-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
An Solution for Crossover Formation
article has not abstract
Published in the journal: An Solution for Crossover Formation. PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003658
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003658Summary
article has not abstract
The MUS-81, SLX-1, and XPF-1 structure-selective endonucleases have been implicated in meiotic crossover (CO) formation in a variety of organisms, but their contributions to C. elegans CO formation have been unclear. In this issue of PLOS Genetics, Agostinho et al., Saito et al., and O'Neil et al. demonstrate that MUS-81 and XPF-1 function in two parallel pathways during the formation of meiotic crossovers in C. elegans and provide important insights into the interplay between endonucleases and the Bloom helicase ortholog during crossover formation.
Meiotic crossovers are important for chromosome segregation. When COs are absent or misplaced, nondisjunction results in aneuploidy, a leading cause of miscarriages and birth defects in humans. Our understanding of the complex process that generates COs has evolved steadily, and the models describing the molecular details have undergone much editing. Key features of the most commonly cited model for meiotic recombination include initiation by a DNA double-strand break (DSB) and a progression of joint molecule (JM) intermediates that link DNA duplexes on homologous chromosomes (reviewed in [1]). In this model, COs are produced by cleavage of a late JM intermediate that has two Holliday junctions (HJs). This cleavage is accomplished by HJ resolvases. A wealth of studies in the yeast Saccharomyces cerevisiae have provided extensive genetic and biochemical support for this model. Although initiation by DSBs appears to be universal (reviewed in [2]), it is unknown how similar subsequent steps are in other organisms. This issue of PLOS Genetics contains a set of three papers that describe progress toward answering this question in C. elegans, particularly with regard to functions of resolvases in generating COs [3]–[5] (Figure 1).
Fig. 1. Venn diagram summarizing the main conclusions shared between the three publications discussed in the text. 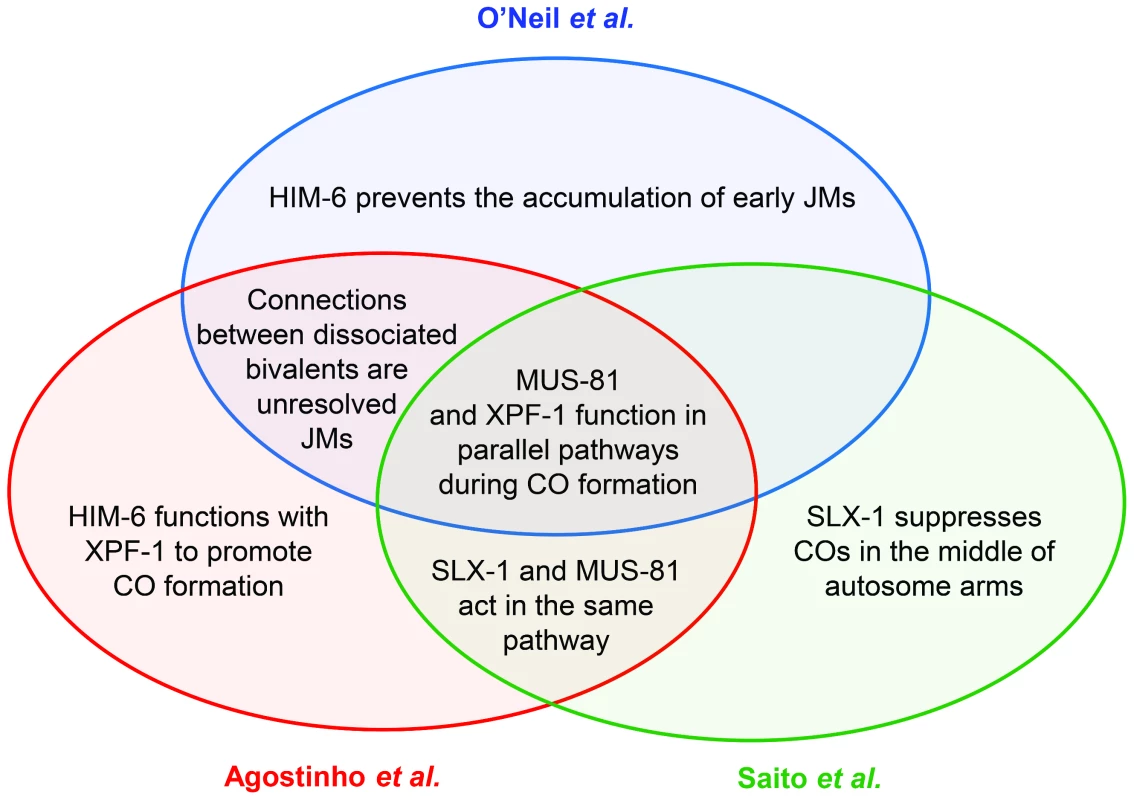
Numerous searches for S. cerevisiae enzymes with in vitro resolvase activity eventually identified three structure-selective endonucleases: Mus81–Mms4, Yen1, and Slx1–Slx4 [6]–[8]. Mus-81–Mms4 and Yen1 are important in generating mitotic COs [9]. Elucidating the functions of these enzymes in generating meiotic crossovers has been more challenging, but substantial progress has been made recently [10], [11]. Our current understanding is that there are at least two CO pathways in S. cerevisiae meiosis (reviewed in [1]). The major pathway produces interfering COs, which are located farther apart than at random. The resolvase that generates these COs is thought to include Exo1 and the MutLγ complex, proteins that are also involved in mismatch repair [11]. Mus81–Mms4, Yen1, and Slx1–Slx4 do function in CO formation, but through a secondary pathway that yields non-interfering COs. Among single mutants, however, only mus81 and mms4 have reduced COs, and even loss of all three confers a relatively modest defect in JM resolution and CO formation, due to redundant and compensatory activities [10], [11].
In the nematode C. elegans, previous work found that SLX-1 and the Slx4 ortholog HIM-18 are required for some meiotic COs [12]. These studies also found a role for the nucleotide excision repair endonuclease XPF-1, which was examined because of the prominent role for its Drosophila ortholog, MEI-9, in generating meiotic COs [13]. To shed additional light on the relationships between the proteins responsible for CO formation in C. elegans, Agostinho et al., O'Neil et al., and Saito et al. [3]–[5] quantified numerous meiotic phenotypes in single, double, triple, and even quadruple mutants. These studies led to the discovery of two parallel, partially redundant pathways—one dependent on MUS-81 and the other on XPF-1. Agostinho et al. and Saito et al. additionally demonstrate that SLX-1 functions with MUS-81 and that HIM-18 functions in both pathways, and Agostinho et al. provide evidence that HIM-6, the ortholog of the Bloom syndrome helicase, collaborates with XPF-1. Saito et al. also extend a previous finding that SLX-1 actually prevents COs in the centers of the chromosomes [12].
What happens in the absence of both pathways? All three groups report multiple chromosome abnormalities. Most strikingly, connections between homologs persist into diakinesis, where they appear as fine DAPI-stained bridges. The researchers hypothesize that these bridges result from unresolved JMs. To test this hypothesis, Agostinho et al. and O'Neil et al. removed SPO-11, the enzyme that generates DSBs that initiate meiotic recombination. This eliminated chromatin bridges. In addition, O'Neil and colleagues elegantly show that the chromatin bridges in mus-81;xpf-1 mutants are efficiently resolved by germline injection of human GEN1, which cleaves HJs in vitro [7], providing further support for the hypothesis that the bridges are due to unresolved HJs.
These studies further highlight the substantial complexity among resolvase functions in meiosis. Although S. cerevisiae resolvase functions are similarly complex, the details are different in many ways. First, in S. cerevisiae, Mus81 generates only non-interfering COs, but C. elegans MUS-81 seems to be involved in making interfering COs. Second, S. cerevisiae Rad1, the ortholog of XPF-1, has no apparent meiotic function [11]. Third, the JM-resolving activity of S. cerevisiae Slx1 and Slx4 is evident only in certain mutant backgrounds [10], [11]. Fourth, in S. cerevisiae, there is no evidence that a resolvase can have an anti-CO role, as is suggested by Saito et al. for SLX-1 at the centers of chromosomes [4].
Additional potential similarities arise from studies of him-6, which encodes the ortholog of human Bloom syndrome helicase (BLM) and S. cerevisiae Sgs1. Sgs1 disassembles early meiotic JMs, both to generate noncrossovers and to prevent formation of aberrant multi-chromatid JMs [10], [11]. O'Neil et al. report that him-6 mutants have defects in processing early intermediates, leading to chromosome fragmentation [3]. Agostinho et al. propose that HIM-6 also has a late function in generating COs in conjunction with XPF-1 [5]. This is an exciting idea, especially given the recent discovery of pro-CO roles for Sgs1 and Drosophila BLM [10], [11], [14], though the authors caution that a full understanding of this function is complicated by the earlier function for HIM-6.
Although the three groups began with similar goals, the different approaches taken in the three papers complement one another to provide numerous important insights into resolvase functions in C. elegans meiosis (Figure 1). The results raise several important questions for future studies. First, what is the source of the COs that occur when both the MUS-81 and XPF-1 pathways are missing? Saito et al. and Agostinho et al. find that COs are reduced by only a little more than one third in mus-81;xpf-1. A simple interpretation is that neither of these enzymes identifies the major meiotic resolvase, but the truth is likely to be more complex. The assays used to measure COs necessarily select for a distinct subset of the progeny: those that had enough COs to ensure proper chromosome segregation. It is possible that the real decrease is more severe, and that MUS-81 and XPF-1 do define the major resolvases. Either way, there must be at least one additional resolvase that hasn't been identified. The authors of these papers discuss several candidates that might be tested.
Another important question concerns the exact nature of the pre-crossover JM. The authors suggest that these resolvases are acting on HJs, and resolution of chromatin bridges by GEN1 supports this suggestion. Agostinho et al. propose intriguing ideas for how the sets of enzymes (MUS-81, SLX-1, and HIM-18; and XPF-1, HIM-18, and HIM-6) might work together to resolve HJs. However, it is possible that the proteins act on a different structure, such as nicked HJs (e.g., see reference [8]). Either way, the groundwork laid in this set of papers will facilitate future experiments aimed at answering these questions and many others.
Zdroje
1. KohlKP, SekelskyJ (2013) Meiotic and mitotic recombination in meiosis. Genetics 194 : 327–334.
2. KeeneyS (2008) Spo11 and the formation of DNA double-strand breaks in meiosis. Genome Dyn Stab 2 : 81–123.
3. O'NeilNJ, MartinJS, YoudsJL, WardJD, PetalcorinMIR, et al. (2013) Joint molecule resolution requires the redundant activities of MUS-81 and XPF-1 during Caenorhabditis elegans meiosis. PLoS Genet 9: e1003582 doi:10.1371/journal.pgen.1003582
4. SaitoTT, LuiDY, KimH-M, MeyerK, ColaiácovoMP (2013) Interplay between structure-specific endonucleases for crossover control during Caenorhabditis elegans meiosis. PLoS Genet 9: e1003586 doi:10.1371/journal.pgen.1003586
5. AgostinhoA, MeierB, SonnevilleR, JagutM, WoglarA, et al. (2013) Combinatorial regulation of meiotic Holliday junction resolution in C. elegans by HIM-6 (BLM) helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 nucleases. PLoS Genet 9: e1003591 doi:10.1371/journal.pgen.1003591
6. KaliramanV, MullenJR, FrickeWM, Bastin-ShanowerSA, BrillSJ (2001) Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev 15 : 2730–2740.
7. IpSC, RassU, BlancoMG, FlynnHR, SkehelJM, et al. (2008) Identification of Holliday junction resolvases from humans and yeast. Nature 456 : 357–361.
8. SchwartzEK, HeyerWD (2011) Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma 120 : 109–127.
9. HoCK, MazonG, LamAF, SymingtonLS (2010) Mus81 and Yen1 promote reciprocal exchange during mitotic recombination to maintain genome integrity in budding yeast. Mol Cell 40 : 988–1000.
10. De MuytA, JessopL, KolarE, SourirajanA, ChenJ, et al. (2012) BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol Cell 46 : 43–53.
11. ZakharyevichK, TangS, MaY, HunterN (2012) Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell 149 : 334–347.
12. SaitoTT, YoudsJL, BoultonSJ, ColaiácovoMP (2009) Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genet 5: e1000735 doi:10.1371/journal.pgen.1000735
13. SekelskyJ, McKimKS, ChinGM, HawleyRS (1995) The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics 141 : 619–627.
14. KohlKP, JonesCD, SekelskyJ (2012) Evolution of an MCM complex in flies that promotes meiotic crossovers by blocking BLM helicase. Science 338 : 1363–1365.
Štítky
Genetika Reprodukčná medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



