-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Effect of India's Total Sanitation Campaign on Defecation Behaviors and Child Health in Rural Madhya Pradesh: A Cluster Randomized Controlled Trial
Background:
Poor sanitation is thought to be a major cause of enteric infections among young children. However, there are no previously published randomized trials to measure the health impacts of large-scale sanitation programs. India's Total Sanitation Campaign (TSC) is one such program that seeks to end the practice of open defecation by changing social norms and behaviors, and providing technical support and financial subsidies. The objective of this study was to measure the effect of the TSC implemented with capacity building support from the World Bank's Water and Sanitation Program in Madhya Pradesh on availability of individual household latrines (IHLs), defecation behaviors, and child health (diarrhea, highly credible gastrointestinal illness [HCGI], parasitic infections, anemia, growth).Methods and Findings:
We conducted a cluster-randomized, controlled trial in 80 rural villages. Field staff collected baseline measures of sanitation conditions, behaviors, and child health (May–July 2009), and revisited households 21 months later (February–April 2011) after the program was delivered. The study enrolled a random sample of 5,209 children <5 years old from 3,039 households that had at least one child <24 months at the beginning of the study. A random subsample of 1,150 children <24 months at enrollment were tested for soil transmitted helminth and protozoan infections in stool. The randomization successfully balanced intervention and control groups, and we estimated differences between groups in an intention to treat analysis. The intervention increased percentage of households in a village with improved sanitation facilities as defined by the WHO/UNICEF Joint Monitoring Programme by an average of 19% (95% CI for difference: 12%–26%; group means: 22% control versus 41% intervention), decreased open defecation among adults by an average of 10% (95% CI for difference: 4%–15%; group means: 73% intervention versus 84% control). However, the intervention did not improve child health measured in terms of multiple health outcomes (diarrhea, HCGI, helminth infections, anemia, growth). Limitations of the study included a relatively short follow-up period following implementation, evidence for contamination in ten of the 40 control villages, and bias possible in self-reported outcomes for diarrhea, HCGI, and open defecation behaviors.Conclusions:
The intervention led to modest increases in availability of IHLs and even more modest reductions in open defecation. These improvements were insufficient to improve child health outcomes (diarrhea, HCGI, parasite infection, anemia, growth). The results underscore the difficulty of achieving adequately large improvements in sanitation levels to deliver expected health benefits within large-scale rural sanitation programs.
Trial Registration: ClinicalTrials.gov NCT01465204
Please see later in the article for the Editors' Summary
Published in the journal: The Effect of India's Total Sanitation Campaign on Defecation Behaviors and Child Health in Rural Madhya Pradesh: A Cluster Randomized Controlled Trial. PLoS Med 11(8): e32767. doi:10.1371/journal.pmed.1001709
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001709Summary
Background:
Poor sanitation is thought to be a major cause of enteric infections among young children. However, there are no previously published randomized trials to measure the health impacts of large-scale sanitation programs. India's Total Sanitation Campaign (TSC) is one such program that seeks to end the practice of open defecation by changing social norms and behaviors, and providing technical support and financial subsidies. The objective of this study was to measure the effect of the TSC implemented with capacity building support from the World Bank's Water and Sanitation Program in Madhya Pradesh on availability of individual household latrines (IHLs), defecation behaviors, and child health (diarrhea, highly credible gastrointestinal illness [HCGI], parasitic infections, anemia, growth).Methods and Findings:
We conducted a cluster-randomized, controlled trial in 80 rural villages. Field staff collected baseline measures of sanitation conditions, behaviors, and child health (May–July 2009), and revisited households 21 months later (February–April 2011) after the program was delivered. The study enrolled a random sample of 5,209 children <5 years old from 3,039 households that had at least one child <24 months at the beginning of the study. A random subsample of 1,150 children <24 months at enrollment were tested for soil transmitted helminth and protozoan infections in stool. The randomization successfully balanced intervention and control groups, and we estimated differences between groups in an intention to treat analysis. The intervention increased percentage of households in a village with improved sanitation facilities as defined by the WHO/UNICEF Joint Monitoring Programme by an average of 19% (95% CI for difference: 12%–26%; group means: 22% control versus 41% intervention), decreased open defecation among adults by an average of 10% (95% CI for difference: 4%–15%; group means: 73% intervention versus 84% control). However, the intervention did not improve child health measured in terms of multiple health outcomes (diarrhea, HCGI, helminth infections, anemia, growth). Limitations of the study included a relatively short follow-up period following implementation, evidence for contamination in ten of the 40 control villages, and bias possible in self-reported outcomes for diarrhea, HCGI, and open defecation behaviors.Conclusions:
The intervention led to modest increases in availability of IHLs and even more modest reductions in open defecation. These improvements were insufficient to improve child health outcomes (diarrhea, HCGI, parasite infection, anemia, growth). The results underscore the difficulty of achieving adequately large improvements in sanitation levels to deliver expected health benefits within large-scale rural sanitation programs.
Trial Registration: ClinicalTrials.gov NCT01465204
Please see later in the article for the Editors' SummaryIntroduction
The practice of open defecation is thought to be a major cause of the persistent worldwide burden of diarrhea and enteric parasite infection among children <5 years old [1]. Reducing open defecation requires access to and use of improved sanitation facilities, which are defined as facilities that prevent human feces from re-entering the environment [2]. In 2010, an estimated 47% of the world's population did not have access to improved sanitation facilities. India alone accounts for a third of those without improved sanitation (814 million), nearly 60% of those who practice open defecation (626 million) [2], and a quarter of the world's deaths from diarrheal diseases among children aged less than 5 years [3].
Observational studies of interventions that prevent human feces from entering the environment have been shown to reduce diarrheal diseases [4],[5] and enteric parasite infections [6]–[8]. Most of this research, however, has focused on the provision of sewerage systems in urban centers. Few studies have been conducted in rural areas of low-income countries where the provision and maintenance of networked sewerage is prohibitively expensive. Consequently, most government and donor financing in the rural sanitation sector focuses on the provision of non-networked toilets. Despite the wide scale deployment of such programs, to our knowledge there have been no published randomized trials to measure the effect of rural sanitation programs on diarrheal diseases, intestinal parasite infections, anemia, or growth in young children.
The objective of this study was to measure the effect of India's Total Sanitation Campaign (TSC) in rural Madhya Pradesh on household availability of improved sanitation facilities as defined by WHO/UNICEF Joint Monitoring Programme (JMP) for water and sanitation [2], open defecation behaviors of household members, water quality, and child health (diarrheal diseases, highly credible gastrointestinal illness [HCGI], enteric parasite infections, anemia, and growth). The TSC, scaled up to all districts in India and deployed to hundreds of millions of people, is possibly the largest rural sanitation program in the world. As a part of their Total Sanitation and Sanitation Marketing (TSSM) project, the Water and Sanitation Program (WSP; the World Bank) provided capacity building support to ten districts of Madhya Pradesh to strengthen the implementation of the program. In two of these ten districts, we studied the effects of the TSC implemented with support from the WSP under the TSSM project using a cluster-randomized controlled trial in 80 rural villages.
We hypothesized that the program would increase availability of individual household latrines (IHLs) and reduce the practice of open defecation in a community through use of IHLs. On the basis of previous research [4]–[8], we further hypothesized that less open defecation would: (i) reduce the quantity of feces in the environment that could contaminate shallow groundwater aquifers, water distribution networks, and soil in the community, and (ii) also reduce enteric pathogen transmission through flies, which are well-established vectors for transmission [9]–[11]. Conditional on improvements in these intermediate outcomes, we hypothesized that children <24 months at enrollment in intervention villages would have a lower prevalence of diarrhea, HCGI, enteric parasite infections, and anemia when measured after the intervention. Finally, we hypothesized that the program would improve average weight-for-age and height-for-age in these young children as a result of fewer symptomatic and asymptomatic enteric infections over longer exposure periods to improved sanitation [12]–[16]. The above hypothesized causal chain between the intervention and health outcomes is depicted in Figure 1.
Fig. 1. Hypothesized causal pathways for intervention impact and measurements. 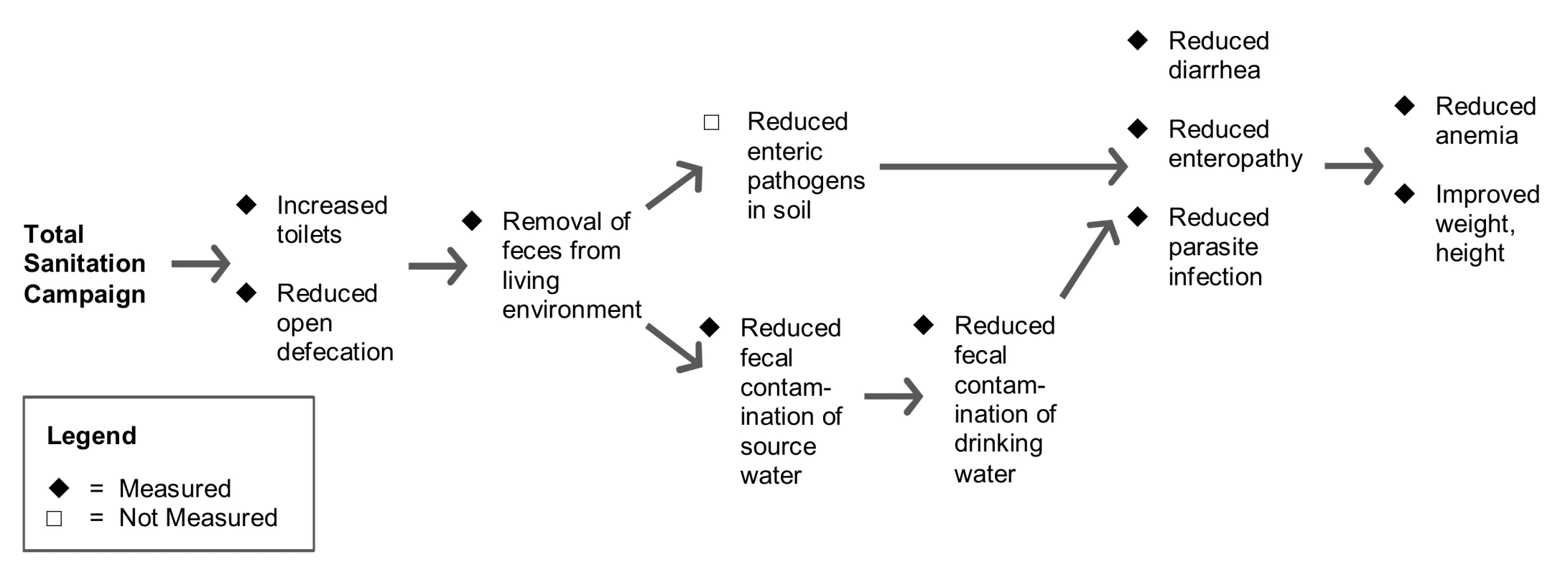
Methods
Ethics Statement
The study is a part of larger six-country study commissioned by the WSP. The study protocol was approved by the Western Institutional Review Board, Olympia, Washington, USA (study number 1095420) and the Independent Ethics Committee, Mumbai, India (IEC/09/11). The survey respondents provided a verbal consent after enumerators apprised them of study objectives, use of collected information, confidentiality, risks, benefits, and respondent rights. Written consent was not obtained because of lower literacy and social norms that would deter women (child caregivers were the main respondents) from signing any document without her husband's or elders' permission.
The protocol for the broader study was formally registered after the completion of fieldwork because at the time the study was conceived pre-registration was not a well-known convention in the field of development economics [17] (the study was originally conceived by PJG and colleagues at the World Bank). The team agreed that a late registration was better than none at all. The original study protocol—established in 2008; before the baseline survey—included another two districts from the state of Himachal Pradesh where the WSP provided support under their TSSM project as well [18]. However, the study in Himachal Pradesh was discontinued because it was impossible to retain a control group for the duration of the study period. The only substantive change to the protocol in Madhya Pradesh after the start of the trial was the increase in sample size planned for follow-up; we provide details in the section on Sample Size. The CONSORT checklist (Text S1) and the follow-up study protocol (Text S2) are provided as supplemental information.
Trial Design
The study design was a cluster randomized controlled trial with randomization at the village level and equal allocation to the two treatment arms. The study population included 80 villages from two neighboring districts in Madhya Pradesh: Dhar and Khargone. The villages randomized to the intervention group received the TSC program and villages in the control group did not receive the TSC until after the study. As a demand driven program, the district administration was duty bound to provide the program to the villages in the control group if they requested it and if the funding was available. The district administration agreed to provide the program to all control villages after the completion of the study. The study measured outcomes, anticipated confounders, and covariates at household and child levels both before and after the intervention in two survey waves. The follow-up survey was administered to the same households who participated in baseline data collection and additional households were included at follow-up (see the section on Sample Size for details).
Study Population
Table 1 describes population characteristics for the study region relative to the state and national population on the basis of India's 2011 Census. Overall, Madhya Pradesh is one of the less developed states of India, including its water and sanitation infrastructure. The study districts are more agricultural, with higher proportion of marginalized population groups and lower literacy than the state average, but with better water supply and drainage infrastructure. IHL coverage (percentage of households with access to IHL) in rural areas of study districts (19.2% in Dhar and 13% in Khargone) is comparable to the state average (13.1%) but much worse than the country average (30.7%). The IHLs are predominantly the types included in the JMP definition of improved sanitation [2]. On average the IHL coverage across India increased by approximately 10% between 2001 and the 2011 Census. However, the change in the IHL coverage between 2001 and 2011 varied widely between states and between districts within each state [19].
Tab. 1. Descriptive statistics for India, Madhya Pradesh, and study districts, Census 2011. 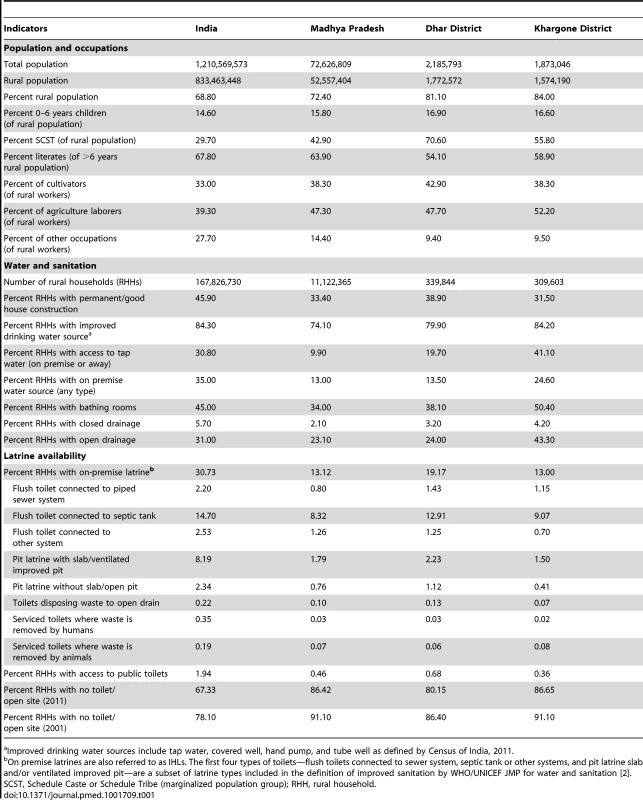
Improved drinking water sources include tap water, covered well, hand pump, and tube well as defined by Census of India, 2011. Study villages were selected in collaboration with the Madhya Pradesh state government. Madhya Pradesh is divided into 50 districts, 313 Blocks, and 23,040 Gram Panchayats (referred to as “villages” in this manuscript). A Gram Panchayat is the smallest Indian administrative unit and has a local elected body. The 80 study villages were the independent units selected in three steps. First, through a series of meetings and site visits, the state government and the WSP selected two of 50 districts in Madhya Pradesh: Dhar and Khargone. Second, 11 of 13 Blocks from Dhar and eight of nine Blocks from Khargone were selected for the study. The remaining Blocks were excluded from the sample frame because all villages from these Blocks were earmarked for the TSC program, precluding the enrollment of control villages. Third, in each administrative Block the government identified villages where they were amendable to randomizing the TSC program.
In each of the 80 study villages, the field team listed and mapped 200 households and randomly selected 25 households with at least one child <24 months of age at enrollment. If a village had multiple sub-villages, then to avoid spreading the sample too thin, the survey team selected the most populous two to three sub-villages for the listing purposes. From the numbered list of eligible households, a random starting number was chosen and thereafter every nth household number was selected where n was determined by dividing eligible number of households by 25. For the follow-up survey we increased the sample size of households per village from 25 to 38 (see section on Sample Size). Additional 100 to 150 households were listed and mapped before the follow-up survey to select additional households. Figure 2 summarizes loss to follow-up in the original cohort and recruitment of new households in the follow-up survey. Because we conducted the follow-up survey 21 months after baseline, the eligibility criteria for newly enrolled households was that they had at least one child between the ages of 21 months and 45 months and were living in the village at the time of the baseline survey to be commensurate with the eligibility criteria for the original cohort. Child caregivers were the main survey respondents, but household heads or other elders occasionally answered questions related to household characteristics.
Fig. 2. CONSORT Flowchart: enrollment, intervention allocation, attrition, and addition of participants. 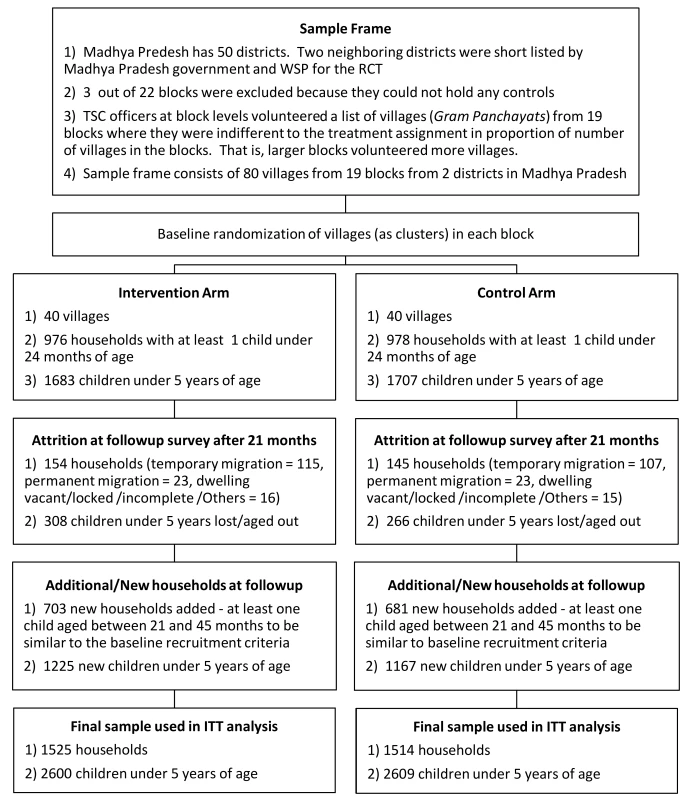
Intervention Program
India's TSC, initiated in 1999, was an ambitious program with a goal to eliminate the practice of open defecation in India by 2012. In 2012, the government transformed TSC into a new program named Nirmal Bharat Abhiyaan (Clean India Campaign). The TSC included subsidies for and promotion of IHLs that can safely confine feces (similar to JMP defined improved sanitation facilities), school sanitation and hygiene education, Anganwadi (preschool) toilets, and community sanitation complexes. The TSC also supported rural sanitary marts and production centers to provide good quality but affordable material for toilet construction. Additionally, the TSC included several features such as ongoing social mobilization and behavior change activities at state, district, and village levels, flexible technology options for toilets, and a community award called the Nirmal Gram Puraskar (NGP) given to communities that were determined to be “open defecation free”—defined as a community where all households have and use IHLs that can safely confine feces—and meet all of the other “total sanitation” requirements defined by the Indian government. The NGP awards ranged from Rs 50,000 (US$1,000) to Rs 500,000 (US$10,000) for villages, up to Rs 2,000,000 (US$40,000) for Blocks, and Rs 5,000,000 (US$100,000) for districts.
In Madhya Pradesh, the TSC was implemented with a concurrent program named Nirmal Vatika (Clean House) under the National Rural Employment Guarantee Scheme to provide additional financial and material subsidies to households. TSC and Nirmal Vatika together provided at least Rs 4,200 (US$84) to below poverty line (BPL) households in the village. The Indian Ministry of Rural Development classifies households as BPL using characteristics such as land holdings, house type, consumer durables, and literacy [20]. BPL households were identified in this study by their ration card color (a document used to access public food and grain distribution system). While the TSC provided subsidy of Rs 2,200 (US$44) to BPL households, Nirmal Vatika provided additional at least Rs 2,000 (US$40) to BPL and non-BPL households both to support IHL construction. These costs were determined by the government to be adequate to construct an offset two-pit latrine with water sealed squat plate and a brick walled room (which will be a JMP defined improved sanitation facility), and this type of latrine was actively promoted in the study districts.
Beginning in 2006, the WSP India office supported the TSC program under the TSSM project in ten districts in Madhya Pradesh. The WSP worked with local authorities to create an enabling environment for the TSC activities, to develop local implementation capacities at the district level, and to support the use of monitoring systems to assess progress towards the TSC goals. WSP promoted and provided capacity building support to implement community-led total sanitation (CLTS) based behavior change methods [21]. The CLTS methodology involves a series of community “triggering” exercises, led by an external facilitator after building rapport with the community in the pre-triggering phase, which highlight the magnitude of the practice of open defecation, elicit shame and disgust, and mobilize community action to end open defecation [21]. These triggering activities are followed by community follow-up actions that are supported by facilitators. Although the intervention used CLTS based tools for behavior change, it cannot be considered as a classical CLTS intervention. CLTS principles require that no hardware subsidies be provided to individual households and specific latrine models not be prescribed [21], whereas the intervention provided hardware subsidies to individual households to build offset pit latrine designs approved under the Nirmal Vatika program. Provision of hardware subsidy as a post-construction incentive was advocated by the WSP, but the mechanisms of the convergence of Nirmal Vatika and the TSC essentially meant that the subsidies were released before and during but rarely after IHL construction.
The TSC program in the study areas was implemented by the village government (Gram Panchayat) with support from district and block administration personnel or consultants. The study investigators and staff were not involved in program implementation.
Outcome Definition and Measurement
The study measured outcomes using a combination of structured questionnaires and observations, sampling and testing of drinking water, child anthropometry and specimen (stool and blood) testing. GfK Mode Pvt Ltd. was contracted to conduct the fieldwork. Training and all field activities were overseen by the study investigators (SRP, ALS). The baseline survey was conducted between 25 May and 18 July, 2009, and the follow-up survey was conducted between 23 February and 25 April, 2011. Questionnaires used in the follow-up survey were the same as those used in the baseline survey with some additional questions to measure program exposure and outcomes. The household questionnaire collected information about household socioeconomics, demographics, exposure to the TSC activities, water and sanitation infrastructure, sanitation - and hygiene-related behaviors, and health/diseases. Interviewers conducted standardized spot-check observations of dwelling sanitation and hygiene facilities. Defecation behavior was reported by adults during private, in-home interviews. Main outcomes were defined as follows.
Toilets, open defecation, hygienic conditions
We classified household sanitation facilities using questions and definitions proposed by the JMP [2]. JMP-defined improved sanitation includes flush/pour flush toilet connected to piped sewer, to septic tank or to offset pit, ventilated improved pit latrine, on-pit latrine with slab and composting toilet that can hygienically separate human excreta from human contact. However, it is possible that the households build rudimentary latrines that are not included in the JMP definition of improved sanitation. For example, in addition to no facility or open defecation, the JMP defined unimproved sanitation facilities include flush toilets disposing waste elsewhere, pit latrine without a slab (open hole), bucket latrine, and hanging latrine. We also report availability of all types of IHLs whether improved or unimproved to assess whether the households moved up the sanitation ladder from no facility to some type of latrine even if unimproved. To assess defecation behavior for men, women, and children (<5 years), interviewers asked households separately for each group whether they openly defecate daily/always, occasionally/seasonally, or never. Interviewers also asked about child feces disposal using the standard JMP question [2]; disposal in a toilet, a confined pit, or buried was classified as hygienic. Field staff also observed whether the IHLs (of any type if present) were being used on the basis of worn path, closable door, odor, anal cleaning material, and water to flush. Field staff also recorded any observed human or animal feces in the household living area.
Caregiver reported illness
The study's primary outcome was diarrhea and HCGI among children <5 years old. We defined diarrhea as ≥3 loose or watery stools in 24 hours, or a single stool with blood/mucus [22] with a 7-day recall period [23] using a previously published instrument [24]. HCGI—a more inclusive measure of enteric infection—was defined as any of the following four conditions: (1) diarrhea; (2) vomiting; (3) soft or watery stool and abdominal cramps occurring together on any day; or (4) nausea and abdominal cramps occurring together on any day [25]–[28]. We measured respiratory symptoms (constant cough, pulmonary congestion, difficulty breathing, breaths per minute) and defined acute lower respiratory illness (ALRI) as constant cough or difficulty breathing and a raised respiratory rate [29]. We also measured bruising/abrasions and itchy skin/scalp to serve as negative control outcomes [30] to check for differential reporting bias in this unblinded trial [31],[32].
Anthropometry
We measured children <24 months at enrollment for height, weight, and mid-upper arm circumference (MUAC) using a standardized anthropometry protocol [33],[34]. Pairs of trained anthropometrists measured child length/height to the nearest 0.1 cm using a portable stadiometer (manufacturer: Seca); children <24 months were measured in the recumbent (lying) position and older children (at follow-up) were measured standing. Weight was measured to the nearest 0.1 kg using an electronic scale (manufacturer: Tanita); children unable to stand were weighed in their caregiver's arms and the caregiver's weight measured separately. MUAC was measured to the nearest 0.1 cm using a pediatric measuring tape. All measurements were collected in duplicate and we used the average of the two measurements in the analysis. We excluded observations if the two measurements differed by >10% (n = 21 [0.48%] for height, n = 85 [1.93%] for weight, n = 23 [0.52%] for MUAC). We converted the anthropometric measurements into Z-scores using the WHO's 2006 growth standards and the WHO publicly available Stata algorithm [35].
Anemia
If the caregiver provided informed consent, trained field staff conducted an in-field test for anemia for children between the ages of 6 and 60 months using HemoCue (HemoCue Ltd). We classified children as severely anemic if their hemoglobin concentration was <7.0 g/dl, moderately anemic if their hemoglobin concentration was 7.0–9.9 g/dl, and mildly anemic if their hemoglobin concentration was 10.0–11.9 g/dl [36]. Parents of children who were severely anemic were advised to visit the nearest health facility for medical attention.
Water quality
We collected 100 ml stored drinking water samples from a random sample of 404 households in the intervention and 403 households in the control groups, and also collected paired samples from the water source from which the households collected their drinking water (511 source samples). The water samples were collected in sterile containers, labeled, and individually packed in a sterile plastic zip-lock cover provided by the laboratory. The sample collectors were provided with sterile gloves and trained to avoid cross-contamination of water and containers. Water samples were stored and transported in ice boxes and tested for Escherichia coli using membrane filtration (100 ml volume filtered) within 36 hours of collection at Envirocare Laboratories Pvt Ltd, Mumbai. The laboratory used HiCrome Agar (M1466) by HiMedia. Each incubation batch included positive and negative control plates. Positive colonies of E. coli were further confirmed with Triple Sugar Iron (TSI) agar test and group of Indole, Methyl red, Voges-Proskauer, and Citrate tests (IMViC). Samples below the lower limit of detection were imputed at 0.5 colony forming units (CFU) per 100 ml (half the limit of detection [37]), and samples beyond the upper limit of detection were imputed at the limit of detection (200 CFU/100 ml).
Child stool parasitology
At the follow-up survey, we selected a random subsample of 1,150 households from 3,039 households and collected a stool specimen from the oldest child between 21 and <60 months of age. All stool samples were preserved in 10% formalin and analyzed at the National Institute for Cholera and Enteric Diseases in Kolkata. Lab technicians tested the samples for soil transmitted helminthes (Ascaris lumbricoides, Trichuris trichiura, Ancylostoma duodenale, and Necator americanus) and tapeworm helminthes (Hymenolepis nana, Taenia sp., Diphyllobothrium latum) using the Kato-Katz technique [38].
A separate aliquot was analyzed to test for protozoan infections (Giardia lamblia, Cryptosporidium sp., Entamoeba histolytica) using a commercially available ELISA kit (TechLab) [39],[40]. All specimens were tested with a combination of microscopy, ELISA, and PCR to achieve high levels of sensitivity and specificity. If a child tested positive for one of the protozoan infections using either microscopy or ELISA, the result was confirmed using isolated DNA from the ELISA positive samples followed by PCR-restriction fragment length polymorphism (RFLP) methods for genotyping local isolates of giardia (β-giardin) [32], Cryptosporidium (18s rRNA) [33], and E. histolytica (SSU rRNA) [34]. If a sample tested positive by microscopy or ELISA but was not confirmed by molecular methods then the sample was classified as negative.
Sample Size
The study was originally designed to have 80% power to detect a 4.5 percentage point reduction in diarrhea prevalence among children <5 years old assuming 15% prevalence in the control group (or a 30% relative reduction) with a two-sided alpha of 5%, and an intra-class correlation of 0.105 [41]. These assumptions led to a design with 40 clusters (villages) per arm and 25 households with children <24 months per cluster. After the commencement of the study but without knowledge of any study outcomes, we decided to additionally power the trial to detect differences between groups in height-for-age Z-scores on the basis of a hypothesis published on the possible effects of improved hygiene and sanitation on child growth [14]. We reviewed measures of variability and within-cluster correlation of height-for-age Z-scores (SD = 2.09, intra-class correlation = 0.17), and chose to increase the within-cluster sample sizes from 25 to 38 households to ensure the study had 80% power to detect differences of +0.2 Z in height-for-age.
Randomization
The village-level randomization was stratified at the administrative Block level because the TSC implementation was coordinated at the Block level and we wanted to ensure that the treatment arms were evenly allocated between districts and geographically stratified within districts. The randomization took place in a public lottery led by study investigators. The Block TSC coordinators or their representatives picked the lottery ticket that assigned villages to treatment groups. Overall, we allocated a total of 20 villages in each district to the intervention and 20 to control (40 villages per arm). The program implementers and researchers were not blinded to the group assignment. Field interviewers were not informed of group assignment, but it was possible for them to identify intervention villages during interviews of Block officers or the village secretary.
Statistical Methods
We checked the baseline balance in the observable characteristics of the randomized groups. Due to highly comparable groups at baseline and the large increase in our within-cluster sample between baseline and follow-up, our analysis focused on group comparisons post-intervention (using follow-up measures only). To evaluate any differential effect of attrition (loss to follow-up) between baseline and follow-up, we compared baseline characteristics of those present at follow-up with those lost to follow-up. We also compared the balance of baseline characteristics across treatment groups for individuals who were present at both baseline and follow-up to determine whether attrition was differential by treatment group.
Our parameter of interest for all outcomes was the mean difference between randomized groups. We conducted the analysis using households and individuals as they were randomized (intention to treat [ITT]). We estimated differences between groups using the following linear regression model:
Where, Yijk is the outcome for individual i in village j and Block k; Tj is the intervention indicator (1 for intervention, 0 for control); Xij are individual, household, and village level characteristics used in adjusted analyses; bk are indicator variables for Blocks since randomization was stratified at the Block level; and εijk is the error term. The parameter β estimates the ITT difference between the randomized groups. In the adjusted analyses, we included the following covariates to improve precision: whether the household head had attended school; whether the government categorized the household as Scheduled Caste or Tribe; child age; and child sex. Additionally, the adjusted models included three baseline characteristics found to be slightly imbalanced between groups despite randomization. These included: percentage of households in the village that used improved water sources; percentage of households in the village that were observed to have soap and water at the hand-washing place used after defecation; and mean height-for-age Z-score of children in the village. To further assess differential impacts of the program by important population subgroups, we re-estimated the effect of the intervention for households with and without IHL (any type) at baseline, and households below the official poverty line and the other households.Since we would expect behaviors and child health outcomes to be correlated within villages, all estimates used Huber-White robust standard errors for the parameter β clustered at the village level [42] and reported p-values for the two sided t-test. Following guidance from Schulz and Grimes [43], we did not adjust p-values or confidence intervals for multiple comparisons because many of the outcomes were highly correlated with one another (for example, correlation between primary outcomes diarrhea and HCGI = 0.78); nominal p-values should be interpreted with this in mind. All analyses were conducted using Stata v12 (Statacorp), and all primary analyses were independently replicated by two investigators (SRP, BFA) from untouched datasets to final estimates.
Access to Protocol and Data
The study protocol, questionnaires, and access to data collected in the study are available upon registration at http://microdata.worldbank.org/.
Results
Enrolment, Baseline Balance, and Attrition
Figure 2 depicts the study participants flow. The baseline survey enrolled a sample of 3,390 children <5 years from 1,954 households from 80 villages. In the follow-up survey the sample size was increased to 5,209 children <5 years from 3,039 households. As reported in Table 2, baseline covariates in intervention and control groups were well balanced with four exceptions. First, 89% of the households in the intervention group had access to improved water sources—tap/piped water, tube well and protected dug wells—compared to 80% of households in the control group. In contrast, a larger proportion of control households (54%) were observed to have soap and water at hand-washing locations used after defecation than in intervention households (44%). On average, more children were found to be anemic in the control group (93%) than in the intervention group (88%). Finally, average height-for-age Z-scores were also slightly imbalanced (−1.38 intervention versus −1.81 control).
Tab. 2. Baseline characteristics by randomized intervention groups, 2009. 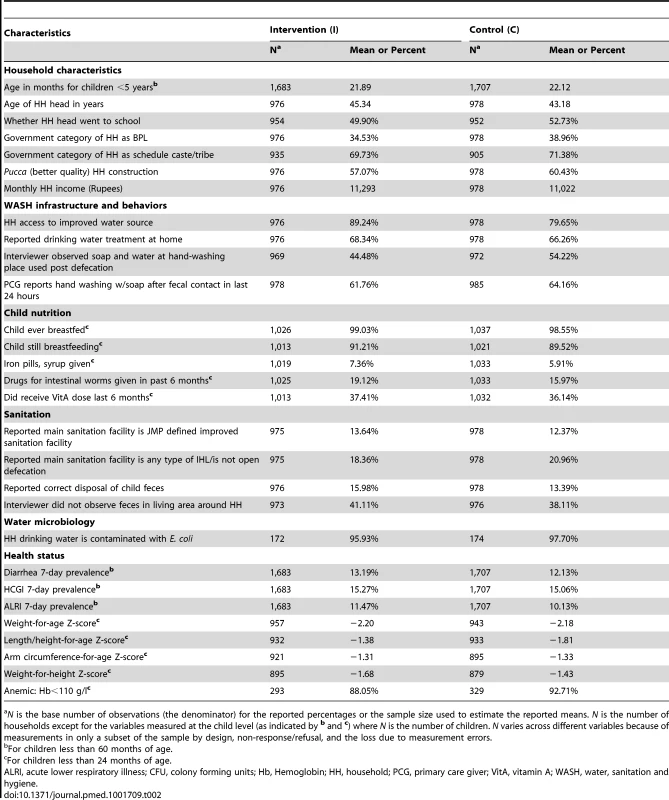
N is the base number of observations (the denominator) for the reported percentages or the sample size used to estimate the reported means. N is the number of households except for the variables measured at the child level (as indicated by b and c) where N is the number of children. N varies across different variables because of measurements in only a subset of the sample by design, non-response/refusal, and the loss due to measurement errors. Attrition was not differential by randomized group on the basis of observable characteristics (see Table S1). Of the 1,954 households enrolled at the baseline, 1,655 were located at the 21-month follow-up survey (15% attrition) without any significant difference between the intervention (16%) and the control (15%) groups. Characteristics remained balanced between intervention and control groups in remaining households.
Compliance to Randomization
The study measured intervention implementation in multiple ways because of the complexity of the TSC program. These measures included: reported implementation by Block coordinators, expenditure of funds documented by official program records, and interviews with local village officials. Out of 40 intervention villages, staff collected administrative information on 39 villages from the TSC Block coordinators (government officers). The coordinators reported that 15/39 intervention villages received some CLTS activities, 33/39 villages applied for a NGP award prior to the follow-up survey. According to Block coordinators' records, 25/39 villages had 100% households with IHLs, 11/39 villages had 80%–99% households with IHLs, and three of 39 villages with 37%–68% households with IHLs. Block coordinators also reported that 21/39 villages received 100% of the funds allocated under the TSC program, 12/39 villages received between 50% and 99%, and six of 39 villages received <50% of their allocated funds. The latest disbursement of the TSC funds was given to 36/39 intervention villages at least 4 to 5 months before the follow-up survey, which would offer sufficient time for IHLs to be constructed and used for 3 or more months.
The study review meetings with Block coordinators also identified that some control villages were contaminated during the study period: TSC activities were initiated in eight control villages within a few months of baseline survey and possibly in two additional control villages a few months prior to the follow-up survey; official records were not available for control villages to ascertain this information objectively. As per the follow-up survey in these ten contaminated villages, the household level coverage of JMP defined improved sanitation facilities increased from 17.4% at baseline to 41.4% at the follow-up, which is similar to the program effect we observed in the intervention group. The household level coverage of JMP defined improved sanitation facilities in uncontaminated control villages increased from 10.7% to 16.2% in the same period. The study's long follow-up period (21 months) and the highly publicized and politicized nature of the TSC program may have contributed to this contamination.
Information from additional sources (village secretaries, school teachers, Anganwadi [pre-school] workers in the village, and the rapid assessment from random sample of households) confirmed that TSC activities translated into a higher recollection and knowledge of the TSC program in the intervention villages compared to the control villages. We also found that households in intervention villages were more aware of CLTS activities, had higher knowledge of TSC, and experienced more personal visits to convince them to build and use IHLs (Table 3).
Tab. 3. Effect of the intervention on program outputs, behavioral outcomes, and water quality, 2011. 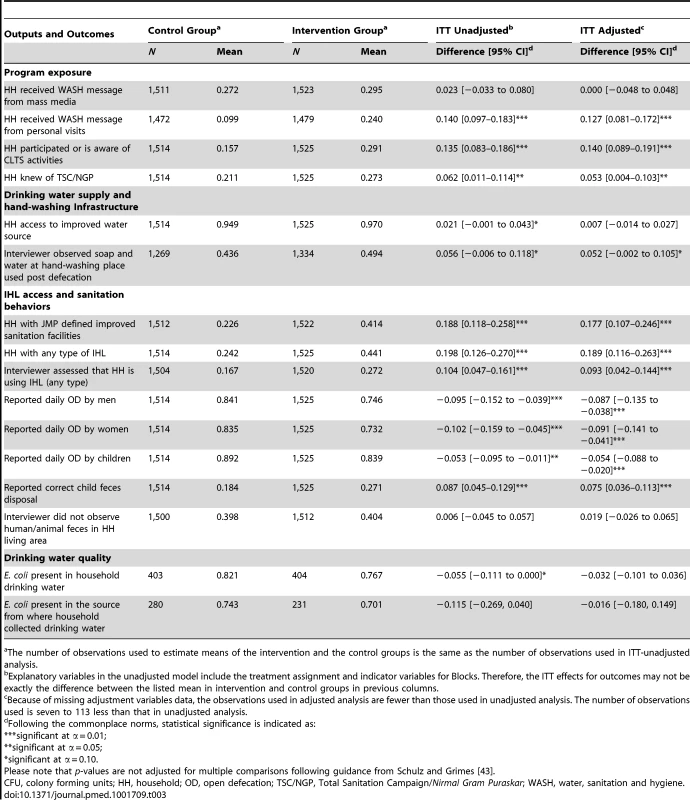
The number of observations used to estimate means of the intervention and the control groups is the same as the number of observations used in ITT-unadjusted analysis. IHL Coverage and Sanitation-Related Behaviors
Table 3 reports the intervention's effect on IHL availability (JMP defined improved sanitation facilities and any type of IHLs) and open defecation behaviors by household members. The intervention increased the coverage of JMP defined improved sanitation facility by average 19 percentage points (95% CI 12%–26%; p-value<0.001) in intervention villages compared to control villages (41.4% intervention versus 22.6% control). The intervention increased the coverage of any type of IHL facility by 20 percentage points (95% CI 13%–27%; p-value<0.001) in intervention villages compared to control villages (44.1% intervention versus 24.2% control). These results indicate that available IHLs were predominantly JMP defined improved sanitation facilities and very few rudimentary latrines or latrines defined as unimproved by the JMP were built. These results are consistent with the TSC design that promoted latrine models that can safely contain the feces.
Although on average fewer households in intervention villages were likely to report daily open defecation compared to control villages for adult men (75% intervention versus 84% control; mean difference: 9.5%; p-value = 0.001), adult women (73% intervention versus 83% control; mean difference: 10%; p-value<0.001), and children <5 years (84% intervention versus 89% control; mean difference: 5%; p-value = 0.014), these reductions in reported open defecation behaviors were smaller than the gains in IHL availability. Amongst the 630 households in intervention villages that had JMP defined improved sanitation facilities at follow-up, 41% reported that adult men or women still practiced daily open defecation; this same figure was 28% among the 339 control village households at follow-up (not reported in results table). A follow-up debriefing question to households who had IHL identified that the main reasons for daily open defecation in spite of having IHL were culture, habit, or preference for defecating in open followed by inadequate water availability.
Drinking Water Quality
In control villages, 82% (331/403) of household drinking water samples tested positive for E. coli compared to 77% (310/404) of samples in intervention villages (mean difference: 5.5%; p-value = 0.050) (Table 3). Table S2 lists the distribution of positive household samples by different E. coli contamination level categories.
Of 511 water source samples tested, 74% (208/280) of the sources in control villages and 70% (162/231) in intervention villages tested positive for E. coli but the difference was not statistically significant (p-value = 0.143).
Caregiver Reported Illness
Diarrhea prevalence did not differ between groups (7.4% intervention versus 7.7% control; p-value = 0.687) (Table 4). HCGI prevalence also did not differ between groups (11.5% intervention versus 12.0% control; p-value = 0.692) (Table 4). We observed no significant differences between groups in negative control caregiver-reported outcomes including bruising/abrasions (1.4% intervention versus 1.3% control) and itchy skin/scalp (2.5% intervention versus 2.2% control) suggesting that differential outcome reporting bias for diarrhea and HCGI was unlikely.
Tab. 4. Effect of the intervention on health outcomes, 2011. 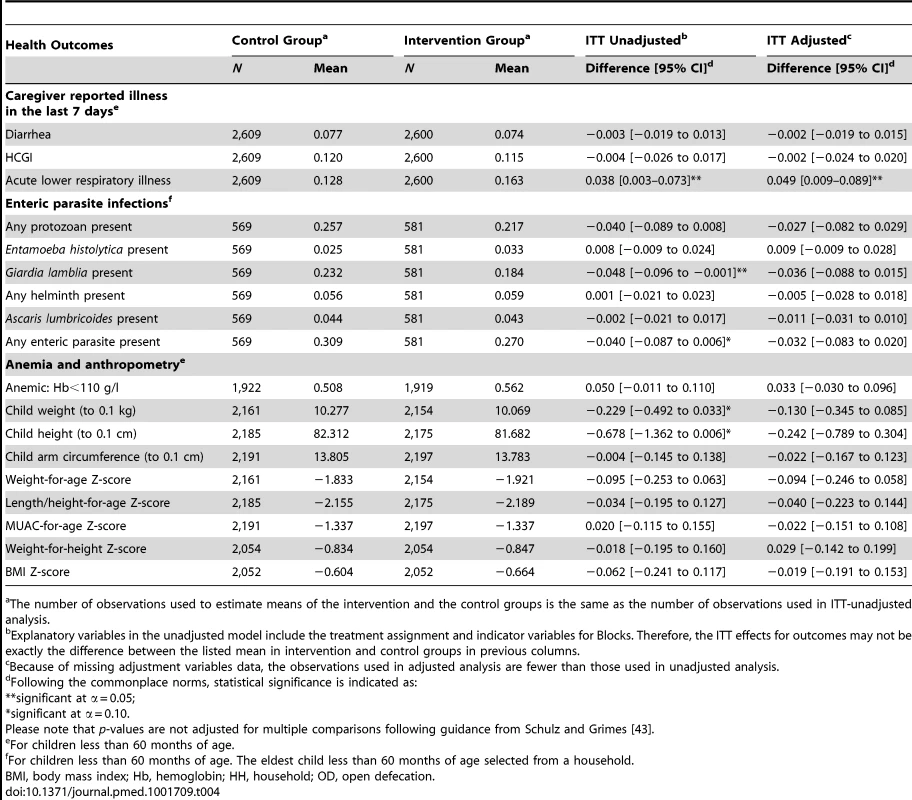
The number of observations used to estimate means of the intervention and the control groups is the same as the number of observations used in ITT-unadjusted analysis. Enteric Parasite Infections
In the subsample of 1,150 children with stool collection, 5.7% (66/1150) had helminth infections and the majority (50/66) were Ascaris infections. All remaining infections were tapeworms; no children were infected with T. trichiura or hookworm. We observed no difference in helminth prevalence between intervention and control groups. Giardia infection was common, and consistent with slightly improved water quality in the intervention group, we found lower Giardia prevalence among children in intervention villages (18%) compared to children in control villages (23%) (mean difference: 4.8%; p-value = 0.047). We detected no Cryptosporidium infections in the study children, and a low prevalence of E. histolytica (33 out of 1,150; 2.9%).
Anemia and Anthropometry
Anemia was prevalent in the study children (54%) and children were small according to international growth standards (Table 4). However, we found no differences between the randomized groups in anemia prevalence or growth outcomes.
Subgroup Results
Table 5 presents the results of subgroup analyses of the effect of the intervention on households with or without any type of IHL at baseline and BPL or non-BPL households. As expected, the program had the largest improvements on JMP defined improved sanitation facilities, IHL use as assessed by enumerators, and reduced reported open defecation by household members in households that did not have IHL (any type) at baseline and in BPL households. This finding is consistent with the TSC design that targeted households without IHLs and offered larger IHL construction subsidies for BPL households. Among BPL households, the intervention increased JMP defined improved sanitation facilities coverage by 30 percentage points (48% intervention versus 18% control; p-value<0.001) and it reduced open defecation among women by 17 percentage points (73% intervention versus 90% control; p-value<0.001). Despite larger improvements in these intermediate outcomes among BPL households or households without IHL at baseline, we did not observe consistent improvement in health outcomes in these subgroups (Table 5).
Tab. 5. Differential effect of the intervention by population subgroups, 2011. 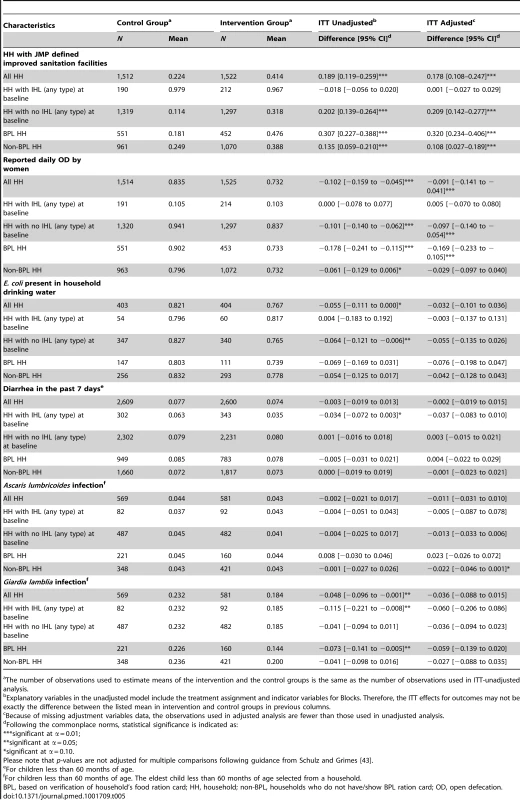
The number of observations used to estimate means of the intervention and the control groups is the same as the number of observations used in ITT-unadjusted analysis. Discussion
The TSC program, implemented with support of the WSP in Dhar and Khargone districts, increased household level coverage of JMP-defined improved sanitation facilities by a modest 19 percentage points in intervention villages compared to control (41% intervention versus 22% control; p-value<0.001). However, the reductions in reported open defecation by adults were even more modest: falling 9 to 10 percentage points (among men: 75% intervention versus 84% control; p-value = 0.001; among women: 73% intervention versus 83% control; p-value<0.001), while reports of correct child feces disposal increased because of intervention by 9 percentage points (27% intervention versus 18% control; p-value<0.001). The availability of IHL and the reductions in open defecation were higher in the BPL household or households without any IHL at the time of baseline but we did not find consistent improvements in the multiple health outcomes in these subgroups. The less than universal or very high levels of IHL coverage in the intervention villages combined with relatively small behavior changes are consistent with our finding of no improvements in child health outcomes including: diarrhea, enteric parasite infection, growth, and anemia.
The study's findings should be viewed as a measure of effectiveness for this specific implementation of India's TSC program in rural Madhya Pradesh. By the end of the study in the intervention group, coverage of JMP defined improved sanitation facilities in a village ranged between 5% and 79% households and percentage of households in a village reporting daily open defecation by adult men ranged between 32% and 97% and that by adult women ranged between 34% and 97%. It is unknown whether enteric pathogen risk is linearly or non-linearly related to the level of improved sanitation in a community, and the intervention did not achieve the goal of universal availability of IHLs or universal elimination of open defecation during the study period. Therefore, our findings cannot speculate the child health outcomes for universal or higher levels of IHL availability or larger open defecation reductions that may be feasible under different contexts, program designs, or implementation efficacy. Additional, forthcoming cluster randomized sanitation intervention trials [44],[45] may generate such evidence if they can achieve adequately high latrine coverage and proportional reductions in open defecation.
This study presents a cautionary tale of how difficult it can be to achieve universal IHL coverage or elimination of open defecation for scaled up rural sanitation programs. The study documented clear evidence of more social mobilization, exposure to behavior change activities, and IHL construction in intervention villages compared to control villages. However, these intermediate outputs of the TSC could not translate into high enough levels of IHL availability and reductions in open defecation practice to deliver the health impacts. This evaluation was a part of a broader six-country effort to also study large-scale sanitation promotion programs in rural Indonesia and Tanzania, as well as large-scale hand-washing promotion programs in Peru, Vietnam, and Senegal. While the Tanzania results are forthcoming, the Indonesia study found even smaller increases in availability of JMP defined improved sanitation facilities and reductions in open defecation following a large-scale sanitation campaign [46] that was similar in design to the classical CLTS approach [21]. A recent cross-sectional survey in Orissa found more optimistic results—72% IHL availability following the TSC [47]—but implementation was heterogeneous. Much less than universal levels of IHL coverage and use were reported in past evaluations of pilot programs and early implementations of India's TSC [48],[49].
Within the broader water-sanitation-hygiene sector, the difficulty of scaling up interventions that are efficacious when widely adopted and properly used across a community is not unique to rural sanitation. Evaluations of large-scale hand-washing promotion campaigns in Peru and Vietnam—part of the broader research effort that included the present trial—found almost no improvements in hand-washing behavior and thus no downstream impacts on child health [50],[51]. Furthermore, the interim evaluation of the national-level Sanitation Hygiene Education and Water supply in Bangladesh program found very small improvements in hygiene and sanitation outcomes, with no impacts on child health [52].
The present evidence from the sector suggests that with few exceptions [53] scaled up sanitation and hygiene programs in rural settings have had difficulty in delivering the health benefits measured in small efficacy studies. Typically, the well-controlled efficacy trials can result in high enough levels of sanitation and hygiene infrastructure and behaviors necessary to deliver the health benefits, but the same levels of infrastructure or behavior change are not guaranteed to accrue to large-scale programs. From a public health perspective, these findings call into question the likelihood of the TSC in its current form to improve child health. Still, the program may be valuable from the policy and development perspective for reasons beyond public health, such as the social benefits of sanitation (dignity, privacy, safety, and reduced burden of coping especially for women) accrued to households that have and use IHLs, and the obligation of the government to provide access to sanitation as a recently recognized human right by the United Nations General Assembly (Resolution number 64/292). As the next iteration of the TSC program—named Nirmal Bharat Abhiyaan (Clean India Campaign)—continues, research efforts that focus on how to significantly increase the access to and use of IHLs would be particularly valuable to guide future program refinement. High levels of IHL coverage and use should be demonstrated in pilot programs before these program refinements are taken to national scale.
Limitations
Like other effectiveness studies that measure the impact of large-scale government programs, we faced the challenges typically not encountered in well-controlled efficacy trials such as imperfect compliance with treatment assignment and poor fidelity of intervention implementation. We found that by 21 months of follow-up, none of the intervention villages achieved the program goal of 100% households having and using IHLs that can safely confine feces; the average household level coverage of JMP defined improved sanitation facilities was 40% (range: 5%–79%). The reasons for the gap between the officials monitoring records of the TSC and the actual status are discussed elsewhere [54]. The Block coordinators also identified that at least eight and possibly ten control villages received the TSC program. ITT estimates of program impacts with imperfect compliance will underestimate the effect possible under perfect compliance.
Another challenge in trials where study investigators have limited control over the program implementation, is significant deviations in the actual implementation timeline compared to the timeline on which the evaluation study is based. While the planned follow-up period from the baseline was 18 months in this study, the actual follow-up measurement at 21 months was the latest possible point we could measure outcomes under the possibility of program expansion into control villages and contractual constraints with the evaluation funding. Although it was possible that impacts on diarrheal diseases could begin relatively soon after intervention, as documented in short-duration efficacy trials [55], we would expect impacts on enteric parasite infection, anemia, and growth to potentially accrue more slowly.
The limited length of follow-up could have also influenced our estimates of the program's effect on IHL availability and use. Longer follow-up could have led to potentially higher levels of IHL coverage or, conversely, lower levels of use (if IHLs are not maintained). Despite this limitation, our estimates of IHL coverage and reported use are broadly consistent with other independent measures following rural sanitation programs in India [47]–[49]. For example, Barnard and colleagues [47] found that 4 to 6 years after TSC implementation in Orissa that 53% of households with an IHL reported some individuals still practiced open defecation. In the present study, 41% of men and 38% of women from the intervention group who have JMP defined improved sanitation facilities reported practicing daily open defecation.
Self-reported outcomes can be subject to differential, biased reporting in unblinded trials [31],[32]. Therefore, in addition to self-reported illnesses, we included several objective child health measurements in this study (parasite infections, anemia, anthropometry). However, we did not include objective measures of sanitation behaviors (disposal of child feces, IHL use, and open defecation). To the extent that our measurements of reported outcomes were subject to courtesy bias, we may have over-estimated IHL use or under-estimated open defecation prevalence in the study population. Furthermore, if the bias was differential by treatment group, then we would expect the study to have over-estimated the improvements due to intervention because we would expect the intervention households to be more sensitized to the stigma of open defecation. Measures of IHL use could be improved in future sanitation studies through the use of passive sensors mounted in the latrine [56],[57].
Generalizability
There is wide variation in TSC implementation within India, and it remains possible that the TSC program was more or less successful in other states [19]. We note, however, that very few Indian states had large growth in IHL availability between 2001 and 2011 when the TSC program was active across India. In Madhya Pradesh, the TSC program was combined with Nirmal Vatika that served to increase the IHL construction subsidies available to all eligible households. Additionally, the districts enrolled in this study received support from the WSP's TSSM project to build capacity for creating an enabling environment, record keeping and monitoring, and implementing CLTS-based behavior change approaches. Therefore, the behavior change approaches in the study districts were arguably more intensive than those in the rest of Madhya Pradesh. However, this study should be not viewed as an evaluation of the CLTS approach as advocated by its practitioners [21] because the intervention only used CLTS behavior change tools and did not follow the key principles of CLTS such as not providing hardware subsidy and not prescribing latrine models.
Conclusions
This 80 village study in rural Madhya Pradesh represents the first published large-scale, randomized evaluation of India's TSC to measure and report outcomes at all stages of the causal chain (Figure 1). While the TSC program in rural Madhya Pradesh implemented with support from the WSP increased the household level availability of JMP defined sanitation facilities (+19%) and to a lesser extent reduced open defecation (−10%), these improvements were insufficient to improve child health outcomes (diarrhea, parasite infections, anemia, growth). Despite the limitations of the present study, including short follow-up and evidence for contamination in the control group, the results underscore the challenge of achieving adequately large levels of improvements in sanitation to deliver the expected health benefits within the scaled-up rural sanitation programs.
Supporting Information
Zdroje
1. MaraD, LaneJ, ScottB, TroubaD (2010) Sanitation and health. PLoS Med 7: e1000363.
2. UNICEF, WHO (2012) World Health Organization and United Nations Children's Fund Joint Monitoring Programme for Water Supply and Sanitation (JMP). Progress on Drinking Water and Sanitation: 2012 Update. New York and Geneva: UNICEF and WHO.
3. Unicef, WHO (2009) Diarrhoea: Why children are still dying and what can be done. New York and Geneva: Unicef/WHO.
4. ClasenTF, BostoenK, SchmidtW-P, BoissonS, FungIC-H, et al. (2010) Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev 6: CD007180.
5. NormanG, PedleyS, TakkoucheB (2010) Effects of sewerage on diarrhoea and enteric infections: a systematic review and meta-analysis. Lancet Infect Dis 10 : 536–544.
6. BarretoML, GenserB, StrinaA, TeixeiraMG, AssisAMO, et al. (2010) Impact of a city-wide sanitation programme in Northeast Brazil on intestinal parasites infection in young children. Environ Health Perspect Available: http://www.ncbi.nlm.nih.gov/pubmed/20705544. Accessed 20 November 2010.
7. Mascarini-SerraLM, TellesCA, PradoMS, MattosSA, StrinaA, et al. (2010) Reductions in the prevalence and incidence of geohelminth infections following a city-wide sanitation program in a Brazilian Urban Centre. PLoS Negl Trop Dis 4: e588.
8. ZiegelbauerK, SpeichB, MäusezahlD, BosR, KeiserJ, et al. (2012) Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 9: e1001162.
9. ChavasseDC, ShierRP, MurphyOA, HuttlySR, CousensSN, et al. (1999) Impact of fly control on childhood diarrhoea in Pakistan: community-randomised trial. Lancet 353 : 22–25.
10. EmersonPM, LindsaySW, AlexanderN, BahM, DibbaS-M, et al. (2004) Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet 363 : 1093–1098.
11. EmersonPM, SimmsVM, MakaloP, BaileyRL (2005) Household pit latrines as a potential source of the fly Musca sorbens–a one year longitudinal study from The Gambia. Trop Med Int Health 10 : 706–709.
12. CheckleyW, BuckleyG, GilmanRH, AssisAM, GuerrantRL, et al. (2008) Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 37 : 816–830.
13. SchmidtW-P, BoissonS, GenserB, BarretoML, BaisleyK, et al. (2010) Weight-for-age z-score as a proxy marker for diarrhoea in epidemiological studies. J Epidemiol Community Health 64 : 1074–1079.
14. HumphreyJH (2009) Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374 : 1032–1035.
15. PrendergastA, KellyP (2012) Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 86 : 756–763.
16. CheckleyW, GilmanRH, BlackRE, EpsteinLD, CabreraL, et al. (2004) Effect of water and sanitation on childhood health in a poor Peruvian peri-urban community. Lancet 363 : 112–118.
17. MiguelE, CamererC, CaseyK, CohenJ, EsterlingKM, et al. (2014) Promoting transparency in social science research. Science 343 : 30–31.
18. Salvatore AL, Patil SR (2011) Scaling up rural sanitation: findings from the impact evaluation baseline survey in Madhya Pradesh, India. The Water and Sanitation Program, The World Bank. Washington (D.C.): The World Bank.
19. GhoshA, CairncrossS (2014) The uneven progress of sanitation in India. J Water, Sanitation & Hygiene for Devel 4 : 15–22.
20. Report of the expert group to recommend the detailed methodology for identifcation of families living below poverty line in the urban areas (2012). Planning Commission, Perspective Planning Division, Government of India. Available: http://planningcommission.nic.in/reports/genrep/rep_hasim1701.pdf. Accessed 14 April 2014.
21. KarK, ChambersR (2008) Handbook on Community-Led Total Sanitation. Plan International (UK) Available: http://www.communityledtotalsanitation.org/resource/handbook-community-led-total-sanitation. Accessed 12 February 2013.
22. BaquiAH, BlackRE, YunusM, HoqueAR, ChowdhuryHR, et al. (1991) Methodological issues in diarrhoeal diseases epidemiology: definition of diarrhoeal episodes. Int J Epidemiol 20 : 1057–1063.
23. ArnoldBF, GalianiS, RamPK, HubbardAE, BriceñoB, et al. (2013) Optimal recall period for caregiver-reported illness in risk factor and intervention studies: a multicountry study. Am J Epidemiol 177 : 361–370.
24. GoldmanN, VaughanB, PebleyAR (1998) The use of calendars to measure child illness in health interview surveys. Int J Epidemiol 27 : 505–512.
25. PaymentP, RichardsonL, SiemiatyckiJ, DewarR, EdwardesM, et al. (1991) A randomized trial to evaluate the risk of gastrointestinal disease due to consumption of drinking water meeting current microbiological standards. Am J Public Health 81 : 703–708.
26. ColfordJM, WadeTJ, SandhuSK, van der LaanMJ, BrookhartMA, et al. (2005) A randomized, controlled trial of in-home drinking water intervention to reduce gastrointestinal illness. Am J Epidemiol 161 : 472–482.
27. ColfordJM, HiltonJF, WrightCC, ArnoldBF, SahaS, et al. (2009) The Sonoma Water Evaluation Trial: a randomized drinking water intervention trial to reduce gastrointestinal illness in older adults. Am J Public Health 99 : 1988–1995.
28. ArnoldB, AranaB, MäusezahlD, HubbardA, ColfordJM (2009) Evaluation of a pre-existing, 3-year household water treatment and handwashing intervention in rural Guatemala. Int J Epidemiol 38 : 1651–1661.
29. GoveS (1997) Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Organ 75 Suppl 1 : 7–24.
30. LipsitchM, TchetgenET, CohenT (2010) Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 21 : 383–388.
31. WoodL, EggerM, GluudLL, SchulzKF, JϋniP, et al. (2008) Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 336 : 601–605.
32. SchmidtW-P, CairncrossS (2009) Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol 43 : 986–992.
33. Cogill B (2003) Anthropometric indicators measurement guide. Washington (D.C.): Food and Nutrition Technical Assistance Project, Academy for Educational Development.
34. De OnisM, OnyangoAW, Broeck JVden, ChumleaWC, MartorellR (2004) Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull 25: S27–S36.
35. WHO (2006) WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and developments. Geneva: WHO.
36. WHO (2011) Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: WHO.
37. HelselDR (1990) Less than obvious - statistical treatment of data below the detection limit. Environ Sci & Technol 24 : 1766–1774.
38. KatzN, ChavesA, PellegrinoJ (1972) A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14 : 397–400.
39. GarciaLS, ShimizuRY (1997) Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol 35 : 1526–1529.
40. Den HartogJ, RosenbaumL, WoodZ, BurtD, PetriWAJr (2013) Diagnosis of multiple enteric protozoan infections by enzyme-linked immunosorbent assay in the guatemalan highlands. Am J Trop Med Hyg 88 : 167–171.
41. LubySP, AgboatwallaM, PainterJ, BillhimerWL, KeswickBH, et al. (2006) Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Trop Med Int Health 11 : 479–489.
42. FreedmanDA (2006) On the so-called “Huber sandwich estimator” and “robust standard errors.”. Am Stat 60 : 299–302.
43. SchulzKF, GrimesDA (2005) Multiplicity in randomised trials I: endpoints and treatments. Lancet 365 : 1591–1595.
44. ClasenT, BoissonS, RoutrayP, CummingO, JenkinsM, et al. (2012) The effect of improved rural sanitation on diarrhoea and helminth infection: design of a cluster-randomized trial in Orissa, India. Emerg Themes Epidemiol 9 : 7.
45. ArnoldBF, NullC, LubySP, UnicombL, StewartCP, et al. (2013) Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits study design and rationale. BMJ Open 3: e003476.
46. Cameron L, Shah M, Olivia S (2013) Impact evaluation of a rural sanitation behavior change project in Indonesia. Washington (D.C.): The World Bank.
47. BarnardS, RoutrayP, MajorinF, PeletzR, BoissonS, et al. (2013) Impact of Indian Total Sanitation Campaign on latrine coverage and use: a cross-sectional study in Orissa three years following programme implementation. PLoS One 8: e71438.
48. PattanayakSK, YangJC, DickinsonKL, PoulosC, PatilSR, et al. (2009) Shame or subsidy revisited: social mobilization for sanitation in Orissa, India. Bull World Health Organ 8 : 580–587.
49. ArnoldBF, KhushRS, RamaswamyP, LondonAG, RajkumarP, et al. (2010) Causal inference methods to study nonrandomized, preexisting development interventions. Proc Natl Acad Sci U S A 107 : 22605–22610.
50. Galiani S, Gertler P, Orsola-Vidal A (2012) Promoting handwashing behavior in peru: the effect of large-scale mass-media and community level interventions. World Bank Policy Research Working Paper 6257. Washington (D.C.): The World Bank.
51. Chase C, Do Q-T (2012) Handwashing behavior change at scale: evidence from a randomized evaluation in Vietnam. World Bank Policy Research Working Paper 6207. Washington (D.C.): The World Bank.
52. HudaTMN, UnicombL, JohnstonRB, HalderAK, SharkerMAY, et al. (2012) Interim evaluation of a large scale sanitation, hygiene and water improvement programme on childhood diarrhea and respiratory disease in rural Bangladesh. Soc Sci Med 75 : 604–611.
53. BiranA, SchmidtW-P, VaradharajanKS, RajaramanD, KumarR, et al. (2014) Effect of a behaviour-change intervention on handwashing with soap in India (SuperAmma): a cluster-randomised trial. Lancet Glob Health 2: e145–e154.
54. HuesoA, BellB (n.d.) An untold story of policy failure: the Total Sanitation Campaign in India. Water Policy 15 : 1001–1017.
55. WaddingtonH, SnilstveitB (2009) Effectiveness and sustainability of water, sanitation, and hygiene interventions in combating diarrhoea. J Dev Eff 1 : 295–335.
56. ClasenT, FabiniD, BoissonS, TanejaJ, SongJ, et al. (2012) Making sanitation count: Developing and testing a device for assessing latrine use in low-income settings. Environ Sci Technol 46 : 3295–3303.
57. ThomasEA, BarstowCK, RosaG, MajorinF, ClasenT (2013) Use of remotely reporting electronic sensors for assessing use of water filters and cookstoves in rwanda. Environ Sci Technol 47 : 13602–13610.
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2014 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Statinová intolerance
- Genetický podklad a screening familiární hypercholesterolémie
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
- DESATORO PRE PRAX: Aktuálne odporúčanie ESPEN pre nutričný manažment u pacientov s COVID-19
-
Všetky články tohto čísla
- Observational Studies: Getting Clear about Transparency
- Scaling up Rural Sanitation in India
- Intervention Synthesis: A Missing Link between a Systematic Review and Practical Treatment(s)
- From Intense Rejection to Advocacy: How Muslim Clerics Were Engaged in a Polio Eradication Initiative in Northern Nigeria
- Ethics, Economics, and the Use of Primaquine to Reduce Falciparum Malaria Transmission in Asymptomatic Populations
- Hand Sanitiser Provision for Reducing Illness Absences in Primary School Children: A Cluster Randomised Trial
- Protective Efficacy and Safety of Three Antimalarial Regimens for the Prevention of Malaria in Young Ugandan Children: A Randomized Controlled Trial
- Heart Failure: Gaps in Knowledge and Failures in Treatment
- Staffing of Healthcare Workers and Patient Mortality: Randomized Trials Needed
- Associations between Stroke Mortality and Weekend Working by Stroke Specialist Physicians and Registered Nurses: Prospective Multicentre Cohort Study
- Women's Access and Provider Practices for the Case Management of Malaria during Pregnancy: A Systematic Review and Meta-Analysis
- Stress Hyperglycaemia in Hospitalised Patients and Their 3-Year Risk of Diabetes: A Scottish Retrospective Cohort Study
- The Effect of India's Total Sanitation Campaign on Defecation Behaviors and Child Health in Rural Madhya Pradesh: A Cluster Randomized Controlled Trial
- Heart Failure Care in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Women's Access and Provider Practices for the Case Management of Malaria during Pregnancy: A Systematic Review and Meta-Analysis
- Observational Studies: Getting Clear about Transparency
- Heart Failure Care in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- Scaling up Rural Sanitation in India
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




