-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Anatomy of the Epidemiological Literature on the 2003 SARS Outbreaks in Hong Kong and Toronto: A Time-Stratified Review
Background:
Outbreaks of emerging infectious diseases, especially those of a global nature, require rapid epidemiological analysis and information dissemination. The final products of those activities usually comprise internal memoranda and briefs within public health authorities and original research published in peer-reviewed journals. Using the 2003 severe acute respiratory syndrome (SARS) epidemic as an example, we conducted a comprehensive time-stratified review of the published literature to describe the different types of epidemiological outputs.Methods and Findings:
We identified and analyzed all published articles on the epidemiology of the SARS outbreak in Hong Kong or Toronto. The analysis was stratified by study design, research domain, data collection, and analytical technique. We compared the SARS-case and matched-control non-SARS articles published according to the timeline of submission, acceptance, and publication. The impact factors of the publishing journals were examined according to the time of publication of SARS articles, and the numbers of citations received by SARS-case and matched-control articles submitted during and after the epidemic were compared. Descriptive, analytical, theoretical, and experimental epidemiology concerned, respectively, 54%, 30%, 11%, and 6% of the studies. Only 22% of the studies were submitted, 8% accepted, and 7% published during the epidemic. The submission-to-acceptance and acceptance-to-publication intervals of the SARS articles submitted during the epidemic period were significantly shorter than the corresponding intervals of matched-control non-SARS articles published in the same journal issues (p<0.001 and p<0.01, respectively). The differences of median submission-to-acceptance intervals and median acceptance-to-publication intervals between SARS articles and their corresponding control articles were 106.5 d (95% confidence interval [CI] 55.0–140.1) and 63.5 d (95% CI 18.0–94.1), respectively. The median numbers of citations of the SARS articles submitted during the epidemic and over the 2 y thereafter were 17 (interquartile range [IQR] 8.0–52.0) and 8 (IQR 3.2–21.8), respectively, significantly higher than the median numbers of control article citations (15, IQR 8.5–16.5, p<0.05, and 7, IQR 3.0–12.0, p<0.01, respectively).Conclusions:
A majority of the epidemiological articles on SARS were submitted after the epidemic had ended, although the corresponding studies had relevance to public health authorities during the epidemic. To minimize the lag between research and the exigency of public health practice in the future, researchers should consider adopting common, predefined protocols and ready-to-use instruments to improve timeliness, and thus, relevance, in addition to standardizing comparability across studies. To facilitate information dissemination, journal managers should reengineer their fast-track channels, which should be adapted to the purpose of an emerging outbreak, taking into account the requirement of high standards of quality for scientific journals and competition with other online resources.
: Please see later in the article for the Editors' Summary
Published in the journal: Anatomy of the Epidemiological Literature on the 2003 SARS Outbreaks in Hong Kong and Toronto: A Time-Stratified Review. PLoS Med 7(5): e32767. doi:10.1371/journal.pmed.1000272
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000272Summary
Background:
Outbreaks of emerging infectious diseases, especially those of a global nature, require rapid epidemiological analysis and information dissemination. The final products of those activities usually comprise internal memoranda and briefs within public health authorities and original research published in peer-reviewed journals. Using the 2003 severe acute respiratory syndrome (SARS) epidemic as an example, we conducted a comprehensive time-stratified review of the published literature to describe the different types of epidemiological outputs.Methods and Findings:
We identified and analyzed all published articles on the epidemiology of the SARS outbreak in Hong Kong or Toronto. The analysis was stratified by study design, research domain, data collection, and analytical technique. We compared the SARS-case and matched-control non-SARS articles published according to the timeline of submission, acceptance, and publication. The impact factors of the publishing journals were examined according to the time of publication of SARS articles, and the numbers of citations received by SARS-case and matched-control articles submitted during and after the epidemic were compared. Descriptive, analytical, theoretical, and experimental epidemiology concerned, respectively, 54%, 30%, 11%, and 6% of the studies. Only 22% of the studies were submitted, 8% accepted, and 7% published during the epidemic. The submission-to-acceptance and acceptance-to-publication intervals of the SARS articles submitted during the epidemic period were significantly shorter than the corresponding intervals of matched-control non-SARS articles published in the same journal issues (p<0.001 and p<0.01, respectively). The differences of median submission-to-acceptance intervals and median acceptance-to-publication intervals between SARS articles and their corresponding control articles were 106.5 d (95% confidence interval [CI] 55.0–140.1) and 63.5 d (95% CI 18.0–94.1), respectively. The median numbers of citations of the SARS articles submitted during the epidemic and over the 2 y thereafter were 17 (interquartile range [IQR] 8.0–52.0) and 8 (IQR 3.2–21.8), respectively, significantly higher than the median numbers of control article citations (15, IQR 8.5–16.5, p<0.05, and 7, IQR 3.0–12.0, p<0.01, respectively).Conclusions:
A majority of the epidemiological articles on SARS were submitted after the epidemic had ended, although the corresponding studies had relevance to public health authorities during the epidemic. To minimize the lag between research and the exigency of public health practice in the future, researchers should consider adopting common, predefined protocols and ready-to-use instruments to improve timeliness, and thus, relevance, in addition to standardizing comparability across studies. To facilitate information dissemination, journal managers should reengineer their fast-track channels, which should be adapted to the purpose of an emerging outbreak, taking into account the requirement of high standards of quality for scientific journals and competition with other online resources.
: Please see later in the article for the Editors' SummaryIntroduction
Emerging infectious diseases have become a major public health concern over the last two to three decades [1],[2]. When such an outbreak occurs, real-time collection, analysis, and dissemination of epidemiological information are key factors contributing to the effective and rapid control of the epidemic [3]. The first challenge for epidemiologists is to develop new surveillance and alert tools to detect in real time the events emerging anywhere in the world, and not just in highly developed countries [4],[5]. Upon detection of the outbreak, appropriate epidemiological studies should be launched immediately to help identify the causative agent, investigate the possible routes and modes of its transmission, define and validate diagnostic criteria, evaluate candidate treatments, forecast the spread of the epidemic, devise and evaluate evidence-based prevention, and monitor policies and strategies [6]–[8]. Hence, the outbreak of an emerging infectious disease causes a heavy epidemiological workload shared by public health specialists from national and international agencies, and academic epidemiologists. The importance of the rapid diffusion of public health information has long been recognized, and specialized international and national papers or Web bulletins are now made available during the course of outbreaks. However, journals remain the primary channel for communication of research. In this study, we analyzed the process according to which the results of academic epidemiological research are submitted and then formally published, during and after the outbreak of an emerging infectious disease. The proposed analysis concerns both the epidemiologists who submit their research to journals, and the journal editors who make decisions about the publication of the submitted research.
The 2003 severe acute respiratory syndrome (SARS) epidemic [9],[10] provided a model of an emerging infectious disease outbreak that occurred in the information-and-computerization age, which already has available real-time information systems, large databases, sophisticated statistical and mathematical analyses, and models. We studied the academic response to the SARS epidemic, and the resulting analysis may be viewed as a dissection of the scientific production on an epidemic—including the dynamics of this production in the course of the outbreak. Our analysis focused on four aspects: (1) distribution of the epidemiologists' workload among the different subspecialties and methodologies; (2) the characteristics of the epidemiological studies published in terms of design, data collection, statistical analysis, quality assurance, and quality control procedures used; (3) the timeline for the publication of the scientific results; and (4) the scientific impact of those publications as compared to control publications issued simultaneously.
Materials and Methods
Literature Search
We searched bibliographic databases for all published articles on epidemiology—defined as the study of the distribution and determinants of health-related states or events in specified populations, and the application of this study to control of health problems [11]—of the SARS epidemic in Hong Kong [12] and Toronto [13]. Hong Kong and Toronto were chosen because both cities, one in Asia and the other in North America, were strongly affected by the epidemic and have highly developed academic and public health infrastructures [14], and the results of the investigations performed on the outbreaks in these cities were almost exclusively published in English-language journals.
The entire literature identification–selection process is presented in Figure S1. Two literature searches were conducted: first, the MEDLINE database via PubMed and, second, the Science Citation Index Expanded and the Social Sciences Citation Index databases that we accessed via the Web of Science (Thomson Reuters). We searched for all journal articles written in English whose main studied subject was the 2003 SARS epidemic in Hong Kong and Toronto. Our study focused on articles published during the SARS epidemic and the 4 y thereafter (e.g., published between 1 January 2003 and 31 July 2007, assuming that most pertinent literature on an epidemic is produced within this period of time). We defined 5 July 2003, when the World Health Organization (WHO) reported that the last human chain of SARS transmission had been broken [15], as the end of the SARS epidemic.
The literature search was done in 2007, and updated on 11 February 2009. First, we used broad search equations to maximize retrieval sensitivity. Then, we finalized the search by removing all articles with at least one of the following seven exclusion criteria: (1) main study objective was not the study of SARS; (2) data analyzed in the study were not collected in Hong Kong or Toronto; (3) not a study on epidemiology (e.g., animal ecology); (4) not an original study (e.g., commentary, summary, review); (5) studies using only qualitative methodology, as defined by Critical Appraisal Skills Programme guidelines for qualitative research [16]; (6) study sample size ≤5; (7) full text of the article was unavailable: a request for a reprint of the article that we sent by e-mail to all corresponding authors of the articles for which we could not retrieve the full text through the Internet (either in open-access journals or in journals for which our institutions or the Inter-University Library of Medicine had a subscription) resulted in an absence of response. Two of the authors (WX and A-JV) independently applied the exclusion criteria to the same random sample of 100 articles. Good agreement was found between the two authors' selections (κ statistic: 0.86) [17]. Subsequently, review of the whole initial set of articles by the first author (WX) resulted in the final bibliographic database that was built with EndNoteX1 software (Thomson Reuters).
To compare the publication timelines of academic research articles with that of public health information, we also searched MEDLINE for public health reports mentioning the 2003 Hong Kong and/or Toronto SARS epidemic published in four public health bulletins during the SARS epidemic and the 4 y thereafter: Morbidity and Mortality Weekly Report for the Centers for Disease Control and Prevention (CDC), The Weekly Epidemiological Record and Bulletin of the World Health Organization for the WHO, and Canada Communicable Disease Report for the Public Health Agency of Canada. At the time of the SARS epidemic, Communicable Diseases Watch, a bulletin of the Centre for Health Protection, Department of Health of Hong Kong was not yet available (its first issue appeared on 13 June 2004). The literature search was done on 15 May 2009.
Data Analyzed
Articles were classified into four large categories (investigation and surveillance, case management, prevention and control, psychobehavior) and in 11 research domains (Table 1) on the basis of the main objective of the study, as stated by the authors. We conducted a detailed analysis of the study designs, and the information given by the authors on the statistical, informatics, and quality-control methods. A data-collection grid was devised (Table S1). For each retained article, we recorded the study type and design, the type and size of the sample population, the software used for data management and statistical analyses, and the quality assurance and quality-control processes described in their Materials and Methods. The collected data were coded in a relational database (Access, Microsoft Office 2003).
Tab. 1. Breakdown of the 11 major epidemiological research domains according to categories 1–4. 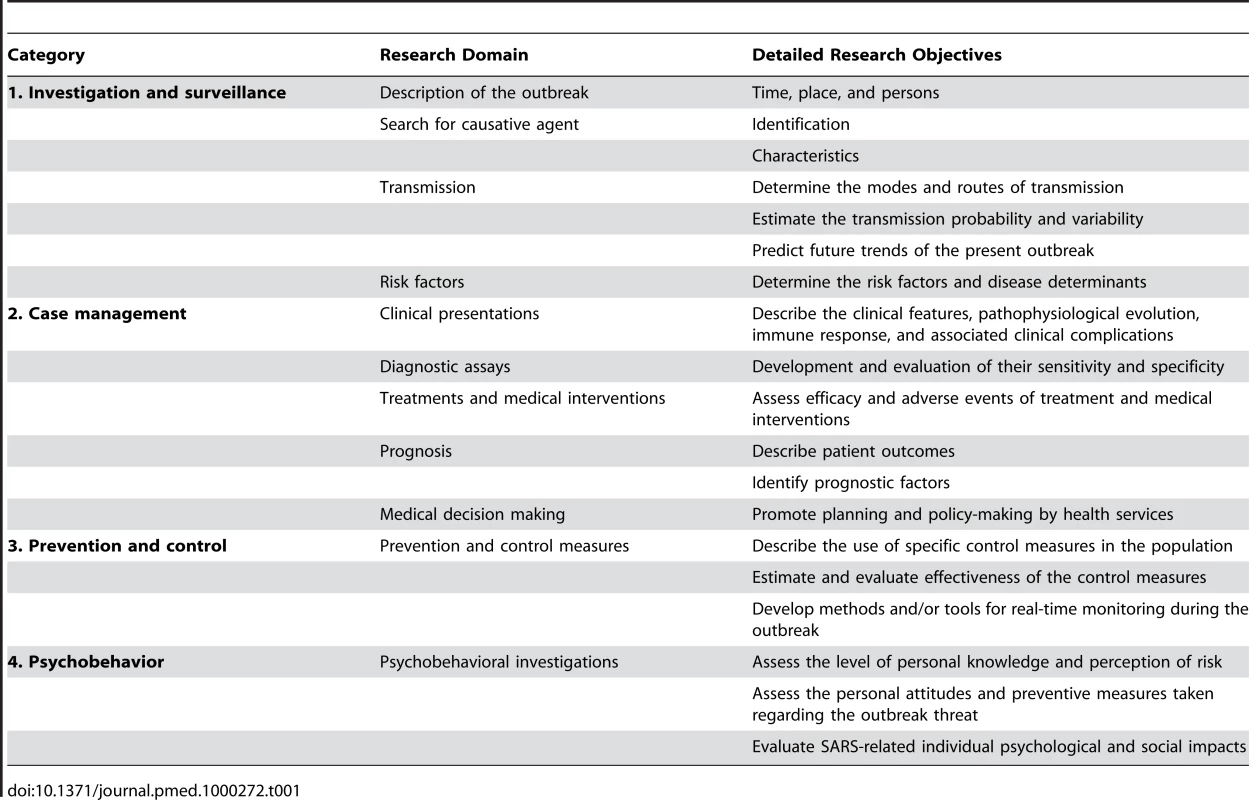
Publication Timeline and Citations of SARS Studies
The timeline of publication process of the articles and their citations were determined for articles whose submission, acceptance, and publication dates were known. Submission and acceptance dates were obtained from the information provided by the journal. The publication date was defined as the date of the article's full text availability: when the date of print publication and the date of online publication were both available, the publication date was defined as the earliest of these. When only the month of publication was available, the publication date was set at the 15th of that month. The numbers of citations of articles were those provided by the Web of Science (Thomson Reuters) on 12–13 January 2009.
In order to find a potential particular pattern of the SARS studies in terms of publication timeline and citations, as compared to comparable studies in other fields, two case-control studies were performed. The first compared the submission-to-acceptance-to-publication sequence for SARS-case versus control articles. The control articles were defined as the two research articles following the SARS-case article published in the same volume and issue of the journal that did not concern SARS. The second case-control study compared the numbers of SARS-case and control article citations. In this analysis, the control articles of a case article were all other research articles published in the same issue and volume of the journal. The two case-control studies were based on the SARS articles submitted within 2 y after the epidemic, and analyses were done separately for articles submitted during and after the epidemic.
In addition, we studied the timeline trend of impact factors of the journals in which the SARS articles had been published. Those impact factors were obtained in the Journal Citation Reports database (Thomson Reuters).
Statistical Analysis
The R statistical freeware (R version 2.6.1, The R Foundation for Statistical Computing) [18] software was used for obtaining all random samples and for all statistical analyses. The Kruskal-Wallis test was used to compare the medians of SARS articles' publication dates for the 11 research domains, and the submission-to-acceptance intervals, the acceptance-to-publication intervals, and the numbers of citations of SARS articles submitted during the epidemic and over the 2 y thereafter. The Kaplan-Meier method was used to represent the time-to-acceptance and the time-to-publication distributions, and then compared by the log-rank test. Cox models with a robust variance estimation that accounts for matching, were fitted to provide hazard ratio (HR) as a measure of the effect size, with 95% confidence interval (CI). The Wilcoxon signed-rank test was used to compare the distributions of the numbers of SARS article citations and of the citations of their corresponding control articles. Dispersions in individual values were expressed with interquartile range (IQR). The Jonckheere test [19] was used to assess the existence of a time trend for the median impact factor of journals in which SARS articles were published. In all tests, the statistical significance was defined as p≤0.05.
Results
Literature Search
The initial literature search provided 932 and 298 articles concerning the epidemic in Hong Kong and Toronto, respectively (see in Figure S1 the flowchart detailing the selection process of SARS articles). Applying the exclusion criteria resulted in the final selection of 263 and 58 articles for Hong Kong and Toronto, respectively. Because ten of the selected articles concerned the epidemic in both Hong Kong and Toronto, the final bibliographic database contained 311 different articles. Dates of online publication were obtained for 164 of the 311 SARS articles. Because ten articles had their online version posted after their print version, analyses involving publication dates were based on 154 online and 157 print publication dates. In addition, we selected a random sample of 100 out of the 263 articles dealing with the Hong Kong epidemic, and retained all articles dealing with the Toronto epidemic (n = 58) for further detailed analyses. These time-consuming analyses were performed on this subsample set of articles in order to facilitate feasibility. Because six studies concerned the epidemic in both cities, this final subset consisted of 152 articles. The detailed list of the whole set and subset articles (n = 311 and n = 152, respectively) is given in the Text S1.
The search of public health bulletins identified 29 SARS reports published on the Hong Kong and/or Toronto epidemic, among which 20 (69%) were published during the epidemic (see Figure S2): 12 in The Weekly Epidemiological Record, zero in Bulletin of the World Health Organization, two in Morbidity and Mortality Weekly Report, three in Canada Communicable Disease Report, and three others published first in Morbidity and Mortality Weekly Report and then in Canada Communicable Disease Report. These reports focused on the description of the outbreak situation (13/20), an update on the number of SARS cases (ten out of 20), prevention and control policies (ten out of 20), and descriptions of the clinical features or the case definition (seven out of 20). Such reports cannot obviously be considered as original peer-reviewed research articles and were not included in the studies related to the research domains or to citation impact.
Typology of Studies
The distributions of the 11 research domains addressed by the 311 articles are shown in Figure 1. The majority (52%) of the published studies were in the “case-management” category, with 16% concerning the assessment of diagnostic tests or criteria. “Investigation and surveillance” represented 23% of the studies; 19% were “psychobehavioral studies”; and 6% concerned “prevention and control.”
Fig. 1. Distribution of the 311 SARS epidemiology papers in the 11 research domains (see Table 1). 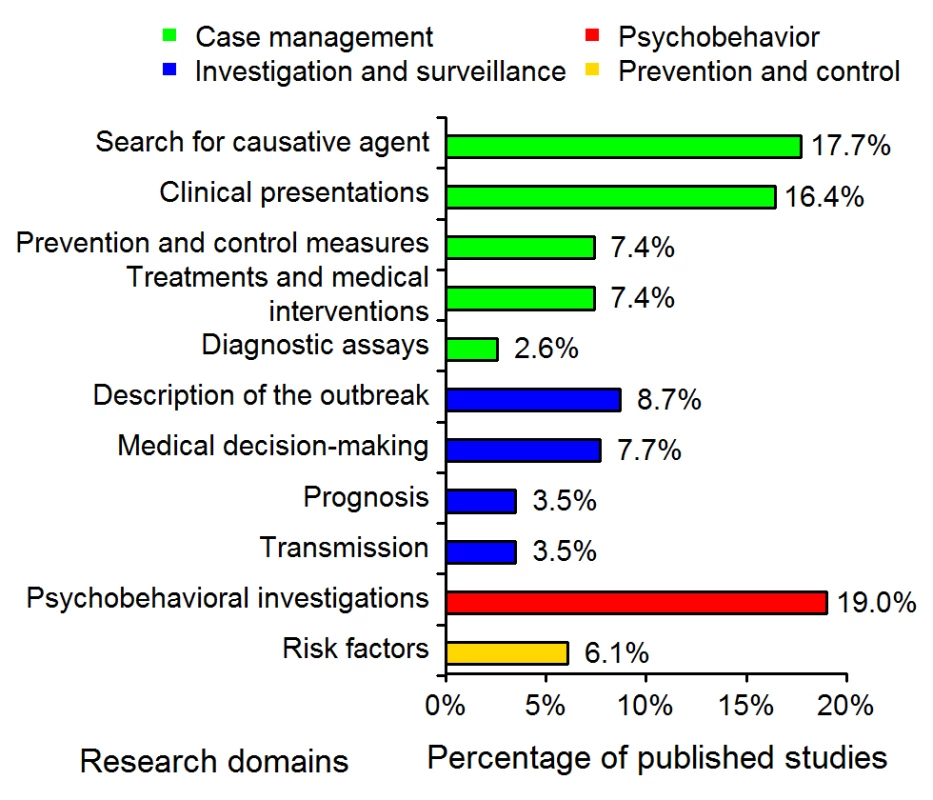
The studies corresponding to the “Case management” and “Investigation and surveillance” categories represented 52% and 23% of the 311 studies, respectively. The detailed analysis (Table 2) performed on the subset of 152 articles showed that the majority of the studies were descriptive epidemiology (54%), 42% were cohort studies, and 20% were cross-sectional studies. Case-control design and experimental studies were rare (5% and 6%, respectively). The majority of studies (70%) were conducted in hospitals. One-third of the studies used a questionnaire to collect data. Post mortem findings were reported in four articles. The use of data initially collected for another reason (hospital records, 13%) or secondary data analysis (i.e., the use of data already analyzed in previous studies, 32%) was frequent.
Tab. 2. Methodological characteristics of the published epidemiological studies on SARS. 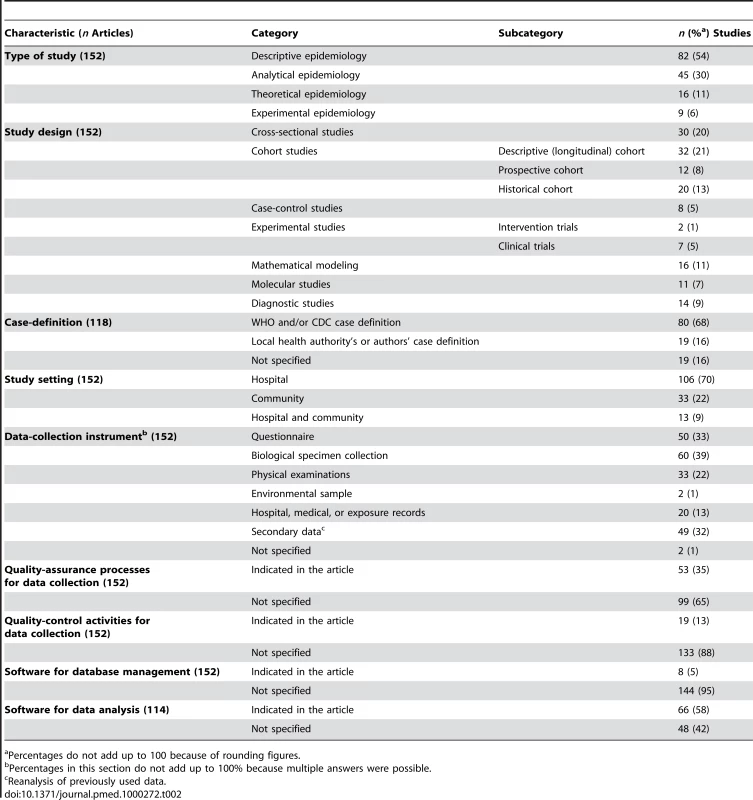
Percentages do not add up to 100 because of rounding figures. Study Population
The study population was composed of SARS patients in 118 (78%) studies, individuals from the general population in 20 (13%), non-SARS health care workers in 27 (18%), and other types (e.g., patients without SARS, households of SARS patients, and quarantined individuals) in 19 (12%) (Figure 2). The largest SARS-patient sample in a single study was 4,536 [20], obtained by pooling Hong Kong and Toronto patients with patients from other affected zones (e.g., Beijing, Singapore) to simulate strategies for controlling SARS outbreaks. The largest sample sizes for the other study population groups were: 12,000 for the general population [21] in a study estimating the seropositivity rate of the SARS coronavirus in the Hong Kong region; 37,174 for health care workers [22] in a study assessing the effectiveness of an herbal formula; and 8,044 for other populations [23] in a study estimating the ability of laboratory tests to discriminate SARS patients and patients with other causes of community-acquired pneumonia.
Fig. 2. Sample sizes used in the epidemiological studies on SARS (152 articles). 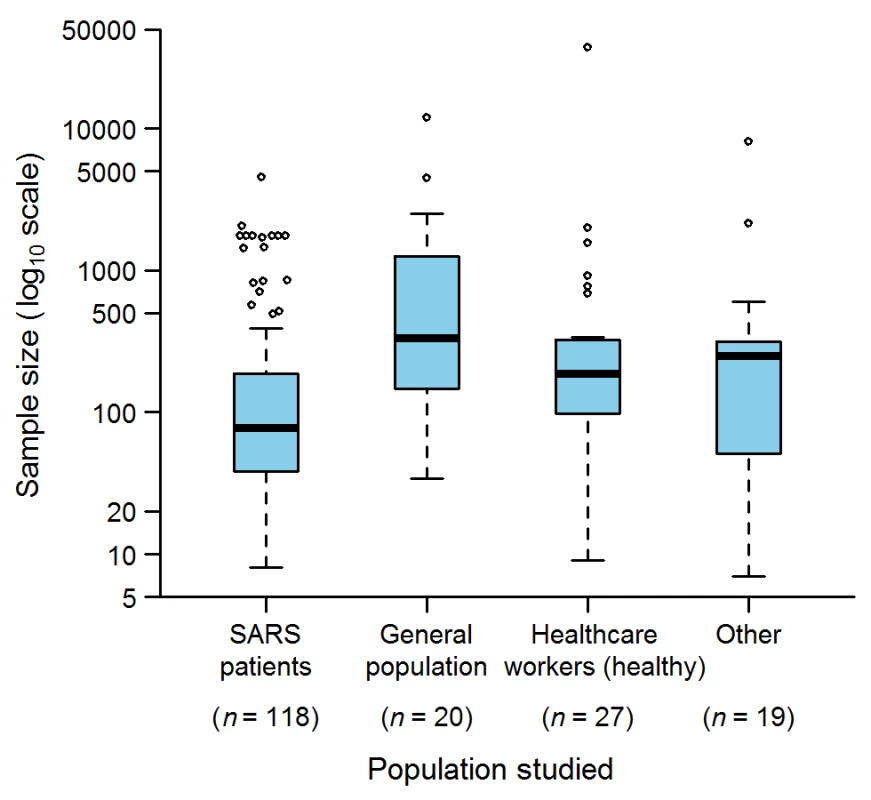
“Other” indicates studies in which patients without SARS, households of SARS patients, and quarantined individuals were studied. The sum of n is greater than 152 because several studies analyzed more than one population. Box-plot representation: The horizontal line inside the box represents the median; the lower and upper borders of the box represent the 25th and 75th percentiles, respectively; the whiskers correspond to extending to 1.5 times the box width (i.e., the IQR) from both ends of the box, and the circles represent values outside that interval. Whenever the minimum or maximum observed value is within the whisker interval, the alternative limit of the corresponding whisker becomes the corresponding minimum or maximum observed value. Quality Assurance and Quality Control
Among the 118 studies including SARS patients, 16% did not specify the SARS case-definition criteria applied, 68% used the WHO and/or CDC criteria, and 16% used the criteria established by authors or local health authorities. Among the 120 (79%) studies that collected original data, only two specified double-data entries and ten stated that they checked the data for errors through manual or systematic inspection. For 70% of the 30 studies with data on chest radiographs, the radiologists' evaluations had been double-blinded. Researchers reported using statistical or mathematical techniques to analyze the data in 114/152 (75%) studies. Among them, statistical methods were not described in seven (6%) articles, and the software used to analyze the data was not indicated in 48 (42%). The database-management software was given in only 5% of the 152 studies.
Journals Publishing SARS Articles
The 311 SARS articles comprising our entire database were published in 137 different journals. Among them, 50 (37%) journals published more than one SARS article. Three journals published more than ten articles: 32 (10%) publications appeared in Emerging Infectious Diseases, 11 (4%) in The Lancet, and 11 (4%) in Radiology. Seventeen studies were published during the epidemic. The first ten published studies appeared in The Lancet (7/10) and The New England Journal of Medicine (3/10). The next seven were published in The Journal of the American Medical Association (2/7), Clinical Chemistry (1/7), Science (1/7), British Medical Journal (2/7), and Canadian Medical Association Journal (1/7).
Publication Timeline
We derived the publication timeline for each of the research domains. Small percentages of articles were submitted or published for each research domain during the epidemic period. The maximum was 38% (21/55) of the articles in the category clinical presentations; the minimum was 3% (2/59) in the category psychobehavioral investigations. Only 10% (5/50), 17% (4/23), and 21% (4/19) of the articles dealing with diagnostic assays, treatments, and prevention and control, respectively, were submitted or published during the epidemic. The median publication dates for the different research domains (see Figure 3) differed significantly (p<0.001).
Fig. 3. Publication dates of SARS papers in the 11 research domains (311 articles). 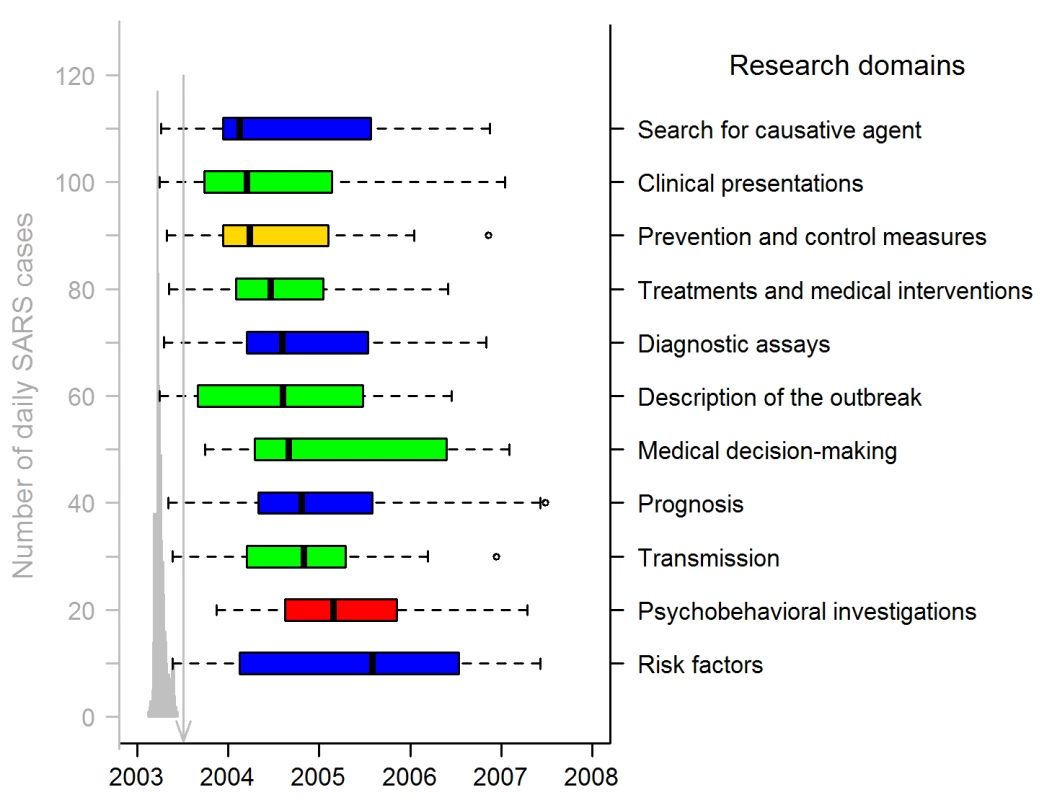
The gray graph on the left shows the timing of the Hong Kong and Canadian epidemics (sum of both daily numbers of SARS cases), with a peak corresponding to 117 and 114 SARS cases on 24 and 25 March 2003. Hong Kong SARS data were from the SARSID database [33]; Canadian data were from the Public Health Agency of Canada [34]. The vertical line points to 5 July 2003, the date WHO declared that the last human chain of transmission had been broken. Box-plot representation: as in Figure 2 except box representation is horizontal; the colors indicate the study categories (see insert in Figure 1; green, case management; blue, investigation and surveillance; red, psychobehavior; yellow, prevention and control). The date-of-submission distribution indicates that the academic response to the epidemic was rapid (Figure 4). In Hong Kong and Toronto, respectively, the 2003 SARS epidemic started on 7 and 15 March, and lasted until 23 June and 2 July [15]. On 31 March, a few weeks after the onset of the epidemic, the first articles reporting on the Hong Kong [24] and Toronto [13] outbreaks were both published online in the same journal. However, only a minority of the total number of SARS articles submitted (34/157, 22%), accepted (14/185, 8%), or published (21/311, 7%, with four, one, and 16 articles published only in an online version, only in a print version, and in both versions, respectively) were available to the scientific community up to and including 5 July, the end of the epidemic. The median date of article submission was 27 February 2004 (IQR: 30 July 2003–15 January 2005); their median acceptance and publication dates were 30 June (IQR: 02 December 2003–02 June 2005) and 16 September 2004 (IQR: 15 February 2004–15 July 2005), respectively, 124 and 202 d later. The median date of article print publication was 31 October 2004 (IQR: 15 February 2004–15 August 2005), 48 d after the median date of the earliest publication.
Fig. 4. Timeline of SARS epidemiology publications on the Hong Kong and Toronto epidemic. 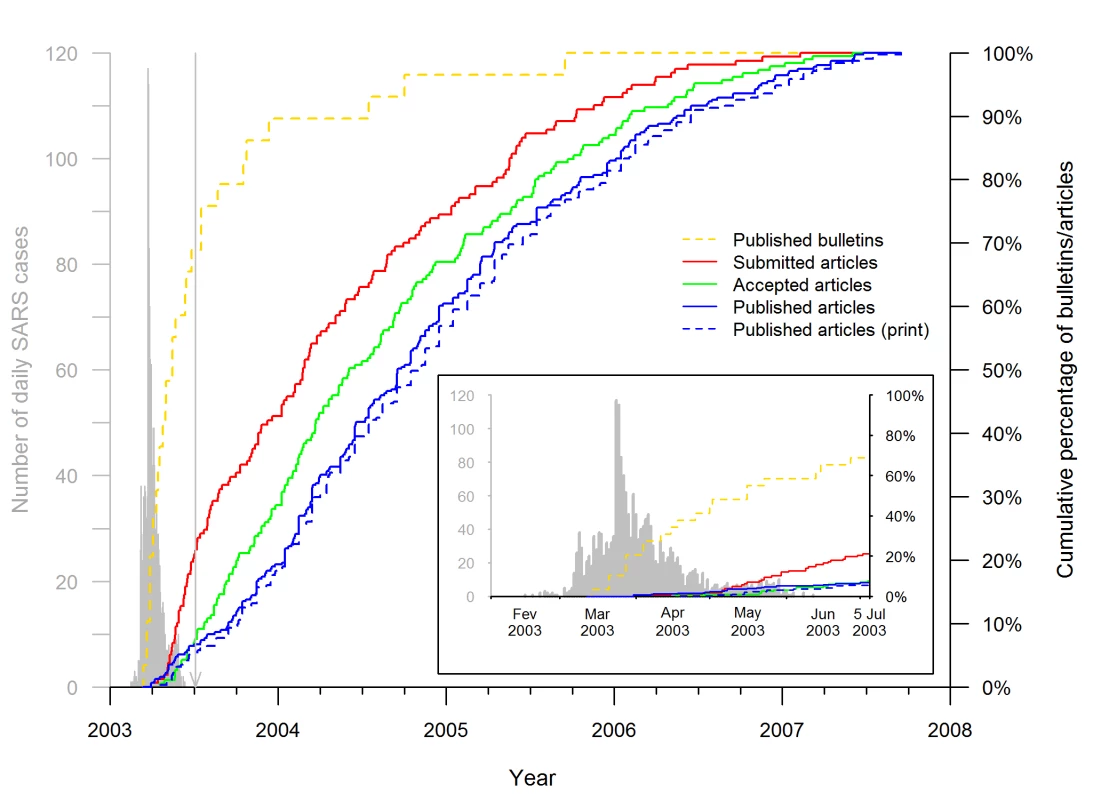
The curves (red, green, and both blue lines) show the cumulative distributions of the 311 articles published by 15 September 2007, according to the publication and acceptance or submission dates for the 185 and 157 articles, respectively, for which the information was available. The solid blue line shows the cumulative distribution of the publication dates, defined as the earliest date of publication, print or online. The dotted blue line shows the cumulative distribution of the print publication dates. The dashed yellow line shows the cumulative distributions of the 29 public health bulletins published by 15 September 2005 according to their publication dates. For comparison, the timing of the Hong Kong and Canadian epidemic is shown in gray on the left, as described in the legend to Figure 3. The vertical line points to 5 July 2003, the date WHO declared that the last human chain of transmission had been broken. The insert is a superposition of the publication timeline and course of the epidemic. Figure 5 shows the Kaplan-Meier curves representing the distributions of the submission-to-acceptance and acceptance-to-publication intervals for the SARS articles submitted during (left, Figure 5A and 5C, respectively) and after the epidemic (right, Figure 5B and 5D, respectively). The SARS articles submitted during the epidemic were accepted and published more rapidly than the non-SARS control articles (HR = 2.7, 95% CI 1.5–4.6, p<0.001 and HR = 1.7, 95% CI 1.1–2.5, p<0.01, respectively). The difference of median submission-to-acceptance intervals between SARS articles and their corresponding control articles was 106.5 d (95% CI 55.0–140.1) (Figure 5A); and the difference of median acceptance-to-publication intervals between SARS articles and their corresponding control articles was 63.5 d (95% CI 18.0–94.1) (Figure 5C). In contrast, the submission-to-acceptance and acceptance-to-publication intervals of the SARS articles submitted after the epidemic did not significantly differ from those of the control articles (p = 0.08 and p = 0.34, respectively). In addition, the submission-to-acceptance and acceptance-to-publication intervals for SARS articles submitted during the epidemic differed significantly from the corresponding intervals of SARS articles submitted over the 2 y thereafter (p<0.001 and p<0.01, respectively).
Fig. 5. Comparison of publication intervals for case and control articles during and after the SARS epidemic. 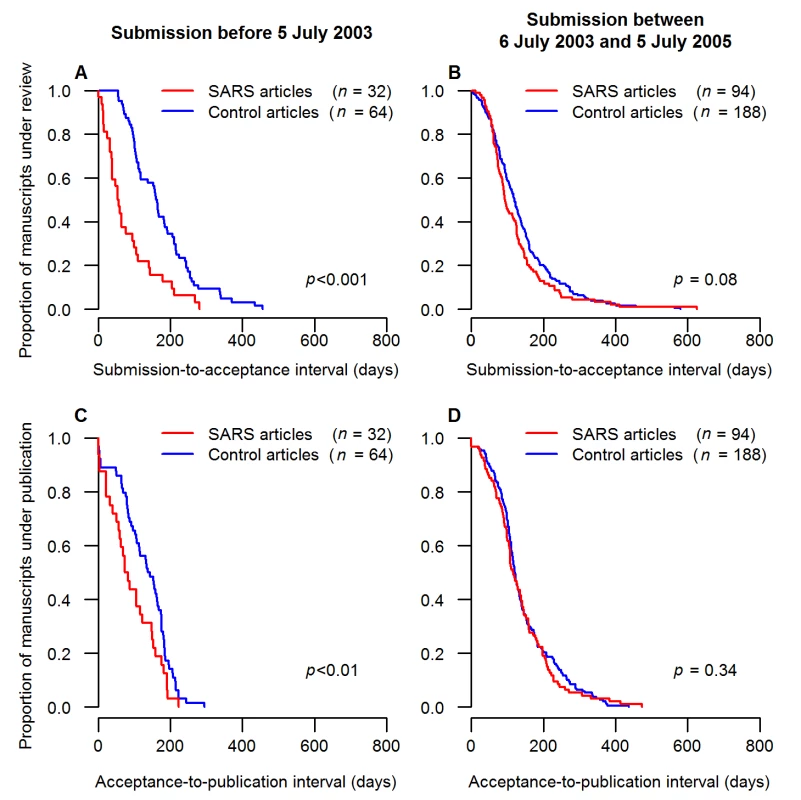
Submission, acceptance, and publication dates were available for 129 SARS articles submitted within 2 y (including 33 submitted during the epidemic), but were unavailable for three out of 129 couples of control. The Kaplan-Meier curves show the proportions of submitted manuscripts (ordinate) that took more than x d (abscissa) to be published. The comparisons of the submission-to-acceptance intervals between SARS articles and their control articles are shown for the SARS articles submitted (A) during the epidemic and (B) within 2 y after the end of the SARS epidemic. The comparisons of the acceptance-to-publication intervals between SARS articles and their control articles are shown for the SARS articles submitted (C) during the epidemic and (D) within 2 y after the end of the SARS epidemic. Journal Impact Factors and Article Citations
The impact factors were available for 130 journals in which 299 SARS articles were published; the remaining 12 articles were published in seven journals that were not indexed in the Journal Citation Report database. The impact factors of these 130 journals ranged between 0.437 and 50.017 (median of 4.131 [IQR: 2.51–6.50]). The impact factors of the journals in which the articles on SARS were published decreased significantly (p<0.0001) with publication dates (Figure 6). The case-control study on the citations received by the articles included 123 of the 129 case articles submitted within 2 y after the epidemic (33 during the epidemic, 90 after the epidemic) and 3,659 control articles, as the number of citations was not available for six of the 129 case articles submitted. The median numbers of citations of the SARS articles submitted during the epidemic and over the 2 y thereafter were 17 (IQR: 8.0–52.0) and eight (IQR: 3.2–21.8), respectively; this difference was significant (p<0.01). Considering these two periods, the number of SARS-article citations was significantly higher than the median number of citations of their control articles (15, IQR 8.5–16.5, p<0.05, and 7, IQR 3.0–12.0, p<0.01, respectively).
Fig. 6. Journal Impact factors according to the time of publication of the 311 selected articles. 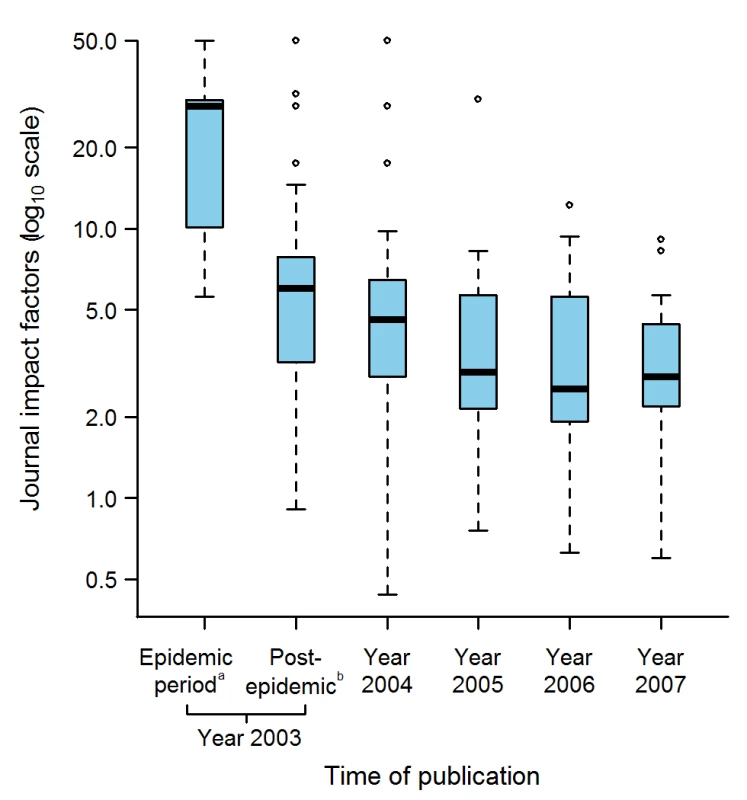
The journal impact factors were obtained for 130 journals in which 299 SARS articles were published, the remaining 12 articles being published in seven journals that were not indexed in the Journal Citation Report database. aUp to and including 5 July 2003, the date WHO declared that the last human chain of transmission had been broken. bBetween 6 July to 31 December 2003. Box-plot representation as described in Figure 2 legend: The horizontal line inside the box represents the median; the lower and upper borders of the box represent the 25th and 75th percentiles, respectively; the whiskers correspond to extending to 1.5 times the box width (i.e., the IQR) from both ends of the box, and the circles represent values outside that interval. Whenever the minimum or maximum observed value is within the whisker interval, the alternative limit of the corresponding whisker becomes the corresponding minimum or maximum observed value. Discussion
Herein we reported our analysis of the scientific literature on the epidemiology of the SARS epidemic in Hong Kong and Toronto taken as a model of an emerging infectious disease epidemic. We outlined the distribution of the workload among the traditional categories of epidemiological studies and methodologies. We showed that the time to disseminate study results could be quite long, in contrast to what would have been expected during a period of high public health alertness. The length of time to publish study results is dependent on several factors, which may classified as author-related (time to prepare the protocol, the questionnaires, to write the paper, and to choose the journal to submit to, and if rejected by this journal, to choose the next journals to submit to) or journal-related (time to find reviewers, to get reviews, to make a decision for publication, to publish). Our results indicate that the SARS articles submitted during the epidemic that were eventually published were more rapidly processed than control articles.
We chose to analyse a subset of the studies made on SARS, and to restrict our analysis to data that might be accurately quantified; in particular we only analyzed published papers. Hong Kong and Toronto accounted for only 2,005 (24%) of SARS cases out of a total of 8,422 cases worldwide. Nevertheless, Hong Kong and Toronto are indeed the cities with the highest number of cases in their respective continents, Asia and America. We also refrained from attempting to obtain qualitative data on each paper, as it could only have been done through surveying authors, editors, and journal managers to learn the numbers of previous submission of their papers (if any) by the authors, the nature of the decision process by editorial boards, etc. This information might be of great interest, but would result in low quality data, exhaustivity, and bias being highly dependent on the qualitative data on the papers. Therefore, we chose to analyse limited information, available in public databases, and set up a design allowing an unbiased comparison of SARS and control articles, and replication of the results.
Our detailed results showed that, during the outbreak of an emerging infectious disease, descriptive epidemiology predominated in the published literature (Table 2). Nearly four-fifths (78%, see Results, “Population Study”) of the published articles concerned studies on SARS patients conducted in a clinical setting. A large number of these investigations were devoted to assessing real-time diagnostic tools. At the same time, 22% of the retrieved articles did not concern SARS patients per se, reflecting the broader need of investigation in the community at-large when mitigation strategies are addressed. In particular, the results of our analysis highlighted the high proportion of published articles on psychobehavioral investigations (Figure 1): during such an outbreak, these investigations are indeed key elements for the implementation of control policies in the hospital and in the general population.
In large measure, the identified articles had relevance for public health authorities during the epidemic. However, only very few of such articles were submitted or published during the SARS epidemic (see Figure 4 and Results, “Publication Timeline”). Some public health information of immediate interest to the general public and health authorities and personnel were provided in a timely manner through specialized international or national public health bulletins: the first report published on the SARS epidemic mentioning Hong Kong and Vietnam was published on 14 March 2003 [25], 2 d after the first WHO alert concerning the SARS threat, and 69% of the public health information was published before the end of the SARS epidemic (Figure 4). However, our analysis of peer-reviewed journal articles (the main target of our study) quantified a worrying fact. Although academic articles providing data on this emerging disease received good impact scores in terms of the numbers of citations, they took a long time to be accepted and published (Figure 5), even though our findings demonstrated that journal managers had already accelerated the publication process during the epidemic (Figure 5A and 5C). The interval between submission and publication, and especially the acceptance-to-publication interval, is an important issue since many academic articles were aimed at solving practical clinical and epidemiological problems. The task of journal managers is difficult because speed to publication, although necessary, should not compromise review or the standard of quality of accepted articles. Our findings might encourage even more scientific journal managers to widely open online sections that could be activated upon detection of an emerging infectious disease outbreak, and possibly use a different review-publication system, so as to enhance the speed of publication. For example, several journals propose a specific section of manuscript submission involving dedicated fast management of those manuscripts that deserve rapid evaluation (e.g., “fast-track publication” in The Lancet, “rapid review” in The New England Journal of Medicine).
Visible changes in the dissemination of scientific information do not only concern speed of publication, they also acknowledge the technological and societal changes in the use of modern communication tools (Web 2.0). In 2007, the Nature Publishing Group launched Nature Precedings, which aims to rapidly disseminate, share, and discuss prepublication data [26]. More recently, the BMJ group launched a Web site on the 2009 H1N1/A pandemic flu [27] where articles, podcasts, learning modules, and other resources are available on a range of issues. PLoS Currents: Influenza is another such recent initiative [28] for rapid and open sharing of new scientific data, analyses, and ideas, the submissions being rapidly screened by a group of moderators who are leading researchers in the field. The rapid progress of information systems is likely to facilitate further similar initiatives. The methodology and the results of our study may be used in the future as a basis for estimating the impact of such changes in the publishing landscape in terms of speed of dissemination and quality.
Our results indicate that the publication delay is also heavily dependent on the researchers themselves: 78% of the articles analyzed in this study (i.e., only those eventually published) were submitted after the epidemic. In some instances, this delay could be explained by a previous unsuccessful submission to a high impact journal during the epidemic before the final submission, which is the only one that could be analyzed herein. However, the primary cause of the delay is undoubtedly the time necessary for the authors to design the study, acquire the data, and finalize the paper. This bottleneck could be reduced by developing a series of ready-to-use information technologies, to improve timeliness and, thus relevance, and further, to improve standardization, and thus comparability across studies in the event of an outbreak. Just as theoretical modelers have shifted to real-time approaches, for example, to estimate the basic/effective reproduction number of an epidemic [29], “field epidemiologists” should benefit from real-time tools for protocol writing and questionnaire design, and have them readily available on the Web so as to be prepared for the next emerging disease outbreak. Others, too, have advocated the need to prepare ready-to-use forms for clinical research on the 2009 H1N1/A pandemic [30]. Specific information systems dedicated to real-time research on the epidemic would provide valuable assistance for designers of future studies. Inspired by the guidelines recommended by STROBE [31] for observational studies, and CONSORT [32] for randomized controlled trials, such systems would offer standardized tools and protocols to assure adherence to good epidemiological practices. Our observations strongly suggest the need for coordinated international action dedicated to the development of such systems that, surprisingly, are not yet available in our information age.
The SARS outbreaks in Hong Kong and Toronto occurred nearly 7 y ago; and this amount of time was required to study the timeline of publications on these outbreaks, especially for analyzing citations of the SARS papers with an appropriate perspective. The approach we present here, which chronicles the academic response to an outbreak, will be useful to assess the changes in information dissemination that are underway.
Supporting Information
Zdroje
1. JonesKE
PatelNG
LevyMA
StoreygardA
BalkD
2008 Global trends in emerging infectious diseases. Nature 451 990 993
2. MorensDM
FolkersGK
FauciAS
2004 The challenge of emerging and re-emerging infectious diseases. Nature 430 242 249
3. ConnollyMA
2005 Communicable disease control in emergencies – a field manual. Geneva: World Health Organization. Available: http://www.who.int/infectious-disease-news/IDdocs/whocds200527/ISBN_9241546166.pdf. Accessed 16 May 2008
4. HeymannDL
RodierGR
2001 Hot spots in a wired world: WHO surveillance of emerging and re-emerging infectious diseases. Lancet Infect Dis 1 345 353
5. MykhalovskiyE
WeirL
2006 The Global Public Health Intelligence Network and early warning outbreak detection: a Canadian contribution to global public health. Can J Public Health 97 42 44
6. GoodmanRA
BuehlerJW
KoplanJP
1990 The epidemiologic field investigation: science and judgment in public health practice. Am J Epidemiol 132 9 16
7. ReingoldAL
1998 Outbreak investigations–a perspective. Emerg Infect Dis 4 21 27
8. U.S. Department of Health and Human Services 2006 Investigating an outbreak. Principles of epidemiology in public health practice. 3rd edition Atlanta Centers for Disease Control and Prevention Available: http://www.ihs.gov/medicalprograms/portlandinjury/pdfs/principlesofepidemiologyinpublichealthpractice.pdf. Accessed 10 March 2007
9. World Health Organization 2003 Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) Geneva Department of Communicable Disease Surveillance and Response, World Health Organization Available: http://www.who.int/csr/sars/en/WHOconsensus.pdf. Accessed 20 January 2007
10. AndersonRM
FraserC
GhaniAC
DonnellyCA
RileyS
2004 Epidemiology, transmission dynamics and control of SARS: the 2002–2003 epidemic. Philos Trans R Soc Lond B Biol Sci 359 1091 1105
11. LastJM
2001 A dictionary of epidemiology. 4th edition New York Oxford University Press
12. DonnellyCA
GhaniAC
LeungGM
HedleyAJ
FraserC
2003 Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 361 1761 1766
13. PoutanenSM
LowDE
HenryB
FinkelsteinS
RoseD
2003 Identification of severe acute respiratory syndrome in Canada. N Engl J Med 348 1995 2005
14. NaylorCD
ChantlerC
GriffithsS
2004 Learning from SARS in Hong Kong and Toronto. JAMA 291 2483 2487
15. World Health Organization. SARS outbreak contained worldwide. Available: http://www.who.int/mediacentre/news/releases/2003/pr56/en/. Accessed 4 February 2007
16. National CASP Collaboration for Qualitative Methodologies 2006 Critical Appraisal Skills Programme: 10 questions to help you make sense of qualitative research Oxford Public Health Resource Unit Available: http://www.phru.nhs.uk/Doc_Links/Qualitative%20Appraisal%20Tool.pdf. Accessed 16 April 2007
17. CohenJ
1960 A coefficient of agreement for nominal scales. Educ Psychol Meas 20 37 46
18. R Development Core Team 2007 R: A language and environment for statistical computing Vienna R Foundation for Statistical Computing Available: http://www.R-project.org
19. JonckheereAR
1954 A distribution-free κ-sample test against ordered alternatives. Biometrika 41 133 145
20. GumelAB
RuanS
DayT
WatmoughJ
BrauerF
2004 Modelling strategies for controlling SARS outbreaks. Proc Biol Sci 271 2223 2232
21. LeungDT
van MarenWW
ChanFK
ChanWS
LoAW
2006 Extremely low exposure of a community to severe acute respiratory syndrome coronavirus: false seropositivity due to use of bacterially derived antigens. J Virol 80 8920 8928
22. LauJT
LeungPC
WongEL
FongC
ChengKF
2005 The use of an herbal formula by hospital care workers during the severe acute respiratory syndrome epidemic in Hong Kong to prevent severe acute respiratory syndrome transmission, relieve influenza-related symptoms, and improve quality of life: a prospective cohort study. J Altern Complement Med 11 49 55
23. MullerMP
TomlinsonG
MarrieTJ
TangP
McGeerA
2005 Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community-acquired pneumonia? Clin Infect Dis 40 1079 1086
24. TsangKW
HoPL
OoiGC
YeeWK
WangT
2003 A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348 1977 1985
25. World Health Organization 2003 Acute respiratory syndrome. China, Hong Kong Special Administrative Region of China, and Viet Nam. Wkly Epidemiol Rec 78 73 74
26. Nature Publishing Group. Nature Precedings. Available: http://precedings.nature.com/. Accessed 16 January 2010
27. British Medical Journal (BMJ) Group. Pandemic flu. Available: http://pandemicflu.bmj.com/. Accessed 16 January 2010
28. Public Library of Science (PLoS). PLoS Currents: Influenza. Available: http://knol.google.com/k/plos/plos-currents-influenza/28qm4w0q65e4w/1%23#. Accessed 28 September 2009
29. CauchemezS
BoellePY
DonnellyCA
FergusonNM
ThomasG
2006 Real-time estimates in early detection of SARS. Emerg Infect Dis 12 110 113
30. TranTH
Ruiz-PalaciosGM
HaydenFG
FarrarJ
2009 Patient-oriented pandemic influenza research. Lancet 373 2085 2086
31. von ElmE
AltmanDG
EggerM
PocockSJ
GotzschePC
2007 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 4 e296 doi:10.1371/journal.pmed.0040296
32. CampbellMK
ElbourneDR
AltmanDG
2004 CONSORT statement: extension to cluster randomised trials. BMJ 328 702 708
33. LeungGM
HedleyAJ
HoLM
ChauP
WongIO
2004 The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med 141 662 673
34. Public Health Agency of Canada 2003 Epidemic curve of a SARS outbreak in Canada, February 23 to 2 July, 2003. Available: http://www.phac-aspc.gc.ca/sars-sras/pdf-ec/ec_20030808.pdf. Accessed 2 March 2008
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2010 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Suicide Prevention for Older Adults in Residential Communities: Implications for Policy and Practice
- Non-Communicable Diseases in Sub-Saharan Africa: The Case for Cohort Studies
- Early Pandemic Influenza (2009 H1N1) in Ho Chi Minh City, Vietnam: A Clinical Virological and Epidemiological Analysis
- Anatomy of the Epidemiological Literature on the 2003 SARS Outbreaks in Hong Kong and Toronto: A Time-Stratified Review
- Prognostic Significance of Subtype Classification for Short- and Long-Term Survival in Breast Cancer: Survival Time Holds the Key
- Negotiating Equitable Access to Influenza Vaccines: Global Health Diplomacy and the Controversies Surrounding Avian Influenza H5N1 and Pandemic Influenza H1N1
- Which New Approaches to Tackling Neglected Tropical Diseases Show Promise?
- Self-Injurious Behavior in Adolescents
- Markers of Dysglycaemia and Risk of Coronary Heart Disease in People without Diabetes: Reykjavik Prospective Study and Systematic Review
- Can Foreign Policy Make a Difference to Health?
- A Population-Based Evaluation of a Publicly Funded, School-Based HPV Vaccine Program in British Columbia, Canada: Parental Factors Associated with HPV Vaccine Receipt
- Genetic Markers of Adult Obesity Risk Are Associated with Greater Early Infancy Weight Gain and Growth
- New Complexities and Approaches to Global Health Diplomacy: View from the U.S. Department of State
- The Impact of Phenotypic and Genotypic G6PD Deficiency on Risk of Infection: A Case-Control Study amongst Afghan Refugees in Pakistan
- The Malawi Developmental Assessment Tool (MDAT): The Creation, Validation, and Reliability of a Tool to Assess Child Development in Rural African Settings
- Journals, Academics, and Pandemics
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Self-Injurious Behavior in Adolescents
- Non-Communicable Diseases in Sub-Saharan Africa: The Case for Cohort Studies
- The Malawi Developmental Assessment Tool (MDAT): The Creation, Validation, and Reliability of a Tool to Assess Child Development in Rural African Settings
- Journals, Academics, and Pandemics
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



