-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
In a document analysis of trial protocols and publications, Erik von Elm and colleagues investigate the potential impact of publication agreements between industry sponsors and academic investigators.
Published in the journal: Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials. PLoS Med 13(6): e32767. doi:10.1371/journal.pmed.1002046
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002046Summary
In a document analysis of trial protocols and publications, Erik von Elm and colleagues investigate the potential impact of publication agreements between industry sponsors and academic investigators.
Introduction
Many randomized clinical trials (RCTs) are designed and sponsored by for-profit companies [1–3]. Companies typically contract academic investigators to identify, recruit, and manage patients. Clinical research under these circumstances is a business transaction that bears the potential for conflicts of interest, including those regarding the publication of trial results [4].
Academic investigators’ careers depend on publication of research results in peer-reviewed journals. For-profit companies aim for approval of new products by regulating agencies or expansion of product indications [5]. Publication of favourable results is also part of companies’ marketing strategy [6–8]. Industry-sponsored trials are less likely to be published than those not sponsored by industry [2,3], the likelihood of publication of outcome data can be related to the direction of the results [1,9,10], and discrepancies between trial reports submitted to regulatory agencies and journal publications occur [11].
To promote transparency in the arrangements between industry and academia, reporting guidelines recommend disclosure of potential conflicts of interest of authors and funders [12,13]. Complete reporting helps readers of journal articles judge how potential conflicts of interest may influence the reporting of trial results. Complete reporting includes details regarding both (i) agreements between industry and study investigators that may affect the publication of results and (ii) co-authorship of industry employees. Several high-impact journals insist that authors of trial reports disclose the sponsor’s role in the study [14–16]. The International Committee of Medical Journal Editors (ICMJE) recommends that investigators should avoid agreements with sponsors that interfere with full access to the dataset and the investigators’ ability to conduct analyses, interpret the results, and submit the manuscript for publication [17]. Further, it has been suggested that journal editors should review protocols or contracts with a focus on publication rights: “Editors may choose not to consider an article if a sponsor has asserted control over the authors’ right to publish” [18].
Previous studies have documented constraints on the publication rights of academic investigators in industry-sponsored RCTs [19,20]. Gøtzsche et al. investigated such constraints in 88 RCT protocols approved by two Danish research ethics committees—44 in 1994/1995 and 44 in 2004—and subsequent journal publications. They found that industry sponsors could have prevented publication in half of the trials. However, this study was restricted to a relatively small sample from a single country [21].
In this article, we consider agreements on publication rights in a cohort of RCT protocols approved by six research ethics committees (RECs) between 13 January 2000 and 25 November 2003 in three countries and the reporting of these agreements in corresponding publications. We also investigate co-authorship by industry employees and the concordance of statements regarding publication rights between trial protocols and corresponding publications.
Methods
Ethical Approval
The participating RECs approved the study or explicitly stated that no ethical approval was necessary.
Aims
We aimed to investigate (i) the existence and types of publication agreements in trial protocols, (ii) the completeness and consistency of the reporting of these agreements in subsequent publications, and (iii) the frequency of co-authorship of industry employees.
Study Design
Previous publications describe in detail the design of this retrospective cohort study [2,22]. In brief, we examined RCT protocols approved between 13 January 2000 and 25 November 2003 by six RECs in Switzerland (Basel, Lucerne, Zurich, and Lausanne), Germany (Freiburg), and Canada (Hamilton) (S1 Table). Of these RECs, all but one (Lucerne) were responsible for human research in large university centres and hospitals in their respective catchment areas. The REC in Lucerne covered an academic teaching hospital and other health research in Central Switzerland. We used our existing contacts to establish this convenience sample of RECs. We determined the completion status and publication history of RCTs as of 27 April 2013 by using information available in REC files and by conducting comprehensive searches for corresponding publications in databases (MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, CINAHL, the Allied and Complementary Medicine Database, Google Scholar, and topic-specific databases) and trial registers (ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform). Two independent investigators determined whether identified publications matched the corresponding protocol. In the case of unclear trial completion or publication status, the REC in charge contacted the investigators using a standardized questionnaire.
Eligibility Criteria for Protocols and Subsequent Publications
In the present analysis, we considered only RCT protocols that clearly documented industry involvement in the design, support, or conduct of the trial (e.g., sponsorship, logistical support, partial funding, or supply of a drug/device). We excluded protocols of studies that (i) compared different doses or routes of administration of the same drug (such as early dose-finding studies), (ii) enrolled only healthy volunteers, (iii) were never started, or (iv) were still ongoing as of April 27, 2013. With respect to multiple corresponding publications, we included only the primary full publication that reported the results from the randomized comparison, and excluded research letters, letters to the editor, and conference abstracts. In the case of more than one full publication, we considered the first publication that included results for the RCT’s primary outcomes.
Definitions
We defined documentation of publication rights as any statement about an agreement between an industry sponsor and the academic investigators regarding the publication of trial results. We classified eligible RCT protocols according to the sponsoring party (investigator or industry) that assumed formal responsibility for the conduct of the trial; the sponsoring party was identified from the following types of information in the protocol: a clearly named sponsor, a prominently displayed company or institution logo, the affiliations of protocol authors, statements about data ownership or publication rights, and statements about full funding by industry or public funding agencies. Disagreements were resolved by consensus or by involving a third investigator as arbitrator (B. K., M. B., or E. v. E.). Investigator-sponsored trials eligible for this study had at least some industry funding, provision of study drugs, or logistical support from industry; industry did not, however, entirely fund these trials.
Information Collected about Publication Agreements
We recorded the presence or absence of any publication agreement between the academic investigators and industry documented in protocols and reported in publications. If reviewers identified such documentation, they assigned it to the most appropriate of the following four mutually exclusive categories: (i) The industry partner retains the right to disapprove any submission for publication (this included any publication using trial data [abstracts or manuscripts for journal publications]). (ii) The industry partner retains the right to at least review and comment on any manuscript/abstract before publication (further constraints may have been possible, but were not clear from the source). (iii) No constraints by the industry partner; in particular, no right to withhold the submission from publication. (iv) Reference to a separate publication agreement document between the industry partner and the investigator, with no further details. Prompted by a reviewer’s comment and considering the considerable ambiguity of original statements, we collapsed categories (i) and (ii) in the analyses herein and labelled the combined category as follows: the industry partner retains the right to disapprove or at least review any abstract or manuscript for publication. Examples of publication agreements in trial protocols are provided in S2 Table.
Data Extraction Process and Search for Publications
Twelve investigators trained in clinical research methodology extracted data from the included trial protocols and correspondence between the RECs and local investigators at the respective centres. To increase consistency in data extraction, pairs of two reviewers extracted the initial 30% of data independently and compared results to achieve consensus; disagreements were resolved by discussion and consultation with an arbitrator (B. K., M. B., or E. v. E.) if necessary. If the available REC files provided no information about the publication status of a trial, we conducted comprehensive searches of electronic databases and surveyed investigators to find any corresponding publications as of April 27, 2013. Twenty-two investigators trained in clinical research methodology extracted data from all corresponding publications, independently and in duplicate; disagreements were resolved by discussion to achieve consensus or by consultation with an arbitrator (B. K., M. B., or E. v. E.). None of the reviewers among the teams extracted data from both a protocol and its corresponding publication.
Statistical Analysis
We summarized binary data as frequencies and proportions and continuous data as medians and interquartile ranges. Publication agreements are described for protocols and corresponding publications separately. To explore differences regarding publication agreements, we stratified by industry versus investigator sponsorship. Prompted by reviewer comments, we additionally explored the differences regarding publication agreements stratified by the extent of industry funding, categorized as (i) provision of medication/device only, (ii) partially funded, beyond medication/device but not whole trial, and (iii) full funding of trial. When analyzing concordance between protocols and publications, we considered only those protocols in which the publication agreement was accessible to us. We used the statistical programme R version 3.1.2 (https://www.r-project.org/) for all analyses. Data are deposited in the Dryad Digital Repository: doi:10.5061/dryad.3s6j7 [23].
Results
Trial Characteristics and Publications
Of 894 RCT protocols involving patients approved by the six RECs between 13 January 2000 and 25 November 2003, 247 (27.6%) had no industry involvement and were excluded. We included 647 (72.4%) protocols, of which 456 (70.5%) mentioned publication agreements (Fig 1). RCTs with protocols that mentioned agreements were on average larger (median sample size 360 versus 222) and more often multicentre trials (439/456 [96.3%] versus 150/191 [78.5%]). Most RCT protocols that mentioned an agreement (417/456 [91.4%]) were industry sponsored (Table 1). In all 39 investigator-sponsored trial protocols that mentioned an agreement, one or more industry partners provided drugs or logistical support. For the included 647 RCTs, we found 388 (60.0%) full journal articles (328 [60.0%] for 547 industry-sponsored RCTs; 60 [60%] for 100 investigator-sponsored RCTs).
Fig. 1. Study flow of RCT protocols and publications. 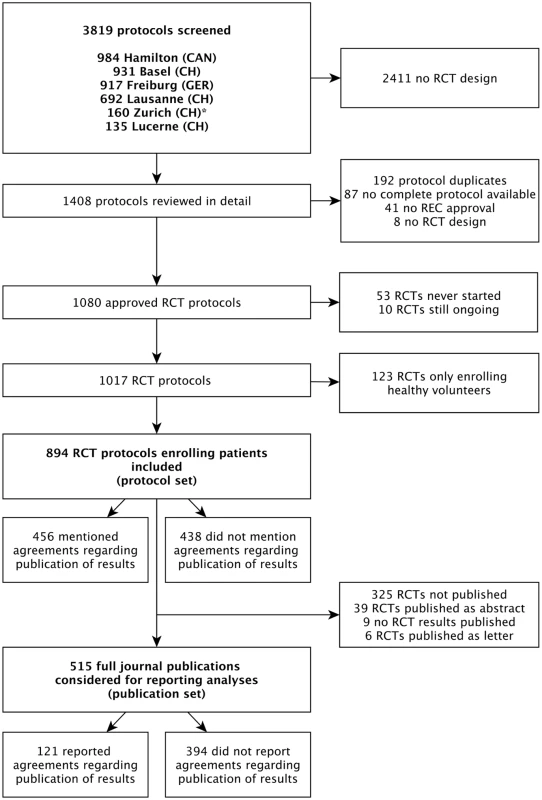
For the Zurich REC, we included RCT protocols only from the two subsidiary RECs responsible for paediatric and surgical RCTs. Tab. 1. Characteristics of included randomized clinical trials as extracted from trial protocols. 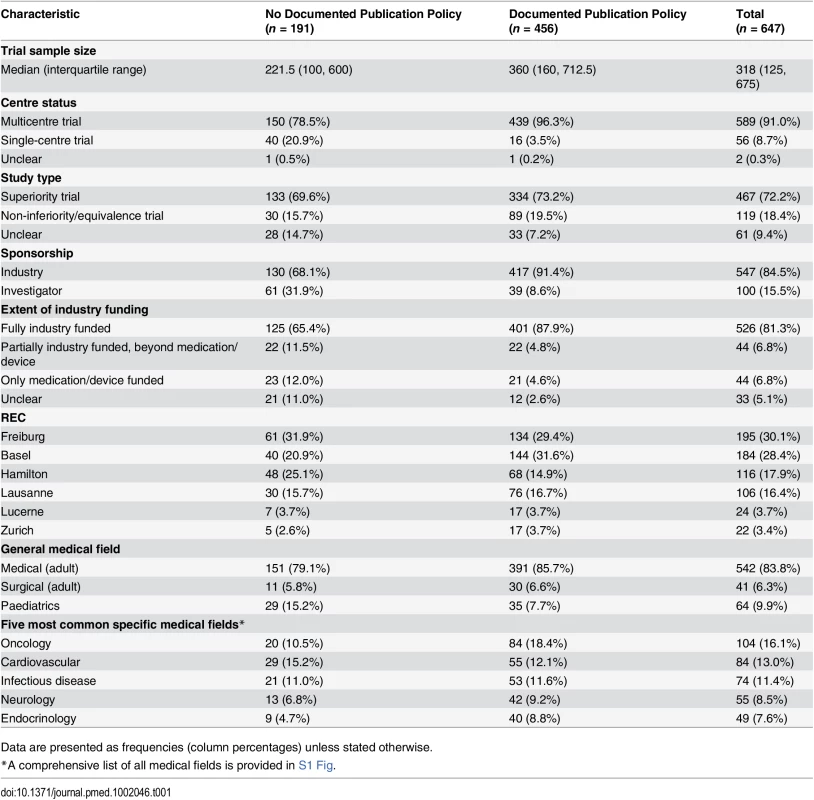
Data are presented as frequencies (column percentages) unless stated otherwise. Types of Agreements Mentioned in Protocols and Publications
In 393 of 456 (86.2%) protocols, the industry partner had the right to disapprove or at least to review publications proposed by academic investigators (Table 2). Publication agreements without any constraints by the industry partner on the academic investigator were documented in 39 (8.6%) RCT protocols (14/417 [3.4%] industry-sponsored RCTs; 25/39 [64.1%] investigator-sponsored RCTs). Twenty-four (5.3%) protocols mentioned separate agreement documents that were not accessible to us.
Tab. 2. Types of publication agreements and industry employee co-authorship as documented in trial protocols and reported in journal publications. 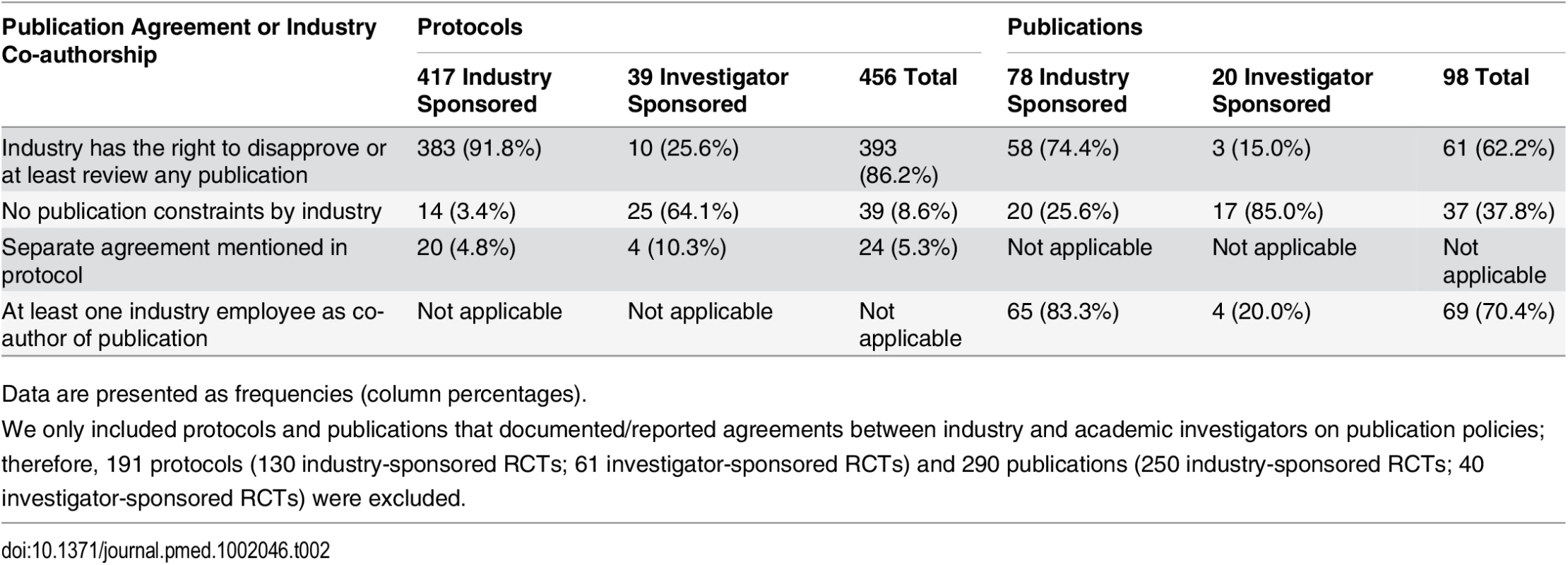
Data are presented as frequencies (column percentages). Of 388 full journal publications, 98 (25.3%) mentioned an agreement regarding the publication of trial results and 290 (74.7%) did not. For 61 of the 98 (62.2%) trials that mentioned an agreement, authors reported that the industry partner had the right to disapprove or at least to review any publication. In 37 (37.8%) publications, the author statement suggested unrestricted publication rights (20/78 [25.6%] industry-sponsored RCTs; 17/20 [85.0%] investigator-sponsored RCTs) (Table 2). The distribution of RCTs with different types of publication agreements by extent of industry funding is displayed in S3 Table.
In 260 of 388 (67.0%) publications, at least one co-author was an industry employee (253/328 [77.1%] industry-sponsored trials, 7/60 [11.7%] investigator-sponsored trials). The median proportion of industry employees among all authors for journal publications was 25% (interquartile range, 17% to 40%); it was 30% for industry-sponsored RCTs and 10% for investigator-sponsored RCTs. In 14 of the 37 (37.8%) publications in which there was a statement that suggested unrestricted publication rights, at least one co-author was an industry employee.
Concordance between Protocols and Publications
Table 3 displays the concordance of information about publication agreements between protocols and subsequent journal publications. For this analysis, we excluded 191 protocols that did not mention a publication agreement and 24 protocols that mentioned a separate publication agreement document that was not accessible to us. This resulted in 432 protocols; of these, 268 had a corresponding journal publication. In 240 of these 268 (89.6%) protocols, the industry partner had the right to disapprove or at least review publications (Table 3). In most cases (177 of 240 [73.8%]), this agreement was not mentioned in the subsequent publication. Of all 71 journal articles that mentioned a publication agreement, 52 (73.2%) included a statement that was in concordance with the documentation in the protocol. In 25 publications, the author statement suggested no constraints, but for 18 of these publications, the corresponding protocol documented a restricting agreement (see S4 Table for excerpts from protocols and publications). In 28 (10.4%) of 268 protocols, the protocol documented that academic investigators were free of any constraints. This was reflected in the corresponding publications in seven instances (25.0%). In six of 268 publications (2.2%), the authors’ statement of unrestricted publication rights was in concordance with what was documented in the protocol and no industry employee was listed as a co-author; five of these were investigator-sponsored RCTs.
Tab. 3. Protocols with publication agreements and the reporting of these agreements in subsequent journal publications. 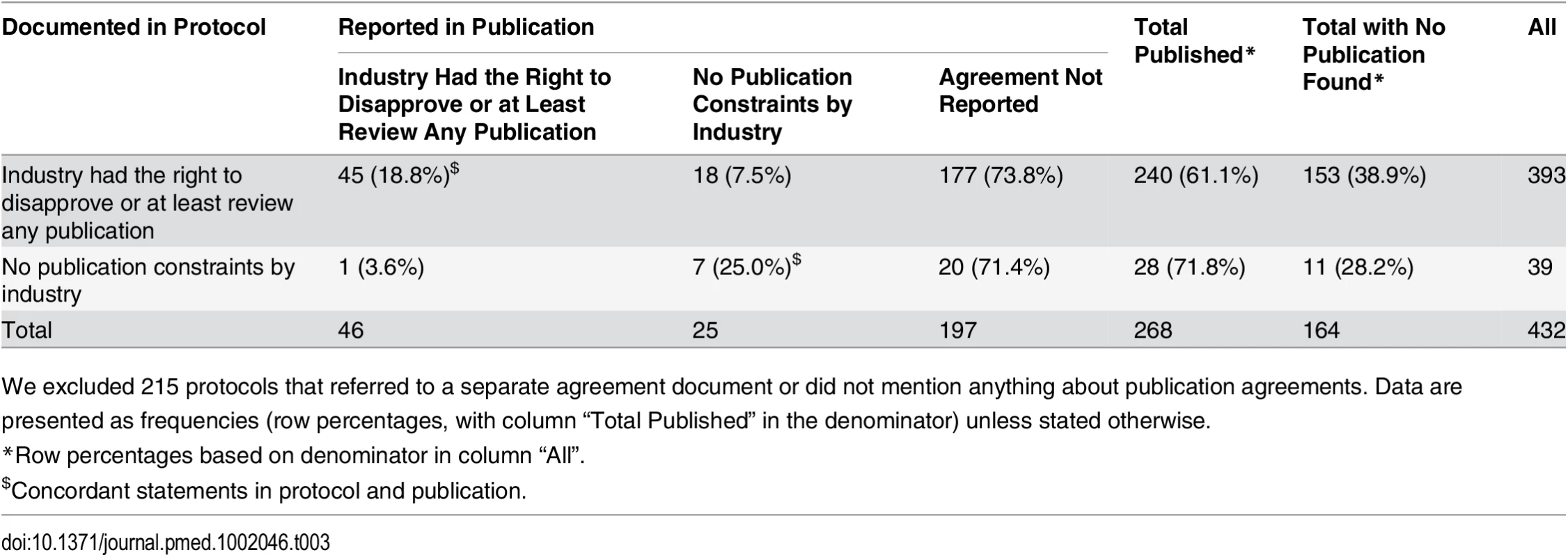
We excluded 215 protocols that referred to a separate agreement document or did not mention anything about publication agreements. Data are presented as frequencies (row percentages, with column “Total Published” in the denominator) unless stated otherwise. Discussion
Summary of Findings
Of 647 RCT protocols that were approved between 13 January 2000 and 25 November 2003 by six RECs in three countries and sponsored or supported by industry, 86% documented publication constraints reserving the right of the industry partner to review the manuscript or allowing the industry partner to disapprove the manuscript. Most agreements (74%) documented in protocols remained unreported in subsequent publications. In 18 instances, author statements in publications suggested no constraints by industry partners while protocols actually documented such constraints. Moreover, at least one co-author was an industry employee in two-thirds of journal publications and in one-third of publications in which statements suggested no publication constraints for academic authors. This suggests that, irrespective of the agreement in the protocol, the industry partner could influence publication content and submission decisions through co-authorship.
Strengths and Limitations
Our data were collected as part of a large international cohort involving six RECs that allowed full access to trial protocols and filed correspondence between the local academic investigator and the REC in charge [2,22]; only investigators’ brochures were exempt from assessment. Because unrestricted access to trial protocols is necessary to maintain scientific rigor [24], we did not ask trialists and sponsors for permission to access their protocols. Doing so could have introduced bias because those with poor reporting practices may not have allowed additional scrutiny. Further strengths of our study include the use of trained methodologists for data collection and independent and duplicate data extraction from identified publications. Finally, our sample included RCTs from various fields of clinical medicine, thus enhancing the generalizability of our results.
Our study has limitations: First, separate agreement documents or legal contracts specifying publication rights were not available to us. However, only 5% of protocols that mentioned publication agreements referred to such separate documents. To the extent that protocols failed to refer to separate documents, we may have underestimated the overall prevalence of publication agreements. Second, we did not approach the local investigators submitting protocols to the REC to inquire whether their publication rights were actually constrained by the industry partner. Therefore, we cannot estimate to what extent the documented agreements impacted the content of publications or led to delays in publication or to non-publication of trial results. Third, the wording of the agreements in protocols was very heterogeneous. We extracted the original text only to provide examples but used our judgement to classify the statements according to four prespecified categories. Because of the considerable ambiguity of the original statements, we eventually collapsed two categories into one. Fourth, we used a convenience sample of six RECs that were—to our knowledge—not in any way particular. Five of them had authority for large university hospitals in their catchment areas, and one was responsible for a large teaching hospital. We cannot say whether these RECs are representative of other RECs in their own or other countries. Fifth, all included protocols were approved from 13 January 2000 and 25 November 2003, and half of the included publications were from the year 2007 or before, leaving the possibility that a substantial proportion of our sample no longer reflects current practices of reporting of publication agreements. In particular, the increasing demand for transparency in the reporting of clinical trials through guidelines and journal policies may have positively influenced current practice. Sixth, exploring the influence of industry co-authors in the writing process would require additional qualitative research, for example, interviews with author teams; however, this was not part of the present study. Seventh, we did not collect information regarding associated data-sharing agreements. The nature of such agreements might have influenced the interpretation of the publication agreements that were the focus of our study. Finally, we previously published the protocol of the overall project but not details about the present sub-study [22]. Therefore, we kept our analysis descriptive while including our data extraction forms detailing all collected variables in S5 Table for interested readers.
Comparison with Other Studies
Gøtzsche et al. reported that 91% of 44 protocols from industry-sponsored trials approved by two RECs in 1994/1995 included constraints on publication rights, but none of the associated publications reported these constraints [21]. In our larger and more recent sample, authors provided statements about agreements on publication rights in about a quarter of journal publications.
Based on survey data from 108 US medical schools, Schulman et al. reported that academic institutions routinely engage in industry-sponsored research that fails to adhere to ICMJE guidelines regarding trial design, access to data, and publication rights [20]. In another survey focusing on institutional policies, Mello et al. approached 122 US medical schools in 2004 [19]. Of the 107 schools that participated, approximately 85% stated that they would not approve contractual provisions giving industry sponsors the authority to revise manuscripts or decide whether results should be published [19]. In the remaining 15%, however, the responsible office would allow such constraints. In our sample, 86% of industry-sponsored trial protocols documented that the sponsor retained the right to disapprove or at least review any resulting publication. The key role of academic medical centres in maintaining scientific integrity has been outlined in a policy proposal regarding their partnership with industry sponsors [25]. This proposal does not, however, explicitly address the issue of publication rights.
Implications
Previous publications have documented misleading presentations of evidence, sometimes referred to as “spin” [26]. In industry-supported trials, spin can be due to conflicts of interest resulting from the funding arrangements [27]. Publication restrictions represent another form of conflict of interest, as reflected in ICMJE’s recent recommendation: “Authors should avoid entering in to agreements with study sponsors, both for-profit and non-profit, that interfere with authors’ access to all of the study’s data or that interfere with their ability to analyze and interpret the data and to prepare and publish manuscripts independently when and where they choose” [17]. Publication agreements may contribute to the presentation of results in misleading ways that favour the interests of a sponsor [28]. This is clearly a possibility when a trial’s industry sponsor has the right of disapproval, which restricts academic freedom in general.
Industry funding disclosed in trial manuscripts may represent a red flag for editors who are to decide about acceptance. If publication restrictions are acknowledged, this may be another. In turn, omission of such statements—or the presence of content that contradicts existing publication restrictions—deprives clinicians, guideline developers, and policy makers of such an alert, and may be deliberate.
We acknowledge that there are instances, in which restricting the investigators’ right to publish is appropriate, e.g., separate publication of subsets of data from a multicentre trial could be confusing. Such restrictions should, however, be limited in time, e.g., until completion of the trial, including publication of its main results. Pending patent applications may be another justification for a time-limited delay of publication, but can certainly not excuse withholding it completely. We suggest that a general reference to confidentiality in the protocol without further explanation is not sufficient to justify restriction or delay of publication.
Besides the issues of publication rights, co-authorship by industry employees on published trial reports allows the industry editorial influence. This issue also needs consideration in the context of possible reporting bias—in the light of our findings, journal editors should be aware of this issue.
The cooperation between industry and academic investigators can be very fruitful and can lead to great improvements in medical care. RECs have a crucial role in ensuring the ethical conduct of clinical research and should therefore also consider whether commercial sponsors’ rights to disapprove publication of trial results hamper the scientific process and erode trust in clinical research. Additionally, mandatory documentation of publication agreements in protocols (as proposed recently [29]) and trial registration could improve transparency. Further studies investigating publication agreements between academia and industry should also consider contracts in addition to approved protocols.
Conclusions
Publication agreements are common in protocols of industry-sponsored RCTs. Journal publications of RCTs rarely provide readers with information about existing agreements, and when they do, statements can be discrepant with information in the corresponding trial protocols. Publication agreements constraining academic authors’ independence, and the incomplete reporting of such agreements in publications, may corrupt the scientific evidence base established by RCTs.
Supporting Information
Zdroje
1. Chan A-W, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291 : 2457–2465. doi: 10.1001/jama.291.20.2457 15161896
2. Kasenda B, von Elm E, You J, Blümle A, Tomonaga Y, Saccilotto R, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311 : 1045–1051. doi: 10.1001/jama.2014.1361 24618966
3. Jones CW, Handler L, Crowell KE, Keil LG, Weaver MA, Platts-Mills TF. Non-publication of large randomized clinical trials: cross sectional analysis. BMJ. 2013;347:f6104. doi: 10.1136/bmj.f6104 24169943
4. Drazen JM. Institutions, contracts, and academic freedom. N Engl J Med. 2002;347 : 1362–1363. doi: 10.1056/NEJMe020122 12397197
5. Bodenheimer T. Uneasy alliance—clinical investigators and the pharmaceutical industry. N Engl J Med. 2000;342 : 1539–1544. doi: 10.1056/NEJM200005183422024 10816196
6. Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2:e138. doi: 10.1371/journal.pmed.0020138 15916457
7. Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med. 2009;361 : 1963–1971. doi: 10.1056/NEJMsa0906126 19907043
8. Vedula SS, Goldman PS, Rona IJ, Greene TM, Dickersin K. Implementation of a publication strategy in the context of reporting biases. A case study based on new documents from Neurontin litigation. Trials. 2012;13 : 136. doi: 10.1186/1745-6215-13-136 22888801
9. Smyth RMD, Kirkham JJ, Jacoby A, Altman DG, Gamble C, Williamson PR. Frequency and reasons for outcome reporting bias in clinical trials: interviews with trialists. BMJ. 2011;342:c7153. doi: 10.1136/bmj.c7153 21212122
10. Chan A-W, Krleza-Jerić K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ. 2004;171 : 735–740. doi: 10.1503/cmaj.1041086 15451835
11. Rising K, Bacchetti P, Bero L. Reporting bias in drug trials submitted to the Food and Drug Administration: review of publication and presentation. PLoS Med. 2008;5:e217. doi: 10.1371/journal.pmed.0050217 19067477
12. DeAngelis CD. Reporting financial conflicts of interest and relationships between investigators and research sponsors. JAMA. 2001;286 : 89. doi: 10.1001/jama.286.1.89 11434832
13. Davidoff F, DeAngelis CD, Drazen JM, Nicholls MG, Hoey J, Højgaard L, et al. Sponsorship, authorship, and accountability. N Engl J Med. 2001;345 : 825–827. doi: 10.1056/NEJMed010093 11556304
14. The Lancet. Statements, permissions, and signatures. 2016 [cited 13 Feb 2016]. Available: http://www.thelancet.com/lancet/information-for-authors/statements-permissions-signatures.
15. The New England Journal of Medicine. About NEJM—editorial policies. 2016 [cited 13 Feb 2016]. Available: http://www.nejm.org/page/about-nejm/editorial-policies.
16. PLoS Medicine. Submission guidelines. 2016 [cited 13 Feb 2016]. Available: http://journals.plos.org/plosmedicine/s/submission-guidelines.
17. International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. 2015 Dec [cited 31 May 2016]. Available: http://icmje.org/icmje-recommendations.pdf.
18. Peddicord D, Covance, Icon Clinical Research, Inveresk Research Group, Kendle International, Parexel International, et al. Sponsorship, authorship, and accountability. N Engl J Med. 2002;346 : 290–292.
19. Mello MM, Clarridge BR, Studdert DM. Academic medical centers’ standards for clinical-trial agreements with industry. N Engl J Med. 2005;352 : 2202–2210. doi: 10.1056/NEJMsa044115 15917385
20. Schulman KA, Seils DM, Timbie JW, Sugarman J, Dame LA, Weinfurt KP, et al. A national survey of provisions in clinical-trial agreements between medical schools and industry sponsors. N Engl J Med. 2002;347 : 1335–1341. doi: 10.1056/NEJMsa020349 12397192
21. Gøtzsche PC, Hróbjartsson A, Johansen HK, Haahr MT, Altman DG, Chan A-W. Constraints on publication rights in industry-initiated clinical trials. JAMA. 2006;295 : 1645–1646. doi: 10.1001/jama.295.14.1645 16609085
22. Kasenda B, von Elm EB, You J, Blümle A, Tomonaga Y, Saccilotto R, et al. Learning from failure—rationale and design for a study about discontinuation of randomized trials (DISCO study). BMC Med Res Methodol. 2012;12 : 131. doi: 10.1186/1471-2288-12-131 22928744
23. Kasenda B. Data from: Agreements between industry and academia on publication rights: a retrospective study of protocols and publications of randomized clinical trials. Dryad Digital Repository. 2016.
24. Chan A-W, Upshur R, Singh JA, Ghersi D, Chapuis F, Altman DG. Research protocols: waiving confidentiality for the greater good. BMJ. 2006;332 : 1086–1089. 16675819
25. Brennan TA, Rothman DJ, Blank L, Blumenthal D, Chimonas SC, Cohen JJ, et al. Health industry practices that create conflicts of interest: a policy proposal for academic medical centers. JAMA. 2006;295 : 429–433. doi: 10.1001/jama.295.4.429 16434633
26. Boutron I, Dutton S, Ravaud P, Altman DG. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA. 2010;303 : 2058–2064. doi: 10.1001/jama.2010.651 20501928
27. Ross JS, Hill KP, Egilman DS, Krumholz HM. Guest authorship and ghostwriting in publications related to rofecoxib: a case study of industry documents from rofecoxib litigation. JAMA. 2008;299 : 1800–1812. doi: 10.1001/jama.299.15.1800 18413874
28. Nieto A, Mazon A, Pamies R, Linana JJ, Lanuza A, Jiménez FO, et al. Adverse effects of inhaled corticosteroids in funded and nonfunded studies. Arch Intern Med. 2007;167 : 2047–2053. doi: 10.1001/archinte.167.19.2047 17954797
29. Chan A-W, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586 23303884
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Direct-to-consumer Marketing to People with Hemophilia
- Geographical Inequalities and Social and Environmental Risk Factors for Under-Five Mortality in Ghana in 2000 and 2010: Bayesian Spatial Analysis of Census Data
- Phosphodiesterase Type 5 Inhibitors and Risk of Malignant Melanoma: Matched Cohort Study Using Primary Care Data from the UK Clinical Practice Research Datalink
- Age, Spatial, and Temporal Variations in Hospital Admissions with Malaria in Kilifi County, Kenya: A 25-Year Longitudinal Observational Study
- Obesity and Multiple Sclerosis: A Mendelian Randomization Study
- Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study
- Impact Evaluation of a System-Wide Chronic Disease Management Program on Health Service Utilisation: A Propensity-Matched Cohort Study
- Early Childhood Developmental Status in Low- and Middle-Income Countries: National, Regional, and Global Prevalence Estimates Using Predictive Modeling
- Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies
- Exclusive Breastfeeding and Cognition, Executive Function, and Behavioural Disorders in Primary School-Aged Children in Rural South Africa: A Cohort Analysis
- Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study
- Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
- Weighing Evidence from Mendelian Randomization—Early-Life Obesity as a Causal Factor in Multiple Sclerosis?
- A Global Champion for Health—WHO’s Next?
- Malaria Epidemiology in Kilifi, Kenya during the 21st Century: What Next?
- Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement
- Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement
- Why Most Clinical Research Is Not Useful
- Delinking Investment in Antibiotic Research and Development from Sales Revenues: The Challenges of Transforming a Promising Idea into Reality
- Novel Three-Day, Community-Based, Nonpharmacological Group Intervention for Chronic Musculoskeletal Pain (COPERS): A Randomised Clinical Trial
- Prediction of Bladder Outcomes after Traumatic Spinal Cord Injury: A Longitudinal Cohort Study
- The Effect of Sitagliptin on Carotid Artery Atherosclerosis in Type 2 Diabetes: The PROLOGUE Randomized Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Why Most Clinical Research Is Not Useful
- Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
- Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study
- Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



