-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa
article has not abstract
Published in the journal: The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa. PLoS Med 8(5): e32767. doi:10.1371/journal.pmed.1001040
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001040Summary
article has not abstract
Summary Points
-
Movement of people is the means by which human pathogens are dispersed, so providing health care to mobile sectors of the community is vital to disease control interventions.
-
Using malaria as a case study, our article examines types of migrant transit and their significance for prevention and treatment of the disease.
-
Asymptomatic untreated infections act as reservoirs for malaria transmission. Malaria control programmes need to identify those migrant streams with potential to transport malaria and to target prevention and treatment measures appropriately.
-
Transit has also played a role in the dispersal of antimalarial drug resistance, with the international transportation of artemisinin-resistant parasites by human migration being the greatest threat to the antimalarial treatments used in Africa today.
-
A geographic framework of human migration at local, national, and international levels is needed so the potential speed and direction of pathogen dispersal can be predicted, and for health policy and services to respond appropriately to the needs of migrants and to threats of pandemic.
This is one article in a six-part PLoS Medicine series on Migration & Health.
Transit Migration
There are many different types of migrants and types of movements and no single commonly agreed definition for transit migration. Papadopoulou [1] describes it as “the stage between emigration and settlement”, while in contrast, the assembly of Inter-Parliamentary Union in Geneva [2] states that “transit migrants are…aliens who stay in the country for some period of time while seeking to migrate permanently to another country”.
Although transit is often defined in terms of international borders, it is highly likely that transit migrants undertake similar patterns of movement within their countries of origin, and within the transit and settlement countries. Within a country people can be moving permanently or multiple times towards urban areas and back home, especially if they are internally displaced people (IDPs). In practice, transitory migration is one component within a broad framework of movement encompassing permanent, temporary, and circulatory migration, all of which can occur in a bidirectional or in a stepwise sequence of moves [3],[4].
This article examines types of transit migration and their significance for the prevention and treatment of malaria in Africa, using a case study of migration in southwest Uganda.
People Movement and Malaria
Plasmodium falciparum malaria—responsible for the most severe malaria—occurs across Africa, although the intensity of transmission varies considerably. Half of Africans live in areas of high endemicity where more than 40% of the population have parasites in their blood [5]; the bulk of these infections are asymptomatic. Transit between different areas exposes people to changing risks of malaria infection, and the burden of infection often falls disproportionately on mobile and migrant sectors of the community [6].
Migrants travelling from low to high transmission areas are at greater risk of acquiring a malarial infection than those travelling in the opposite direction, where also having no acquired immunity means they are much more vulnerable to disease. Evidence from large-scale population resettlement programs in Ethiopia [7], Indonesia, and Brazil [8] show sharp increases in malaria morbidity and mortality across all age groups in migrants from low to high transmission areas.
As such, migration has enormous significance for patterns of malaria infection and disease, and for malaria control. When large groups of people move from high to low transmission areas, the immediate result, as measurable by parasite prevalence, would be an overall increase in transmission [9]. But more common than unidirectional permanent migrations are the regular and cyclical movements of migrants or return migrants to areas of low transmission. In either case, a migrant infected with malaria can serve as a reservoir and seed localised outbreaks or epidemics in those areas, and thus migrants become “active transmitters” of infection in low transmission areas [10].
Migration, Malaria, and Drug Resistance
International transit of people with malaria played a significant role in the global dispersal of resistance to both chloroquine and sulphadoxine-pyrimethamine (also known as SP or Fansidar), two drugs that were the mainstay of malaria treatment in Africa for 30 years. In both cases, resistance mutations arose in Southeast Asia and were subsequently imported into Africa during the 1970s for chloroquine, and during the 1980s and 1990s for pyrimethamine and sulphadoxine, respectively [11],[12]. While there was a 17-year lag between the appearance of chloroquine resistance in Southeast Asia and its introduction to Africa, once established in Africa the dispersal of resistance has been shown to follow the predictable process of incremental diffusion [12].
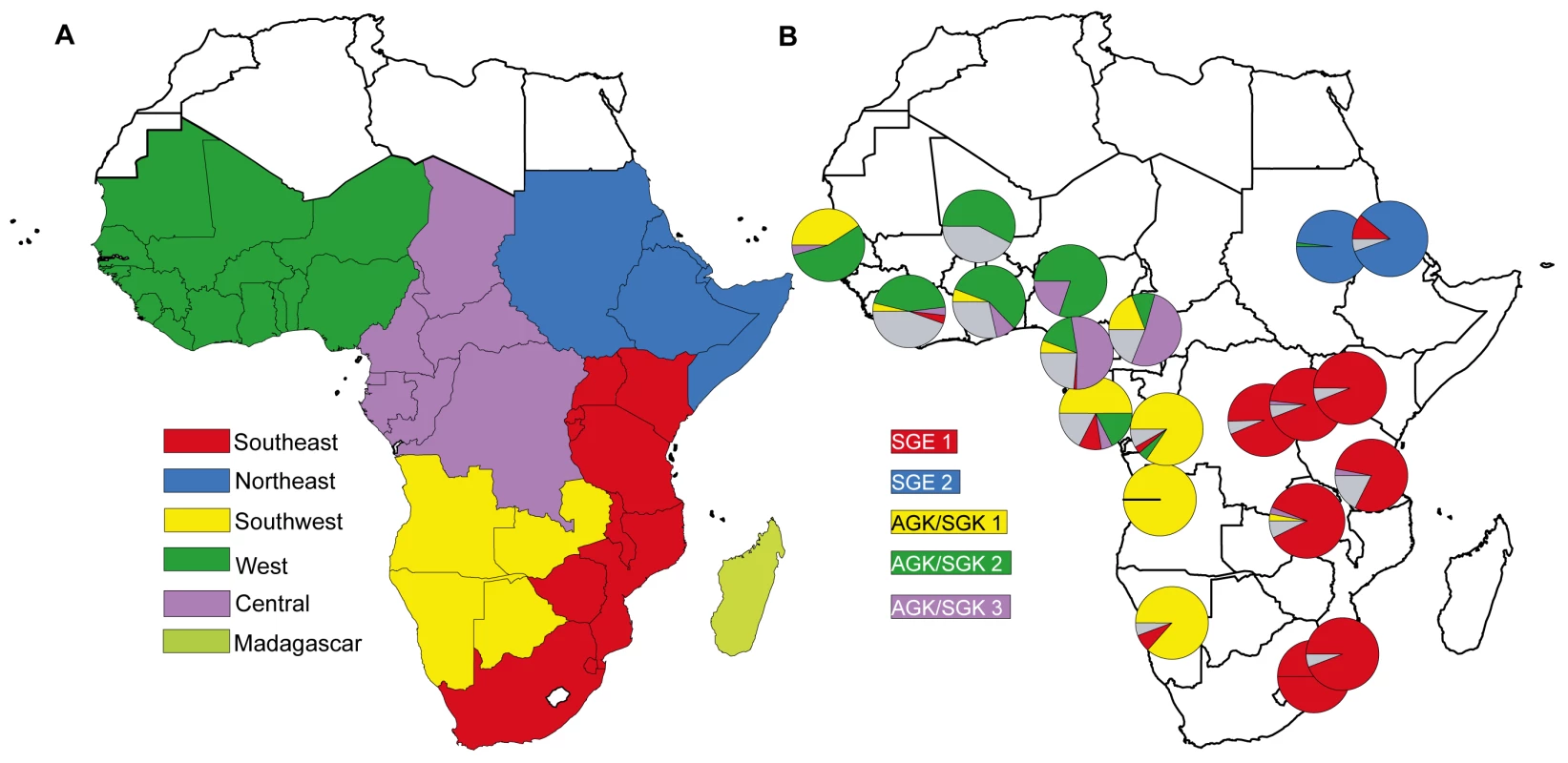
In 2010, Tatem et al. [13] used permanent migration data derived from national census statistics and measures of malaria endemicity to describe communities of malaria-endemic African countries linked by higher levels of infection movement (see Figure 1A). Their analysis highlights “natural” migration regions where high levels of malaria infection are interchanged. A comparison of malaria migration data (Figure 1A) with the geographic distribution of parasite drug resistance lineages (Figure 1B) shows there is broad correspondence between migration and resistance patterns.
The description of the geographic patterns of drug resistance dispersal comes from an analysis [14] that described five major lineages of sulphadoxine resistance at the dhps gene in P. falciparum. These emerged during the 1990s when the SP drug was widely used for treatment of malaria. Each lineage occurred via a single mutation event, and has a distinctive geographical distribution that reflects circulation and dispersal from their site of initial emergence [14]. The map in Figure 1b is a snapshot of the distribution of these resistance lineages among 20 African populations sampled during 2000–2007. The degree to which parasite populations share resistance lineages is an indication of the extent of parasite mixing during the 10–15 years they have been in circulation. The major regional distinctions between parasite populations on the African mainland are between populations in the east and west, and between the northern and southern populations in the east and the west.
The similarities between the geographical pattern of resistance mutation dispersal in Africa and the levels of permanent migration between countries support the view that parasite circulation through regionally distinct migration streams was central to the spread of drug resistance mutations. However, permanent migration reported in census data as “place of birth” captures only a fraction of the migration and transit taking place. Furthermore, having been collated at the national level, these data lack the spatial resolution to examine migration and mobility within countries. Patterns of human circulation change according to the dictates of war, trade, and transport infrastructure. These maps suggest the existence of networks of high volume migration, whose boundaries are unlikely to be national borders.
Case Study: Complex Migration Patterns and Drug-Resistant Malaria in Southwest Uganda
There has emerged a highly resistant form of P. falciparum in the Kabale and Rukungiri districts of southwest Uganda [15] and in bordering areas of Rwanda [16]. The form carries a mutation in the dhfr gene, which confers high level resistance to a number of antifolate drugs in general use (including sulfadoxine-pyrimethamine and chlorproguanil-dapsone). To predict the likely path of dispersal of this resistant form of the parasite, we examined the types and frequency of population movements in Uganda. The region of resistance emergence is shown in red in Figure 2.
Fig. 2. The complex patterns of migration flow in Uganda. 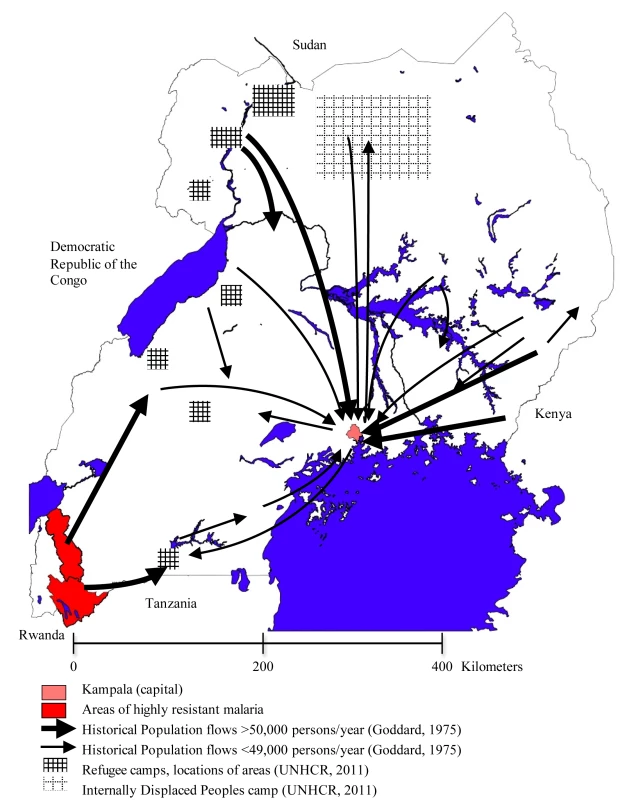
In 2005, Uganda hosted an international migrant pool of 2.25% of its total population, with a reported net migration of 5,000 people [17]. Internal migration is much higher: nationally, 19.7% of the population of Uganda report to have lived in another village, town, or district for more than 6 months at any one time in the last 5 years [18]. And sub-national or district-level studies have shown that only 18% of heads of households were born in their current areas of residence, and this proportion is higher in rural areas (29%) [19].
Uganda also has a long history of hosting refugees from neighbouring countries. In 2008, the country was home to nearly 250,000 refugees from Sudan, the Democratic Republic of the Congo, and Rwanda in camps containing upwards of 3,000 people each [20], and an additional 915,000 IDPs still remain after being displaced during the ongoing conflict in northern Uganda [21]. The location of refugee and IDP camps is illustrated by shaded regions in Figure 2.
The interregional migrant streams in Figure 2 illustrate the central influence of the main city of Kampala. Urbanization is a major component of permanent migration, although it has progressed more slowly in East Africa than in other parts of the world. In 2002, just over 12% of the total Ugandan population was estimated to live in an urban area [18]. Migration for labour is now accepted as part of a household strategy to improve traditional livelihood [19],[22] and has been relatively well-documented in Uganda. Migrant labour is characterised by circulatory rather than permanent movements, with migrants returning to their areas of origin at the end of a formal contract or wage-paying period [23]. This migration system was disrupted with the economic collapse in the 1970s and 1980s, with reports of reverse migration from major urban areas, such as Kampala, back to rural areas [24]. This reverse flow continued until the mid-1980s [25], when urbanisation began to slowly increase again.
Apart from labour migration, the amount of circulatory migration associated with other activities is difficult to establish. Return migration appears to be frequent with an estimated 10% of urban dwellers making return visits to their home areas of origin annually [26],[27].
Migration in Southwest Uganda
The southwest of Uganda in particular has always suffered from scarcity of land, as well as erosion and soil degradation [28], which has led to out-migration from the region [29]. More recently, improved roads and increased vehicle numbers, as well as market liberalisation, has led to a further increase in temporary mobility between rural and urban areas [22].
Another result of high population density has been migration and resettlement, both programmed and spontaneous, into neighbouring areas. The first official resettlement schemes took place in the late 1940s, and involved the transfer of approximately 15,000 people from South Kigezi (now Kabale) into North Kigezi (now Rukungiri) [30]. Further resettlement schemes were undertaken to relocate labour from Kabale and Rukungiri to Bunyoro in order to clear 500 square miles of bush as part of a tsetse barrier scheme [31].
While these schemes ended in the 1960s, migration between Kabale, Rukungiri, and the Bunyoro region continued and the original scheme resulted in a strong migration route opening between these areas [32]. Around the same time (mid to late 1950s) in Kabale, people had begun to encroach into the Bwindi forests in the district [33]. Migration data, based on birthplace information taken during the 1969 population census in Uganda, clearly showed a large migration stream from southwestern districts (Kigezi) into the western Toro and Ankole regions as well as into Kampala and its surrounding areas.
These large northward migrant streams from the affected area of southwest Uganda indicate that the highly drug-resistant malaria parasites have the means to spread quickly within Southern Uganda. The maps in Figures 1 and 2 indicate that the future dispersal of resistance has the potential to extend rapidly throughout east and southeast Africa provided that selection in the form of continuing use of SP for treatment is maintained. The relative importance of different types of travel for the dispersal of malaria is difficult to quantify individually, but it is clear from patterns of dispersal of drug resistance that the most significant migration streams carry huge volumes of traffic and that these should be incorporated into thinking about malaria control.
Geographical Framework of Migration for Malaria Elimination
Programmes for control and eradication of malaria in Africa during the 1950s were undermined by failure to take account of population mobility, and by the difficulties of access to and adequate health care provision for mobile sectors of the population [3]. A geographical framework of malaria dispersal is needed for contemporary national planning and regional coordination of malaria control measures directed towards elimination.
To build such a framework, there is an urgent need for better data on circulation within countries and short-term circulation of populations around porous borders. Countries that have multiple parasite populations, such as the Democratic Republic of the Congo, require detailed surveys of travel behavior across their territory, while countries with large populations of IDPs such as Uganda and Sudan (including Darfur) are also key.
Data on permanent internal migration can be used to describe migration routes and possible migration streams but cannot inform as to the frequency of travel between different places. Permanent migration data from census are informative, but the national scale of the data is not sufficiently high resolution to begin to disentangle the movement of disease within national borders. In particular, permanent migration data will not allow us to capture information on movements between countries with leaky borders, or illegal movement between countries. Some types of circulation in these situations are not captured in surveys because people do not wish to disclose travel across border areas. One example is when IDPs may make a series of return visits to their areas of origin for planting and harvesting but report being present in IDP camps in order to fully avail themselves of food vouchers.
Mapping the dispersal of drug resistance mutations has potential utility in defining regions that encompass significant population mobility and quantifying population connectivity. Such measures summarise the circulation of parasites through all types of migration and have value in this respect, but they are not informative about which migrant sectors of the population should be prioritized for malaria prevention and treatment intervention.
Need for Migration Data in the Containment of Artemisinin Resistance
As we have noted, migration has historically played a pivotal role in the global dissemination of drug resistance. Therefore, the recent confirmation of resistance to artemisinins on the Cambodia–Thailand border [34] has triggered a concerted effort to contain it. In the policy document “Global Planning Artemisinin Resistance Containment” [35], the World Health Organization identifies three tiers of risk, and implies that migration data are central to their definition. Tier I areas have credible evidence of artemisinin resistance; Tier II areas have significant inflows of people from Tier I; and Tier III areas have no evidence of artemisinin resistance and limited contact with Tier I.
Although most of Africa can be comfortably classified as Tier III, the risk posed by incoming migrants cannot be assessed without knowing the number and final destination of travelers, together with their likelihood of carrying a malaria infection. There is currently no policy for screening travelers to Africa and no interventions to prevent the importation of artemisinin-resistant malaria into Africa. Gathering data on malaria infection rates among incoming travelers should be a priority for African national malaria control programmes.
Policy Needs and Recommendations
Clearly the current data on transit in Africa are limited, and more migration data are needed. A geographic framework of human migration at local, national, and international levels is particularly essential. It is only through the establishment of such a framework that policy and services can respond appropriately to the needs of migrants and at the same time sustain the gains made in malaria control initiatives. For example, we need to:
-
1. Identify networks of high volume transit and migration within Africa. This should include non-permanent and cyclical transit both within and between countries.
-
2. Apply malaria control and elimination interventions across areas that encompass significant volumes of migration.
-
3. Identify mobile communities and mobile sectors of the population who need to be prioritized in the provision of malaria treatment and prevention measures.
-
4. Provide targeted health care to these communities.
Transit at the inter-continental, national, and local level have all played a role in the dispersal of antimalarial drug resistance in the past. Arguably, the international transportation of artemisinin-resistant parasites by human migration is the greatest threat to the antimalarial treatments used in Africa today. There are currently no policy measures in place to prevent the importation of artemisinin-resistant parasites to Africa. As a result, we also need to:
-
5. Gather data on the volume of migration between areas of confirmed artemisinin resistance and African destinations.
-
6. Establish what the rate of malaria infection among such travelers is and what proportion will travel to areas where transmission can potentially occur. Once the risks of importation are quantified “a speedy, scientifically sound, and coordinated response from affected countries, donors, and international organisations” is needed [36].
-
7. When artemisinin resistance eventually emerges in Africa, apply migration frameworks to guide strategies for the containment or management of resistance through use of alternative treatment combinations.
Molecular markers for resistance to drugs of the past provide insight into the drivers of resistance dispersal. If the rules governing the spread of resistance can be elucidated now, then policy interventions to protect the efficacy of artesimin combination therapies can be designed proactively rather than reactively and the threat of resistance to new treatments managed with foresight.
Zdroje
1. PapadopoulouA
2005
Exploring the asylum-migration nexus: A case study of transit migrants
in Europe
Geneva
Global Commission on International Migration
2. IPU
2005
Migration and Development
Geneva
Inter-Parliamentary Union (IPU)
3. ProtheroRM
1977
Disease and mobility: a neglected factor in
epidemiology.
Int J Epidemiol
6
259
267
4. ZimmermanCKissLHossainM
2011
Migration and health: A framework for 21st century
policy-making.
PLoS Medicine
In press
5. HaySIGuerraCAGethingPWPatilAPTatemAJ
2009
A world malaria map: Plasmodium falciparum endemicity in
2007.
PLoS Medicine
6
e1000048
6. NajeraJA
1996
Malaria Control among Refugees and Displaced persons
Geneva
7. ProtheroRM
2001
Migration and malaria risk.
Health, Risk & Society
3
19
38
8. SawyerD
1993
Economic and social consequences of malaria in new colonization
projects in Brazil.
Social Science and Medicine
37
1131
1131
9. KazmiJHPanditK
2001
Disease and dislocation: the impact of refugee movements on the
geography of malaria in NWFP, Pakistan.
Social Science & Medicine
52
1043
1055
10. ProtheroRM
1987
Populations on the Move.
Third World Quarterly
9
1282
1310
11. AndersonTJRoperC
2005
The origins and spread of antimalarial drug resistance: lessons
for policy makers.
Acta Trop
94
269
280
12. NaidooIRoperC
2010
Following the path of most resistance: dhps K540E dispersal in
African Plasmodium falciparum.
Trends Parasitol
26
447
456
13. TatemAJSmithDL
2010
International population movements and regional Plasmodium
falciparum malaria elimination strategies.
Proceedings of the National Academy of Sciences
107
12222
12227
14. PearceRJPotaHEveheMSBa elHMombo-NgomaG
2009
Multiple origins and regional dispersal of resistant dhps in
African Plasmodium falciparum malaria.
PLoS Med
6
e1000055
15. LynchCPearceRPotaHCoxJAbekuTA
2008
Emergence of a dhfr mutation conferring high-level drug
resistance in Plasmodium falciparum populations from southwest
Uganda.
J Infect Dis
197
1598
1604
16. CorineKImwongMFanelloCIStepniewskaKUwimanaA
2009
Molecular correlates of high level antifolate resistance in
Rwandan children with Plasmodium falciparum malaria.
Antimicrob Agents Chemother
AAC.00498-00409
17. ParsonsCSkeldonRWalmsleyTWintersL
2007
A database of bilateral migrant stocks.
Available at http://www.migrationdrc.org/research/typesofmigration/global_migrant_origin_database.html
18. UBOS
2007
Uganda Demographic and Health Survey 2006. Kampala, Uganda and
Calverton, Maryland, USA
Kampala, Uganda
Uganda Bureau of Statistics (UBOS) and Macro International
Inc
19. BrycesonDFMaunderDACMbaraTCKibomboRDavisASC
2003
Sustainable livelihoods, mobility and access needs
Crowthorne, UK
Transport Research Laboratory
20. UNHCR
2009
United Nations High Commissioner for Refugees Global Appeal
Report 2008–2009.
http://www.unhcr.org/474ac8d00.html. Kampala
21. IDMC
2010
Internal Displacement Monitoring Centre-Country Profile
Uganda
Internal Displacement Monitoring Centre.
http://www.internal-displacement.org/idmc/website/countries.nsf/(httpEnvelopes)/2439C2AC21E16365C125719C004177C7?OpenDocument
22. DeshingkarPGrimmS
2004
Voluntary Internal Migration: An Update.
Paper commissioned jointly by Urban Rural Change Team and the Migration
Team DFID Overseas Development Institute: London
23. GouldWTS
1995
Regional Labour Migration Systems in East Africa: Continuity and
Change.
The Cambridge Survey of World Migration
183
24. PottsD
1997
Urban lives: Adopting new strategies and adapting rural
links.
RakodiC
The urban challenge in Africa: Growth and management of its large
cities
Tokyo - New York - Paris
United Nations University Press
25. JamalVWeeksJ
1993
Africa misunderstood: Or whatever happened to the rural-urban
gap?
London
Macmillan
26. OuchoJO
1996
Urban migrants and rural development in Kenya
Nairobi: University Press
27. TacoliC
2001
Urbanisation and migration in Sub-Saharan Africa: Changing
patterns and trends.
Mobile Africa: changing patterns of movement in Africa and
beyond
141
28. CarswellG
2002
Farmers and Following: Agricultural Change in Kigezi District,
Uganda.
Geographical Journal
130
140
29. OlsonJBerryL
2003
Land degradation in Uganda: its extent and
impact.
available at lada virtualcentre org/eims/download asp
30. SwanzyH
1951
Quarterly Notes.
Afr Aff (Lond)
50
89
123
31. SwanzyH
1947
Quarterly Notes.
Afr Aff (Lond)
46
181
197
32. BelshawDGR
1984
Planning and agrarian change in East Africa: appropriate and
inappropriate models for land settlement schemes.
Understanding Green Revolutions: Agrarian Change and Development
Planning in South Asia
270
33. JelliffeDBBennettFJStroudCENovotnyMEKarrachHA
1961
Field Survey of the Health of Bachiga Children in the Kayonza
District of Kigezi, Uganda.
The American Journal of Tropical Medicine and Hygiene
10
435
34. DondorpAMNostenFPoravuthYDDP. P.A
2009
Artemisinin Resistance in Plasmodium falciparum
malaria.
New England Journal of Medicine
361
455
467
35. WHO
2011
Global Planning for Artemisinin Resistance Containment (GPARC)
Geneva
36. WhiteNJ
2010
Artemisinin resistance–the clock is
ticking.
Lancet
376
2051
2052
37. GoddardADGouldWTSMasserFI
1975
Census data and migration analysis in tropical
Africa.
Geografiska Annaler Series B Human Geography
26
41
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2011 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Statinová intolerance
- Genetický podklad a screening familiární hypercholesterolémie
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
- DESATORO PRE PRAX: Aktuálne odporúčanie ESPEN pre nutričný manažment u pacientov s COVID-19
-
Všetky články tohto čísla
- Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial
- Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Characterizing the Epidemiology of the 2009 Influenza A/H1N1 Pandemic in Mexico
- The Joint Action and Learning Initiative: Towards a Global Agreement on National and Global Responsibilities for Health
- Let's Be Straight Up about the Alcohol Industry
- Advancing Cervical Cancer Prevention Initiatives in Resource-Constrained Settings: Insights from the Cervical Cancer Prevention Program in Zambia
- The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa
- Health Aspects of the Pre-Departure Phase of Migration
- Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
- Threshold Haemoglobin Levels and the Prognosis of Stable Coronary Disease: Two New Cohorts and a Systematic Review and Meta-Analysis
- If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
- Migration and Health: A Framework for 21st Century Policy-Making
- Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study
- The Impact of Retail-Sector Delivery of Artemether–Lumefantrine on Malaria Treatment of Children under Five in Kenya: A Cluster Randomized Controlled Trial
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy