-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Mapping the Risk of Anaemia in Preschool-Age Children: The Contribution of Malnutrition, Malaria, and Helminth Infections in West Africa
Background:
Childhood anaemia is considered a severe public health problem in most countries of sub-Saharan Africa. We investigated the geographical distribution of prevalence of anaemia and mean haemoglobin concentration (Hb) in children aged 1–4 y (preschool children) in West Africa. The aim was to estimate the geographical risk profile of anaemia accounting for malnutrition, malaria, and helminth infections, the risk of anaemia attributable to these factors, and the number of anaemia cases in preschool children for 2011.Methods and Findings:
National cross-sectional household-based demographic health surveys were conducted in 7,147 children aged 1–4 y in Burkina Faso, Ghana, and Mali in 2003–2006. Bayesian geostatistical models were developed to predict the geographical distribution of mean Hb and anaemia risk, adjusting for the nutritional status of preschool children, the location of their residence, predicted Plasmodium falciparum parasite rate in the 2 - to 10-y age group (Pf PR2–10), and predicted prevalence of Schistosoma haematobium and hookworm infections. In the four countries, prevalence of mild, moderate, and severe anaemia was 21%, 66%, and 13% in Burkina Faso; 28%, 65%, and 7% in Ghana, and 26%, 62%, and 12% in Mali. The mean Hb was lowest in Burkina Faso (89 g/l), in males (93 g/l), and for children 1–2 y (88 g/l). In West Africa, severe malnutrition, Pf PR2–10, and biological synergisms between S. haematobium and hookworm infections were significantly associated with anaemia risk; an estimated 36.8%, 14.9%, 3.7%, 4.2%, and 0.9% of anaemia cases could be averted by treating malnutrition, malaria, S. haematobium infections, hookworm infections, and S. haematobium/hookworm coinfections, respectively. A large spatial cluster of low mean Hb (<80 g/l) and maximal risk of anaemia (>95%) was predicted for an area shared by Burkina Faso and Mali. We estimate that in 2011, approximately 6.7 million children aged 1–4 y are anaemic in the three study countries.Conclusions:
By mapping the distribution of anaemia risk in preschool children adjusted for malnutrition and parasitic infections, we provide a means to identify the geographical limits of anaemia burden and the contribution that malnutrition and parasites make to anaemia. Spatial targeting of ancillary micronutrient supplementation and control of other anaemia causes, such as malaria and helminth infection, can contribute to efficiently reducing the burden of anaemia in preschool children in Africa.
: Please see later in the article for the Editors' Summary
Published in the journal: Mapping the Risk of Anaemia in Preschool-Age Children: The Contribution of Malnutrition, Malaria, and Helminth Infections in West Africa. PLoS Med 8(6): e32767. doi:10.1371/journal.pmed.1000438
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000438Summary
Background:
Childhood anaemia is considered a severe public health problem in most countries of sub-Saharan Africa. We investigated the geographical distribution of prevalence of anaemia and mean haemoglobin concentration (Hb) in children aged 1–4 y (preschool children) in West Africa. The aim was to estimate the geographical risk profile of anaemia accounting for malnutrition, malaria, and helminth infections, the risk of anaemia attributable to these factors, and the number of anaemia cases in preschool children for 2011.Methods and Findings:
National cross-sectional household-based demographic health surveys were conducted in 7,147 children aged 1–4 y in Burkina Faso, Ghana, and Mali in 2003–2006. Bayesian geostatistical models were developed to predict the geographical distribution of mean Hb and anaemia risk, adjusting for the nutritional status of preschool children, the location of their residence, predicted Plasmodium falciparum parasite rate in the 2 - to 10-y age group (Pf PR2–10), and predicted prevalence of Schistosoma haematobium and hookworm infections. In the four countries, prevalence of mild, moderate, and severe anaemia was 21%, 66%, and 13% in Burkina Faso; 28%, 65%, and 7% in Ghana, and 26%, 62%, and 12% in Mali. The mean Hb was lowest in Burkina Faso (89 g/l), in males (93 g/l), and for children 1–2 y (88 g/l). In West Africa, severe malnutrition, Pf PR2–10, and biological synergisms between S. haematobium and hookworm infections were significantly associated with anaemia risk; an estimated 36.8%, 14.9%, 3.7%, 4.2%, and 0.9% of anaemia cases could be averted by treating malnutrition, malaria, S. haematobium infections, hookworm infections, and S. haematobium/hookworm coinfections, respectively. A large spatial cluster of low mean Hb (<80 g/l) and maximal risk of anaemia (>95%) was predicted for an area shared by Burkina Faso and Mali. We estimate that in 2011, approximately 6.7 million children aged 1–4 y are anaemic in the three study countries.Conclusions:
By mapping the distribution of anaemia risk in preschool children adjusted for malnutrition and parasitic infections, we provide a means to identify the geographical limits of anaemia burden and the contribution that malnutrition and parasites make to anaemia. Spatial targeting of ancillary micronutrient supplementation and control of other anaemia causes, such as malaria and helminth infection, can contribute to efficiently reducing the burden of anaemia in preschool children in Africa.
: Please see later in the article for the Editors' SummaryIntroduction
The most up-to-date global estimates of childhood anaemia indicate that 293.1 million children aged <5 y are anaemic worldwide, and 28.5% of those are located in sub-Saharan Africa (SSA) [1]. Childhood anaemia is considered a severe public health problem in SSA, reaching 67% prevalence, or 83.5 million children, in the region [1]. Anaemia in infancy and childhood is associated with reduced cognitive development [2], growth [3], immune function [4], and survival.
Anaemia is usually multifactorial in origin, and malnutrition, infectious diseases, inherited haemoglobinopathies [5], and thalassemias [6] are thought to be the major contributors.
The main micronutrient deficiency contributing to anaemia is iron deficiency [7], but other micronutrients, such as vitamin A [8], vitamin C [9], and folate [10] are important in the pathophysiology of anaemia. Among the most common infectious diseases in SSA, malaria [11], HIV [12], bacteraemia caused by organisms such as Steptococcus pneumoniae, non-typhi Salmonella species, and Haemophilus influenzae type b [13],[14], and helminth infections caused by hookworm and Schistosoma haematobium (the aetiological agent of urinary schistosomiasis) [15]–[17] are known to cause anaemia. The general mechanisms by which these infections lead to anaemia include blood loss, sequestration of red blood cells by the spleen, haemolysis by antibodies, and anaemia of inflammation (via TNF-alpha and IL-6 production) [18],[19]. In the case of parasite infections, synergisms between multiple species infections (coinfection) and high parasite burden (infection intensity) are known to exacerbate anaemia [20]–[22].
The most common form of anaemia is caused by low levels of iron (or iron-deficiency anaemia), and efforts to mitigate its effects include the population delivery of iron supplements and food fortification with iron [23]. However, in addition to undernutrition, immune responses to infections can lead to infection-induced hypoferremia, which prevents the growth of pathogens and can be anti-inflammatory by reducing a potential prooxidant. This well-recognised phenomenon shows that iron deficiency can protect against common infectious agents, and recent empirical evidence suggests iron supplementation is linked to increased severity of infectious disease in the presence of malaria and/or undernutrition in certain subgroups [24]. Anaemia cases in which blood haemoglobin concentration (Hb) falls below 70 g/l are potentially life-threatening situations, and control can be achieved by providing hospital emergency treatment, which includes iron and folate supplementation and blood transfusions [25].
Anaemia control can also focus on infectious disease causes of anaemia. In the case of malaria control programmes, the adoption of artemisinin-based combination therapies for the treatment of malaria patients and the large-scale deployment of long-lasting insecticide-treated bed nets among high-risk groups, especially young children and pregnant women, are currently being promoted [26],[27]. An alternative strategy is chemoprophylaxis with antimalarial drugs: intermittent preventive treatment for women in pregnancy [28] and once-a-term mass administration of a full therapeutic course of antimalarial drugs to schoolchildren [29] are effective at reducing malaria parasitaemia and halving the rates of anaemia among these high-risk groups. The fundamental aim of the helminth control programmes is morbidity control, and the prevalence of anaemia has been used as a measurable target in control programmes for schistosomiasis and soil-transmitted helminths [30]. The basis of control of helminth infections is mass administration of single-dose antihelminthics [31]. In areas with high prevalence of helminth infection, treatment of severe anaemia cases generally includes deworming [32]. However, supplementation has been found to be inefficient in the presence of inflammation due to iron sequestration, and deworming is warranted when anaemia coexists with high parasite prevalence [25]. Mass deworming has been shown to cause a small increase in Hb in preschool and school-age children in SSA [33]. With the aim of alleviating the anaemia burden of endemic populations, micronutrients are also being distributed as part of deworming programmes [34]. For example, vitamin A supplementation is being given to preschool and school-age children in many countries in Africa as a single dose immediately after deworming [35].
Targeting the correct set of interventions to population subgroups at increased risk of anaemia would have important implications in more efficient delivery of limited national resources. Modern spatial risk prediction methods are being used as control tools for targeting malaria [36] and helminth infection [37] interventions in SSA. To date there are no studies that have predicted spatially the risk of anaemia. Furthermore, the contribution that malnutrition and infections make to the overall anaemia burden is largely unknown. Population attributable fractions (PAFs) are useful for translating surveillance data on risk factor prevalence and disease occurrence into numbers that can help policymakers and the public appreciate the potential benefits to be gained by risk factor reduction and health promotion [38]. This information could provide an important evidence base to work out the best delivery balance between micronutrient supplementation and food fortification versus deworming and malaria control.
In this paper, we describe unique preschool anaemia data from national surveys in three contiguous countries in the West African region (Burkina Faso, Ghana, and Mali) and predict, to our knowledge for the first time, the prevalence of anaemia and mean Hb across the region. In doing so, we adjust for malnutrition and the prevalence of infection of the major parasitic contributors and estimate the attributable risk of anaemia due to malnutrition, malaria, and helminth infections. We aimed to develop a predictive decision-support tool for quantifying the overall burden of anaemia, spatial heterogeneity in the anaemia burden, and the contribution that malnutrition and parasitic infections make to preschool anaemia.
Methods
Data
The preschool anaemia data used in this study were collected within the Demographic and Health Surveys (DHS) programme. These datasets are in the public domain and are available from Measure DHS (http://www.measuredhs.com/login.cfm) on demand. Anaemia data were collected by the MEASURE DHS+ programme using standardised protocols and anaemia testing procedures [39]. Capillary blood samples in young children were obtained by heel prick and were tested using the Hemocue blood haemoglobin testing system, which is considered a durable and reliable system under field conditions [39]. More detailed information on DHS survey design and anaemia testing data are available online (http://www.measuredhs.com) and is summarised in Text S1.
Anthropometric measures (height-for-age Z-score, an indicator of stunting; weight-for-height Z-score, an indicator of wasting; and weight-for-age Z-score, an indicator of underweight) and data on anaemia status for children aged 1–4 y were extracted from the DHS household survey datasets for Burkina Faso (2003), Ghana (2003), and Mali (2006). Although these surveys included data for children aged <1 y, we selected children aged 1–4 y only since children aged <1 y are known to experience a physiological decrease of Hb [11] and Hb in children 0–1 is dependent on maternal iron provisioning and therefore is likely to be confounded by maternal anaemia status—these physiologic factors would inhibit accurate estimation of anaemia risk in infants. To classify the undernutrition of preschool-age children we used composite index of anthropometric failure (CIAF) groupings, which provide a summary statistic of anthropometric failures [40]. The CIAF is a method of partitioning undernutrition in children into seven mutually exclusive categories including no anthropometric failure (CIAF Group A), single anthropometric failures (stunting only, CIAF Group F; wasting only, CIAF Group B; and underweight only, CIAF Group Y), and multiple anthropometric failures (stunting and underweight, CIAF Group E; wasting and underweight, CIAF Group C; and wasting, stunting, and underweight, CIAF Group D).
The geographical unit of the DHS surveys is the sample “cluster”. These are usually census enumeration areas, sometimes villages in rural areas or city blocks in urban areas. Coordinates taken at the centre of each cluster were used to geo-locate clusters in the three study countries. We extracted spatially predicted values of P. falciparum parasite rate in the 2 - to 10-y age group (Pf PR2–10) for each DHS cluster using the geographical information system ArcView version 9.3 (ESRI). These spatial predictions were created by the Malaria Atlas Project (http://www.map.ox.ac.uk/) using model-based geostatistics (MBG); the Pf PR2–10 was estimated based on data from microscopy (approximately 80%) and rapid diagnostic tests (approximately 20%) [36]. We used previously reported parasitological survey data of hookworm and S. haematobium infections in Burkina Faso, Ghana, and Mali and MBG [37],[41],[42] to predict helminth infection risk across the region. Data for preschool-age children were not collected in these parasitic surveys, and predictions specific to the 1 - to 4-y age group were, therefore, not available. We age-standardised the spatial prediction maps available for the 5 - to 9-y age group to the 1 - to 4-y age group based on age-prevalence profiles of these infections (more detail in Text S1). Spatially predicted values of prevalence of infection and coinfection with S. haematobium and hookworm in children aged 1–4 y were then extracted for each DHS cluster in the geographical information system for spatial modelling. A 5×5 km resolution rural/urban surface derived from the Global Rural-Urban Mapping Project beta product was obtained from the Center for International Earth Science Information Network of the Earth Institute at Columbia University (http://sedac.ciesin.columbia.edu/gpw/global.jsp). The values of this surface were extracted for each DHS survey cluster in the geographical information system to define whether the residence was urban or rural.
Spatial Risk Prediction
Blood Hb is the key indicator for anaemia, and different age groups have different cut-off points for the haemoglobin level below which an individual is classified as anaemic [23]. A cut-off of <110 g/l was used to define anaemia in children aged 1–4 y, based on altitude-adjusted Hb available in a continuous scale. Within the group of anaemic individuals, the severity level can also be defined by clinically relevant altitude-adjusted Hb cut-offs: mild anaemia, 100–109 g/l; moderate anaemia, 70–99 g/l; and severe anaemia, < 70 g/l [23]. The initial candidate set of predictor variables included gender, age in months, number of members in the household, residence (rural/urban), CIAF group, and the cluster-level ecological variables of Pf PR2–10, prevalence of S. haematobium infection, prevalence of hookworm infection, and prevalence of S. haematobium and hookworm mono - and coinfection.
We developed spatial prediction models using the Bayesian statistical software WinBUGS version 1.4 (Medical Research Council Biostatistics Unit and Imperial College London). All models had the individual covariates plus a geostatistical random effect, in which spatial autocorrelation between locations was modelled using an exponentially decaying autocorrelation function (Text S1). Model selection for the prediction stage was based on the evaluation of the deviance information criteria (DIC) of each model (the lower the DIC, the better the model fit). Spatial prediction was based on MBG, using the model with the lowest DIC [43]. Statistical notation of Bayesian geostatistical models and spatial interpolation procedures are presented in Text S1.
Model Validation
To assess the predictive performance of the final models of prevalence of anaemia and Hb, a single validation dataset was generated by random selection of 25% of the data (more detail in Text S1). The ability of the models to predict known mean prevalence of anaemia and mean Hb was assessed by three summary statistics: mean prediction error, mean absolute prediction error, and the correlation coefficient between the predicted and the actual values. The mean prediction error provides a measure of the bias of the predictor, the mean absolute prediction error provides a measure of the mean accuracy of individual predictions, and the correlation coefficient provides a measure of association between the observed data and prediction sets. The correlation between the observed and prediction data were visualised using scatter plots with a least-squares best fitting line and 95% confidence intervals. The ability of the final model to predict anaemia endemicity class membership was assessed by comparing the predicted prevalence of anaemia to the observed prevalence, dichotomised at 80%. Following the same procedure, the predicted mean Hb was compared to the observed mean Hb, dichotomised at 90 g/l. The area under curve (AUC) statistic of the receiver operating characteristic curve was used for the comparison [44]. An AUC value of 0.7 was taken to indicate acceptable predictive performance.
Estimation of the Number of Children Aged under 5 y with Anaemia and the Population Attributable Fraction of Anaemia Due to Different Contributors
We extracted population density data (total heads per 2.5 arc-minute grid cell) for Burkina Faso, Ghana, and Mali from the Gridded Population of the World (GPWv3) map for 2009 [45]. The population structure and population growth rate of each country was obtained from the World Population Prospects 2008 Revision Population Database (Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat; http://esa.un.org/unpd/wpp/unpp/panel_population.htm). However, the age categories were slightly different to those used in our analysis. The proportion aged 0–4 y obtained from the World Population Prospects database was discounted by a factor of 0.2 to obtain the proportion aged 1–4 y (the age group in our study). The population density map was multiplied by the proportion of the population aged 1–4 y in each country and by the estimated population growth rate for the period 2005–2011 to derive a map of the number of children aged 1–4 y in 2011 in each grid cell.
Estimates of the PAF for specific predictors are used to guide policymakers in planning public health interventions [46]. Estimation procedures for PAF of anaemia for helminth infections in the 1 - to 4-y age group are presented in Text S1.
Results
Survey Results
A total of 7,147 children aged 1–4 y, including 3,477 girls and 3,670 boys, in Burkina Faso (2,096 children), Ghana (2,360 children), and Mali (2,691 children) were included in the analysis. We included in the analysis all children with complete geographical (i.e., DHS cluster coordinates), demographical (i.e., age, gender, and number of members in household), and morbidity (i.e., Hb and malnutrition status) information. The mean age in months was 34.4 (standard deviation [SD]: 13.7), and the mean number of members per household was 7.7 (SD: 4.4). The spatial distribution of the raw prevalence of anaemia at 1,192 locations in the study area is presented in Figure 1. Results from the DHS data used show that the prevalence of mild, moderate, and severe anaemia was 21%, 66%, and 13% in Burkina Faso; 28%, 65%, and 7% in Ghana, and 26%, 62%, and 12% in Mali (Table 1). There was a significant difference in the proportion of mild anaemia between Burkina Faso and the other countries (p = 0.015), and there was also a significant difference in the proportion of severe anaemia between Burkina Faso and Ghana (p = 0.021), but not between Burkina Faso and Mali. None of the remainding geographical differences in anaemia levels were significant. The results indicate that prevalence of anaemia is highest in children aged 1–2 y and decreases with increasing age (Figure 2). By contrast, Hb steadily increases with age (Figure 3). The mean Hb was lower in Burkina Faso (89 g/l) than in Ghana (97 g/l) (p = 0.027) and Mali (94 g/l) (p = 0.047). It was lower in males (94 g/l) than females (96 g/l) (p>0.05), and for children aged 1–2 y (87 g/l) than for children aged 2+ y (99 g/l) (p<0.001). The prevalence of stunting, wasting, and being underweight in the study area was 87.8%, 89.7%, and 71.2%, respectively. The prevalence of anthropometric failures based on CIAF groupings was the following: 3.3% for no anthropometric failures (Group A), 7.1% for single failures (Groups Y and F), 7.0% for wasting and underweight (Group C), and 62.4% for wasting and stunting and underweight (Group D). The mean Pf PR2–10 and rates of S. haematobium infection, hookworm infection, and S. haematobium/hookworm coinfection for the study area were 52.0% (SD: 12.5), 26.8% (SD: 19.1), 8.2% (SD: 10.0), and 3.6% (SD: 5.7), respectively.
Fig. 1. Mean prevalence of anaemia at 1,192 DHS survey sites. 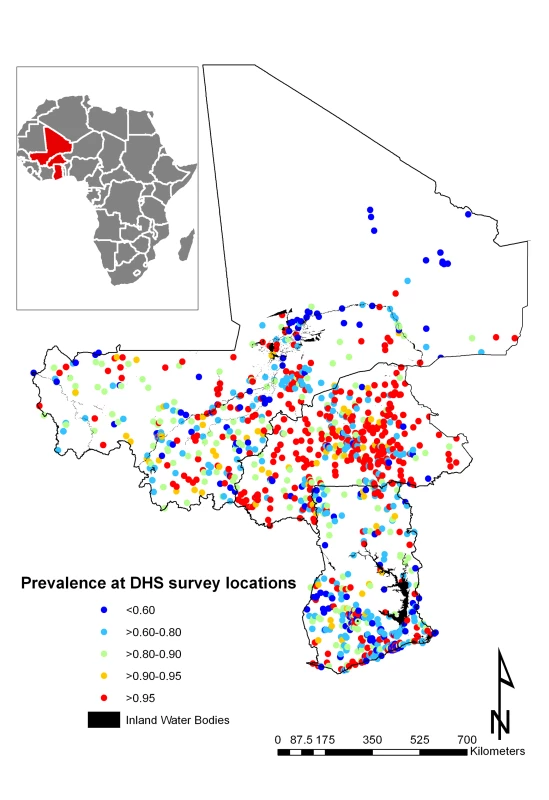
Surveys conducted in Burkina Faso (2003), Ghana (2003), and Mali (2006). Fig. 2. Profile of anaemia by age in Burkina Faso, Ghana, and Mali. 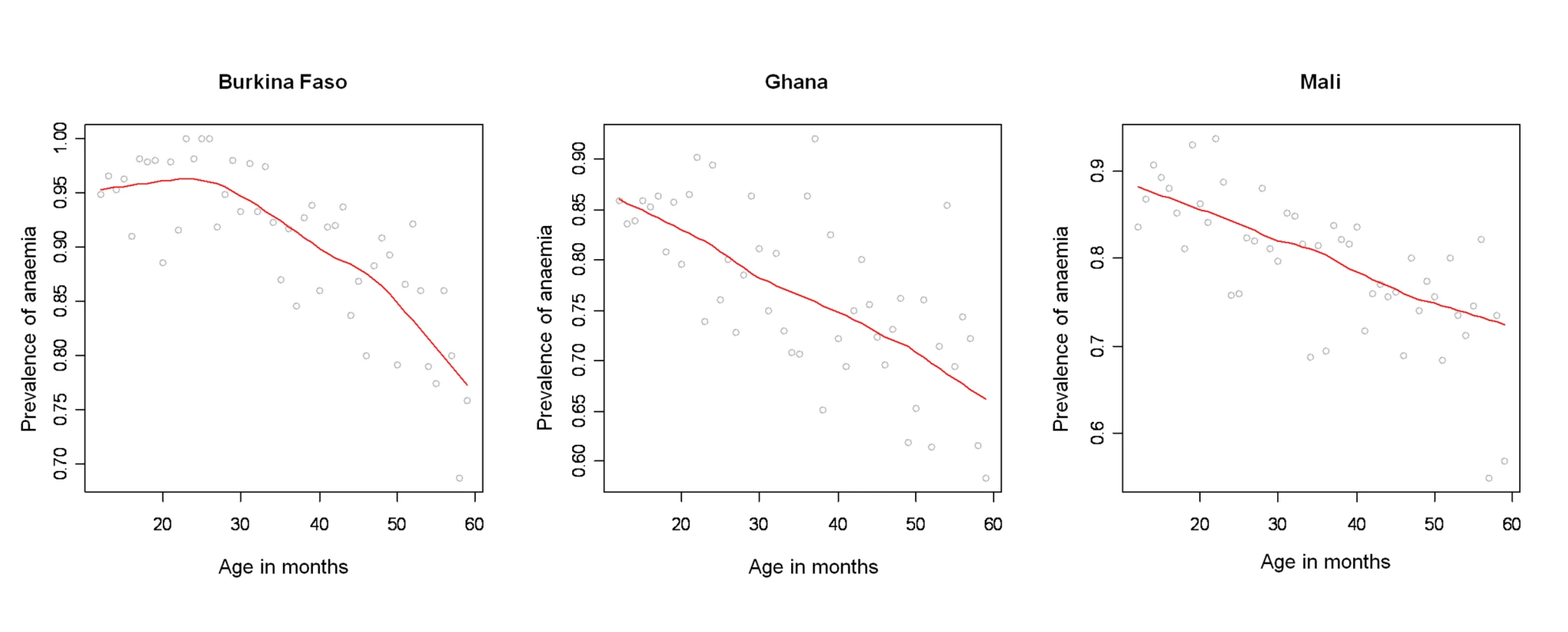
Anaemia (y-axis; Hb<110 g/l) by age in months (x-axis; in months) with smooth fit line (red line) generated by a loess smoother, in children aged 1–4 y in the DHS surveys for Burkina Faso (2003), Ghana (2003), and Mali (2006). Fig. 3. Age patterns of mean haemoglobin concentration. 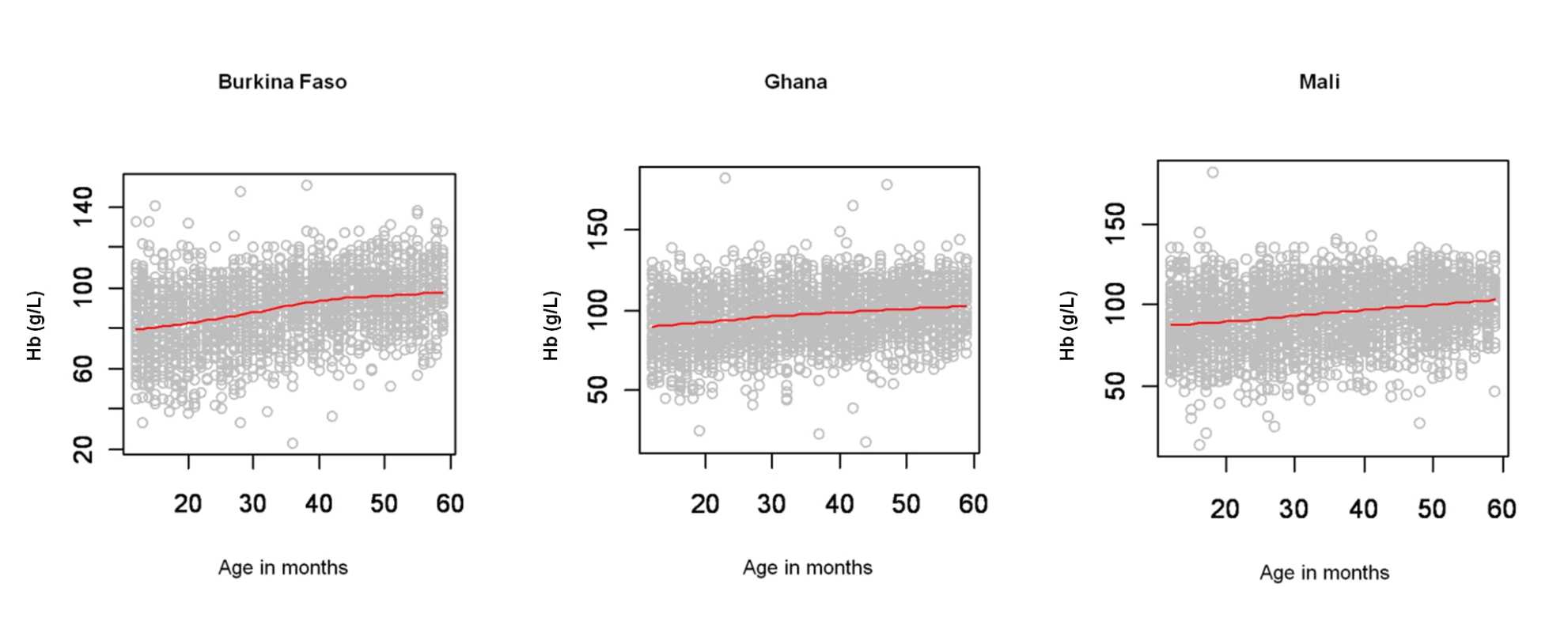
Age (x-axis; in months) patterns of mean Hb (y-axis; g/l) with smooth fit line (red line) generated by a loess smoother, in children aged 1–4 y in the DHS surveys for Burkina Faso (2003), Ghana (2003), and Mali (2006). Tab. 1. Number and proportion of children aged 1–4 y with mild anaemia, moderate anaemia, and severe anaemia in 5,888 anaemic children in the West Africa region. 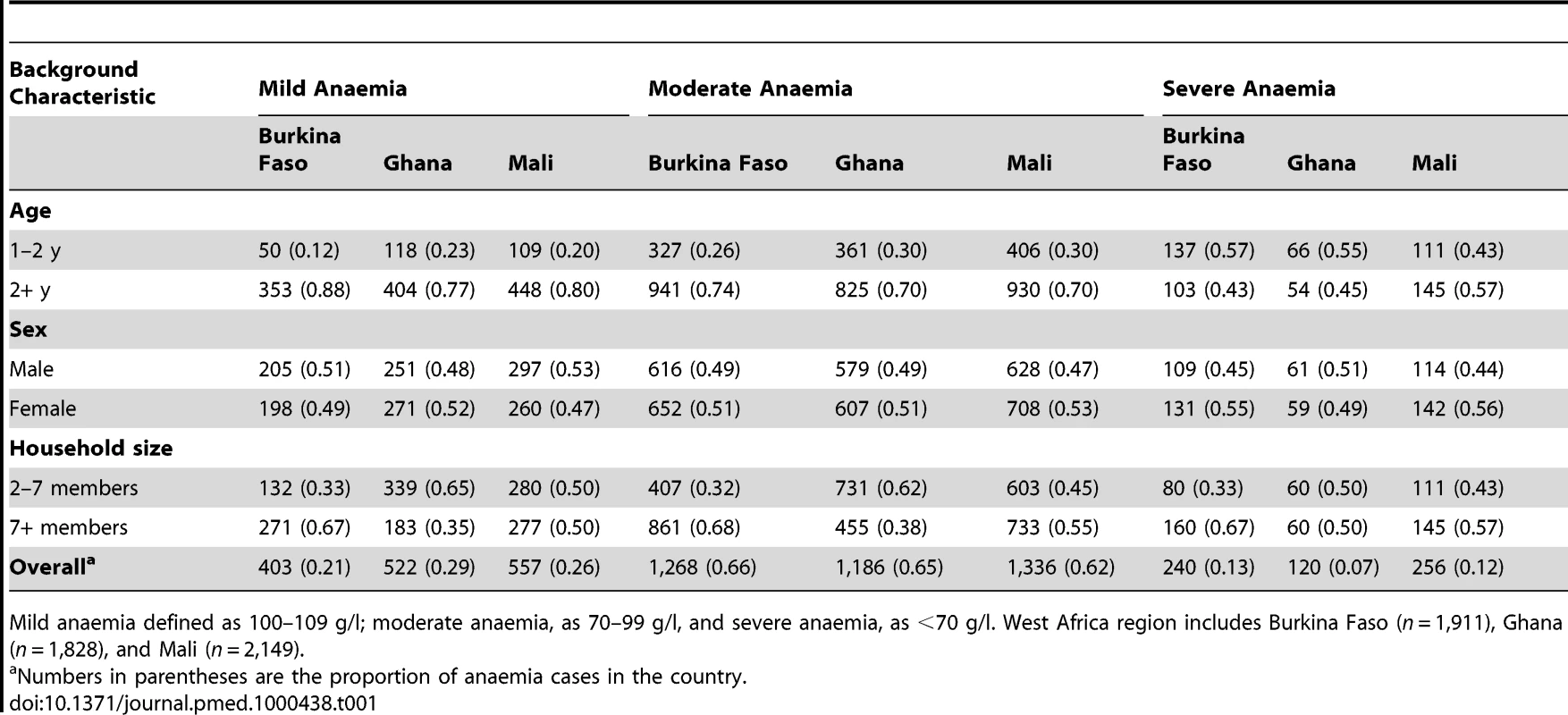
Mild anaemia defined as 100–109 g/l; moderate anaemia, as 70–99 g/l, and severe anaemia, as <70 g/l. West Africa region includes Burkina Faso (n = 1,911), Ghana (n = 1,828), and Mali (n = 2,149). Predicted Risk of Childhood Anaemia
It can be seen from the 95% credible interval, that individual-level variables significantly associated with risk of anaemia in all models tested are age in months, residence (rural versus urban), and having three anthropometric failures (CIAF Group D); gender, the number of members in the household, and other CIAF groupings were not associated with anaemia risk (Table 2). In all models tested, the Pf PR2–10 was significantly and positively associated with anaemia risk. In model 6, the fixed effect of prevalence of hookworm and the product term between the prevalence of S. haematobium and the prevalence of hookworm infection were significantly and positively associated with risk of anaemia. While not significant at the 5% level, prevalence of S. haematobium (models 4 and 6) and the prevalence of coinfection (model 3) were positively associated with anaemia. The model with the lowest DIC was model 6, and, therefore, this model was used in the prediction phase. This model was able to predict prevalence of anaemia being greater that 80% with an AUC>0.8 (Table 3). The risk of anaemia in children aged 1–4 y was consistently high across the entire study area, with maximal prevalence (>95%) in a large focus straddling the borders of Burkina Faso and Mali (Figure 4). Smaller sized foci of high prevalence of anaemia were also predicted for southern areas of Mali, central areas of Burkina Faso, northern areas in Ghana, and areas adjacent to Volta Lake in Ghana. Phi (φ) indicates the rate of decay of spatial autocorrelation and varied from 13.68 in model 3 to 14.80 in model 5. Therefore, after accounting for the effect of covariates in model 6, the radii of the foci were approximately 23 km (note, φ is measured in decimal degrees and 3/φ determines the cluster size; one decimal degree is approximately 111 km at the equator).
Fig. 4. Predictive geographical risk of anaemia in children aged 1–4 y, based on a model-based geostatistical Bernoulli model. 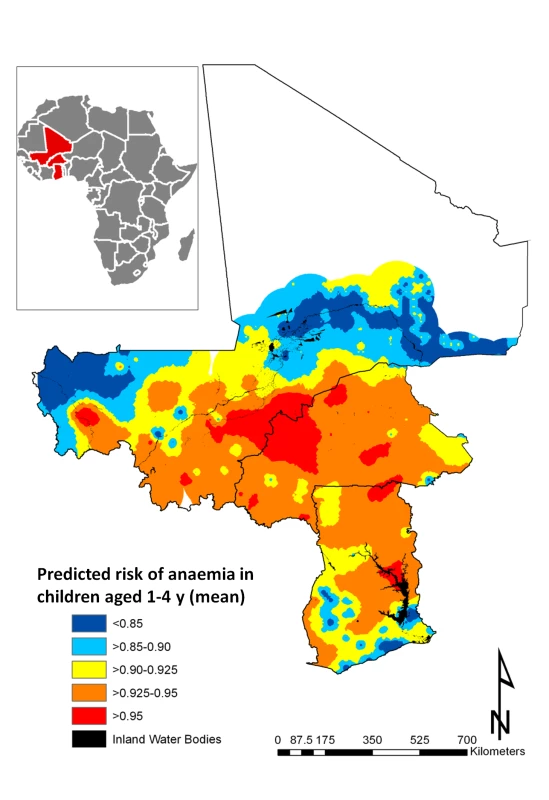
Tab. 2. Associations with anaemia risk, based on model-based geostatistical Bernoulli models. 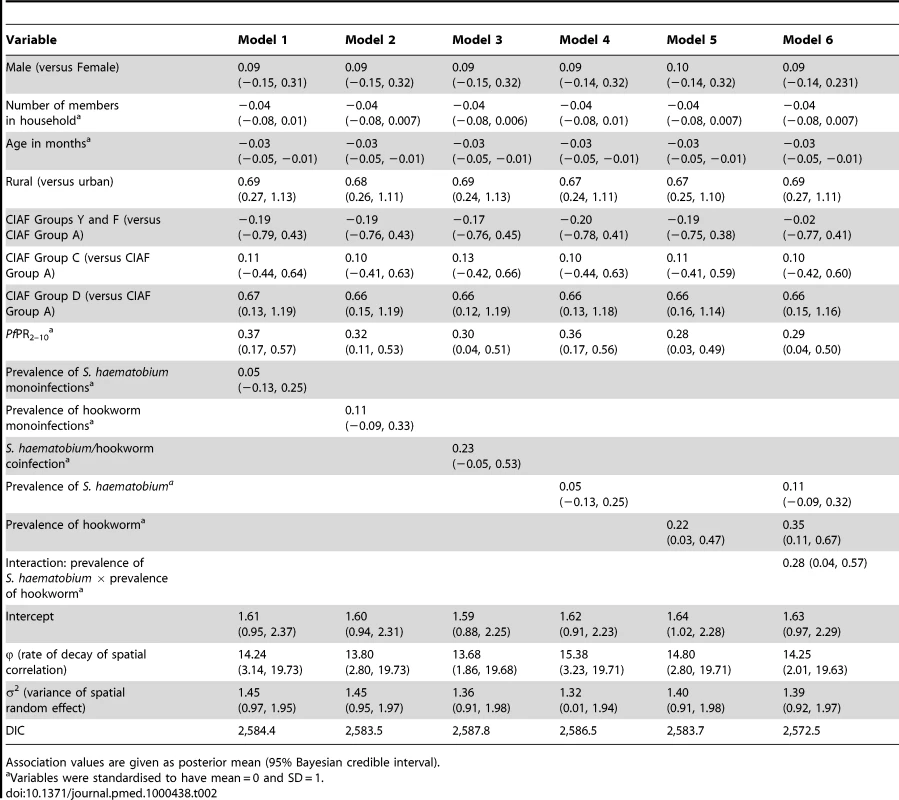
Association values are given as posterior mean (95% Bayesian credible interval). Tab. 3. Summary of validation statistics for predictive models of anaemia prevalence and haemoglobin concentration in Burkina Faso, Ghana, and Mali. 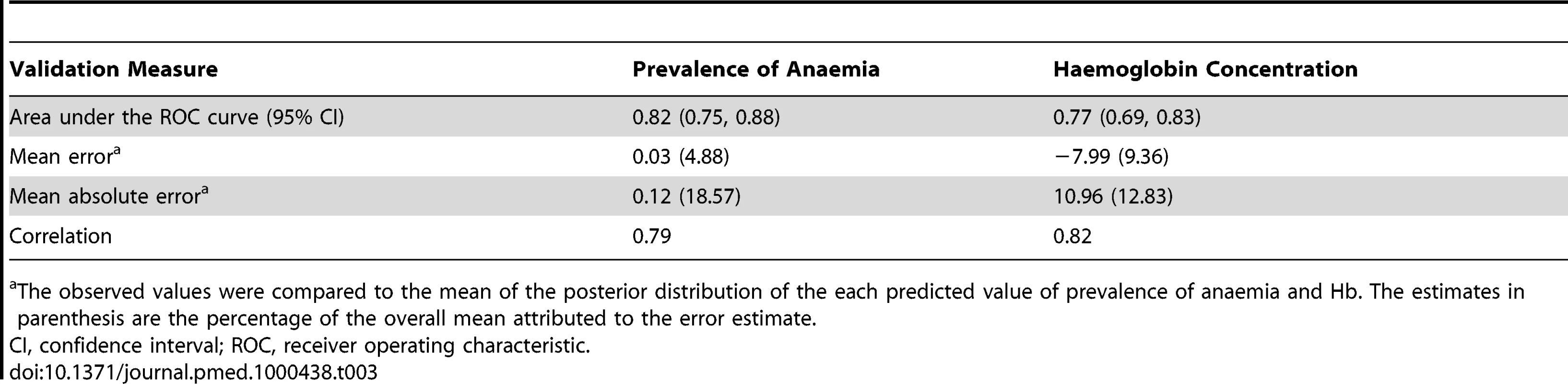
The observed values were compared to the mean of the posterior distribution of the each predicted value of prevalence of anaemia and Hb. The estimates in parenthesis are the percentage of the overall mean attributed to the error estimate. Predicted Mean Haemoglobin Concentration
All individual-level variables except number of members in household, age in months, and single anthropometric failures (CIAF Groups Y and F) were significantly associated with mean Hb in all models tested (Table 4). While rural residences and two or more anthropometric failures were significantly and negatively associated with the mean Hb, there was a significant positive association with mean Hb in male children. As with the models of risk of anaemia, Pf PR2–10 was significantly associated with mean Hb in all models tested. At the 5% level neither S. haematobium nor hookworm infection was significantly associated with mean Hb; however, S. haematobium/hookworm coinfections, hookworm monoinfections, and hookworm prevalence of infection were negatively associated with mean Hb. Estimates presented in Figure 5 are the mean posterior predicted mean Hb values from model 6 (the model that yielded the lowest DIC); this model was able to predict Hb greater than 90 g/l with an AUC >0.7 (Table 3). Figure 5 shows overlapping similarities to the map showing the predicted risk of anaemia (Figure 4) in that areas where Hb was predicted to be lowest (<80 g/l) for children 1–4 y are localised in a large focus straddling the borders of Burkina Faso and Mali. After accounting for the effect of covariates in model 6, the radii of the foci were approximately 22 km (Table 4).
Fig. 5. Predictive geographical variation of mean haemoglobin concentration in children aged 1–4 y, based on a model-based geostatistical Gaussian model. 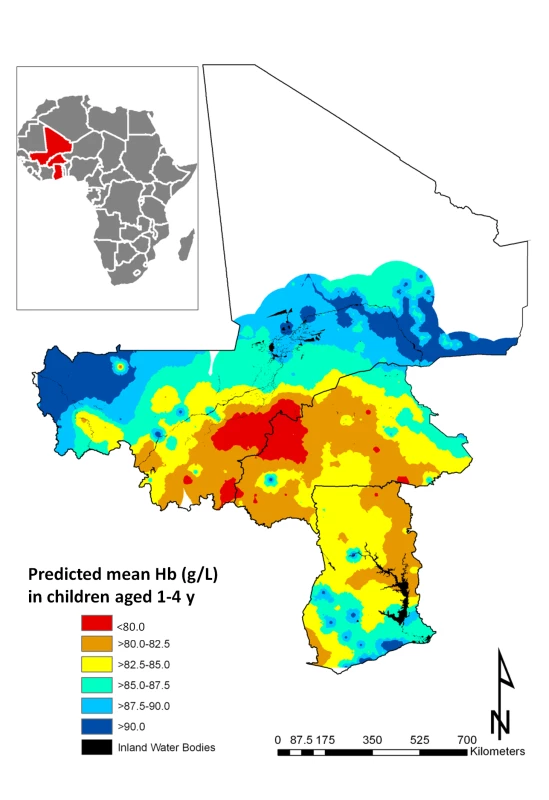
Tab. 4. Associations with altitude-adjusted haemoglobin concentration, based on model-based geostatistical Gaussian models. 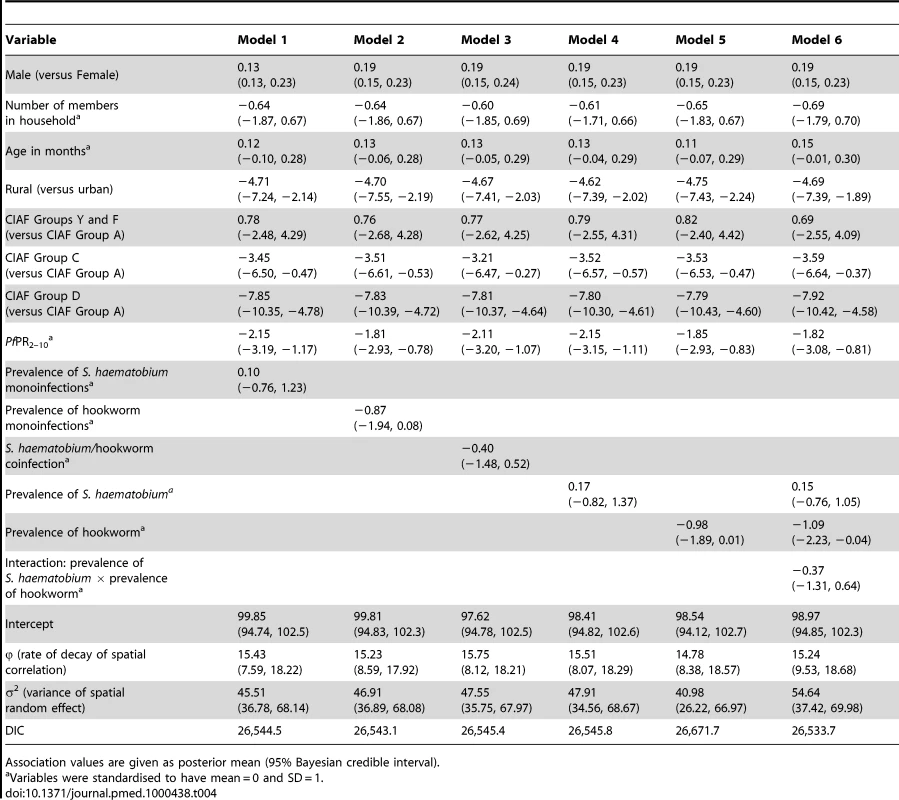
Association values are given as posterior mean (95% Bayesian credible interval). Risk of anaemia attributable to S. haematobium and hookworm infections
We estimated the PAF of anaemia due to CIAF Group D, P. falciparum, S. haematobium, and hookworm separately from model 6, and that due to coinfections from Model 3. Our results indicate that the estimated risk of anaemia attributable to CIAF Group D, P. falciparum, S. haematobium, hookworm, and S. haematobium/hookworm coinfection is 36.8%, 14.9%, 3.7%, 4.2%, and 0.9%, respectively.
Number of children and geographical distribution of childhood anaemia
The predicted total number of children aged 1–4 y with anaemia in Burkina Faso, Ghana, and Mali for 2011 is presented in Table 5. Our results indicate that in the three countries, approximately 6.7 million children aged 1–4 y are anaemic. Severe malnutrition, P. falciparum infection, hookworm infection, S. haematobium infection, and S. haematobium/hookworm coinfection were responsible for an estimated 2.5 million, 1.0 million, 250,000, 285,000, and 61,000 anaemia cases, respectively, in 2011. The areas with the greatest predicted number of anaemic children are located central Burkina Faso and southern Ghana (Figure 6). Figure 6 shows the number of children with anaemia in each 5×5 km pixel.
Fig. 6. Predictive geographical variation of number of children aged 1–4 y with anaemia, for 2011. 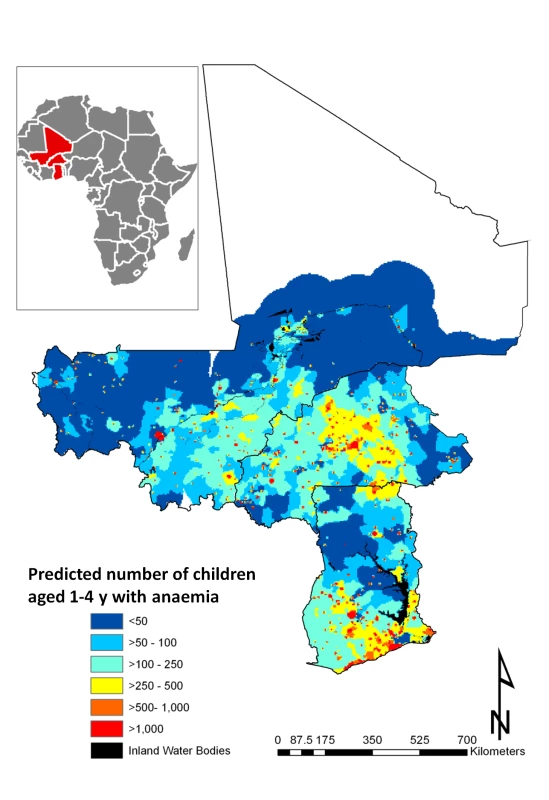
Tab. 5. Predicted number of children aged 1–4 y with anaemia in Burkina Faso, Ghana, and Mali in 2011. 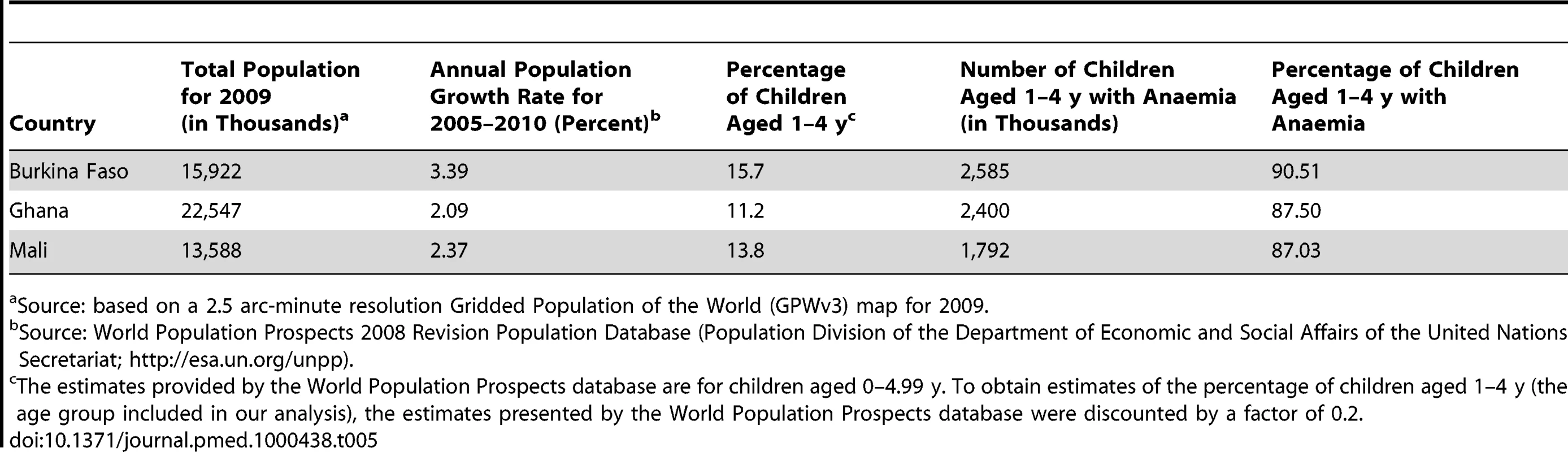
Source: based on a 2.5 arc-minute resolution Gridded Population of the World (GPWv3) map for 2009. Discussion
This study presents new cartographic resources that shed new light on the ranking of anaemia prevalence and anaemia severity within the countries studied by depicting important sub-national geographical heterogeneities, representing an added value over and above what could be achieved directly from national-level summary statistics of the DHS data alone. The approach addresses important operational constraints for anaemia control in the African continent, and the resulting maps could provide the next step needed for efficient and effective anaemia control in preschool children in the following ways. First, they could be used by national programme managers as decision-support tools for targeting the delivery of ancillary micronutrient supplementation and fortified food, with the aim of reducing iron-deficiency anaemia. Second, empirical maps of anaemia in this age group would allow the identification of subgroups where the secondary effects of micronutrient supplementation could be minimised. For example, the main concern about iron supplementation is the fact that it has been linked to increased severity of infectious disease in the presence of malaria and/or undernutrition in preschool children [24]. Finally, anaemia maps would allow the monitoring and evaluation of the impact of anaemia control programmes and, in the case of severe anaemia, planning resource allocation to combat life-threatening anaemia [37].
Burden of Childhood Anaemia for West Africa in 2011
Based on the World Health Organization classification system for anaemia prevalence [23], it is clear that anaemia is a severe public health problem in the study area. We demonstrated that anaemia risk in children aged 1–4 y is high throughout the study area, with the highest risk in a large region extending across the borders of Burkina Faso and Mali. We predicted that the number of childhood anaemia cases is highest in Burkina Faso, followed by Ghana and Mali, and the magnitude of our predictions is consistent with estimates recently reported by the World Health Organization [1]. Using a predictive map of mean Hb we have shown that areas of severe anaemia are much smaller but overlap with areas where the prevalence of anaemia was predicted to be highest (>95%). These results suggest that resources for the treatment of moderate to severe anaemia, such as iron supplementation, deworming, and blood for emergency transfusion, should be prioritised towards populations located in the clusters of high anaemia risk identified in this study.
This study reveals that malnutrition plays a central role in preschool anaemia burden in West Africa. The model including malnutrition, PfPR2–10, and helminth coinfection (Model 6) indicates that almost 40% of anaemia cases in preschool children in 2011 would have been averted by improving the nutritional status of children. Socio-economic status is a well-known risk factor for anaemia and infection at small spatial scales [47], and our results show that rural households are at significantly increased risk of anaemia compared to urban households. The same model also underlines the role of malaria infection in preschool children anaemia burden in the West African region in that the proportion of anaemia attributable to malaria was approximately 15%. These results are supported by earlier findings in Kenya using individual-level data (14% for infected preschool-age children and 7% for the whole population) [17]. The risk of anaemia attributable to hookworm infection (4.2%) is comparable to that estimated for S. haematobium (3.7%) and is significantly increased in hookworm/S. haematobium coinfections. This is consistent with evidence suggesting that morbidity associated with these infections is more pronounced in individuals with multiple infections [21]. Hookworm and S. haematobium infections have the smallest attributable risks both because the relative risk for these factors is modest and more importantly because the frequency of their mean prevalence in the population is low compared to malnutrition and malaria. Nevertheless, these results suggest that hookworm and S. haematobium infections are also important in the aetiology of anaemia in preschool children in West Africa, and deworming should be included in programmes aimed at controlling anaemia in this age group.
We calculated that a total of 6.7 million children aged 1–4 y in Burkina Faso, Ghana, and Mali are anaemic. Our regional - and country-level estimates of number of children with anaemia are in line with estimates recently put forward by the World Health Organization in the three study countries [1]. In that regard, our study generated an important cartographic resource, providing important new information about sub-national priority areas for targeting anaemia control in the region and the quantity of resources needed in those areas (Figure 6).
Using Predictive Parasite Infection Maps to Model Anaemia
Important uncertainties should be noted from the anaemia DHS datasets and the prediction surfaces for parasite infection used in our models, which are likely to be propagated through the modelling framework. The outcome input data from the DHS surveys (anaemia and Hb) were collected in different years (2003 for Burkina Faso and Ghana and 2006 for Mali), and the covariate input prediction surfaces for parasite infection (malaria and helminth predictive surfaces) were for 2007. In order to assess relationships between anaemia indicators and potential contributors, we assumed that there was no contraction in anaemia cases in the three countries between the year anaemia data was collected and 2007. Although this temporal disparity may not be so problematic in the case of the DHS data for Mali, it may be problematic for Burkina Faso and Ghana; an overestimation of effects in those countries could be observed particularly in areas where the effects of intervention efforts to control anaemia were substantial. However, the degree to which the observed relationships are obscured by past spatially variable intervention efforts is not quantified in the literature.
A rigorous assessment of the uncertainty associated with the mapped outputs of the input African malaria map was undertaken by [36]. This assessment provides great confidence about the input surface for the countries in our study in that the probability of correct endemicity class prediction was highest in West Africa. In this region, uncertainty was most important in small areas in southwest Ghana and northwest Mali. These latter estimates adjust for population density (using the population-weighted index in uncertainty) and reflect the co-occurrence of both low density of PfPR2–10 surveys and large populations in these regions. Despite the fact that point predictions generated by the malaria model are reasonably accurate, the model was shown to underestimate the probability of PfPR2–10 taking low values. This means that in low endemicity areas the PfPR2–10 may be overestimated [36]. However, our study is located in countries where malaria endemicity is high, and therefore we do not expect this suboptimal performance to significantly affect the point values of malaria endemicity used in our models. Similarly, the results of uncertainty assessment for the helminth infection covariate surfaces give us great confidence about their use in our models. The predictive ability of endemicity class membership (<50% for S. haematobium infection, 10% for hookworm infection, and 5% for S. haematobium/hookworm coinfection) was moderately good, with all AUC values above 0.7 [37].
Finally, by using existing continental-level and other mapped layers as proxies of parasite infection, we have adopted an ecological approach to modelling anaemia prevalence and Hb. This approach was chosen because comparable individual-level infection data were not available for the study area. Instead, the mean prevalence of parasite infection was used as a proxy for the true infection status of preschool children included in the analysis. This approach provides a somewhat imprecise measurement of exposure to P. falciparum and helminth infection and therefore may result in regression dilution bias arising from imprecise exposure measurement, which is most likely to lead to underestimation of the observed effects of parasite infections [48]. Although the observed relationships are biologically plausible, in the absence of individually collected data it is not possible to know to what extent the magnitude of relationships represent an artefact introduced by ecological fallacy.
Using Population Attributable Fractions to Determine the Role of Competing Factors in Anaemia
We used PAFs to represent the fraction of the total anaemia risk in the population that would not have occurred if the effect associated with the contributor of interest were absent while distributions of other contributors in the population remained unchanged [48],[49]. The PAF estimates attributable outcome and not necessarily preventable outcome numbers, as it may not be possible to remove the risk factor from the population altogether. Hence the numbers may overestimate achievable impact and are therefore measures of potential impact. An alternative statistic could have been used, namely, the population impact of eliminating a risk factor (the potential number of disease events prevented in a population over the next t years by eliminating a risk factor) [50].
PAF estimation is of public health significance when the risk factors being investigated are clearly the most proximal in the causal pathway and when there is consensus that the exposure is amenable to intervention [38],[51]. The nutritional factors and infections included in our anaemia model are well known to be causally related to anaemia, but as outlined above, these do not represent the complete multifactorial nature of anaemia. Haemoglobinopathies and thalassemias are importance inherited haematological conditions, particularly in the population of West Africa [52], but predictive surfaces for the sickle cell trait have only recently become available [53]. This study adopted an ecological approach to anaemia modelling in that the true infectious status of children is assigned by spatially overlaying available mapped parasite endemicity surfaces. In doing so, the estimated relative risks for these factors are prone to regression dilution bias, which may contribute to more conservative PAF estimates. In the absence of comparable individual-level data, the practical and logical limitations of including surrogate factors in PAF estimation are not trivial to assess, but our results are consistent to the only study available using individual-level data [17].
Another issue related to the interpretation and public health relevance of a PAF concerns specification of the exposure group [51]. For PAF estimation we have retained the continuous nature of the parasite surfaces to enable spatial prediction across all the areas and to avoid arbitrary categorisation of parasite endemicity surfaces, which could yield reference levels with few or no observations, resulting in PAF estimates with low power. We calculated the PAF for the mean of each parasite surface in the region, which corresponds to the fraction of total anaemia risk in the population that would have been reduced had the children been living in areas where the mean prevalence of the risk factors was very close to zero. Full consideration of continuous covariates is theoretically possible and is a matter of statistical modelling, and PAF estimates (model-based) have been developed for continuous exposures [54]. Our PAF estimation may be extended in future work to estimate a more general measure than PAF, namely, the generalised impact fraction (the fraction reduction of anaemia risk that would result from changing the current distribution of the contributing factors to some modifiable distribution) [55]. However, to set the level of reduction of the risk factor would require evidence of the effectiveness of malnutrition and parasite interventions, which is not objectively available.
Accuracy of the PAF estimates also depends on the representativeness of the input data from the population of interest and the completeness of the multivariable model. The DHS anaemia data are to the best of our knowledge the most complete and representative anaemia data available in the public domain. The anaemia data were collected using standardised methods and quality control protocols (see http://www.measuredhs.com/start.cfm). The input data used to produce smooth maps of malaria included 3,384 geo-positioned records where parasite rates had been diagnosed either using microscopy (2,764 [81.7%]) or rapid diagnostic tests (n = 587 [17.3%]) [36]. The schistosomiasis and hookworm data were obtained in nationally representative surveys using Kato Katz and urine filtration methods [41],[42]. In PAF estimation the multivariable model needs to be as complete as possible; if one or several factors act as true confounders of the association between exposure and disease, then the crude PAF estimates are in general biased and there is a need for adjustment when estimating the PAFs [55]. Regression models allow one to take into account adjustment factors as well as interaction of exposure with some or all adjustment factors [54]. We are confident in our statistical control of confounding by adjusting our analysis for age, sex, and socio-economic factors; we also considered interactions between proximal parasite infections. However, even if one uses adjusted estimates of the relative risk, PAF estimates can be biased in the presence of unaccounted confounding factors, and overestimation of PAFs can occur [49],[56]. Malaria endemicity values may be confounded by the presence of bed net usage, which in turn is known to be influenced by socio-economic status. We found collinearity between bed net usage and socio-economic indicators in the DHS data, which provided statistical support for the inclusion of socio-economic indicators only. Furthermore, these indicators are also related to a broader group of distal factors contributing indirectly to anaemia (e.g., water, sanitation, and deworming).
The order of a variable in the causal pathway and the way it is entered in a multivariable model influence its PAF estimation [57]. The impact of different combinations of proximal infection contributors on the observed relationships with anaemia indicators was assessed by building different models (Tables 2 and 4). In so doing, we noticed the effect of variable order on the resulting coefficients, and PAF estimation was conducted based on the model with best statistical support for model complexity and fit to the data. Furthermore, indirect effects can be noticed when more distal factors impact proximal risk factors by increasing their rate or prevalence. Some of the anthropometric failures used in our models as proxies of malnutrition, and stunting, in particular, can be the result of an indirect effect of both parasite infections and malnutrition, but collinearity between these factors was not identified at variable screening.
Finally, the PAFs refer not to the general population but rather to the study population in West Africa. The results generated from an adjusted PAF model for a specific population may not fit settings in other populations [49]. PAFs in other populations may differ because of varying prevalence of risk factors and the impact of additional socio-demographic factors that were not included in the original sample [56].
Accuracy of Geostatistical Anaemia Modelling and Potential Refinements
The frequency distributions for the predicted anaemia and Hb surfaces cover substantially smaller ranges of values than those of the DHS input data. The resulting anaemia and Hb predictive surfaces are certainly smoother than the raw data from which they are predicted because the MBG modelling approach makes predictions at unsampled locations using linear associations between covariates and the DHS survey data. This smoothing effect (or interpolation) has important repercussions on the models' ability to accurately predict anaemia endemicity over very short distances.
The models performed satisfactorily when predicting point values and endemicity classes of anaemia indicators. However, certain aspects of the uncertainty statistics are suboptimal in that the anaemia risk model tends to overestimate prevalence by 5% and the Hb model tends to underestimate Hb by 10 g/l. Nevertheless, despite the different sources of uncertainty that are embedded in the MBG modelling approach, the resulting predictive maps represent an important evidence base for operational managers of anaemia control in the region.
The computational demands of the MBG modelling approach restricted the range of modelling procedures we could utilise to improve the predictive ability of the anaemia and Hb models. A number of potential improvements to the geostatistical approach could be employed in the following ways. First, future iterations of these maps should consider the incorporation of other covariates, particularly the assessment of the additional influence of inherited blood disorders (haemoglobinopathies and thalassemias) once these become available. Second, our approach could be updated once the existing mapped surfaces have been revised with the inclusion of diagnostic uncertainty into their modelling frameworks. This is particularly important for schistosomiasis in low transmission settings [58]. Third, prediction surface uncertainty around the predicted mean of infection covariates could be incorporated in the modelling framework by modelling the distribution of probable values using a beta distribution parameterized by the predicted posterior mean and the posterior standard deviation for each parasitological survey location. Fourth, the inclusion of spatial variation of spatial dependency in anaemia risk (non-stationarity) could be another possible refinement but was considered computationally infeasible. Future iterations of the present models could incorporate non-stationarity: models could assume separate regional fixed coefficients and include a series of random coefficient models incorporating different correlation structures. Fifth, the 5×5 km resolution may not have been sufficiently precise to classify exposures, and a reduced resolution could have been chosen at the expense of computational run time. For example, an urban-rural map of 5×5 km resolution may not be sufficiently precise to define clusters as rural or urban, since settlements may vary in size across the study area. Finally, infections considered here are known to cause multiple competing morbidities, and the methods presented here could be extended to investigate spatial heterogeneity of co-morbidities attributable to malaria and helminth infections. This would involve applying a multinomial analogue of the present model. Although analysis of the spatial variation in other childhood morbidity indicators, such as stunting, fever, pneumonia, and diarrhoea, has been attempted at the national scale in Malawi [59],[60] and Burkina Faso [61], and at the continental scale in the case of paediatric fevers associated to malaria infection [62], none of these studies have investigated the differential role of malnutrition and parasite infection metrics in prevalence of co-morbidities at a regional or continental scale.
Conclusions
The combination of anaemia and mean Hb predictive maps has allowed the identification of communities in West Africa where preschool-age children are at increased risk of morbidity. The use of anaemia maps as an alternative to aggregated country-level estimates has important practical implications for targeted control in the region and could contribute to the efficient allocation of nutrient supplementation programmes and delivery of fortified foods as well as the planning and evaluation of resource needs for geographical delivery of transfusion services for severe anaemia cases. This study shows that existing continental-level disease and other mapped layers can be used to predict anaemia risk. The development of maps indicating the geographical risk profile of anaemia controlling for malnutrition and major infections would allow assessment of the risk of anaemia due to different causes, which would in turn constitute an important evidence base to work out the best balance between interventions. In the future, these maps could be updated in subsequent methodological iterations to incorporate further modelling refinements.
Supporting Information
Zdroje
1. World Health Organization 2008 Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva World Health Organization. Available: http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf. Accessed 25 April 2011
2. Grantham-McGregorS
AniC
2001 A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr 131 649S 666S; discussion 666S-668S
3. LawlessJW
LathamMC
StephensonLS
KinotiSN
PertetAM
1994 Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr 124 645 654
4. OppenheimerSJ
2001 Iron and its relation to immunity and infectious disease. J Nutr 131 616S 633S; discussion 633S-635S
5. MorrisCR
SingerST
WaltersMC
2006 Clinical hemoglobinopathies: iron, lungs and new blood. Curr Opin Hematol 13 407 418
6. WambuaS
MwangiTW
KortokM
UyogaSM
MachariaAW
2006 The effect of α+-thalassaemia on the incidence of malaria and other diseases in children living on the coast of Kenya. PLoS Med 3 e158 doi:10.1371/journal.pmed.0030158
7. KraemerK
ZimmermannMB
2007 Nutritional anemia. Basel Sight and Life Press
8. SembaRD
BloemMW
2002 The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr 56 271 281
9. FishmanSM
ChristianP
WestKP
2000 The role of vitamins in the prevention and control of anaemia. Public Health Nutr 3 125 150
10. AllenLH
PeersonJM
2009 Impact of multiple micronutrient versus iron-folic acid supplements on maternal anemia and micronutrient status in pregnancy. Food Nutr Bull 30 S527 S532
11. CrawleyJ
2004 Reducing the burden of anemia in infants and young children in malaria-endemic countries of Africa: from evidence to action. Am J Trop Med Hyg 71 25 34
12. AdiasTC
UkoE
ErhaborO
2006 Anaemia in human immunodeficiency virus infection: a review. Niger J Med 15 203 206
13. WilliamsTN
UyogaS
MachariaA
NdilaC
McAuleyCF
2009 Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet 374 1364 1370
14. MeansRT
2000 The anaemia of infection. Best Pract Res Clin Haematol 13 151 162
15. BatesI
McKewS
SarkinfadaF
2007 Anaemia: a useful indicator of neglected disease burden and control. PLoS Med 4 e231 doi:10.1371/journal.pmed.0040231
16. StoltzfusRJ
ChwayaHM
MontresorA
AlbonicoM
SavioliL
2000 Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0 - to 5-y old Zanzibari children and these relationships change with age. J Nutr 130 1724 1733
17. BrookerS
PeshuN
WarnPA
MosoboM
GuyattHL
1999 The epidemiology of hookworm infection and its contribution to anaemia among pre-school children on the Kenyan coast. Trans R Soc Trop Med Hyg 93 240 246
18. FriedmanJF
KanzariaHK
McGarveyST
2005 Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol 21 386 392
19. HotezPJ
BrookerS
BethonyJM
BottazziME
LoukasA
2004 Hookworm infection. N Engl J Med 351 799 807
20. GhoshK
2007 Pathogenesis of anemia in malaria: a concise review. Parasitol Res 101 1463 1469
21. EzeamamaAE
McGarveyST
AcostaLP
ZierlerS
ManaloDL
2008 The synergistic effect of concomitant schistosomiasis, hookworm, and trichuris infections on children's anemia burden. PLoS Negl Trop Dis 2 e245 doi:10.1371/journal.pntd.0000245
22. BrookerS
ClementsAC
HotezPJ
HaySI
TatemAJ
2006 The co-distribution of Plasmodium falciparum and hookworm among African schoolchildren. Malar J 5 99
23. World Health Organization 2001 Iron deficiency anaemia: assessment, prevention, and control: a guide for programme managers. Geneva World Health Organization. Available: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. Accessed 25 April 2011
24. SazawalS
BlackRE
RamsanM
ChwayaHM
StoltzfusRJ
2006 Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367 133 143
25. StoltzfusRJ
DreyfussML
1998 Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Washington (District of Columbia) ILSI Press
26. MendisK
RietveldA
WarsameM
BosmanA
GreenwoodB
2009 From malaria control to eradication: The WHO perspective. Trop Med Int Health 14 802 809
27. World Health Organization 2007 Malaria elimination: a field manual for low and moderate endemic countries. Geneva World Health Organization. Available: http://whqlibdoc.who.int/publications/2007/9789241596084_eng.pdf. Accessed 25 April 2011
28. BriandV
CottrellG
MassougbodjiA
CotM
2007 Intermittent preventive treatment for the prevention of malaria during pregnancy in high transmission areas. Malar J 6 160
29. BrookerS
ClarkeS
SnowRW
BundyDA
2008 Malaria in African schoolchildren: options for control. Trans R Soc Trop Med Hyg 102 304 305
30. BrookerS
WhawellS
KabatereineNB
FenwickA
AndersonRM
2004 Evaluating the epidemiological impact of national control programmes for helminths. Trends Parasitol 20 537 545
31. FenwickA
WebsterJP
Bosque-OlivaE
BlairL
FlemingFM
2009 The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002-2008. Parasitology 136 1719 1730
32. StoltzfusRJ
DreyfussML
ChwayaHM
AlbonicoM
1997 Hookworm control as a strategy to prevent iron deficiency. Nutr Rev 55 223 232
33. GulaniA
NagpalJ
OsmondC
SachdevHP
2007 Effect of administration of intestinal anthelmintic drugs on haemoglobin: systematic review of randomised controlled trials. BMJ 334 1095
34. World Health Organization 2006 Iron supplementation of young children in regions where malaria transmission is intense and infectious disease highly prevalent. Geneva World Health Organization. Available: http://www.who.int/entity/child_adolescent_health/documents/pdfs/who_statement_iron.pdf. Accessed 25 April 2011
35. World Health Organization, United Nations Children's Fund 2004 How to add deworming to vitamin A distribution. Geneva World Health Organization. Available: http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE_PVC_2004.11.pdf. Accessed 25 April 2011
36. HaySI
GuerraCA
GethingPW
PatilAP
TatemAJ
2009 A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med 6 e1000048 doi:10.1371/journal.pmed.1000048
37. MagalhaesRJ
ClementsAC
PatilAP
GethingPW
BrookerS
2011 The applications of model-based geostatistics in helminth epidemiology and control. Adv Parasitol 74 267 296
38. RoweAK
PowellKE
FlandersWD
2004 Why population attributable fractions can sum to more than one. Am J Prev Med 26 243 249
39. SharmanA
2000 Anemia testing in population-based surveys: general information and guidelines for country monitors and program managers. Calverton (Maryland) ORC Macro
40. NandyS
IrvingM
GordonD
SubramanianSV
SmithGD
2005 Poverty, child undernutrition and morbidity: new evidence from India. Bull World Health Organ 83 210 216
41. ClementsAC
FirthS
DembeleR
GarbaA
ToureA
2010 Use of Bayesian geostatistical prediction to estimate local variations in Schistosoma haematobium infection in West Africa. Bull World Health Organ 87 921 929
42. ClementsAC
GarbaA
SackoM
ToureS
DembeleR
2008 Mapping the probability of schistosomiasis and associated uncertainty, West Africa. Emerg Infect Dis 14 1629 1632
43. DigglePJ
MoyeedRA
TawnJA
1998 Model-based geostatistics. Appl Stat 47 299 350
44. BrookerS
HaySI
BundyDA
2002 Tools from ecology: useful for evaluating infection risk models? Trends Parasitol 18 70 74
45. HaySI
NoorAM
NelsonA
TatemAJ
2005 The accuracy of human population maps for public health application. Trop Med Int Health 10 1073 1086
46. RothmanKJ
GreenlandS
LashTL
2008 Modern epidemiology, 3rd edition. Philadelphia Lippincott, Williams, & Wilkins
47. RasoG
VounatsouP
SingerBH
N'GoranEK
TannerM
2006 An integrated approach for risk profiling and spatial prediction of Schistosoma mansoni-hookworm coinfection. Proc Natl Acad Sci U S A 103 6934 6939
48. HutcheonJA
ChioleroA
HanleyJA
2010 Random measurement error and regression dilution bias. BMJ 340 c2289
49. BruzziP
GreenSB
ByarDP
BrintonLA
SchairerC
1985 Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 122 904 914
50. HellerRF
BuchanI
EdwardsR
LyratzopoulosG
McElduffP
2003 Communicating risks at the population level: application of population impact numbers. BMJ 327 1162 1165
51. RockhillB
NewmanB
WeinbergC
1998 Use and misuse of population attributable fractions. Am J Public Health 88 15 19
52. WeatherallDJ
CleggJB
2001 Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ 79 704 712
53. PielFB
PatilAP
HowesRE
NyangiriOA
GethingPW
2010 Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun 1 104
54. BenichouJ
2001 A review of adjusted estimators of attributable risk. Stat Methods Med Res 10 195 216
55. BenichouJ
1991 Methods of adjustment for estimating the attributable risk in case-control studies: a review. Stat Med 10 1753 1773
56. RuckingerS
von KriesR
ToschkeAM
2009 An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med Res Methodol 9 7
57. MasonCA
TuS
2008 Partitioning the population attributable fraction for a sequential chain of effects. Epidemiol Perspect Innov 5 5
58. LeonardoLR
RiveraP
SanielO
VillacorteE
CrisostomoB
2008 Prevalence survey of schistosomiasis in Mindanao and the Visayas, The Philippines. Parasitol Int 57 246 251
59. KazembeLN
MuulaAS
SimoongaC
2009 Joint spatial modelling of common morbidities of childhood fever and diarrhoea in Malawi. Health Place 15 165 172
60. KazembeLN
NamangaleJJ
2007 A Bayesian multinomial model to analyse spatial patterns of childhood co-morbidity in Malawi. Eur J Epidemiol 22 545 556
61. MargaiFM
2007 Geographical targeting of risk zones for childhood stunting and related health outcomes in Burkina Faso. World Health Popul 9 64 82
62. GethingPW
KiruiVC
AleganaVA
OkiroEA
NoorAM
2010 Estimating the number of paediatric fevers associated with malaria infection presenting to Africa's public health sector in 2007. PLoS Med 7 e1000301 doi:10.1371/journal.pmed.1000301
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2011 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- The First Model-Based Geostatistical Map of Anaemia
- The Dynamics of Health and Return Migration
- Life Course Trajectories of Systolic Blood Pressure Using Longitudinal Data from Eight UK Cohorts
- Evaluation of Coseasonality of Influenza and Invasive Pneumococcal Disease: Results from Prospective Surveillance
- Energy Density, Portion Size, and Eating Occasions: Contributions to Increased Energy Intake in the United States, 1977–2006
- Scaling Up Global Health Interventions: A Proposed Framework for Success
- The Effect of Highly Active Antiretroviral Therapy on the Survival of HIV-Infected Children in a Resource-Deprived Setting: A Cohort Study
- Cardiac Complications in Patients with Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis of Observational Studies
- Epidemiological Characteristics of 2009 (H1N1) Pandemic Influenza Based on Paired Sera from a Longitudinal Community Cohort Study
- Mapping the Risk of Anaemia in Preschool-Age Children: The Contribution of Malnutrition, Malaria, and Helminth Infections in West Africa
- More and Better Information to Tackle HIV Epidemics: Towards Improved HIV Incidence Assays
- Human Trafficking: The Shameful Face of Migration
- Global Protection and the Health Impact of Migration Interception
- Migration and "Low-Skilled" Workers in Destination Countries
- The Effect of Handwashing at Recommended Times with Water Alone and With Soap on Child Diarrhea in Rural Bangladesh: An Observational Study
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Migration and "Low-Skilled" Workers in Destination Countries
- Mapping the Risk of Anaemia in Preschool-Age Children: The Contribution of Malnutrition, Malaria, and Helminth Infections in West Africa
- More and Better Information to Tackle HIV Epidemics: Towards Improved HIV Incidence Assays
- Energy Density, Portion Size, and Eating Occasions: Contributions to Increased Energy Intake in the United States, 1977–2006
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



