-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
article has not abstract
Published in the journal: Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo. PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002859
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002859Summary
article has not abstract
In vivo modeling of tumor suppressor p53 functions and regulation has a history of unexpected and even enigmatic outcomes [1], despite the status of p53 as the most frequently mutated gene or dysfunctional pathway in human cancers [2], [3]. Beginning with the surprising viability of the first mice deleted for Trp53 [4], [5], various hypotheses of compensation, cell type–specificity, stimulus-dependent response, or modifier influences were posed to explain how an exquisitely regulated transcription factor, implicated in a vast array of pathways [6], appeared to have no impact on development. Limited background-specific developmental and fertility problems do occur, especially in female p53-null mice [7], [8], and deletion of potentially compensatory p53 family members, p63 and p73 isoforms, leads to profound developmental and tissue-specific phenotypes [9], [10]. But overall, the most striking result of p53 loss in vivo is early tumor predisposition in p53−/− mice, which lack genomic surveillance provided by p53-mediated regulation of cell cycle arrest, apoptosis, and senescence.
As reported by Concepcion et al. in this issue of PLoS Genetics [11], expectations built on cell-based studies of p53 response are again unrealized in mouse models. Previously, multiple in vitro analyses suggested that microRNA (miR)-34 family members are important players in a p53-regulated network of genomic surveillance [12]–[17] (Table 1). Together, these studies strongly supported the view that p53 response to multiple stimuli depended on miR-34, and that ectopic expression of miR-34 was sufficient to elicit p53 response, consistent with miR-34 functioning as a bonafide tumor suppressor. However, Concepcion et al. report that complete inactivation of the entire family of miR-34 genes (miR-34a/b/c) or knockout of each individual miR-34 gene in mice leads to little or no change in p53-mediated functions in tumor suppression [11].
Tab. 1. A list of different in vitro and in vivo model systems used to study miR-34 functions. 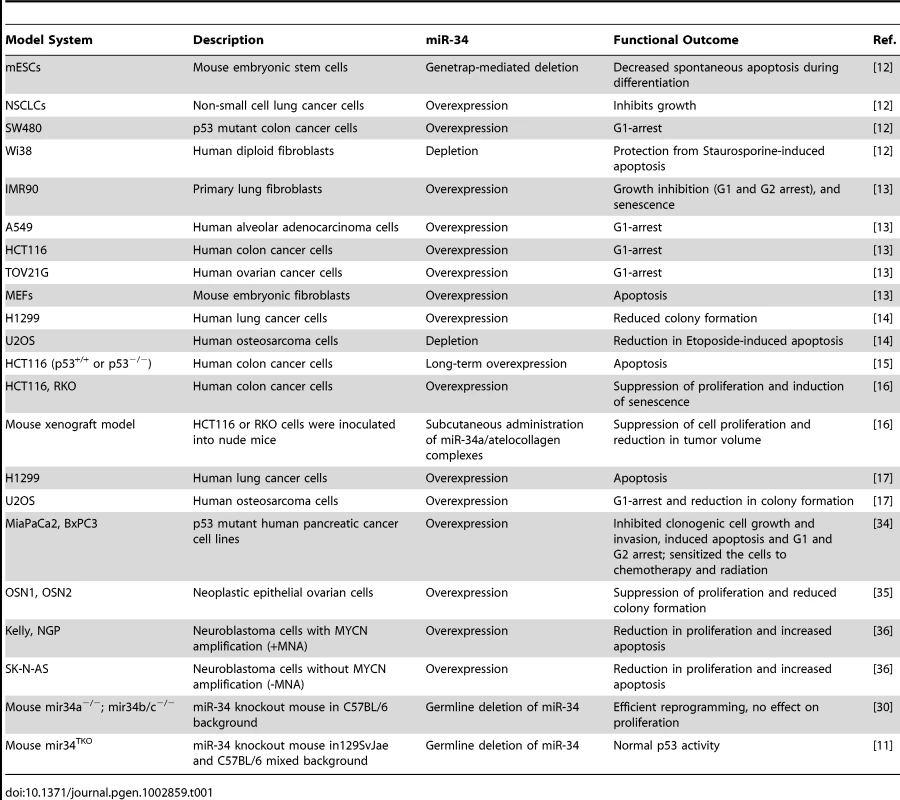
Interest in a miR-34 axis as mediator of p53-response begins with the niche that miRNAs fill in regulation of RNA expression. miRNAs are small, regulatory non-coding RNAs that generally mediate post-transcriptional silencing of a number of specific target mRNAs [18]. More than 50% of human miRNA genes are found within cancer-associated or fragile sites of the genome, which suggests that miRNAs play essential roles in tumorigenesis [19]. The identification of miRNAs as regulatory targets of p53 [20] suggested their potential involvement in tumor suppression, and expanded the repertoire of p53 downstream targets to both coding and non-coding genes. Further, the view that p53 both positively and negatively regulates gene expression could now rely on increased expression of miRNAs as a mechanism for p53-mediated, indirect repression of gene expression [13], [20], in addition to the few documented cases of direct repression by p53 binding to chromatin [21]–[25].
The members of the evolutionarily conserved miR-34 family, which arise from three different transcripts at two different gene loci in vertebrates, were the first of several non-coding RNAs identified as directly activated by p53 in response to genotoxic stress [13], [26]. miR-34a is at 1p36, a region commonly deleted in tumors, and miR-34b and miR-34c share a common primary transcript arising from 11q23 [27], [28]. miR-34a, b, and c are expressed at very low levels in several types of cancers [28]. Previous reports show that p53 directly activates miR-34a/b/c expression and, dependent on cellular context, they act downstream of p53 in mediating cell cycle arrest or apoptosis [29]. The current list of validated miR-34 downstream targets includes several genes that are repressed during cell cycle arrest or apoptosis when p53 is activated [28].
Given the rationale provided by these studies in cultured cells (Table 1), multiple laboratories created genetic knockout models of either miR-34a or miR-34b/c, or a compound mutant animal harboring homozygous deletion of all three miR-34 family members (miR-34TKO) [11], [30]. Surprisingly, mice bearing the miR-34 deletion(s) developed normally, are born at the expected Mendelian ratio, and are fertile [11]. The authors subjected the mice and derived mouse embryonic fibroblasts (MEFs) to a battery of tests to assess any impact on p53-dependent tumor suppression. MEFs obtained from mir-34TKO mice have a slightly higher proliferation rate, but reach senescence with kinetics similar to wild-type MEFs. In response to genotoxic threats, miR-34–deficient MEFs are indistinguishable from wild type: they undergo p53-dependent cell cycle arrest and apoptosis. With ectopic expression of oncogenic K-Ras, p53-deficient MEFs are readily transformed, which is not true of K-Ras–expressing miR-34−/− MEFs.
In the intact mouse, the story is similar: aging cohorts of mir-34TKO mice remain healthy with no spontaneous tumors, in contrast to p53-null mice [4]. In fact, miR-34–deficient mice remain remarkably healthy and tumor-free for at least 60 weeks after irradiation. Assays of apoptosis in response to irradiation proved positive in tissues of these mice, which additionally exhibited no acceleration of tumor progression in Eμ-models of B-cell lymphomagenesis. All of these assessments of p53 functions in vivo undermine the view that miR-34 functions as a tumor suppressor or is an essential component of the p53-tumor suppression network.
Although miR-34 proved nonessential in the most highly studied examples of p53 function (senescence, cell cycle arrest, apoptosis, and tumor suppression), it remains possible that miR-34 is involved in other p53-influenced processes, such as metabolism, autophagy, stem cell quiescence, differentiation, and embryogenesis [6]. For example, specific links between miR-34 – and p53-regulated functions have been forged in stem cells [26]. miR-34–deficient MEFs are more efficiently reprogrammed to induced pluripotent stem cells (iPSCs), by expression of pluripotency factors and c-myc [30], compared to wild-type counterparts. While this study of miR-34 as a barrier to reprogramming does not establish a direct tie to p53, it complements multiple reports that depletion of p53 or dysfunctional p53 pathways enhance the efficiency of reprogramming differentiated, somatic cells to iPSCs [31]. Recently, we showed that p53 promotes human embryonic stem cell differentiation by direct activation of p21 and miRNAs, including miR-34a, which repress pluripotency factors and SIRT1 [32]. Taken together, these results indicate that miR-34 has pro-differentiation effects in maintenance of nontransformed, somatic cells, some of which are p53-dependent.
In the future, miR-34–deficient mouse models will be valuable in addressing whether miR-34 functions downstream of p53 in a tissue - and/or context-specific manner. miR-34a, miR-34b, and miR-34c share the same seed sequence and target the same RNAs, although differences in target accessibility or binding affinities may dictate their effectiveness. Genome-wide expression analysis may be needed to determine family member–specific effects, such as the reported regulation of c-MYC by miR-34b/c and not miR-34a [33]. Questions of specificity in gene targets for each member of a miRNA family and potential compensation by other miRNAs may be addressed by studies in these and other miRNA mouse models, perhaps still under development. Non-coding RNAs are thought to act in networks that impact diverse cellular pathways, suggesting considerable challenges ahead in asking the right questions and understanding the functional significance of these RNAs.
Zdroje
1. SpikeBT, WahlGM (2011) p53, stem cells, and reprogramming: tumor suppression beyond guarding the genome. Genes Cancer 2 : 404–419.
2. SoussiT (2007) p53 alterations in human cancer: more questions than answers. Oncogene 26 : 2145–2156.
3. HollsteinM, SidranskyD, VogelsteinB, HarrisCC (1991) p53 mutations in human cancers. Science 253 : 49–53.
4. HarveyM, McArthurMJ, MontgomeryCAJr, ButelJS, BradleyA, et al. (1993) Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet 5 : 225–229.
5. JacksT, RemingtonL, WilliamsBO, SchmittEM, HalachmiS, et al. (1994) Tumor spectrum analysis in p53-mutant mice. Curr Biol : CB 4 : 1–7.
6. VousdenKH, PrivesC (2009) Blinded by the light: the growing complexity of p53. Cell 137 : 413–431.
7. SahVP, AttardiLD, MulliganGJ, WilliamsBO, BronsonRT, et al. (1995) A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 10 : 175–180.
8. HuW, FengZ, TereskyAK, LevineAJ (2007) p53 regulates maternal reproduction through LIF. Nature 450 : 721–724.
9. MillsAA, ZhengB, WangXJ, VogelH, RoopDR, et al. (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398 : 708–713.
10. YangA, WalkerN, BronsonR, KaghadM, OosterwegelM, et al. (2000) p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404 : 99–103.
11. ConcepcionCP, HanY-C, MuP, BonettiC, YaoE, et al. (2012) Intact p53-dependent responses in miR-34-deficient mice. PloS Genet 8: e1002792 doi:10.1371/journal.pgen.1002797.
12. BommerGT, GerinI, FengY, KaczorowskiAJ, KuickR, et al. (2007) p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 17 : 1298–1307.
13. HeL, HeX, LimLP, de StanchinaE, XuanZ, et al. (2007) A microRNA component of the p53 tumour suppressor network. Nature 447 : 1130–1134.
14. Raver-ShapiraN, MarcianoE, MeiriE, SpectorY, RosenfeldN, et al. (2007) Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26 : 731–743.
15. ChangTC, WentzelEA, KentOA, RamachandranK, MullendoreM, et al. (2007) Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 26 : 745–752.
16. TazawaH, TsuchiyaN, IzumiyaM, NakagamaH (2007) Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A 104 : 15472–15477.
17. TarasovV, JungP, VerdoodtB, LodyginD, EpanchintsevA, et al. (2007) Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6 : 1586–1593.
18. BartelDP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116 : 281–297.
19. CalinGA, SevignaniC, DumitruCD, HyslopT, NochE, et al. (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 101 : 2999–3004.
20. HeL, HeX, LoweSW, HannonGJ (2007) microRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat Rev Cancer 7 : 819–822.
21. SpurgersKB, GoldDL, CoombesKR, BohnenstiehlNL, MullinsB, et al. (2006) Identification of cell cycle regulatory genes as principal targets of p53-mediated transcriptional repression. J Biol Chem 281 : 25134–25142.
22. LeeKC, CroweAJ, BartonMC (1999) p53-mediated repression of alpha-fetoprotein gene expression by specific DNA binding. Mol Cell Biol 19 : 1279–1288.
23. LinT, ChaoC, SaitoS, MazurSJ, MurphyME, et al. (2005) p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 7 : 165–171.
24. RileyT, SontagE, ChenP, LevineA (2008) Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Bio 9 : 402–412.
25. HoJ, BenchimolS (2003) Transcriptional repression mediated by the p53 tumour suppressor. Cell Death Differ 10 : 404–408.
26. LinCP, ChoiYJ, HicksGG, HeL (2012) The emerging functions of the p53-miRNA network in stem cell biology. Cell Cycle 11 : 2063–72.
27. VersteegR, CaronH, ChengNC, van der DriftP, SlaterR, et al. (1995) 1p36: every subband a suppressor? Eur J Cancer 31A: 538–541.
28. HermekingH (2010) The miR-34 family in cancer and apoptosis. Cell Death Differ 17 : 193–199.
29. HermekingH (2007) p53 enters the microRNA world. Cancer Cell 12 : 414–418.
30. ChoiYJ, LinCP, HoJJ, HeX, OkadaN, et al. (2011) miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol 13 : 1353–1360.
31. KrizhanovskyV, LoweSW (2009) Stem cells: The promises and perils of p53. Nature 460 : 1085–1086.
32. JainAK, AlltonK, IacovinoM, MahenE, MilczarekRJ, et al. (2012) p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol 10: e1001268 doi:10.1371/journal.pbio.1001268.
33. LeucciE, CoccoM, OnnisA, De FalcoG, van CleefP, et al. (2008) MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol 216 : 440–450.
34. JiQ, HaoX, ZhangM, TangW, YangM, et al. (2009) MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PloS ONE 4: e6816 doi:10.1371/journal.pone.0006816.
35. CorneyDC, Flesken-NikitinA, GodwinAK, WangW, NikitinAY (2007) MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res 67 : 8433–8438.
36. WelchC, ChenY, StallingsRL (2007) MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene 26 : 5017–5022.
Štítky
Genetika Reprodukčná medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



