-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
In budding yeast, transcriptional silencing, which is important to regulate gene expression and maintain genome integrity, requires silent information regulator (Sir) proteins. In addition, Rtt106, a histone chaperone involved in nucleosome assembly, functions in transcriptional silencing. However, how transcriptional silencing is regulated during mitotic cell division is not well understood. We show that cells lacking Dia2, a component of the SCFDia2 E3 ubiquitin ligase involved in DNA replication, display defects in silencing at the telomere and HMR locus and that the F-box and C-terminal regions of Dia2, two regions important for Dia2's ubiquitylation activity, are required for proper transcriptional silencing at these loci. In addition, we show that Sir proteins are mislocalized in dia2Δ mutant cells. Mutations in Dia2 and Rtt106 result in a synergistic loss of silencing at the HMR locus and significant elevation of Sir4 proteins at the HMR locus, suggesting that silencing defects in dia2Δ mutant cells are due, at least in part, to the altered levels of Sir4 at silent chromatin. Supporting this idea, we show that SCFDia2 ubiquitylates Sir4 in vitro and in vivo. Furthermore, Sir4 binding to silent chromatin is dynamically regulated during the cell cycle, and this regulation is lost in dia2Δ mutant cells. These results demonstrate that the SCFDia2 complex is involved in transcriptional silencing, ubiquitylates Sir4, and regulates transcriptional silencing during the cell cycle.
Published in the journal: The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing. PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002846
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002846Summary
In budding yeast, transcriptional silencing, which is important to regulate gene expression and maintain genome integrity, requires silent information regulator (Sir) proteins. In addition, Rtt106, a histone chaperone involved in nucleosome assembly, functions in transcriptional silencing. However, how transcriptional silencing is regulated during mitotic cell division is not well understood. We show that cells lacking Dia2, a component of the SCFDia2 E3 ubiquitin ligase involved in DNA replication, display defects in silencing at the telomere and HMR locus and that the F-box and C-terminal regions of Dia2, two regions important for Dia2's ubiquitylation activity, are required for proper transcriptional silencing at these loci. In addition, we show that Sir proteins are mislocalized in dia2Δ mutant cells. Mutations in Dia2 and Rtt106 result in a synergistic loss of silencing at the HMR locus and significant elevation of Sir4 proteins at the HMR locus, suggesting that silencing defects in dia2Δ mutant cells are due, at least in part, to the altered levels of Sir4 at silent chromatin. Supporting this idea, we show that SCFDia2 ubiquitylates Sir4 in vitro and in vivo. Furthermore, Sir4 binding to silent chromatin is dynamically regulated during the cell cycle, and this regulation is lost in dia2Δ mutant cells. These results demonstrate that the SCFDia2 complex is involved in transcriptional silencing, ubiquitylates Sir4, and regulates transcriptional silencing during the cell cycle.
Introduction
Chromatin structure governs a host of cellular processes, including gene expression, DNA replication, and DNA repair [1]. In higher eukaryotic cells, chromatin is classified into two major forms, euchromatin and heterochromatin, based on cytological staining. Euchromatin, the less dense and gene-rich form of chromatin, is associated with active gene transcription, whereas heterochromatin has a more compact structure and is associated with gene silencing. Though poor in genes, heterochromatin is important for development, centromere formation and the maintenance of genome integrity [2]–[4]. Therefore, it is important to understand how heterochromatin is formed and inherited during S phase of the cell cycle.
The budding yeast, Saccharomyces cerevisiae, has three heterochromatin-like loci: telomeres, the HM cryptic mating type loci (HMR and HML) and ribosomal DNA (rDNA) repeats. The initiation and maintenance of silent chromatin in budding yeast require Silent Information Regulator (Sir) proteins. While silencing at telomeres and the HM loci is regulated by many of the same factors, including Sir2, Sir3 and Sir4, only Sir2 is required for rDNA silencing. A stepwise model has been proposed for silent chromatin formation [5], [6]. For instance, at the HMR locus, Sir1 and Sir4 are recruited to the E silencer, a DNA sequence element containing binding sites for the origin-recognition complex (ORC) and transcription factors Rap1 and Abf1, through protein-protein interactions. Sir4 then recruits Sir2, a NAD+-dependent histone deacetylase, which deacetylates lysine residues on histones H3 and H4, including histone H4 lysine 16 (H4K16). This leads to the recruitment and binding of Sir3 and Sir4 to the adjacent nucleosome, as Sir3 and Sir4 bind hypoacetylated histones with higher affinity. This cycle of histone deacetylation and Sir protein binding to hypoacetylated nucleosomes leads to the spread of Sir proteins across the entire silent chromatin domain [5]–[7]. Despite the fact that protein factors and histone modifications involved in silent chromatin formation and maintenance in budding yeast are different from those in mammalian cells, this mechanism of step-wise formation of silent chromatin is likely to be conserved in higher eukaryotic cells [5], [8]. Importantly, despite advances made in understanding chromatin structure and transcriptional silencing, how silent chromatin is inherited and maintained during S phase of the cell cycle remains elusive.
During S phase of the cell cycle, nucleosomes ahead of the replication fork are disassembled to facilitate access of DNA replication machinery to DNA. Immediately following DNA replication, replicated DNA is reassembled into nucleosomes using both newly-synthesized histones and parental histones in a process called DNA replication-coupled nucleosome assembly. It is known that deposition of newly-synthesized H3–H4 requires histone H3–H4 chaperones, including CAF-1, Asf1 and Rtt106 [9]. Various studies in budding yeast indicate that these histone chaperones function in two parallel pathways in transcriptional silencing: an Asf1 dependent pathway and a CAF-1 dependent pathway [10], [11]. For instance, asf1Δ or rtt106Δ cells exhibit reduced silencing at both telomeres and the HM loci when combined with mutations in Cac1, the large subunit of CAF-1 [10]–[13]. In addition, Sir proteins are mislocalized in cells lacking both Rtt106 and CAF-1 [11]. On the other hand, rtt106Δ asf1Δ double mutant cells do not exhibit synergistic silencing defects, suggesting that Rtt106 and Asf1 function in the same genetic pathway in transcriptional silencing [13]. These results support the idea that nucleosome assembly factors are important for proper formation and inheritance of silent chromatin structure in budding yeast [6].
Dia2 is an F-box containing protein that serves as the substrate recognition component of a SCF (Skp1/Cullin/F-box protein) ubiquitin E3 ligase. Cells lacking Dia2 exhibit gross chromosomal rearrangements and sensitivity to cytotoxic agents, indicative of a role for Dia2 in maintaining genome integrity [14]–[16]. Furthermore, Dia2 has been shown to be important during DNA replication [15]–[17]. The F-box domain in each of 11 known F-box containing proteins in budding yeast interacts with the SCF component Skp1, enabling interactions between the ubiquitylation machinery and substrate [18]. In addition to the F-box domain, Dia2 has two additional important domains: a tetratricopeptide repeat (TPR) domain at the N-terminus involved in mediating Dia2's interaction with replisome components [14], [17] and a leucine rich repeat (LRR) region at the C-terminus. The LRR domain in other F-box containing proteins is known to be involved in substrate binding [19].
In a genetic screen designed to identify genes that function in parallel to RTT106 in silencing at the HMR locus, we discovered that DIA2, when deleted, enhances rtt106Δ silencing defects at the HMR locus. Structure-function studies revealed that the Dia2 F-box and LRR regions are important for transcriptional silencing. Furthermore, both Sir3 and Sir4 are mislocalized in dia2Δ cells, and Sir4 binding to the HMR locus is significantly elevated in dia2Δ rtt106Δ mutant cells. In addition, we show that Sir4 is ubiquitylated in yeast cells in a Dia2-dependent manner and that Sir4 levels on chromatin are cell cycle regulated, and this regulation is lost in dia2Δ mutant cells. Therefore, we suggest that the SCFDia2 E3 ligase functions in transcriptional silencing, in part through the regulation of Sir4 ubiquitylation.
Results
SCFDia2 functions in transcriptional silencing
We identified RTT106 in a screen for genes that function in parallel with PCNA in silencing at the HMR silent mating type locus [13]. Using a similar approach, we set out to identify genes that functioned in parallel with RTT106 in transcriptional silencing. Briefly, we used the synthetic genetic array (SGA) approach [20], [21] to combine the rtt106Δ single mutant containing the HMR::GFP reporter gene with each of ∼4700 yeast deletion mutants. The HMR::GFP reporter contains the green fluorescent protein (GFP) integrated at the HMR silent mating type locus within the a1 gene; thus, GFP is silenced. Once double mutants containing the HMR::GFP reporter gene were selected, flow cytometry was used to identify those genes from the collection of mutants that when combined with rtt106Δ, resulted in a significant elevation in the percentage of GFP expressing cells.
We identified five genes (CAC1, CAC2, SIR1, ARD1, and DIA2) that enhanced the silencing defects of rtt106Δ cells. Cac1 and Cac2 are two subunits of the histone chaperone CAF-1, and Sir1 is necessary for initiation of silent chromatin formation at the HM loci [22], [23]. Deletion of SIR1, CAC1, or CAC2 is known to enhance the silencing defects of rtt106Δ mutant cells [11], [13]. In addition, ARD1 is predicted to have a role in transcriptional silencing [24], [25]. These results affirm that our screen was effective for identifying factors that enhance silencing defects of rtt106Δ cells.
Dia2 is the F-box containing protein of the SCFDia2 ubiquitin E3 ligase involved in DNA replication, and it may also have a role in transcriptional regulation [16], [26]. Therefore, we decided to focus our studies on Dia2. To confirm our results, we deleted DIA2 from our standard genetic background (W303) and assessed transcriptional silencing at the HMR locus using the HMR::GFP reporter. Cells defective for transcriptional silencing at the HMR locus express GFP and exhibit a rightward shift in the flow cytometry profile as observed for sir3Δ cells (Figure 1A). Mutating DIA2 in rtt106Δ cells resulted in a rightward shift compared to single mutant cells (Figure 1A), indicating an increase in the percentage of GFP expressing cells, which was quantified in Figure 1B. Moreover, dia2Δ single mutant cells exhibited an elevated percentage of cells expressing GFP compared to wild-type cells, suggesting a role for Dia2 in HMR silencing. To validate the flow cytometry analysis, the percentage of cells expressing GFP was also determined using fluorescence microscopy. Among the strains tested, a similar trend was observed using both methods of determining the percentage of cells expressing GFP (Figure S1). To confirm the effect of dia2Δ on HMR silencing, we determined how the loss of Dia2 affected the expression of the silenced a1 gene at the HMR locus. RNA was collected from single and double mutant cells of the alpha mating type, reverse transcribed, and cDNA analyzed using real-time PCR. Upon normalizing the expression of a1 to ACT1, dia2Δ cells exhibited elevated a1 gene expression compared to wild-type cells (Figure 1C). Furthermore, dia2Δ rtt106Δ had an even greater a1 gene expression level compared to either the dia2Δ or rtt106Δ single mutant. These results are consistent with the idea that DIA2 and RTT106 function in parallel to regulate transcriptional silencing at the HMR locus.
Fig. 1. Dia2 functions in parallel with Rtt106 in HMR silencing. 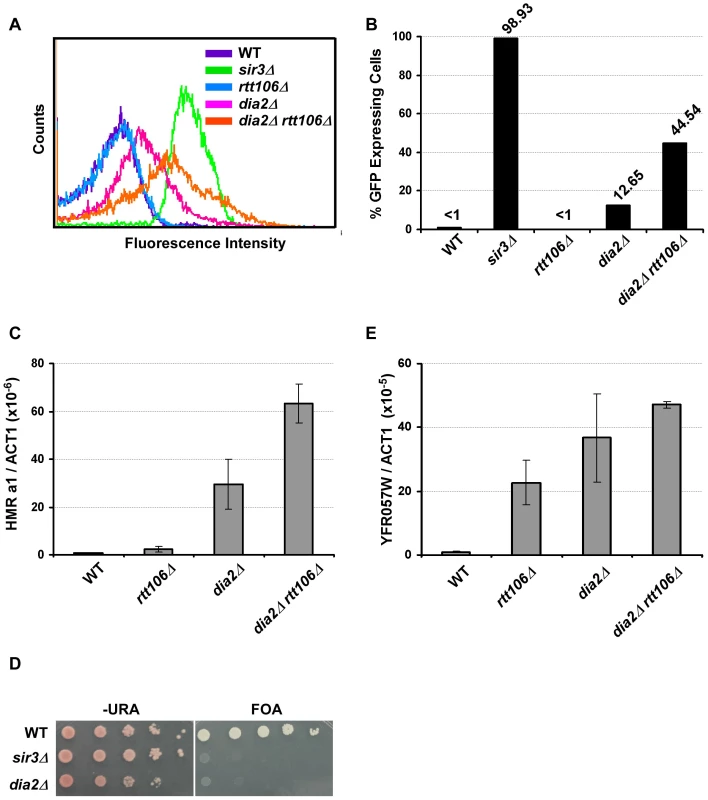
(A–C) Mutant dia2Δ cells have defects in transcriptional silencing at the HMR locus. Cells with the indicated genotype were analyzed for GFP expression using flow cytometry (A), and the percentage of cells expressing GFP was reported (B). Wild-type (WT) cells (less than 1%) and cells with SIR3 deleted (sir3Δ, over 95%) were used as standards to set the gate for GFP expression. The flow cytometry profile and percentage of cells expressing GFP are reported from one of three independent experiments. (C) HMR a1 gene expression is elevated in dia2Δ and dia2Δ rtt106Δ mutants. RNA was collected from cells of the indicated genotype and reverse transcribed. Expression of the HMR a1 gene was analyzed via real-time PCR, normalized against the expression of the ACT1 gene and reported as the average ± standard deviation (s.d.) of two independent experiments. (D–E) Telomere silencing is compromised in dia2Δ cells. (D) Telomere silencing was assayed using the URA3 gene integrated at the left end telomere of chromosome VII and growth on medium containing 5-fluoroorotic acid (FOA). (E) YFR057W expression levels were determined by real time RT-PCR and analyzed as described above with the average ± s.d. of two independent experiments shown. Transcriptional silencing at telomeres and the HMR locus utilize similar mechanisms [6]. Moreover, it has been shown that Pof3, the homolog of Dia2 in S. pombe, is required for maintaining telomere length and telomeric silencing [27]. We, therefore, determined whether Dia2 in S. cerevisiae had a role in telomeric silencing using cells containing the reporter gene, URA3, integrated at the left arm of telomere VII. When plated on media containing 5-fluoroorotic acid (FOA) that is toxic to cells that express URA3, wild-type cells survive because of telomeric silencing of the URA3 gene. Cells with telomeric silencing defects (such as sir3Δ, used as a control) exhibit growth defects in media containing FOA [24]. We found that dia2Δ cells exhibited defects in telomeric silencing compared to wild-type cells (Figure 1D). We confirmed these observed telomeric silencing defects using RT-PCR to assess the expression of YFR057W, a gene found at the telomere on the right arm of chromosome VI and known to be silenced via a Sir-mediated mechanism [28]. Compared to wild-type and rtt106Δ cells, dia2Δ cells exhibited higher YFR057W gene expression, suggesting a telomeric silencing defect (Figure 1E). Interestingly, deletion of RTT106 in dia2Δ mutant cells did not significantly increase expression of YFR057W, suggesting that deletion of RTT106 does not exacerbate the telomeric silencing defect of dia2Δ cells. These results demonstrate that Dia2 is required for efficient silencing at both the HMR locus and telomeres and suggest that Dia2 functions in a pathway parallel to Rtt106 in transcriptional silencing at the HMR locus, but not at telomeres.
The dia2Δ mutant exhibits a synthetic interaction with mutations at genes encoding histone chaperones and at histone lysine residues implicated in nucleosome assembly
In addition to RTT106, mutations in ASF1, HIR1 and CAF-1 also result in silencing defects [12], [13]. We, therefore, tested how loss of DIA2 affected silencing at the HMR locus in the absence of each of these H3–H4 histone chaperones. Each double mutant, dia2Δ asf1Δ, dia2Δ cac1Δ or dia2Δ hir1Δ, had a higher percentage of cells expressing GFP compared to the respective single mutants (Figure 2A). These results suggest that Dia2 impacts transcriptional silencing at the HMR locus in a pathway parallel to each of the known H3–H4 histone chaperones.
Fig. 2. DIA2 genetically interacts with genes encoding histone chaperones and histone H3–H4 lysine mutants with defects in nucleosome assembly. 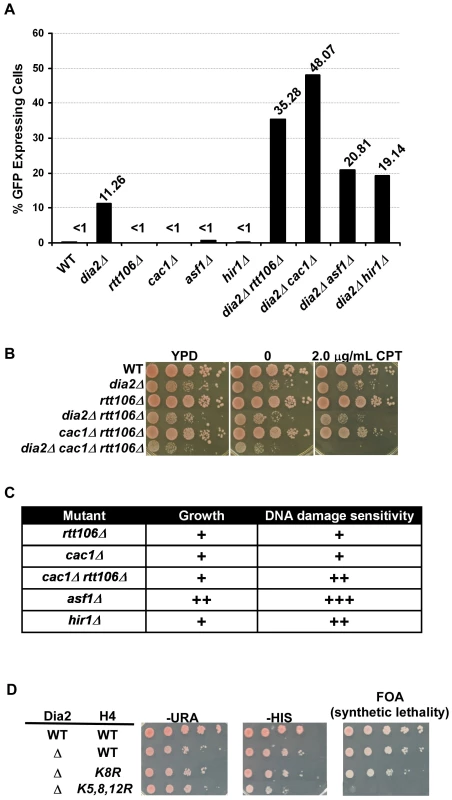
(A) Dia2 functions in parallel with known H3–H4 histone chaperones in transcriptional silencing at the silent HMR locus. Cells of the indicated genotype were assessed for HMR silencing as described in Figure 1A–1B. The percentage of cells expressing GFP from one of three independent experiments was reported. (B–C) Dia2 functions in parallel with known H3–H4 histone chaperones in growth and DNA damage sensitivity. (B) Genetic interactions among DIA2, RTT106, and CAC1 were assessed by spotting a ten fold series dilution of cells of the indicated genotype onto regular growth media (YPD), media containing DMSO (0) or media with the indicated concentration of camptothecin (CPT). (C) A summary of the genetic analysis of growth and DNA damage sensitivity of histone chaperone mutations combined with deletion of DIA2. A ‘+’ indicates a synthetic effect in the double mutant compared to either single mutant alone. Spot assays were performed as described above using different concentrations of CPT and methyl methanesulfonate (MMS). Representative images are shown in Figure S2. (D) The dia2Δ mutant is synthetic lethal with mutations at histone H4 lysine residues 5, 8 and 12 (H4K5,8,12R). A ten fold serial dilution of cells was spotted onto plates of the indicated media. Both copies of histone genes (HHT1-HHF1 and HHT2-HHF2) were deleted. Wild type H3–H4 was expressed from a URA3 containing plasmid, whereas the histone H4 mutant (as indicated) was expressed from a plasmid containing the HIS3 gene. Media containing FOA was used to select for the loss of the URA3-containing plasmid and detect synthetic lethality. In addition to defects in transcriptional silencing, histone chaperone mutants are sensitive to DNA damaging agents [10], [29], [30]. Moreover, dia2Δ cells also exhibit sensitivity to a number of DNA damaging agents [15], [16]. To determine whether the parallel action observed for DIA2 and H3–H4 histone chaperones extended beyond their function in transcriptional silencing, we tested the growth and DNA damage sensitivity of dia2Δ cells containing a mutation at each H3–H4 histone chaperone. The DNA damaging agents camptothecin (CPT) and methyl methanesulfonate (MMS) were used to assess genetic interactions in response to DNA damage. Synthetic interactions in growth and DNA damage sensitivity were observed for dia2Δ with each of the histone chaperone mutants tested (Figure 2B, 2C and Figure S2). The most dramatic effects were observed in dia2Δ asf1Δ and dia2Δ cac1Δ rtt106Δ mutants, with the interaction between DIA2 and ASF1 being the most pronounced. Furthermore, dia2Δ cac1Δ rtt106Δ cells grew slower on regular growth media (YPD) than dia2Δ and cac1Δ rtt106Δ cells and showed an increased sensitivity to CPT over dia2Δ, cac1Δ rtt106Δ and dia2Δ rtt106Δ cells. Taken together, these genetic interactions provide support for a role for Dia2 and the SCFDia2 complex in processes linked to nucleosome assembly.
Post-translational modifications on newly synthesized histones work in concert with histone chaperones to regulate nucleosome assembly [9]. For instance, histone H3 lysine 56 acetylation (H3K56Ac), catalyzed by the histone acetyltransferase Rtt109, is important for regulating the interaction between histones and the histone chaperones CAF-1 and Rtt106, and thus, H3K56Ac is a critical regulator of histone deposition [29], [31]. H3 N-terminal tail acetylation, catalyzed by Gcn5 and Rtt109, also serves as an important regulator of nucleosome assembly [32]. Finally, acetylation of histone H4 lysines 5, 8 and 12 (H4K5,8,12Ac), catalyzed by Hat1 and Elp3, has also been implicated in nucleosome assembly [33], [34]. Given the observed genetic interactions between dia2Δ and the H3–H4 histone chaperones involved in nucleosome assembly, we, therefore, determined how mutations in these important histone lysine residues affected the growth and CPT sensitivity of dia2Δ cells. For each histone mutant, the acetylated lysine residues were mutated to arginine to mimic the unacetylated state. We observed that dia2Δ cells showed significant growth defects and increased sensitivity towards CPT when combined with H3K56R, H4K5,12R and H3K9,14,18,23,27R (Table 1). Notably, we were unable to construct the dia2Δ H4K5,8,12R mutant via plasmid shuffling, suggesting that dia2Δ exhibited a synthetic lethal interaction with mutations at H4 lysines 5, 8 and 12 (Table 1 and Figure 2D). To confirm this result, wild-type and dia2Δ cells expressing wild-type histones H3–H4 from a uracil (URA3) containing plasmid were transformed with either wild-type or mutant forms of H3-H4K5, 8, 12 on a plasmid with a histidine (HIS) selection marker. The wild-type H3–H4 histone plasmid (URA) could not be lost in dia2Δ H4K5,8,12R cells (as no growth was observed when these cells were plated on FOA medium), whereas this plasmid was readily lost in dia2Δ or dia2Δ H4K8R cells (Figure 2D). This suggests that Dia2 functions in parallel with acetylation of H4 lysine residues 5, 8 and12 in maintaining cell viability. Together, these genetic analyses provide further evidence supporting the idea that the SCFDia2 E3 ligase has a role in a process linked to nucleosome assembly.
Tab. 1. Effect of histone lysine mutations on the growth and DNA damage sensitivity of dia2Δ cells. 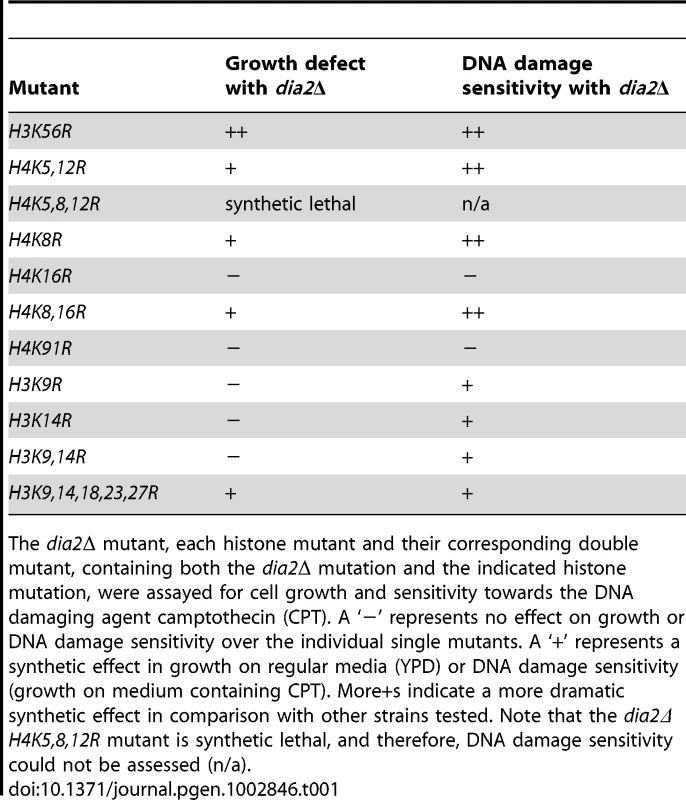
The dia2Δ mutant, each histone mutant and their corresponding double mutant, containing both the dia2Δ mutation and the indicated histone mutation, were assayed for cell growth and sensitivity towards the DNA damaging agent camptothecin (CPT). A ‘−’ represents no effect on growth or DNA damage sensitivity over the individual single mutants. A ‘+’ represents a synthetic effect in growth on regular media (YPD) or DNA damage sensitivity (growth on medium containing CPT). More+s indicate a more dramatic synthetic effect in comparison with other strains tested. Note that the dia2Δ H4K5,8,12R mutant is synthetic lethal, and therefore, DNA damage sensitivity could not be assessed (n/a). The F-box and LRR domain of Dia2 are required for transcriptional silencing
Dia2 contains three primary functional domains: an N-terminal tetratricopeptide repeat (TPR) region, an F-box domain and a C-terminal leucine rich repeat (LRR) domain (Figure 3A). The TPR region is important for Dia2's localization to the replisome [14], [17]. The F-box domain is required for Dia2 ubiquitylation activity, as the F-box, in general, facilitates interactions with other SCFDia2 components [15], [16]. The LRR region is proposed to be an interaction motif for substrates of the SCFDia2 complex [17]. To gain further insight into Dia2's role in transcriptional silencing, we deleted each of the three domains of Dia2, expressed these dia2 mutants in dia2Δ cells and analyzed transcriptional silencing at the silent HMR locus and the telomere. Expression of Dia2 in dia2Δ cells restored the silencing at the HMR locus, as the percentage of cells expressing GFP was similar to wild-type cells (Figure 3B). Expression of the Dia2-TPRΔ domain mutant also restored HMR silencing. Interestingly, deletion of the Dia2 F-box (F-boxΔ) or LRR domain (LRRΔ) did not rescue the HMR silencing defect. These results suggest that the Dia2 F-box and LRR domains are indispensable for silencing at the HMR locus.
Fig. 3. Dia2's F-box and LRR domains are indispensable for transcriptional silencing. 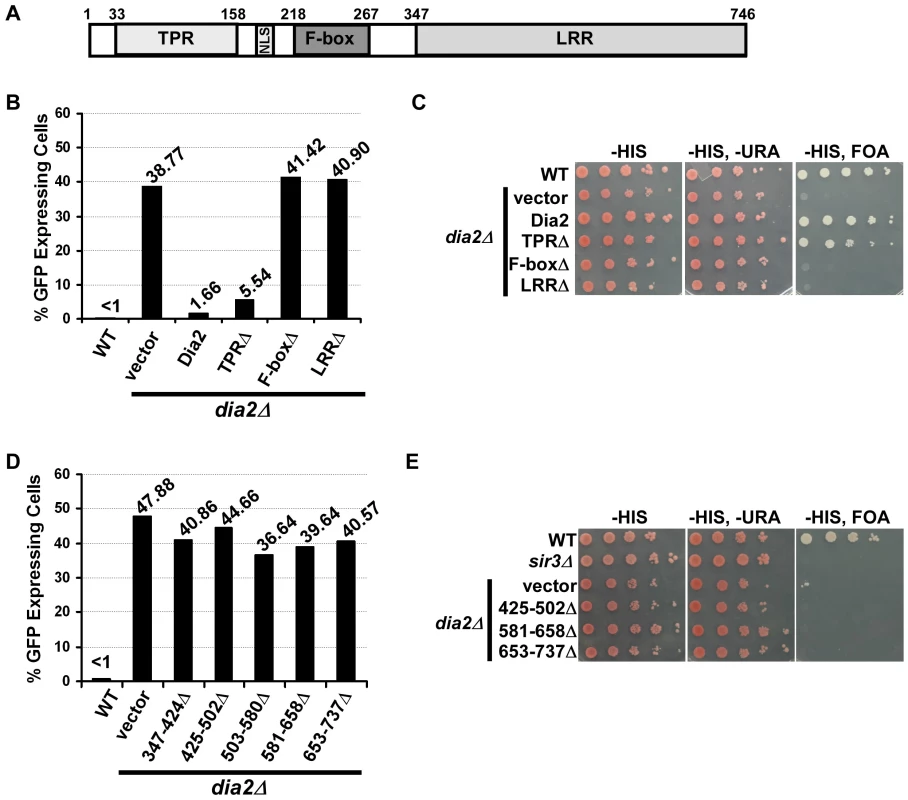
(A) A schematic drawing of the domain structure of Dia2. Dia2 contains a tetratricopeptide repeat (TPR) region, nuclear localization signal (NLS), F-box and leucine rich repeat (LRR) domain. (B) The Dia2 F-box and LRR domains are important for silencing at the HMR locus. The dia2Δ mutant cells containing the HMR::GFP reporter were transformed with empty vector or a plasmid expressing WT or different dia2 mutants, and the percentage of cells expressing GFP was determined as in Figure 1B. Note that the percentage of cells expressing GFP in dia2Δ cells was higher when using selective media compared to cells growing in YPD. (C) The Dia2 F-box and LRR domains are important for telomere silencing. The dia2Δ mutants containing the VIIL-URA3 reporter were transformed with empty vector or a plasmid expressing the indicated dia2 mutant and spotted onto selective media (-HIS) and media containing FOA (-HIS, FOA) to assay silencing. (D–E) The Dia2 LRR domain is important for HMR silencing (D) and telomere silencing (E). Assays were performed as described in B and C. Similar to the effects observed at the HMR locus, the expression of full-length Dia2 in dia2Δ cells containing the URA3 reporter at telomere VIIL rescued the telomere silencing defects of dia2Δ cells to that of wild-type cells, and expression of Dia2 lacking the TPR region (TPRΔ) resulted in an almost complete rescue of dia2Δ telomere silencing defects (Figure 3C). In contrast, expression of Dia2 lacking the F-box (F-boxΔ) or the LRR region (LRRΔ) was unable to rescue the telomere silencing defects of dia2Δ cells.
While the expression of the TPRΔ and F-boxΔ mutants was similar to that of full length Dia2 in dia2Δ cells (Figure S3), expression of the Dia2 LRRΔ mutant was much less than the other mutants (data not shown), most likely due to instability of the shortened form of the protein. Therefore, we made five additional Dia2 mutants, each with deletion of approximately 75 amino acids of the LRR domain: LRR (amino acids 347–424)Δ, 425–502Δ, 503–580Δ, 581–658Δ, and 653–737Δ. Expression of these five mutants was, for the most part, similar to full length Dia2 and other Dia2 mutant forms (Figure S3). Importantly, dia2Δ cells expressing each of the LRR mutants exhibited defects in HMR (Figure 3D) and telomere silencing (Figure 3E) similar to dia2Δ cells transformed with empty vector. Together, these results suggest that silencing defects displayed in cells expressing dia2 mutants lacking the LRR or F-box are unlikely due to reduced expression of these mutant proteins. Instead, the F-box domain, essential for Dia2's role in protein ubiquitylation, and LRR region, predicted to be important for substrate recognition, are indispensable for SCFDia2's role in transcriptional silencing, suggesting that SCFDia2's role in silencing is likely mediated through its ability to ubiquitylate a substrate involved in transcriptional silencing.
Sir3 and Sir4 are mis-localized in dia2Δ cells
Sir proteins serve as structural components of yeast silent chromatin [6]. Sir3 and Sir4 form four to five foci at the nuclear periphery, which reflects the clustering of yeast telomeres [35]–[37]. Thus, we tested whether loss of Dia2 affected the localization of Sir3 and Sir4, and therefore, silent chromatin structure, using fluorescence microscopy. As reported, wild-type cells expressing Sir3-GFP or GFP-Sir4 formed foci at the nuclear periphery [11], [37]. While some dia2Δ cells had Sir3-GFP or Sir4-GFP foci patterns similar to wild-type cells (Figure 4A, dia2Δ, panel 1), a considerable percentage of dia2Δ mutant cells lost proper localization of Sir3 (Figure 4B) and Sir4 (Figure 4C) in dia2Δ or dia2Δ rtt106Δ cells. In the case of Sir3-GFP foci, some mutant cells had large areas of fluorescence without distinct foci (Figure 4A, dia2Δ panels 2 and 3). A commonly observed Sir4 pattern was multiple small foci (>8) that were relatively non-distinct (Figure 4A, dia2Δ panel 2), in addition to cells containing areas of fluorescence with no distinct foci (Figure 4A, dia2Δ, panel 3). Because the dia2Δ mutation did not affect the protein levels of Sir3 and Sir4 to a significant degree (Figure S4), the mis-localization of Sir3 and Sir4 observed in dia2Δ mutant cells is likely due to changes in telomeric chromatin structure.
Fig. 4. Sir3 and Sir4 are mislocalized in dia2Δ cells and cells expressing dia2 mutants lacking the Dia2 F-box or LRR domain. 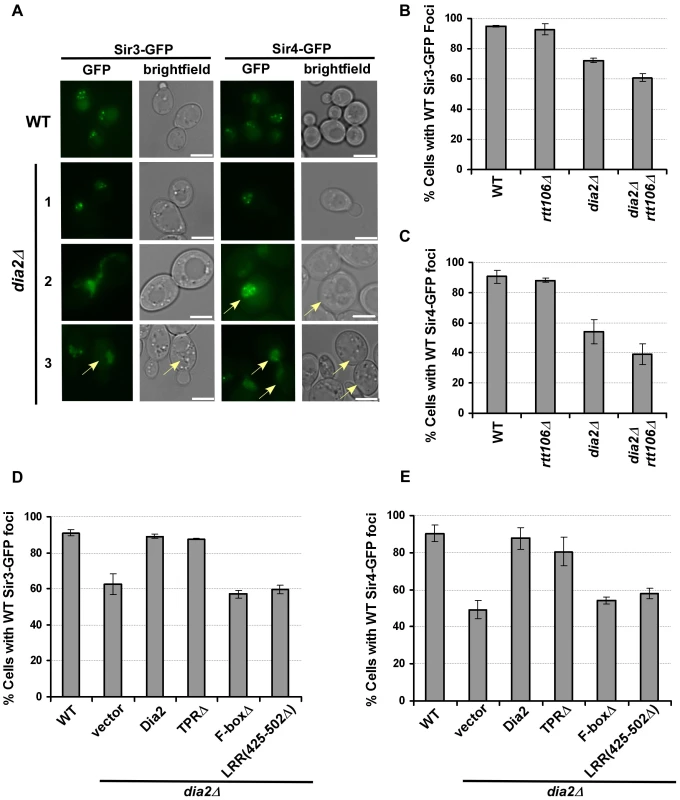
(A–C) Sir3 and Sir4 are mislocalized in dia2Δ and dia2Δ rtt106Δ cells. (A) Representative images of Sir3-GFP or Sir4-GFP foci in WT and dia2Δ cells. Cells expressing Sir3-GFP and GFP-Sir4 exhibited nuclear foci in WT cells, and this pattern is lost in some dia2Δ cells. Note that dia2Δ cells are larger in size than wild-type cells (images were taken under the same magnification). The scale bar represents 5 µm. Cells that exhibited Sir protein localization defects as described in the text were marked by arrows. (B–C) Quantification of the percentage of dia2Δ cells that had WT-like Sir3-GFP foci (B) and WT-like Sir4-GFP foci (C). (D–E) Cells expressing dia2 mutants lacking the Dia2 F-box and LRR domains exhibit defects in the localization of Sir3 (D) and Sir4 (E). For each experiment, at least 100 cells were counted for each genotype, and the average ± s.d. percentage of cells expressing WT-like foci from at least two independent experiments was reported. To determine whether the localization of Sir3-GFP and Sir4-GFP was dependent upon particular Dia2 domains, we expressed the Dia2 mutants lacking specific domains (see Figure 3) in dia2Δ cells. Expression of full-length Dia2 or Dia2 TPRΔ restored the percentage of cells containing wild-type Sir3-GFP or Sir4-GFP foci closer to the percentage observed for wild-type cells, whereas cells expressing dia2 mutants lacking the F-box (F-boxΔ) or a portion of the LRR domain [LRR(425–502Δ) and LRR(581–658Δ)] did not (Figure 4D–4E and data not shown). Thus, the F-box and LRR regions are important for proper localization of Sir3 and Sir4, consistent with the idea that Dia2's role in transcriptional silencing is dependent upon its ubiquitylation activity.
Sir4 binding at the HMR a1 gene locus is elevated in dia2Δ rtt106Δ cells
To further analyze how the dia2Δ mutation affects the binding of Sir proteins to chromatin, chromatin immunoprecipitation (ChIP) assays were performed in unsynchronized wild-type, dia2Δ, dia2Δ rtt106Δ and control cells (sir3Δ) using antibodies against Sir4, Sir2, and Sir3. ChIP DNA was analyzed using real-time PCR with primers amplifying different positions at the HMR locus or the telomere region on the right arm of chromosome VI (Tel-VIR). Consistent with published results, Sir protein binding at silent chromatin loci was lost in sir3Δ cells, and more Sir proteins bound to silent chromatin than the corresponding active chromatin loci tested at both the HMR locus and telomere (Figure 5 and Figure S5) [11]. Compared to wild-type cells, dia2Δ cells exhibited a slight elevation in Sir4 binding at HMR silent chromatin (a1 gene, HMR silent) (Figure 5A), whereas dia2Δ rtt106Δ cells had a much larger and significant increase in Sir4 binding at the silent HMR locus than wild-type and dia2Δ cells (Figure 5A). Similarly, we also observed significantly more Sir2 binding at the HMR locus in dia2Δ rtt106Δ mutant cells compared to wild-type and dia2Δ mutant cells (Figure 5B). In contrast, Sir3 binding to the silent HMR locus was not altered to a significant degree in either dia2Δ or dia2Δ rtt106Δ cells when chIP was performed using an antibody against endogenous Sir3 (Figure S5A) or performed using IgG beads in strains in which Sir3 was tagged with a tandem affinity purification (TAP) tag (Sir3-TAP)(Figure 5C). Notably, the dia2Δ or dia2Δ rtt106Δ mutation did not affect mRNA levels of Sir4 or Sir2 (Figure S6), suggesting that the increase in Sir4 and Sir2 proteins at the HMR locus is not likely due to increased gene transcription in mutant cells. In addition, the overall protein levels of Sir3 and Sir4 were not altered to a detectable degree in cell extracts (Figure S4). Therefore, the observed elevation in Sir4 and Sir2 at the silent HMR locus in dia2Δ cells is not likely due to increased steady-state levels of Sir proteins and is likely due to elevated Sir2 and Sir4 levels on chromatin.
Fig. 5. Sir protein binding at the HMR locus is altered in dia2Δ rtt106 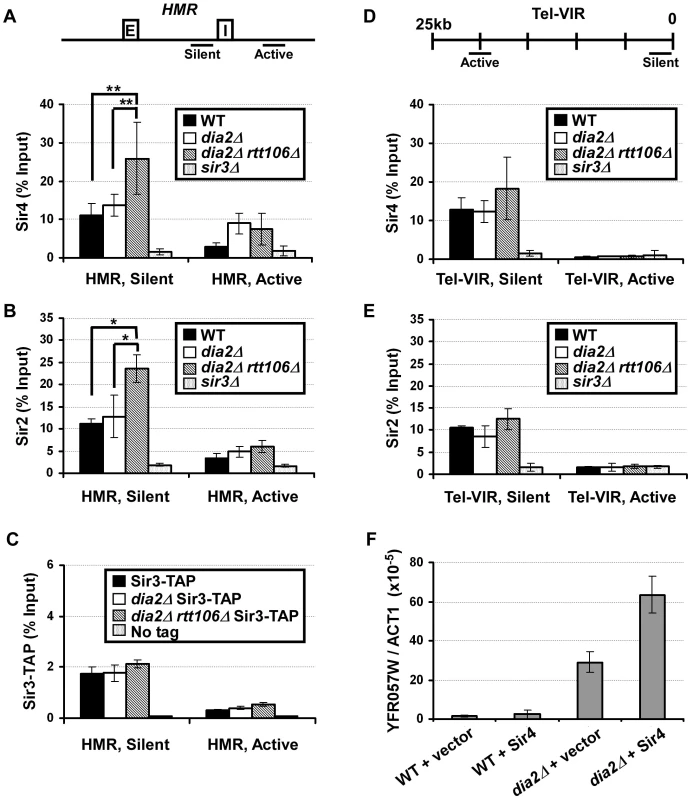
Δ cells. (A) Sir4 binding at the HMR silent chromatin locus is increased in dia2Δ rtt106Δ cells. ChIP assays were performed using an antibody against Sir4, and the resulting DNA was quantified via real time PCR using primers amplifying the a1 gene located at the HMR silent mating type cassette (HMR, silent,) and a region outside of the silent chromatin boundary (HMR, active). The upper panel indicates the location of primers at the HMR locus, and E and I represent the E and I silencers at the HMR locus. (B) Sir2 binding is increased at HMR silent chromatin in dia2Δ rtt106Δ cells. ChIP assays were performed as described in (A) using antibodies against Sir2. (C) Sir3 binding at the HMR locus is not altered in dia2Δ mutants. Sir3-TAP ChIP was performed using IgG sepharose. (D) Sir4 binding to telomeric silent chromatin is not significantly altered in dia2Δ rtt106Δ cells. ChIP assays were performed as described in (A), and the ChIP DNA was analyzed using PCR primers amplifying a fragment 0.77 kb from the telomere end of the right arm of chromosome VI right (Tel-VIR, silent) or 20 kb from the telomere end (Tel-VIR, active). The PCR primer locations are indicated in the upper panel. (E) Sir2 binding to telomeric heterochromatin is not significantly altered. ChIP samples from (B) were analyzed for the telomere positions. ChIP data is presented as the average ChIP signal (as the percentage of input) ± s.d. from at least three independent experiments. * represents a p-value <0.05, ** indicates a p-value <0.01 obtained using the student's t-test. (F) Ectopic expression of Sir4 further compromises telomeric silencing in dia2Δ cells. RNA was extracted from WT and dia2Δ cells transformed with empty vector or a centromere plasmid for expression of Sir4, and the expression of YFR057W was analyzed as described in Figure 1E. Sir protein binding was also assessed at telomeric silent chromatin. No significant change in Sir4 binding was detected at telomeric silent chromatin (Tel-VIR, silent) in dia2Δ cells compared to wild-type cells (Figure 5D). Sir4 binding to the telomeric silent chromatin in dia2Δ rtt106Δ cells was not increased significantly compared to wild-type (p-value for three independent experiments is 0.1). Thus, levels of Sir4 proteins are unlikely altered at telomeres in dia2Δ rtt106Δ mutant cells compared to wild-type cells. These results are consistent with the result in Figure 1 showing that deletion of RTT106 synergistically increases the silencing defect of dia2Δ cells at the HMR locus, but not the telomere. Sir2 (Figure 5E) and Sir3 (Figure S5B and S5C) binding to telomeric silent chromatin was not altered to a detectable degree in either dia2Δ or dia2Δ rtt106Δ cells compared to wild-type cells. Taken together, it is likely that mutations in Dia2 alter the chromatin binding of Sir4 and Sir2, but not Sir3, in dia2Δ rtt106Δ cells, and these imbalanced alterations in Sir protein binding to silent chromatin may contribute to the silencing defects observed for dia2Δ and dia2Δ rtt106Δ cells, especially at the silent HMR locus.
Expression of Sir4 in dia2Δ mutant cells leads to synergistic loss of telomeric silencing
Overexpression of Sir4 is known to result in silencing defects, most likely due to disruptions in Sir protein stoichiometry [38], [39]. To further test whether the effect of dia2Δ on silencing is due to altered Sir4 protein levels on chromatin, we investigated the effects of exogenous expression of Sir4 on telomeric silencing, as transcriptional silencing at telomeres is perturbed more easily than that at the HMR locus [40]. Wild-type and dia2Δ cells were transformed with a centromere plasmid for expression of Sir4 under the control of its own promoter. RNA was then extracted for quantitative RT-PCR analysis of YFR057W expression. While ectopic expression of Sir4 in wild-type cells did not result in obvious changes in expression of YFR057W, Sir4 expression in dia2Δ cells resulted in over a two-fold increase in YFR057W expression compared to dia2Δ cells transformed with empty vector (Figure 5F). This suggests that telomeric silencing in dia2Δ cells is more sensitive to changes in Sir4 levels than that of wild-type cells. These results are consistent with the idea that altered Sir4 levels at chromatin contribute to the silencing defects observed in dia2Δ cells.
SCFDia2 ubiquitylates Sir4
Since dia2Δ rtt106Δ cells exhibited elevated Sir4 binding at silent chromatin, we hypothesized that SCFDia2 may ubiquitylate Sir4. To test this idea, we first determined whether cell extracts prepared from cells expressing MYC-Dia2 or MYC-Dia2 F-boxΔ integrated at the endogenous DIA2 locus (Figure 6A, left panel) could ubiquitylate Sir4 purified from dia2Δ cells using TAP purification. Briefly, Flag–ubiquitin (Flag-Ub), E1, E2 (Cdc34) and the respective whole cell extract (E3) were incubated with Sir4-CBP (calmodulin binding peptide, part of the TAP tag). As negative controls, reaction mixtures containing all components except E1, E3 (cell extract) or substrate were assembled. Following ubiquitylation, Sir4 was pulled down using calmodulin beads, and ubiquitylated species were detected via Western blot using antibodies against the Flag epitope. More Sir4-associated ubiquitylated species were detected in the reactions using the extracts from full length Dia2 than from those using extracts from cells expressing Dia2 lacking the F-box domain (Figure 6A, right panel). No ubiquitylated species were detected in control reactions lacking E1, E3 or Sir4 (substrate). Thus, SCFDia2 ubiquitylates Sir4 and/or a Sir4-associated protein in vitro.
Fig. 6. SCFDia2 ubiquitylates Sir4 in vitro and in vivo. 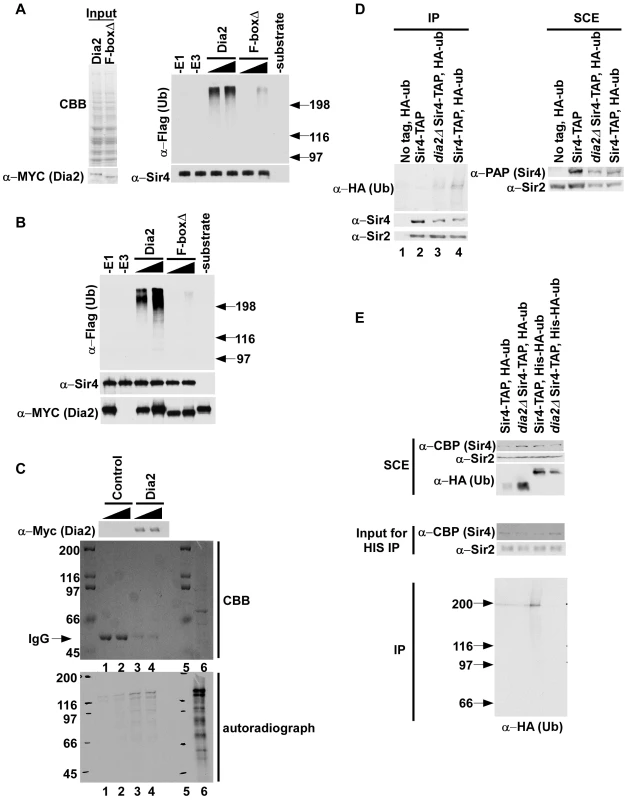
(A–B) Dia2 ubiquitylates Sir4 in vitro (A) (left panel) Cell extracts prepared from full length MYC-Dia2 or MYC-Dia2 F-boxΔ (F-boxΔ) cells were normalized using Coomassie staining (CBB, upper panel) and Western blot analysis using antibodies against the MYC epitope (bottom panel). (right panel) Two different amounts of extract were used for in vitro ubiquitylation assays using Sir4-TAP purified from dia2Δ cells as substrate, Flag-ubiquitin (Flag-ub), E1, and E2 (Cdc34). Following the ubiquitylation reaction, Sir4-CBP proteins were purified using calmodulin beads, and ubiquitylated species were detected via Western blot using Flag antibody. Sir4 were detected by Western blot using antibody against Sir4. (B) Ubiquitylation reactions were carried out as described in A except purified MYC-Dia2 or MYC-Dia2 F-boxΔ was used as E3. (C) Sir4 binds the SCFDia2 complex in vitro. Binding studies were performed using control beads, prepared by incubating beads with antibodies against the Myc epitope, or beads containing the MYC-Dia2 complex prepared as in B and two different amounts of in vitro translated 35S-methione labeled Sir4 (lane 6, 35S-Sir4 input). The presence of the SCFDia2 in binding samples was detected by Western blot (upper panel) using antibodies against the Myc epitope. In addition, binding samples were resolved by SDS-PAGE, stained with Coomassie (CBB, middle panel) and bound 35S-Sir4 was detected via autoradiography (lower panel); Lane 5 is a protein marker. (D–E) Sir4 is ubiquitylated in vivo, and this ubiquitylation is decreased in dia2Δ cells. (D) Sir4-TAP was purified from cells of the indicated genotype either with or without expression of HA-ubiquitin (HA-ub) using tandem affinity purification. Ubiquitylated species were detected using antibodies against the HA epitope. Right panel: SCE, soluble cell extract. (E). Sir4-TAP was purified from wild-type or dia2Δ mutant cells expressing either HA-ubiquitin or His-HA-Ubiquitin using IgG sepharose. After cleavage with TEV protease, the eluted proteins were denatured and purified using Ni-NTA beads. Purified ubiquitylated species were detected by Western blot using antibodies against HA. To test this idea further, the SCFDia2 complex was purified from yeast cells expressing MYC-Dia2 and MYC-Dia2 F-boxΔ and used to ubiquitylate Sir4 purified from yeast cells as described in Figure 6A. More ubiquitylated species were detected in reactions containing purified SCFDia2 (Dia2) than those containing the complex purified using the Dia2 F-box mutant (F-boxΔ) (Figure 6B). Taken together, these experiments indicate that SCFDia2 ubiquitylates Sir4, or a protein associated with Sir4, in vitro, and this ubiquitylation depends on the F-box domain of Dia2.
To provide additional evidence that SCFDia2 ubiquitylates Sir4, we tested whether Sir4 bound to SCFDia2 in vitro. Beads containing the MYC-Dia2 complex purified for in vitro ubiquitylation reactions in Figure 6B or control beads were incubated with two different amounts of in vitro translated 35S-methionine labeled Sir4. After the beads were washed, bound 35S-Sir4 was detected via autoradiography, and the presence of the MYC-Dia2 was detected via Western blot using antibodies against the Myc epitope. Compared to control reactions, more 35S-Sir4 signal was detected in samples containing the MYC-Dia2 complex (Figure 6C, compare lanes 3–4 to lanes 1–2 of lower panel) despite the fact that more MYC antibodies (IgG) could be detected by CBB staining in control samples (Figure 5C, middle panel) than samples containing Myc-Dia2 (Figure 5C, upper panel) These data further support the idea that Sir4 is a substrate of the SCFDia2 complex.
Next, we asked whether Sir4 is ubiquitylated in vivo and whether this ubiquitylation depends on Dia2 using our published procedures [41]. Briefly, Sir4-TAP was purified from wild type or dia2Δ cells transformed with a plasmid expressing HA-tagged ubiquitin (HA-Ub). Following TAP purification, ubiquitylated species were detected by Western blot with antibodies against the HA epitope. In wild-type (Sir4-TAP) cells, ubiquitylated protein species co-purified with Sir4, and these immunoprecipitated species were specific as no HA signal could be detected in controls (a strain without Sir4-TAP but containing HA-Ub or a strain with Sir4-TAP but no HA-Ub)(Figure 6D, lanes 1 and 2, respectively). Importantly, these ubiquitylated species were notably reduced in dia2Δ cells compared to wild-type cells, despite equal levels of purified Sir4 (Figure 6D, compare lane 4 to lane 3). These results suggest that the SCFDia2 complex ubiquitylates Sir4 in vivo. Interestingly, we observed similar amounts of Sir2 co-purified with Sir4 in wild-type cells and dia2Δ cells, suggesting that the Sir2-Sir4 interaction is not affected to a detectable degree in dia2Δ mutant cells (Figure S7).
To further confirm that Sir4 is ubiquitylated in vivo, we purified ubiquitylated species using a two-step purification procedure. First, Sir4-TAP was purified from wild-type and dia2Δ mutant cells expressing either HA-tagged ubiquitin or His-HA tagged ubiquitin (Figure 6E). The associated ubiquitylated species were then purified under denaturing conditions using Ni-NTA beads that bound to His-ubiquitin. A band with a similar size to Sir4 was detected from wild-type cells expressing His-HA ubiquitin, but not from dia2Δ cells expressing His-HA tagged ubiquitin. In addition, little signal was detected from cells expressing HA-ubiquitin (lanes 1 and 2), suggesting that co-purification of Sir4 with ubiquitin under denatured conditions was specific (Figure 6E). Together, these data strongly support the conclusion that Sir4 is a substrate for SCFDia2.
Sir4 binding and transcriptional silencing at telomeres is altered during the cell cycle in a Dia2-dependent manner
It has been shown that heterochromatin is dynamically regulated during S phase of the cell cycle in mammalian cells. During mitosis, phosphorylation of histone H3 serine 10 by Aurora B kinase displaces HP1 from heterochromatin [42], [43]. In S. pombe, this dynamic loss of HP1 protein from heterochromatin is proposed to facilitate transcription of siRNA required for the re-establishment of heterochromatin during S phase [44]. Therefore, we asked whether Sir4 proteins at chromatin are also dynamically regulated during mitotic cell division and whether this regulation depends on Dia2. Briefly, wild-type and dia2Δ mutant cells were arrested at G1 using α-factor and then released into the cell cycle. Cells were collected at 0, 30 and 60 minutes following release for analysis of DNA content by flow cytometry (Figure 7B), for ChIP assays using Sir4 antibodies (Figure 7C) and for analysis of telomere gene expression by RT-PCR (Figure 7D). Flow cytometry analysis indicated that both wild-type and dia2Δ mutant cells collected at 0, 30 and 60 minutes were predominantly at G1, S and G2/M phase of the cell cycle, respectively (Figure 7B). In wild-type cells, Sir4 binding at telomeric silent chromatin was significantly reduced as the cells proceeded from G1 to S and G2/M phases (Figure 7C). In contrast, Sir4 binding was not altered at telomeric silent chromatin in dia2Δ cells as the cells progressed through the cell cycle.
Fig. 7. Sir4 binding at telomeric silent chromatin and expression of a telomeric gene are regulated during the cell cycle in a Dia2-dependent manner. 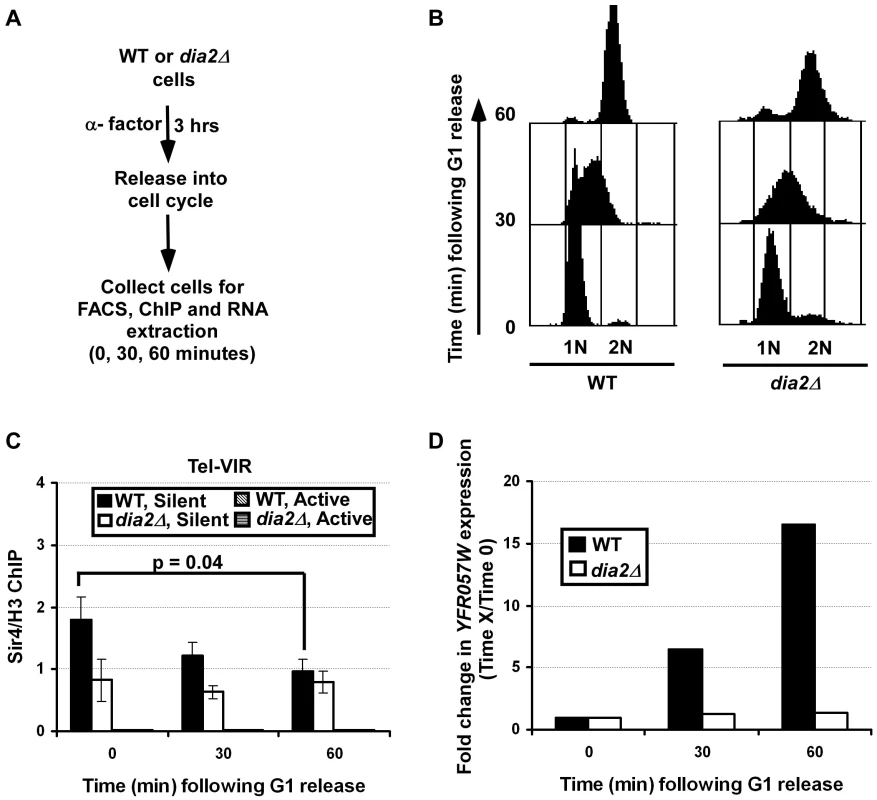
(A) Schematic representation of the experiments to test Sir4 binding and gene expression at telomeric silent chromatin during the cell cycle. Briefly, WT or dia2Δ cells were arrested at G1 with α-factor. Samples were collected for analysis of DNA content (B), Sir4 ChIP (C) and (D) gene expression at 0, 30 and 60 minutes following released into cell cycle. (B) Cell cycle analysis of WT and dia2Δ cells collected in the experiment described in A. DNA was stained using propidium iodide and analyzed using flow cytometry. (C) Sir4 binding is reduced at telomere silent chromatin as cells progress through the cell cycle, whereas Sir4 levels in dia2Δ mutant cells are not changed during the cell cycle. At each time point, cells were collected for ChIP assay using antibodies against Sir4 and histone H3. ChIP DNA was analyzed by real time PCR using PCR primers amplifying both silent and active chromatin loci as described in Figure 5D. The Sir4 ChIP signal was normalized against that of H3. The data presented is the average ± s.d. of three independent experiments with the p-value indicated as determined using the student's t-test. (D) The expression of the YFR057W increases as the cell cycle progresses. RNA was isolated from cells collected at each time point and reverse transcribed. The expression of YFR057W was analyzed using quantitative real-time PCR and was normalized against the expression of ACT1 as described in Figure 1E. Data is presented as the ratio of the relative expression of YFR057W at each indicated time point to the YFR057W expression at the G1 phase (0) time point for the respective cell type (thus, YFR057W expression at time 0 is 1 for both genotypes). The average calculated from two independent experiments is shown. To determine whether the observed changes in Sir4 binding affect silencing at telomeres, RT-PCR was performed to assess the expression of the telomere gene, YFR057W, as the cells progressed through the cell cycle. Consistent with Figure 1E, dia2Δ cells exhibited higher expression of YFR057W at all time points compared to wild-type cells (data not shown). When normalized against the expression of YFR057W at G1, we observed that there was an increase in gene expression of the telomere gene, YFR057W as cells progressed from G1 through to G2/M phase in wild-type cells (Figure 7D). Interestingly, only minor fluctuations in YFR057W expression were observed in dia2Δ cells. Together, these results suggest that Sir4 proteins at silent chromatin in budding yeast are regulated during the cell cycle, and this regulation is dependent on Dia2. Given our observation of SCFDia2's role in Sir4 ubiquitylation, we suggest that Sir4 ubiquitylation, mediated by SCFDia2, regulates the binding of Sir4 at silent chromatin during the cell cycle.
Discussion
In a yeast genetic screen for genes that function in parallel with RTT106 in transcriptional silencing, we found that deletion of DIA2, which encodes the F-box protein of the SCFDia2 E3 ubiquitin ligase complex with a role in DNA replication, enhances silencing defects of rtt106Δ mutant cells. Epistasis analysis suggests that DIA2 functions in parallel with CAF-1, Hir1 and Asf1, three other H3–H4 histone chaperones, in transcriptional silencing at the HMR locus, growth and the response to DNA damaging agents. In addition, localization of Sir proteins to silent chromatin loci is altered in dia2Δ mutant cells, and this alteration depends on Dia2's role in protein ubiquitylation. Furthermore, we show that SCFDia2 ubiquitylates Sir4 in vitro and in vivo and that Sir4 levels at silent chromatin are dynamically regulated during the cell cycle. Remarkably, this dynamic regulation is compromised in dia2Δ mutant cells. These results reveal a novel role for SCFDia2 in transcriptional silencing and suggest that SCFDia2 functions in silencing in part by ubiquitylating Sir4, which could serve as a mechanism to regulate Sir4 chromatin binding during the cell cycle.
A role for SCFDia2 in nucleosome assembly
The SCFDia2 complex is known to have a role in DNA replication [16]. However, how SCFDia2 functions in DNA replication is not clear. Our genetic analysis suggests that SCFDia2 may function in DNA replication-coupled nucleosome assembly. First, we show that the dia2Δ mutation exhibits synthetic defects in growth and sensitivity towards DNA damaging agents when combined with mutations in CAF-1, Asf1 and Rtt106, histone H3–H4 chaperones known to be involved in replication-coupled nucleosome assembly. Second, we show that the dia2Δ mutation exhibits synthetic defects with mutations at lysine residues of histones H3 and H4 known to be involved in the regulation of replication-coupled nucleosome assembly. Surprisingly, the dia2Δ mutation is synthetic lethal with mutations at H4 lysine residues 5, 8 and 12. Acetylation of these lysine residues occurs on newly synthesized H4 and is conserved from yeast to human cells [9]. Interestingly, DIA2 shares many genetic interactions with RTT101 [45], another ubiquitin ligase functioning in DNA replication [41], [46]. Genetic analysis using the epistatic miniarray profile (E-MAP) approach indicates that Rtt101 functions in the same genetic pathway as Rtt109, the histone H3 lysine 56 acetyltransferase known to be involved in DNA replication-coupled nucleosome assembly [29], [31], [47]. These results, combined with ours presented here, suggest that the SCFDia2 ubiquitin E3 ligase may function in nucleosome assembly. Further investigation is needed to determine Dia2's exact role in this process.
A role for SCFDia2 in transcriptional silencing
Using RT-PCR and reporter genes integrated at the HMR locus and telomeres, we show that Dia2 is needed for efficient transcriptional silencing at both the HMR locus and telomeres. Cells with defects in nucleosome assembly are known to affect transcriptional silencing [11], [12], [48]. Because genetic studies presented here suggest that Dia2 has a role in nucleosome assembly, it is possible that Dia2 impacts silencing through its role in nucleosome assembly. However, we have presented several lines of evidence supporting the idea that SCFDia2 impacts transcriptional silencing, at least partly through ubiquitylation of Sir4. First, we show that the role of SCFDia2 in transcriptional silencing depends on the Dia2 functional domains (the F-box and LRR) involved in protein ubiquitylation. Second, SCFDia2 ubiquitylates Sir4 in vitro and in vivo and interacts with Sir4 in vitro. Third, in wild-type cells, the levels of Sir4 at the silent telomere locus are reduced when cells proceed from G1 to S and G2/M phase of the cell cycle, and this regulation is not observed in dia2Δ mutant cells. Importantly, the reduction of Sir4 at telomere silent chromatin correlates with increased transcription of YFR057W, a gene located near a telomere, during the cell cycle. Thus, we suggest that SCFDia2 functions in transcriptional silencing in budding yeast, at least partly through ubiquitylation of Sir4.
It is not unprecedented for an E3 ubiquitin ligase to be involved in transcriptional silencing. In fact, several ubiquitin ligases and hydrolases have been shown to have roles in transcriptional silencing in budding yeast and other organisms. In S. pombe, the Rik1-Cul4 E3 ligase complex is important for the recruitment of the Clr4 histone methyltransferase for RNAi-mediated heterochromatin formation. Deletion of the Rik1-Cul4 complex results in silencing defects [49]–[51]. In budding yeast, Rtt101, a cullin protein of a ubiquitin E3 ligase complex, is important for telomeric silencing [52]. Rad6, involved in ubiquitylation of H2B, also impacts transcriptional silencing in budding yeast [53], [54]. Finally, Sir4 is known to interact with two ubiquitin hydrolases, Ubp3 and Dot4/Ubp10 [55], [56]. Ubp10 is proposed to regulate transcriptional silencing by deubiquitylating H2B [57]. How Ubp3 is involved in silencing is still unknown. Interestingly, loss of Upb3 results in improved silencing at telomeres and the HM loci (HML) [55], the opposite effect as that of loss of Dia2.
How does Sir4 ubiquitylation affect transcriptional silencing? Protein ubiquitylation, in general, is known to mediate two distinct functions. First, protein ubiquitylation marks proteins for degradation by the 26S proteasome. Second, protein ubiquitylation can also regulate protein-protein interactions [58], [59]. Interestingly, we did not detect significant changes in the steady-state levels of Sir4 in dia2Δ cells, suggesting that Sir4 ubiquitylation by SCFDia2 is not likely involved in regulating the steady-state level of Sir4. However, we did observe a significant increase in Sir4 proteins at the HMR silent locus in dia2Δ rtt106Δ double mutant cells. It has been reported that β-catenin levels on chromatin, but not steady state levels, are regulated by ubiquitylation through a protein complex containing the histone acetyltransferase component TRRAP and Skp1 [60]. Therefore, it is possible that a SCF ubiquitin ligase can regulate protein levels on chromatin. We have shown previously that Rtt106 binds Sir4 [11]. While the functional significance of the Rtt106-Sir4 interaction is not clear, it is possible that this interaction regulates Sir4 binding to silent chromatin. This could explain why loss of Rtt106 leads to aberrant accumulation of Sir4 at silent chromatin in dia2Δ rtt106Δ mutant cells. Therefore, we suggest that Sir4 ubiquitylation by SCFDia2 regulates Sir4 levels at chromatin, which in turn regulates silencing. Supporting this idea, we show that elevation of Sir4 levels using a centromere plasmid, while having no apparent effect on telomeric silencing in wild-type cells, reduces telomeric silencing in dia2Δ mutant cells.
It has been observed that heterochromatin proteins such as HP1 are dynamically regulated during mitotic cell division. For instance, in human cells, phosphorylation of serine 10 of histone H3 (H3S10ph) during mitosis reduces the binding affinity of HP1 towards H3K9me3, which propels the dissociation of HP1 from heterochromatin [42]. In S. pombe, dissociation of the sequence homolog of HP1, Swi6, from heterochromatin via H3S10ph results in transcription of siRNA during S phase. This, in turn, helps to maintain heterochromatin during S phase of the cell cycle [44]. These results highlight the fact that heterochromatin in S. pombe and mammalian cells is dynamically regulated during mitotic cell division. It was previously unknown whether silent chromatin in budding yeast was also regulated during S phase of the cell cycle. We observed that Sir4 binding to telomeric silent chromatin was significantly reduced as cells progressed from G1 to S and G2/M phase of the cell cycle. Concomitant with the reduction of Sir4 binding, the transcription of the telomere gene, YFR057W, increased when cells entered S phase. These results demonstrate that budding yeast silent chromatin is also dynamically regulated during S phase of the cell cycle. Because HP1 and histone modifications equivalent to H3K9me3 and H3S10ph are not present in budding yeast, we propose that perhaps SCFDia2-mediated ubiquitylation of Sir4 serves as a mechanism to regulate Sir4 proteins and silent chromatin structure during the cell cycle. Further investigation is warranted to address such a role for the SCFDia2 complex and Sir4 ubiquitylation.
In summary, our studies reveal a role for the SCFDia2 E3 ligase in transcriptional silencing. In addition, we show that the SCFDia2 E3 ligase binds and ubiquitylates Sir4. Furthermore, Dia2 is required for the regulation of Sir4 binding to chromatin during S phase of the cell cycle. These studies reveal a novel mechanism by which yeast silent chromatin is regulated during S phase of the cell cycle.
Materials and Methods
Yeast strains and plasmids
All yeast strains, except those for the SGA screen, were derived from W303 (leu2-3, 112 ura3-1, his3-11, trp1-1, ade2-1 can1-100) and constructed using standard methods and can be found listed in Table S1. The synthetic genetic array method to screen 4,700 viable yeast deletion mutants has been previously described in detail, along with the assay used to screen specifically for mutants that exhibit defects in HMR silencing [13], [21]. Detailed methods for the screen can be found in Text S1. Plasmids for Dia2 and Sir4 expression were constructed using standard methods in the vector, pRS313. Oligos used to construct the mutant Dia2 plasmids, as well as those used for analysis of mRNA expression and ChIP DNA using real-time PCR, are listed in Table S2.
Silencing assays
Telomeric silencing was analyzed as described previously [13]. Briefly, yeast cells containing the URA3 gene at the left arm of chromosome VII (URA3-VIIL) were plated onto the indicated media in a 10 fold series dilution with a starting OD600 of 6.0 for growth on 5-fluoroorotic acid (FOA) and OD600 0.6 on media not containing FOA. Images were taken after four days of incubation at 30°C.
Assays for silencing at the HMR silent mating type locus were performed as described [13] in cells containing a GFP reporter integrated at the silent HMR locus. Cells were grown at 25°C to OD600 0.6–0.8 and washed three times with synthetic complete (SC) –TRP media. The percentage of cells expressing GFP was determined using flow cytometry with the GFP populations in wild-type and sir3Δ cells as standards.
Localization of Sir3-GFP and Sir4-GFP using fluorescence microscopy
Cells in which Sir3 or Sir4 were tagged with GFP were grown in YPD or selective media (SC-HIS) at 25°C and collected at OD600 0.6–0.8. Cells were washed three times with SC-TRP media and analyzed using a Zeiss fluorescence microscope. Images were taken with z-stack images captured at every 0.3 µm, and one z-stack image was shown in Figure 4A. At least 100 cells of each genotype were counted from at least two independent experiments, and the percentage of cells exhibiting foci similar to wild-type cells was reported.
Chromatin immunoprecipitation assay (ChIP)
ChIP assays were performed as described [11]. Cells were first fixed with 1% formaldehyde and then quenched with glycine. Cells were collected and homogenized using glass beads. Chromatin DNA was sheared to an average size of 0.5 to 1 kb by sonication and immunoprecipitated with specific antibodies against the protein of interest. The co-precipitated DNA was analyzed by real-time PCR using primers whose sequences are listed in Table S1. To analyze Sir4 levels during the cell cycle, cells were arrested for 3 hours with α-factor. After washing away α-factor with cold water three times, cells were released into fresh medium and collected at different time points for analysis of DNA content, gene expression and ChIP assays as described above.
Ubiquitylation assays
Ubiquitylation assays were performed as described [41]. Briefly, Flag-ubiquitin, E1, E2, E3 (whole cell extracts prepared from cells expressing full length MYC-Dia2 or MYC-Dia2 F-boxΔ or purified MYC-Dia2 (full length and F-boxΔ). More details for both the in vitro and in vivo ubiquitylation assays can be found in Text S1.
Supporting Information
Zdroje
1. GrothARochaWVerreaultAAlmouzniG 2007 Chromatin challenges during DNA replication and repair. Cell 128 721 733
2. ElginSCGrewalSI 2003 Heterochromatin: silence is golden. Curr Biol 13 R895 898
3. ProbstAVAlmouzniG 2008 Pericentric heterochromatin: dynamic organization during early development in mammals. Differentiation 76 15 23
4. GrunsteinM 1998 Yeast heterochromatin: regulation of its assembly and inheritance by histones. Cell 93 325 328
5. MoazedD 2001 Common themes in mechanisms of gene silencing. Mol Cell 8 489 498
6. RuscheLNKirchmaierALRineJ 2003 The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem 72 481 516
7. GrewalSIMoazedD 2003 Heterochromatin and epigenetic control of gene expression. Science 301 798 802
8. MoazedD 2011 Mechanisms for the inheritance of chromatin states. Cell 146 510 518
9. LiQBurgessRZhangZ 2012 All roads lead to chromatin: Multiple pathways for histone deposition. Biochim Biophys Acta 1819 238 246
10. TylerJKAdamsCRChenSRKobayashiRKamakakaRT 1999 The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 402 555 560
11. HuangSZhouHTararaJZhangZ 2007 A novel role for histone chaperones CAF-1 and Rtt106p in heterochromatin silencing. Embo J 26 2274 2283
12. SharpJAFoutsETKrawitzDCKaufmanPD 2001 Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr Biol 11 463 473
13. HuangSZhouHKatzmannDHochstrasserMAtanasovaE 2005 Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc Natl Acad Sci U S A 102 13410 13415
14. MorohashiHMaculinsTLabibK 2009 The amino-terminal TPR domain of Dia2 tethers SCF(Dia2) to the replisome progression complex. Curr Biol 19 1943 1949
15. BlakeDLukeBKanellisPJorgensenPGohT 2006 The F-box protein Dia2 overcomes replication impedance to promote genome stability in Saccharomyces cerevisiae. Genetics 174 1709 1727
16. KoeppDMKileACSwaminathanSRodriguez-RiveraV 2006 The F-box protein Dia2 regulates DNA replication. Mol Biol Cell 17 1540 1548
17. MimuraSKomataMKishiTShirahigeKKamuraT 2009 SCF(Dia2) regulates DNA replication forks during S-phase in budding yeast. Embo J 28 3693 3705
18. DeshaiesRJ 1999 SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol 15 435 467
19. KipreosETPaganoM 2000 The F-box protein family. Genome Biol 1 REVIEWS3002
20. TongAHBooneC 2006 Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol 313 171 192
21. TongAHEvangelistaMParsonsABXuHBaderGD 2001 Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294 2364 2368
22. KaufmanPDKobayashiRStillmanB 1997 Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev 11 345 357
23. TrioloTSternglanzR 1996 Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature 381 251 253
24. AparicioOMBillingtonBLGottschlingDE 1991 Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66 1279 1287
25. WangXConnellyJJWangCLSternglanzR 2004 Importance of the Sir3 N terminus and its acetylation for yeast transcriptional silencing. Genetics 168 547 551
26. AndressEJHolicREdelmannMJKesslerBMYuVP 2011 Dia2 controls transcription by mediating assembly of the RSC complex. PLoS One 6 e21172
27. KatayamaSKitamuraKLehmannANikaidoOTodaT 2002 Fission yeast F-box protein Pof3 is required for genome integrity and telomere function. Mol Biol Cell 13 211 224
28. Vega-PalasMAMartin-FigueroaEFlorencioFJ 2000 Telomeric silencing of a natural subtelomeric gene. Mol Gen Genet 263 287 291
29. LiQZhouHWurteleHDaviesBHorazdovskyB 2008 Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 134 244 255
30. LingerJTylerJK 2005 The yeast histone chaperone chromatin assembly factor 1 protects against double-strand DNA-damaging agents. Genetics 171 1513 1522
31. HanJZhouHHorazdovskyBZhangKXuRM 2007 Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 315 653 655
32. BurgessRJZhouHHanJZhangZ 2010 A role for Gcn5 in replication-coupled nucleosome assembly. Mol Cell 37 469 480
33. AiXParthunMR 2004 The nuclear Hat1p/Hat2p complex: a molecular link between type B histone acetyltransferases and chromatin assembly. Mol Cell 14 195 205
34. LiQFazlyAMZhouHHuangSZhangZ 2009 The elongator complex interacts with PCNA and modulates transcriptional silencing and sensitivity to DNA damage agents. PLoS Genet 5 e1000684
35. PalladinoFLarocheTGilsonEAxelrodAPillusL 1993 SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell 75 543 555
36. GottaMLarocheTFormentonAMailletLScherthanH 1996 The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J Cell Biol 134 1349 1363
37. HoppeGJTannyJCRudnerADGerberSADanaieS 2002 Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol 22 4167 4180
38. MarshallMMahoneyDRoseAHicksJBBroachJR 1987 Functional domains of SIR4, a gene required for position effect regulation in Saccharomyces cerevisiae. Mol Cell Biol 7 4441 4452
39. CockellMPalladinoFLarocheTKyrionGLiuC 1995 The carboxy termini of Sir4 and Rap1 affect Sir3 localization: evidence for a multicomponent complex required for yeast telomeric silencing. J Cell Biol 129 909 924
40. LooSRineJ 1995 Silencing and heritable domains of gene expression. Annu Rev Cell Dev Biol 11 519 548
41. HanJLiQMcCulloughLKettelkampCFormosaT 2010 Ubiquitylation of FACT by the cullin-E3 ligase Rtt101 connects FACT to DNA replication. Genes Dev 24 1485 1490
42. HirotaTLippJJTohBHPetersJM 2005 Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 438 1176 1180
43. FischleWTsengBSDormannHLUeberheideBMGarciaBA 2005 Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438 1116 1122
44. ChenESZhangKNicolasECamHPZofallM 2008 Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 451 734 737
45. CollinsSRMillerKMMaasNLRoguevAFillinghamJ 2007 Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446 806 810
46. ZaidiIWRabutGPovedaAScheelHMalmstromJ 2008 Rtt101 and Mms1 in budding yeast form a CUL4(DDB1)-like ubiquitin ligase that promotes replication through damaged DNA. EMBO Rep 9 1034 1040
47. PanXYePYuanDSWangXBaderJS 2006 A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124 1069 1081
48. ZhangZShibaharaKStillmanB 2000 PCNA connects DNA replication to epigenetic inheritance in yeast. Nature 408 221 225
49. HongEJVillenJGeraceELGygiSPMoazedD 2005 A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol 2 106 111
50. HornPJBastieJNPetersonCL 2005 A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev 19 1705 1714
51. JiaSKobayashiRGrewalSI 2005 Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat Cell Biol 7 1007 1013
52. MimuraSYamaguchiTIshiiSNoroEKatsuraT 2010 Cul8/Rtt101 forms a variety of protein complexes that regulate DNA damage response and transcriptional silencing. J Biol Chem 285 9858 9867
53. HuangHKahanaAGottschlingDEPrakashLLiebmanSW 1997 The ubiquitin-conjugating enzyme Rad6 (Ubc2) is required for silencing in Saccharomyces cerevisiae. Mol Cell Biol 17 6693 6699
54. SunZWAllisCD 2002 Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418 104 108
55. MoazedDJohnsonD 1996 A deubiquitinating enzyme interacts with SIR4 and regulates silencing in S. cerevisiae. Cell 86 667 677
56. KahanaAGottschlingDE 1999 DOT4 links silencing and cell growth in Saccharomyces cerevisiae. Mol Cell Biol 19 6608 6620
57. KahanaA 2001 The deubiquitinating enzyme Dot4p is involved in regulating nutrient uptake. Biochem Biophys Res Commun 282 916 920
58. DeshaiesRJJoazeiroCA 2009 RING domain E3 ubiquitin ligases. Annu Rev Biochem 78 399 434
59. FinleyD 2009 Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78 477 513
60. FinkbeinerMGSawanCOuzounovaMMurrRHercegZ 2008 HAT cofactor TRRAP mediates beta-catenin ubiquitination on the chromatin and the regulation of the canonical Wnt pathway. Cell Cycle 7 3908 3914
Štítky
Genetika Reprodukčná medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



