-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Uptake and Population-Level Impact of Expedited Partner Therapy (EPT) on and : The Washington State Community-Level Randomized Trial of EPT
Matthew Golden and colleagues report on the effects of promoting treatment without testing for sexual partners of patients with chlamydia or gonorrhea.
Published in the journal: Uptake and Population-Level Impact of Expedited Partner Therapy (EPT) on and : The Washington State Community-Level Randomized Trial of EPT. PLoS Med 12(1): e32767. doi:10.1371/journal.pmed.1001777
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001777Summary
Matthew Golden and colleagues report on the effects of promoting treatment without testing for sexual partners of patients with chlamydia or gonorrhea.
Introduction
Chlamydia trachomatis and Neisseria gonorrhoeae cause the two most commonly reported infections in the United States [1], and new approaches to preventing these sexually transmitted infections (STIs) are needed.
Increasing the number of exposed sex partners of persons with gonorrhea or chlamydial infection who receive treatment could decrease the incidence and prevalence of infection. However, few US health departments currently devote substantial resources to partner services for bacterial STIs other than syphilis, and over 80% of patients with gonorrhea or chlamydial infection are left to notify their partners without assistance [2]. Randomized controlled trials conducted in the late 1990s and early 2000s found that expedited partner therapy (EPT)—the practice of treating the sex partners of persons with curable STIs without requiring the partners to first undergo a medical evaluation—increases partner treatment and decreases rates of reinfection with gonorrhea or chlamydia [3–6]. In the wake of these trials, many US states made EPT legal, and the practice is now lawful in most of the country [7]. In most instances, EPT involves medical practitioners giving patients medication to give to their sex partners, a practice called patient-delivered partner therapy (PDPT).
Many physicians at least sometimes give patients medication to give to a sex partner [8–10], but relatively few persons with gonorrhea in the US currently receive PDPT [11], and the proportion of patients with chlamydial infection who receive PDPT is not well defined. Working in King County, Washington (WA), we previously reported that a population-based program could significantly increase medical providers’ use of PDPT [12]. However, the scale of PDPT promotion and evaluation in King County has been an exception, and most efforts to institute and assess PDPT programs have focused on individual clinics or small groups of clinics [13–16]. The feasibility of promoting EPT more widely and the intervention’s population-level effect are unknown.
We conducted a stepped-wedge, community-level randomized trial to test the hypothesis that a public health program could increase the population-level use of EPT, and through this effort decrease the prevalence of chlamydial infection and incidence of gonorrhea in women. Stepped-wedge trials are a type of cluster randomized trial in which clusters of clinics or communities receive an intervention in a randomly assigned order; outcome analyses then compare variance both between communities and within a community before and after the intervention. We have previously described this study design and its use in the context of this community-level trial [17]. The study employed cluster randomization in order to measure the population-level impact of our intervention, and used a stepped-wedge design because it allowed all areas of the state to eventually adopt the intervention, which promotes the use of EPT, a clinically beneficial intervention.
Methods
Ethics Statement
The University of Washington Institution Review Board approved study procedures. Local health officers in WA State agreed to their jurisdictions’ participation in the trial. Because the trial did not enroll individuals as part of a study, persons receiving interventions through the study did not undergo a consent process.
Study Design and Participating Communities
Local health jurisdictions (LHJs)—administrative units that usually correspond to a single county—were the study’s unit of randomization and analysis. All WA State LHJs except King County were eligible to participate in the trial. We excluded King County because the study intervention was developed there and could not practically be discontinued and then restarted [18]. The trial initiated the study intervention in groups of LHJs (i.e., waves) at four time points (i.e., steps) separated by intervals of 6–8 mo between October 2007 and August 2009. A computer randomly assigned LHJs into the four waves, with randomization stratified by region and LHJ size. The allocation of LHJs into waves was concealed from state and local public health department staff and from all investigators except the study biostatistician until 1 mo before initiation of the intervention in each wave. No blinding was done after the study intervention was initiated.
Intervention
The intervention was designed to increase the treatment of sex partners of individual heterosexual patients and included two components: (1) promotion of PDPT use and (2) targeted provision of public health partner services. In each LHJ, the study sought to increase PDPT use by supplying free PDPT packs to clinicians and by making free packs available through commercial pharmacies for clinicians to prescribe for their patients’ partners. When the intervention was initiated in each LHJ, the research team and local health department staff sent a letter to every clinician who had reported one or more cases of bacterial STI in the prior year. (Clinicians treating STIs in WA State include a diverse array of physicians [family medicine practitioners, internal medicine practitioners, obstetricians and gynecologists] and mid-level practitioners.) This letter informed clinicians that state guidelines recommend that medical providers offer PDPT to all heterosexual individuals with gonorrhea or chlamydial infection when the clinician cannot “otherwise assure” that all of a patient’s potentially exposed partners will be treated [19]. The letter also included information about how to obtain free PDPT to stock in their office or clinic, and how to prescribe free PDPT through local pharmacies.
In each LHJ, research team and public health staff members visited clinics and offices that had reported large numbers of cases of gonorrhea or chlamydial infection in the year prior to program initiation; these clinics were chosen in collaboration with local staff and were not defined using fixed criteria. During these visits, public health staff supplied clinics with PDPT packets and educated clinic staff and medical providers about the program.
Although all clinicians were eligible to receive free PDPT packs to stock in their offices or clinics, the study also developed a mechanism for medical providers to prescribe free PDPT packs through local commercial pharmacies. Only selected pharmacies stocked PDPT packs for use as part of the study. These pharmacies typically belonged to one of several large commercial chains. The study chose participating pharmacies in collaboration with local public health staff in each LHJ with the goal of assuring widespread access to PDPT packs. A total of 157 pharmacies participated in the study.
Additional efforts to promote PDPT use in intervention communities included annual letters to medical providers reporting at least one case of gonorrhea or chlamydial infection, letters to medical providers whose patients received partner services but not PDPT informing them about the program, information on STD case report forms outlining WA State’s PDPT guidelines and how to obtain free PDPT, discussions with clinic or office staff during telephone calls to complete incomplete case reports, and inclusion of information about PDPT in continuing medical education trainings. The study also worked with the largest health maintenance organization in the state and with large family planning organizations to promote PDPT use. All medications for PDPT were distributed in premade packs that met state legal requirements and included condoms, information about STIs, and a medication allergy warning in English and Spanish that asked recipients to call the study coordinator if they experienced an adverse drug reaction. All packs included 1 g of azithromycin; packs for gonorrhea also included a 400-mg dose of cefixime. Both medications were to be taken as a single dose.
The study sought to target the provision of partner services to persons at high risk for failing to ensure a partner’s treatment. Diagnosing medical providers and laboratories in WA State are required to report gonorrhea or chlamydial infection cases to the local and state public health department. Medical providers do this using a case report form. As part of the trial, we modified that form to ask providers to indicate whether they wanted the public health department to provide their patient with partner services. The form recommended that providers refer patients with any of the following risks for partner services: (1) >1 sex partner in the 60 d prior to diagnosis, (2) ≥1 sex partner in the prior 60 d whom the patient did not anticipate having sex with again, (3) the patient indicated he/she could not or would not notify a partner, or (4) the patient was a man who has sex with men. Prior studies have associated the first two of these factors with the failure of partners to receive treatment [12,18].
Partner services consisted of a structured interview that guided public health staff (usually disease intervention specialists) to define a partner treatment plan for a patient’s most recent partner and all partners from the prior 60 d. Staff providing partner services offered patients free PDPT to treat up to three partners, and offered to directly contact partners whom patients did not want to notify themselves. When contacting partners, staff advised them to seek medical evaluation, but also offered them treatment without such an evaluation. Both patients and partners could obtain free PDPT through commercial pharmacies or via the mail. The study intervention did not include efforts to find index cases or partners through field investigations (e.g., going to persons’ homes or places of work).
Although the study sought to phase in partner services according to a prespecified design, in 2007 the WA State legislature allocated new funds for communicable disease that some LHJs used to increase partner services in 2008 and 2009, a change beyond the control of the study. The study did not measure which LHJs increased the provision of partner services using new state funding. No public health department funds were used to purchase or promote the use of PDPT outside of the study’s randomization sequence.
Study Outcomes and Statistical Methods
The study’s two primary, prespecified outcomes measured for each LHJ were (1) chlamydia test positivity among women ages 14–25 y tested through Infertility Prevention Project (IPP) or Planned Parenthood of Western Washington clinics and (2) the incidence of reported gonorrhea in women. Prespecified secondary outcomes included use of PDPT by medical providers, adverse reactions, and recurrent gonorrhea and recurrent chlamydial infection; these last two outcomes will be presented in a separate report.
The study’s chlamydia positivity endpoint used results for all women ages 14–25 y tested for C. trachomatis in IPP clinics, including tests performed in the Planned Parenthood of Western Washington laboratory, during five prespecified time periods (analysis intervals; 7/1/07–9/30/07, 3/1/08–5/31/08, 10/15/08–1/14/09, 5/1/09–7/31/09, 6/1/10–8/31/10). These intervals correspond to the last 3 mo of data in each intervention period (when the intervention should have reached maximal effect). The IPP was a national chlamydia testing program funded by the Centers for Disease Control and Prevention (CDC), and data from the IPP have previously been used to monitor chlamydial prevalence in young women [20–22]. During the study period, IPP testing occurred in 219 clinics in WA State, including 98 family planning, women’s health, or reproductive health clinics; 36 community clinics; 29 STD or HIV testing clinics; 17 adolescent or school-based clinics; and 39 clinics of other types. In 2008, Planned Parenthood of Western Washington began testing women with health insurance at a separate laboratory, while continuing to send specimens from uninsured women to the IPP laboratory. Because this change could have altered the IPP population, the study included tests performed in both the Planned Parenthood of Western Washington and the IPP laboratories in the outcome analysis. (Throughout the paper we refer to the clinics providing chlamydia outcome data as IPP clinics, which includes the Planned Parenthood of Western Washington laboratory.) All chlamydial testing was done using nucleic acid amplification tests.
The outcome analyses focused on women because men are not typically screened for STIs—meaning that no sentinel population of tested men existed in the state—and because reported STI incidence in men includes outcomes in both heterosexual men and men who have sex with men (MSM); CDC and WA State guidelines recommend against the use of EPT in MSM [23], and our study did not seek to increase EPT use in MSM. We elected to use chlamydia positivity in sentinel populations of women as our outcome because we did not believe that testing a random sample of the entire state population of young women would be feasible, and believed that positivity in a describable population would be a better outcome than incidence of reported infections, which reflects both testing patterns and true incidence. Because gonorrhea has become an infrequent infection in women in WA State, it was not possible to power the study to use gonorrhea positivity within IPP clinics as an outcome. As a result, we used the incidence of reported gonorrhea in women as our gonorrhea outcome.
The primary analysis of the study’s chlamydia outcome used a mixed effects generalized linear model with a log link and included covariates for intervention status andtime period; random effects for LHJ and clinic within LHJ were used to adjust for the fact that not all clinics contributed to the IPP database during all time periods. The study design assumed 162 women testing in each of 24 LHJs in each analysis interval, 5% chlamydia positivity, a coefficient of variation between LHJs of 0.33 (based on preliminary data), and α = 0.05 (two-tailed). We anticipated 80% power to detect a prevalence ratio of 0.70 [17]. The effect size anticipated was consistent with preliminary mathematical modeling work undertaken in preparing the study grant application. The study gonorrhea outcome was the number of cases of gonorrhea reported among women in each participating LHJ in each prespecified time period. This analysis used a marginal Poisson regression model with covariates for intervention status and time period, an offset for female population size within the LHJ, clustering by LHJ, and an exchangeable working correlation structure. Robust variances are reported. These analysis models were prespecified. The study results also include a single post hoc analysis assessing the intervention’s impact on a combined gonorrhea and chlamydia endpoint. This analysis was similar to the analysis of gonorrhea but included two outcomes for each LHJ for each time period: (1) gonorrhea cases, as described above, and (2) chlamydia cases from the IPP clinics with offset equal to the number of women tested in the LHJ during the analysis interval. The model included separate time trends for each disease but a common intervention effect. All analyses were carried out using Stata version 12.1 (StataCorp, College Station, Texas).
In order to assess the comparability of the population of women in which we evaluated our C. trachomatis outcome to all sexually active women in the communities participating in the trial, we compared the age and race/ethnicity distributions of women tested in IPP clinics to US census data for women in the WA State LHJs included in the study. Because many younger women are not sexually active, and consequently would not be at risk for STIs or tested for C. trachomatis, we used US national data from the National Survey of Family Growth 2006–2010 [24] to define the proportion of women at each age who have ever had sex, and multiplied the number of women in each age group by that proportion to define the number of sexually active women in each age group in the state. The proportion of black versus white young women who were sexually active in the National Survey of Family Growth varied by only 3%, and therefore we did not adjust our statewide population estimate for race/ethnicity.
The study was also designed to assess the scalability of a public health EPT intervention. This effort initially included measurement of four outcomes: (1) the proportion of persons receiving PDPT from their medical provider, (2) the proportion of persons receiving public health partner services, (3) the proportion of persons receiving either PDPT from their medical provider or partner services from public health staff, and (4) the proportion of persons receiving PDPT from either their medical provider or from public health partner services staff. In September 2009, we added a question to routine partner services interviews to measure the proportion of persons who were offered PDPT by their medical provider, a fifth outcome related to the intervention’s uptake; data on this final outcome were not available for the pre-intervention periods in study waves 1–3, but were available for at least part of the intervention period for all four study waves. As a result, we report this outcome only for the intervention periods. To measure these outcomes, the study attempted to interview random samples of patients comprising 20% of reported cases of gonorrhea and 20% of reported cases of chlamydial infection; random samples were automatically drawn by the public health department computer case registry as cases were entered into that system. Staff interviewed patients starting 4–6 mo prior to each wave’s initiation of the intervention and throughout the intervention period; interviewers made a minimum of three attempts to contact each selected interviewee by telephone. Staff did not attempt field visits to interview patients. The analysis of PDPT use excluded MSM, who were identified using information collected on the case report and during partner services interviews. Of 9,690 randomly selected women and men not identified as MSM, 5,741 (59%) had complete interview data. The median time from patient treatment to interview was 11 d (interquartile range 5–22 d), and 92% of interviews were conducted by telephone. To adjust for nonresponse, estimates of the proportion of persons offered and receiving PDPT from their medical provider used post-stratification weights based on gender, race/ethnicity, STI diagnosis, and, during the intervention period, whether providers referred patients for partner services. Confidence intervals for proportions were computed on the logit scale and backtransformed using standard methods for random samples, adjusting for the weighting. To estimate the proportion of persons who received PDPT from either their provider or from public health staff, we summed the estimated proportion receiving PDPT from medical providers with the directly measured proportion of all cases in patients who were not MSM who received PDPT from public health staff and who did not also receive PDPT from their medical provider.
Adverse drug reactions were ascertained based on calls to the study coordinator; information sheets included in each partner pack asked recipients to call the coordinator if they experienced a drug reaction. The study did not conduct follow-up interviews with patients to ascertain the effect of the intervention on partnership dissolution or intimate partner violence.
The trial’s sponsor did not require it to be registered in ClinicalTrials.gov, and the registration website was not designed to accommodate information on community-level trials that do not enroll individuals. Consequently, we did not register the trial prior to the study’s initiation. We did register the study in ClinicalTrials.gov after study initiation.
We are not aware of other ongoing community-level trials related to EPT, though several individual-level trials have evaluated the effect of EPT (ClinicalTrials.gov NCT01720654; [3–5,25,26]).
Results
Populations Participating in the Trial
Of WA State’s 25 LHJs, 23 participated in the study (Figs. 1 and 2). Table 1 presents data from clinics participating in the WA State IPP program in the year prior to the start of the study; these clinics include all clinics in which we measured chlamydia positivity, one of our main study outcomes, and is stratified by study wave. Chlamydia outcome data were available at baseline and for each of the four steps for 22 of the 23 participating LHJs (one LHJ had no clinical site providing data). Table 2 compares the age, race, and ethnicity of women tested for chlamydial infection in clinics participating in the IPP program with those of women ages 14–25 y in the areas of WA State participating in the study. Women tested at sentinel clinical sites were younger than the overall population of sexually active women in the areas served by the clinics. The study had data on the incidence of newly diagnosed and reported gonorrhea for all LHJs at each step.
Fig. 1. Study flow diagram. 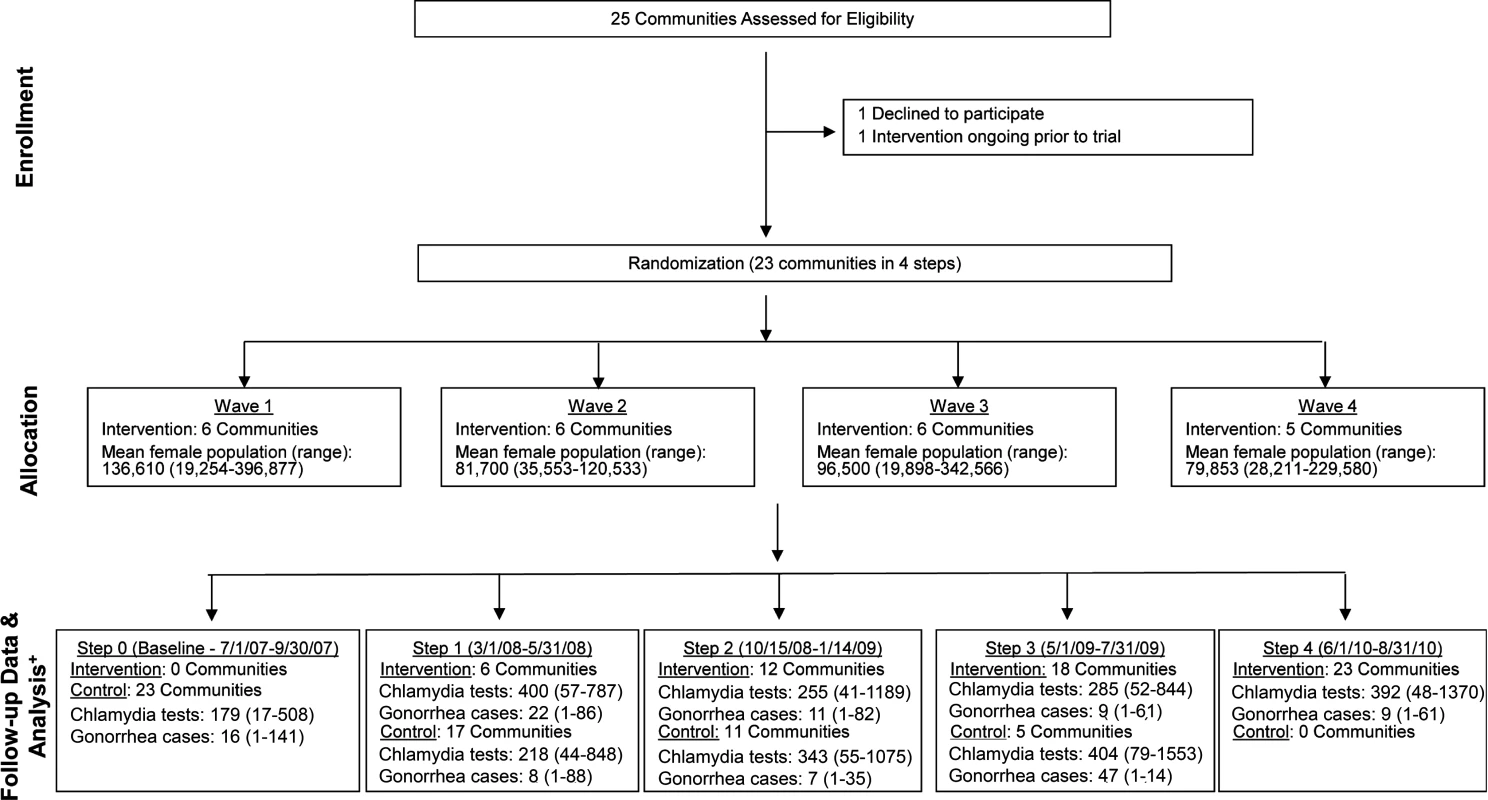
Modified for stepped-wedge design from suggested CONSORT criteria format for cluster randomized trials [47]. +Numbers of tests and cases presented as means with ranges. Fig. 2. Washington State local health jurisdictions instituting an expedited partner therapy intervention in four temporally defined waves. 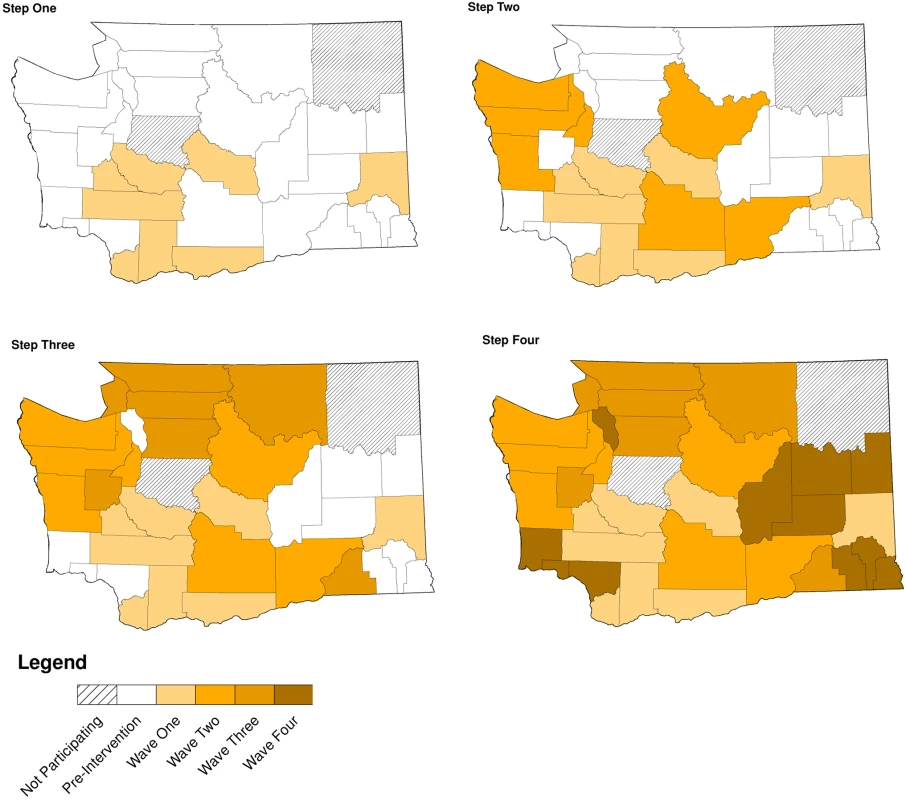
Tab. 1. Study population size and characteristics in local health jurisdictions in each study wave during the year prior to the intervention (October 2006–September 2007). 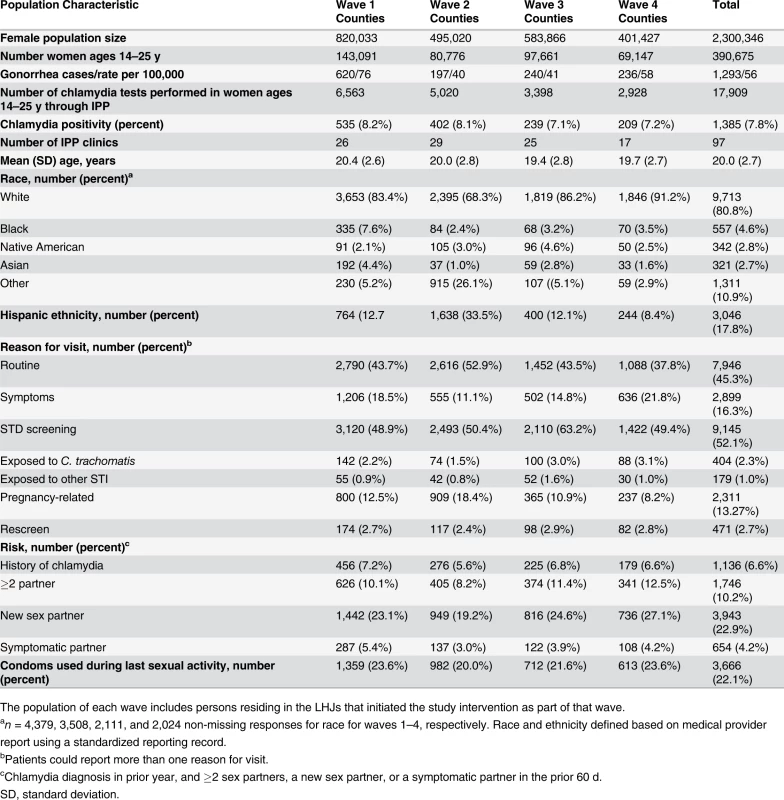
The population of each wave includes persons residing in the LHJs that initiated the study intervention as part of that wave. Tab. 2. Characteristics of women tested for C. trachomatis in clinics providing outcome data for the trial compared to all women in areas of WA State participating in the trial. 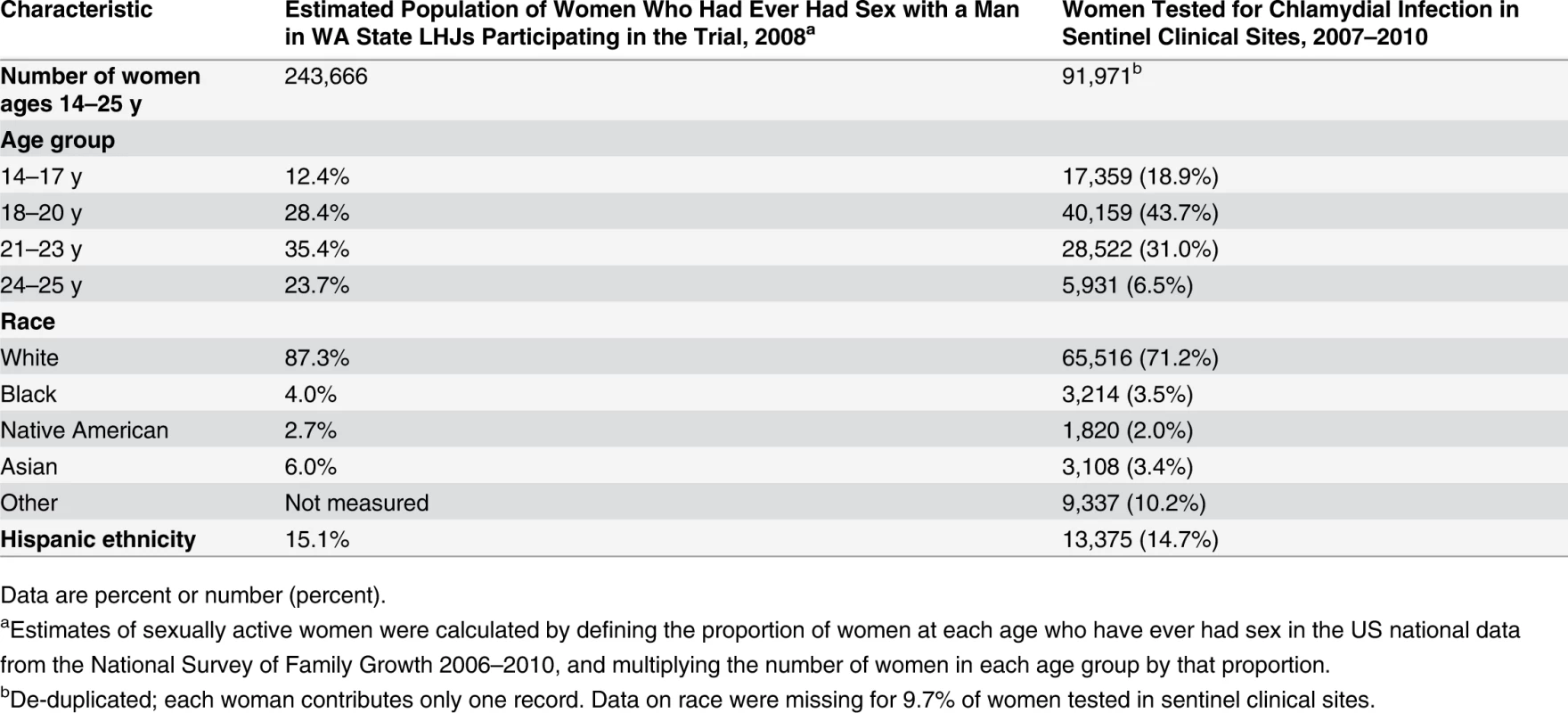
Data are percent or number (percent). Intervention Uptake
Between May 2007 and December 2010, participating LHJs reported 5,164 and 52,945 cases of gonorrhea and chlamydial infection, respectively, excluding cases in MSM. The percentage of cases of gonorrhea or chlamydial infection in patients who were not MSM across all study waves who received partner services increased from 25.2% in the pre-intervention periods to 44.9% during the intervention periods (p < 0.001), and this increase was evident in diverse groups defined by demographic factors and sources of care (Fig. 3; Table 3). However, this increase was apparent only in the first two study waves that instituted the intervention, before the impact of new, unanticipated state funding for partner services was fully realized.
Fig. 3. Percentage of persons with gonorrhea or chlamydial infection who received components of the study intervention in periods before and during the study intervention, by study wave. 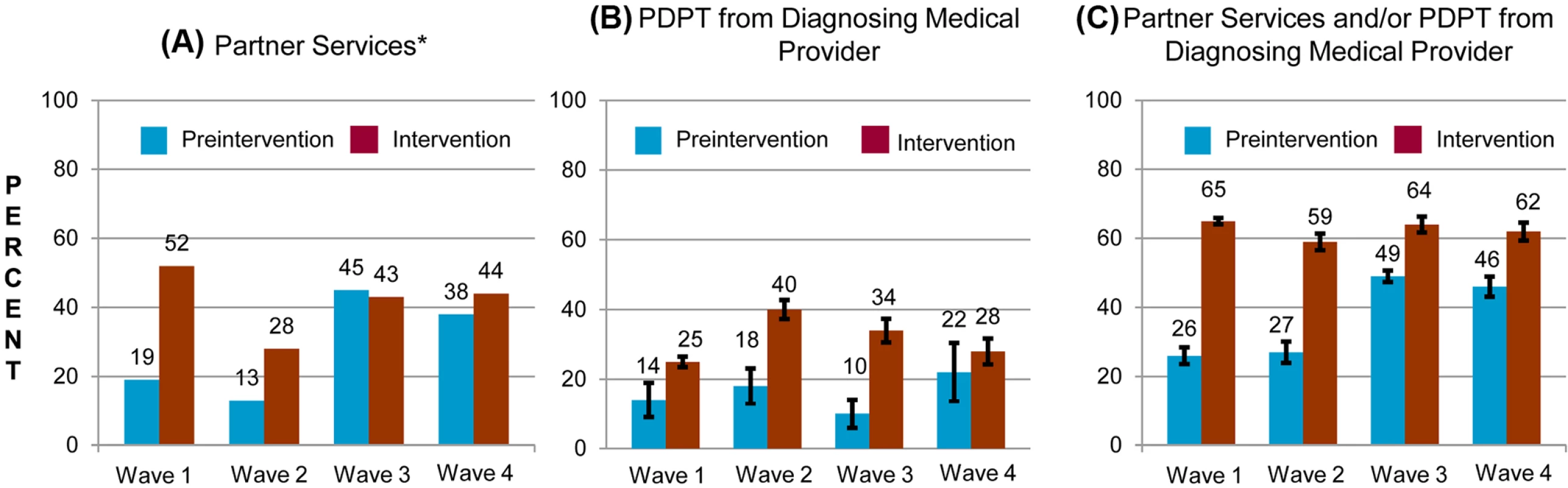
Percentage of persons receiving (A) PDPT from their diagnosing clinician, (B) public health partner services, or (C) either PDPT or public health partner services. Tab. 3. Receipt of public health partner services within subgroups of patients during baseline and intervention periods. 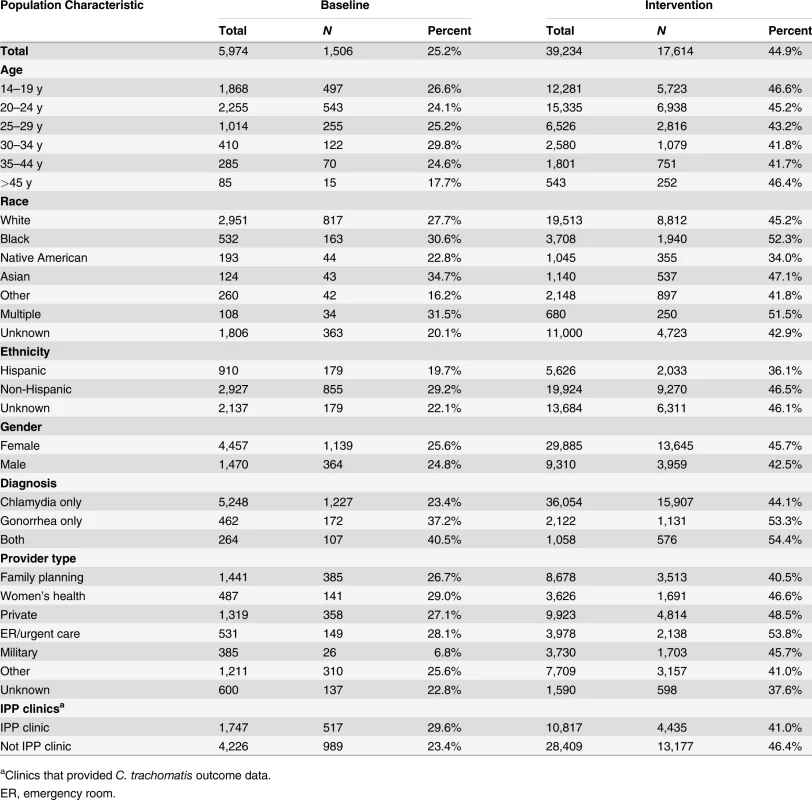
aClinics that provided C. trachomatis outcome data. The study intervention significantly increased the use of PDPT by diagnosing medical providers. As was the case for the increase in partner services, this increase occurred across subgroups defined by demographic factors, STI diagnosis, and type of medical provider (Table 4). Over the study period, public health and research staff distributed 19,299 PDPT packs to 472 different medical clinics, offices, and pharmacies. Pharmacies dispensed 7,110 PDPT packs. (The study measured medication dispensing only through pharmacies; the number of packs dispensed by medical provider offices was not measured.) Based on interviews with randomly selected patients, the percentage of patients receiving PDPT from their diagnosing provider increased from 18.3% in the pre-intervention periods to 34.0% during the intervention periods (p < 0.001). Including PDPT prescribed by either medical providers or by public health staff providing partner services, the estimated proportion of patients who received PDPT increased from 18.3% in the pre-intervention periods to 43.9% in the intervention periods. In 2010, the final year of the study, providers offered PDPT to an estimated 38.1% and 51.9% of all heterosexual individuals with gonorrhea and chlamydial infection, respectively, and 31.8% and 44.7% of patients received PDPT from either their medical provider or from public health staff providing partner services. Among 1,670 persons randomly selected for study interviews who reported receiving PDPT from a medical provider during the intervention period, 1,550 (92.6%) stated that they gave a PDPT pack to at least one sex partner. Providers offered PDPT more frequently to patients who they did not refer for partner services than to those they did refer for such services (80.9% versus 29.3% p < 0.001), and patients who did not receive partner services were much more likely to receive PDPT from medical providers than those who received partner services (62.5% versus 12.0%, p < 0.001). Persons referred for partner services were more likely to have >1 sex partner or a partner they did not anticipate having sex with again in the future, our criteria for referral for public health partner services (32.4% versus 20.1%, p < 0.001).
Tab. 4. Medical providers’ use of patient delivered partner therapy within subgroups of patients during baseline and intervention periods. 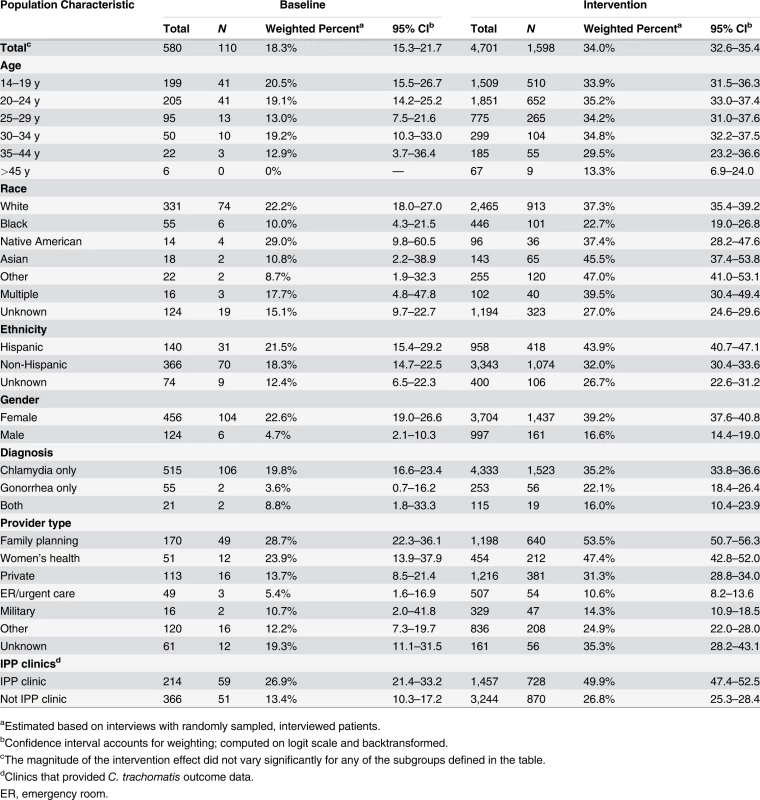
aEstimated based on interviews with randomly sampled, interviewed patients. Effect of the Intervention on Chlamydia Positivity and Reported Gonorrhea Incidence in Women, and Adverse Effects
Fig. 4 shows patterns of chlamydia positivity and gonorrhea incidence overall (Fig. 4A) and in each wave of LHJs receiving the intervention (Fig. 4B), with red lines and open symbols representing pre-intervention time periods, and black lines and solid symbols representing intervention time periods. Chlamydia positivity among women ages 14–25 y tested in IPP clinics in LHJs participating in the trial decreased over the study period from 8.2% to 6.5% (p < 0.001), while the annualized incidence of gonorrhea in women declined dramatically from 59.6 to 26.4 per 100,000 (p < 0.001). After adjusting for temporal trends, the intervention was associated with an approximately 10% reduction in both chlamydia positivity and gonorrhea incidence, though the confidence bounds on these outcomes both included one (Table 5). A post hoc analysis that combined the study’s two primary outcomes resulted in narrower confidence bounds, suggesting that the intervention was associated with at least a small population-level effect. The estimated coefficient of variation between LHJs for chlamydia positivity was 0.24 and for gonorrhea incidence was 0.40, close to the estimate used for the study’s initial power calculations (0.33).
Fig. 4. Trends in chlamydia test positivity and gonorrhea incidence for 2007–2010 among women in 23 local health jurisdictions in Washington State. 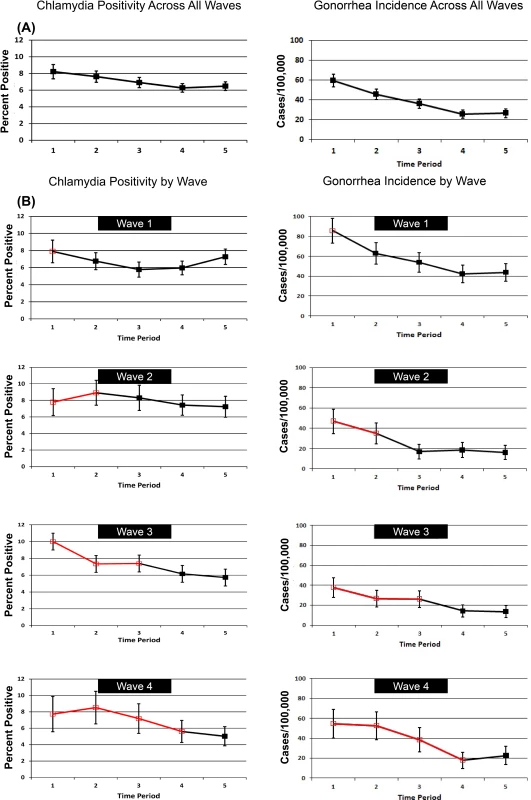
Tab. 5. Association of the study intervention with chlamydia test positivity and reported gonorrhea incidence in women. 
No episodes of anaphylaxis or other major adverse drug reactions were reported to the study coordinator during the trial. The study did not record the number of instances of nausea or vomiting associated with study medications.
Discussion
We found that a public health intervention could significantly increase both medical providers’ use of PDPT and the targeted provision of partner services in a state with a population over 6.5 million. Chlamydial test positivity among women evaluated in sentinel clinics and the incidence of gonorrhea among women significantly declined during the study period, and although the confidence bounds for our primary study outcomes included one, the relative risk and rate ratios associated with the study intervention were consistent with the intervention resulting in an approximately 10% reduction in our population-level measures of gonorrhea and chlamydial infection in women.
We believe that the best interpretation of our findings is that our intervention likely resulted in a modest reduction in STI morbidity. Our study was powered to identify a 30% reduction in chlamydia positivity and gonorrhea incidence, effect sizes that substantially exceeded the 10% reduction observed. In a post hoc analysis that combined gonorrhea and chlamydia outcomes, the upper bound of the confidence interval was 1.01, suggesting that that our intervention likely had at least some small population-level effect. Of course, it is possible that our intervention was truly ineffective and that the decline in chlamydia test positivity and gonorrhea incidence observed during the study period was a consequence of a secular trend that was independent of our study. Rates of reported gonorrhea among women declined by 28% in California and 17% in Oregon during the study period, suggesting that a regional decrease in gonococcal incidence was occurring, though these declines were much smaller than the 53% decline observed in our study communities.
Several factors may have diminished our intervention’s impact, thereby decreasing our study’s statistical power. First, the intervention might not have reached a high enough proportion of people with gonorrhea or chlamydia, or might not have reached the persons most important in STI transmission. Second, the intervention’s effect may have been attenuated as a result of the non-independence of STI transmission between LHJs. Insofar as residents of study communities had sex with partners from LHJs other than their own or obtained care in LHJs outside their own, interventions in early wave communities could have lowered rates of STIs in other areas, leading to a general decline in gonorrhea and chlamydial infection that was not in step with our community randomization sequence. We do not have data on what proportion of partnerships involve persons in different LHJs. Third, our intervention could have leaked into areas that were still awaiting its institution, thereby diminishing our intervention effect. We observed relatively high levels of PDPT use at baseline in wave 4 (22%), but there was no clear trend toward increasing use of PDPT outside of the trial, as evidenced by the low level of PDPT use at baseline in wave 3 LHJs (10%). Fourth, the state’s unanticipated increase in the provision of partner services outside of the study may have contributed to observed decreases in STIs. Because this increase in the provision of partner services did not occur in synchrony with the study’s randomization sequence, it may have diminished our study’s power to see an effect and the intervention’s effect size. Lastly, the fact that only 23 LHJs participated in the trial (compared to 24 in the design) and that the effective average (harmonic mean) sample size per cluster per time period was 107 (compared to 162 in the design) resulted in a modest reduction in power relative to that anticipated in our study design (67% versus 80%).
The relatively small population-level effect we observed is consistent with findings from a recent mathematical model that suggested that increasing partner treatment may have only a small impact on chlamydia and gonorrhea prevalence [27]. However, prior models have suggested the opposite conclusion [28,29], and no gonorrhea or chlamydia model has been validated, making the predictive value of these models’ findings uncertain.
Our study demonstrates that a public health program that provides widespread access to free PDPT can lead to very large increases in PDPT use. The CDC and numerous US states currently have guidelines that include recommendations on the use of PDPT for gonorrhea and chlamydial infection [19,30–35]. The extent to which the intervention is used is not precisely known. A recent CDC study reported that only approximately 9.5% of persons diagnosed with gonorrhea in the US in 2010 received PDPT [11]. That study included MSM, a population in which PDPT is used less frequently and that we did not include in our study, and excluded from analysis persons whose partners were treated in the absence of PDPT, inflating estimates of PDPT use by an amount that is unknown. Despite these differences in study design, the use of PDPT for gonorrhea in WA State during our intervention period was clearly very much higher than that observed in any other area of the US for which data are available. How often medical providers outside of WA State use PDPT for chlamydial infection is not well defined. Studies surveying providers in the US have typically found that half or more report ever using PDPT, with 15%-50% reporting that they use it routinely, usually, or always [8–10]. However, the validity of these surveys is uncertain. To our knowledge, only one study including patients from more than a single clinic has evaluated PDPT use among persons with chlamydial infection outside of WA State; it found that 19% of 743 women diagnosed with chlamydial infection in nine California family planning clinics in 2005–2006 received PDPT [13]. This number is somewhat lower than the baseline level of 29% PDPT use by WA State family planning providers in our pre-intervention period, and well below the 53.5% observed following implementation of our study intervention.
We believe that our findings should promote increased efforts to expand the use of EPT. The effectiveness of EPT is supported by randomized trials demonstrating an individual-level benefit [3–6] and by a cost-effectiveness analysis [36]. Our study showed that a public health intervention could lead to a very large increase in PDPT use and, although not definitive, suggests that the increased use of EPT resulted in a modest decrease in population-level measures of gonorrhea and chlamydial infection in women. The goal of the trial, to definitively impact population-level measures of STIs over a relatively short period of time, was ambitious. Few data exist from community-level randomized trials, and the study designs are themselves experimental. Although several community-level randomized trials have evaluated interventions to prevent STIs in lower income nations [37]—some of which observed population level effects [38–41]—to date, only one such trial has been completed in a high-income nation [42]. That study, which was conducted in the Netherlands to evaluate the effect of chlamydial screening, was not successful in increasing chlamydial screening (the study intervention) and did not demonstrate an effect on STI prevalence. There are no community-level trials demonstrating the population-level benefits of STI clinical care, STI screening, traditional partner services, or the promotion of condom use, all of which are mainstays of STI control in high-income nations. Thus, while community-level randomized trials are a good aspirational evidentiary standard and additional trials of this type should ideally be undertaken, they are not currently a practical standard for defining which STI prevention interventions should be adopted.
The role of EPT in the control of gonorrhea merits specific comment. Current STD treatment guidelines in both the US and Europe do not recommend the routine use of cefixime as a therapy for gonorrhea [43,44]. At the same time, US guidelines continue to recommend the use of cefixime and azithromycin as an EPT regimen and as an alternative therapy for gonorrhea [43,45]. N. gonorrhoeae with diminished susceptibility to cefixime is uncommon in the US, causing fewer than 0.5% of all gonococcal infections in heterosexual individuals in 2013 (R. Kirkcaldy, personal communication). In Seattle, as of July 2014, we have not identified any N. gonorrhoeae isolates with decreased susceptibility to cefixime for more than 2 y. While additional research on this issue is needed, simple mathematical models suggest that eliminating the use of EPT could increase the transmission of gonorrhea overall, and under some instances might increase the transmission of gonococci with diminished susceptibility to oral third-generation cephalosporins [46]. Randomized trials have found that EPT decreases the absolute risk of gonococcal reinfection by 9% or more [4,5]. Given this very large effect size, the current rarity of gonococci with diminished susceptibility to cefixime among heterosexual individuals in the US, and the potential deleterious effect of eliminating EPT on gonococcal transmission, we believe that, at least at present, public health authorities in the US should continue to promote the use of EPT.
Our chlamydial outcome measure was based on test positivity in a sentinel population, and trends in that population may not have been representative of trends among all women in WA State. Women in IPP clinics were younger than the population of sexually active women ages 14–25 y in WA State overall, and it seems likely that the population in which we measured outcomes was at higher risk for STIs than the total population of women in the state. Also, the composition of the IPP population may have changed over time in ways that could have influenced the study outcome. Our analysis included random effects for LHJs and for clinics within LHJs to adjust for changes in the study population, but this adjustment may not have completely eliminated confounding. Ideally, our trial would have measured chlamydial prevalence in a random sample of women in each LHJ. However, such a design was not feasible given the resources available for the study.
Our measures of PDPT use by medical providers were based on surveys conducted with randomly selected patients being treated for gonorrhea or chlamydia, not all of whom were successfully interviewed. Although we weighted our estimates to adjust for nonresponse, our estimates could still be affected by bias if factors other than those used to weight the data affected our response rate. While this limitation affects the precision of our estimates of PDPT use, the use of PDPT in WA State during the study period was several times higher than that observed in the US as a whole [11], strongly suggesting that our intervention had a large population-level effect on PDPT use.
In conclusion, we found that a public health program that promoted use of free PDPT substantially increased clinicians’ PDPT use. Although our trial did not definitively demonstrate that EPT decreases chlamydia test positivity or gonorrhea incidence, we did observe substantial population-level declines in both infections concurrent with the introduction of our intervention. WA State has continued to make free PDPT packs available statewide over the 3 y since our study ended, and the number of packs distributed in the state has remained relatively stable in the post-study period, demonstrating that a state-wide system of EPT provision is sustainable. To our knowledge, no other US state or area outside of the US has developed a system similar to WA State’s. Given existing evidence and guidelines in support of EPT [3–5,23], we believe that continued and expanded use of the intervention is warranted, and that the design of our program can be a model for health departments seeking to increase EPT use.
Supporting Information
Zdroje
1. Centers for Disease Control and Prevention (2013) Sexually transmitted disease surveillance 2012. Atlanta: Centers for Disease Control and Prevention. 25512673
2. Golden MR, Hogben M, Potterat JJ, Handsfield HH (2004) HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis 31 : 709–712. 15608584
3. Schillinger JA, Kissinger P, Calvet H, Whittington WL, Ransom RL, et al. (2003) Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis 30 : 49–56. 12514443
4. Golden MR, Whittington WL, Handsfield HH, Hughes JP, Stamm WE, et al. (2005) Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med 352 : 676–685. 15716561
5. Kissinger P, Mohammed H, Richardson-Alston G, Leichliter JS, Taylor SN, et al. (2005) Patient-delivered partner treatment for male urethritis: a randomized, controlled trial. Clin Infect Dis 41 : 623–629. 16080084
6. Ferreira A, Young T, Mathews C, Zunza M, Low N (2013) Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 10: CD002843. doi: 10.1002/14651858.CD002843.pub2 24092529
7. Centers for Disease Control and Prevention (2014) Legal status of expedited partner therapy (EPT). Atlanta: Centers for Disease Control and Prevention 23859917
8. Hogben M, McCree DH, Golden MR (2005) Patient-delivered partner therapy for sexually transmitted diseases as practiced by U.S. physicians. Sex Transm Dis 32 : 101–105. 15668616
9. Guerry SL, Bauer HM, Packel L, Samuel M, Chow J, et al. (2005) Chlamydia screening and management practices of primary care physicians and nurse practitioners in California. J Gen Intern Med 20 : 1102–1107. 16423098
10. Rogers ME, Opdyke KM, Blank S, Schillinger JA (2006) Patient-delivered partner treatment and other partner management strategies for sexually transmitted diseases used by New York City healthcare providers. Sex Transm Dis 34 : 88–92. 16810120
11. Cramer R, Leichliter JS, Stenger MR, Loosier PS, Slive L, et al. (2013) The legal aspects of expedited partner therapy practice: do state laws and policies really matter? Sex Transm Dis 40 : 657–662. doi: 10.1097/01.OLQ.0000431358.18959.d4 23859917
12. Golden MR, Hughes JP, Brewer DD, Holmes KK, Whittington WL, et al. (2007) Evaluation of a population-based program of expedited partner therapy for gonorrhea and chlamydial infection. Sex Transm Dis 34 : 598–603. 17413683
13. Yu YY, Frasure-Williams JA, Dunne EF, Bolan G, Markowitz L, et al. (2011) Chlamydia partner services for females in California family planning clinics. Sex Transm Dis 38 : 913–918. doi: 10.1097/OLQ.0b013e3182240366 21934563
14. Stephens SC, Bernstein KT, Katz MH, Philip SS, Klausner JD (2010) The effectiveness of patient-delivered partner therapy and chlamydial and gonococcal reinfection in San Francisco. Sex Transm Dis 37 : 525–529. doi: 10.1097/OLQ.0b013e3181d8920f 20502392
15. Mickiewicz T, Al-Tayyib A, Thrun M, Rietmeijer C (2012) Implementation and effectiveness of an expedited partner therapy program in an urban clinic. Sex Transm Dis 39 : 923–929. doi: 10.1097/OLQ.0b013e3182756f20 23169171
16. Kerns JL, Jones HE, Pressman EJ, Fratarelli LA, Garth J, et al. (2011) Implementation of expedited partner therapy among women with chlamydia infection at an urban family planning clinic. Sex Transm Dis 38 : 722–726. doi: 10.1097/OLQ.0b013e318214bb83 21844724
17. Hussey MA, Hughes JP (2007) Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 28 : 182–191. 16829207
18. Golden MR, Whittington WL, Handsfield HH, Malinski C, Clark A, et al. (2001) Partner management for gonococcal and chlamydial infection: expansion of public health services to the private sector and expedited sex partner treatment through a partnership with commercial pharmacies. Sex Transm Dis 28 : 658–665. 11677389
19. Washington State Department of Health (2005) Washington State provider guidelines for patient delivered therapy. Olympia: Washington State Department of Health. 15838410
20. Satterwhite CL, Grier L, Patzer R, Weinstock H, Howards PP, et al. (2011) Chlamydia positivity trends among women attending family planning clinics: United States, 2004–2008. Sex Transm Dis 38 : 989–994. doi: 10.1097/OLQ.0b013e318225f7d7 21992972
21. Centers for Disease Control and Prevention (2010) Sexually transmitted disease surveillance 2009. Atlanta: Centers for Disease Control and Prevention.
22. Fine D, Dicker L, Mosure D, Berman S (2008) Increasing chlamydia positivity in women screened in family planning clinics: do we know why? Sex Transm Dis 35 : 47–52. 17700377
23. Centers for Disease Control and Prevention (2009) CDC expert consultation on the effect of antiretroviral therapy on risk of sexual transmission of HIV infection and superinfection. Atlanta: Centers for Disease Control and Prevention.
24. Groves RM, Mosher WD, Lepkowski JM, Kirgis NG (2009) Planning and development of the continuous National Survey of Family Growth. Vital Health Stat 1 : 1–64. 20141029
25. Kissinger P, Schmidt N, Mohammed H, Leichliter JS, Gift TL, et al. (2006) Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sex Transm Dis 33 : 445–450. 16531939
26. Cameron ST, Glasier A, Scott G, Young H, Melvin L, et al. (2009) Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod 24 : 888–895. doi: 10.1093/humrep/den475 19136481
27. Althaus CL, Turner KM, Mercer CH, Auguste P, Roberts TE, et al. (2014) Effectiveness and cost-effectiveness of traditional and new partner notification technologies for curable sexually transmitted infections: observational study, systematic reviews and mathematical modelling. Health Technol Assess 18 : 1–100. doi: 10.3310/hta18020 24411488
28. Kretzschmar M, van Duynhoven YT, Severijnen AJ (1996) Modeling prevention strategies for gonorrhea and Chlamydia using stochastic network simulations. Am J Epidemiol 144 : 306–317. 8686700
29. Hethcote H, Yorke J, Nold A (1982) Gonorrhea modeling. Math Biosci 58 : 93–109.
30. US Department of Health and Human Services Public Health Service, Centers for Disease Control and Prevention National Center for HIV, STD, and TB Prevention (2006) Expedited partner therapy in the management of sexually transmitted diseases: review and guidance.Washington (District of Columbia): US Department of Health and Human Services.
31. New Mexico Department of Health (2007) New Mexico Department of Health guidelines for expedited partner treatment (EPT) of sexually transmitted diseases. Santa Fe: New Mexico Department of Health.
32. Minnesota Department of Health (2008) Expedited partner therapy (EPT) for Chlamydia trachomatis and Neisseria gonorrhoeae: guidance for medical providers in Minnesota. Minneapolis: Minnesota Department of Health. doi: 10.1097/OLQ.0b013e31815b0158 18166849
33. Bauer HM, Wohlfeiler D, Klausner JD, Guerry S, Gunn RA, et al. (2008) California guidelines for expedited partner therapy for Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Dis 35 : 314–319. doi: 10.1097/OLQ.0b013e31815b0158 18166849
34. New York State Department of Health (2011) Expedited partner therapy guidelines for health care providers in NYS for Chlamydia trachomatis. Albany: New York State Department of Health.
35. Tennessee Department of Health (2013) Expedited STD management implementation guide. Nashville: Tennessee Department of Health.
36. Gift TL, Kissinger P, Mohammed H, Leichliter JS, Hogben M, et al. (2011) The cost and cost-effectiveness of expedited partner therapy compared with standard partner referral for the treatment of Chlamydia or gonorrhea. Sex Transm Dis 38 : 1067–1073. doi: 10.1097/OLQ.0b013e31822e9192 21992986
37. Ng BE, Butler LM, Horvath T, Rutherford GW (2011) Population-based biomedical sexually transmitted infection control interventions for reducing HIV infection. Cochrane Database Syst Rev 2011: CD001220. doi: 10.1002/14651858.CD001220.pub3 21412869
38. Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, et al. (1995) Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 346 : 530–536. 7658778
39. Kamali A, Quigley M, Nakiyingi J, Kinsman J, Kengeya-Kayondo J, et al. (2003) Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet 361 : 645–652. 12606175
40. Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, et al. (1999) Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet 353 : 525–535. 10028980
41. Garcia PJ, Holmes KK, Carcamo CP, Garnett GP, Hughes JP, et al. (2012) Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multicomponent community-randomised controlled trial. Lancet 379 : 1120–1128. doi: 10.1016/S0140-6736(11)61846-1 22341824
42. van den Broek IV, van Bergen JE, Brouwers EE, Fennema JS, Gotz HM, et al. (2012) Effectiveness of yearly, register based screening for chlamydia in the Netherlands: controlled trial with randomised stepped wedge implementation. BMJ 345: e4316. doi: 10.1136/bmj.e4316 22767614
43. Centers for Disease Control and Prevention (2012) Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61 : 590–594. 22874837
44. Unemo M, European STI Guidelines Editorial Board (2012) The 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults recommends dual antimicrobial therapy. Euro Surveill 17 : 20323. 23231859
45. Centers for Disease Control and Prevention (2013) Guidance on the use of expedited partner therapy in the treatment of gonorrhea. Atlanta: Centers for Disease Control and Prevention. 18987617
46. Golden MR, Barbee LA, Kerani R, Dombrowski JC (2014) Potential deleterious effects of promoting the use of ceftriaxone in the treatment of Neisseria gonorrhoeae. Sex Transm Dis 41 : 619–625. doi: 10.1097/OLQ.0000000000000174 25211259
47. Campbell MK, Elbourne DR, Altman DG, CONSORT Group (2005) CONSORT statement: extension to cluster randomised trials. BMJ 328 : 702–708. 22951546
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2015 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Randomized Controlled Trials in Environmental Health Research: Unethical or Underutilized?
- Uptake and Population-Level Impact of Expedited Partner Therapy (EPT) on and : The Washington State Community-Level Randomized Trial of EPT
- Supporting Those Who Go to Fight Ebola
- Evaluation of a Minimally Invasive Cell Sampling Device Coupled with Assessment of Trefoil Factor 3 Expression for Diagnosing Barrett's Esophagus: A Multi-Center Case–Control Study
- Hormonal Contraception and the Risk of HIV Acquisition: An Individual Participant Data Meta-analysis
- Association between Respiratory Syncytial Virus Activity and Pneumococcal Disease in Infants: A Time Series Analysis of US Hospitalization Data
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Association between Respiratory Syncytial Virus Activity and Pneumococcal Disease in Infants: A Time Series Analysis of US Hospitalization Data
- Randomized Controlled Trials in Environmental Health Research: Unethical or Underutilized?
- Supporting Those Who Go to Fight Ebola
- Evaluation of a Minimally Invasive Cell Sampling Device Coupled with Assessment of Trefoil Factor 3 Expression for Diagnosing Barrett's Esophagus: A Multi-Center Case–Control Study
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



