-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The ITA.LI.CA Staging System: A Novel Staging System for Hepatocellular Carcinoma
In this Perspective, Neehar Parikh and Amit Singal discuss the advantages of the ITA.LI.CA staging system for prognoses of liver cancer developed by Alessandro Vitale and colleagues.
Published in the journal: The ITA.LI.CA Staging System: A Novel Staging System for Hepatocellular Carcinoma. PLoS Med 13(4): e32767. doi:10.1371/journal.pmed.1002005
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.1002005Summary
In this Perspective, Neehar Parikh and Amit Singal discuss the advantages of the ITA.LI.CA staging system for prognoses of liver cancer developed by Alessandro Vitale and colleagues.
Hepatocellular carcinoma (HCC) is an increasingly common and highly morbid malignancy. Appropriate tumor staging is crucial to inform prognosis and guide treatment decisions in clinical practice; however, staging for HCC can be complex because of many patient-level factors that can impact prognosis and treatment eligibility. HCC is a unique malignancy in that it typically occurs in the setting of underlying organ dysfunction (i.e., cirrhosis), so most staging systems take both tumor burden and the degree of underlying liver dysfunction into account for prognostication. The most commonly used staging systems for HCC include the Barcelona Clinic Liver Cancer (BCLC) system [1], the Hong Kong Liver Cancer (HKLC) system [2], the Cancer of the Liver Italian Program (CLIP) system [3], the Japan Integrated Staging (JIS) system [4], and the Model to Estimate Survival in Ambulatory HCC patients (MESIAH) (Table 1) [5]. However, there has been a lack of consensus regarding the optimal staging system, and none is universally accepted. Most existing systems were derived from single-center data, lack prospective or external validation, and lack granularity in intermediate - and advanced-stage patients. In this issue of PLOS Medicine, Alessandro Vitale and colleagues detail the derivation and validation of a novel staging system for HCC, the ITA.LI.CA system [6]. The ITA.LI.CA system attempts to address many deficiencies of prior staging systems and demonstrates better discriminant ability for predicting survival than existing HCC staging systems in both internal and external validation cohorts.
Tab. 1. Existing staging systems for hepatocellular carcinoma. 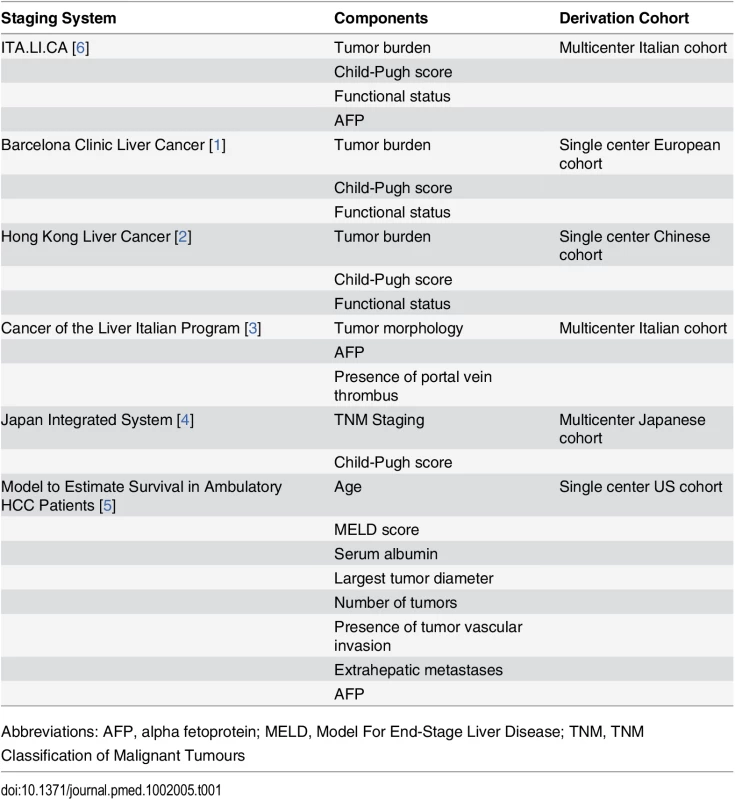
Abbreviations: AFP, alpha fetoprotein; MELD, Model For End-Stage Liver Disease; TNM, TNM Classification of Malignant Tumours The ITA.LI.CA system was derived from a prospective multicenter database of over 5,000 HCC patients from Italy. The majority of patients in the cohort had hepatitis C infection, nearly all (97%) had good performance status, and three-fourths had well-compensated cirrhosis. External validation was performed using data from a Taiwanese cohort of over 2,600 patients, with the primary etiology of liver disease for patients in this cohort being chronic hepatitis B infection. Using a priori variable selection based on prior staging systems and a literature review, the authors derived a model that uses a prognostic score based upon tumor burden (categories of 0, A, B1–3, and C), functional status, Child-Pugh score, and alpha fetoprotein (AFP) concentration (≤1,000 or >1,000 ng/ml). The model had better discriminant ability than any of the existing staging systems in the training, internal validation, and external validation cohorts (c-statistic values being 0.72, 0.71, and 0.78, respectively).
The BCLC staging system is currently the most widely accepted staging system and has been endorsed by the American Association for the Study of Liver Disease (AASLD) and European Association for the Study of the Liver (EASL) [7,8]. Though some aspects of the ITA.LI.CA system are rooted in the BCLC, it is distinct in several important ways: first, in subclassifying BCLC stage B patients into B1, B2, and B3 categories based on degree of intrahepatic tumor burden; second, in differentiating patients with intrahepatic and extrahepatic metastases; and finally, by incorporating the serum biomarker AFP. In the BCLC system, all patients with liver-isolated disease, without metastases or vascular invasion, are grouped together as BCLC stage B [1]. However, differential survival and locoregional treatment allocation for BCLC stage B patients has been demonstrated in several studies [9,10]. For example, distinguishing whether BCLC stage B patients are within (B2) or beyond (B3) Milan criteria is important when considering liver transplantation. Similarly, recent data suggest prognosis in patients with extrahepatic metastases is worse than those with intrahepatic metastases, so the differentiation in the ITA.LI.CA system, essentially subclassifying the BCLC stage C patients, adds further granularity to estimating prognosis [11]. Finally, AFP is not part of the BCLC staging system but can serve as a surrogate for occult vascular invasion, distant metastases, or aggressive tumor biology. Patients with an AFP > 500 ng/ml have a higher risk of recurrence post-transplant as well as a lower likelihood of response to locoregional therapy [12]. These three important distinctions as compared to the BCLC system likely explain, in part, the higher prognostic accuracy of the ITA.LI.CA staging system in derivation and validation cohorts.
Although the model demonstrated good prognostic discrimination among study patients, it should be noted that most patients in both cohorts had good performance status, compensated cirrhosis, and early or intermediate stage tumors. It is unclear if the ITA.LI.CA staging system would perform as well in cohorts with high rates of hepatic decompensation, poor performance status, and/or advanced tumor stage—subgroups that currently account for the majority of HCC patients in several countries, including the United States. Further, very few patients in this study—less than 2% in the derivation cohort and none in the external validation cohort—underwent liver transplantation, a curative therapy for both the tumor and underlying cirrhosis that plays a crucial role in the management of HCC patients.
Potential future steps in further refinement and validation of the ITA.LI.CA staging system include prospectively assigning treatment allocation recommendations to patients in different stages and validation in more contemporary cohorts, in which transplantation or systemic therapies are utilized to a greater extent.
Conclusions
The authors of the ITA.LI.CA staging system have introduced a novel staging system for HCC, building on existing staging systems. This system helps in better differentiation of intermediate and advanced stage patients, and the prognostic model contains several important factors that are clinically relevant in the care of patients with HCC. This system is an important iteration in the evolution of staging for HCC, and, while it enters a crowded field, the ITA.LI.CA staging system is a worthy entrant.
Zdroje
1. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–38. Epub 1999/10/13. doi: 10.1055/s-2007-1007122 10518312.
2. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–700.e3. doi: 10.1053/j.gastro.2014.02.032 24583061.
3. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28(3):751–5. doi: 10.1002/hep.510280322 9731568.
4. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38(3):207–15. doi: 10.1007/s005350300038 12673442.
5. Yang JD, Kim WR, Park KW, Chaiteerakij R, Kim B, Sanderson SO, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56(2):614–21. doi: 10.1002/hep.25680 22370914; PubMed Central PMCID: PMC3564594.
6. Farinati F, Vitale A, Spolverato G, Pawlik TM, Huo T-l, Lee Y-H, et al. Development and validation of a new prognostic system for patients with hepatocellular carcinoma. PLoS Med. 2016;13(4): e1002006.
7. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43. doi: 10.1016/j.jhep.2011.12.001 22424438.
8. Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. doi: 10.1002/hep.24199 21374666; PubMed Central PMCID: PMC3084991.
9. Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–59. doi: 10.1055/s-0032-1329906 23397536.
10. Arizumi T, Ueshima K, Iwanishi M, Minami T, Chishina H, Kono M, et al. Validation of a Modified Substaging System (Kinki Criteria) for Patients with Intermediate-Stage Hepatocellular Carcinoma. Oncology. 2015;89 Suppl 2 : 47–52. doi: 10.1159/000440631 26584036.
11. Yoo JJ, Lee JH, Lee SH, Lee M, Lee DH, Cho Y, et al. Comparison of the effects of transarterial chemoembolization for advanced hepatocellular carcinoma between patients with and without extrahepatic metastases. PLoS ONE. 2014;9(11):e113926. doi: 10.1371/journal.pone.0113926 25427152; PubMed Central PMCID: PMC4245068.
12. Mehta N, Sarkar M, Dodge JL, Fidelman N, Roberts JP, Yao FY. Intention to treat outcome of T1 hepatocellular carcinoma with the "wait and not ablate" approach until meeting T2 criteria for liver transplant listing. Liver Transpl. 2016;22(2):178–87. doi: 10.1002/lt.24360 26479422.
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Why Are Some Population Interventions for Diet and Obesity More Equitable and Effective Than Others? The Role of Individual Agency
- Risk of Bias in Systematic Reviews of Non-Randomized Studies of Adverse Cardiovascular Effects of Thiazolidinediones and Cyclooxygenase-2 Inhibitors: Application of a New Cochrane Risk of Bias Tool
- The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control
- The Association between Sulfonylurea Use and All-Cause and Cardiovascular Mortality: A Meta-Analysis with Trial Sequential Analysis of Randomized Clinical Trials
- Disentangling the Association between Statins, Cholesterol, and Colorectal Cancer: A Nested Case-Control Study
- Gender Differences in Homicide of Neonates, Infants, and Children under 5 y in South Africa: Results from the Cross-Sectional 2009 National Child Homicide Study
- Mobile Phones As Surveillance Tools: Implementing and Evaluating a Large-Scale Intersectoral Surveillance System for Rabies in Tanzania
- Building Learning Health Systems to Accelerate Research and Improve Outcomes of Clinical Care in Low- and Middle-Income Countries
- The Future of the RTS,S/AS01 Malaria Vaccine: An Alternative Development Plan
- Birth “Out-of-Hours”: An Evaluation of Obstetric Practice and Outcome According to the Presence of Senior Obstetricians on the Labour Ward
- A Nested Case–Control Study of Metabolically Defined Body Size Phenotypes and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC)
- Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma
- Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries
- The Impact of Hotspot-Targeted Interventions on Malaria Transmission in Rachuonyo South District in the Western Kenyan Highlands: A Cluster-Randomized Controlled Trial
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
- Clinical Implications of Cancer Genomics: A Call for Papers
- The ITA.LI.CA Staging System: A Novel Staging System for Hepatocellular Carcinoma
- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Child Homicide: A Global Public Health Concern
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Observational Evidence of For-Profit Delivery and Inferior Nursing Home Care: When Is There Enough Evidence for Policy Change?
- Experimental Treatment of Ebola Virus Disease with TKM-130803: A Single-Arm Phase 2 Clinical Trial
- The Chernobyl Disaster and Beyond: Implications of the Sendai Framework for Disaster Risk Reduction 2015–2030
- Is There Evidence of Poorer Birth Outcomes for Mothers and Babies When the Most Senior Obstetrician Is Not On Site?
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



