-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Evaluation of a Minimally Invasive Cell Sampling Device Coupled with Assessment of Trefoil Factor 3 Expression for Diagnosing Barrett's Esophagus: A Multi-Center Case–Control Study
Background:
Barrett's esophagus (BE) is a commonly undiagnosed condition that predisposes to esophageal adenocarcinoma. Routine endoscopic screening for BE is not recommended because of the burden this would impose on the health care system. The objective of this study was to determine whether a novel approach using a minimally invasive cell sampling device, the Cytosponge, coupled with immunohistochemical staining for the biomarker Trefoil Factor 3 (TFF3), could be used to identify patients who warrant endoscopy to diagnose BE.Methods and Findings:
A case–control study was performed across 11 UK hospitals between July 2011 and December 2013. In total, 1,110 individuals comprising 463 controls with dyspepsia and reflux symptoms and 647 BE cases swallowed a Cytosponge prior to endoscopy. The primary outcome measures were to evaluate the safety, acceptability, and accuracy of the Cytosponge-TFF3 test compared with endoscopy and biopsy.
In all, 1,042 (93.9%) patients successfully swallowed the Cytosponge, and no serious adverse events were attributed to the device. The Cytosponge was rated favorably, using a visual analogue scale, compared with endoscopy (p < 0.001), and patients who were not sedated for endoscopy were more likely to rate the Cytosponge higher than endoscopy (Mann-Whitney test, p < 0.001). The overall sensitivity of the test was 79.9% (95% CI 76.4%–83.0%), increasing to 87.2% (95% CI 83.0%–90.6%) for patients with ≥3 cm of circumferential BE, known to confer a higher cancer risk. The sensitivity increased to 89.7% (95% CI 82.3%–94.8%) in 107 patients who swallowed the device twice during the study course. There was no loss of sensitivity in patients with dysplasia. The specificity for diagnosing BE was 92.4% (95% CI 89.5%–94.7%). The case–control design of the study means that the results are not generalizable to a primary care population. Another limitation is that the acceptability data were limited to a single measure.Conclusions:
The Cytosponge-TFF3 test is safe and acceptable, and has accuracy comparable to other screening tests. This test may be a simple and inexpensive approach to identify patients with reflux symptoms who warrant endoscopy to diagnose BE.
Published in the journal: Evaluation of a Minimally Invasive Cell Sampling Device Coupled with Assessment of Trefoil Factor 3 Expression for Diagnosing Barrett's Esophagus: A Multi-Center Case–Control Study. PLoS Med 12(1): e32767. doi:10.1371/journal.pmed.1001780
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001780Summary
Background:
Barrett's esophagus (BE) is a commonly undiagnosed condition that predisposes to esophageal adenocarcinoma. Routine endoscopic screening for BE is not recommended because of the burden this would impose on the health care system. The objective of this study was to determine whether a novel approach using a minimally invasive cell sampling device, the Cytosponge, coupled with immunohistochemical staining for the biomarker Trefoil Factor 3 (TFF3), could be used to identify patients who warrant endoscopy to diagnose BE.Methods and Findings:
A case–control study was performed across 11 UK hospitals between July 2011 and December 2013. In total, 1,110 individuals comprising 463 controls with dyspepsia and reflux symptoms and 647 BE cases swallowed a Cytosponge prior to endoscopy. The primary outcome measures were to evaluate the safety, acceptability, and accuracy of the Cytosponge-TFF3 test compared with endoscopy and biopsy.
In all, 1,042 (93.9%) patients successfully swallowed the Cytosponge, and no serious adverse events were attributed to the device. The Cytosponge was rated favorably, using a visual analogue scale, compared with endoscopy (p < 0.001), and patients who were not sedated for endoscopy were more likely to rate the Cytosponge higher than endoscopy (Mann-Whitney test, p < 0.001). The overall sensitivity of the test was 79.9% (95% CI 76.4%–83.0%), increasing to 87.2% (95% CI 83.0%–90.6%) for patients with ≥3 cm of circumferential BE, known to confer a higher cancer risk. The sensitivity increased to 89.7% (95% CI 82.3%–94.8%) in 107 patients who swallowed the device twice during the study course. There was no loss of sensitivity in patients with dysplasia. The specificity for diagnosing BE was 92.4% (95% CI 89.5%–94.7%). The case–control design of the study means that the results are not generalizable to a primary care population. Another limitation is that the acceptability data were limited to a single measure.Conclusions:
The Cytosponge-TFF3 test is safe and acceptable, and has accuracy comparable to other screening tests. This test may be a simple and inexpensive approach to identify patients with reflux symptoms who warrant endoscopy to diagnose BE.Introduction
It is estimated that 5% to 15% of adults in the Western world suffer from reflux symptoms [1], and this is the commonest physician diagnosis for gastrointestinal consultations, accounting for 9 million physician consultations in the US in 2009 [2]. On the other hand, many individuals with recurrent reflux symptoms do not consult their doctor and hence remain uninvestigated. Endoscopy is performed to justify and tailor the prescription of acid-suppressant medications, to identify early esophageal adenocarcinoma that has not yet advanced sufficiently to cause weight loss or other alarm symptoms, and to diagnose the pre-malignant precursor for esophageal adenocarcinoma called Barrett's esophagus (BE). A diagnosis of BE will be found in up to 25% of those undergoing endoscopy, depending on the severity of the symptoms and the age and sex of the individual [3–7]. The majority of individuals with BE are undiagnosed [8].
The importance of BE lies in its potential to progress to esophageal adenocarcinoma through intermediate dysplastic stages. Although the absolute risk of malignant progression is low [9,10], this is a cancer that, according to most recent data from the US Surveillance, Epidemiology, and End Results registries, has shown a more than 9-fold increase among white men, with about half succumbing to their cancer within a year. On the other hand, survival rates are high (>80%) for superficial cancers confined to the mucosa [11].
Thresholds for endoscopy referral have been the subject of vigorous debate. The major US medical societies and British Society of Gastroenterology recommend endoscopic screening for patients with multiple risk factors for esophageal adenocarcinoma, i.e., reflux symptoms, male, age above 50, high body mass index (BMI), and high waist-to-hip ratio [12,13]. Once diagnosed, patients with BE generally enter a surveillance program. Although the merits of surveillance are controversial [12,14–16], there are now strong data to support endoscopic treatment for individuals with low and high grade dysplasia, as well as intra-mucosal carcinoma, as a cancer prevention strategy [17–19].
Hence, there is a renewed interest in determining whether a more systematic diagnosis of BE coupled with endoscopic therapy could lead to lower mortality from esophageal adenocarcinoma. Endoscopic screening for all patients with reflux symptoms would not be affordable or justifiable for most health care systems. There is therefore a need for a more systematic, cost-effective, and patient-friendly diagnostic test for BE. We have developed a minimally invasive non-endoscopic cell collection device, called the Cytosponge, coupled with immunohistochemical staining for a single biomarker, Trefoil Factor 3 (TFF3), that could provide such a test. The device was piloted previously and consists of a 30-mm polyurethane sponge, contained within a capsule, which is attached to a string (S1 Fig.). The capsule is swallowed and dissolves within the stomach after 3–5 min. The sponge is then retrieved by pulling on the string, thus collecting cells on its return passage along the esophagus [20,21]. Analysis of the cell specimen for TFF3 expression provides an objective, binary read-out of the presence or absence of BE. This biomarker was ascertained from a gene expression study designed to distinguish between BE cells and those from the gastric cardia, squamous esophagus, and oropharynx, which are serially sampled by the Cytosponge as it is retrieved [21]. The current study described herein takes the previous work from a proof of concept to a case–control study powered to obtain accurate estimates of sensitivity and specificity while ensuring that the procedure remains well accepted and safe in a larger cohort of patients.
Methods
Study Design and Oversight
We conducted a case–control study to determine the sensitivity and specificity of the Cytosponge-TFF3 test for the detection of BE compared with endoscopy and biopsy as the reference standard (see S1 Protocol). The study was conducted according to the standards of the STARD statement for reporting studies of diagnostic accuracy (http://www.stard-statement.org/; see S1 Checklist). The UK Medicines and Healthcare Products Regulatory Agency approved use of the Cytosponge (Medical Research Council, London, UK) for this trial provided that we continued to collect safety data. Ethics approval was obtained from the East of England–Cambridge Central Research Ethics Committee (No: 10/H0308/71) and registered in the UK Clinical Research Network Study Portfolio (9461). Individual written informed consent was obtained for each patient.
Study Participants and Setting
Consecutive patients due to attend endoscopy for a clinically indicated procedure were recruited. Cases were individuals with a previous diagnosis of BE attending for their monitoring endoscopy. BE was defined as endoscopically visible columnar-lined esophagus that measured at least 1 cm circumferentially or at least 3 cm in non-circumferential tongues (Prague classification ≥C1 or ≥M3), with documented histopathological evidence of intestinal metaplasia (IM) on at least one biopsy in the course of their endoscopic history. Controls were individuals referred to endoscopy because of dyspepsia and/or reflux symptoms. Participants who were initially enrolled as control patients but were then diagnosed with BE at endoscopy (n = 13) were crossed over to the case arm. Participants were recruited from across 11 centers that were geographically dispersed across the UK (S1 Table). We included four tertiary referral centers for BE (Cambridge, University College London, Newcastle, and Nottingham) to enrich for cases of BE with dysplasia, in case dysplasia adversely affected the sensitivity of the Cytosponge-TFF3 assay. Exclusion criteria were patients with bleeding diatheses or on anticoagulant medication, and those with known cirrhosis, varices, or dysphagia.
Overview of Study Procedures
During a single office visit, we collected data on demographics, clinical exposures (alcohol, tobacco, drugs), and symptoms; administered the Cytosponge; and then performed an endoscopy procedure. Following each procedure, acceptability measures were collected. The Cytosponge was administered by a research nurse, and the whole process, including instructing the patient, took less than 10 min. Twenty-seven nurses were involved in administering the device over the course of the study across the different sites. After retrieval, the Cytosponge was placed in SurePath Preservative Fluid (TriPath Imaging, Burlington, North Carolina, US) and kept at 4°C until transportation to the laboratory.
BE patients who happened to undergo a second surveillance endoscopy for clinical purposes during the study period were invited to swallow the Cytosponge again.
Endpoints
The primary outcomes were sensitivity and specificity of the Cytosponge-TFF3 assay for detecting BE compared with endoscopy, as well as the safety and acceptability of the device. Secondary endpoints were the sensitivity of the TFF3 screen in patients with dysplasia and in patients with BE who swallowed the Cytosponge on more than one occasion.
Cytosponge Sample Processing
Anonymized samples were processed to paraffin blocks and cut into consecutively numbered sections. Immunostaining was carried out on slides 2 and 15 for TFF3 (proprietary monoclonal antibody; BD Diagnostics, Durham, North Carolina, US) using standard protocols on a BOND-MAX autostainer (Leica Biosystems, Newcastle upon Tyne, UK) according to GCP/GLP standards in the Tissue Bank laboratory housed within Addenbrooke's Hospital, Cambridge. One of two independent researchers (E. W. or C. S. R.) and a gastrointestinal histo-cytopathologist (M. O.) scored the slides in a binary fashion as either positive (≥1 TFF3-positive goblet cell) or negative. The expert histo-cytopathologist had the final say on TFF3 status. The scorers were unaware of the clinical diagnosis of the patient.
Endoscopy
The gastroscopies were carried out by a local study endoscopist within an hour of the Cytosponge procedure, and patients had the choice of sedation or local anesthetic spray. A strict protocol was followed. Biopsy samples were taken from the cardia as well as 2 cm above the squamocolumnar junction in all participants, and in BE cases diagnostic biopsies were collected following the recommended Seattle surveillance protocol [22]. Diagnostic biopsies were reviewed locally. Biopsies with a diagnosis of dysplasia were reviewed in an expert consensus meeting (M. O., M. N., B. D., and P. K.). When reviewing the biopsy data, all of the histo-cytopathologists were blinded to the result of the Cytosponge test.
Acceptability Measures
Acceptability for both the Cytosponge and endoscopy was recorded by the participant using a visual analogue scale in which 0 represented the “worst experience ever,” 10 represented the “best experience ever,” and 5 indicated “neither pleasant nor unpleasant” [20]. This acceptability measure was completed after swallowing the Cytosponge and also after the endoscopy procedure.
Statistical Analysis
Statistical analyses were conducted by the Cancer Prevention Trials Unit (B. V. N. and P. D. S.). Assuming a sensitivity of 80% and a specificity of 90% for the TFF3 screen, we needed to recruit at least 600 BE patients and 450 controls to ensure a 95% confidence interval of approximately ±3%. Statistics for continuous variables were expressed as medians and interquartile ranges (IQRs). The Mann-Whitney test and Kruskal-Wallis test were used to compare continuous and ordinal variables, respectively, between groups, and Fisher's exact test and the Cochran-Armitage trend test [23] were used to compare counts between categorical and ordinal variables, respectively. The κ statistic for agreement between the two TFF3 scorers was calculated. Accuracy of the Cytosponge-TFF3 test was reported using Clopper-Pearson exact 95% CIs [24]. There were no missing data for the Cytosponge-TFF3 test. For the acceptability data, patients who were unable to swallow the Cytosponge and those who failed to fill in the visual analogue scale were excluded from the analysis. All reported p-values were two-sided. Statistical analyses were carried out using Prism V5.01, SPSS version 19, or R version 3.
Modeling of Risk Stratification of TFF3-Positive Patients
We recently demonstrated that analysis for TP53 mutations in Cytosponge samples can be used to diagnose high grade dysplasia (HGD) when compared with samples from patients without BE and patients with BE who had never developed dysplasia over a median follow-up of 6 y [25]. To understand the broader context of the Cytosponge-TFF3 test and stratified risk using detection of TP53 mutations, we have modeled this in a hypothetical population of 10,000 individuals attending primary care for investigation of reflux symptoms. We used a sensitivity of 79.9% and a specificity of 92.4% for detection of any length of BE; for TP53 mutation detection we performed the calculations using a sensitivity of 86% (95% CI 65%–96%) and a specificity of 100% (95% CI 94.6%–100%) for detection of HGD.
Results
Patient Characteristics and Device Safety
In all, 463 controls with dyspepsia and reflux symptoms and 647 BE cases were recruited; Fig. 1 summarizes the flow of these patients through the study. The cases were generally older than the controls, with a male predominance (male:female ratio 4.0 : 1.0), as expected for this disease (Table 1). The median length of the BE segment for the cases was 5 cm (IQR 3–8), and 30% (176/596) of the cases had a circumferential BE segment of ≤1 cm.
Fig. 1. Study CONSORT diagram detailing the flow of control and Barrett's esophagus patients (cases) through the study. 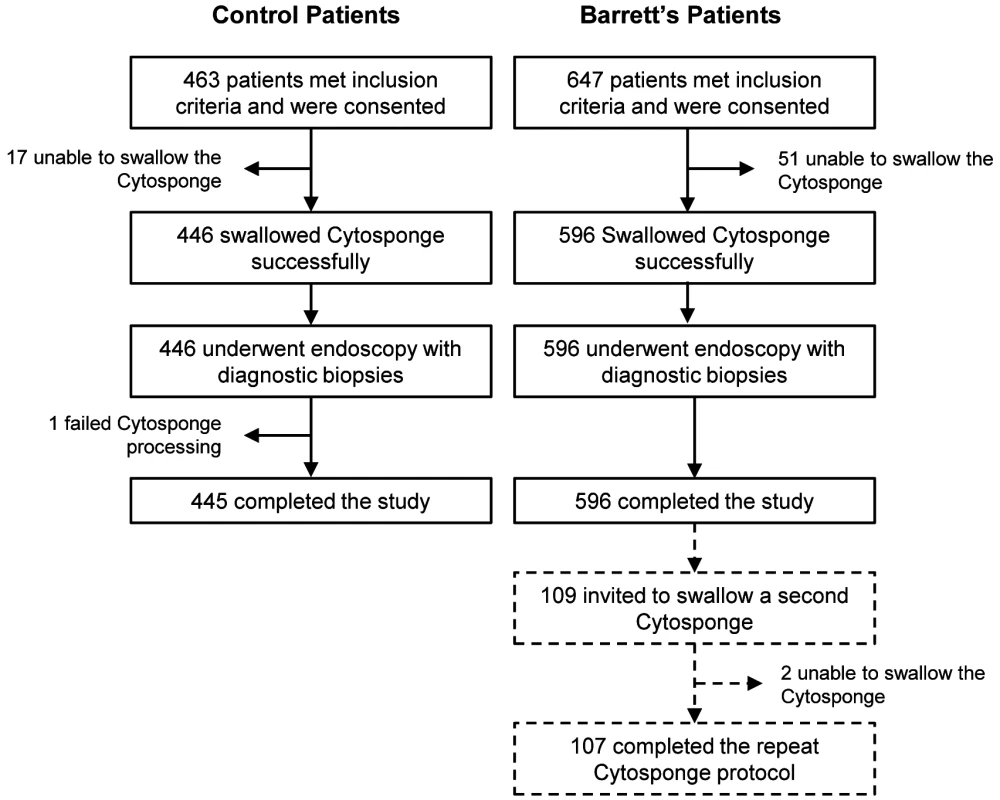
Patients who were unable to swallow the Cytosponge or whose Cytosponge sample failed processing were excluded from the study. Exact numbers of control and BE patients who successfully completed the study are noted. Tab. 1. Demographics of the BEST2 Study patient cohorts. 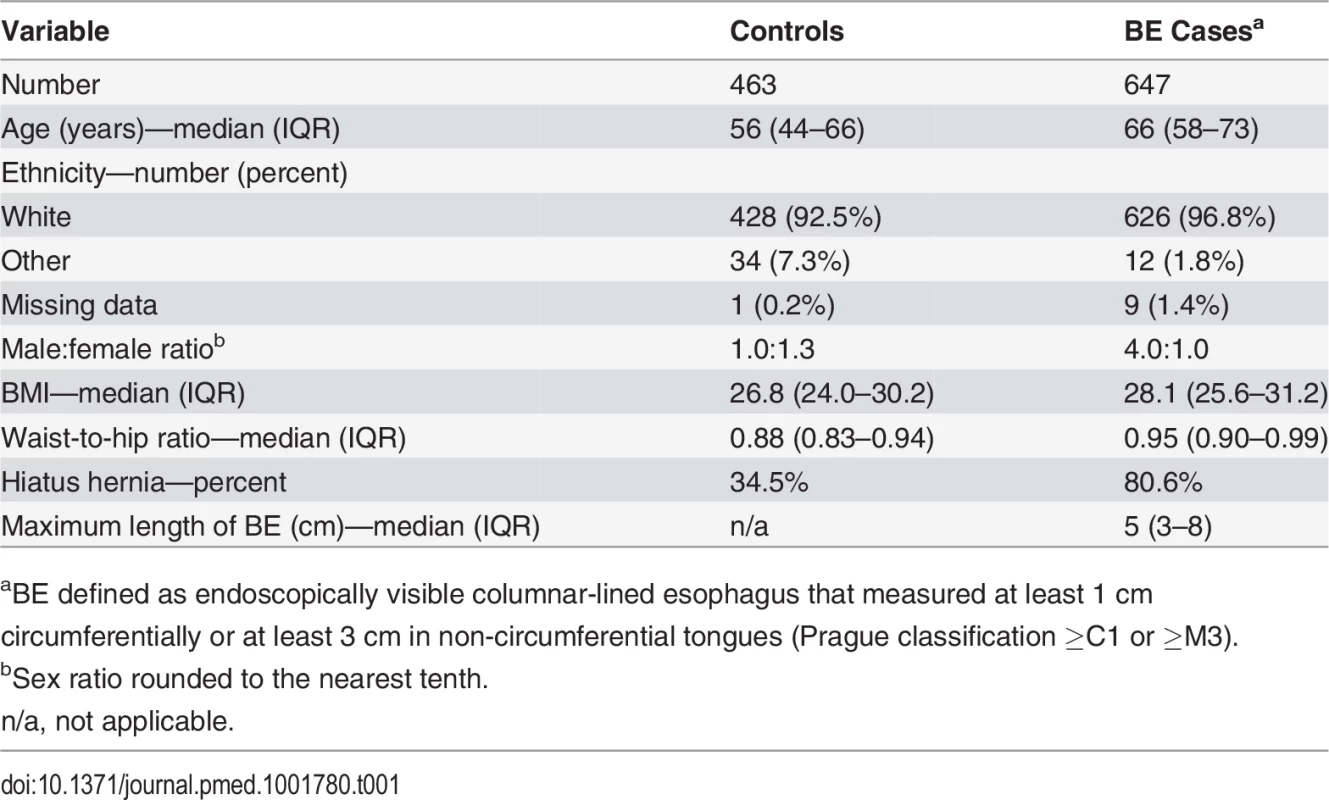
aBE defined as endoscopically visible columnar-lined esophagus that measured at least 1 cm circumferentially or at least 3 cm in non-circumferential tongues (Prague classification ≥C1 or ≥M3). Three serious adverse events were reported under the Good Clinical Practice guidelines, none of which were attributed to the Cytosponge. One patient had onset of atrial fibrillation during radiofrequency ablation that was performed at the same time as the study endoscopy. One patient was admitted for a bleed from a biopsy site, which stopped spontaneously. One patient was found to have unsuspected esophageal varices during the endoscopy, but no evidence of bleeding or Cytosponge abrasions were seen. Three days later the patient had a hematemesis, and the varices were banded. It was not possible to determine the trigger for bleeding in this case.
In terms of adverse events, 16.7% of patients had oozing of blood from a Cytosponge abrasion site (noted by the endoscopist); however, these abrasions did not require any intervention. The oozing was at worst similar to that seen at a biopsy collection site. There were anecdotal reports of a short-lived sore throat.
Procedure Acceptability
93.9% of patients (1,042/1,110) swallowed the Cytosponge successfully. The proportion of patients who were unable to swallow the device was significantly higher in the case arm than in the control arm (7.9% versus 3.9%, p = 0.003). Using a fully adjusted logistic regression, increasing BMI was associated with more difficulty swallowing the Cytosponge; however, the age and gender of the patients had no impact. Of the 6% (68/1,110) of patients who were unable to swallow the device, 11.8% (8/68) were also unable to tolerate endoscopy.
When asked to rate the Cytosponge procedure using a visual analogue scale for acceptability with 0 representing the worst experience ever, 5 representing a neutral score, and 10 representing the best experience ever, 689/746 patients (92.4%) scored the experience as 4 or more, and 729/746 patients (97.7%) gave a score of 3 or more (mildly unpleasant or better), with a median value of 6 (IQR 5–8; Fig. 2). Overall, the Cytosponge procedure was rated significantly higher than the endoscopy procedure (Wilcoxon signed rank test, p< 0.001). In all, 258/748 patients (34.5%) rated the procedures equally, and 276/732 patients (37.8%) preferred the Cytosponge. On the whole, men rated the Cytosponge better than endoscopy (Mann-Whitney test, p = 0.029), and patients who were not sedated for endoscopy were more likely to rate the Cytosponge higher than endoscopy (Mann-Whitney test, p < 0.001).
Fig. 2. Acceptability of endoscopy and the Cytosponge test. 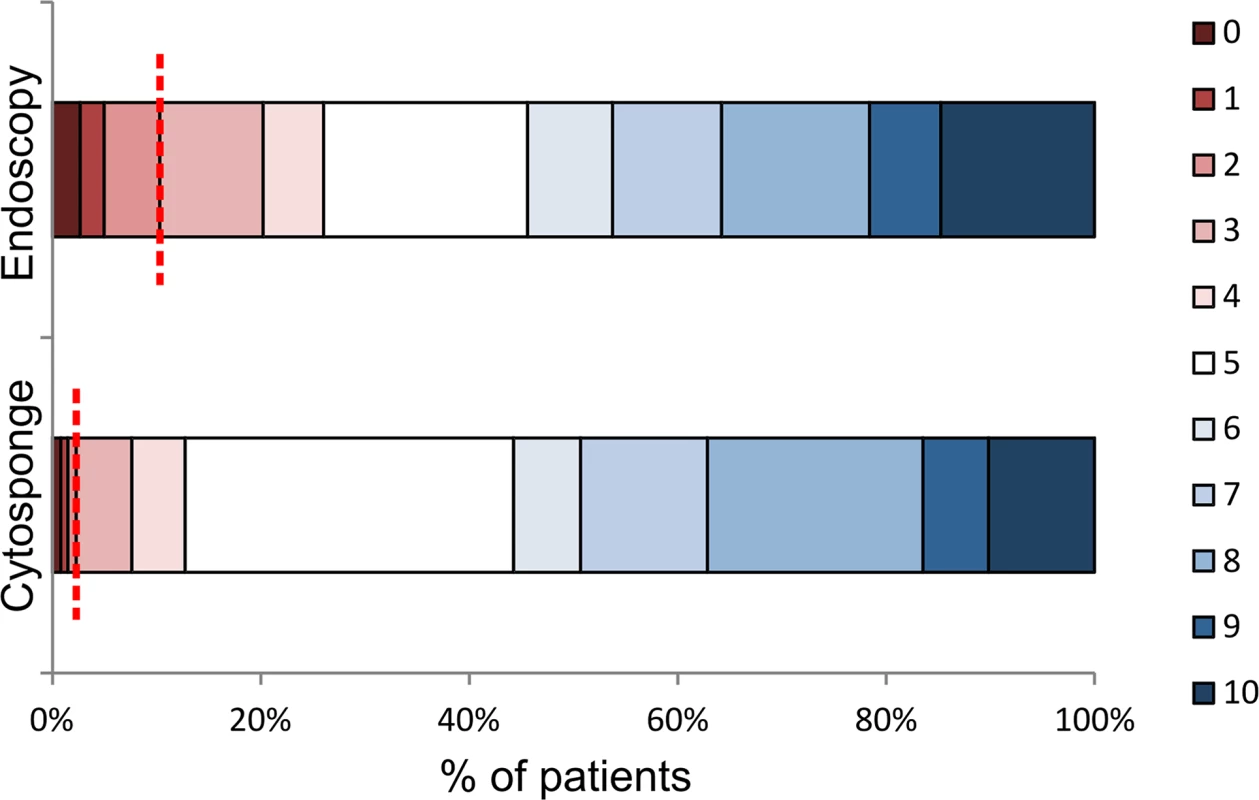
Patients were asked to rate the procedures using a visual analogue acceptability scale after swallowing the Cytosponge and after endoscopy. The colors representing the different acceptability scores are shown on the right-hand side, with 0 representing the worst experience ever, 5 representing a neutral experience, and 10 representing the best experience ever. The dotted red line marks the boundary between mildly unpleasant or worse (left of the line, score of 0–3) and acceptable scores (right of the line, score of 4 or more), for ease of comparison between the two procedures. Diagnostic Accuracy
A diagnosis of BE using the Cytosponge relies on adequate sampling of the esophageal mucosa and the presence of TFF3 positivity. Fig. 3 shows examples of TFF3 staining for a case with widespread TFF3 positivity and for a case with a single TFF3-positive goblet cell, which was also categorized as positive. A negative case is shown for comparison. The κ statistic for agreement between the two TFF3 scorers was 0.945 (95% CI 0.933–0.958), indicating substantial agreement. Overall the sensitivity and specificity for diagnosing BE according to our definition (at least 1 cm circumferentially or at least 3 cm in non-circumferential tongues, with IM in any diagnostic biopsy) were 79.9% (95% CI 76.4%–83.0%; Table 2) and 92.4% (95% CI 89.5%–94.7%), respectively (raw data available in S2 Table). There were 34 false positives for diagnosing BE (positive according to the Cytosponge-TFF3 test but negative according to endoscopy) within the 445 control patients who completed the study. None of these patients with false-positive results had IM of the gastric cardia.
Fig. 3. TFF3 immunohistochemical staining of Cytosponge samples. 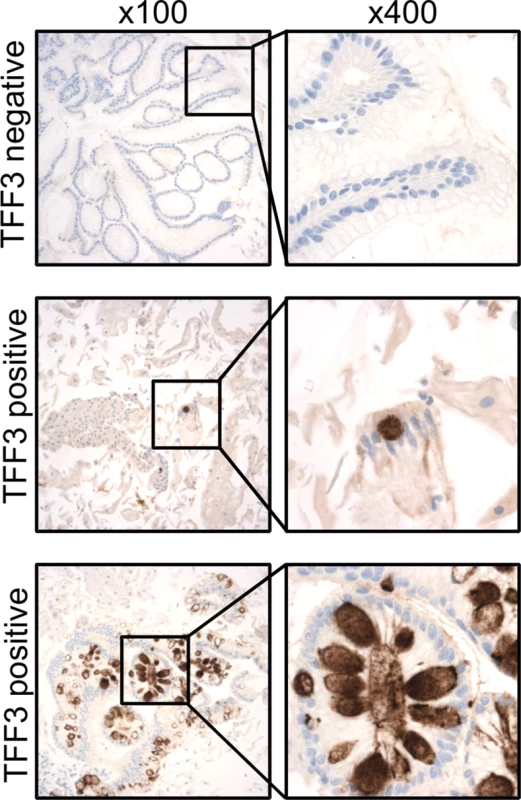
TFF3 staining was performed on all Cytosponge samples to test the sensitivity and specificity of the Cytosponge-TFF3 test for diagnosing BE. TFF3 was scored in a binary fashion, with samples with one or more TFF3-positive goblet cells being classed as positive. Shown are immunohistochemical images illustrating examples of TFF3-negative and-positive staining at low magnification (100×) and high magnification (400×). Tab. 2. Sensitivity of the Cytosponge-TFF3 in different groups of patients (full dataset in S2 and S3 Tables). 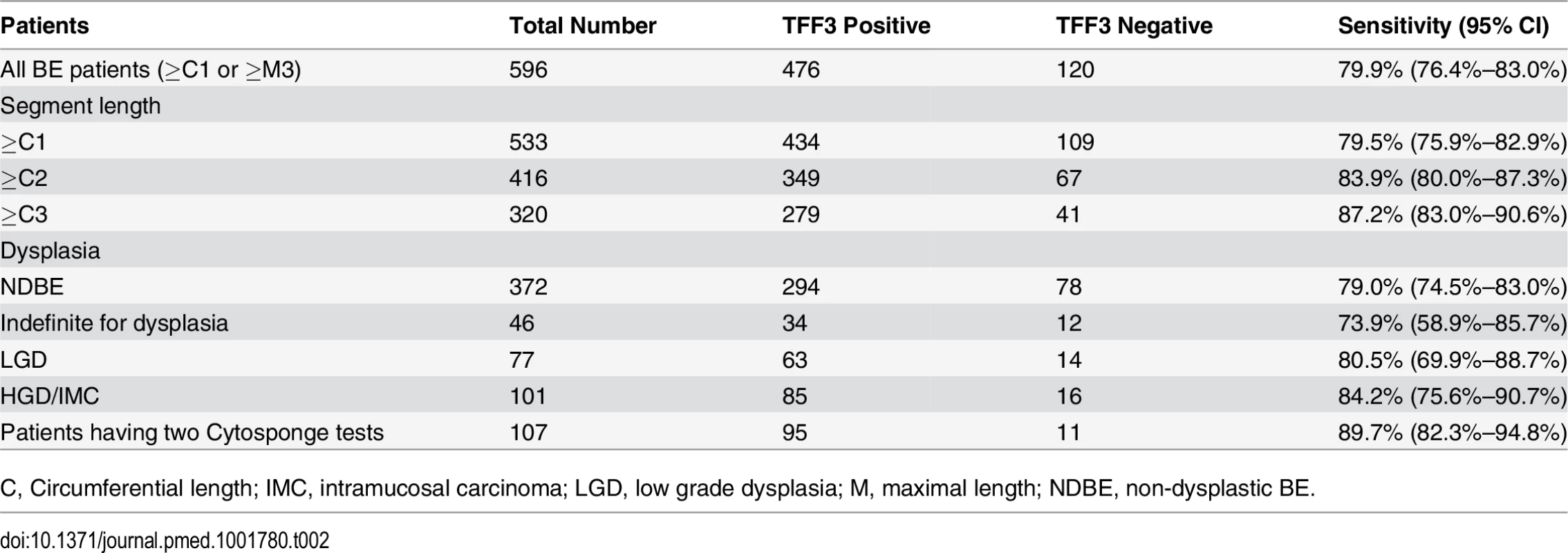
C, Circumferential length; IMC, intramucosal carcinoma; LGD, low grade dysplasia; M, maximal length; NDBE, non-dysplastic BE. The sensitivity for diagnosing BE increased with the circumferential length of the BE segment up to 87.2% (95% CI 83.0%–90.6%, Cochran-Armitage trend test, p < 0.001; Table 2), and TFF3 positivity was not reduced in the presence of dysplasia (Cochran-Armitage trend test, p = 0.253; Table 2). Furthermore, the sensitivity and specificity were not affected by any of the clinical covariates, namely, age, gender, BMI, and waist-to-hip ratio (Table 3). A significant difference in sensitivity, but not specificity, was seen between centers (p < 0.001; S3 Table). This difference seems to be attributable to different proportions of cases with no columnar cells on their sample. Once cases with five or fewer columnar cell groups were excluded, the sensitivity was uniformly high (p = 0.24, sensitivity range 91% to 98%).
Tab. 3. Clinical covariates have no impact on sensitivity and specificity of the test. 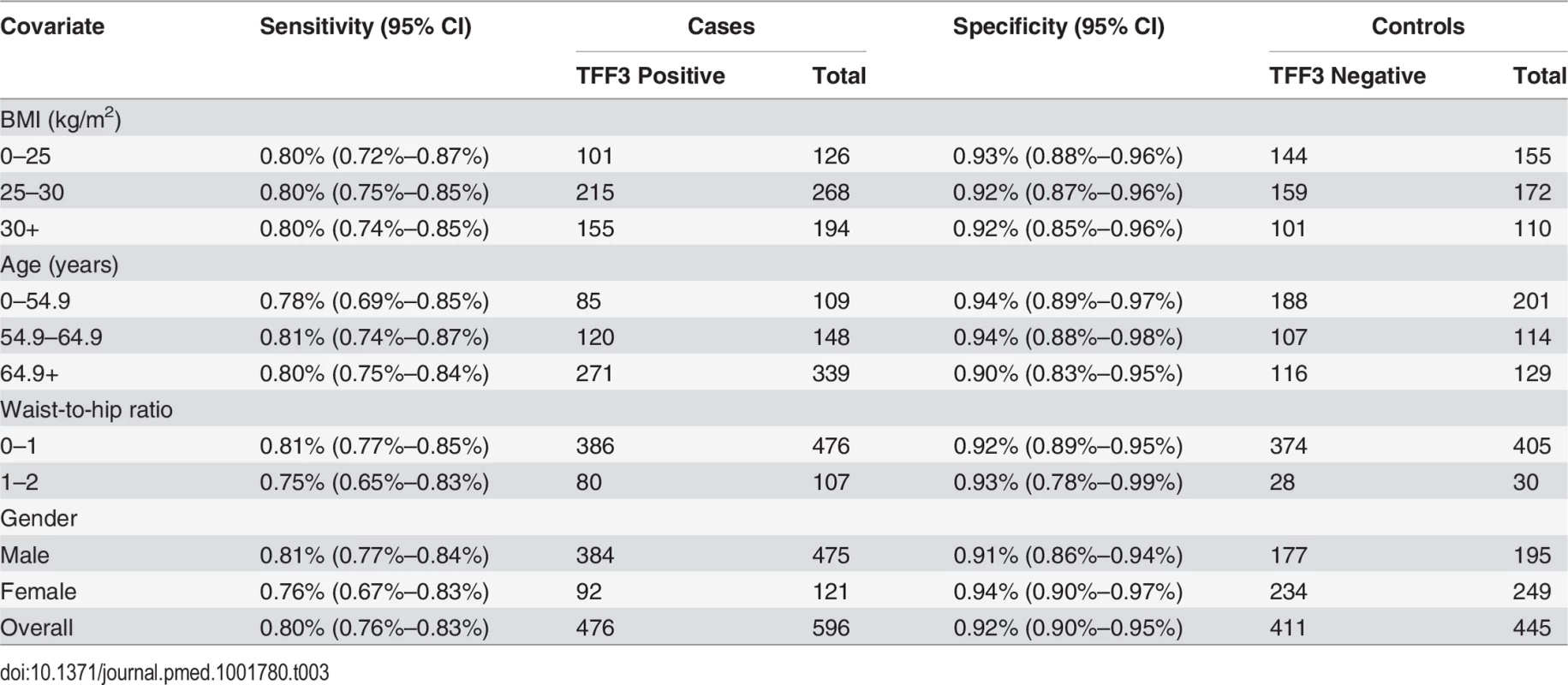
The study design allowed for patients with BE to swallow a second Cytosponge if they attended for a further clinically indicated endoscopy during the time course of the study. As shown in Fig. 1, 107/109 patients (98.2%) successfully swallowed the device on a second occasion, and when both Cytosponge-TFF3 test results were considered, the sensitivity increased to 89.7% (95% CI 82.3%–94.8%; Table 2). The time interval between procedures varied depending on the clinical indication, but this did not obviously affect the result (Mann-Whitney test, p = 0.139).
Modeling of Risk Stratification of TFF3-Positive Patients
Risk stratification of TFF3-positive patients could be achieved through the addition of screening for further molecular markers in the same Cytosponge sample, and this strategy has the potential to significantly reduce the proportion of TFF3-positive patients who would require endoscopy. Indeed, using an exquisitely sensitive (down to an allele fraction of 0.6%) next-generation sequencing approach, we recently demonstrated that screening for TP53 mutations in Cytosponge samples can be used to diagnose HGD [25]. We modeled risk stratification in a population of 10,000 individuals offered the Cytosponge-TFF3 test (Fig. 4). In this population, we would expect 974 (9.8%) patients to test TFF3 positive. Of these, 737 would be false positives, and following TP53 testing, between zero and 40 patients would require endoscopy. Those testing negative for TP53 mutations would be scheduled for a repeat Cytosponge, expected to be between 2 and 5 y later, but the optimal interval is still to be determined. Similarly, for the true TFF3-positive patients with BE (prevalence 3%), between two and 14 cases would test positive for TP53 mutations, and approximately two of these would be confirmed dysplastic at endoscopy. Hence, following risk stratification, the endoscopy burden in the TFF3-positive arm would be significantly reduced from 974 to between two and 54 cases. On the other hand, one would expect 9,026 patients to test TFF3 negative, of which 63 would be false negatives. If sensitivity was felt to be paramount and these patients were given a second Cytosponge-TFF3 test, then one would find another 33 true positives at the expense of 681 false positives (based on our repeat Cytosponge-TFF3 test data). However, after risk stratification of these positive cases using TP53 sequencing, again the number of endoscopies would be dramatically reduced to between two and 39 (numbers not shown in Fig. 4).
Fig. 4. Modeling of Cytosponge-TFF3 testing and risk stratification in the primary care population with reflux symptoms. 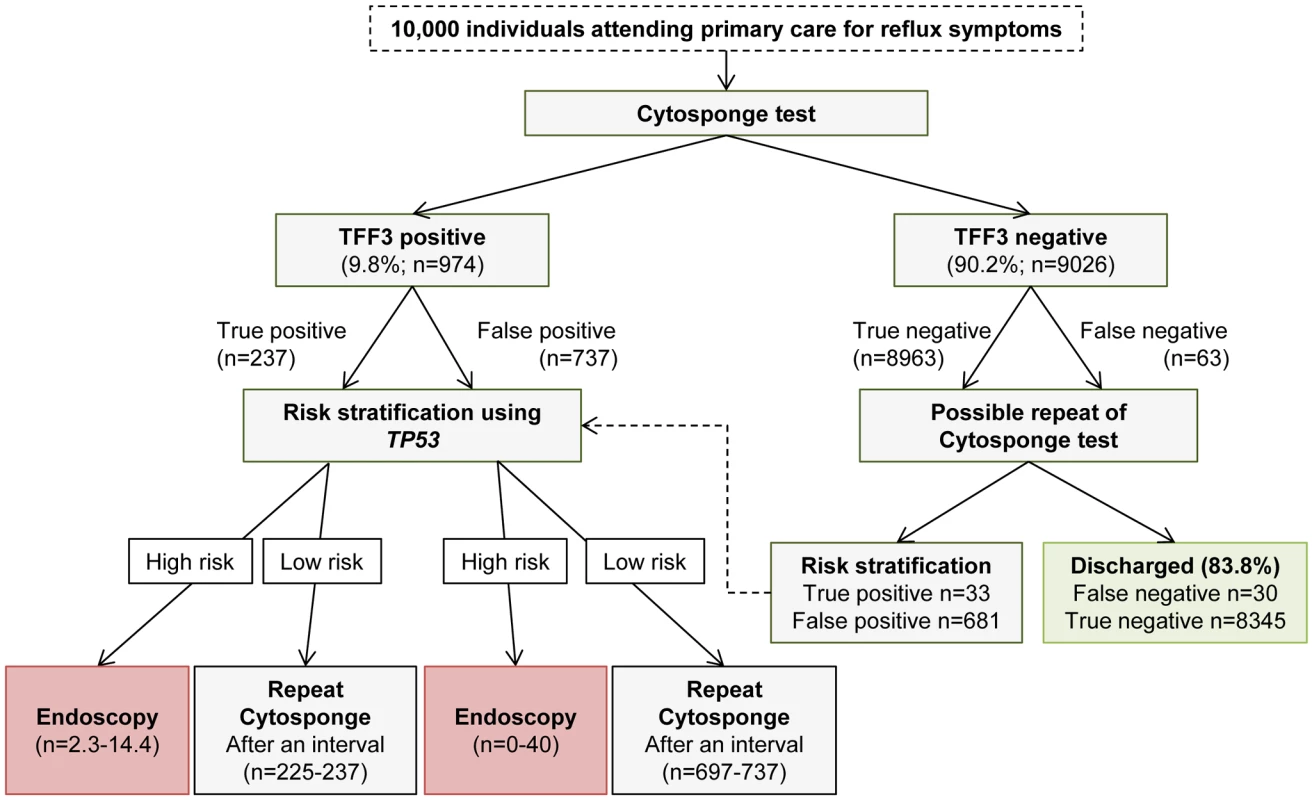
Extrapolation of findings to a hypothetical population of 10,000 individuals with reflux symptoms using a sensitivity and specificity of 79.9% and 92.4%, respectively, for the TFF3 screen, and a sensitivity and specificity of 86% (95% CI of 65%–96%) and 100% (95% CI of 94.6%–100%), respectively, for TP53 mutation screening for detection of HGD. The assumed prevalence of BE was 3%. In patients found to be high risk, endoscopy within 6–8 wk would be recommended. For low-risk patients, a repeat Cytosponge-TFF3 test would be performed at an interval of several years (exact timing to be determined) in case they had become TP53 positive over this time period. In the TFF3-negative arm, the repeat Cytosponge testing might not be necessary. If it took place, repeat testing would be recommended within 6 to 8 wk of the delivery of the TFF3-negative results. Discussion
The Cytosponge-TFF3 test is safe and generally acceptable to patients with symptomatic reflux or dyspepsia undergoing investigation, and for those patients with BE undergoing surveillance. The test has a sensitivity of around 80% for diagnosing BE; sensitivity increases with BE segment length and is not compromised in the presence of dysplasia. The specificity of the test is 92%.
Sensitivity of the Cytosponge-TFF3 Test
In the previous pilot study in which the primary endpoints were feasibility, safety, and acceptability, 504 patients were recruited [20]. Although not powered for accuracy, the sensitivity and specificity obtained in this initial study were 73.3% (95% CI 44.9%–92.2%) and 93.8% (95% CI 91.3%–95.8%) for ≥1 cm circumferential columnar epithelium for 15/501 patients with BE. Hence, it is very encouraging that in this adequately powered study, the overall sensitivity of the test has improved, with tighter confidence intervals (79.9%, 95% CI 76.4%–83.0%) and a similar specificity of 92.4% (95% CI 89.5%–94.7%). In both studies, the sensitivity increased with the length of the BE segment. This is highly relevant given the documented increase in cancer risk with longer BE segments [26,27]. This finding has been further confirmed by an analysis of over 1,000 cases that demonstrated an annual incidence of esophageal adenocarcinoma of 0.32% per year in patients with BE segments of 3 cm or more, compared with an annual esophageal adenocarcinoma risk of 0.04% for patients with BE segments <3 cm and 0.01% for patients with ultra-short BE segments [28].
The Cytosponge-TFF3 test sensitivity for diagnosing BE increased to nearly 90% when the device was swallowed a second time. The commonest reason for a false-negative test (76%) was the absence of columnar cells on the specimen, rather than failure of the TFF3 antibody to recognize BE when columnar cells were present. Hence, in clinical practice, it would be possible to advise patients with a TFF3-negative test and no or very few columnar cells seen on the specimen to attend for a repeat Cytosponge-TFF3 test, similar to being recalled for a repeat cervical smear because of an inadequate specimen. Alternatively, the Cytosponge-TFF3 test could be performed twice in everyone, in a manner analogous to the serial fecal occult blood testing undertaken in some countries as part of a colorectal cancer screening program. It is also possible that the device could be engineered to maximize collection of cells at the gastroesophageal junction.
False Positives
False positives are also an important consideration for any screening or diagnostic test. Analysis of our Cytosponge-TFF3 test data in which there were 34/445 (7.6%) false positives suggests that these were not due to IM at the gastric cardia, nor were they due to very short tongues of BE. Patients with BE tongues of 1 cm or less with no IM present on biopsies were included in the control cohort as these patients are likely to have irregular Z lines, but there was no correlation between these types of patients with very short Barrett tongues and false-positive TFF3 results (p = 1.0). However, the correlation between false-positive TFF3 results and the presence of Helicobacter pylori nearly reached significance, with a p-value of 0.0825. H. pylori infection may lead to the presence of IM within the stomach, which may have been missed on the gastric cardia biopsy used to assess the presence of cardia IM, but which may nevertheless have been sampled by the Cytosponge. It is also known that up to 10% of patients presenting to endoscopy for dyspepsia have a diagnosis of gastric IM, a known precursor lesion to gastric cancer [29]. If a portion of these false-positive results are indeed due to gastric IM, it may actually be beneficial to the patients to be made aware of this, especially since an estimated 10% of these patients will develop gastric cancer. To put these data in context, our false-positive rate of 7.6% compares with the false positive rate of 2%–9% for colorectal screening. The new multi-target stool DNA test for colorectal cancer screening [30] has an even higher false-positive rate of 10.2%–13.4%, compared with that of the current fecal immunochemical test, which is 3.6%–5%, although the authors of the multi-target stool DNA test article argue that sensitivity is more important than specificity. Another relevant comparison is cervical cancer screening, which has a false-positive rate of 6%–15% [31], where the over-diagnosis of micro-invasive cancer is considered more than compensated by the prevention of cancer through treatment of cervical intra-epithelial neoplasia. With the advent of outpatient-based endoscopic therapy for BE, a similar argument can be applied to BE. Risk stratification could lead to the equivalent grading as for cervical intra-epithelial neoplasia 1–3.
Acceptability and Implementation of the Test
The acceptability data are very encouraging. The same scale was used in the community-based pilot study [20], in which the median score was 7 (IQR 5–8) compared with the median of 6 (IQR 5–8) described here. It is likely that the clinical setting may alter patient preferences. The community setting is usually more familiar and less daunting for patients, and in this study the individuals were about to have an endoscopy procedure a few minutes later, which would have influenced their experience.
When considering implementation of a new technology, the logistical considerations are also important. The Cytosponge procedure was administered in 11 centers by a total of 27 nurses; training occurred via one training visit by the nurse coordinator (I. D.). In terms of sample logistics, the samples were stored at 4°C and could be kept for several weeks prior to processing. The sample processing and antibody staining were performed in a laboratory housed within the hospital pathology department, and as such followed clinical laboratory standards. Because of the binary nature of the TFF3 scoring, this test would be amenable to automated image scanning. Overall, this study was conducted using methods relevant to routine clinical practice, and nothing emerged that would seem to preclude the implementation of such a diagnostic test.
The Cytosponge-TFF3 test could be used as a first-line diagnostic test in patients with multiple risk factors for BE (as recommended by the major medical society guidelines [13,32,33]) who would otherwise be referred for endoscopy to rule out BE. However, since a previous modeling study suggested that the Cytosponge-TFF3 test combined with endotherapy for treating early cancer had cost advantages over endoscopy [34], this approach may open up the possibility for more systematic diagnosis of a broader subgroup of the population in the future. Indeed, since only 12% of patients with gastroesophageal reflux disease are currently being endoscoped, it follows that only a small fraction of BE cases are being diagnosed through the current referral routes. While the sensitivity of the Cytosponge-TFF3 test is not perfect at 80% to 90%, depending on length of BE segment and repeat Cytosponge testing, it would allow a diagnosis in the vast majority of the currently undiagnosed pool of patients, thus enabling the required step-change to improve mortality of esophageal adenocarcinoma. However, our modeling is limited to the expected number of BE patients diagnosed and does not estimate the expected number of patients who would progress to esophageal adenocarcinoma. Further modeling would be required to obtain this degree of information. Based on a 5% prevalence of BE and sensitivity of 80%, the modeled negative and positive predictive values of the test would be ~98.9% and 34.7%, respectively. However, the positive predictive value depends heavily on disease prevalence and would decrease to 25.1% for a population with a 3% BE prevalence and increase to 44.9% for patient populations with more severe reflux symptoms. Hence, the population at risk could be tailored further using simple symptoms nomograms [35,36]. These modeled values are favorable compared with current screening tests such as fecal occult blood testing, cervical smears, and mammography [31,37–38].
While the case–control design was appropriate to obtain accuracy data with narrow confidence intervals, this design has known limitations in terms of the ability to generalize the data to the primary care population consulting for reflux symptoms. Care must be taken not to extrapolate the negative and positive predictive values directly. In addition, the acceptability data were limited to a single measure. In the future it will be important to collect more in-depth qualitative data on acceptability.
Conclusions
The Cytosponge-TFF3 test can diagnose BE in a manner that is acceptable to patients and logistically feasible across multiple centers. This test may substantially lower the threshold for investigating patients with reflux, as part of a strategy to reduce population mortality from esophageal adenocarcinoma. Analysis of a full panel of risk-stratification biomarkers enriched further for dysplasia and applied to this cohort is ongoing, and a randomized controlled trial of the Cytosponge-TFF3 test compared with the current standard of care for patients with reflux and dyspepsia symptoms in primary care is in preparation. The data from such studies will be required prior to implementation of the Cytosponge-TFF3 test on a population level.
Supporting Information
Zdroje
1. Dent J, El-Serag HB, Wallander MA, Johansson S (2005) Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 54 : 710–717. 15831922
2. Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, et al. (2012) Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143 : 1179–1187. doi: 10.1053/j.gastro.2012.08.002 22885331
3. Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, et al. (2005) Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology 129 : 1825–1831. 16344051
4. Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, et al. (2008) Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut 57 : 1354–1359. doi: 10.1136/gut.2007.145177 18424568
5. Gerson LB, Shetler K, Triadafilopoulos G (2002) Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology 123 : 461–467. 12145799
6. Rex DK, Cummings OW, Shaw M, Cumings MD, Wong RKH, et al. (2003) Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology 125 : 1670–1677. 14724819
7. Ward EM, Wolfsen HC, Achem SR, Loeb DS, Krishna M, et al. (2006) Barrett's esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 101 : 12–17. 16405528
8. Lao-Sirieix P, Fitzgerald RC (2012) Screening for oesophageal cancer. Nat Rev Clin Oncol 9 : 278–287. doi: 10.1038/nrclinonc.2012.35 22430857
9. Desai TK, Krishnan K, Samala N, Singh J, Cluley J, et al. (2012) The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 61 : 970–976. doi: 10.1136/gutjnl-2011-300730 21997553
10. Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P (2011) Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 365 : 1375–1383. doi: 10.1056/NEJMoa1103042 21995385
11. Zehetner J, DeMeester SR, Hagen JA, Ayazi S, Augustin F, et al. (2011) Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 141 : 39–47. doi: 10.1016/j.jtcvs.2010.08.058 21055772
12. Spechler SJ (2013) Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA 310 : 627–636. doi: 10.1001/jama.2013.226450 23942681
13. Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, et al. (2014) British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 63 : 7–42. doi: 10.1136/gutjnl-2013-305372 24165758
14. Sharma P, McQuaid K, Dent J, Fennerty MB, Sampliner R, et al. (2004) A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop. Gastroenterology 127 : 310–330. 15236196
15. Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, et al. (2013) Impact of endoscopic surveillance on mortality From Barrett's esophagus–associated esophageal adenocarcinomas. Gastroenterology 145 : 312–319. doi: 10.1053/j.gastro.2013.05.004 23673354
16. Bhat SK, McManus DT, Coleman HG, Johnston BT, Cardwell CR, et al. (2014) Oesophageal adenocarcinoma and prior diagnosis of Barrett's oesophagus: a population-based study. Gut 64 : 20–25. doi: 10.1136/gutjnl-2013-305506 24700439
17. Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, et al. (2014) Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 311 : 1209–1217. doi: 10.1001/jama.2014.2511 24668102
18. Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, et al. (2009) Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 360 : 2277–2288. doi: 10.1056/NEJMoa0808145 19474425
19. Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, et al. (2013) Radiofrequency ablation and endoscopic mucosal resection for dysplastic Barrett's esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology 145 : 87–95. doi: 10.1053/j.gastro.2013.03.045 23542069
20. Kadri SR, Lao-Sirieix P, O'Donovan M, Debiram I, Das M, et al. (2010) Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ 341: c4372. doi: 10.1136/bmj.c4372 20833740
21. Lao-Sirieix P, Boussioutas A, Kadri SR, O'Donovan M, Debiram I, et al. (2009) Non-endoscopic screening biomarkers for Barrett's oesophagus: from microarray analysis to the clinic. Gut 58 : 1451–1459. doi: 10.1136/gut.2009.180281 19651633
22. Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, et al. (1993) An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology 105 : 40–50. 8514061
23. Armitage P (1955) Tests for linear trends in proportions and frequencies. Biometrics 11 : 375–386.
24. Clopper C, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26 : 404–413.
25. Weaver JMJ, Ross-Innes C, Shannon N, Lynch AG, Forshew T, et al. (2014) Ordering of mutations in preinvasive disease stages of oesophageal carcinogenesis. Nat Genet 46 : 837–843. doi: 10.1038/ng.3013 24952744
26. Wani S, Falk G, Hall M, Gaddam S, Wang A, et al. (2011) Patients with nondysplastic Barrett's esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 9 : 220–227. doi: 10.1016/j.cgh.2010.11.008 21115133
27. Weston AP, Sharma P, Mathur S, Banerjee S, Jafri AK, et al. (2004) Risk stratification of Barrett's esophagus: updated prospective multivariate analysis. Am J Gastroenterol 99 : 1657–1666. 15330898
28. Pohl H, Arash H, Ell C, Manner H, May A, et al. (2014) Length of Barrett's esophagus and cancer risk—implications from a population based study [abstract]. Digestive Disease Week 2014; 3–6 May 2014; Chicago, Illinois, US. Gastroenterology 146: S-122. doi: 10.1053/j.gastro.2014.04.029 25284828
29. Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW (2002) The long term results of endoscopic surveillance of premalignant gastric lesions. Gut 50 : 378–381. 11839718
30. Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, et al. (2014) Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 370 : 1287–1297. doi: 10.1056/NEJMoa1311194 24645800
31. Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, et al. (2011) Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 155 : 687–697. doi: 10.7326/0003-4819-155-10-201111150-00376 22006930
32. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ (2011) American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 140 : 1084–1091. doi: 10.1053/j.gastro.2011.01.030 21376940
33. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ (2011) American Gastroenterological Association technical review on the management of Barrett's esophagus. Gastroenterology 140: e18–52. doi: 10.1053/j.gastro.2011.01.031 21376939
34. Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G (2013) Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett's esophagus. Gastroenterology 144 : 62–73.e66. doi: 10.1053/j.gastro.2012.09.060 23041329
35. Thrift AP, Kendall BJ, Pandeya N, Vaughan TL, Whiteman DC, et al. (2012) A clinical risk prediction model for Barrett esophagus. Cancer Prev Res (Phila) 5 : 1115–1123. 22787114
36. Liu X, Wong A, Kadri SR, Corovic A, O'Donovan M, et al. (2014) Gastro-esophageal reflux disease symptoms and demographic factors as a pre-screening tool for Barrett's esophagus. PLoS ONE 9: e94163. doi: 10.1371/journal.pone.0094163 24736597
37. Steele RJC, McClements PL, Libby G, Black R, Morton C, et al. (2009) Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 58 : 530–535. doi: 10.1136/gut.2008.162883 19036949
38. Kavanagh AM, Giles GG, Mitchell H, Cawson JN (2000) The sensitivity, specificity, and positive predictive value of screening mammography and symptomatic status. J Med Screen 7 : 105–110. 11002452
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2015 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Randomized Controlled Trials in Environmental Health Research: Unethical or Underutilized?
- Uptake and Population-Level Impact of Expedited Partner Therapy (EPT) on and : The Washington State Community-Level Randomized Trial of EPT
- Supporting Those Who Go to Fight Ebola
- Evaluation of a Minimally Invasive Cell Sampling Device Coupled with Assessment of Trefoil Factor 3 Expression for Diagnosing Barrett's Esophagus: A Multi-Center Case–Control Study
- Hormonal Contraception and the Risk of HIV Acquisition: An Individual Participant Data Meta-analysis
- Association between Respiratory Syncytial Virus Activity and Pneumococcal Disease in Infants: A Time Series Analysis of US Hospitalization Data
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Association between Respiratory Syncytial Virus Activity and Pneumococcal Disease in Infants: A Time Series Analysis of US Hospitalization Data
- Randomized Controlled Trials in Environmental Health Research: Unethical or Underutilized?
- Supporting Those Who Go to Fight Ebola
- Evaluation of a Minimally Invasive Cell Sampling Device Coupled with Assessment of Trefoil Factor 3 Expression for Diagnosing Barrett's Esophagus: A Multi-Center Case–Control Study
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



