-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
Successful completion of meiosis requires the induction and faithful repair of DNA double-strand breaks (DSBs). DSBs can be repaired via homologous recombination (HR) or non-homologous end joining (NHEJ), yet only repair via HR can generate the interhomolog crossovers (COs) needed for meiotic chromosome segregation. Here we identify COM-1, the homolog of CtIP/Sae2/Ctp1, as a crucial regulator of DSB repair pathway choice during Caenorhabditis elegans gametogenesis. COM-1–deficient germ cells repair meiotic DSBs via the error-prone pathway NHEJ, resulting in a lack of COs, extensive chromosomal aggregation, and near-complete embryonic lethality. In contrast to its yeast counterparts, COM-1 is not required for Spo11 removal and initiation of meiotic DSB repair, but instead promotes meiotic recombination by counteracting the NHEJ complex Ku. In fact, animals defective for both COM-1 and Ku are viable and proficient in CO formation. Further genetic dissection revealed that COM-1 acts parallel to the nuclease EXO-1 to promote interhomolog HR at early pachytene stage of meiotic prophase and thereby safeguards timely CO formation. Both of these nucleases, however, are dispensable for RAD-51 recruitment at late pachytene stage, when homolog-independent repair pathways predominate, suggesting further redundancy and/or temporal regulation of DNA end resection during meiotic prophase. Collectively, our results uncover the potentially lethal properties of NHEJ during meiosis and identify a critical role for COM-1 in NHEJ inhibition and CO assurance in germ cells.
Published in the journal: COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining. PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003276
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003276Summary
Successful completion of meiosis requires the induction and faithful repair of DNA double-strand breaks (DSBs). DSBs can be repaired via homologous recombination (HR) or non-homologous end joining (NHEJ), yet only repair via HR can generate the interhomolog crossovers (COs) needed for meiotic chromosome segregation. Here we identify COM-1, the homolog of CtIP/Sae2/Ctp1, as a crucial regulator of DSB repair pathway choice during Caenorhabditis elegans gametogenesis. COM-1–deficient germ cells repair meiotic DSBs via the error-prone pathway NHEJ, resulting in a lack of COs, extensive chromosomal aggregation, and near-complete embryonic lethality. In contrast to its yeast counterparts, COM-1 is not required for Spo11 removal and initiation of meiotic DSB repair, but instead promotes meiotic recombination by counteracting the NHEJ complex Ku. In fact, animals defective for both COM-1 and Ku are viable and proficient in CO formation. Further genetic dissection revealed that COM-1 acts parallel to the nuclease EXO-1 to promote interhomolog HR at early pachytene stage of meiotic prophase and thereby safeguards timely CO formation. Both of these nucleases, however, are dispensable for RAD-51 recruitment at late pachytene stage, when homolog-independent repair pathways predominate, suggesting further redundancy and/or temporal regulation of DNA end resection during meiotic prophase. Collectively, our results uncover the potentially lethal properties of NHEJ during meiosis and identify a critical role for COM-1 in NHEJ inhibition and CO assurance in germ cells.
Introduction
DNA double-strand breaks (DSBs) are toxic DNA lesions that, if not repaired correctly, can cause gross chromosomal alterations. For this reason, DSBs are potent inducers of cell death as well as malignant transformation [1]. Two major DSB repair mechanisms have evolved that are able to repair DSBs: an error-free pathway called homologous recombination (HR) and an efficient but error-prone pathway called non-homologous end joining (NHEJ) [2], [3]. Together NHEJ and HR safeguard genome integrity, however, on a mechanistic level, they are mutually exclusive. NHEJ is based on DNA end protection: the Ku70/Ku80 heterodimer stabilizes the double-strand (ds) DNA ends and prepares the DSB for direct ligation by DNA ligase IV [2]. In contrast, HR is based on DNA end resection: nucleases degrade the dsDNA ends to expose 3′ single strand (ss) DNA tails, which then form a nucleoprotein filament with the recombinase RAD51 that promotes strand invasion and subsequent DNA synthesis reactions [3]. Because of its conservative nature, HR is better suited for maintaining genome stability, but it requires an undamaged DNA template (i.e. the sister chromatid or homologous chromosome), which is not always available. As a result, most human cells (especially non-cycling somatic cells) typically rely on NHEJ for DSB repair [2], [4].
DSB repair fidelity is particularly important in germ cells, as they harbor the genetic material that is passed on to the next generation. Germ cells create haploid gametes via a specialized program of cell division called meiosis, in which a single round of DNA replication is followed by two subsequent rounds of chromosome segregation (named meiosis I and meiosis II). Separation of the parental/homologous chromosomes during meiosis I requires the induction of programmed DSBs [5]. Meiotic DSBs are introduced by SPO11, a highly conserved topoisomerase-like protein that, after cutting, remains covalently bound to the 5′ ends of the DSB. Loss of SPO11 function leads to severe chromosome missegregation and aneuploid gametes in many model systems, highlighting the importance of meiotic DSB formation for successful gametogenesis and species survival [5], [6].
Meiotic DSBs need to be repaired via HR, as only this pathway creates repair products known as crossovers (COs), which are required for the establishment of chiasmata, the transient links between homologous chromosomes. Chiasmata are essential for proper chromosome alignment and segregation during meiosis I [7]. Given that NHEJ competes with HR and does not lead to COs, this activity should be restricted in order to assure chromosome stability during gametogenesis. Previous studies have revealed that Caenorhabditis elegans (C. elegans) germ cells posses NHEJ activity, yet in wild-type germ cells this error-prone pathway seems to be inhibited very efficiently [8], [9], [10], [11].
Recent insights on mitotic DSB repair have led to the identification of several factors that are able to block NHEJ activity, including the tumor suppressor CtIP [12]. Studies on DSB repair pathway choice in meiotic cells are hampered by the fact that crucial regulators like CtIP are required for mammalian development, which precludes analysis of CtIP-deficient gametes [13]. Here, we exploited the C. elegans model system to explore if the CtIP homolog COM-1 is responsible for the robust HR bias in metazoan germ cells; maternal contribution of com-1 gene products enables com-1 mutant embryos to develop into adults that produce COM-1-deficient germ cells [14]. COM-1/CtIP is well conserved throughout evolution and homologous counterparts named Sae2 and Ctp1 have also been identified in the unicellular organisms S. cerevisiae and S. pombe, respectively [14], [15], [16]. In yeast, Sae2/Ctp1 is required for Spo11 removal and therefore is crucial for the initiation of meiotic DSB repair [17], [18].
Here, we show that COM-1 is dispensable for meiotic recombination per se, yet it is crucial to complete meiosis: COM-1 is required to block toxic Ku activity at meiotic DSBs and therefore is needed to prevent chromosome aggregation and CO failure. In addition, we reveal a role for COM-1 in interhomolog HR: COM-1 acts parallel to the nuclease EXO-1 to generate RAD-51-coated recombination intermediates at early/mid pachytene stage. We thus identified a dual role for COM-1 during metazoan meiosis: it blocks toxic NHEJ activity and guarantees the timely formation of interhomolog COs.
Results
COM-1–deficient germ cells bear chromosomal aggregates and univalents
In order to study the meiotic functions of COM-1 we obtained two different com-1 mutant alleles previously identified by Penkner and colleagues (Figure S1) and [14]. In C. elegans, defects in repair of meiotic DSBs can be detected relatively easily, as these often manifest as chromosomal abnormalities in diakinesis nuclei of maturing oocytes (Figure 1A). Wild-type diakinesis nuclei typically have six rod-shaped DAPI-stained bodies named bivalents, which represent the six pairs of homologous chromosomes, each held together by chiasmata (Figure 1A and Figure 2A). In the absence of meiotic DSBs (e.g. in spo-11 mutants) chiasmata are not formed, which can be detected by the presence of 12 DAPI-stained bodies, i.e. univalents [6]. When meiotic DSBs are induced but not repaired, chromosomal fragmentation occurs, typically resulting in ≥12 irregularly shaped DAPI-stained bodies at diakinesis [19], [20]. Surprisingly, com-1 mutant oocytes exhibited a different chromosomal pattern: the diakinesis nuclei contained 1 to 12 DAPI-stained entities [14]. We validated this finding by careful inspection of COM-1-deficient diakinesis nuclei (Figure 1C and 1D). These diakinesis nuclei occasionally showed chromosomal fragments, albeit only in 2% of the oocytes (Figure 3C). We argued that the low frequency of chromosomal fragmentation in com-1 mutants is inconsistent with a conserved role for COM-1 in SPO-11 removal, given that SPO-11-bound DSBs are refractory to repair. Based on the diakinesis studies we envisaged a different scenario in which com-1 mutants are able to repair meiotic DSBs, yet do so in an error-prone manner, ultimately resulting in chromosomal aggregates and failed chiasmata formation. Several observations supported this hypothesis: Firstly, unlike spo-11 mutants, com-1 mutant oocytes hardly ever contained exactly 12 univalents, which indicated that DSBs were induced. Secondly, all diakinesis nuclei had fewer than 12 DAPI-stained bodies and rarely contained small chromosomal fragments, arguing that most programmed DSBs are repaired. Thirdly, the diakinesis nuclei often contained more than 6 DAPI-stained bodies and frequently exhibited DAPI bodies that morphologically resembled univalents, which implied that chiasmata formation was impaired. Finally, many diakinesis nuclei had fewer than six DAPI-stained bodies, potentially reflecting chromosomal entanglements and/or fusions between non-homologous chromosomes.
Fig. 1. Loss of cku-80 prevents chromosomal aggregation and restores chiasmata formation and embryonic survival in com-1(t1626) mutants. 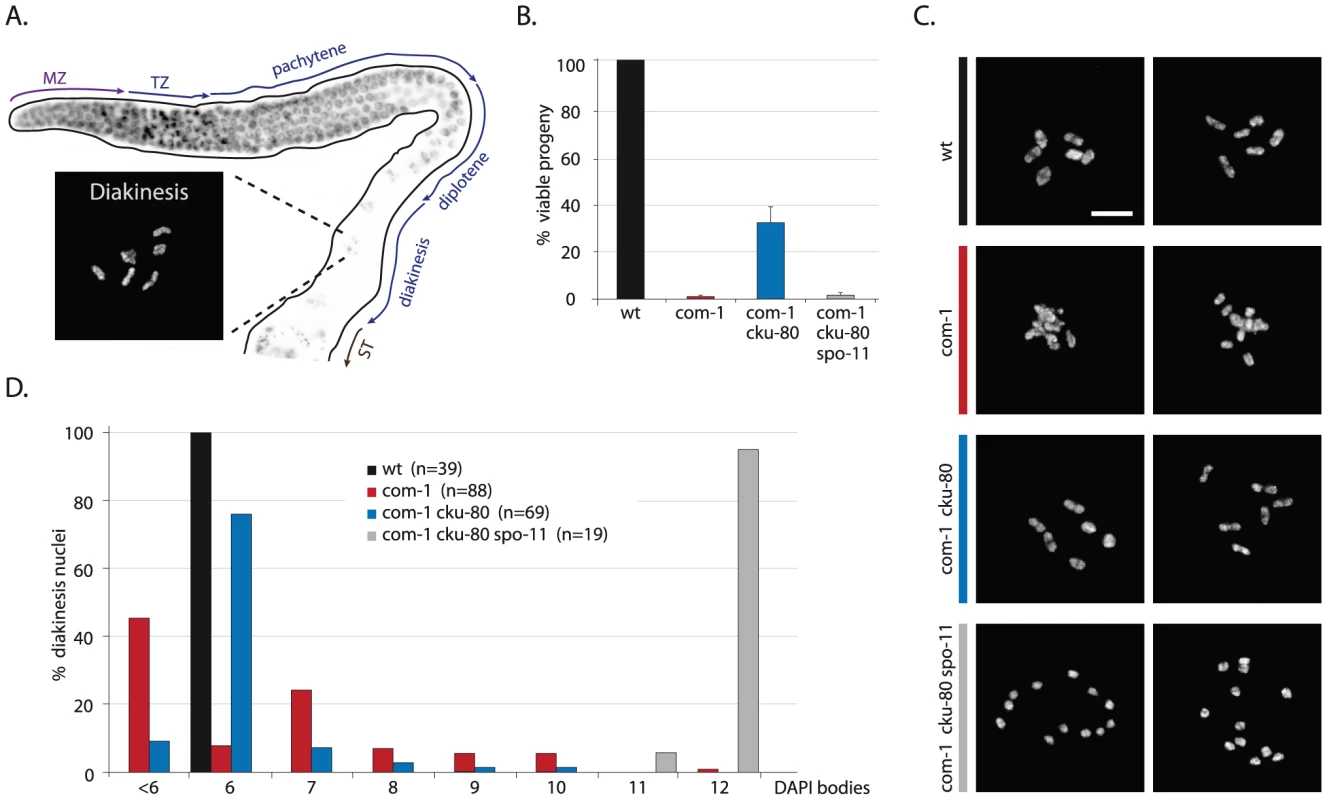
(A) Schematic overview of the C. elegans germline, in which different zones correspond to the successive stages of meiotic prophase. MZ: mitotic zone; TZ: transition zone; ST: spermatheca. Blow-up shows a typical wild-type diakinesis nucleus with six bivalents. (B) Percentage progeny survival of animals of the indicated genotype; values are the average of 3 independent experiments, error bars represent S.E.M. (C) Two representative pictures of diakinesis nuclei of animals of the indicated genotype (D) Frequency distribution of DAPI-stained entities at diakinesis. n = number of germlines analyzed. Scale bars, 5 µm. Fig. 2. Loss of cku-80 as well as γ-irradiation rescues the CO defect of com-1 mutants. 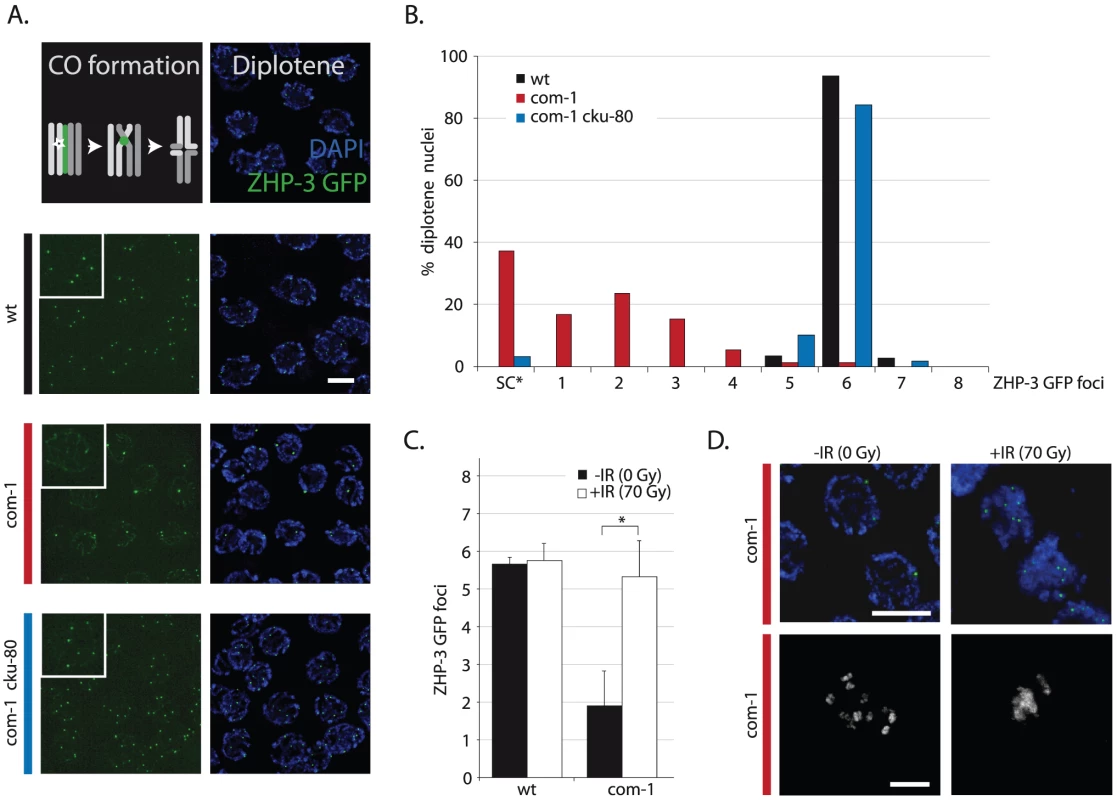
(A) Localization pattern of ZHP-3::GFP at diplotene stage. Upper panel shows schematic overview of dynamic ZHP-3 re-localization (green) during CO formation; lower panels show representative pictures of diplotene nuclei of animals of the indicated genotype that express a ZHP-3::GFP transgene (left: GFP signal only, inset = blow-up of single nucleus; right: merge of GFP and DAPI signal). (B) Frequency distribution of ZHP-3::GFP foci in diplotene nuclei of animals of the indicated genotype; SC* = ZHP-3::GFP signal along the synaptonemal complex, no distinct foci (C) Average number of ZHP-3::GFP foci in diplotene nuclei of wild-type or com-1 mutant animals 24 hours after mock/IR-treatment: 0 Gy (black bars)/70 Gy (white bars); Error bars represent SD, *the difference between mock- and IR-treated com-1 mutants was highly significant (p<0.001 by Student's t-test, two tailed) (D) Upper panel: representative pictures of diplotene nuclei of mock/IR-treated com-1 mutants that express a ZHP-3::GFP transgene (merge of GFP and DAPI signal); Lower panel: representative pictures of diakinesis nuclei of mock/IR-treated com-1 mutants that express a ZHP-3::GFP transgene (DAPI signal only). Scale bars, 5 µm. Fig. 3. Loss of lig-4 prevents chromosomal fusion in com-1 mutants, but does not restore viability. 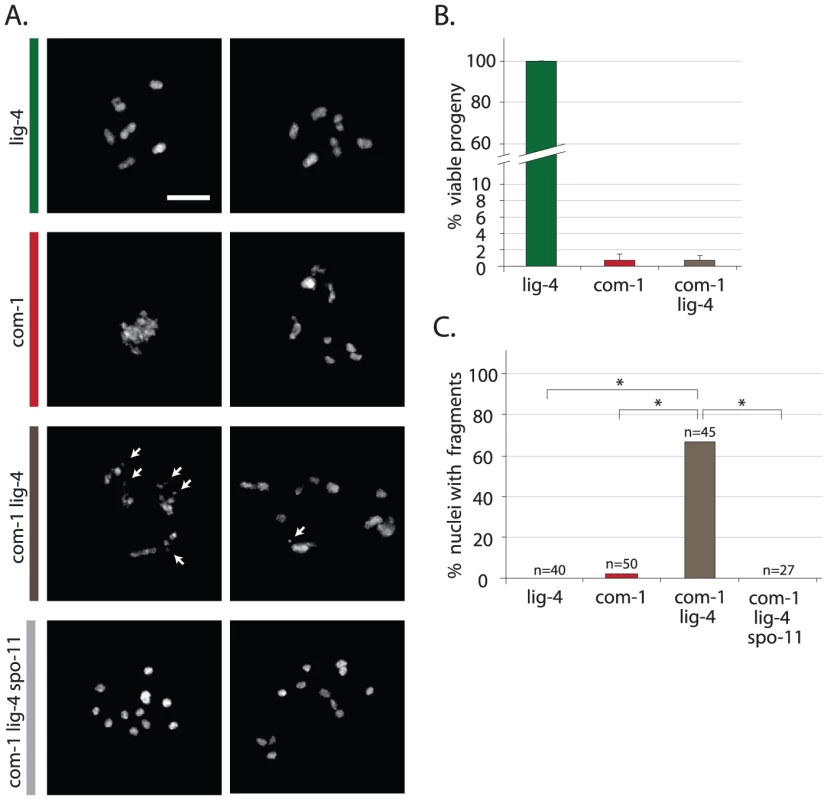
(A) Two representative pictures of diakinesis nuclei of animals of the indicated genotype. White arrows point out chromosomal fragments (B) Percentage progeny survival; values are the average of 3 independent experiments, error bars represent S.E.M. (C) Percentage of diakinesis nuclei that show chromosomal fragments; n = number of germlines analyzed. Scale bars, 5 µm. *The difference between these genotypes was highly significant (p<0.001 by Fisher's exact test, two tailed). Loss of Ku restores chiasmata formation and viability in com-1 mutant animals
To test if the chromosomal aggregation events in com-1 mutant oocytes were due to inappropriate NHEJ activity, we crossed com-1 mutants with worms lacking the NHEJ factor CKU-80. Strikingly, cku-80 deficiency led to a >20 fold increase in viability among com-1 mutant progeny: while com-1 single mutants produced 0–2% viable embryos, com-1 cku-80 double mutants produced 30–40% viable progeny (Figure 1B). Moreover, nearly all hatchlings of com-1 cku-80 double mutants successfully developed into adults, while com-1 single mutant hatchlings typically died as arrested L1/L2 larvae.
To verify these observations we crossed animals carrying another allele of com-1 to worms lacking the other well-conserved Ku subunit CKU-70. The resultant com-1(t1489) cku-70 double mutants showed identical phenotypes as the aforementioned com-1(t1626) cku-80 double mutants, including elevated embryonic survival and restored larval development as compared to com-1(t1489) single mutants (Figure S1). We therefore conclude that com-1 deficient animals suffer from toxic Ku activity and that in the absence of Ku, COM-1 is dispensable for C. elegans development and gametogenesis.
In contrast to the diakinesis nuclei of com-1 single mutants, which hardly ever contain six DAPI-stained bodies, 70% of diakinesis nuclei of com-1 cku-80 double mutants had the wild-type set of six bivalents (Figure 1C and 1D). We obtained similar results for com-1 cku-70 double mutants (Figure S1). The fact that Ku deficiency restored bivalent formation in com-1 mutant animals implies that both the univalents and the chromosomal aggregates in com-1 deficient oocytes were due to Ku-mediated NHEJ. These observations also demonstrate that COM-1 is not required for chiasma formation per se. Notably, both bivalent formation and embryonic viability in com-1 cku-80 double mutants were completely spo-11-dependent (Figure 1), which indicates that chiasma formation in com-1 mutants occurs at programmed DSBs and not at spontaneous DSBs.
Based on these diakinesis studies we conclude that i) COM-1 is crucial to prevent NHEJ activity in meiotic cells; ii) Ku can act efficiently on meiotic DSBs (at least when COM-1 activity is perturbed); iii) a com-1-independent mechanism exists that is able to convert SPO-11-induced DSBs into proper chiasmata, and iv) in contrast to Sae2/Ctp1 in yeast, COM-1 is not required for SPO-11 removal in C. elegans.
Ku prevents CO formation in com-1 mutant germlines
Since com-1 single mutants fail to adequately form chiasmata and this defect can be restored by Ku loss (Figure 1), we reasoned that Ku might obstruct CO formation. In C. elegans, exactly one CO occurs per homolog pair and these presumptive CO sites can be visualized by specific recruitment of the fusion protein ZHP-3::GFP at late pachytene/diplotene stage [21], [22]. As shown in Figure 2B, wild-type animals had six ZHP-3::GFP foci in nearly all diplotene nuclei. In contrast, com-1 single mutants on average had only two ZHP-3::GFP foci per diplotene nucleus (Figure 2A and 2B) and often exhibited persistent ZHP-3::GFP localization along the full length of the synaptonemal complex (SC) – a localization pattern characteristic of CO failure [21].
Importantly, loss of cku-80 alleviated the ZHP-3::GFP localization defect of com-1 mutant germlines: virtually all diplotene nuclei of com-1 cku-80 double mutants had the normal complement of six ZHP-3::GFP foci (Figure 2A and 2B). We conclude that COM-1 is not needed for CO formation per se, yet COM-1 is essential to prevent interference by Ku and hence is critical for CO assurance.
The CO defect of com-1 mutants is due to a scarcity of accessible DSBs
We hypothesized that Ku binds DSB ends and blocks DNA end resection and subsequent meiotic recombination. In order to test if the CO defect observed in com-1 mutants is due to an insufficient number of DSBs available for HR, we subjected these animals to ionizing radiation (IR) to introduce additional DSBs. 70 Gy of IR did not alter the number of COs in wild-type animals: six ZHP-3::GFP foci were present per diplotene nucleus, irrespective of IR treatment (Figure 2C). Strikingly, 70 Gy of IR substantially increased CO formation in com-1 mutant animals: while mock-treated com-1 mutants had on average only two ZHP-3::GFP foci per diplotene nucleus, irradiated com-1 mutants commonly contained six foci (Figure 2C).
Previous studies have shown that IR can increase CO frequencies only when meiotic DSBs are limiting, e.g. in spo-11 mutants [6], [23], [24]. This effect is attributed to CO homeostasis mechanisms that ensure that meiotic cells receive at least one and only one CO per homolog pair [22], [24]. Our results imply that in the absence of com-1 CO homeostasis mechanisms are still active and encourage the formation of the obligate COs, yet the substrates to do so are limited. A recent dose-response study estimated that 10 Gy of IR resulted in ∼4 DSBs per chromosome pair, which was sufficient to consistently induce six CO foci in spo-11 animals [23]. We exposed com-1 mutants to 10 Gy, 50 Gy and 70 Gy of IR and found that only 70 Gy resulted in a robust induction of six ZHP-3::GFP foci (Figure 2 and Figure S2). The observation that 10 Gy of IR was not sufficient to induce six CO foci in com-1 mutants, suggests that Ku can also hijack SPO-11-independent DSBs. In support of this notion, IR resulted in increased levels of chromosomal aggregation in com-1 deficient oocytes (Figure 2D) and [14]. Given the relatively high IR dose needed to allow six CO foci to be formed in COM-1-deficient animals, we propose that IR alleviates the CO defect, not because it introduces SPO-11-independent DSBs, but because it can introduce a total number of DSBs that exceeds the capacity of available Ku, leaving a subset of DSBs unblocked and available for HR.
We conclude that both IR treatment and Ku deletion alleviated the CO deficit in com-1 mutant animals, yet only Ku deletion restored the bias towards HR-mediated DSB repair.
Loss of LIG-4 does not restore viability of com-1 mutants
In com-1 mutant animals Ku causes two problems: defective CO formation and chromosomal aggregation. We next set out to determine how Ku exerts these toxic effects. In classical NHEJ, Ku blocks DNA end resection, stabilizes the break ends and recruits the downstream factor LIG-4, which subsequently seals the DSB [2]. To assess if the Ku complex could be toxic independent of LIG-4-mediated ligation, we made com-1 lig-4 double mutants and compared those to com-1 cku-80 and com-1 cku-70 double mutants. Interestingly, unlike cku-70 and cku-80, the introduction of a lig-4 null allele did not rescue progeny survival of com-1 mutants (Figure 3B). Since either lig-4 or cku-70/cku-80 loss prevents NHEJ, blocking NHEJ per se is not sufficient to restore viability in com-1 mutants. We therefore infer that Ku has toxic activities that are independent of NHEJ-mediated fusion.
Consistent with that notion, diakinesis nuclei of com-1 lig-4 double mutants often showed more than six DAPI-stained bodies, indicating that CO formation remained perturbed (Figure 3A). While lig-4 deletion did not restore the CO defect, it did prevent chromosomal aggregation: in contrast to com-1 single mutants, the diakinesis nuclei of com-1 cku-80 and com-1 lig-4 double mutants rarely had fewer than six DAPI-stained bodies (Figure 1C and Figure 3A). These observations indicate that chromosomal aggregation in com-1 mutants mainly depends on classical NHEJ.
Notably, diakinesis nuclei of com-1 lig-4 double mutants frequently contained small DAPI-stained fragments, which are indicative of persistent DSBs (Figure 3A and 3C). We next established that these chromosomal fragments were the consequence of defective repair of programmed SPO-11-induced DSBs (and not of spontaneous DSBs): com-1 lig-4 spo-11 triple mutant animals exhibited 12 intact univalents at diakinesis and no fragmentation (Figure 3A and 3C). Together, these results strongly suggest that in COM-1-deficient animals, Ku promotes LIG-4-mediated fusions and that in the absence of LIG-4 the Ku-bound DSBs remain unrepaired. We therefore propose that COM-1 needs to prevent Ku activity not only because Ku promotes classical NHEJ at meiotic DSBs, but mainly because Ku forestalls meiotic recombination directly.
Ku acts at early/mid pachytene stage and blocks the formation of RAD-51 foci
We next determined how and when Ku prevents meiotic recombination. Based on their homologous counterparts, we expect CKU-70/CKU-80 to block DNA end resection. This scenario is consistent with the reported defect in RAD-51 recruitment in COM-1-deficient germlines [14]. Meiotic recombination is initiated in the transition zone where RAD-51-coated recombination intermediates become visible as distinct foci [25], [26]. In wild-type worms, the number of RAD-51 foci peaks at early/mid pachytene stage (Figure 4, zone 4+5) and as repair progresses, these RAD-51 foci disappear by late pachytene stage (Figure 4, zone 6+7) [27]. In com-1 single mutants, however, we could not detect the typical rise of RAD-51 foci in early/mid pachytene nuclei, suggestive of a defect early in meiotic recombination (Figure 4C, zone 4+5). Strikingly, this defect was relieved by cku-80 loss: com-1 cku-80 double mutants did show the strong increase in RAD-51 foci at early/mid pachytene stage (Figure 4D, zone 4+5). These results demonstrate that, in the absence of COM-1, CKU-80 prevents efficient formation of RAD-51-coated HR intermediates, likely by inhibiting DNA end resection. Moreover, they reveal that CKU-80 can already act at early pachytene stage, which paradoxically is the stage where programmed DSBs need to be channeled into HR.
Fig. 4. Loss of cku-80 restores RAD-51 recruitment to meiotic DSBs in com-1 mutant germlines. 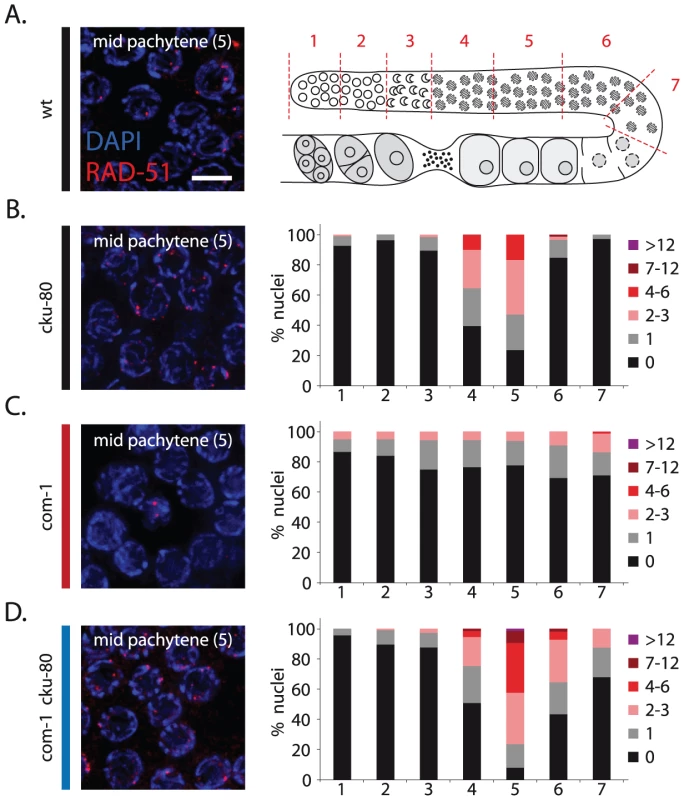
(A) Left: representative image of mid-pachytene nuclei in wild-type germlines stained with RAD-51 antibody; merge of RAD-51 (red) and DAPI signal (blue); Right: schematic overview of the C. elegans germline with indicated zones (1–7) used for RAD-51 foci analysis. (B)(C)(D) RAD-51 foci analysis of cku-80, com-1 and com-1 cku-80 double mutant germlines, respectively. Left: representative images of mid-pachytene nuclei (zone 5) stained with RAD-51 antibody, merge of RAD-51 (red) and DAPI signal (blue) Right: Stacked histograms depict the quantification of RAD-51 foci in germlines of the indicated genotypes. The number of RAD-51 foci per nucleus is categorized by the color code shown on the right. The percent of nuclei observed for each category (y-axis) are depicted for each zone along the germline axis (x-axis). Three independent gonads were scored for each genotype. Scale bars, 5 µm. While com-1 cku-80 double mutant germlines were proficient in RAD-51 loading, we noted a mild delay in RAD-51 focus formation compared to cku-80 single mutant controls (Figure 4, zone 4+6). This delay suggests that COM-1 may also be required for efficient DNA end resection and thus the timely formation of interhomolog COs.
COM-1 and EXO-1 act redundantly to promote meiotic recombination
To find the factors responsible for COM-1-independent meiotic recombination, we searched for genes known to have overlapping functions with COM-1 or its homologs. In yeast, the sensitivity of Sae2-deficient mitotic cells to DSB-inducing agents can be rescued by overexpressing the 5′-3′ exonuclease Exo1 [28]. Furthermore, Exo1 transcription is highly induced during yeast meiosis and Exo1 promotes CO formation [29], [30], making Exo1 a suitable candidate for enabling com-1-independent CO formation.
A clear Exo1 homolog is present in C. elegans, F45G2.3, which we named exo-1. We used a deletion mutant of exo-1, which is predicted to express a severely truncated protein lacking the conserved nuclease domain (Figure 5A), to show that EXO-1 has a conserved role in HR-mediated DSB repair in germ cells. Firstly, exo-1 mutant germlines were hypersensitive to IR, in a manner epistatic with the well-studied HR factor brc-1 (Figure 5B) and secondly, exo-1 mutants were hypersensitive to transposon-induced DSBs, i.e. exo-1 deficiency significantly reduced embryonic survival in animals that have elevated levels of transposition in the germline (Figure S3). Despite the need for exo-1 in repair of ectopic DSBs, unchallenged exo-1 single mutants did not display major meiotic defects (Figure 5C and 5D), which suggests that EXO-1 does not act on SPO-11-induced DSBs or it operates in a redundant fashion.
Fig. 5. EXO-1 promotes DSB repair in germ cells. 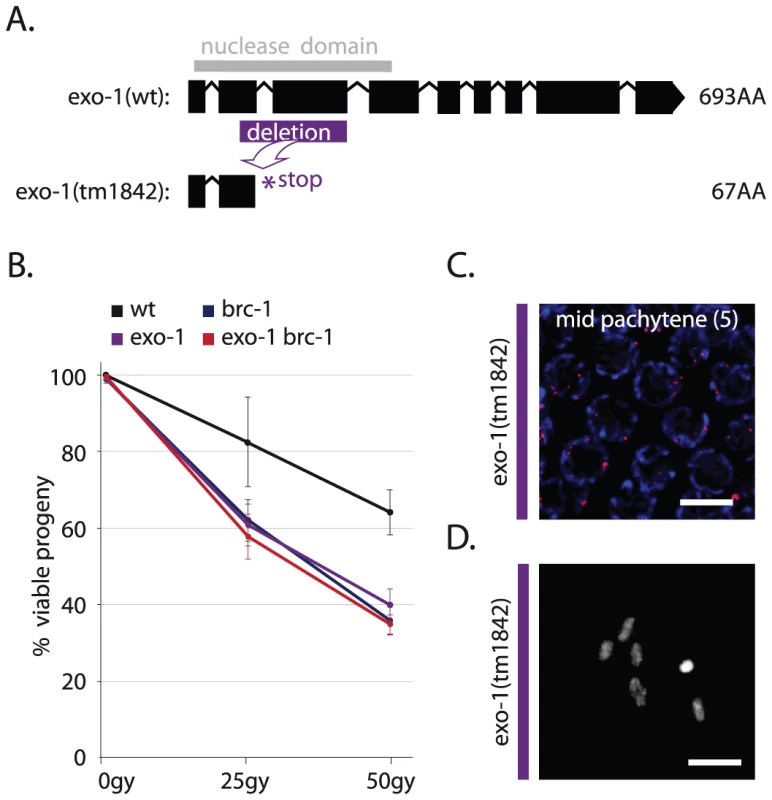
(A) Gene model of wild-type F45G2.3 (exo-1) with the position of its catalytic domain (gray) and below its truncation allele tm1842; a 559 bp deletion (purple) results in a premature stop (B) Percentage progeny survival of animals of the indicated genotype treated with the indicated dose of IR; values are the average of 3 independent experiments, error bars represent S.E.M. (C) RAD-51 immunostaining of mid-pachytene nuclei (zone 5) in exo-1 deficient germlines; merge of RAD-51 (red) and DAPI signal (blue) (D) Representative picture of a diakinesis nucleus of exo-1 deficient animals. To assess if EXO-1 is responsible for COM-1-independent meiotic recombination, we created com-1 cku-80 exo-1 triple mutants and analyzed CO formation and progeny survival. In contrast to com-1 cku-80 double mutants, which have robust CO formation (Figure 1C), com-1 cku-80 exo-1 triple mutants fail to adequately produce COs, as illustrated by the scarcity of ZHP-3::GFP foci at diplotene (Figure 6A) and the lack of chiasmata at diakinesis (Figure 6B). Consequently, com-1 cku-80 exo-1 animals typically produce aneuploid gametes and hardly any viable progeny (Figure 6D and 6E).
Fig. 6. EXO-1 is required for meiotic recombination in absence of COM-1. 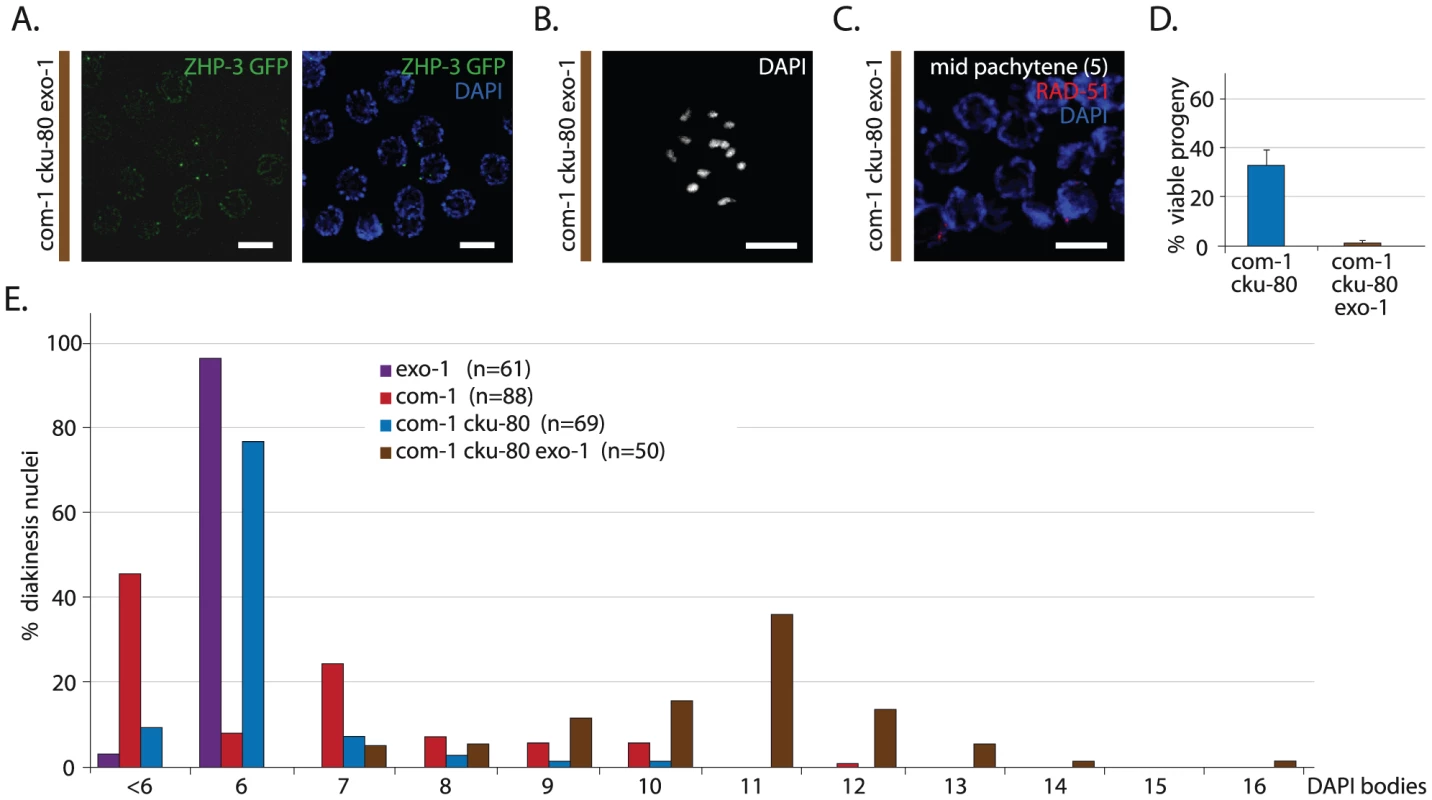
(A) Representative image of diplotene nuclei of com-1 cku-80 exo-1 triple mutant animals that express a ZHP-3::GFP transgene (left: GFP signal only, right: merge of GFP and DAPI signal) (B) Representative picture of a diakinesis nucleus in com-1 cku-80 exo-1 triple mutants germlines (C) RAD-51 immunostaining of mid-pachytene nuclei (zone 5) in com-1 cku-80 exo-1 mutant germlines; merge of RAD-51 (red) and DAPI signal (blue) (D) Percentage progeny survival of animals of the indicated genotype; values are the average of 3 independent experiments*, error bars represent S.E.M. (E) Frequency distribution of DAPI-stained entities at diakinesis*. n = number of germlines analyzed. The com-1 cku-80 exo-1 triple mutants occasionally showed >12 DAPI bodies due to chromosomal fragmentation. See Figure 7E for quantification. Scale bars, 5 µm. *These experiments were performed in parallel to those depicted in Figure 1B and 1D; reference values are depicted again here. We next investigated how EXO-1 promotes CO formation in com-1 deficient germlines. Recently, yeast Exo1 has been shown to promote CO formation via two distinct activities: i) by performing DNA end resection and ii) by resolving CO intermediates named double Holliday Junctions (dHJs) [30]. These two Exo1 activities affect HR at different steps: DNA end resection promotes the formation of RAD-51 intermediates, whereas dHJ resolution supports the clearance of RAD-51 intermediates. We found that early/mid pachytene nuclei of com-1 cku-80 exo-1 triples contained hardly any foci (Figure 6C), which contrasts the many RAD-51 foci observed in com-1 cku-80 double mutants (Figure 4D). This implies that EXO-1 promotes com-1-independent CO formation mainly via its role in DNA end resection.
From these results it can be deduced that i) EXO-1 can act on meiotic DSBs and ii) EXO-1 and COM-1 act in parallel pathways to promote RAD-51 recruitment at early/mid pachytene stage and individually can assure timely CO formation. Furthermore, both COM-1 and EXO-1 are not essential for SPO-11 removal because we did not observe substantial chromosome fragmentation in the diakinesis nuclei of com-1 cku-80 exo-1 triple mutants. Instead, we detected six to twelve regularly shaped DAPI-stained bodies (Figure 6B and 6E), which suggests some degree of DSB repair.
Homolog-independent HR does not depend on COM-1 and EXO-1
C. elegans germ cells switch between different DSB repair modes as they progress through meiosis [31]. In the early stages of meiotic prophase, the majority of meiotic DSBs are repaired using the homologous chromosome as a template [31], [32]. At late pachytene stage this dominance is thought to be relieved, allowing homolog-independent mechanisms to repair the meiotic DSBs [32], [33]. One example that supports this notion is that mutant animals defective in interhomolog HR (e.g. syp-2 mutants) show persistent meiotic DSBs that are eventually repaired late in meiotic prophase in a rad-51-dependent manner [32]. Subsequent studies suggest that these remaining DSBs are repaired efficiently via intersister HR, ultimately giving rise to intact chromosomes at diakinesis [19], [34].
To investigate the contribution of COM-1 and EXO-1 to homolog-independent HR, we quantified RAD-51 focus formation throughout the germline. com-1 cku-80 double mutants had many RAD-51 foci at early/mid pachytene stage (Figure 7A, zone 4+5), but very few RAD-51 foci at late pachytene stage (Figure 7A, zone 7), indicating that the majority of RAD-51 intermediates were resolved by that point. Conversely, com-1 cku-80 exo-1 triple mutant germlines had very few RAD-51 foci at early/mid pachytene stage (Figure 7B, zone 4+5), but showed many RAD-51 foci at late pachytene stage (Figure 7B, zone 7). This abundance of RAD-51-coated recombination intermediates at late pachytene implies that COM-1 and EXO-1 are dispensable for DNA end resection at these later stages, which suggests further redundancy and/or temporal regulation of DNA end resection during meiotic prophase. Moreover, these findings imply that intersister HR may not be affected by com-1 and exo-1 loss.
Fig. 7. EXO-1 and COM-1 are needed for efficient interhomolog HR, but dispensable for intersister HR. 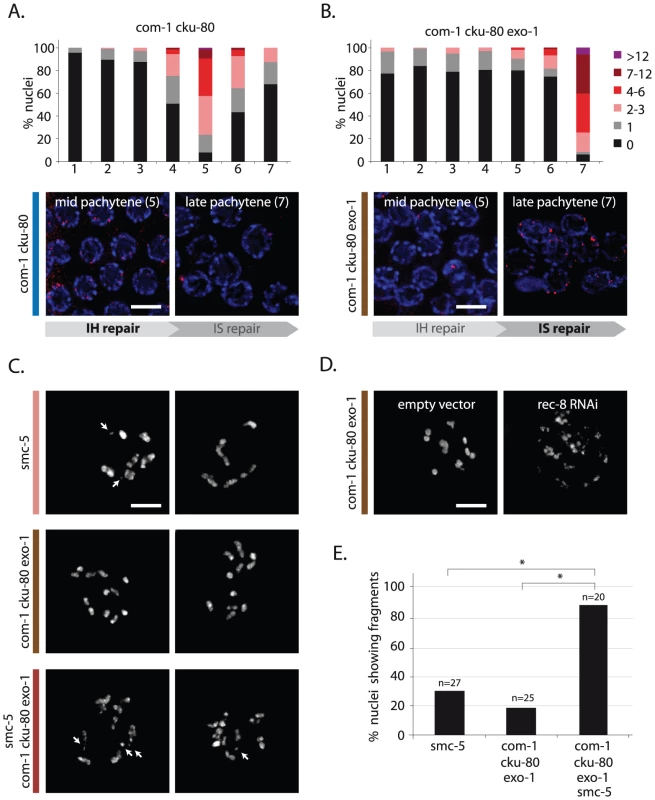
(A,B) RAD-51 foci analysis of com-1 cku-80 double and com-1 cku-80 exo-1 triple mutant germlines, respectively. Stacked histograms depict the quantification of RAD-51 foci in germlines of the indicated genotypes. See Figure 4 for details. Representative images of mid-pachytene nuclei (zone 5) and late pachytene nuclei (zone 7) stained with RAD-51 antibody (red). IH = interhomolog, IS = intersister (C) Two representative pictures of diakinesis nuclei of animals of the indicated genotype. White arrows point out chromosomal fragments (D) Representative picture of a diakinesis nucleus of com-1 cku-80 exo-1 triple mutant animals, which are fed on E. coli strains carrying either a control- or rec-8 RNAi vector. (E) Percentage of diakinesis nuclei that show chromosomal fragments; n = number of germlines analyzed. *The difference between these genotypes was highly significant (p<0.001 by Fisher's exact test, two tailed). Scale bars, 5 µm. To test if intersister HR is responsible for the residual repair activity in the triple mutant, we depleted the cohesin factor REC-8, which is proposed to promote both interhomolog as well as intersister HR [9], [20], [35]. REC-8 depletion caused extensive chromosomal fragmentation in com-1 cku-80 exo-1 triple mutants (Figure 7D), implying that REC-8-dependent HR is active in the absence of COM-1 and EXO-1. REC-8 depletion, however, has documented pleiotropic effects, including altered SPO-11 activity, which may affect the levels of chromosome fragmentation [31]. We therefore substantiated these findings by deleting Structural Maintenance of Chromosomes 5 (smc-5) in com-1 cku-80 exo-1 animals. Smc-5 has recently been shown to be specifically required for homolog-independent (presumably intersister) HR during C. elegans meiosis [34]. Analogous to REC-8 depletion, deletion of smc-5 in com-1 cku-80 exo-1 triple mutants resulted in high levels of chromosome fragmentation at diakinesis (Figure 7C and 7E). Similar results were obtained when deleting the SMC-5 complex partner SMC-6 (Figure S3). Together these observations strongly suggest that, while COM-1 and EXO-1 redundantly promote RAD-51 recruitment and subsequent CO formation at early/mid pachytene stage, at late pachytene stage both proteins are dispensable for RAD-51-mediated intersister HR.
Ku deficiency does not fully restore genome stability in com-1 mutants
Despite the observation that HR is active and COs are formed in germlines lacking both com-1 and cku-80, progeny survival of com-1 cku-80 double mutants was not restored to wild-type levels. In fact, ∼70% of com-1 cku-80 double mutant progeny died during embryonic development (Figure 1B). Moreover, the mutant animals that survived frequently displayed developmental abnormalities, including altered body morphology and faulty vulval development (Figure S4). These phenotypes suggest that Ku-deficient com-1 mutants still suffered from genomic instability. In support of this notion, com-1 cku-80 and com-1 cku-70 double mutants exhibited high levels of X-chromosome non-disjunction, as revealed by a 50-fold increase in XO males among the surviving progeny (Figure S4). Careful analysis of com-1 cku-80 deficient germlines revealed that the fidelity of meiotic DSB repair is incomplete: diakinesis nuclei of com-1 cku-80 double mutants occasionally showed chromosomal abnormalities, including unstable bivalent attachments and chromosomal aggregates (Figure 1D and Figure S5). We detected similar chromosomal aberrations in com-1 lig-4 double mutants (Figure S5), supporting the notion that an alternative mutagenic repair pathway exists that can provoke chromosomal aggregates in germ cells devoid of classical NHEJ [8]. We propose that Ku-deficient com-1 mutants still suffer from (NHEJ-independent) error-prone repair events, which cause substantial chromosomal instability and embryonic lethality.
We next addressed whether these aberrant repair events in com-1 cku-80 double mutants induced germ cell apoptosis. Interestingly, despite the high degree of chromosomal instability, the level of apoptosis was not observed to be increased in com-1 single mutant germlines [14]. Although we cannot formally exclude that COM-1 by itself is required for the signaling of apoptosis, our cytological data argue that Ku blocks end resection in these animals and thus precludes the formation of ssDNA - a major trigger for the DNA damage checkpoint [36], [37]. To test this hypothesis further, we counted apoptotic cells, marked by transgenic CED-1::GFP, in com-1 cku-80 deficient germlines. We observed a mild but statistically significant increase as compared to com-1 single mutants (Figure S4). This result may reflect inefficient repair of a fraction of DSBs in com-1 cku-80 double mutants, as was also suggested by the subtle delay in RAD-51 focus resolution during meiotic prophase (Figure 4, zone 6). These phenotypes are however very mildly different from wild-type behavior [14], [27], [38]. We thus conclude that the vast majority of meiotic DSBs are repaired effectively in com-1 cku-80 mutant germ cells, without activating the DNA damage checkpoint. The fidelity of repair, however, is clearly affected by com-1 and cku-80 loss.
Discussion
The conserved C-terminus of COM-1 counteracts Ku activity and thereby supports efficient meiotic recombination
We identified COM-1 as a crucial factor in preventing toxic Ku activity at meiotic DSBs. Both com-1 alleles used in this study (t1626 and t1489) are loss-of-function alleles and encode for C-terminally truncated proteins [14]. Although sequence analysis of the t1489 allele revealed a different mutation than previously annotated, both alleles still contain a premature stop that prohibits expression of the conserved C-terminus (Figure S1).
Previously, Penkner and colleagues claimed that COM-1 was required specifically to repair SPO-11-induced DSBs but not IR-induced DSBs – suggestive of a conserved role for COM-1 in SPO-11 removal [14]. However, the data we present here reveals that COM-1-deficient germlines are able to repair SPO-11-induced DSBs both via NHEJ and HR. Moreover, we show that COM-1 is not required for meiotic recombination per se, but instead is needed to prevent Ku activity at early pachytene stage to allow DNA end resection and CO formation to take place.
Despite the high conservation of the C-terminal domain of Sae2/COM-1, the contribution of these proteins to SPO-11 removal has clearly diverged between yeast and metazoans: while in yeast a single point mutation in the C-terminus of Sae2 can block Spo11 removal and subsequent HR reactions [39], removal of the entire C-terminus of COM-1 does not prohibit meiotic recombination in C. elegans.
Spo11 removal in yeast not only requires Sae2, but also the highly conserved nuclease Mre11 [18], [40]. Perhaps metazoan MRE-11 is able to remove SPO-11 independently of COM-1. In that scenario, MRE-11 would create free DSB ends that could act as a substrate for both HR and NHEJ (Figure 8). In C. elegans, MRE-11 is needed for meiotic DSB formation, however, this requirement can be bypassed by the depletion of meiotic cohesin [41], [42]. Meiotic DSB induction in absence of MRE-11 results in severe chromosome fragmentation, suggesting that MRE-11 is also required for SPO-11 removal in C. elegans [42].
Fig. 8. Model for meiotic recombination in C. elegans. 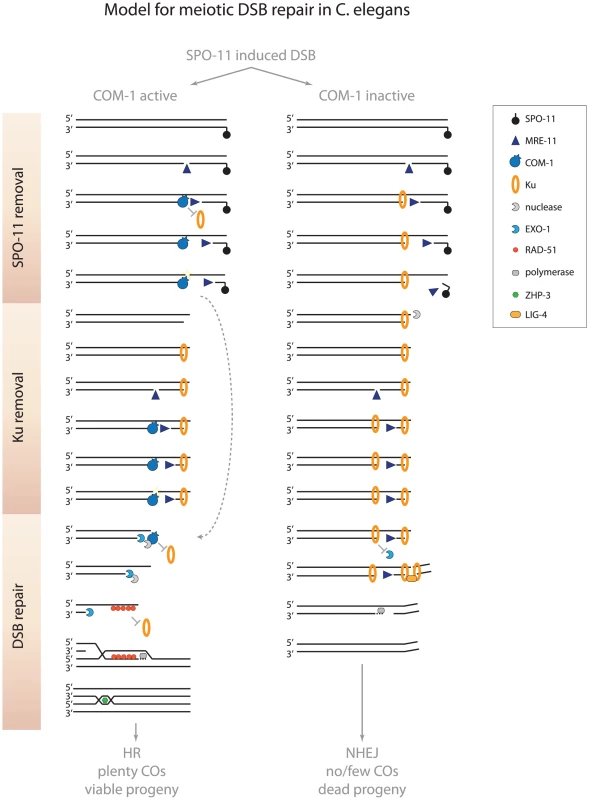
In wild-type germlines, MRE-11 may create substrates at meiotic DSBs that allow COM-1 to efficiently remove Ku (and SPO-11). When COM-1 function is perturbed, MRE-11 mediated processing may still release SPO-11 bound oligos. However, MRE-11 activity alone is not sufficient to counteract Ku binding and prevent toxic NHEJ activity. Without COM-1 and Ku, SPO-11 is removed and EXO-1 promotes DNA end resection and allows the obligate COs to be formed. See text for further details. We show here that COM-1 is not required for the initiation of meiotic DSB repair, but is needed to channel the programmed DSBs into HR. When COM-1 function is perturbed, Ku blocks EXO-1-mediated resection and promotes LIG-4-mediated fusion. How COM-1 prevents Ku activity on a molecular level is unknown to date, but based on the current models of DNA end resection at meiotic DSBs and the observations described here, we propose that COM-1 cleaves off Ku-bound DSB ends and thereby enables EXO-1 to perform DNA end resection (Figure 8).
A model for COM-1–dependent Ku removal
Recent work on yeast meiosis has led to a new model for initiation of meiotic recombination that is based on bidirectional DNA end resection [43]. In this model Mre11 creates a single-strand nick up to 300 nucleotides from the meiotic DSB end. This nick then acts as a substrate for both Exo1 and Mre11: Exo1 starts resection in the 5′-3′ direction (away from the DSB) and Mre11 initiates resection in the 3′-5′ direction (towards the DSB end). Accordingly, the 3′-5′ exonuclease activity of Mre11 is critical for the efficient release of Spo11 oligos and subsequent meiotic recombination [43]. Mre11 is proposed to also remove Ku from DSB ends, since Ku (like Spo11) blocks DSB ends and prevents HR-mediated repair [43]. However, recent in vitro studies have revealed that human MRE11 cannot compete with Ku for DNA binding nor is able to displace Ku from DSB ends [44]. In these reactions, Ku efficiently prevented EXO1 from performing DNA end resection, even in the presence of MRE11. Our in vivo model is consistent with such an interaction, as MRE-11-proficient, but COM-1-deficient, germlines are able to remove SPO-11, but are not able to prevent Ku from hijacking meiotic DSBs (Figure 1 and Figure 2). Our observations imply that SPO-11 removal and Ku exclusion are two distinct activities. Based on the bidirectional nature of DNA end resection and the fact that the affinities of Ku to ssDNA nicks and dsDNA ends are almost equal [45], [46], we propose that Ku may act at the upstream nick to prevent EXO-1-mediated end resection. In such a scenario, MRE-11 may still be able to progress towards the DSB end to remove SPO-11, thus creating a free DSB end that allows NHEJ-mediated repair (Figure 8). The notion that Ku may block 5′-3′ resection by EXO-1, but not 3′-5′ resection by MRE-11 is supported by the fact that the 3′-5′ exonuclease activity of mammalian MRE11 promotes deletion formation during classical NHEJ [47].
We hypothesize that COM-1 prevents Ku occupancy at meiotic DSBs and therefore safeguards proper 5′-3′ DNA end resection and CO formation (Figure 8). While our study reveals that MRE-11, in the absence of COM-1, is not sufficient to prevent Ku activity at meiotic DSBs, we cannot exclude that COM-1 requires MRE-11 activity to counteract Ku. In fact, COM-1 may cut the gapped DNA structure that arises when MRE-11 progresses towards the DSB end, which would release both MRE-11 and Ku from the break site (Figure 8). In support of this model, Sae2 has been shown to possess intrinsic endonuclease activity on gapped DNA substrates in vitro and this activity is proportional to the length of exposed ssDNA [48]. Moreover, Sae2 mutants accumulate both Mre11 and Ku at DSB ends [49], [50].
Ku can act at early pachytene stage and competes with interhomolog HR
Several studies have found evidence of NHEJ-mediated chromosomal aggregates in C. elegans germ cells, however, the biological relevance of these NHEJ events has been uncertain, since they were evident only when meiotic recombination was completely abolished (e.g. by rad-51, brc-2 or msh-4 mutation) and were detected only at diakinesis, the final stage of meiotic prophase [8], [9], [10]. Here we report that Ku can act even when meiotic recombination is proficient and that it does so early in meiosis, at early/mid pachytene stage. Moreover, we reveal that meiotic Ku activity can result in toxic chromosomal aggregates and a fatal lack of obligate COs.
The capacity of Ku to block meiotic recombination is maybe best illustrated by the low levels of ZHP-3::GFP foci observed in COM-1-deficient animals – a phenotype that can be completely alleviated by Ku loss (Figure 2). ZHP-3::GFP localizes to presumptive CO sites and forms six distinct foci in wild-type diplotene nuclei [21]. A recent study by Rosu and colleagues revealed that although each C. elegans meiotic nucleus may undergo up to 40 programmed DSBs, a single DSB per chromosome pair is largely sufficient to assure CO formation [51]. Given that more than a third of the COM-1-deficient nuclei are not able to form a single ZHP-3::GFP focus, and the ones that do only form on average 2–3 ZHP-3::GFP foci, we predict that in the absence of COM-1 nearly all meiotic DSBs are blocked by Ku. To shed more light on this subject, we tried to outcompete Ku by creating many extra DSBs using IR. Only when com-1 mutants were treated with a relatively high dose of IR (estimated to inflict ∼170 DSBs per nucleus [23]) the majority of diplotene nuclei had six CO foci. Based on these experiments we estimate that Ku is able to block ∼97% of all meiotic DSBs when COM-1 function is impaired.
Despite this high toxic capacity of Ku, wild-type worms exhibit very robust CO formation and at least a hundred-fold bias towards HR over NHEJ-mediated repair of germline DSBs [51], [52]. This suggests that COM-1 is very potent in either blocking or removing Ku at meiotic DSBs. Given the striking affinity of Ku towards DNA ends [45] and the detrimental effects of meiotic NHEJ on species survival [this study], additional levels of regulation might be necessary to guarantee the strong HR bias in germ cells. In mouse spermatocytes, Ku protein levels drop significantly at early/mid pachytene stage, revealing that Ku activity can be prevented both by COM-1 activity and at the level of transcription/translation [53]. Interestingly, recently identified COM1 mutants in rice also displayed many non-homologous chromosome entanglements in meiotic cells, indicating that COM-1-mediated NHEJ inhibition may be a common phenomenon among eukaryotes [54].
COM-1 and EXO-1 promote the timely formation of CO substrates
In addition to its role in NHEJ inhibition, COM-1 also supports DNA end resection during early/mid pachytene stage (Figure 4 and Figure 7). In yeast, DNA end resection at meiotic DSBs is performed by Exo1 and Sgs1/Dna2, with Exo1 having the major role [39]. Accordingly, Exo1 mutants show subtle but significant meiotic defects including reduced spore viability and a two-fold decrease in CO recombination [55]. We show here that COM-1-proficient worms do not rely on EXO-1 for meiotic recombination, as exo-1 single mutants form both meiotic RAD-51 foci and bivalents normally (Figure 5). When COM-1 is absent however, EXO-1 becomes essential for RAD-51 loading at early/mid pachytene stage and subsequent CO formation (Figure 6). Thus meiotic germ cells require either COM-1 or EXO-1 to perform timely DNA end resection. How COM-1 promotes extensive DNA end resection is still unclear, as COM-1 homologs are implicated only in the onset of resection [49]. COM-1 may be needed for the recruitment of other nucleases to meiotic DSBs. For instance, recruitment of the nuclease DNA2 to DSBs strongly depends on CtIP in human cells [56]. In line with this suggestion, we found a strong synthetic lethal interaction between exo-1 and dna-2 (unpublished observations). We also demonstrated that HR via the sister chromatid is not abolished by com-1 and exo-1 mutation, revealing another activity that is able to resect meiotic DSBs independent of COM-1 and EXO-1, but only in late pachytene nuclei (Figure 7). Why this activity does not support meiotic recombination and CO formation at early pachytene stage is still an open question.
Implications of mutagenic NHEJ activity in germ cells
COM-1 is dispensable for meiotic recombination per se, however without it, many meiotic DSBs will be repaired via NHEJ, a mutagenic DSB repair pathway that generates non-CO products. The scarcity of COs, combined with the extensive chromosomal aggregation, provides a cogent explanation for the poor fertility of com-1 mutant animals and reveals the deleterious nature of unscheduled NHEJ during meiosis.
How NHEJ is kept in check during human meiosis remains to be addressed. Recent studies have revealed that a subclass of so-called Seckel and Jawad syndrome patients express truncated CtIP variants that typically lack the conserved C-terminus [57], which are very reminiscent of the com-1 alleles used for this study (Figure S1). Although these patients suffer from severe mental retardation and skeletal abnormalities, it is unknown to date if they also have fertility defects.
Multicellular animals rely heavily on NHEJ to maintain genome stability in somatic tissues, but the efficacy of this repair pathway seems to come with a price: uncontrolled NHEJ activity has been shown to drive tumorigenesis in mice [58] and the data presented here uncover its toxic properties during meiosis. Recent advances in genome-wide sequencing have revealed that many complex chromosomal rearrangements that occur de novo in human germlines show typical NHEJ footprints [59], [60], which suggests that incomplete inhibition of NHEJ during gametogenesis may affect genome evolution in many organisms, including humans, and could lead to pathogenic chromosomal alterations that cause serious inborn diseases.
Materials and Methods
Worm strains and culture conditions
All strains were maintained at 15°C using standard C. elegans techniques [61]. The wild-type background was Bristol N2. In case of mutant strains that carried a linked unc-32(e189) allele, matched unc-32(e189) homozygotes served as controls. The following mutations, transgenes and genetic balancers were used:
LGII: smc-5(ok2421), smc-6(ok3294), dna-2 [62], mln1[dpy-10 mIs14].
LGIII: com-1(t1626) [14], com-1(t1489) [14], unc-32(e189), cku-80(ok861), cku-70(tm1524), lig-4(ok716), exo-1(tm1842), brc-1(tm1145), hT2[let-? qIs48].
LG IV: spo-11(ok79), jfIs2[ZHP-3::GFP] [21].
Y-irradiation and progeny survival/him assays
Synchronized L4 worms were either left unchallenged or irradiated using an x-ray generator (200 kV; 10 mA; 11 Gy/min dose rate; YXLON International) to create germline DSBs. Three (irradiated) hermaphrodites were pooled on an OP50 seeded NGM plate and cultured at 20°C to produce progeny. After 40 hrs mothers were removed and the ratio between dead eggs/hatched larvae was assessed 24 hrs later. For Him assay, the percentage of males among the hatched progeny was determined. For all survival/him assays, at least three independent plates were scored per condition. Figures provide mean values of three independent experiments.
DAPI staining and ZHP-3::GFP analysis
Synchronized L4 worms were picked and allowed to age 20–24 hrs. Gonad dissection was carried out in 1× EBT (25 mM HEPES-Cl pH 7.4, 118 mM NaCl, 48 mM KCl, 2 mM CaCl, 2 mM MgCl, 0.1% Tween 20 and 20 mM sodium azide). An equal volume of 4% formaldehyde in EBT was added (final concentration is 2% formaldehyde) and allowed to incubate for 5 min. The dissected worms were freeze-cracked in liquid nitrogen for 10 min, incubated in methanol at −20°C for 10 min, transferred to PBS/0.1% Tween (PBST), washed 3×10 min in PBS/1%Triton-X and stained 10 min in 0.5 µg/ml DAPI/PBST. Finally samples were de-stained in PBST for 1 h and mounted with Vectashield. Diakinesis nuclei of −1 position oocytes (closest to the spermatheca) were analyzed using Leica DM6000 microscope. To examine CO formation, ZHP-3::GFP foci were analyzed in ∼15 most proximal pachytene/diplotene nuclei of at least six independent germlines (∼100 nuclei).
Immunofluorescence and RAD-51 focus quantification
RAD-51 protein was detected by indirect immunofluorescence. Germlines were dissected and fixed for whole-mount staining as described above, then blocked with 1% BSA in PBST and incubated overnight at 4°C with rabbit anti-RAD-51 antibody (Novus Biologicals) diluted 1∶200. Primary antibody was detected using Alexa488 Goat anti-rabbit antibody (Invitrogen) diluted 1∶1000 and DNA was counter-stained with 0.5 µg/ml DAPI. RAD-51 foci were imaged using a Leica DM6000 deconvolution microscope collecting 0.5 µm Z-sections. The number of foci per nucleus was counted for each of the seven zones of the germline [27]. Three to five germlines were quantified per condition.
Supporting Information
Zdroje
1. McKinnonPJ, CaldecottKW (2007) DNA strand break repair and human genetic disease. Annu Rev Genomics Hum Genet 8 : 37–55.
2. LieberMR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79 : 181–211.
3. SymingtonLS, GautierJ (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45 : 247–271.
4. ShaharOD, RamEV, ShimshoniE, HareliS, MeshorerE, et al. (2011) Live imaging of induced and controlled DNA double-strand break formation reveals extremely low repair by homologous recombination in human cells. Oncogene
5. KeeneyS, GirouxCN, KlecknerN (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88 : 375–384.
6. DernburgAF, McDonaldK, MoulderG, BarsteadR, DresserM, et al. (1998) Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94 : 387–398.
7. Hunter N (2006) Meiotic recombination; Aguilera A, and Rothstein, R., eds., editor. Heidelberg: Springer-Verlag.
8. MartinJS, WinkelmannN, PetalcorinMI, McIlwraithMJ, BoultonSJ (2005) RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol 25 : 3127–3139.
9. SmolikovS, EizingerA, HurlburtA, RogersE, VilleneuveAM, et al. (2007) Synapsis-defective mutants reveal a correlation between chromosome conformation and the mode of double-strand break repair during Caenorhabditis elegans meiosis. Genetics 176 : 2027–2033.
10. AdamoA, CollisSJ, AdelmanCA, SilvaN, HorejsiZ, et al. (2010) Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol Cell 39 : 25–35.
11. ClejanI, BoerckelJ, AhmedS (2006) Developmental modulation of nonhomologous end joining in Caenorhabditis elegans. Genetics 173 : 1301–1317.
12. YunMH, HiomK (2009) CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 459 : 460–463.
13. ChenPL, LiuF, CaiS, LinX, LiA, et al. (2005) Inactivation of CtIP leads to early embryonic lethality mediated by G1 restraint and to tumorigenesis by haploid insufficiency. Mol Cell Biol 25 : 3535–3542.
14. PenknerA, Portik-DobosZ, TangL, SchnabelR, NovatchkovaM, et al. (2007) A conserved function for a Caenorhabditis elegans Com1/Sae2/CtIP protein homolog in meiotic recombination. EMBO J 26 : 5071–5082.
15. LimboO, ChahwanC, YamadaY, de BruinRA, WittenbergC, et al. (2007) Ctp1 is a cell-cycle-regulated protein that functions with Mre11 complex to control double-strand break repair by homologous recombination. Mol Cell 28 : 134–146.
16. SartoriAA, LukasC, CoatesJ, MistrikM, FuS, et al. (2007) Human CtIP promotes DNA end resection. Nature 450 : 509–514.
17. KeeneyS, KlecknerN (1995) Covalent protein-DNA complexes at the 5′ strand termini of meiosis-specific double-strand breaks in yeast. Proc Natl Acad Sci U S A 92 : 11274–11278.
18. HartsuikerE, MizunoK, MolnarM, KohliJ, OhtaK, et al. (2009) Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, but Rec12Spo11 removal is dispensable for other MRN-dependent meiotic functions. Mol Cell Biol 29 : 1671–1681.
19. AdamoA, MontemauriP, SilvaN, WardJD, BoultonSJ, et al. (2008) BRC-1 acts in the inter-sister pathway of meiotic double-strand break repair. EMBO Rep 9 : 287–292.
20. SeversonAF, LingL, van ZuylenV, MeyerBJ (2009) The axial element protein HTP-3 promotes cohesin loading and meiotic axis assembly in C. elegans to implement the meiotic program of chromosome segregation. Genes Dev 23 : 1763–1778.
21. BhallaN, WynneDJ, JantschV, DernburgAF (2008) ZHP-3 acts at crossovers to couple meiotic recombination with synaptonemal complex disassembly and bivalent formation in C. elegans. PLoS Genet 4: e1000235 doi:10.1371/journal.pgen.1000235.
22. HillersKJ, VilleneuveAM (2003) Chromosome-wide control of meiotic crossing over in C. elegans. Curr Biol 13 : 1641–1647.
23. YokooR, ZawadzkiKA, NabeshimaK, DrakeM, ArurS, et al. (2012) COSA-1 Reveals Robust Homeostasis and Separable Licensing and Reinforcement Steps Governing Meiotic Crossovers. Cell 149 : 75–87.
24. YoudsJL, MetsDG, McIlwraithMJ, MartinJS, WardJD, et al. (2010) RTEL-1 enforces meiotic crossover interference and homeostasis. Science 327 : 1254–1258.
25. AlpiA, PasierbekP, GartnerA, LoidlJ (2003) Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112 : 6–16.
26. MetsDG, MeyerBJ (2009) Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell 139 : 73–86.
27. SaitoTT, YoudsJL, BoultonSJ, ColaiacovoMP (2009) Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genet 5: e1000735 doi:10.1371/journal.pgen.1000735.
28. MimitouEP, SymingtonLS (2010) Ku prevents Exo1 and Sgs1-dependent resection of DNA ends in the absence of a functional MRX complex or Sae2. EMBO J 29 : 3358–3369.
29. SzankasiP, SmithGR (1995) A role for exonuclease I from S. pombe in mutation avoidance and mismatch correction. Science 267 : 1166–1169.
30. ZakharyevichK, MaY, TangS, HwangPY, BoiteuxS, et al. (2010) Temporally and biochemically distinct activities of Exo1 during meiosis: double-strand break resection and resolution of double Holliday junctions. Mol Cell 40 : 1001–1015.
31. HayashiM, ChinGM, VilleneuveAM (2007) C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression. PLoS Genet 3: e191 doi:10.1371/journal.pgen.0030191.
32. ColaiacovoMP, MacQueenAJ, Martinez-PerezE, McDonaldK, AdamoA, et al. (2003) Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell 5 : 463–474.
33. Martinez-PerezE, VilleneuveAM (2005) HTP-1-dependent constraints coordinate homolog pairing and synapsis and promote chiasma formation during C. elegans meiosis. Genes Dev 19 : 2727–2743.
34. BickelJS, ChenL, HaywardJ, YeapSL, AlkersAE, et al. (2010) Structural maintenance of chromosomes (SMC) proteins promote homolog-independent recombination repair in meiosis crucial for germ cell genomic stability. PLoS Genet 6: e1001028 doi:10.1371/journal.pgen.1001028.
35. PasierbekP, JantschM, MelcherM, SchleifferA, SchweizerD, et al. (2001) A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev 15 : 1349–1360.
36. ZouL, ElledgeSJ (2003) Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300 : 1542–1548.
37. BaillyA, GartnerA (2013) Germ cell apoptosis and DNA damage responses. Adv Exp Med Biol 757 : 249–276.
38. Jaramillo-LambertA, EngebrechtJ (2010) A single unpaired and transcriptionally silenced X chromosome locally precludes checkpoint signaling in the Caenorhabditis elegans germ line. Genetics 184 : 613–628.
39. ManfriniN, GueriniI, CitterioA, LucchiniG, LongheseMP (2010) Processing of meiotic DNA double strand breaks requires cyclin-dependent kinase and multiple nucleases. J Biol Chem 285 : 11628–11637.
40. MoreauS, FergusonJR, SymingtonLS (1999) The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol 19 : 556–566.
41. ChinGM, VilleneuveAM (2001) C. elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev 15 : 522–534.
42. BaudrimontA, PenknerA, WoglarA, MamnunYM, HulekM, et al. (2011) A new thermosensitive smc-3 allele reveals involvement of cohesin in homologous recombination in C. elegans. PLoS ONE 6: e24799 doi:10.1371/journal.pone.0024799.
43. GarciaV, PhelpsSE, GrayS, NealeMJ (2011) Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature 479 : 241–244.
44. SunJ, LeeKJ, DavisAJ, ChenDJ (2011) Human Ku70/80 blocks Exonuclease1-mediated DNA resection in the presence of human Mre11 or Mre11/Rad50 complex. J Biol Chem
45. BlierPR, GriffithAJ, CraftJ, HardinJA (1993) Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem 268 : 7594–7601.
46. FalzonM, FewellJW, KuffEL (1993) EBP-80, a transcription factor closely resembling the human autoantigen Ku, recognizes single - to double-strand transitions in DNA. J Biol Chem 268 : 10546–10552.
47. ZhuangJ, JiangG, WillersH, XiaF (2009) Exonuclease function of human Mre11 promotes deletional nonhomologous end joining. J Biol Chem 284 : 30565–30573.
48. LengsfeldBM, RattrayAJ, BhaskaraV, GhirlandoR, PaullTT (2007) Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol Cell 28 : 638–651.
49. ShimEY, ChungWH, NicoletteML, ZhangY, DavisM, et al. (2010) Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J 29 : 3370–3380.
50. KimHS, VijayakumarS, RegerM, HarrisonJC, HaberJE, et al. (2008) Functional interactions between Sae2 and the Mre11 complex. Genetics 178 : 711–723.
51. RosuS, LibudaDE, VilleneuveAM (2011) Robust crossover assurance and regulated interhomolog access maintain meiotic crossover number. Science 334 : 1286–1289.
52. RobertVJ, DavisMW, JorgensenEM, BessereauJL (2008) Gene conversion and end-joining-repair double-strand breaks in the Caenorhabditis elegans germline. Genetics 180 : 673–679.
53. GoedeckeW, EijpeM, OffenbergHH, van AalderenM, HeytingC (1999) Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet 23 : 194–198.
54. JiJ, TangD, WangK, WangM, CheL, et al. (2012) The role of OsCOM1 in homologous chromosome synapsis and recombination in rice meiosis. Plant J
55. KirkpatrickDT, FergusonJR, PetesTD, SymingtonLS (2000) Decreased meiotic intergenic recombination and increased meiosis I nondisjunction in exo1 mutants of Saccharomyces cerevisiae. Genetics 156 : 1549–1557.
56. PetersonSE, LiY, ChaitBT, GottesmanME, BaerR, et al. (2011) Cdk1 uncouples CtIP-dependent resection and Rad51 filament formation during M-phase double-strand break repair. J Cell Biol 194 : 705–720.
57. QvistP, HuertasP, JimenoS, NyegaardM, HassanMJ, et al. (2011) CtIP Mutations Cause Seckel and Jawad Syndromes. PLoS Genet 7: e1002310 doi:10.1371/journal.pgen.1002310.
58. BuntingSF, CallenE, WongN, ChenHT, PolatoF, et al. (2010) 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141 : 243–254.
59. KloostermanWP, GuryevV, van RoosmalenM, DuranKJ, de BruijnE, et al. (2011) Chromothripsis as a mechanism driving complex de novo structural rearrangements in the germline. Hum Mol Genet 20 : 1916–1924.
60. ChiangC, JacobsenJC, ErnstC, HanscomC, HeilbutA, et al. (2012) Complex reorganization and predominant non-homologous repair following chromosomal breakage in karyotypically balanced germline rearrangements and transgenic integration. Nat Genet 44 : 390–397.
61. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
62. LeeMH, HollisSE, YooBH, NykampK (2011) Caenorhabditis elegans DNA-2 helicase/endonuclease plays a vital role in maintaining genome stability, morphogenesis, and life span. Biochem Biophys Res Commun 407 : 495–500.
Štítky
Genetika Reprodukčná medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 2- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



