-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview
Background:
There is considerable debate as to the relative merits of using randomised
controlled trial (RCT) data as opposed to observational data in systematic
reviews of adverse effects. This meta-analysis of meta-analyses aimed to
assess the level of agreement or disagreement in the estimates of harm
derived from meta-analysis of RCTs as compared to meta-analysis ofobservational studies.
Methods and Findings:
Searches were carried out in ten databases in addition to reference checking,
contacting experts, citation searches, and hand-searching key journals,
conference proceedings, and Web sites. Studies were included where a pooled
relative measure of an adverse effect (odds ratio or risk ratio) from RCTs
could be directly compared, using the ratio of odds ratios, with the pooled
estimate for the same adverse effect arising from observational studies.
Nineteen studies, yielding 58 meta-analyses, were identified for inclusion.
The pooled ratio of odds ratios of RCTs compared to observational studies
was estimated to be 1.03 (95% confidence interval 0.93–1.15).
There was less discrepancy with larger studies. The symmetric funnel plot
suggests that there is no consistent difference between risk estimates from
meta-analysis of RCT data and those from meta-analysis of observational
studies. In almost all instances, the estimates of harm from meta-analyses
of the different study designs had 95% confidence intervals that
overlapped (54/58, 93%). In terms of statistical significance, in
nearly two-thirds (37/58, 64%), the results agreed (both studies
showing a significant increase or significant decrease or both showing no
significant difference). In only one meta-analysis about one adverse effectwas there opposing statistical significance.
Conclusions:
Empirical evidence from this overview indicates that there is no difference
on average in the risk estimate of adverse effects of an intervention
derived from meta-analyses of RCTs and meta-analyses of observational
studies. This suggests that systematic reviews of adverse effects should notbe restricted to specific study types.
:
Please see later in the article for the Editors' Summary
Published in the journal: Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview. PLoS Med 8(5): e32767. doi:10.1371/journal.pmed.1001026
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001026Summary
Background:
There is considerable debate as to the relative merits of using randomised
controlled trial (RCT) data as opposed to observational data in systematic
reviews of adverse effects. This meta-analysis of meta-analyses aimed to
assess the level of agreement or disagreement in the estimates of harm
derived from meta-analysis of RCTs as compared to meta-analysis ofobservational studies.
Methods and Findings:
Searches were carried out in ten databases in addition to reference checking,
contacting experts, citation searches, and hand-searching key journals,
conference proceedings, and Web sites. Studies were included where a pooled
relative measure of an adverse effect (odds ratio or risk ratio) from RCTs
could be directly compared, using the ratio of odds ratios, with the pooled
estimate for the same adverse effect arising from observational studies.
Nineteen studies, yielding 58 meta-analyses, were identified for inclusion.
The pooled ratio of odds ratios of RCTs compared to observational studies
was estimated to be 1.03 (95% confidence interval 0.93–1.15).
There was less discrepancy with larger studies. The symmetric funnel plot
suggests that there is no consistent difference between risk estimates from
meta-analysis of RCT data and those from meta-analysis of observational
studies. In almost all instances, the estimates of harm from meta-analyses
of the different study designs had 95% confidence intervals that
overlapped (54/58, 93%). In terms of statistical significance, in
nearly two-thirds (37/58, 64%), the results agreed (both studies
showing a significant increase or significant decrease or both showing no
significant difference). In only one meta-analysis about one adverse effectwas there opposing statistical significance.
Conclusions:
Empirical evidence from this overview indicates that there is no difference
on average in the risk estimate of adverse effects of an intervention
derived from meta-analyses of RCTs and meta-analyses of observational
studies. This suggests that systematic reviews of adverse effects should notbe restricted to specific study types.
:
Please see later in the article for the Editors' SummaryIntroduction
There is considerable debate regarding the relative utility of different study designs in generating reliable quantitative estimates for the risk of adverse effects. A diverse range of study designs encompassing randomised controlled trials (RCTs) and non-randomised studies (such as cohort or case-control studies) may potentially record adverse effects of interventions and provide useful data for systematic reviews and meta-analyses [1],[2]. However, there are strengths and weaknesses inherent to each study design, and different estimates and inferences about adverse effects may arise depending on study type [3].
In theory, well-conducted RCTs yield unbiased estimates of treatment effect, but there is often a distinct lack of RCT data on adverse effects [2],[4]–[7]. It is often impractical, too expensive, or ethically difficult to investigate rare, long-term adverse effects with RCTs [5],[7]–[16]. Empirical studies have shown that many RCTs fail to provide detailed adverse effects data, that the quality of those that do report adverse effects is poor [6],[17]–[31], and that the reporting may be strongly influenced by expectations of investigators and patients [32].
In general RCTs are designed and powered to explore efficacy [1],[3],[9],[30],[33]. As the intended effects of treatment are more likely to occur than adverse effects and to occur within the trial time frame, RCTs may not be large enough, or have a sufficient follow-up to identify rare, long-term adverse effects, or adverse effects that occur after the drug has been discontinued [1]–[3],[9],[13],[15],[16],[18],[19],[21],[26],[30],[33]–[53]. Moreover, generalisability of RCT data may be limited if, as is often the case, trials specifically exclude patients at high risk of adverse effects, such as children, the elderly, pregnant women, patients with multiple comorbidities, and those with potential drug interactions [1]–[3],[15],[38],[39],[41],[45],[46],[54]–[56].
Given these limitations it may be important to evaluate the use of data from non-randomised studies in systematic reviews of adverse effects. Owing to the lack of randomisation, all types of observational studies are potentially afflicted by an increased risk of bias (particularly from confounding) [8],[57] and may therefore be a much weaker study design for establishing causation [12]. Nevertheless, observational study designs may sometimes be the only available source of data for a particular adverse effect, and are commonly used in evaluating adverse effects [1],[9],[13],[52],[58],[59]. It is also debatable how important it is to control for confounding by indication for unanticipated adverse effects. Authors have argued that confounding is less likely to occur when an outcome is unintended or unanticipated than when the outcome is an intended effect of the exposure. This is because the potential for that adverse effect is not usually associated with the reasons for choosing a particular treatment, and therefore does not influence the prescribing decision [52],[59]–[62]. For instance, in considering the risk of venous thrombosis from oral contraceptives in healthy young women, the choice of contraceptive may not be linked to risk factors for deep venous thrombosis (an adverse effect that is not anticipated). Thus, any difference in rates of venous thrombosis may be due to a difference in the risk of harm between contraceptives [52],[62].
As both RCTs and observational studies are potentially valuable sources of adverse effects data for meta-analysis, the extent of any discrepancy between the pooled risk estimates from different study designs is a key concern for systematic reviewers. Previous research has tended to focus on differences in treatment effect between RCTs and observational studies [63]–[69]. However, estimates of beneficial effects may potentially be prone to different biases to estimates of adverse effects amongst the different study designs. Can the different study designs provide a consistent picture on the risk of harm, or are the results from different study designs so disparate that it would not be meaningful to combine them in a single review? This uncertainty has not been fully addressed in current methodological guidance on systematic reviews of harms [46], probably because the existing research has so far been inconclusive, with examples of both agreement and disagreement in the reported risk of adverse effects between RCTs and observational studies [1],[11],[15],[48],[51],[70]–[78]. In this meta-analysis of meta-analyses, we aimed to compare the estimates of harm (for specific adverse effects) reported in meta-analysis of RCTs with those reported in meta-analysis of observational studies for the same adverse effect.
Methods
Search Strategy
Broad, non-specific searches were undertaken in ten electronic databases to retrieve methodology papers related to any aspect of the incorporation of adverse effects into systematic reviews. A list of the databases and other sources searched is given in Text S1. In addition, the bibliographies of any eligible articles identified were checked for additional references, and citation searches were carried out for all included references using ISI Web of Knowledge. The search strategy used to identify relevant methodological studies in the Cochrane Methodology Register is described in full in Text S2. This strategy was translated as appropriate for the other databases. No language restrictions were applied to the search strategies. However, because of logistical constraints, only non-English papers for which a translation was readily available were retrieved.
Because of the limitations of searching for methodological papers, it was envisaged that relevant papers may be missed by searching databases alone. We therefore undertook hand-searching of selected key journals, conference proceedings, and Web sources, and made contact with other researchers in the field. In particular, one reviewer (S. G.) undertook a detailed hand search focusing on the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) to identify systematic reviews that had evaluated adverse effects as a primary outcome. A second reviewer (Y. K. L.) checked the included and excluded papers that arose from this hand search.
Inclusion Criteria
A meta-analysis or evaluation study was considered eligible for inclusion in this review if it evaluated studies of more than one type of design (for example, RCTs versus cohort or case-control studies) on the identification and/or quantification of adverse effects of health-care interventions. We were principally interested in meta-analyses that reported pooled estimates of the risk of adverse effects according to study designs that the authors stated as RCTs, as opposed to analytic epidemiologic studies such as case-control and controlled cohort studies (which authors may have lumped together as a single “observational” category). Our review focuses on the meta-analyses where it was possible to compare the pooled risk ratios (RRs) or odds ratios (ORs) from RCTs against those from other study designs.
Data Extraction
Information was collected on the primary objective of the meta-analyses; the adverse effects, study designs, and interventions included; the number of included studies and number of patients by study design; the number of adverse effects in the treatment and control arm or comparator group; and the type of outcome statistic used in evaluating risk of harm.
We relied on the categorisation of study design as specified by the authors of the meta-analysis. For example, if the author stated that they compared RCTs with cohort studies, we assumed that the studies were indeed RCTs and cohort studies.
Validity assessment and data extraction were carried out by one reviewer (S. G.), and checked by a second reviewer (Y. K. L.). All discrepancies were resolved after going back to the original source papers, with full consensus reached after discussion.
Validity Assessment
The following criteria were used to consider the validity of comparing risk estimates across different study designs. (1) Presence of confounding factors: Discrepancies between the results of RCTs and observational studies may arise because of factors (e.g., differences in population, administration of intervention, or outcome definition) other than study design. We recorded whether the authors of the meta-analysis checked if the RCTs and observational studies shared similar features in terms of population, interventions, comparators, and measurement of outcomes and whether they used methods such as restriction or stratification by population, intervention, comparators, or outcomes to improve the comparability of pooled risk estimates arising from different groups of studies. (2) Heterogeneity by study design: We recorded whether the authors of the meta-analysis explored heterogeneity of the pooled studies by study design (using measures such as Chi2 or I2). We assessed the extent of heterogeneity of each meta-analysis using a cut-off point of p < 0.10 for Chi2 test results, and we specifically looked for instances where I2 was reported as above 50%. In the few instances where both statistics were presented, the results of I2 were given precedence [79]. (3) Statistical analysis comparing study designs: We recorded whether the authors of the meta-analysis described the statistical methods by which the magnitude of the difference between study designs was assessed.
Data Analysis
A descriptive summary of the data in terms of confidence interval (CI) overlap between pooled sets of results by study design, and any differences in the direction of effect between study designs, were presented. The results were said to agree if both study designs identified a significant increase, a significant decrease, or no significant difference in the adverse effects under investigation.
Quantitative differences or discrepancies between the pooled estimates from the respective study designs for each adverse effect were illustrated by taking the ratio of odds ratios (ROR) from meta-analysis of RCTs versus meta-analysis of observational studies. We calculated ROR by using the pooled OR for the adverse outcome from RCTs divided by the pooled OR for the adverse outcome from observational studies. If the meta-analysis of RCTs for a particular adverse effect yielded exactly the same OR as the meta-analysis of observational studies (i.e., complete agreement, or no discrepancy between study designs), then the ROR would be 1.0 (and ln ROR = 0). Because adverse events are rare, ORs and RRs were treated as equivalent [80].
The estimated ROR from each “RCT versus observational study” comparison was then used in a meta-analysis (random effects inverse variance method; RevMan 5.0.25) to summarize the overall ROR between RCTs and observational studies across all the included reviews. The standard error (SE) of ROR can be estimated using the SEs for the RCT and observational estimates:(1)
SEs pertaining to each pooled OR(RCT) and OR(Observ) were calculated from the published 95% CI.
Statistical heterogeneity was assessed using I2 statistic, with I2 values of 30%–60% representing a moderate level of heterogeneity [81].
Results
Included Studies
In total, 52 articles were identified as potentially eligible for this review. On further detailed evaluation, 33 of these articles either compared different types of observational studies to one another (for example, cohort studies versus case control studies) or compared only the incidence of adverse effects (without reporting the RR/OR) in those receiving the intervention according to type of study [57],[82]–[113].
We finally selected 19 eligible articles that compared the relative risk or ORs from RCTs and observational studies (Figure 1) [6],[114]–[131]. These 19 articles covering meta-analysis of 58 separate adverse effects will be the focus of this paper. The 58 meta-analyses included a total of over 311 RCTs and over 222 observational studies (comprising 57 cohort studies, 75 case-control studies, and at least 90 studies described as “observational” by the authors without specifying the exact type) (Table S1). (Exact numbers of RCTs and observational studies cannot be calculated as overlap in the included studies in McGettigan and Henry [127] could not be ascertained.)
Fig. 1. Flow chart for included studies. 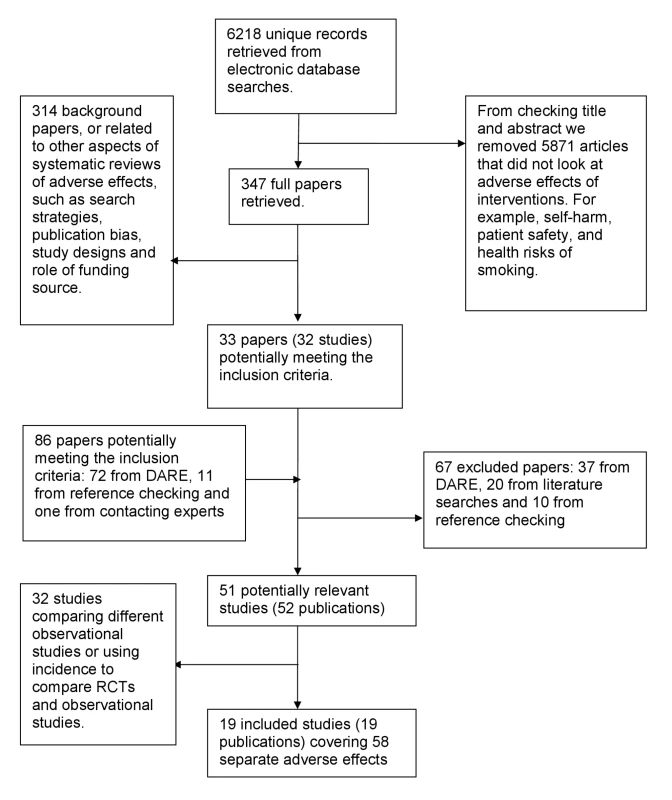
Two of the 19 articles were methodological evaluations with the main aim of assessing the influence of study characteristics (including study design) on the measurement of adverse effects [6],[127], whereas the remaining 17 were systematic reviews within which subgroup analysis by study design was embedded [114]–[126],[128]–[131] (Table S1).
Adverse Effects
The majority of the articles compared the results from RCTs and observational studies using only one adverse effect (11/19, 58%) [114],[115],[117]–[119],[121],[122],[124],[125],[129],[130], whilst three included one type of adverse effect (such as cancer, gastrointestinal complications, or cardiovascular events) [116],[127],[128], and five articles included a number of specified adverse effects (ranging from two to nine effects) or any adverse effects [6],[120],[123],[126],[131].
Interventions
Most (17/19, 89%) of the articles included only one type of intervention (such as hormone replacement therapy [HRT] or nonsteroidal anti-inflammatory drugs) [114]–[120],[122]–[131], whilst one article looked at two interventions (HRT and oral contraceptives) [121] and another included nine interventions [6]. Most of the analyses focused on the adverse effects of pharmacological interventions; however, other topics assessed were surgical interventions (such as bone marrow transplantation and hernia operations) [6],[120] and a diagnostic test (ultrasonography) [131].
Excluded Studies
Text S3 lists the 67 studies that were excluded from this systematic review during the screening and data extraction phases, with the reasons for exclusion.
Summary of Methodological Quality
Role of confounding factors
Although many of the meta-analyses acknowledged the potential for confounding factors that might yield discrepant findings between study designs, no adjustment for confounding factors was reported in most instances [6],[114]–[116],[118]–[122],[124]–[126],[128]–[131]. However, a few authors did carry out subgroup analysis stratified for factors such as population characteristics, drug dose, or duration of drug exposure.
There were two instances where the authors of the meta-analysis performed some adjustment for potential confounding factors: one carried out meta-regression [123], and in the other methodological evaluation the adjustment method carried out was unclear [127].
Heterogeneity by study design
Thirteen meta-analyses measured the heterogeneity of at least one set of the included studies grouped by study design using statistical analysis such as Chi2 or I2 [6],[115]–[117],[119],[121],[123]–[125],[127],[129]–[131].
The pooled sets of RCTs were least likely to exhibit any strong indication of heterogeneity; only five (15%) [6],[117],[130],[131] of the 33 [6],[115]–[117],[119],[121],[123],[124],[129]–[131] sets of pooled RCTs were significantly heterogeneous, and in two of these sets of RCTs the heterogeneity was only moderate, with I2 = 58.9% [117] and I2 = 58.8% [130].
Three of the four case-control studies, one of the four cohort studies, and 14 of the 25 studies described as “observational studies” also exhibited substantial heterogeneity.
Statistical analysis comparing study designs
Authors of one meta-analysis explicitly tested for a difference between the results of the different study designs [6]. Two other analyses reported on the heterogeneity of the pooled RCTs, the pooled observational studies, and the pooled RCTs and observational studies, which can indicate statistical differences where the pooled study designs combined are significantly heterogenous but no significant heterogeneity is seen when the study designs are pooled separately.
Data Analysis
Text S4 documents the decisions made in instances where the same data were available in more than one format.
Size of studies
In ten methodological evaluations the total number of participants was reported in each set of pooled studies by study design [114],[116],[118]–[120],[123],[125],[126],[130],[131], and in another five methodological evaluations the pooled number of participants was reported for at least one type of study design [6],[124],[127]–[129]. Studies described as “observational” by the authors contained the highest number of participants per study, 34,529 (3,798,154 participants/110 studies), followed by cohort studies, 33,613 (1,378,131 participants/41 studies). RCTs and case-control studies had fewer participants, 2,228 (821,954 participants/369 studies) and 2,144 (105,067 participants/49 studies), respectively.
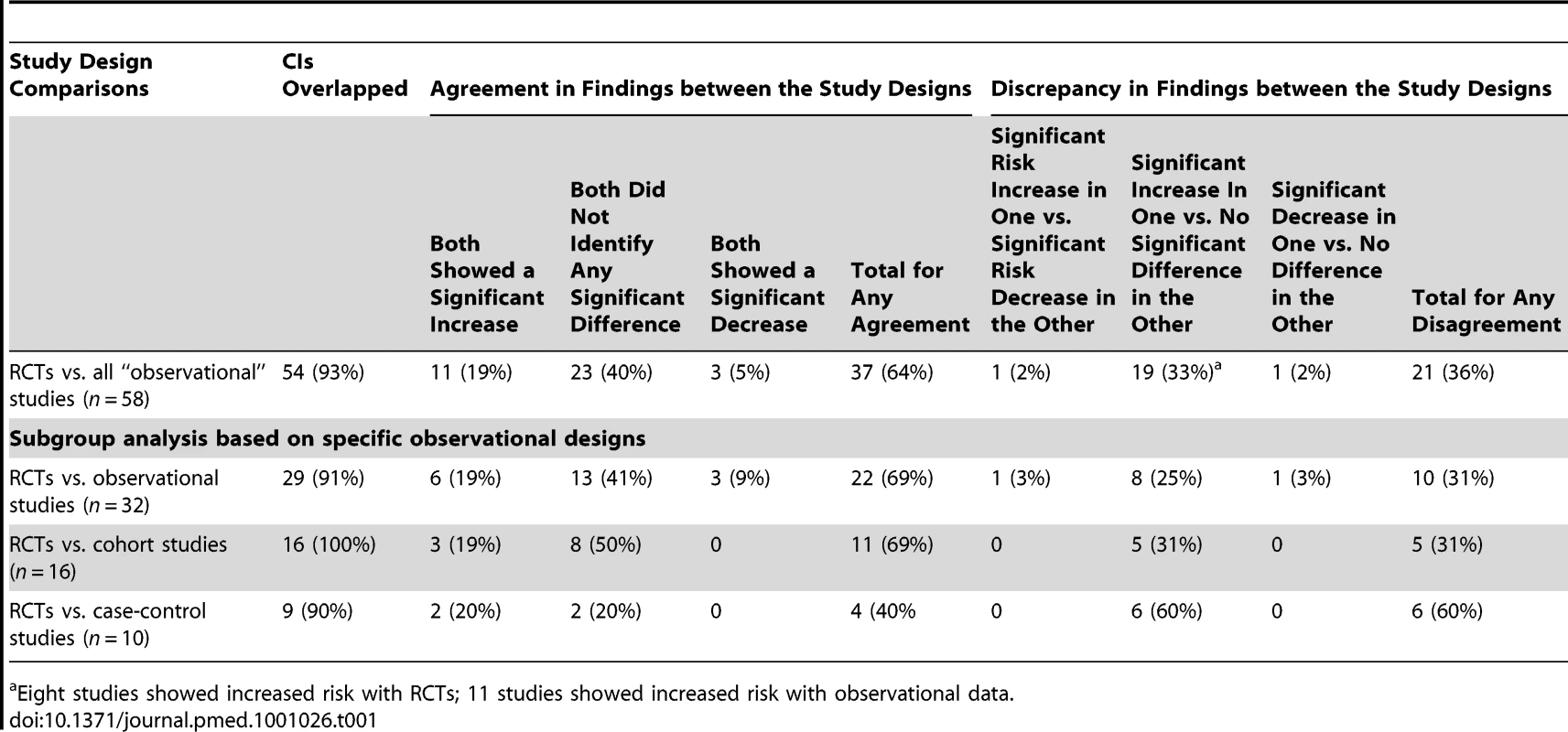
Confidence interval overlap
In almost all instances the CIs for the pooled results from the different study designs overlapped (Table 1). However, there were four pooled sets of results in three methodological evaluations where the CIs did not overlap [6],[119],[121].
Agreement and disagreement of results
In most of the methodological evaluations the results of the treatment effect agreed between types of study design [6],[116],[118],[120],[121],[123]–[131]. Most studies that showed agreement between study designs did not find a significant increase or significant decrease in the adverse effects under investigation (Table 1).
There were major discrepancies in one pooled set of results. Col et al. [119] found an increase in breast cancer with menopausal hormone therapy in RCTs but a decrease in observational studies.
There were other instances where although the direction of the effect was not in opposing directions, apparently different conclusions may have been reached had a review been restricted to either RCTs or observational studies, and undue emphasis was placed on statistical significance tests. For instance, a significant increase in an adverse effect could be identified in an analysis of RCT data, yet pooling the observational studies may have identified no significant difference in adverse effects between the treatment and control group. Table 1 shows that the most common discrepancy between study types occurred when one set of studies identified a significant increase whilst another study design found no statistically significant difference. Given the imprecision in deriving estimates of rare events, this may not reflect any real difference between the estimates from RCTs and observational studies, and it would be more sensible to concentrate on the overlap of CIs rather than the variation in size of the p-values from significance testing.
Ratio of risk ratio or odds ratios estimates
RRs or ORs from the RCTs were compared to those from the observational studies by meta-analysis of the respective ROR for each adverse effect.
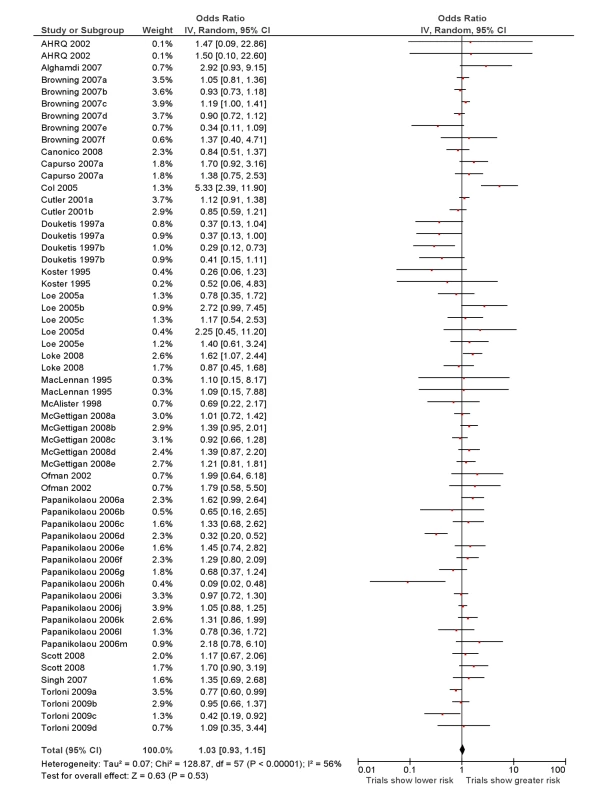
RCTs versus all “observational” studies
The overall ROR from meta-analysis using the data from all the studies that compared RCTs with either cohort studies or case-control studies, or that grouped studies under the umbrella of “observational” studies was estimated to be 1.03 (95% CI 0.93–1.15) with moderate heterogeneity (I2 = 56%, 95% CI 38%–67%) (Figure 2).
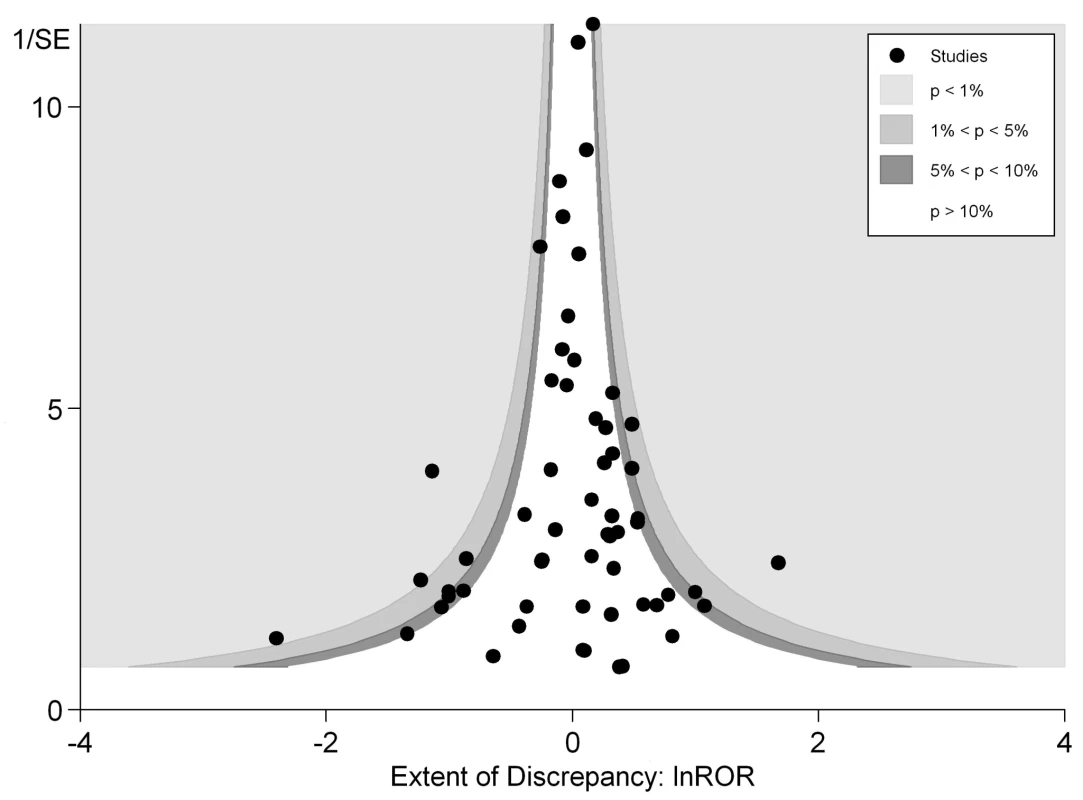
In Figure 3 we plotted the magnitude of discrepancy (ROR) from each meta-analysis against the precision of its estimates (1/SE), with the contour lines showing the extent of statistical significance for the discrepancy. Values on the x-axis show the magnitude of discrepancy, with the central ln ROR of zero indicating no discrepancy, or complete agreement between the pooled OR estimated from RCTs and observational studies. The y-axis illustrates the precision of the estimates (1/SE), with the data points at the top end having greater precision. This symmetrical distribution of the RORs of the various meta-analyses around the central ln ROR value of zero illustrates that random variation may be an important factor accounting for discrepant findings between meta-analyses of RCTs versus observational studies. If there had been any systematic and consistent bias that drove the results in a particular direction for certain study designs, the plot of RORs would likely be asymmetrical. The vertically tapering shape of the funnel also suggests that the discrepancies between RCTs and observational studies are less apparent when the estimates have greater precision. This may support the need for larger studies to assess adverse effects, whether they are RCTs or observational studies.
Both figures can be interpreted as demonstrating that there are no consistent systematic variations in pooled risk estimates of adverse effects from RCTs versus observational studies.
Sensitivity analysis: limiting to one review per adverse effect examined
There are no adverse effects for which two or more separate meta-analyses have used exactly the same primary studies (i.e., had complete overlap of RCTs and observational studies) to generate the pooled estimates. This reflects the different time periods, search strategies, and inclusion and exclusion criteria that have been used by authors of these meta-analyses such that even though they were looking at the same adverse effect, they used data from different studies in generating pooled overall estimates. As it turns out, the only adverse effect that was evaluated in more than one review was venous thromboembolism (VTE). There was some, but not complete, overlap of primary studies in three separate reviews of VTE with HRT (involving three overlapping case-control studies from a total of 18 observational studies analysed) and two separate reviews of VTE with oral contraceptives (one overlapping RCT, six [of 13] overlapping cohort studies, and two [of 20] overlapping case-control studies).
For the sensitivity analysis, we removed the three older meta-analyses pertaining to VTE so that the modest overlap could be further reduced, with only one review per specific adverse effect for the sensitivity analysis. The most recent meta-analyses for VTE (Canonico et al. [117] for VTE with HRT, Douketis et al. [121] for VTE with oral contraceptives) were used for analysis of the RORs. This yields RORs that are very similar to the original estimates: 1.06 (95% CI 0.96–1.18) for the overall analysis RCTs versus all observational studies, 1.00 (95% CI 0.71–1.42) for RCTs versus case-control studies and 1.07 (95% CI 0.86–1.34) for RCTs versus cohort studies.
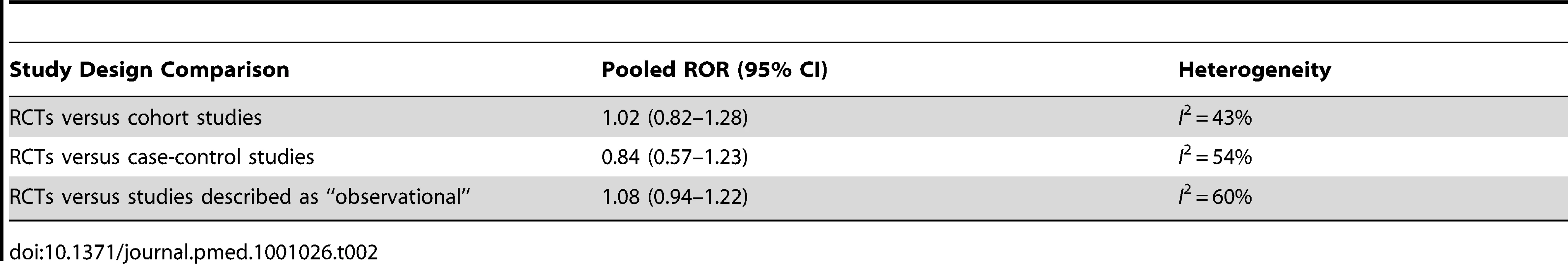
Subgroup analysis
Subgroup analysis for comparison of RCTs against specific types of “observational” studies was carried out and is summarised in Table 2. Forest plots for each of these comparisons can be viewed in Figure S1.
Discussion
Our analyses found little evidence of systematic differences in adverse effect estimates obtained from meta-analysis of RCTs and from meta-analysis of observational studies. Figure 3 shows that discrepancies may arise not just from differences in study design or systematic bias, but possibly because of the random variation, fluctuations or noise, and imprecision in attempting to derive estimates of rare events. There was less discrepancy between the study designs in meta-analyses that generated more precise estimates from larger studies, either because of better quality, or because the populations were more similar (perhaps because large, long-term RCTs capture a broad population similar to observational studies). Indeed, the adverse effects with discrepant results between RCTs and observational studies were distributed symmetrically to the right and left of the line of no difference, meaning that neither study design consistently over - or underestimates risk of harm as compared to the other. It is likely that other important factors such as population and delivery of intervention are at play here—for instance, the major discrepancy identified in Col et al. [119] for HRT and breast cancer is already well documented. This discrepancy has also been explained by the timing of the start of treatment relative to menopause, which was different between trials and observational studies. After adjustment, the results from the different study designs have been found to no longer differ [132],[133].
Most of the pooled results from the different study designs concurred in terms of identifying a significant increase or decrease, or no significant difference in risk of adverse effects. On the occasions where a discrepancy was found, the difference usually arose from a finding of no significant risk of adverse effects with one study design, in contrast to a significant increase in adverse effects from the other study design. This may reflect the limited size of the included studies to identify significant differences in rare adverse effects.
The increased risk in adverse effects in some studies was not consistently related to any particular study design—RCTs found a significant risk of adverse effects associated with the intervention under investigation in eight instances, while observational studies showed a significantly elevated risk in 11 cases.
Although reasons for discrepancies are unclear, specific factors which may have led to differences in adverse effect estimates were discussed by the respective authors. The differences between observational studies and RCTs in McGettigan and Henry's meta-analysis of cardiovascular risk were thought to be attributable to different dosages of anti-inflammatory drugs used [127]. Differences in Papanikolaou et al. [6] and Col et al. [119] were attributed to differing study populations. Other methodological evaluations discussed the nature of the study designs themselves being a factor that may have led to differences in estimates. For example, some stated that RCTs may record a higher incidence of adverse effects because of closer monitoring of patients, longer duration of treatment and follow-up, and more thorough recording, in line with regulatory requirements [6],[128]. Where RCTs had a lower incidence of adverse effects, it was suggested that this could be attributed to the exclusion of high-risk patients [119] and possibly linked to support by manufacturers [6].
The overall ROR did not suggest any consistent differences in adverse effects estimates from meta-analysis of RCTs versus meta-analysis of observational studies. This interpretation is supported by the funnel plot in Figure 3, which shows that differences between the results of the two study designs are equally distributed across the range. Some discrepancies may arise by chance, or through lack of precision from limited sample size for detecting rare adverse effects. While there are a few instances of sizeable discrepancies, the pooled estimates in Figure 2 and Table 2 indicate that in the scheme of things (particularly where larger, more precise primary studies are available), meta-analysis of observational studies yield adverse effects estimates that broadly match those from meta-analysis of RCTs.
Limitations
This systematic review of reviews and methodological evaluations has a number of limitations. When comparing the pooled results from different study designs it is important to consider any confounding factors that may account for any differences identified. For instance, if one set of studies was carried out on a younger cohort of patients, with a lower drug dosage, or with shorter duration of use, or relied on passive ascertainment of adverse effects data [6],[17],[52],[134], it might be expected that the magnitude of any adverse effects recorded would be lower. However, most of the methodological evaluations were not conducted with the primary aim of assessing differences in study design, but were systematic reviews with some secondary comparative evaluation of study design embedded.
Another constraint of our overview is that we accepted information and data as reported by the authors of the included meta-analyses. We did not attempt to source the primary studies contained in each meta-analysis, as this would have required extracting data from more than 550 papers. For instance, we relied on the authors' categorisation of study design but are aware that authors may not all have used the same definitions. This is a particular problem with observational studies, where it is often difficult to determine the methodology used in the primary study and categorise it appropriately. In order to overcome this limitation, we chose to base our analysis on RCTs compared to “all” observational studies (either cohort studies, case-control studies, or “observational” studies as defined by the author), with a subgroup analysis based on different types of observational designs.
Another important limitation to this review is the potentially unrepresentative sample used. Systematic reviews with embedded data comparing different study designs may have been missed. The search strategy used was limited to a literature search to identify methodological papers whose primary aim was to assess the influence of study design on adverse effects and to a sift of the full text of systematic reviews of adverse effects (as a primary outcome) from the Cochrane Database of Systematic Reviews and DARE. Nevertheless, it should be noted that the Cochrane Database of Systematic Reviews and DARE databases cover a large proportion of all systematic reviews and that systematic reviews in which adverse effects are included as a secondary aim are unlikely to present subgroup analysis by study design for the adverse effects data.
There was considerable heterogeneity between the comparisons of different studies, suggesting that any differences may be specific to particular types of interventions or adverse effects. It may be that particular types of adverse effects can be identified more easily via particular types of study designs [3],[14],[135],[136]. However, it was difficult to assess the methodological evaluations by type of adverse effects. This would be of interest, given that the literature suggests that RCTs may be better at identifying some types of adverse effects (such as common, anticipated, and short-term) than observational studies.
Future Research
Where no randomized data exist, observational studies may be the only recourse [137]. However, the potential value of observational data needs to be further demonstrated, particularly in specific situations where existing RCTs are short-term or based on highly selected populations. Comparisons of risk estimates from different types of observational studies (e.g., case-control as opposed to cohort) merit further assessment.
In addition, it would be useful (based on a case-control type of design) to carry out an in-depth examination of the meta-analyses (and their included primary studies) with substantial discrepancy amongst the RCTs and observational studies, as compared to other meta-analyses where RCTs and observational studies had close agreement. Any future research in this area should look into the role of confounding factors (such as different population selection and duration of drug exposure) between studies, and lack of precision in point estimates of risk for rare events that could have accounted for discrepant findings amongst RCTs and observational studies.
Conclusions
Our findings have important implications for the conduct of systematic reviews of harm, particularly with regards to selection of a broad range of relevant studies. Although there are strengths and weaknesses to each study design, empirical evidence from this overview indicates that there is no difference on average between estimates of the risk of adverse effects from meta-analyses of RCTs and of observational studies. Instead of restricting the analysis to certain study designs, it may be preferable for systematic reviewers of adverse effects to evaluate a broad range of studies that can help build a complete picture of any potential harm and improve the generalisability of the review without loss of validity.
Supporting Information
Zdroje
1. ChouRHelfandM
2005
Challenges in systematic reviews that assess treatment
harms.
Ann Intern Med
142
1090
1099
2. MittmannNLiuBAKnowlesSRShearNH
1999
Meta-analysis and adverse drug reactions.
CMAJ
160
987
988
3. IoannidisJPMulrowCDGoodmanSN
2006
Adverse events: the more you search, the more you
find.
Ann Intern Med
144
298
300
4. LevineMWalterSLeeHHainesTHolbrookA
1994
User's guides to the medical literature, IV: how to use an
article about harm.
JAMA
271
1615
1619
5. MeadeMOCookDJKernermanPBernardG
1997
How to use articles about harm: the relationship between high
tidal volumes, ventilating pressures, and ventilator-induced lung
injury.
Crit Care Med
25
1915
1922
6. PapanikolaouPNChristidiGDIoannidisJPA
2006
Comparison of evidence on harms of medical interventions in
randomized and nonrandomized studies.
CMAJ
174
635
641
7. PriceDJeffersonTDemicheliV
2004
Methodological issues arising from systematic reviews of the
evidence of safety of vaccines.
Vaccine
22
2080
2084
8. AnelloC
1996
Does research synthesis have a place in drug regulatory policy?
synopsis of issues: assessment of safety and postmarketing
surveillance.
Clin Res Reg Aff
13
13
21
9. JacobRFLloydPM
2000
How to evaluate a dental article about harm.
J Prosthet Dent
84
8
16
10. KallenBAJ
2005
Methodological issues in the epidemiological study of the
teratogenicity of drugs.
Congenit Anom (Kyoto)
45
44
51
11. PedersenATOttesenB
2003
Issues to debate on the Women's Health Initiative (WHI)
study. Epidemiology or randomized clinical trials—time out for hormone
replacement therapy studies?
Hum Reprod
18
2241
2244
12. SackettDLHaynesRBGuyattGHTugwellP
1991
Clinical epidemiology.
London
Little, Brown and Company
13. SkeggDC
2001
Evaluating the safety of medicines, with particular reference to
contraception.
Stat Med
20
3557
3569
14. BrewerTColditzGA
1999
Postmarketing surveillance and adverse drug reactions: current
perspectives and future needs.
JAMA
281
824
829
15. EggerMSchneiderMDavey SmithG
1998
Spurious precision? Meta-analysis of observational
studies.
BMJ
316
140
144
16. StrausSERichardsonWSGlasziouPHaynesRB
2005
Evidence-based medicine: how to practice and teach
EBM.
London
Elsevier
17. RothwellPM
2005
External validity of randomised controlled trials: “to whom
do the results of this trial apply?”.
Lancet
365
82
93
18. EdwardsJEMcQuayHJMooreRACollinsSL
1999
Reporting of adverse effects in clinical trials should be
improved: lessons from acute postoperative pain.
J Pain Symptom Manage
18
427
437
19. PapanikolaouPNIoannidisJP
2004
Availability of large-scale evidence on specific harms from
systematic reviews of randomized trials.
Am J Med
117
582
589
20. IoannidisJPLauJ
2001
Completeness of safety reporting in randomized trials: an
evaluation of 7 medical areas.
JAMA
285
437
443
21. LokeYKDerryS
2001
Reporting of adverse drug reactions in randomised controlled
trials—a systematic survey.
BMC Clin Pharmacol
1
3
22. IoannidisJPEvansSJGotzschePCO'NeillRTAltmanDG
2004
Better reporting of harms in randomized trials: an extension of
the CONSORT statement.
Ann Intern Med
141
781
788
23. LevyJH
1999
Adverse drug reactions in hospitalized patients. The Adverse Reactions
Source. Available: http://adversereactions.com/reviewart.htm. Accessed 17 March
2011
24. PapanikolaouPNChurchillRWahlbeckKIoannidisJP
2004
Safety reporting in randomized trials of mental health
interventions.
Am J Psychiatry
161
1692
1697
25. IoannidisJPContopoulos-IoannidisDG
1998
Reporting of safety data from randomised trials.
Lancet
352
1752
1753
26. CuervoGLClarkeM
2003
Balancing benefits and harms in health care.
BMJ
327
65
66
27. NuovoJSatherC
2007
Reporting adverse events in randomized controlled
trials.
Pharmacoepidemiol Drug Saf
16
349
351
28. EthgenMBoutronIBaronGGiraudeauBSibiliaJ
2005
Reporting of harm in randomized, controlled trials of
nonpharmacologic treatment for rheumatic disease.
Ann Intern Med
143
20
25
29. LeePEFischerbHDRochonbPAGillbSSHerrmanngN
2008
Published randomized controlled trials of drug therapy for
dementia often lack complete data on harm J Clin Epidemiol
61
1152
1160
30. YaziciY
2008
Some concerns about adverse event reporting in randomized
clinical trials.
Bull NYU Hosp Jt Dis
66
143
145
31. GartlehnerGThiedaPHansenRAMorganLCShumateJA
2009
Inadequate reporting of trials compromises the applicability of
systematic reviews.
Int J Technol Assess Health Care
25
323
330
32. RiefWNestoriucYvon Lilienfeld-ToalADoganISchreiberF
2009
Differences in adverse effect reporting in placebo groups in SSRI
and tricyclic antidepressant trials: a systematic review and
meta-analysis.
Drug Saf
32
1041
1056
33. HenryDHillS
1999
Meta-analysis-its role in assessing drug safety.
Pharmacoepidemiol Drug Saf
8
167
168
34. BuekensP
2001
Invited commentary: rare side effects of obstetric interventions:
are observational studies good enough?
[comment] Am J Epidemiol
153
108
109
35. ClarkeADeeksJJShakirSA
2006
An assessment of the publicly disseminated evidence of safety
used in decisions to withdraw medicinal products from the UK and US
markets.
Drug Saf
29
175
181
36. DieppePBartlettCDaveyPDoyalLEbrahimS
2004
Balancing benefits and harms: the example of non-steroidal
anti-inflammatory drugs.
BMJ
329
31
34
37. EtminanMCarletonBRochonPA
2004
Quantifying adverse drug events: are systematic reviews the
answer?
Drug Saf
27
757
761
38. GuttermanEM
2004
Pharmacoepidemiology in safety evaluations of newly approved
medications.
Drug Inf J
38
61
67
39. HallWDLuckeJ
2006
How have the selective serotonin reuptake inhibitor
antidepressants affected suicide mortality?
Aust N Z J Psychiatry
40
941
950
40. HughesMDWilliamsPL
2007
Challenges in using observational studies to evaluate adverse
effects of treatment.
New Engl J Med
356
1705
1707
41. HyrichKL
2005
Assessing the safety of biologic therapies in rheumatoid
arthritis: the challenges of study design.
J
Rheumatol
Suppl 72
48
50
42. JeffersonTDemicheliV
1999
Relation between experimental and non-experimental study designs.
HB vaccines: a case study.
J Epidemiol Comm Health
53
51
54
43. JeffersonTTraversaG
2002
Hepatitis B vaccination: risk-benefit profile and the role of
systematic reviews in the assessment of causality of adverse events
following immunisation.
J Med Virol
67
451
453
44. KaufmanDWShapiroS
2000
Epidemiological assessment of drug-induced
disease.
Lancet
356
1339
1343
45. BeardKLeeA
2006
Introduction.
LeeA
Adverse drug reactions, 2nd ed
London
Pharmaceutical Press
46. LokeYKPriceDHerxheimerA
Cochrane Adverse Effects Methods Group
2007
Systematic reviews of adverse effects: framework for a structured
approach.
BMC Med Res Methodol
7
32
47. OlssonSMeyboomR
2006
Pharmacovigilance.
MulderGJDenckerL
Pharmaceutical toxicology: safety sciences of drugs
London
Pharmaceutical Press
229
241
48. RayWA
2003
Population-based studies of adverse drug effects.
N Engl J Med
349
1592
1594
49. VitielloBRiddleMAGreenhillLLMarchJSLevineJ
2003
How can we improve the assessment of safety in child and
adolescent psychopharmacology?
J Am Acad Child Adolesc Psychiatry
42
634
641
50. VandenbrouckeJP
2004
Benefits and harms of drug treatments.
BMJ
329
2
3
51. VandenbrouckeJP
2004
When are observational studies as credible as randomised
trials?
Lancet
363
1728
1731
52. VandenbrouckeJP
2006
What is the best evidence for determining harms of medical
treatment?
CMAJ
174
645
646
53. AagaardLHansenEH
2009
Information about ADRs explored by pharmacovigilance approaches:
a qualitative review of studies on antibiotics, SSRIs and
NSAIDs.
BMC Clin Pharmacol
9
1
31
54. AhmadSR
2003
Adverse drug event monitoring at the food and drug
administration: your report can make a difference.
J Gen Intern Med
18
57
60
55. RavaudPTubachF
2005
Methodology of therapeutic trials: lessons from the late evidence
of the cardiovascular toxicity of some coxibs.
Joint Bone Spine
72
451
455
56. Hordijk-TrionMLenzenMWijnsWde JaegerePSimoonsML
2006
Patients enrolled in coronary intervention trials are not
representative of patients in clinical practice: results from the Euro Heart
Survey on Coronary Revascularization.
Eur Heart J
27
671
678
57. TramerMRMooreRAReynoldsDJMcQuayHJ
2000
Quantitative estimation of rare adverse events which follow a
biological progression: a new model applied to chronic NSAID
use.
Pain
85
169
182
58. McDonaghMPetersonKCarsonS
2006
The impact of including non-randomized studies in a systematic
review: a case study [abstract].
Abstract P084. 14th Cochrane Colloquium; 2006 October 23-26; Dublin,
Ireland
59. PsatyBMKoepsellTDLinDWeissNSSiscovickDS
1999
Assessment and control for confounding by indication in
observational studies.
J Am Geriatr Soc
47
749
754
60. ReevesBC
2008
Principles of research: limitations of non-randomized
studies.
Surgery
26
120
124
61. StrickerBHPsatyBM
2004
Detection, verification, and quantification of adverse drug
reactions.
BMJ
329
44
47
62. VandenbrouckeJP
2008
Observational research, randomised trials, and two views of
medical science.
PLoS Med
5
e67
doi:10.1371/journal.pmed.0050067
63. BrittonAMcKeeMBlackNMcPhersonKSandersonC
1998
Choosing between randomised and non-randomised studies: a
systematic review.
Health Technol Assess
2
1
124
64. ConcatoJShahNHorwitzRI
2000
Randomised, controlled trials, observational studies, and the
hierarchy of research designs.
N Engl J Med
342
1887
1892
65. IoannidisJPHaidichABPappaMPantazisNKokoriSI
2001
Comparison of evidence of treatment effects in randomised and
non-randomised studies.
JAMA
286
821
830
66. MacLehoseRRReevesBCHarveyIMSheldonTARussellIT
2000
A systematic review of comparisons of effect sizes derived from
randomised and non-randomised studies.
Health Technol Assess
4
67. ShepherdJBagnallA-MColquittJDinnesJDuffyS
2006
Sometimes similar, sometimes different: a systematic review of
meta-analyses of random and non-randomized policy intervention
studies.
Abstract P085. 14th Cochrane Colloquium; 2006 October 23-26; Dublin,
Ireland
68. ShikataSNakayamaTNoguchiYTajiYYamagishiH
2006
Comparison of effects in randomized controlled trials with
observational studies in digestive surgery.
Ann Surg
244
668
676
69. OliverSBagnallAMThomasJShepherdJSowdenA
2010
Randomised controlled trials for policy interventions: a review
of reviews and meta-regression.
Health Technol Assess
14
1
192
70. BerlinJAColditzGA
1999
The role of meta-analysis in the regulatory process for foods,
drugs and devices.
JAMA
28
830
834
71. KramerBSWilentzJAlexanderDBurklowJFriedmanLM
2006
Getting it right: being smarter about clinical
trials.
PLoS Med
3
e144
doi:10.1371/journal.pmed.0030144
72. HenryDMoxeyAO'ConnellD
2001
Agreement between randomized and non-randomized studies: the
effects of bias and confounding [poster
presentation].
9th Chochrane Colloqium. 2001 October 9-13; Lyon, France
73. MartinG
2005
Conflicting clinical trial data: a lesson from
albumin.
Crit Care
9
649
650
74. KimJEvansSSmeethLPocockS
2006
Hormone replacement therapy and acute myocardial infarction: a
large observational study exploring the influence of age.
Int J Epidemiol
35
731
738
75. McPhersonKHemminkiE
2004
Synthesising licensing data to assess drug
safety.
BMJ
328
518
520
76. PetittiDB
1994
Coronary heart disease and estrogen replacement therapy. Can
compliance bias explain the results of observational
studies?
Ann Epidemiol
4
115
118
77. PosthumaWFWestendorpRGVandenbrouckeJP
1994
Cardioprotective effect of hormone replacement therapy in
postmenopausal women: is the evidence biased?
[comment].
BMJ
308
1268
1269
78. JickHRodriguezGPerez-GuthannS
1998
Principles of epidemiological research on adverse and beneficial
drug effects.
Lancet
352
1767
1770
79. PereraRHeneghanC
2008
Interpretating meta-analysis in systematic
reviews.
Evid Based Med
13
67
69
80. DaviesHTO
1998
Interpreting measures of treatment effect.
Hosp Med
59
499
501
81. DeeksJHigginsJAltmanDG
2008
Analysing data and undertaking meta-analyses.
HigginsJPGreenS
Cochrane handbook for systematic reviews of interventions
Chichester
John Wiley and Sons
82. BagerPWhohlfahrtJWestergaardT
2008
Caesarean delivery and risk of atopy and allergic disease:
meta-analysis.
Clin Exp Allergy
38
634
642
83. BergendalAOdlindVPerssonIKielerH
2009
Limited knowledge on progestogen-only contraception and risk of
venous thromboembolism.
Acta Obstet Gynecol Scand
88
261
266
84. BolliniPGarciaRLAPérezGSWalkerAM
1992
The impact of research quality and study design on epidemiologic
estimates of the effect of nonsteroidal anti-inflammatory drugs on upper
gastrointestinal tract disease.
Arch Int Med
152
1289
1295
85. ChanWSRayJWaiEKGinsburgSHannahME
2004
Risk of stroke in women exposed to low-dose oral contraceptives:
a critical evaluation of the evidence.
Arch Intern Med
164
741
747
86. ChouRFuRCarsonSSahaSHelfandM
2006
Empirical evaluation of the association between methodological
shortcomings and estimates of adverse events.
Rockville (Maryland)
Agency for Healthcare Research and Quality
Technical Reviews, No. 13
87. ChouRFuRCarsonSSahaSHelfandM
2007
Methodological shortcomings predicted lower harm estimates in one
of two sets of studies of clinical interventions.
J Clin Epidemiol
60
18
28
88. CosmiBCastelvetriCMilandriMRubboliAConfortiA
2000
The evaluation of rare adverse drug events in Cochrane reviews:
the incidence of thrombotic thrombocytopenic purpura after ticlopidine plus
aspirin for coronary stenting [abstract].
Abstract PA13. 8th Cochrane Colloquium; 2000 October; Cape Town, South
Africa
89. DolovichLRAddisAVaillancourtJMPowerJDKorenG
1998
Benzodiazepine use in pregnancy and major malformations or oral
cleft: meta-analysis of cohort and case-control studies.
BMJ
317
839
843
90. GargPPKerlikowskeKSubakLGradyD
1998
Hormone replacement therapy and the risk of epithelial ovarian
carcinoma: a meta-analysis.
Obstet Gynecol
92
472
479
91. GillumLAMamidipudiSKJohnstonSC
2000
Ischemic stroke risk with oral contraceptives: a
meta-analysis.
JAMA
284
72
78
92. GradyDGebretsadikTKerlikowskeKErnsterVPetittiD
1995
Hormone replacement therapy and endometial cancer risk: a
meta-analysis.
Obstet Gynecol
85
304
313
93. HenryDMcGettiganP
2003
Epidemiology overview of gastrointestinal and renal toxicity of
NSAIDs.
Int J Clin
Pract
Suppl 135
43
49
94. JensenPMikkelsenTKehletH
2002
Postherniorrhaphy urinary retention—effect of local,
regional, and general anesthesia: a review.
Reg Anesth Pain Med
27
612
617
95. JohnstonSCColfordJMJrGressDR
1998
Oral contraceptives and the risk of subarachnoid
hemorrhage.
Neurology
51
411
418
96. JonesGRileyMCouperDDwyerT
1999
Water fluoridation, bone mass and fracture: a quantitative
overview of the literature.
Aust N Z J Public Health
23
34
40
97. LeipzigRMCummingRGTinettiME
1999
Drugs and falls in older people: a systematic review and
meta-analysis: I. Psychotropic drugs.
J Am Geriatr Soc
47
30
39
98. LeipzigRMCummingRGTinettiME
1999
Drugs and falls in older people: a systematic review and
meta-analysis: II. Cardiac and analgesic drugs.
J Am Geriatr Soc
47
40
50
99. LokeYKDerrySAronsonJK
2004
A comparison of three different sources of data in assessing the
frequencies of adverse reactions to amiodarone.
Br J Clin Pharmacol
57
616
621
100. McGettiganPHenryD
2006
Cardiovascular risk and inhibition of cyclooxygenase: a
systematic review of the observational studies of selective and nonselective
inhibitors of cyclooxygenase 2.
JAMA
296
1633
1644
101. NalysnykLFahrbachKReynoldsMWZhaoSZRossS
2003
Adverse events in coronary artery bypass graft (CABG) trials: a
systematic review and analysis.
Heart
89
767
772
102. OgerEScarabinPY
1999
Assessment of the risk for venous thromboembolism among users of
hormone replacement therapy.
Drugs Aging
14
55
61
103. RossSDDiGeorgeAConnellyJEWhittingGWMcDonnellN
1998
Safety of GM-CSF in patients with AIDS: A review of the
literature.
Pharmacotherapy
18
1290
1297
104. SalhabMAl SarakbiWMokbelK
2005
In vitro fertilization and breast cancer risk: a
review.
Int J Fertil Womens Med
50
259
266
105. SchwarzEBMorettiMENayakSKorenG
2008
Risk of hypospadias in offspring of women using loratadine during
pregnancy: a systematic review and meta-analysis.
Drug Saf
31
775
788
106. ScottPAKingsleyGHSmithCMChoyEHScottDL
2007
Non-steroidal anti-inflammatory drugs and myocardial infarctions:
comparative systematic review of evidence from observational studies and
randomised controlled trials.
Ann Rheum Dis
66
1296
1304
107. SiegelCAMardenSMPersingSMLarsonRJSandsBE
2009
Risk of lymphoma associated with combination anti-tumor necrosis
factor and immunomodulator therapy for the treatment of Crohn's
disease: a meta-analysis.
Clin Gastroenterol Hepatol
7
874
881
108. SmithJSGreenJBerrington de GonzalezAApplebyPPetoJ
2003
Cervical cancer and use of hormonal contraceptives: a systematic
review.
Lancet
36
1159
1167
109. TakkoucheBMontes-MartínezAGillSSEtminanM
2007
Psychotropic medications and the risk of fracture: a
meta-analysis.
Drug Saf
30
171
184
110. TramerMRMooreRAMcQuayHJ
1997
Propofol and bradycardia: causation, frequency and
severity.
Br J Anaesth
78
642
651
111. VohraSJohnstonBCCramerKHumphreysK
2007
Adverse events associated with pediatric spinal manipulation: a
systematic review.
Pediatrics
119
e275
e283
112. WangTColletJPShapiroSWareMA
2008
Adverse effects of medical cannabinoids: a systematic
review.
CMAJ
178
1669
1678
113. WoolcottJCRichardsonKJWiensMOPatelBMarinJ
2009
Meta-analysis of the impact of 9 medication classes on falls in
elderly persons.
Arch Int Med
169
1952
1960
114. Agency for Healthcare Research and Quality
2002
Hormone replacement therapy and risk of venous
thromboembolism.
Rockville (Maryland)
Agency for Healthcare Research and Quality
115. AlghamdiAAMoussaFFremesSE
2007
Does the use of preoperative aspirin increase the risk of
bleeding in patients undergoing coronary artery bypass grafting surgery?
Systematic review and meta-analysis.
J Card Surg
22
247
256
116. BrowningDRMartinRM
2007
Statins and risk of cancer: a systematic review and
metaanalysis.
Int J Cancer
120
833
843
117. CanonicoMPlu-BureauGLoweGDOScarabinPY
2008
Hormone replacement therapy and risk of venous thromboembolism in
postmenopausal women: systematic review and meta-analysis.
BMJ
336
1227
1231
118. CapursoGSchünemannHJTerrenatoIMorettiAKochM
2007
Meta-analysis: the use of non-steroidal anti-inflammatory drugs
and pancreatic cancer risk for different exposure
categories.
Aliment Pharmacol Ther
26
1089
1099
119. ColNFKimJAChlebowskiRT
2005
Menopausal hormone therapy after breast cancer: a meta-analysis
and critical appraisal of the evidence.
Breast Cancer Res
7
R535
R540
120. CutlerCGiriSJeyapalanSPaniaguaDViswanathanA
2001
Acute and chronic graft-versus-host disease after allogeneic
peripheral-blood stem-cell and bone marrow transplantation: a
meta-analysis.
J Clin Oncol
19
3685
3691
121. DouketisJDGinsbergJSHolbrookACrowtherMDukuEK
1997
A reevaluation of the risk for venous thromboembolism with the
use of oral contraceptives and hormone replacement therapy.
Arch Int Med
157
1522
1530
122. KosterTSmallRARosendaalFRHelmerhorstFM
1995
Oral contraceptives and venous thromboembolism: a quantitative
discussion of the uncertainties.
J Intern Med
238
31
37
123. LoeSMSanchez-RamosLKaunitzAM
2005
Assessing the neonatal safety of indomethacin tocolysis: a
systematic review with meta-analysis.
Obstet Gynecol
106
173
179
124. LokeYKSinghSFurbergCD
2008
Long-term use of thiazolidinediones and fractures in type 2
diabetes: A meta-analysis.
CMAJ
180
32
39
125. MacLennanSCMacLennanAHRyanP
1995
Colorectal cancer and oestrogen replacement therapy. A
meta-analysis of epidemiological studies.
Med J Aust
162
491
493
126. McAlisterFAClarkHDWellsPSLaupacisA
1998
Perioperative allogenic blood transfusion does not cause adverse
sequelae in patients with cancer: a meta-analysis of unconfounded
studies.
Br J Surg
85
171
178
127. McGettiganPHenryD
2008
Cardiovascular ischaemia with anti-inflammarory drugs [oral
presentation]
128. OfmanJJMacLeanCHStrausWLMortonSCBergerML
2002
A meta-analysis of severe upper gastrointestinal complications of
non-steroidal anti-inflammatory drugs.
J Rheumatol
29
804
812
129. ScottPAKingsleyGHScottDL
2008
Non-steroidal anti-inflammatory drugs and cardiac failure:
meta-analysis of observational studies and randomised controlled
trials.
Eur J Heart Fail
10
1102
1107
130. SinghSLokeYKFurbergCD
2007
Thiazolidinediones and heart failure: A
teleo-analysis.
Diabetes Care
30
2148
2153
131. TorloniMRVedmedovskaNMerialdiMBetranAPAllenT
2009
Safety of ultrasonography in pregnancy: WHO systematic review of
the literature and meta-analysis.
Ultrasound Obstet Gynecol
33
599
608
132. PrenticeRChlebowskiRStefanickMMansonJLangerR
2008
Conjugated equine estrogens and breast cancer risk in the
Women's Health Initiative clinical trial and observational
study.
Am J Epidemiol
167
1407
1415
133. VandenbrouckeJ
2009
The HRT controversy: observational studies and RCTs fall in
line.
Lancet
373
1233
1235
134. LevineMAHametPNovoselSJolainB
1997
A prospective comparison of four study designs used in assessing
safety and effectiveness of drug therapy in hypertension
management.
Am J Hypertens
10
1191
1200
135. RossSD
2001
Drug-related adverse events: a readers' guide to assessing
literature reviews and meta-analyses.
Arch Int Med
161
1041
1046
136. SuttonAJCooperNJLambertPCJonesDRAbramsKR
2002
Meta-analysis of rare and adverse event data.
Expert Rev Pharmacoecon Outcomes Res
2
367
379
137. LokeYKTrivediANSinghS
2008
Meta-analysis: gastrointestinal bleeding due to interaction
between selective serotonin uptake inhibitors and non-steroidal
anti-inflammatory drugs.
Aliment Pharmacol Ther
27
31
40
Štítky
Interné lekárstvo
Článek The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in AfricaČlánek If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2011 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Nech brouka žít… Ať žije astma!
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
-
Všetky články tohto čísla
- Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial
- Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Characterizing the Epidemiology of the 2009 Influenza A/H1N1 Pandemic in Mexico
- The Joint Action and Learning Initiative: Towards a Global Agreement on National and Global Responsibilities for Health
- Let's Be Straight Up about the Alcohol Industry
- Advancing Cervical Cancer Prevention Initiatives in Resource-Constrained Settings: Insights from the Cervical Cancer Prevention Program in Zambia
- The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa
- Health Aspects of the Pre-Departure Phase of Migration
- Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
- Threshold Haemoglobin Levels and the Prognosis of Stable Coronary Disease: Two New Cohorts and a Systematic Review and Meta-Analysis
- If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
- Migration and Health: A Framework for 21st Century Policy-Making
- Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study
- The Impact of Retail-Sector Delivery of Artemether–Lumefantrine on Malaria Treatment of Children under Five in Kenya: A Cluster Randomized Controlled Trial
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy