-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
Leprosy is a chronic infectious disease caused by Mycobacterium Leprae, where the host genetic background plays an important role toward the disease pathogenesis. Various studies have identified a number of human genes in association with leprosy or its clinical forms. However, non-replication of results has hinted at the heterogeneity among associations between different population groups, which could be due to differently evolved LD structures and differential frequencies of SNPs within the studied regions of the genome. A need for systematic and saturated mapping of the associated regions with the disease is warranted to unravel the observed heterogeneity in different populations. Mapping of the PARK2 and PACRG gene regulatory region with 96 SNPs, with a resolution of 1 SNP per 1 Kb for PARK2 gene regulatory region in a North Indian population, showed an involvement of 11 SNPs in determining the susceptibility towards leprosy. The association was replicated in a geographically distinct and unrelated population from Orissa in eastern India. In vitro reporter assays revealed that the two significantly associated SNPs, located 63.8 kb upstream of PARK2 gene and represented in a single BIN of 8 SNPs, influenced the gene expression. A comparison of BINs between Indian and Vietnamese populations revealed differences in the BIN structures, explaining the heterogeneity and also the reason for non-replication of the associated genomic region in different populations.
Published in the journal: Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups. PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003578
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003578Summary
Leprosy is a chronic infectious disease caused by Mycobacterium Leprae, where the host genetic background plays an important role toward the disease pathogenesis. Various studies have identified a number of human genes in association with leprosy or its clinical forms. However, non-replication of results has hinted at the heterogeneity among associations between different population groups, which could be due to differently evolved LD structures and differential frequencies of SNPs within the studied regions of the genome. A need for systematic and saturated mapping of the associated regions with the disease is warranted to unravel the observed heterogeneity in different populations. Mapping of the PARK2 and PACRG gene regulatory region with 96 SNPs, with a resolution of 1 SNP per 1 Kb for PARK2 gene regulatory region in a North Indian population, showed an involvement of 11 SNPs in determining the susceptibility towards leprosy. The association was replicated in a geographically distinct and unrelated population from Orissa in eastern India. In vitro reporter assays revealed that the two significantly associated SNPs, located 63.8 kb upstream of PARK2 gene and represented in a single BIN of 8 SNPs, influenced the gene expression. A comparison of BINs between Indian and Vietnamese populations revealed differences in the BIN structures, explaining the heterogeneity and also the reason for non-replication of the associated genomic region in different populations.
Introduction
Mycobacterium leprae is the causative agent of chronic granulomatous infectious disease, known as Leprosy. The disease affects skin, the peripheral nerves and can cause irreversible impairment of the nerve function with consequent chronic disabilities [1]. The prevalence of leprosy which declined dramatically after the introduction of Multidrug therapy in 1980s, however, continues to survive as a major public health problem with more than 200,000 new cases reported globally every year, especially in China and India [2]. Our understanding about the mechanism underlying infection and how it leads to different clinical forms is limited; because M. leprae only infects humans and cannot be cultured in vitro [3]. Only a limited number show clinically recognizable lesions [4], and a simultaneous spectrum of the disease symptoms that depends upon the interaction between host immune system and the pathogen. Tuberculoid and lepromatous leprosy are at opposite ends of the spectrum, associated with an immune response mediated either by type 1 helper T (Th1) or type 2 helper T (Th2) cells [5]. The limited genetic diversity between different isolates of M. leprae strains [6] illustrates that the differences in susceptibility towards the disease or its clinical manifestations among patients are governed by host genetic factors, which have been implicated from studies of familial clustering [7], studies of twins [8], complex segregation analysis [9], [10], and test of analysis with the HLA genes [11]. Recent genome-wide association studies [12], [13] have further supported the involvement of host genetic background in inter-individual variability. Several studies have identified a number of human genes, such as HLA-DR [14], [15], LTA [16], TLRs [17], [18]; and genomic regions like 10p13 [19], 6p21 [20], 17q11–q21 [21], 20p13 [22] and 6q25-26 harbouring variants in the common regulatory region of PARK2 and PACRG genes [23] to be associated with the disease or its clinical forms. The results have suggested a polygenic nature of the disease with a high degree of heterogeneity among different populations and only a few unequivocal replications.
PARK2 and PACRG genes both share a common regulatory region and encode the proteins that are involved in cellular ubiquitination. Little is known about the specific function of the PACRG gene. PARK2 protein product-parkin, however, has been identified as an ubiquitin E3 ligase involved in delivery of polyubiquinated proteins to the proteasomal complex [24]. Only experimental evidence for the involvement of the PARK2 and its co-regulated gene PACRG with the host responses to M. leprae was provided by positional cloning in Vietnamese and Brazilian populations [23]. Different pathway analyses also showed the importance of these genes in pathogenesis of the disease [13], [25]. However, attempts to replicate the results in other populations failed in the past [26], [27], suggesting the possible involvement of different variants in diverse populations providing susceptibility towards leprosy. This possibility could arise due to a change in LD structures across the populations for the SNPs distributed in the specific genomic regions.
The present study with this rationale selected a group of SNPs, saturating the regulatory region of PARK2 and PACRG genes, to find out the variant LD structure, if any, in Indian population as compared to Brazilian and Vietnamese; and study the unexplored variants that may be responsible for an association with leprosy or its sub-types in the studied population.
Results
PARK2 and PACRG gene regulatory region was saturated with 96 SNPs with approximately 1 SNP per Kb for PARK2 gene regulatory region to perform a population based case-control study in two unrelated Indian population groups. To rule out population stratification in the studied groups which confounds a disease association study, the MDS (multi-dimensional scaling) plot based on IBS (identity by state) pair-wise distances for 61 individual identifying autosomal SNPs not associated with the disease [28] was carried out. The results showed a compact cluster indicating the populations under study to be homogeneous (Figure S1). Locus wise FST was also calculated for the SNPs associated with Leprosy in the Indian populations. All the polymorphisms showed a very low locus-wise FST value, indicating that the patients and controls belonged to the same population group.
Figure 1 provides a schematic picture of the distribution of 11 significantly associated SNPs out of a total of 96 SNPs studied for the region (criteria details provided in the Materials and Methods section) in two geographically distinct and unrelated population groups, using a MassArray platform. Detailed distribution, minor allele frequencies, HWE status and BIN structure information for all studied SNPs in controls and patients is provided in Table S1; and the information on 11 significantly associated SNPs along with their ORs and P values are presented in Table 1.
Fig. 1. A partial map of Chromosome-6q26 expanded to show the position and distribution of 11 significant SNPs (shown with rs numbers) in the regulatory region of the PARK2 and PACRG genes. 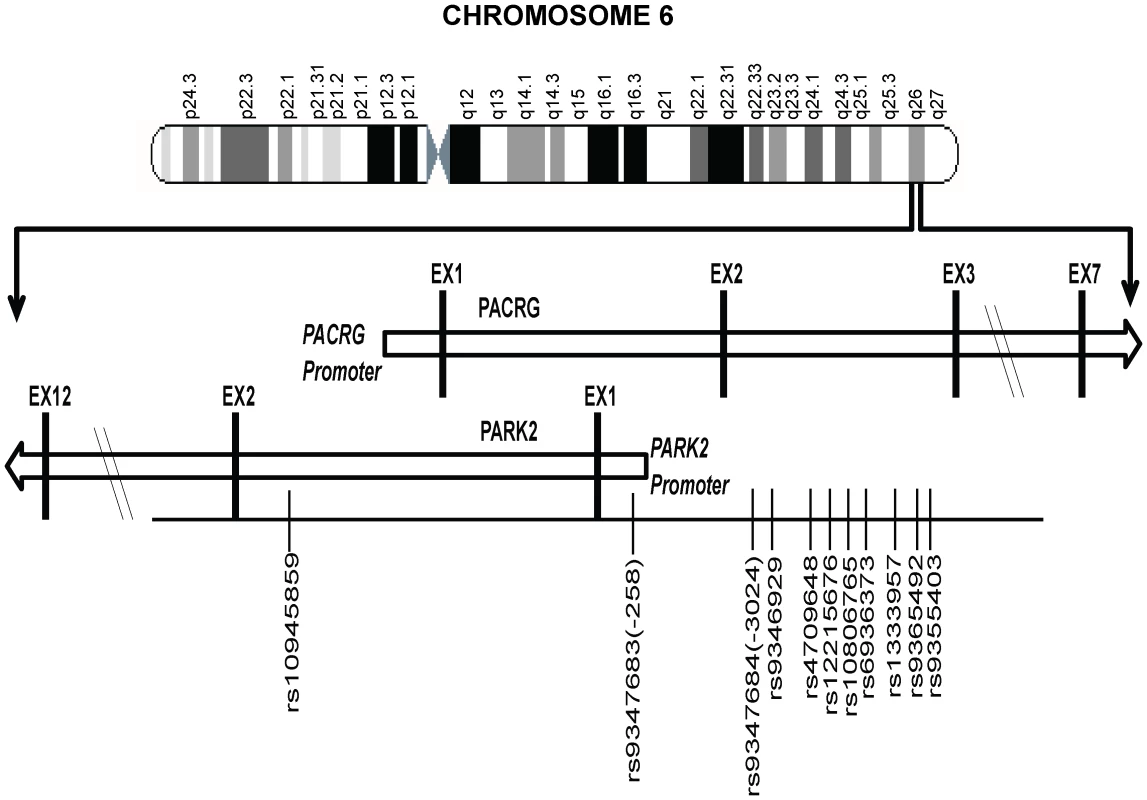
Tab. 1. Allele and genotype frequencies for 11 significant SNPs within PARK2 and PACRG gene regulatory region in two different cohorts of patients with Leprosy. 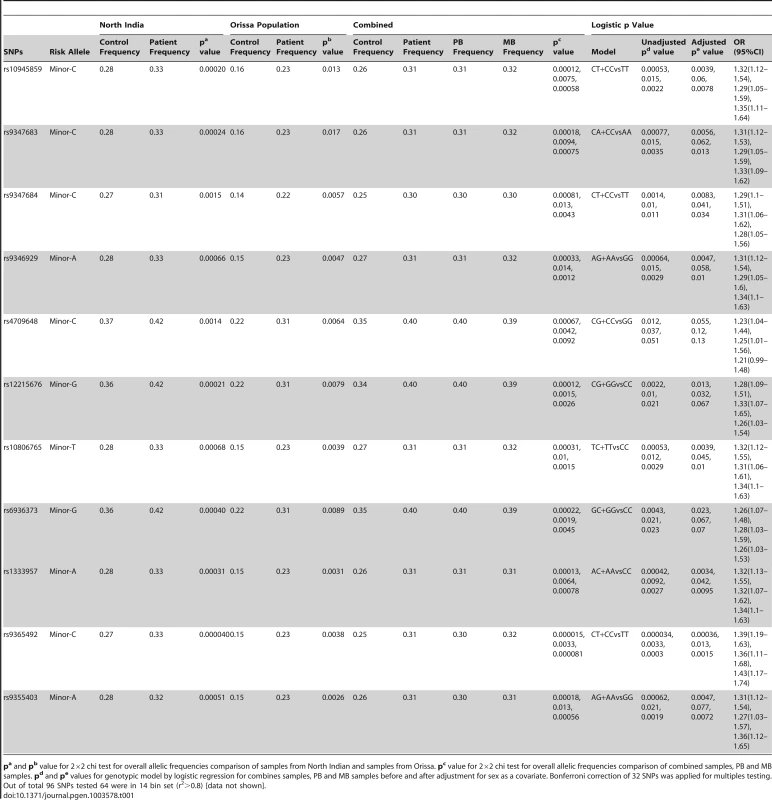
pa and pb value for 2×2 chi test for overall allelic frequencies comparison of samples from North Indian and samples from Orissa. pc value for 2×2 chi test for overall allelic frequencies comparison of combined samples, PB and MB samples. pd and pe values for genotypic model by logistic regression for combines samples, PB and MB samples before and after adjustment for sex as a covariate. Bonferroni correction of 32 SNPs was applied for multiples testing. Out of total 96 SNPs tested 64 were in 14 bin set (r2>0.8) [data not shown]. Eleven of the studied 96 SNPs showed a consistent and strong association with leprosy susceptibility, both in the North Indian and the East Indian-Orissa population groups. Ten out of 11 SNPs were located in the regulatory region of the PARK2 gene and a single SNP within the regulatory region of the PACRG gene (Figure 1). The observation made for the 11 SNPs on 2305 samples (829 leprosy patients and 1476 controls) from northern India was also made in a geographically unrelated Indian population of 380 individuals (184 leprosy patients and 196 controls) from Orissa in East India with a consistent association for SNP rs10945859, located 6.67 kb upstream of PACRG gene, rs9347683 (−258) within the core promoter region of PARK2 gene and SNPs rs9347684 (−3024), rs9346929, rs4709648, rs12215676, rs10806765, rs6936373, rs1333957, rs9365492, rs9355403, located within 63.8 kb upstream region of the PARK2 gene.
A combined analysis of the North Indian and East Indian-Orissa population groups confirmed the strong association for these 11 SNPs: rs10945859 (CC+CT vs. TT, OR = 1.32, 95% CI = 1.12–1.54, p = 5.30E-04); rs9347683(−258) (CC+CA vs. AA, OR = 1.31, 95% CI = 1.12–1.53), p = 7.70E-04); rs9347684 (CC+CT vs. TT, OR = 1.29, 95% CI = 1.10–1.51, p = 1.40E-03), rs9346929 (AA+GA vs. GG, OR = 1.31, 95% CI = 1.12–1.54, p = 6.40E-04), rs4709648 (CC+CG vs. GG, OR = 1.23, 95% CI = 1.04–1.44, p = 1.20E-02), rs12215676 (GG+CG vs. CC, OR = 1.28, 95% CI = 1.09–1.51, p = 2.20E-03), rs10806765 (TT+TC vs. CC, OR = 1.32, 95% CI = 1.12–1.55, p = 5.30E-04), rs6936373 (GG+GC vs. CC, OR = 1.26, 95% CI = 1.07–1.48, p = 4.30E-043), rs1333957 (AA+CA vs. CC, OR = 1.32, 95% CI = 1.13–1.55, p = 4.20E-04), rs9365492 (CC+TC vs. TT, OR = 1.39, 95% CI = 1.19–1.63, p = 3.40E-05), rs9355403 (AA+GA vs. GG, OR = 1.31, 95% CI = 1.12–1.54, p = 6.20E-04). The association of all 11 SNPs, involving the minor allele for the risk, was strong even after adjustment with sex as a covariate and the Bonferroni correction for multiple testing. A stepwise multivariate logistic regression analysis for eleven significantly associated SNPs along with the sex as a covariate in combined population showed retention of 2 out of 11 SNPs (rs9365492, p = 0.0033 and rs9355403, p = 0.024) in the model. In addition, analysis after dividing the patients in two known sub-types of the disease, i.e., pauci-bacillary (PB) and multi-bacillary (MB), both within North Indian and East Indian-Orissa population, showed a strong association of all the 11 SNPs with PB and MB form of the leprosy with a power >98%, MAF = 0.27 and OR = 1.44 in the North Indian and >50%, MAF = 0.15 and OR = 1.55 for East Indian-Orissa population (Table S2). The association with the MB sub-type in comparison to the PB form of the disease showed higher significance values. However, the heterogeneity testing between the PB and MB form of the leprosy did not show any significant difference between the two groups.
LD and Bin structure of studied SNPs in Indian population
Linkage Disequilibrium (LD) analysis of the studied SNPs in regulatory region of PARK2 and PACRG was performed using Haploview v4.2 in controls of North Indian and East Indian-Orissa population and compared with the Vietnamese. The detailed distribution of 96 SNPs in different BINs (for r2 cut off value ≥0.80) within North Indian and East Indian-Orissa population is provided in Table S1, which also includes 11 significantly associated SNPs as part of two BINs (BIN-1 with 8 and BIN-2 with 3 significantly associated SNPs) (Figure 2). The 3 significant SNPs (rs1333955, rs10806768, rs6915128) within our (North & East Indian–Orissa) and recently published North Indian (Agra) [29] study, grouped together in a single BIN-6 (Figure 3; Table S1), however, the significance in both the studies was marginal; and in our case was lost after Bonferroni correction.
Fig. 2. The association statistics of the 11 significant SNPs in the regulatory region of the PARK2 and PACRG genes; presented as negative logarithm of the <i>P</i>-Value and their linkage disequilibrium (LD) plot based on pairwise LD for r<sup>2</sup> cut off value ≥0.80. 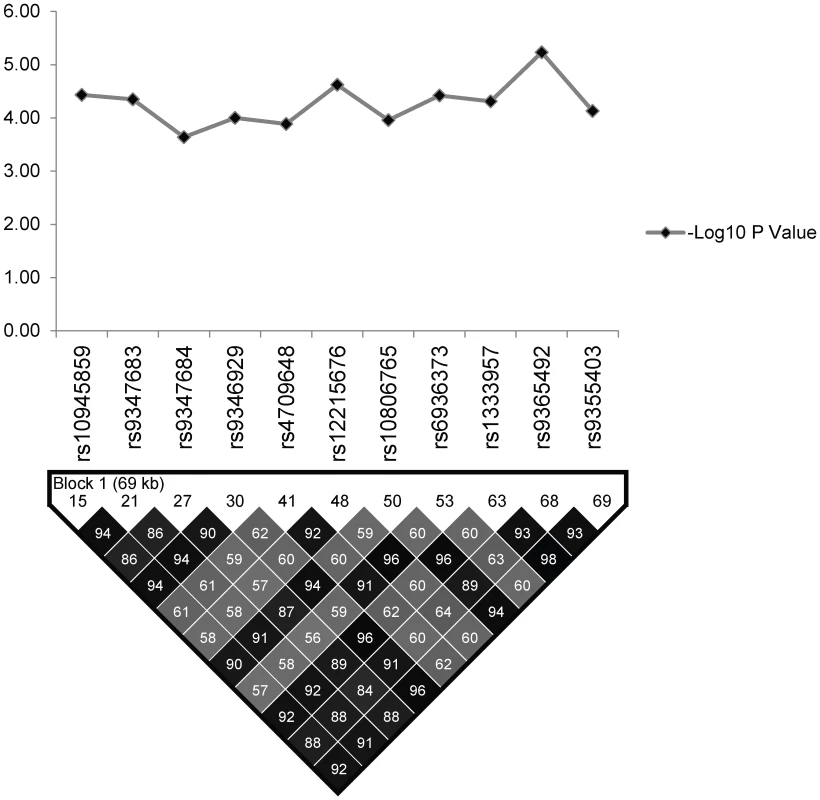
Fig. 3. A schematic lay-out of the BIN structure (r2≥0.80) in the regulatory region of the PARK2 and PACRG genes in North Indian and East Indian-Orissa and Vietnamese population for 41 SNPs spanning 148 Kb region of Chromosome 6q26, where 36 SNPs are common to both Vietnamese and Indian population and 5 significant SNPs (No. 20, 22, 23, 26, 32) are exclusively studied in the Indian population. 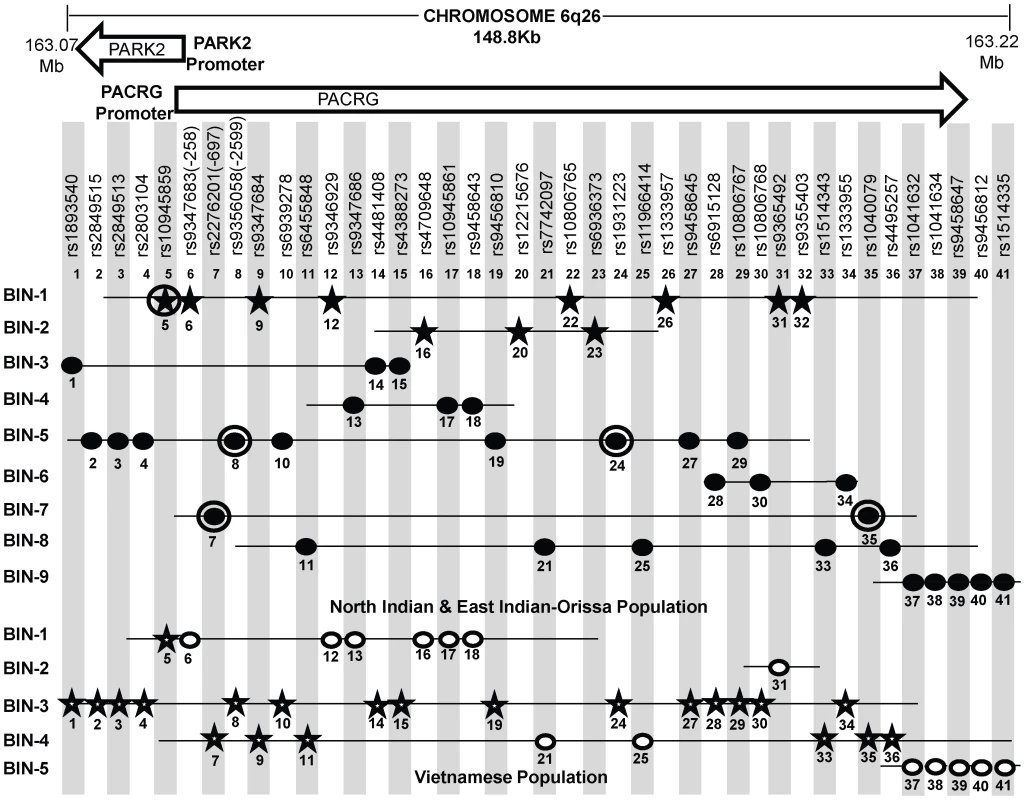
[It may be noted that a similar BIN structure was observed in the North-Indian and East-Indian-Orissa populations]. Physical location of the studied chromosomal region is given in Mb on top. Vietnamese population information of Mira et al, 2004 and common SNPs between Indian and Vietnamese population (Alter et al, 2012) was shared by Prof. Schurr. Rest of the SNPs & BIN structure information was retrieved from Alter et al (2012). SNPs in star shape indicate the significant association (in two respective populations-Indian and Vietnamese). 11 significantly associated SNPs in studied Indian populations are distributed in two BINs (BIN 1 with 8 and BIN 2 with 3 SNPs). Distribution of significant SNPs in Vietnamese population is shown in BIN 1, BIN 2 and BIN3. SNPs, rs10945859 (No. 1) and rs9347684 (No. 9), although shared significance in both the Indian and Vietnamese population, but these showed no significant difference in expression in in vitro reporter assay for the alternative alleles. Each SNP is designated by a No. ranging from 1 to 41 according to increasing order of the chromosomal position. Filled Black Star - Significant SNPs in Indian (North and East Indian-Orissa) population, Unfilled Star - Significant SNPs in Vietnamese population, Filled Black Dot - Non-Significant SNPs in North and East Indian-Orissa population, Unfilled Dot - Non-Significant SNPs in Vietnamese population, Black Circled Dot and Black Circled Star SNPs (No. 5, 7, 8, 24, 35) studied by us earlier [26]. In order to draw a parity between the studied SNPs for the overlapping regulatory region between PARK2 and PACRG genes in the Vietnamese and both groups of Indian populations (North and East Indian-Orissa), detailed information was sought for the Vietnamese samples. Information of 81 SNPs studied in the Vietnamese population [23] and 41 SNPs common between Indian (North, East Indian-Orissa) and Vietnamese as studied by Alter et al [29], was made available (courtesy Dr. Schurr) and rest of the studied SNP Bin structure information was retrieved from the supplementary files provided in the article. A comparison with 96 SNPs studied in Indians showed 36 SNPs common to both Vietnamese and Indian population and 5 significant SNPs exclusive to Indian population and not studied in the Vietnamese. The 5 SNPs were part of the 11 significantly associated SNPs observed in Indian samples; and the remaining 6 SNPs were part of the group of 36 SNPs common between Vietnamese and Indians. This allowed us to generate the BIN structure for 41 SNPs, which included 41 SNPs in Indian population and 36 SNPs for Vietnamese. The 11 significant SNPs observed in our (North & East Indian-Orissa) study were distributed in two BINs (8 in one BIN and 3 in another BIN) and rest of the 30 non-significant SNPs were distributed in seven other BINs. The BIN structure generated with the available information (Table S1) did not differ between our studied (North & East Indian-Orissa) samples and that of the North Indian (Agra) samples studied by the Alter et al [29] (data not shown).
However, the BIN structure generated for 36 SNPs in Vietnamese were distributed in five BINs (Figure 3). BIN-3 and BIN-4 in Vietnamese contained 15 and 8 SNPs, respectively to add up to 23, where 21 out of 23 SNPs were significantly associated in this population. However, 20 of these 21 SNPs were observed to be non-significant in Indian population groups studied, and constituted different BIN structures (BIN-3 to BIN-9). BIN-1 in Vietnamese population contained 7 SNPs, including the SNP rs10945859 located 6.67 kb upstream of PACRG gene, that was significantly associated both in Vietnamese and Indian population and BIN-2 of the Vietnamese population contained only single SNP, rs9365492. The 3 out of the 6 SNPs within the promoter region of PARK2 gene, located in BIN-1 and the single SNP, rs9365492 in BIN-2 were non-significant in Vietnamese population and showed significance in Indian population. Thus, comparing BIN-1 and BIN-2 in Vietnamese population with BIN-1 in Indian population, carrying 8 significantly associated SNPs; we found that 1 SNP in the BIN in Vietnamese and all the 8 SNPs in Indians showed a significant association with leprosy. However, the functional significance of the 2 common significant SNPs (rs10945859, rs9347684) between the two populations (Vietnamese and Indian) did not show any significant difference in expression in in vitro reporter assay for the alternative alleles (data not shown).
Haplotype analysis
Haplotype analysis (Tables 2–4), using haplostats software-the Haplotype 4, with risk alleles at all the 11 significantly associated SNP positions, showed an increased risk (OR = 1.36, p = 2.46E-06, Freqcontrols = 23%, Freqpatients = 29%) when compared to other haplotypes, generated for the 11 significantly associated SNPs in the combined Indian population (Table 2). A stepwise multivariate logistic regression analysis for 11 significantly associated SNPs (distributed in 2 BINs), keeping the sex as a covariate in combined Indian population, showed that 2 out of 8 SNPs (rs9365492, rs9355403) of BIN-1 were significant in the model. Thus BIN-1 remained most strongly associated with susceptibility to leprosy. Subsequently, we performed the phased analysis of SNPs in BIN-1 and BIN-2 to identify the haplotypes showing stronger association with leprosy (Tables 2–4). This was done to assay for combination of SNPs in either of the BINs providing more risk towards leprosy susceptibility. Haplotype 3 with risk alleles at all the 8 significantly associated positions provided an increased risk (OR = 1.34, p = 2.88E-06, Freqcontrols = 23%, Freqpatients = 29%) in comparison to other haplotypes generated in the combined Indian population (Table 3). Similarly, BIN-2 representing the Haplotypes of 3 significantly associated SNPs showed Haplotype 2 with risk alleles at all the 3 significantly associated positions, providing an increased risk (OR = 1.29, p = 7.56E-06, Freqcontrols = 34%, Freqpatients = 40%) in comparison to other haplotypes generated for the 3 significantly associated SNPs in the combined Indian population (Table 4).
Tab. 2. Haplotype structure, haplotype frequencies, significant p values and odds ratio between patients versus healthy controls of 11 significantly associated SNPs. 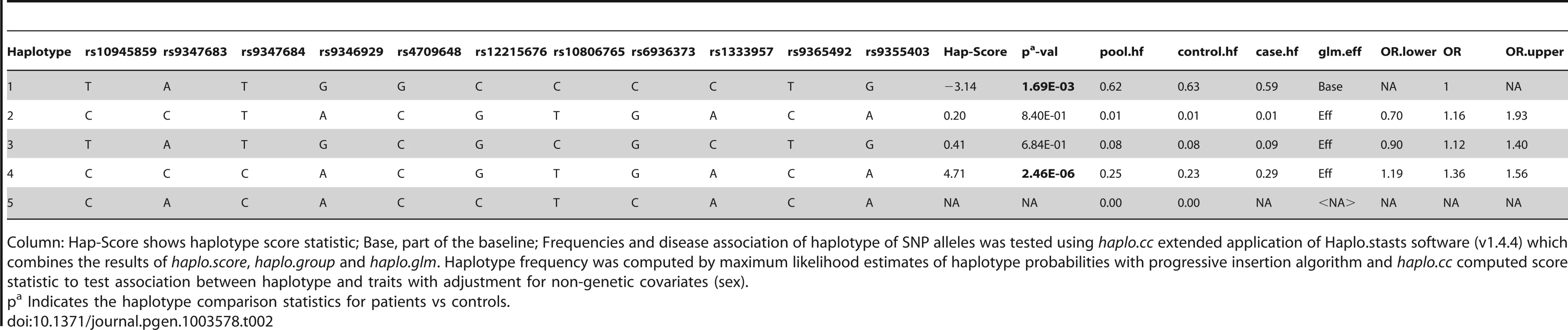
Column: Hap-Score shows haplotype score statistic; Base, part of the baseline; Frequencies and disease association of haplotype of SNP alleles was tested using haplo.cc extended application of Haplo.stasts software (v1.4.4) which combines the results of haplo.score, haplo.group and haplo.glm. Haplotype frequency was computed by maximum likelihood estimates of haplotype probabilities with progressive insertion algorithm and haplo.cc computed score statistic to test association between haplotype and traits with adjustment for non-genetic covariates (sex). Tab. 3. Haplotype structure, haplotype frequencies, significant p values and odds ratio between patients versus healthy controls of 8 SNPs representing BIN-1 of Indian population. 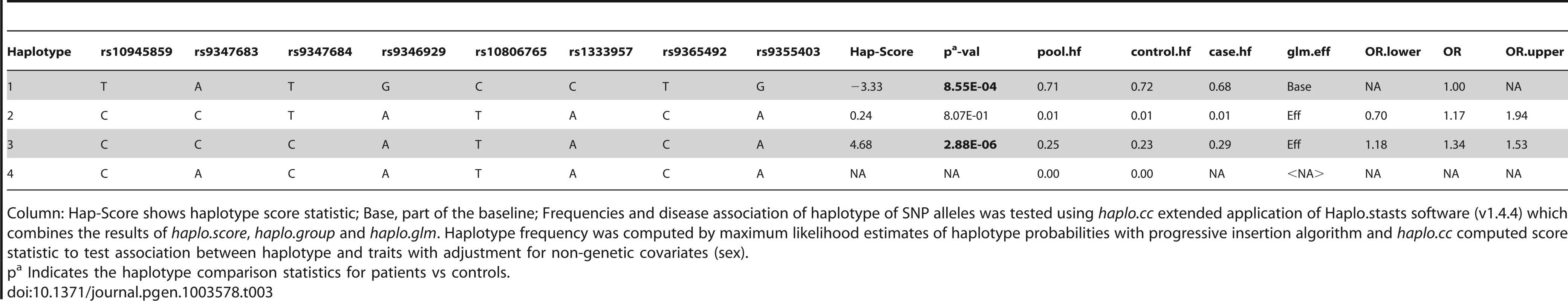
Column: Hap-Score shows haplotype score statistic; Base, part of the baseline; Frequencies and disease association of haplotype of SNP alleles was tested using haplo.cc extended application of Haplo.stasts software (v1.4.4) which combines the results of haplo.score, haplo.group and haplo.glm. Haplotype frequency was computed by maximum likelihood estimates of haplotype probabilities with progressive insertion algorithm and haplo.cc computed score statistic to test association between haplotype and traits with adjustment for non-genetic covariates (sex). Tab. 4. Haplotype structure, haplotype frequencies, significant p values and odds ratio between patients versus healthy controls of 3 SNPs representing BIN-2 of Indian population. 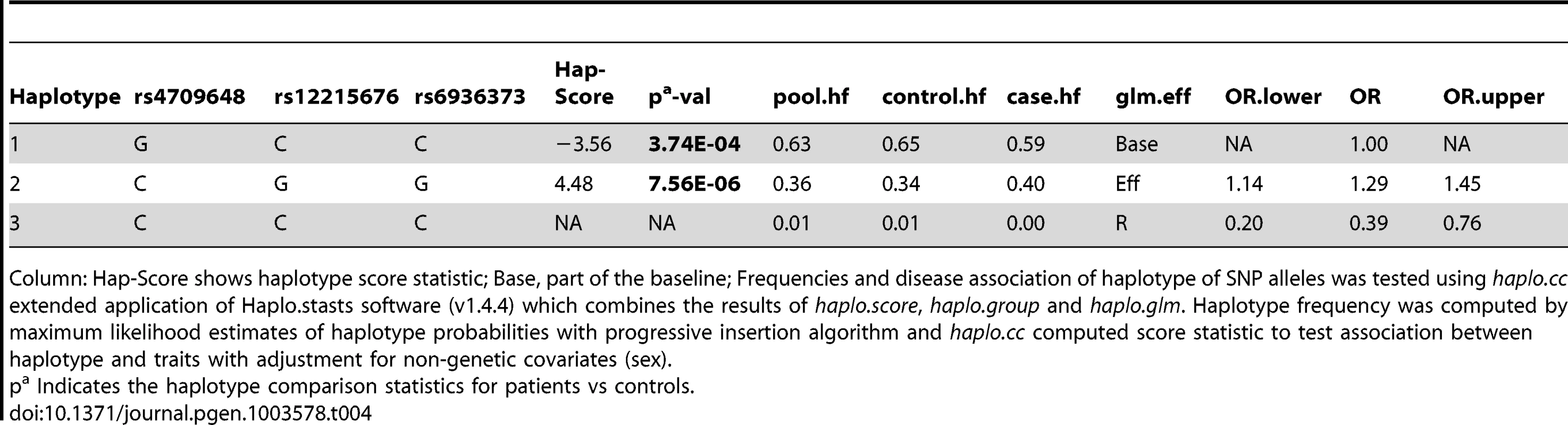
Column: Hap-Score shows haplotype score statistic; Base, part of the baseline; Frequencies and disease association of haplotype of SNP alleles was tested using haplo.cc extended application of Haplo.stasts software (v1.4.4) which combines the results of haplo.score, haplo.group and haplo.glm. Haplotype frequency was computed by maximum likelihood estimates of haplotype probabilities with progressive insertion algorithm and haplo.cc computed score statistic to test association between haplotype and traits with adjustment for non-genetic covariates (sex). Luciferase expression study for the SNPs significantly associated with the disease
Out of 11 significantly associated SNPs with leprosy in Indian population, only one core promoter SNP rs9347683 (−258) of PARK2 gene had been analysed functionally and documented in literature [30], [31]. None of the other SNPs in the region were studied earlier for their functional implication. The 2 SNPs (rs9365492 and rs9355403), 113 bp apart, lying within 63.8 kb upstream region of PARK2 gene; and two SNPs found significant in both Indian and Vietnamese population, SNP rs9347684 located within the 3.5 kb upstream region of the PARK2 gene and another SNP rs10945859 located 6.67 kb upstream of PACRG gene were chosen to assay their functional role and were cloned in the pGL3 promoter bearing luciferase-reporter expressing vector.
To test the enhancer activity of the SNPs, rs9365492 and rs9355403, the region bearing both the SNPs were cloned in pGL3 promoter vector in 4 allele combinations (Table S3). All 4 clones were transfected in 3 different cell lines: HepG2, MCF7 and HeLa. The result showed a lower expression for Clone2, Clone3 and Clone4 compared to Clone1 containing both SNPs as protective alleles (Figure 4). The expression was lowest in Clone 3 with rs9365492(T)-rs9355403(A), representing protective allele for SNP rs9365492 and risk allele for rs9355403. Bioinformatics analysis, using Tansfac-AliBaba2 tool [32] and HaploReg [33] (Collection from TRANSFEC, JASPER and protein-binding microarray experiments) databases revealed that the minor Risk alleles for both the SNPs, rs9365492 and rs9355403, affected the transcription binding site (Table S3).
Fig. 4. Luciferase expression assay of upstream SNPs of PARK2 gene (rs9365492 (T/C) and rs9355403 (G/A)): C & A respectively represent risk allele for the SNP. 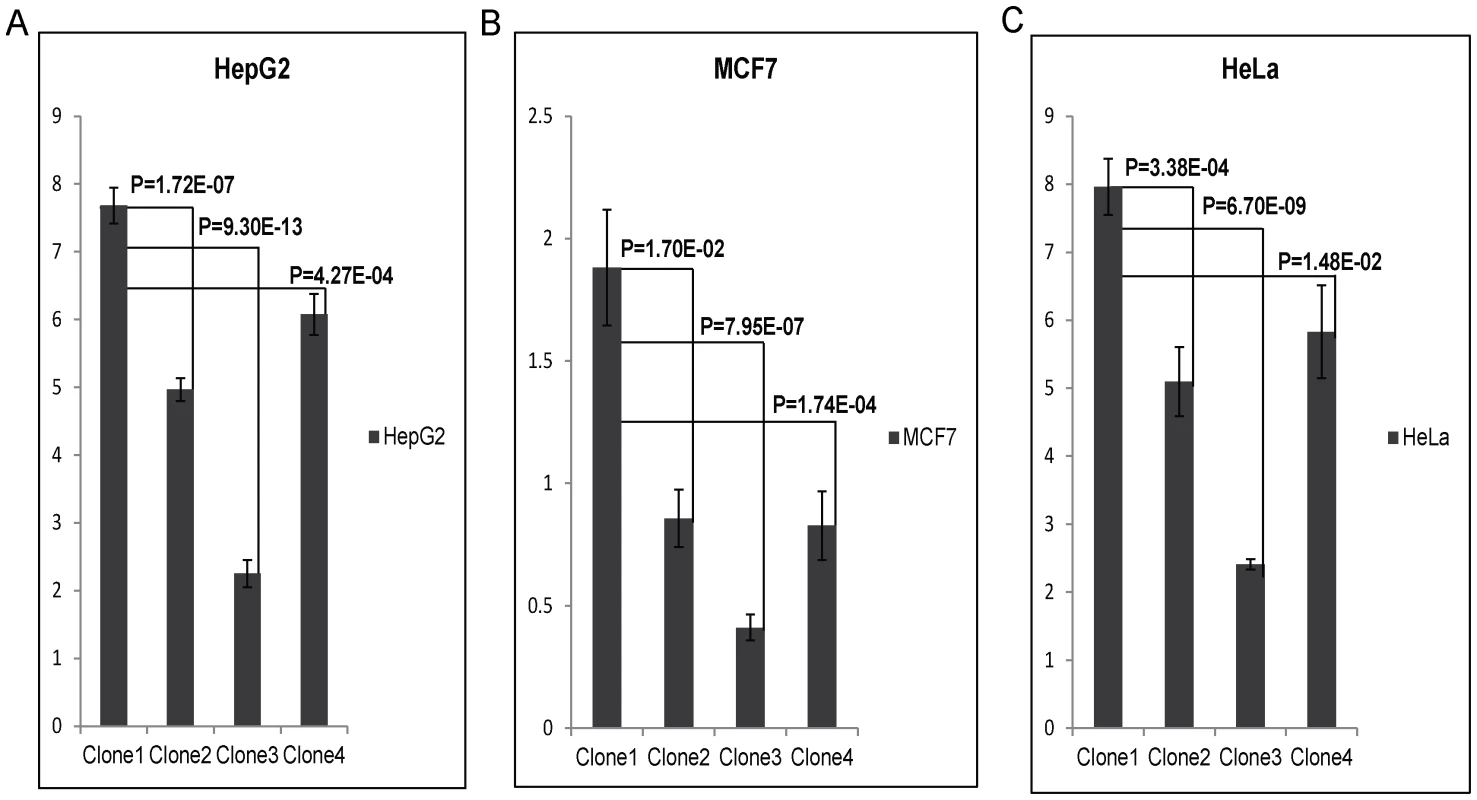
Bar with standard error shows the mean expression values in three different cell lines (HepG2, MCF7 and HeLa) for different Clones in PGL3 promoter vector: Clone1, with protective allele combination - rs9365492(T)-rs9355403(G); Clone2, with risk and protective allele combination - rs9365492(C)-rs9355403(G); Clone3, with protective and risk allele combination - rs9365492(T)-rs9355403(A) and Clone4, with risk allele combination - rs9365492(C)-rs9355403(A). P-Values for comparison of mean (one way ANOVA) expression between clones with different allele combination of 2 SNPs is also shown. SNP-rs9347684, located 3.5 kb upstream region of the PARK2 gene; and SNP-rs10945859, located within the 6.67 kb upstream region of PACRG gene, were cloned in pGL3 promoter vector to test for enhancer activity. Clone1 with rs9347684 protective T allele, Clone2 with risk C allele and similarly Clone1 with rs10945859 protective T allele and Clone2 risk C allele, did not show any significant change in the reporter gene expression in any of the 3 cell lines (data not shown).
Discussion
Leprosy continues to remain a major health problem in many parts of the world, regardless of long history of research, advances in the medical field and the introduction of Multidrug therapy (MDT) in 1980s. The inability to grow the bacterium in vitro has been one of the inadequacies to unravel the intricacies of the biology of the disease. Yet efforts have been made to identify the role of host genetic factors to understand susceptibility mechanisms, especially in the background of limited genetic diversity between different isolates of M. leprae. Research has progressed over the years in identifying many candidates as risk providers, using genome wide linkage, association and candidate gene studies. However, search for common genetic variants across the afflicted population groups in the world has emerged equivocal. Looking for genes and its variants which are proposed either by genome wide linkage or association studies with an assumed importance in the pathway biology of the disease does provide a window for re-search. More so when the LD maps for the relevant genomic regions are expected to differ from one population group to another, explaining the heterogeneity among associations.
The present study fine mapped the overlapping PARK2 and PACRG gene regulatory region to detect the variant(s) associated with Leprosy susceptibility in geographically distinct and unrelated Indian population groups. Since earlier studies did not succeed in replicating [26], [27] the association of the studied variants within this shared region of the genes with Leprosy; as was observed in Vietnamese and Brazilian population [23], it was pertinent to re-visit the region with sufficiently saturated number of SNPs. The purpose was to unravel any difference in LD structures and the heterogeneity in association in-between population groups. This assumption was based on the fact that involvement of PARK2/PACRG which made some relevance in understanding the patho-biology of leprosy in two unrelated populations of the world, i.e. Brazilians and Vietnamese, should have shown its involvement in the disease even in different ethnic groups of India, despite heterogeneity in association. If this were true, the nature of heterogeneity could be explained through differential LD structures, involving variants within the same gene. To answer this question it was appropriate to study the overlapping regulatory region saturated with 96 SNPs (nearly 1 SNP/Kb for PARK2 regulatory region) and compare the LD structure between the Indian and Vietnamese population.
The LD map of 96 SNPs (Table S1) in two geographically distinct and unrelated populations of India, included 2 BINs of the 11 Significant SNPs (Figure 2). Further, a comparison of Haplotypes generated with 11 significant SNPs associated with leprosy in Indians showed that Haplotype 4 (Table 2) with risk alleles at all the 11 SNP loci provided an increased risk (OR = 1.36, p = 2.46E-06) when compared to the Haplotypes generated (Table 3 and Table 4) after categorizing the 11 significant SNPs on the basis of BINs; BIN-1 with 8 and BIN-2 with 3 SNPs. The haplotype analysis and the expression profile for the studied significant SNPs in the PARK2 gene regulatory region confirmed that the risk allele for the significantly associated SNPs were responsible for an increased risk towards leprosy and the same risk SNP allele disrupted the transcription factor binding site in a bioinformatics analysis, confirmed further by a reduction in expression in an in-vitro reporter (luciferase) expression analysis.
In order to compare the SNP distribution within the overlapping regulatory region in Vietnamese and Indians, an LD map was generated of 41 SNPs, with 36 common to Indians and Vietnamese and 5 exclusive to Indians and not studied in Vietnamese (Figure 3, Table S1). Confining to the number of these SNPs, instead of what actually could have been compared, was due to the availability of the information in Vietnamese (courtesy Dr. Schurr) [23], [29]. Most of the significant SNPs in Vietnamese population were located in the region far below 3′ side of the PARK2 gene [29] and not located in the regulatory region of the PARK2 and the PACRG, the focus of our study. However, Alter et al [29] in their study found 3 SNPs (rs1333955, 10806768, rs6915128) located in the regulatory region of the PARK2 to be significantly associated both in Vietnamese and Indian (Agra) population. The same SNPs were found significant by us as well but the significance was marginal (Table S1) and was lost after Bonferroni correction. Also, the 2 SNPs rs10945859 and rs9365492 studied by Alter et al [29], representing Indian population of Agra, were common to our 11 significant SNPs in north and east India-Orissa populations, however, these did not turn out to be significant in Agra population studied from India. The reason possibly is the small sample size of their studied Indian (Agra) population or presumably some unknown methodological reason. We have confronted a similar experience earlier where we could not replicate the significant association of rs10945859 (Malhotra et. al. [26]) in leprosy susceptibility; and do find its involvement in a larger sample set using MassArray genotyping procedure. Further, having an information on missing SNPs in Vietnamese would provide in future an exact BIN structure for the regulatory region for comparison with information available from other and diverse Indian populations; which would throw additional light on the evolution of LD structures and the differences in unrelated populations, such as Vietnamese, Brazilian, Chinese, Indians, where heterogeneity among association for the genes have been reported for Leprosy disease. Incidentally, as expected all the studied samples from India either by us (North Indian comprising Delhi, U.P., Bihar and East Indian-Orissa) or Alter et al (Agra) showed an overlapping BIN structure with the available SNP information (Table S1) which differed from that of Vietnamese [23], [29] (Figure 3). Thus, there are no discrepancy in-between population groups within Northern part of India at least. The observations also replicated in East Indian-Orissa population with a power >50% of association, which could further increase with the increase in sample size. The homogeneity check using 61 individual identifying autosomal SNP markers [28] for the studied North Indian and East Indian-Orissa populations showed a compact cluster, suggesting the homogeneity between the studied populations (Figure S1). Moreover, a similar BIN structure was observed in the North-Indian and East Indian-Orissa populations. However, interestingly the variation in LD structure between the Indian and the Vietnamese population was apparent as one of the causes of genetic heterogeneity.
A comparison of the 36 common SNPs between Indian and Vietnamese population for the region, generated different BIN structures in the two populations (Figure 3). The 20 significant SNPs in Vietnamese population could not be replicated in Indians (Figure 3), supporting the heterogeneity in association in the two unrelated populations of the world. Also, the analysis of 2 common significant SNPs in-between Indian and Vietnamese populations, rs9347684 (3.5 kb upstream of the PARK2 gene) and rs10945859 (6.67 kb upstream of the PACRG gene), both part of 8 significant SNPs in BIN-1 in Indians, failed to show any functional significance in in-vitro reporter (luciferase) expression profiles obtained for the alternative variants. This probably suggests that the two potential SNPs common to the two populations do not have any functional bearing on the biological process critical to the disease development. The remaining 4 SNPs (rs9347683, rs9346929, rs4709648, rs9365492) out of 36 common SNPs with a significant association only in Indian population were part of BIN-1 and BIN-2 containing 11 significantly associated SNPs. Among these, 1 SNP of BIN-1 has been functionally defined as a core promoter SNP rs9347683 (−258) [30], [31]. The functional importance of this SNP was also reflected in the HaploReg database [33] (collection from TRANSFEC, JASPER and protein-binding microarray experiments). To find out if there was any other functional SNP within BIN-1 in Indian population to explain the heterogeneity among the populations, we selected most significant SNP, rs9365492 and another SNP 113 bp apart, rs9355403, one of these rs9365492 located in a separate BIN-2 in Vietnamese population and the other rs9355403 not studied by them [29], [30]. When checked through Bioinformatics analysis, the SNP positions were involved in the transcription factor binding (Table S3). Further comparison of both these SNPs with the close primates (Chimpanzee, Orangutan, Rhesus, Gorilla, Gibbon, Baboon), showed that the risk allele was absent in all the organisms and evaluation of allele frequencies between different population groups of the world showed the lowest frequency of the risk allele in the ancestral African population which kept increasing from European to Indians and Japanese (Table S4). In vitro reporter assays confirmed the involvement of the risk alleles in an enhancer like activity. The four possible haplotypes (Clones-1 to 4) of the two SNPs (rs9365492 and rs9355403) showed lower expression of the reporter gene for Clones 2 to 4 possessing risk alleles for either of the SNPs, when compared to Clone-1 (with protective alleles at both the SNP positions) (Figure 4). Lowest expression was observed for Clone-3. However, the expected combinations as designed in Clone-2 and Clone-3 of the haplotypes, were not observed in the patient and control samples studied. The overall analysis indicated a stronger repressing effect of the risk SNP allele rs9355403 in presence of the protective SNP allele rs9365492 in a haplotype when compared to other haplotype combinations. It is apparent from the differential expression results expected of PARK2 gene due to the SNP variations, how important it could turn out in driving immunological response against the bacterium in the primary host within Schwann cells and monocyte derived macrophages; by involving specific transcription factors in regulating the gene expression [23], which could further be validated in future studies by carrying out mobility/gel shift assays that would establish the exact role of theses SNPs in affecting transcription binding unequivocally.
Researchers have demonstrated parkin protein as a multi-functional protein with a likely role in proteolysis of damaged proteins. Other functions include its role in general protein turnover and several cellular functions as divergent as, cell cycle control, apoptosis and maintenance of mitochondrial function [34], [35]. Microarray expression of Drosophila parkin k/o model [36] has shown an increased expression of innate immune response genes. This indicates that parkin also plays an important role as an immune-regulatory molecule that contributes to down regulation of the immune responsiveness. In our study variant allele in the regulatory region of PARK2 gene is expected to reduce the expression of parkin protein, which in turn could contribute to the higher expression of the immune regulatory molecules [36]. The role of parkin protein in regulating the degradation of proteins involved in the immune response to M. leprae [37]–[39], support the preferential involvement in the susceptibility to multi-bacillary form of leprosy, as observed by us. Also various E3 ubiquitin ligase proteins act as suppressor molecules that limit IL-2 production and proliferation in anergic T-cell [40]. This conclusion is further supported by the fact that ubiqutin protein involved in the ubiqutination process is known to inhibit the production of the pro-inflammatory cytokine TNF-alpha and enhance the production of IL-4, IL-10, and IL-13 [41]–[46] leading to decreased CMI response towards the infectious agent. However, the mechanism underlying these effects need further work.
Materials and Methods
Ethics statement
The study was approved by the Institutional Ethics Review Board of JNU, as per the guidelines of Indian Council of Medical Research, India.
Subjects
A study was carried out in 2685 samples from two different cohorts (including 829 Leprosy patients from North India; 184 Patients from Orissa, in Eastern India; 1476 unrelated healthy control subjects from northern India; and 196 unrelated healthy control subjects from Orissa, Eastern India). Northern Indian samples were collected from Lok Nayak Jai Prakash Hospital, New Delhi, and from Guru Teg Bahadur Hospital, Delhi, and the Orissa (Eastern Indian) samples were collected from Cuttack Leprosy Home and Hospital, Orissa. Diagnosis of Leprosy was made by at least 2 independent leprologists after a physical examination of each patient and standard histological and pathological examination of the affected skin lesions. The patients group was classified as pauci-bacillary (PB) or multi-bacillary (MB) according to the Ridley and Jopling criteria [47]. The present study includes 452 Pauci-bacillary patients and 560 Multi-bacillary patients, with a mean age of 32.30±3.2 years (range 6–80 years). All these patients were under treatment with multidrug therapy (MDT) specific for multibacillary (MB) and paucibacillary (PB) leprosy, as recommended by the World Health Organization.
The study included the Control group with mean age of 35.97 years (range 3–82 years). None of the controls had any family history of tuberculosis, leprosy or any other related disease. A pre-informed written consent form, following the Indian Council of Medical Research (ICMR) norms, was obtained from all individuals whose blood sample was collected.
SNP selection and genotyping
To rule out the population stratification, we selected 61 individual identifying autosomal SNP markers [28] based on threshold heterogeneity >35%; Fst valve <0.06; Linkage Disequilibrium value (D')<0.011 and distribution among 52 different world populations.
To unravel the role of PARK2 and PACRG genes and to determine the contributory functional variants for leprosy susceptibility in the Indian population, we selected 96 SNPs from the shared regulatory genomic region of both the genes with a saturation of nearly 1 SNP per Kb for PARK2 gene regulatory region. SNP selection was carried out based on their minor allele frequency (>5%) in the publicly available database from the National Center of Biotechnology Information (NCBI) EntrezSNP (build 36) and the International HapMap project: [Han Chinese, Japanese (Asian populations), and African (Ancestral)] populations. SNPs were also included from the promoter, exonic, intronic boundary; and also chosen on the basis of their functional role as reported in literature.
The flanking sequences for all the SNPs were downloaded from the National Center of Biotechnology Information (NCBI) site. High-throughput genotyping of the SNPs was performed by the iPLEX Gold chemistry on the matrix-assisted laser desorption, ionization time of flight mass spectrometer (MALDI-TOF-Sequenom). SNPs with a call rate <90% were removed from the analysis. All the Significant SNPs had a call rate of >95%.
Statistical analysis
SNP genotype frequencies were subjected to Hardy-Weinberg equilibrium (HWE) analysis in patients and controls. SNPs with deviation (p<0.01) from HWE were removed from the study. Significant association of SNPs was tested by 3×2 and 2×2 Chi-square test for overall genotype and allele frequencies between leprosy patients and controls. SNPs with overall significance (p<0.05) were also confirmed by unconditional logistic regression analysis for different genotype models (recessive, dominant and co-dominant) and then corrected for age and sex. Bonferroni correction was also applied for multiple testing. SPSS software, version 17 (SPSS) was used for statistical analysis.
Frequencies and disease association of haplotypes was tested using haplo.cc extended application of Haplo.stasts software (v1.4.4). Linkage disequilibrium (LD) structure was determined using Haploview software, (version 4.2) [48]. To ensure adequate quality in statistical results in an association study, power of the study was calculated by Quanto software (v1.2.4.0) for the combined samples from Delhi and Orissa based on allele frequency and the effective size of the respective polymorphism.
To lower the risk of population stratification, MDS (multi-dimensional scaling) analysis was carried out, using Plink software, version 1.06 [49], [50]. For population differentiation analysis, Fst was calculated by the formula {FST = (HT−HS)/HT}, where HS and HT are the global heterozygosity indices over subpopulations (patients, control subjects, and 4 HapMap populations) and total population.
In vitro reporter expression analysis
Out of total 11 significantly associated SNPs, SNP rs10945859 located 6.67 kb upstream of the regulatory region of the PACRG gene, SNP rs9347684 located within the 3.5 kb upstream region of the PARK2 gene and two SNPs (113 bp apart) located within a 63.8 Kb upstream region of PARK2 gene, were assessed for their enhancer like activity. Amplicons of 633 bp bearing SNP rs10945859, 608 bp region containing SNP rs9347684 and 760 bp region containing the two SNPs (rs9365492 and rs9355403) were cloned into PGL3 promoter vector (Promega) carrying SV40 promoter and luciferase expression unit. Different combinations of SNP alleles were created into the PCR product and cloned into the vector to test for the functional analysis. SDM (site directed mutagenesis) was performed by the Stratagene mutagenesis kit. Phusion DNA polymerase (Finnzymes, Keilaranta, Espoo, Finland) was used for PCR amplification as well as for SDM of the cloned regions. Sequences of all the cloned inserts were confirmed by direct sequencing (Table S5). Plasmid DNA was isolated using the plasmid maxi kit (Qiagen Inc., Valencia, CA, USA) for transient transfection. ESCORT transfecting reagent was used to transfect HepG2, MCF7 and HeLa cells at a density of 1×105 cells per well in twelve-well plates and grown in Dulbecco-modified Eagle medium with 10% bovine calf serum overnight, prior to transfection. A total of 1 µg of vector construct and 0.1 µg of pRL-TK Renilla luciferase vector (Promega Corporation) with 2 µL of Escort (Sigma) were used for each transfection. Cells were collected 48 h after transfection and analyzed using the Dual-Luciferase Reporter Assay System (Promega). Luciferase activity was detected by luminometer (TD-20/20, DLReady; Turner Designs, Inc., Sunnyvale, CA, USA, and Promega Corporation). The pRL-TK vector that provided the constitutive expression of Renilla luciferase was co-transfected as an internal control to correct the differences in both transfection and harvest efficiencies. Transfections were carried out in triplicates and repeated at least thrice in independent experiments. Mean luciferase activity for the alleles of SNP was compared by one way ANOVA and presented in a bar diagram along with standard error.
Supporting Information
Zdroje
1. Hasting R, Opromolla D (1994) Pathology of Leprosy. 2nd edition. Edinburgh: Churchill Livingstone. pp 291.
2. WHO (2010) Global leprosy situation. Wkly Epidemiol Rec 85 : 337–348.
3. FinePE (1983) Natural history of leprosy–aspects relevant to a leprosy vaccine. Int J Lepr Other Mycobact Dis 51 : 553–555.
4. Quintana-MurciL, AlcaisA, AbelL, CasanovaJL (2007) Immunology in natura: clinical, epidemiological and evolutionary genetics of infectious diseases. Nat Immunol 8 : 1165–1171.
5. ModlinRL (1994) Th1–Th2 paradigm: insights from leprosy. J Invest Dermatol 102 : 828–832.
6. MonotM, HonoreN, GarnierT, AraozR, CoppeeJY, et al. (2005) On the origin of leprosy. Science 308 : 1040–1042.
7. ShieldsED, RussellDA, Pericak-VanceMA (1987) Genetic epidemiology of the susceptibility to leprosy. J Clin Invest 79 : 1139–1143.
8. ChakravarttiM, VogelF (1973) A twin study on leprosy. Topics in human genetics. Stuttgart, Germany: Georg Thieme Verlag 1 : 1–123.
9. AbelL, DemenaisF (1988) Detection of major genes for susceptibility to leprosy and its subtypes in a Caribbean island: Desirade island. Am J Hum Genet 42 : 256–266.
10. AbelL, VuDL, ObertiJ, NguyenVT, VanVC, et al. (1995) Complex segregation analysis of leprosy in southern Vietnam. Genet Epidemiol 12 : 63–82.
11. ToddJR, WestBC, McDonaldJC (1990) Human leukocyte antigen and leprosy: study in northern Louisiana and review. Rev Infect Dis 12 : 63–74.
12. WongSH, GochhaitS, MalhotraD, PetterssonFH, TeoYY, et al. (2010) Leprosy and the adaptation of human toll-like receptor 1. PLoS Pathog 6: e1000979.
13. ZhangFR, HuangW, ChenSM, SunLD, LiuH, et al. (2009) Genomewide association study of leprosy. N Engl J Med 361 : 2609–2618.
14. MoraesMO, CardosoCC, VanderborghtPR, PachecoAG (2006) Genetics of host response in leprosy. Lepr Rev 77 : 189–202.
15. ZhangF, LiuH, ChenS, WangC, ZhuC, et al. (2009) Evidence for an association of HLA-DRB1*15 and DRB1*09 with leprosy and the impact of DRB1*09 on disease onset in a Chinese Han population. BMC Med Genet 10 : 133.
16. AlcaisA, AlterA, AntoniG, OrlovaM, NguyenVT, et al. (2007) Stepwise replication identifies a low-producing lymphotoxin-alpha allele as a major risk factor for early-onset leprosy. Nat Genet 39 : 517–522.
17. SchuringRP, HamannL, FaberWR, PahanD, RichardusJH, et al. (2009) Polymorphism N248S in the human Toll-like receptor 1 gene is related to leprosy and leprosy reactions. J Infect Dis 199 : 1816–1819.
18. BochudPY, HawnTR, SiddiquiMR, SaundersonP, BrittonS, et al. (2008) Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J Infect Dis 197 : 253–261.
19. SiddiquiMR, MeisnerS, ToshK, BalakrishnanK, GheiS, et al. (2001) A major susceptibility locus for leprosy in India maps to chromosome 10p13. Nat Genet 27 : 439–441.
20. MiraMT, AlcaisA, Van ThucN, ThaiVH, HuongNT, et al. (2003) Chromosome 6q25 is linked to susceptibility to leprosy in a Vietnamese population. Nat Genet 33 : 412–415.
21. JamiesonSE, MillerEN, BlackGF, PeacockCS, CordellHJ, et al. (2004) Evidence for a cluster of genes on chromosome 17q11–q21 controlling susceptibility to tuberculosis and leprosy in Brazilians. Genes Immun 5 : 46–57.
22. ToshK, MeisnerS, SiddiquiMR, BalakrishnanK, GheiS, et al. (2002) A region of chromosome 20 is linked to leprosy susceptibility in a South Indian population. J Infect Dis 186 : 1190–1193.
23. MiraMT, AlcaisA, NguyenVT, MoraesMO, Di FlumeriC, et al. (2004) Susceptibility to leprosy is associated with PARK2 and PACRG. Nature 427 : 636–640.
24. ShimuraH, HattoriN, KuboS, MizunoY, AsakawaS, et al. (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25 : 302–305.
25. SchurrE, AlcaisA, de LeseleucL, AbelL (2006) Genetic predisposition to leprosy: A major gene reveals novel pathways of immunity to Mycobacterium leprae. Semin Immunol 18 : 404–410.
26. MalhotraD, DarvishiK, LohraM, KumarH, GroverC, et al. (2006) Association study of major risk single nucleotide polymorphisms in the common regulatory region of PARK2 and PACRG genes with leprosy in an Indian population. Eur J Hum Genet 14 : 438–442.
27. LiJ, LiuH, LiuJ, FuX, YuY, et al. (2012) Association study of the single nucleotide polymorphisms of PARK2 and PACRG with leprosy susceptibility in Chinese population. Eur J Hum Genet 20 : 488–489.
28. PakstisAJ, SpeedWC, FangR, HylandFC, FurtadoMR, et al. (2010) SNPs for a universal individual identification panel. Hum Genet 127 : 315–324.
29. AlterA, FavaVM, HuongNT, SinghM, OrlovaM, et al. (2012) Linkage disequilibrium pattern and age-at-diagnosis are critical for replicating genetic associations across ethnic groups in leprosy. Hum Genet
30. TanEK, PuongKY, ChanDK, YewK, Fook-ChongS, et al. (2005) Impaired transcriptional upregulation of Parkin promoter variant under oxidative stress and proteasomal inhibition: clinical association. Hum Genet 118 : 484–488.
31. WestAB, MaraganoreD, CrookJ, LesnickT, LockhartPJ, et al. (2002) Functional association of the parkin gene promoter with idiopathic Parkinson's disease. Hum Mol Genet 11 : 2787–2792.
32. MatysV, FrickeE, GeffersR, GosslingE, HaubrockM, et al. (2003) TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31 : 374–378.
33. WardLD, KellisM (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 40: D930–934.
34. MarinI, LucasJI, GradillaAC, FerrusA (2004) Parkin and relatives: the RBR family of ubiquitin ligases. Physiol Genomics 17 : 253–263.
35. Abou-SleimanPM, MuqitMM, WoodNW (2006) Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci 7 : 207–219.
36. GreeneJC, WhitworthAJ, AndrewsLA, ParkerTJ, PallanckLJ (2005) Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Hum Mol Genet 14 : 799–811.
37. ChenZJ (2005) Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol 7 : 758–765.
38. ChuangTH, UlevitchRJ (2004) Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol 5 : 495–502.
39. LiuYC, PenningerJ, KarinM (2005) Immunity by ubiquitylation: a reversible process of modification. Nat Rev Immunol 5 : 941–952.
40. MuellerDL (2004) E3 ubiquitin ligases as T cell anergy factors. Nat Immunol 5 : 883–890.
41. Garcia-CovarrubiasL, ManningEW3rd, SorellLT, PhamSM, MajetschakM (2008) Ubiquitin enhances the Th2 cytokine response and attenuates ischemia-reperfusion injury in the lung. Crit Care Med 36 : 979–982.
42. MajetschakM (2011) Extracellular ubiquitin: immune modulator and endogenous opponent of damage-associated molecular pattern molecules. J Leukoc Biol 89 : 205–219.
43. MajetschakM, KrehmeierU, BardenheuerM, DenzC, QuintelM, et al. (2003) Extracellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood 101 : 1882–1890.
44. PatelMB, MajetschakM (2007) Distribution and interrelationship of ubiquitin proteasome pathway component activities and ubiquitin pools in various porcine tissues. Physiol Res 56 : 341–350.
45. SainiV, RomeroJ, MarcheseA, MajetschakM (2010) Ubiquitin receptor binding and signaling in primary human leukocytes. Commun Integr Biol 3 : 608–610.
46. SinghM, RoginskayaM, DalalS, MenonB, KaverinaE, et al. (2010) Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res 86 : 20–28.
47. RidleyDS, JoplingWH (1966) Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 34 : 255–273.
48. BarrettJC, FryB, MallerJ, DalyMJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 : 263–265.
49. AggarwalS, AliS, ChopraR, SrivastavaA, KalaiarasanP, et al. (2011) Genetic variations and interactions in anti-inflammatory cytokine pathway genes in the outcome of leprosy: a study conducted on a MassARRAY platform. J Infect Dis 204 : 1264–1273.
50. AliS, ChopraR, AggarwalS, SrivastavaAK, KalaiarasanP, et al. (2012) Association of variants in BAT1-LTA-TNF-BTNL2 genes within 6p21.3 region show graded risk to leprosy in unrelated cohorts of Indian population. Hum Genet 131 : 703–716.
Štítky
Genetika Reprodukčná medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



