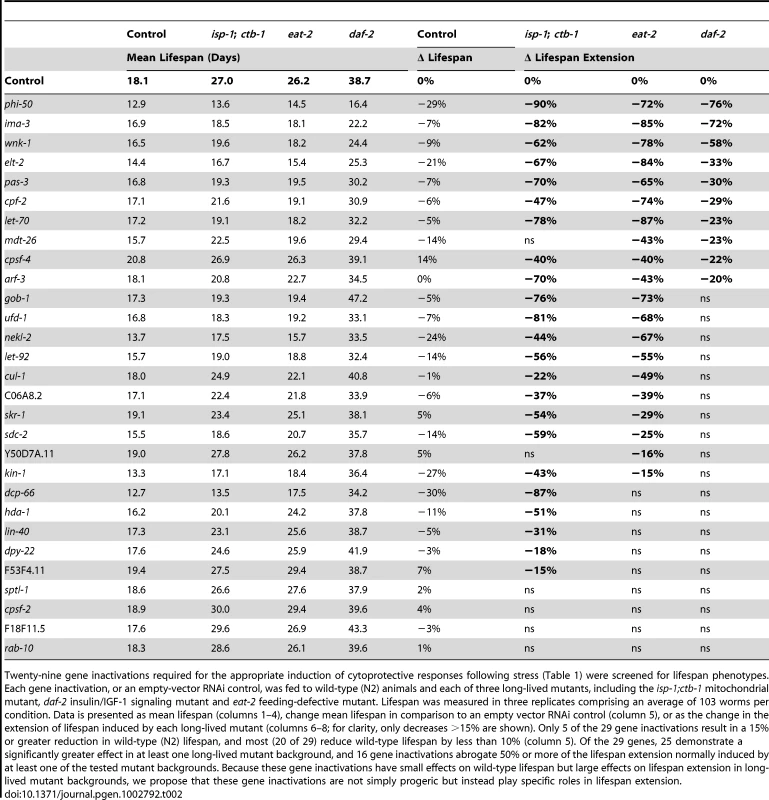
Twenty-nine gene inactivations required for the appropriate induction of cytoprotective responses following stress (Table 1) were screened for lifespan phenotypes. Each gene inactivation, or an empty-vector RNAi control, was fed to wild-type (N2) animals and each of three long-lived mutants, including the isp-1;ctb-1 mitochondrial mutant, daf-2 insulin/IGF-1 signaling mutant and eat-2 feeding-defective mutant. Lifespan was measured in three replicates comprising an average of 103 worms per condition. Data is presented as mean lifespan (columns 1–4), change mean lifespan in comparison to an empty vector RNAi control (column 5), or as the change in the extension of lifespan induced by each long-lived mutant (columns 6–8; for clarity, only decreases >15% are shown). Only 5 of the 29 gene inactivations result in a 15% or greater reduction in wild-type (N2) lifespan, and most (20 of 29) reduce wild-type lifespan by less than 10% (column 5). Of the 29 genes, 25 demonstrate a significantly greater effect in at least one long-lived mutant background, and 16 gene inactivations abrogate 50% or more of the lifespan extension normally induced by at least one of the tested mutant backgrounds. Because these gene inactivations have small effects on wild-type lifespan but large effects on lifespan extension in long-lived mutant backgrounds, we propose that these gene inactivations are not simply progeric but instead play specific roles in lifespan extension. Many gene inactivations that inhibit lifespan extension also decrease stress tolerance. To reveal the role of cytoprotective response regulatory genes in the tolerance of xenobiotic stress, we inactivated the 29 genes identified in the screen and challenged animals with sublethal (LD30) doses of antimycin, sodium azide, cadmium chloride and paraquat. These toxins parallel the conditions utilized in the screen. Antimycin targets the function of mitochondrial ETC complex III and activates phsp-6::gfp. Sodium azide disrupts the final step of electron transport, blocking energy production and releasing reactive oxygen species, leading to the induction of pgst-4::gfp [58]. Cadmium, like tunicamycin, induces the ER stress response and cadmium tolerance is dependent upon a functional ER UPR [62]. Paraquat survival has been utilized as a measure of ROS tolerance, known to result from the activation of cytoprotective genes downstream of insulin/IGF-1 signaling, such as the superoxide dismutase sod-3 [53].
Inactivation of 16 of 29 genes that disrupt induction of the cytoprotective GFP fusion genes, also disrupted the ability of animals to survive exposure to xenobiotics (Figure 3). Eleven of the sixteen xenobiotic-sensitive gene inactivations (phi-50, wnk-1, nekl-2, mdt-26, let-70, arf-3, elt-2, dpy-22, let-92, F18F11.5, and C06A8.2) enhance sensitivity to the xenobiotic that pairs with the compromised cytoprotective response. The strongest examples of this correlation include phi-50 and nekl-2 (pgst-4::gfp/sodium azide), elt-2, wnk-1 and mdt-26 (phsp-4::gfp/cadmium chloride), let-92, elt-2 and mdt-26 (phsp-6::gfp/antimycin) and dpy-22 (psod-3::gfp/sodium azide). Of the eleven total gene inactivations that pair in this way, seven are also susceptible to additional xenobiotics, suggesting that the pathways examined serve cytoprotection more extensively than previously predicted. In addition, five gene inactivations (dcp-66, pas-3, kin-1, cpf-2 and cul-1) are sensitive only to xenobiotics that do not directly pair with the observed deficit in cytoprotective gene induction, further demonstrating the complexity of stress responsive gene networks and their protective functions. None of the 29 gene inactivations significantly decreased survival following treatment with drug solvent controls alone (Figure S4).
Fig. 3. Regulation of cytoprotective gene activation is required for xenobiotic stress tolerance. 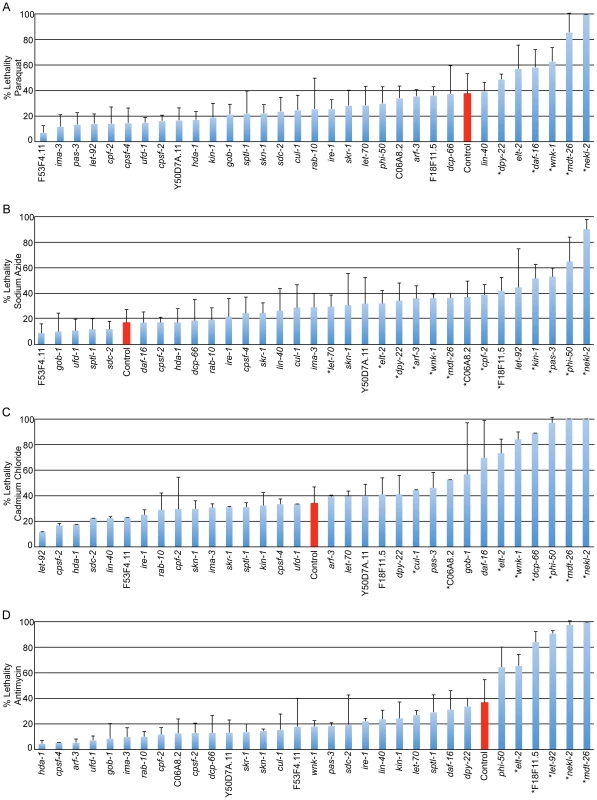
Genes found to regulate cytoprotective responses in our screen were inactivated in rrf-3ts sterile mutant animals. Animals were raised to day 3 adulthood on solid media and transferred to solution containing sublethal (∼LD30) treatments of 24 mg/ml paraquat (A), 22 µg/ml sodium azide (B), 5.2 mg/ml cadmium chloride (C), 696 µg/ml antimycin (D) or solvent alone (Figure S4). Animals were incubated in solution for 18 hours and survival was measured by spontaneous movement. An average of 94 animals were scored for each condition. Control samples treated with empty vector (L4440) RNAi are shown in red, with all other samples treated with gene inactivations that disrupt cytoprotective responses. Gene inactivations are ordered by decreasing survival within each panel. Phenotypes demonstrated by inactivation of nekl-2, mdt-26, wnk-1, phi-50 and elt-2 are amongst the most robust. Gene inactivations do not reduce survival in the presence of solvent alone (Figure S4). Error bars display S.D., asterisks indicate significantly decreased survival, p<0.05. Cumulatively, phi-50, ima-3, elt-2, nekl-2, wnk-1, let-92, mdt-26, and let-70 stand out amongst the 29 genes that disrupt cytoprotective response (Table S6). wnk-1, phi-50 and elt-2 are severely sensitive to multiple xenobiotic stress conditions, but not control conditions, and modulate lifespan extension in all three tested axes (insulin/IGF-1 signaling, mitochondrial function and caloric restriction). While ima-3 and let-92 do not demonstrate xenobiotic sensitivity under the tested conditions, and let-70 only a subtle sensitivity to sodium azide, they are amongst the most robust suppressors of cytoprotective transcription and suppress lifespan extension in all three long-lived mutants. mdt-26 and nekl-2 are sensitive to all four xenobiotic treatments and suppress lifespan extension in two of the three long-lived mutants tested (daf-2 and eat-2 or isp-1;ctb-1 and eat-2, respectively). In total, we identify 15 regulators of cytoprotection that are required for tolerance of xenobiotic stress and lifespan extension (Table S6).
Discussion
Stress tolerance and lifespan extension are remarkably correlated. The contradictory extension of lifespan by ostensibly deleterious conditions, and the concomitant induction of stress tolerance, suggests that lifespan extension may occur through the hormetic induction of damage-buffering cytoprotective mechanisms. We have identified the cytoprotective pathways that are upregulated by conditions that extend lifespan. In a screen of 160 gene inactivations that increase lifespan, the most potent lifespan extension phenotypes were defined by the induction of suites of cytoprotective genes (Figure S2) [32], [35], [63]. To identify upstream regulatory genes in xenobiotic responses, we designed an RNAi screen to detect gene inactivations that disrupt the normal induction of phsp-6::gfp, pgst-4::gfp and phsp-4::gfp by toxins, and the activation of psod-3::gfp by low insulin/IGF-1 signaling. The induction of cytoprotective longevity-modulatory pathways by toxins may be the normal biological context in which these pathways function, having evolved as countermeasures to the xenobiotic and environmental challenges that animals encounter. Because xenobiotics and lifespan extending gene inactivations engage the same cytoprotective, physiological and behavioral responses, xenobiotic responses may be triggered by direct surveillance of cell functions, which would provide broad, adaptive utility in toxin detection [64], [65], [66].
We identified 29 gene inactivations that decouple normal transcriptional responses to toxins and environmental stress. While some gene inactivations were specific to one toxic modality, such as mitochondrial dysfunction, others affected multiple, distinct toxin response pathways. The identified genes may act in damage surveillance, signaling, or the transcriptional coordination of cytoprotective responses by acting either within cells and tissues or across tissues by an as-yet-undefined endocrine mechanism. Gene inactivations specific to one toxin may act in dedicated surveillance pathways, while those that affect multiple, distinct responses may identify points of signal convergence.
Many of the gene inactivations that disrupt the coupling of cellular dysfunction and the transcription of cytoprotective responses in the screen are annotated phosphorylation or transcription factors. The kinase nekl-2, which we identify in the regulation of gst-4 expression, is necessary for the nuclear localization of skn-1 following oxidative stress [67]. The kinase wnk-1, which we identify as a regulator of the ER stress response, has previously been placed upstream of effector genes in the osmotic stress response [68]. let-92, the catalytic subunit of protein phosphatase 2A, stands out as a potent regulator of gst-4 expression, suggesting it may play a critical role in the regulation of skn-1 activity. The transcription factor elt-2 is expressed exclusively in the intestine, a critical tissue in xenobiotic detection and detoxification in C. elegans. Targets of elt-2 include osmoprotective and innate immune responses, detoxification and oxidative defenses and metal detoxification, as well as the transcription factor pha-4 and, potentially, skn-1. This multitude of key cytoprotective functions is consistent with our finding that elt-2 is required for appropriate expression of hsp-6, hsp-4, sod-3 and gst-4.
We identified five components of the ubiquitin proteasome system (pas-3, let-70, ufd-1, skr-1, cul-1). Proteasome regulatory components like aip-1, and potentially skn-1, are believed to prolong lifespan by stimulating the degradation of damaged proteins [69]. Others, such as the E3 ubiquitin ligase vhl-1, serve regulatory functions by degrading key signaling components [70]. The E2 ubiquitin conjugation factor let-70 regulates all four tested cytoprotective responses (phsp-6::gfp, phsp-4::gfp, psod-3::gfp, pgst-4::gfp), but not the heat shock response (phsp-16.2::gfp) or a constitutively expressed control (psur-5::gfp). It has been previously shown that let-70 interacts with numerous chaperones, contributes to the DNA damage response, and that inactivation of let-70 increases the size of aggregates in a polyglutamine model of protein aggregation [71]. The diverse functions of let-70, one of 22 E2 ubiquitin conjugation factors in C. elegans, are consistent with its interaction with a much larger pool of target-specifying ubiquitin-protein ligases (E3s).
We identify three deacetylases (hda-1, dcp-66, lin-40), all of which regulate the expression of psod-3::gfp downstream of insulin/IGF-1 signaling. Studies of chromatin modification have demonstrated that both silencing and desilencing epigenetic marks regulate lifespan [72], [73].
Other genes of interest include ima-3 and phi-50 (Table 1, Table 2). ima-3, one of three importin alphas that channel NLS-tagged molecules into the nucleus, stands out as the most potent regulator of the mitochondrial UPR we identify. Our results suggest that ima-3 may participate in the transport of stress regulatory factors into the nucleus. phi-50, which regulates psod-3::gfp and pgst-4::gfp, is orthologous to the human hydroxymethylglutaryl-CoA synthases 1 and 2 (HMGCS1, 2) [74]. HMGCS1 is required for the prenylation of proteins such as GTPases, which are essential to organelle homeostasis and many signaling cascades, while HMGCS2 mediates the response to fasting [75], [76].
Eighteen genes found to disrupt the induction of cytoprotective responses serve putative microRNA or RNAi functions, suggesting that small RNAs regulate stress response. As most microRNAs in C. elegans are not individually essential for viability under standard laboratory conditions, the possibility that they fulfill conditional functions such as stress response is particularly appealing [77]. Small RNAs are attractive candidates for stress response regulation, as they are rapidly inducible and the suppression of protein levels they mediate is rapidly reversible [78], [79]. Small RNAs may be generated without translation and spread easily amongst cellular compartments or from cell to cell [79], [80]. Roles for small RNAs in stress responsive gene regulation are emerging in bacterial, plant and mammalian systems [81], [82], [83], [84], [85], [86], [87]. The capacity of small RNA pathways to mediate the expression of duplicated and genetically linked genes may contribute to their potential to act as key regulators of xenobiotic response, as detoxification genes are known to form long tandem arrays by duplication [88]. Like canonical stress response regulatory genes, some small RNAs have been found to regulate longevity [89]. Regulation of cytoprotective genes by siRNA - or miRNA-mediated silencing is likely indirect under the tested conditions because inactivation of genes required for silencing would be expected to increase, not decrease, the expression of a direct target. Additionally, the transgenes utilized were promoter fusions.
The complexity of stress response is increasingly evident, challenging the presumption that cytoprotective pathways are genetically independent. Translation initiation factor 2 (eIF2α) integrates signals from four stress-activated kinases, each responding to diverse stress stimuli including oxidative damage, amino acid starvation, infection and ER stress. Another example is found in the transcription factor slr-2, which co-regulates a suite of diverse stress response genes, including hsp-16.2 (heat shock), hif-1 (oxidative stress) and gpdh-1 [90]. The insulin/IGF-1 signaling pathway contributes to the tolerance of heat, radiation, osmotic stress, oxidative damage and heavy metals, as well as pathogens. It is not surprising that some of the genes identified in our study engage more than one cytoprotective mechanism.
The regulators of cytoprotection we identified contribute to lifespan extension in three distinct long-lived mutants: isp-1 (mitochondrial function), eat-2 (caloric intake) and daf-2 (insulin/IGF-1 signaling). Nearly all of the identified regulators of cytoprotection contributed to lifespan extension in at least one of these mutants and many modulate lifespan extension in at least two conditions. Because previously identified positive regulators of lifespan, such as daf-16 and hsf-1, manifest the cumulative benefit of large suites of co-regulated genes, we suggest that the stress response and lifespan regulatory genes identified here similarly abrogate the induction of many downstream cytoprotective effectors. Our finding that nearly all gene inactivations that disrupt the induction of cytoprotective pathways by toxins also disrupt longevity extension suggests a tight coupling of these pathways. Increased longevity may be the cumulative result of cytoprotective pathway induction or, alternatively, a coregulated output of xenobiotic response analogous to the hormonal pathways of lifespan regulation engaged by insulin/IGF-1 signaling mutants. Our results suggest that xenobiotic and environmental response mechanisms underpin diverse models of longevity extension, with the potential to unify the study of long-lived animals.
Lifespan poses an evolutionary conundrum, as the genetic determination of lifespan ostensibly suggests post-reproductive selection. Our data suggests that lifespan-determining genes do not specify lifespan per se, but rather the activity of damage-buffering cytoprotective pathways normally engaged only in response to stress stimuli, such as toxins. Cytoprotective programs must be subject to Darwinian evolution, selected pre-reproductively to maintain the viability of larval and young adult animals in the presence of xenobiotic and environmental challenges. Post-reproductive adults could engage the same programs. The functions of these pathways are expected to be highly regulated, since they marshal essential resources, such as iron for cytochrome p450s or ATP for chaperones and transporters, away from anabolic pathways and reproduction; organisms that upregulate these pathways continuously would be outcompeted by those who regulate them conditionally [91].
We have identified upstream regulatory components of longevity and xenobiotic response pathways, the overlap of which supports our hypothesis that longevity pathways evolved as xenobiotic and environmental stress response programs. Our results reveal the complex networking of cytoprotective gene regulation. The genes we have identified may act in the detection of stress stimuli, the transduction of a resulting signal or the direct regulation of the transcription of stress response effectors. We find that these upstream regulators play central roles in both xenobiotic stress tolerance and the extension of lifespan in several canonical long-lived strains, including eat-2, isp-1 and daf-2. Xenobiotic and environmental stress response pathways may underpin many current models of longevity extension. The xenobiotic hypothesis of aging invokes hormesis, a phenomenon observed from microorganisms to humans, highlighting the possibility of a xenobiotic approach to longevity extension in humans.
Materials and Methods
Strains
Fluorescent strains phsp-6::gfp(sj4100), phsp-60::gfp(sj4058), phsp-4::gfp(sj4005), psod-3::gfp(cf1553), pgst-4::gfp(cl2166)., phsp-16.2::gfp(tj375) and pfat-7::gfp(bc15777) were obtained from the C. elegans genome center (CGC). pgpdh-1::gfp(vp198) was obtained courtesy of Kevin Strange. Bristol(N2), rrf-3(pk1426) and daf-16::gfp(gr1352) were obtained from the Ruvkun laboratory. pF55G11.7::gfp(hd92), plys-1::gfp(hd102), plys-7::gfp(hd100) and pnlp-29::gfp(hd101) were obtained courtesy of Scott Alper. Long lived strains were daf-2(e1370), eat-2(ad465) and isp-1;ctb-1(mq989).
Activation of GFP Fusion Genes by RNAi
RNAi clones were grown overnight in LB with 100 µg/ml ampicillin and seeded 100 µl/well to 24-well 5 mM IPTG worm plates. Clones were induced overnight at room temperature. Synchronized L1 worms were raised on RNAi at 20°C, 50 animals/well. Fluorescence was assayed at 48, 72 and 96 hours. Scores were recorded from 0 (no expression) to 4 (strong expression). For lethal clones, worms were grown to young adulthood at 20°C on empty-vector RNAi (L4440) before treatment, with subsequent scoring after 24, 48 and 72 hours. Each clone was scored in three trials. Resulting data was clustered using the open source software Cluster 3.0 with hierarchical uncentered correlation of average linkage and visualized using Java TreeView.
Activation of GFP Fusion Genes by Stress
RNAi clones were cultured overnight in LB with 100 µg/ml ampicillin and seeded 100 µl/well to 24-well 5 mM IPTG worm plates, each well containing 1.5 mL agar. Synchronized L1 transgenic strains (SJ4100, sj4005, cf1553;e1370, cl2166) were distributed to RNAi, 50 animals/well, and raised to young adulthood (56 hours) at 20°C. At this time, each well was treated with 0.5 µl 20 mg/ml antimycin in EtOH (sj4100), 1.8 µl10 mg/ml tunicamycin in DMSO (sj4005), 17 µl1 mg/ml sodium azide in water (cl2166) or 1 h 37°C heat shock (tj375). Toxins were diluted in water to 20 µl/well and applied directly to the agar wells. Expression was assayed after 8 hours (tunicamycin, heat), or 24 hours (antimycin, sodium azide). For sod-3(cf1553), psod-3::gfp;daf-2(e1370)ts was raised to young adulthood at 15°C and shifted to 25°C with GFP expression assayed after 12 hours. All clones in the primary screen were scored in three replicates. Candidate stress response regulatory genes were subsequently verified in five or more additional replicates.
Quantification of Fluorescence
Treated worms (see activation of fusion genes by stress in materials and methods) were washed into 200 µl M9 containing 0.3 mg/ml lavamisole and 0.005% Triton X-100, and concentrated in a 96-well pate by centrifugation for 1 minute at 500 RPM, then transferred to 96-well glass slides with a final liquid volume of 5 µl/well. Imaging of slides was automated using Molecular Devices ImageXpress Micro imaging platform with MetaXpress software. Device captures four images per well, which are tiled to construct full wells. Images are captured in both in both GFP and bright field channels. Custom MATLAB (The Mathworks, Natick, MA) scripts distinguish well boundaries by blurring the image and applying a threshold of L*F where L is determined by Otsu's method and F = 0.9. To identify worms, bright field images are bottom hat filtered to decrease variability in background intensity. Otsu's method and a size filter are applied to distinguish objects from background and debris. An outlier method is applied in place of Otsu's when effectiveness is low (<0.7). Fluorescence of worm objects is averaged from the median intensity of each well after background subtraction. Results were averaged from four to eight replicates for each experimental condition and the psur-5::gfp control, with two replicates for the phsp-16.2::gfp control. Significance was determined by a one-tailed t-test, p = 0.05, and fold decreased expression >1.5× without multiple tests correction. Venn diagrams were generated using the online BioInfoRx Venn diagram tool available at <bioinforx.com/free/bxarrays/venndiagram.php>.
Quantification of Endogenous Gene Expression by qPCR
Wild-type N2 animals were raised on HT115 bacteria on 10 cm agarose plates at 20°C and treated with cytoprotective response-inducing toxins (see activation of fusion genes by stress in materials and methods). Animals were harvested after 8 hours (antimycin, tunicamycin) or 24 hours (sodium azide). In the case of sod-3, e1370 animals were raised to young adulthood at 15°C and shifted to 25°C and harvested after 12 hours. To isolate total RNA, animals were washed, resuspended in trizol, frozen and homogenized by grinding. RNA was isolated by chloroform extraction followed by ethanol precipitation. Reverse transcription was carried out with the Ambion AM1710 Retroscript RT-PCR kit. Quantitative PCR reactions utilized 12.5 µl Bio-Rad iQ SYBR Green PCR Supermix (170-8880) with 2 µl template, 5.5 µl water and 5 µl each of two of the following paired oligos: hsp-4 GAGAACACAATTTTCGACGCC/GACTTGTCGACGATCTTGAACGG; hsp-6 GATAAGATCATCGCTGTCTACG/GTGATCGAAGTCTTCTCCTCCG; sod-3 CACTATTAAGCGCGACTTCGG/CAATATCCCAACCATCCCCAG; gst-4 GCCAATCCGTATCATGTTTGC/CAAATGGAGTCGTTGGCTTCAG. Fold change was measured in comparison to Y45F10D.4 using oligos GTCGCTTCAAATCAGTTCAGC/GTTCTTGTCAAGTGATCCGACA as described by Hoogewijs et al. 2008 [92]. Quantitative RT-PCR was carried out using a Bio-Rad C1000-CFX96RT thermocycler (3 m 95°C; 44 cycles of 95°C 10 s, 60°C 30 s, 72°C 30 s; 5 m 72°C). Experiments were carried out with four replicates of approximately 4,000 animals per replicate.
Lifespan Analysis
Long-lived strains daf-2(e1370), eat-2(ad465) and isp-1;ctb-1(mq989) were raised to young adulthood at 20°C on 10 cm worm plates with HT115 E. coli. RNAi clones were cultured overnight in LB with 100 µg/ml ampicillin, seeded 400 µl/well to 6-well 5 mM IPTG worm plates and induced overnight. The young adult animals were transferred to the prepared IPTG worm plates, 40 animals/well, and FuDR was immediately applied to the plate to a final concentration of 80 µg/ml agar. On day 4 adulthood, wells were supplemented with 400 µl of additional bacteria concentrated to 10× in 5 mM IPTG M9 with 100 mg/ml ampicillin and induced for 2 hours at room temperature before application to worm plates. Lifespan was scored by touch response on alternate days with censoring. Survival statistics were calculated using SPSS Kaplan-Meier. All analyses are based upon mean lifespan. Experiments were performed with three replicates per condition and an average of 103 worms scored per condition. Significance was held to p = 0.05 within each tested strain; significance of differences in lifespan extension are based upon a threshold of 15% decrease. The DNA and RNA synthesis inhibitor 5-fluoro-2′-deoxyuridine (FUdR) was used to inhibit progeny production. Although the use of FUdR is well established and does not affect the lifespan of wild-type animals, FUdR can affect lifespan of particular mutants [93], [94], [95], [96]. For example, mutation of tub-1 or gas-1 extends lifespan in the presence of FUdR, but not in its absence [93], [97]. Our experiments included FUdR in both control and experimental trials, so that any FUdR effects on lifespan were controlled.
Stress Tolerance
L1 rrf-3(pk1426)ts worms were synchronized overnight in M9 and raised to young adulthood at 25°C on 10 cm worm plates with HT115 E. coli. RNAi clones were cultured overnight in LB with 100 µg/ml ampicillin, seeded to 6-well 5 mM IPTG worm plates and induced overnight. Young adult rrf-3 worms were distributed to RNAi, 50 animals/well, and raised for 3 days at 25°C. Worms were transferred to wells of M9 containing 24 mg/ml paraquat, 5.2 mg/ml cadmium chloride, 22 µg/ml sodium azide, or 696 µg/ml antimycin, with a total volume of 230 µl per well in a 24-well format. Animals were incubated in solution for 16 hours. Survival was then analyzed by scoring for spontaneous movement. Experiments were conducted with three replicates and an average of 94 animals scored per condition. Significance of proportion survival was determined by a one-tailed t-test, p = 0.05 without multiple tests correction. Error bars display S.D.
Supporting Information
Zdroje
1. CurranSPRuvkunG 2007 Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet 3 e56 doi:10.1371/journal.pgen.0030056
2. JohnsonTEde CastroEHegi de CastroSCypserJHendersonS 2001 Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol 36 1609 1617
3. JohnsonTEHendersonSMurakamiSde CastroEde CastroSH 2002 Longevity genes in the nematode Caenorhabditis elegans also mediate increased resistance to stress and prevent disease. J Inherit Metab Dis 25 197 206
4. de CastroEHegi de CastroSJohnsonTE 2004 Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Radic Biol Med 37 139 145
5. DoonanRMcElweeJJMatthijssensFWalkerGAHouthoofdK 2008 Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 22 3236 3241
6. BokovAChaudhuriARichardsonA 2004 The role of oxidative damage and stress in aging. Mechanisms of ageing and development 125 811 826
7. GoldenTRHinerfeldDAMelovS 2002 Oxidative stress and aging: beyond correlation. Aging cell 1 117 123
8. LarsenP 1993 Aging and resistance to oxidative damage in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 90 8905 8914
9. HarmanD 1956 Aging: a theory based on free radical and radiation chemistry. Journal of gerontology 11 298 300
10. PerezVIBokovAVan RemmenHMeleJRanQ 2009 Is the oxidative stress theory of aging dead? Biochimica et biophysica acta 1790 1005 1014
11. VanfleterenJR 1993 Oxidative stress and ageing in Caenorhabditis elegans. The Biochemical journal 292 Pt 2 605 608
12. PhillipsJPCampbellSDMichaudDCharbonneauMHillikerAJ 1989 Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proceedings of the National Academy of Sciences of the United States of America 86 2761 2765
13. LithgowGJWhiteTMMelovSJohnsonTE 1995 Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proceedings of the National Academy of Sciences of the United States of America 92 7540 7544
14. ReaSWuDCypserJVaupelJJohnsonT 2005 A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nature genetics 37 894 902
15. HsuA-LMurphyCKenyonC 2003 Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science (New York, NY) 300 1142 1147
16. FargnoliJKunisadaTFornaceAJJrSchneiderELHolbrookNJ 1990 Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proceedings of the National Academy of Sciences of the United States of America 87 846 850
17. UdelsmanRBlakeMJStaggCALiDGPutneyDJ 1993 Vascular heat shock protein expression in response to stress. Endocrine and autonomic regulation of this age-dependent response. The Journal of clinical investigation 91 465 473
18. Ben-ZviAMillerEAMorimotoRI 2009 Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proceedings of the National Academy of Sciences of the United States of America 106 14914 14919
19. MunozMRiddleD 2003 Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 163 171 251
20. CalfonMZengHUranoFTillJHHubbardSR 2002 IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415 92 96
21. DurieuxJWolffSDillinA 2011 The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144 79 91
22. Henis-KorenblitSZhangPHansenMMcCormickMLeeSJ 2010 Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A 107 9730 9735
23. YonedaTBenedettiCUranoFClarkSGHardingHP 2004 Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci 117 4055 4066
24. SykiotisGPBohmannD 2010 Stress-activated cap'n'collar transcription factors in aging and human disease. Science signaling 3 re3
25. SykiotisGPHabeosIGSamuelsonAVBohmannD 2011 The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Current opinion in clinical nutrition and metabolic care 14 41 48
26. TulletJMHertweckMAnJHBakerJHwangJY 2008 Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132 1025 1038
27. WalkerAKSeeRBatchelderCKophengnavongTGronnigerJT 2000 A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap'n'Collar-related basic leucine zipper proteins. The Journal of biological chemistry 275 22166 22171
28. ZubovychIOStraudSRothMG 2010 Mitochondrial dysfunction confers resistance to multiple drugs in Caenorhabditis elegans. Mol Biol Cell 21 956 969
29. McElweeJJSchusterEBlancEThomasJHGemsD 2004 Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. The Journal of biological chemistry 279 44533 44543
30. GemsDMcElweeJJ 2005 Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mechanisms of ageing and development 126 381 387
31. TaweWNEschbachMLWalterRDHenkle-DuhrsenK 1998 Identification of stress-responsive genes in Caenorhabditis elegans using RT-PCR differential display. Nucleic acids research 26 1621 1627
32. MurphyCTMcCarrollSABargmannCIFraserAKamathRS 2003 Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277 283
33. CristinaDCaryMLuncefordAClarkeCKenyonC 2009 A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans. PLoS Genet 5 e1000450 doi:10.1371/journal.pgen.1000450
34. RogersANChenDMcCollGCzerwieniecGFelkeyK 2011 Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in C. elegans. Cell Metab 14 55 66
35. McElweeJJSchusterEBlancEThomasJHGemsD 2004 Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem 279 44533 44543
36. LiXMatilainenOJinCGlover-CutterKMHolmbergCI 2011 Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet 7 e1002119 doi:10.1371/journal.pgen.1002119
37. OliveiraRPPorter AbateJDilksKLandisJAshrafJ 2009 Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 8 524 541
38. HsuALMurphyCTKenyonC 2003 Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300 1142 1145
39. ChenDThomasELKapahiP 2009 HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet 5 e1000486 doi:10.1371/journal.pgen.1000486
40. GarsinDAVillanuevaJMBegunJKimDHSifriCD 2003 Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300 1921
41. KurzCLTanMW 2004 Regulation of aging and innate immunity in C. elegans. Aging cell 3 185 193
42. LawsTRHardingSVSmithMPAtkinsTPTitballRW 2004 Age influences resistance of Caenorhabditis elegans to killing by pathogenic bacteria. FEMS microbiology letters 234 281 287
43. Mohri-ShiomiAGarsinD 2008 Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. The Journal of biological chemistry 283 194 395
44. ChavezVMohri-ShiomiAMaadaniAVegaLAGarsinDA 2007 Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics 176 1567 1577
45. SinghVAballayA 2006 Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proceedings of the National Academy of Sciences of the United States of America 103 13092 13099
46. EvansEAKawliTTanMW 2008 Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 4 e1000175 doi:10.1371/journal.ppat.1000175
47. MilletACEwbankJJ 2004 Immunity in Caenorhabditis elegans. Curr Opin Immunol 16 4 9
48. ShaoZZhangYYeQSaldanhaJNPowell-CoffmanJA 2010 C. elegans SWAN-1 Binds to EGL-9 and regulates HIF-1-mediated resistance to the bacterial pathogen Pseudomonas aeruginosa PAO1. PLoS Pathog 6 e1001075 doi:10.1371/journal.ppat.1001075
49. ShiversRPPaganoDJKooistraTRichardsonCEReddyKC 2010 Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet 6 e1000892 doi:10.1371/journal.pgen.1000892
50. HamiltonBDongYShindoMLiuWOdellI 2005 A systematic RNAi screen for longevity genes in C. elegans. Genes Dev 19 1544 1555
51. HansenMHsuALDillinAKenyonC 2005 New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet 1 e17 doi:10.1371/journal.pgen.0010017
52. LeiersBKampkotterAGreveldingCGLinkCDJohnsonTE 2003 A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic Biol Med 34 1405 1415
53. HondaYHondaS 1999 The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J 13 1385 1393
54. AlperSMcBrideSJLackfordBFreedmanJHSchwartzDA 2007 Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol Cell Biol 27 5544 5553
55. McKaySJJohnsenRKhattraJAsanoJBaillieDL 2003 Gene expression profiling of cells, tissues, and developmental stages of the nematode C. elegans. Cold Spring Harbor symposia on quantitative biology 68 159 169
56. OggSParadisSGottliebSPattersonGILeeL 1997 The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389 994 999
57. GuhaThakurtaDPalomarLStormoGDTedescoPJohnsonTE 2002 Identification of a novel cis-regulatory element involved in the heat shock response in Caenorhabditis elegans using microarray gene expression and computational methods. Genome Res 12 701 712
58. AnJHBlackwellTK 2003 SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev 17 1882 1893
59. KimJKGabelHWKamathRSTewariMPasquinelliA 2005 Functional genomic analysis of RNA interference in C. elegans. Science 308 1164 1167
60. ParryDHXuJRuvkunG 2007 A whole-genome RNAi Screen for C. elegans miRNA pathway genes. Curr Biol 17 2013 2022
61. Samuelson AVCCRuvkunG 2007 Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes and Development 21 2976 2994
62. UranoFCalfonMYonedaTYunCKiralyM 2002 A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J Cell Biol 158 639 646
63. Perez-CampoRLopez-TorresMCadenasSRojasCBarjaG 1998 The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. Journal of comparative physiology B, Biochemical, systemic, and environmental physiology 168 149 158
64. MeloJARuvkunG 2012 Inactivation of Conserved C. elegans Genes Engages Pathogen - and Xenobiotic-Associated Defenses. Cell 149 452 466
65. McEwanDLKirienkoNVAusubelFM 2012 Host Translational Inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an Immune Response in Caenorhabditis elegans. Cell host & microbe 11 364 374
66. DunbarTLYanZBallaKMSmelkinsonMGTroemelER 2012 C. elegans Detects Pathogen-Induced Translational Inhibition to Activate Immune Signaling. Cell host & microbe 11 375 386
67. KellAVenturaNKahnNJohnsonTE 2007 Activation of SKN-1 by novel kinases in Caenorhabditis elegans. Free Radic Biol Med 43 1560 1566
68. ChoeKPStrangeK 2007 Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol 293 C915 927
69. YunCStanhillAYangYZhangYHaynesCM 2008 Proteasomal adaptation to environmental stress links resistance to proteotoxicity with longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A 105 7094 7099
70. MehtaRSteinkrausKASutphinGLRamosFJShamiehLS 2009 Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science 324 1196 1198
71. HowardRASharmaPHajjarCCaldwellKACaldwellGA 2007 Ubiquitin conjugating enzymes participate in polyglutamine protein aggregation. BMC Cell Biol 8 32
72. GreerELMauresTJHauswirthAGGreenEMLeemanDS 2010 Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature 466 383 387
73. MauresTJGreerELHauswirthAGBrunetA 2011 The H3K27 demethylase UTX-1 regulates C. elegans lifespan in a germline-independent, insulin-dependent manner. Aging Cell
74. BoukaftaneYDuncanAWangSLabudaDRobertMF 1994 Human mitochondrial HMG CoA synthase: liver cDNA and partial genomic cloning, chromosome mapping to 1p12-p13, and possible role in vertebrate evolution. Genomics 23 552 559
75. MorckCOlsenLKurthCPerssonAStormNJ 2009 Statins inhibit protein lipidation and induce the unfolded protein response in the non-sterol producing nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 106 18285 18290
76. BuhaescuIIzzedineH 2007 Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem 40 575 584
77. MiskaEAAlvarez-SaavedraEAbbottALLauNCHellmanAB 2007 Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet 3 e215 doi:10.1371/journal.pgen.0030215
78. AmbrosV 2003 MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113 673 676
79. PocockR 2011 Invited review: decoding the microRNA response to hypoxia. Pflugers Arch 461 307 315
80. ChitwoodDHTimmermansMC 2010 Small RNAs are on the move. Nature 467 415 419
81. AltuviaSWeinstein-FischerDZhangAPostowLStorzG 1997 A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90 43 53
82. GottesmanSMcCullenCAGuillierMVanderpoolCKMajdalaniN 2006 Small RNA regulators and the bacterial response to stress. Cold Spring Harb Symp Quant Biol 71 1 11
83. LeungAKCalabreseJMSharpPA 2006 Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci U S A 103 18125 18130
84. LeungAKSharpPA 2006 Function and localization of microRNAs in mammalian cells. Cold Spring Harb Symp Quant Biol 71 29 38
85. PhillipsJRDalmayTBartelsD 2007 The role of small RNAs in abiotic stress. FEBS Lett 581 3592 3597
86. SunkarRChinnusamyVZhuJZhuJK 2007 Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci 12 301 309
87. SunkarRZhuJK 2004 Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16 2001 2019
88. ThomasJH 2006 Analysis of homologous gene clusters in Caenorhabditis elegans reveals striking regional cluster domains. Genetics 172 127 143
89. de LencastreAPincusZZhouKKatoMLeeSS 2010 MicroRNAs both promote and antagonize longevity in C. elegans. Curr Biol 20 2159 2168
90. KirienkoNVFayDS 2010 SLR-2 and JMJC-1 regulate an evolutionarily conserved stress-response network. EMBO J 29 727 739
91. JenkinsNLMcCollGLithgowGJ 2004 Fitness cost of extended lifespan in Caenorhabditis elegans. Proc Biol Sci 271 2523 2526
92. HoogewijsDHouthoofdKMatthijssensFVandesompeleJVanfleterenJR 2008 Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC molecular biology 9 9
93. AitlhadjLSturzenbaumS 2010 The use of FUdR can cause prolonged longevity in mutant nematodes. Mechanisms of ageing and development 131 364 369
94. GruberJNgLPoovathingalSHalliwellB 2009 Deceptively simple but simply deceptive–Caenorhabditis elegans lifespan studies: considerations for aging and antioxidant effects. FEBS letters 583 3377 3464
95. HosonoR 1978 Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine. Experimental gerontology 13 369 374
96. MitchellDHStilesJWSantelliJSanadiDR 1979 Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. Journal of gerontology 34 28 36
97. Van RaamsdonkJHekimiS 2011 FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mechanisms of ageing and development 132 519 540
Štítky
Genetika Reprodukčná medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



